Abstract
Befitting oxygen's key role in life's processes, hypoxia engages multiple signaling systems that evoke pervasive adaptations. Using surrogate genetics in a powerful biological model, we dissect a poorly understood hypoxia-sensing and signal transduction system. Hypoxia triggers NO-dependent accumulation of cyclic GMP and translocation of cytoplasmic GFP-Relish (an NFκB/Rel transcription factor) to the nucleus in Drosophila S2 cells. An enzyme capable of eliminating NO interrupted signaling specifically when it was targeted to the mitochondria, arguing for a mitochondrial NO signal. Long pretreatment with an inhibitor of nitric oxide synthase (NOS), L-NAME, blocked signaling. However, addition shortly before hypoxia was without effect, suggesting that signaling is supported by the prior action of NOS and is independent of NOS action during hypoxia. We implicated the glutathione adduct, GSNO, as a signaling mediator by showing that overexpression of the cytoplasmic enzyme catalyzing its destruction, GSNOR, blocks signaling, whereas knockdown of this activity caused reporter translocation in the absence of hypoxia. In downstream steps, cGMP accumulated, and calcium-dependent signaling was subsequently activated via cGMP-dependent channels. These findings reveal the use of unconventional steps in an NO pathway involved in sensing hypoxia and initiating signaling.
INTRODUCTION
Oxygen supply can limit the performance of an athlete or the growth of a microorganism. Not surprisingly, evolution has produced remarkable specializations for oxygen delivery and developed adaptive responses to oxygen insufficiency. The limitations of these mechanisms are seen in cardiac infarct and stroke when debilitating damage results from temporary interruption in oxygen supply. Here we explore the activation of a poorly understood hypoxia response pathway that is likely to make important contributions to adaptation to and survival of hypoxic stress.
In mammals, nitric oxide (NO) plays numerous signaling roles contributing to neuronal function, immune defenses and importantly to vascular tone in the circulatory system. Its identification as a vasodilatory molecule (EDRF: endothelial-derived relaxation factor) uncovered the role of NO as a biological regulator, and linked it to physiological activities of tremendous importance to human health (Moncada and Higgs, 2006). Biochemical investigation identified nitric oxide synthase (NOS) and elucidated a signaling pathway in which NO binds to the heme group of the beta subunit of soluble guanylyl cyclase (sGC) to activate cGMP synthesis and subsequently reduce blood vessel tone and blood pressure (Krumenacker et al., 2004). However, this canonical pathway represents only the tip of the iceberg: identification of widespread roles of NO in diverse organisms has led to the realization that it is a general and ancient biological regulator, and multiple pathways of NO generation and action have been suggested. Much has yet to be learned about these other aspect of NO biology.
In addition to activating sGC through a heme group, NO exhibits other types of interaction that are candidates for mediating aspects of its physiological action. Notably, in a process known as nitrosylation, or nitrosation, NO can modify free sulfydryl (thiol) groups of cysteines in proteins to produce nitrosothiols, SNOs. Transfer of the NO adduct from one sulfydryl to another (transnitrosylation) is likely to play a signal transduction role (reviewed in Stamler et al., 2001). Study of this posttranslational modification, which is proposed to be a widespread mediator of signaling, is a relatively new field, and the list of proteins that are modified through nitrosylation is expanding rapidly. Because NO is highly reactive, transport of an NO signal in tissues can be facilitated through reaction with glutathione and movement of the resulting S-nitrosoglutathione (GSNO), which can subsequently signal by modifying thiol groups on target proteins by transnitrosylation (Lipton et al., 2001; Foster et al., 2003). The discovery of GSNO reductase (GSNOR), which reduces GSNO to restore GSH and to eliminate the NO adduct as NH4+ (Jensen et al., 1998), revealed the importance of the control of this NO metabolite. GSNOR-deficient mice show enhanced sensitivity to both damaging consequences of an excessive NO signal, such as increased sensitivity to endotoxic challenge (Liu et al., 2004), and beneficial effects, such as reduced infarct size after occlusion of a coronary artery (Lima et al., 2009). These findings suggest that GSNO participates in NO signaling and that GSNOR down-regulates signaling. Additionally, stimulation of ventilation by a further metabolite of GSNO (Lipton et al., 2001) and pharmacological activities of S-nitrosothiols (Palmer et al., 2007) revealed the hypoxia-mimetic activity of these compounds and suggested that they have an important role as hypoxia signals.
NO contributes to homeostasis of local oxygen levels in mammals and influences the extent of damage after interruption in blood supply. Local hypoxia caused by metabolic demand triggers increased blood perfusion to match oxygen delivery with need. This is accomplished by sophisticated adjustments in the involved capillary bed and in the upstream vessels (Segal, 2005). Investigations into this important phenomenon identified multiple signals contributing to this response, including NO, but the signaling mechanisms are imprecisely defined. NO is involved in the shear-dependent vasodilation that appears to contribute to increases in blood flow to hypoxic tissues (Ungvari et al., 2001) and may make additional contributions. However, the means of NO generation during hypoxia remains enigmatic. One enzyme that generates NO, NOS, uses O2 as a substrate and cannot produce significant NO in severe hypoxia (Leone et al., 1991; Rengasamy and Johns, 1991). In the presence of O2, NOS activity generates NO, which is unstable and is rapidly oxidized to nitrite (NO2− (Ignarro et al., 1993). One intriguing proposal avoids the difficulty of synthesizing NO in low oxygen by suggesting that hemoglobin carries a reservoir of NO in the form of an SNO adduct, generated on the free sulfhydryl group (−SH) on a particular cysteine. This NO is released when oxygen tension is low (reviewed in Stamler et al., 2001). However, a recent genetic test failed to identify obvious defects in mice lacking the cysteine residue of hemoglobin that forms the SNO adduct (Isbell et al., 2008). An alternative proposal suggests that nitrite serves as the intravascular storage molecule for NO and that it is converted to NO in hypoxia (Kim-Shapiro et al., 2006; Feelisch et al., 2008). This proposal is in accord with a long-appreciated possibility that NO can be produced by biological reduction of nitrite (Walters and Taylor, 1965; Fulop et al., 1995; Zweier et al., 1995; Millar et al., 1998). Exogenous addition of nitrite contributes to alleviation of hypoxic symptoms (reviewed in Lundberg et al., 2008), but the mechanism by which this occurs remains unclear.
Despite the usefulness of model organisms for genetic dissection of signaling systems, there has been relatively little analysis of NO signaling in these tractable systems. Such models have much to offer because the myriad of signaling cascades influenced by hypoxia complicate analyses and put a premium on the ability to isolate particular signaling events, as can readily be done in model systems. In Drosophila, exposure to hypoxia has been associated with events as diverse as changes in gene expression (Zhou et al., 2008), a behavioral response (Wingrove and O'Farrell, 1999), activation of Hypoxia-inducible Factor (Lavista-Llanos et al., 2002), inhibition of transcription and translation (Teodoro and O'Farrell, 2003), and suspended animation of Drosophila embryos (Foe and Alberts, 1985; Wingrove and O'Farrell, 1999; Teodoro and O'Farrell, 2003). Although some of these events involve NO (Wingrove and O'Farrell, 1999; Teodoro and O'Farrell, 2003), the pleiotropy of the in vivo response confounded analysis of regulation of this signal. Therefore, we sought a simpler experimental context in which to explore its mechanisms of action. We uncovered a role for NO in the signaling process that activates Relish-dependent innate immune responses in Drosophila (Foley and O'Farrell, 2003). Because aspects of the immune response can be induced in Drosophila S2 cells (Foley and O'Farrell, 2004) and these cells have been successfully used to genetically dissect the inhibition of translation that occurs in hypoxia, using RNA interference (RNAi; Lee et al., 2008), we explored use of these cells to examine the role of NO in hypoxia.
Drosophila S2 cells expressing green fluorescent protein (GFP)-tagged Relish (GFP-Rel) responded to NO donors in culture by translocating this tagged Rel/nuclear factor κB (NF-κB)-type transcription factor from the cytoplasm to the nucleus (Dijkers and O'Farrell, 2007). This NO-induced translocation depends on calcium mobilization and subsequent activation of a calcium-dependent phosphatase, calcineurin. We showed that this pathway also operates in vivo: inhibition of calcineurin in Drosophila larvae interfered with Rel-dependent antimicrobial peptide induction in response to either infection or exposure to an NO donor. This previous work showed that this cell culture offers an assay suited to the functional identification of players downstream of NO. The system also allows convenient use of RNAi, pharmacological modulators, and transgenes (Foley and O'Farrell, 2004; Armknecht et al., 2005). Here, we show that hypoxia also induces GFP-Rel translocation to the nucleus, allowing us to use this model to dissect a cascade of signal transduction from oxygen sensing to molecular output.
MATERIALS AND METHODS
Cells, Flies, Reagents, and Antibodies
Drosophila S2 cells were cultured in Drosophila Schneider's medium (Invitrogen, San Diego, CA) supplemented with 10% heat-inactivated fetal calf serum, penicillin, and streptomycin. The S2 cell line stably expressing N-terminally GFP-tagged Rel under the control of the copper-inducible metallothionine promoter has been described (Foley and O'Farrell, 2004).
Transient expression in S2 cells by the Invitrogen protocol (Invitrogen, Carlsbad, CA) used pAc5/V5HisB expression vector. The Fusarium oxysporum NOR cDNA (N-terminally modified) was a gift from H. Shoun (University of Tokyo, Tokyo, Japan; Nakahara et al., 1993). NOR constructs were amplified and C-terminally hemagglutinin (HA)-tagged. Amplification of a fragment encoding the first 50 aa of Drosophila citrate synthase provided a mitochondrial (mt) localization signal when fused to the N-terminus of NOR (mtNOR). Point mutations (S286V/T243V; Okamoto et al., 1997) were introduced in NOR using the following forward primers: T243: gctggcaacgcagtcatggtaaacatgattgct; S286: tgtcgctaccataccgcggttgcactag. GSNOR (Fdh, Formaldehyde dehydrogenase) was amplified from cDNA with corresponding primers without the stop codon and cloned with a C-terminal HA tag. All constructs were verified by sequencing.
We used COXIV mAb from Abcam (Cambridge, MA), rabbit anti-cGMP has been described (de Vente et al., 1987), HA mAb from BabCo (Richmond, CA).
RNAi Experiments
RNAi treatments of gene-specific regions were carried out according to (Clemens et al., 2000) but without serum-starving the cells, using the Drosophila RNAi library described in Foley and O'Farrell (2004). For generation of double-strand RNA (dsRNA), a Promega T7 Ribomax kit was used (Madison, WI). Primer pairs that were used for the generation of dsRNA: NOS: GATCATGTGGGCATCTTTCC/CCAGGACCAATCAGAATAATGG; GSNOR: AGTGGTGTTGGGACATGAGG/ATGCATTCGAAGGTGTAATCG; cGMP-dependent channels: CG17922: CAAAGAGGAGGAGGAGGAGG/GTGTTCTCCTTGGTTTGAAAGG; Cng: AGCAGAACACACTGTAAGTCGC/TGCCCATTTCAATTATTTCAGC; CG42260: catagatggtgatgcagatgc/cacttgggctgaagtaccacc; cngl: GATACAAGGACTCCGTTATGGG/GAGGGTAGTCAAACCTCAAAGC; Ih: CATATCCAAGCAGAACTCATCG/GGTGTTGATGGAACTCGACG. For the analysis of efficient knockdown of transcripts, total RNA was isolated using TRIzol (Invitrogen).
GFP-Rel Translocation Experiments and Immunofluorescence
Copper sulfate (0.1 mM) was added to normoxic cells for 6 h to induce GFP-Rel. SNAP (Calbiochem, La Jolla, CA) or 8-Br-cGMP (Sigma, St. Louis, MO) were added to normoxic GFP-Rel cells. After appropriate incubations (e.g., hypoxia), cells were allowed to adhere to Superfrost Plus Gold slides (Fisher Scientific, Hampton, NH) for 2 min, before fixation with 2% formaldehyde, and the percentage of cells displaying nuclear GFP fluorescence was counted using a Leica microscope (Wetzler, Germany). Samples were randomized before counting. The minimum number of cells counted in each individual experiment was 800. Data represent the average of at least three independent experiments. For hypoxic treatments, a 1% O2 atmosphere was created by continuously perfusing a box with nitrogen under the control of an O2 sensor (BioSpherics, Redfield, NY), and cells were analyzed after overnight (o/n) incubation. Ca2+ scavenger BAPTA-AM, NO scavenger PTIO (2-phenyl-4,4,5,5,-tetramethylinidazoline-1-oxyl-3-oxide), IBMX (3-isobutyl-1-methylxanthine; Sigma), or sGC inhibitor ODQ (1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one) were added for 30 min before subjecting the cells to 1% O2. NOS inhibitor nitro-l-arginine methyl ester (L-NAME) or the inactive isomer N-nitro-d-arginine-methylester (D-NAME) was added either 30 min or 48 h before subjecting cells to hypoxia. For antibody stainings, S2 cells adhered to concanavalin A–coated coverslips, fixed in PBS containing 4% formaldehyde (Sigma) for 10 min. Mitochondria were stained using mitotrackerRed (Molecular Probes, Eugene, OR; 50 nM for 30 min) before fixation. DNA was visualized using Hoechst 33258 (Molecular Probes). Images were taken using an Olympus (Tokyo, Japan) microscope driven with Deltavision software (Applied Precision, Issaquah, WA). Images were processed using Adobe Photoshop 7.0 (San Jose, CA).
Western Blotting
Western blotting procedure was previously described in Dijkers and O'Farrell (2007).
RESULTS
NO Is Involved in Transducing Responses to Hypoxia
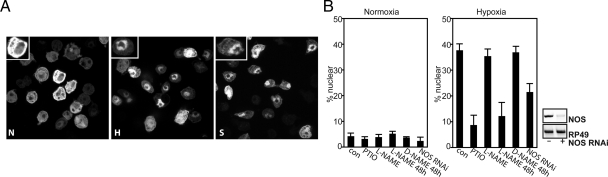
Previous work demonstrated that GFP-Rel expressed in S2 cells translocates to the nucleus in response to NO donor S-nitroso-N-acetylpenicillamine (SNAP; Dijkers and O'Farrell, 2007), and here we show that it also translocates after reduction in O2 levels (Figure 1A). The fraction of cells with GFP-Rel predominantly in the nucleus is easily scored (Figure 1, A and B), providing a robust assay for the contributions of different players to the response.
Figure 1.
NO is involved in transducing responses to hypoxia. (A) S2 cells expressing GFP-Rel were either left untreated (normoxia, N) or cultured in 1% O2 for 8 h (hypoxia, H) or in the presence of SNAP (2 mM, S) for 3 h before fixation and analysis for GFP localization. For illustration, insets represent a higher magnification of a cell in the image. (B) Left, GFP-Rel cells were either left untreated (con), treated with PTIO (0.2 mM, 30 min), L-NAME (1 mM, 30 min), L-NAME (1 mM, 48 h), D-NAME (1 mM, 48 h), or with dsRNAi against NOS (5 d) before and during o/n incubation under normoxic circumstances (normoxia), or hypoxia (1% O2). In this and subsequent figures, the bars are averages of at least three independent experiments; error bars, ±SEM. Right, RNAi against NOS knocks down NOS mRNA. Cells were treated without or with NOS dsRNA, and expression of NOS was analyzed by RT-PCR. RP49 was used as a control for equal input.
We tested whether NO is involved in hypoxia-induced translocation (HIT) of GFP-Rel. Exposure of S2 cells to the NO scavenger PTIO inhibited HIT (Figure 1B), suggesting a requirement for NO. Because NOS requires O2 to generate NO, a role for NOS in hypoxia was in doubt. Indeed, the NOS inhibitor L-NAME had no effect when added immediately before hypoxia or even when added 24 h earlier (not shown). However, after prolonged pretreatment (48 h), L-NAME, but not its inactive isomer D-NAME, inhibited HIT (Figure 1B). In addition, RNAi, a technique routinely and effectively exploited in Drosophila S2 cells (Clemens et al., 2000), was used to effectively knock down NOS levels (Figure 1B, right). A 5-d pretreatment with NOS dsRNA reduced HIT (Figure 1B, left). In support of the specificity of this effect, we also obtained similar reduction of HIT when a nonoverlapping region of NOS was used for RNAi (not shown). Thus, NOS is required, but the requirement for its prolonged inactivation to have an effect in hypoxia suggests that NOS acts before hypoxia. These results and additional findings reported below suggest that HIT is mediated by NO and provides an experimental setting to examine signal generation and transduction.
Scavenging of NO in the Mitochondria Attenuates Responses to Hypoxia
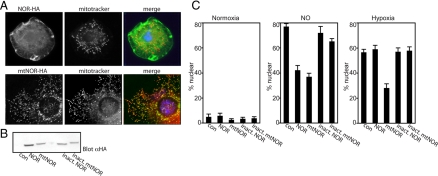
Because the majority of oxygen use occurs in the mitochondria, it seemed likely that this organelle would participate in the response to reductions in oxygen. To explore this possibility, we wanted to locate the site of NO generation or signaling within the cell during hypoxia. To target NO, we developed a novel tool that uses a fungal NOR (Nakahara et al., 1993) to eliminate NO. Neither Drosophila nor mammals possess this enzyme. To analyze NO function in the cytoplasm and mitochondria, we expressed cytoplasmic NOR and mitochondrially targeted NOR (mtNOR) transgenes (Figure 2A). Expression of either of these constructs, but not their catalytically inactive forms (Figure 2B), attenuated nuclear translocation of GFP-Rel in response to SNAP (Figure 2C), demonstrating that fungal NOR is active in Drosophila cells. The failure to completely block the NO response might be quantitative, where NOR activity was not sufficient, or some of the NO might have been in an inaccessible form (e.g., SNO; see below). We then examined the influence of NOR on the response to hypoxia. Strikingly, mtNOR attenuated HIT, whereas cytoplasmic NOR and the inactive NOR controls did not. The selective inhibition of the hypoxic response by mtNOR suggests that NO production or signaling occurs in or in association with the mitochondria. This signal needs to be transduced from the mitochondria to the cytoplasm to promote GFP-Rel translocation. Our result suggests that the NO-dependent signal in the cytosol that promotes GFP-Rel translocation is present in a form other than NO itself because it is not sensitive to cytoplasmic NOR.
Figure 2.
(A) Scavenging of nitric oxide in the mitochondria attenuates responses to hypoxia. Localization of NOR constructs. S2 cells were transfected with NOR-HA or mtNOR-HA construct and stained with mitotracker-Red (50 nM) for 30 min before fixation and staining with anti-HA (green) and DNA staining (Hoechst, blue). (B) S2 cells were cotransfected with GFP-Rel and either empty vector (con), NOR-HA, mtNOR-HA, inactive NOR-HA or inactive mtNOR-HA and analyzed for HA expression by Western blot. (C) Analysis of samples in B for GFP-Rel localization in normoxia or after NO treatment (SNAP, 2 mM, 3 h) or hypoxia (8 h, 1% O2).
GSNO Modulates the Response to Hypoxia
One possible means of transporting the highly reactive NO in tissues is in association with glutathione. Nitrosylation of glutathione produces GSNO, which can diffuse and subsequently modify thiol groups on proteins by transnitrosylation or can be broken down to again produce NO (reviewed in Lipton, 2001). GSNOR (also known as glutathione-dependent formaldehyde dehydrogenase) was found to metabolically reduce GSNO (Steffen et al., 2001). In yeast, deletion of GSNOR is associated with an increase of nitrosylated proteins in the cell (Liu et al., 2001).
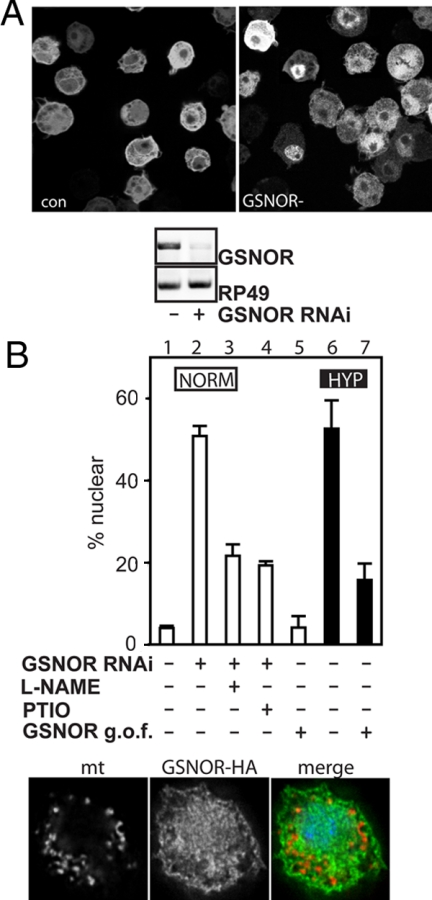
We tested a putative involvement of GSNOR in hypoxia. First we investigated Drosophila GSNOR (Fdh) RNAi, which should elevate GSNO. Five days of GSNOR dsRNA treatment knocked down GSNOR RNA, and, upon GFP-Rel induction, was sufficient to drive GFP-Rel into the nucleus in the absence of hypoxia (Figure 3A, top). Similar results were found when another RNAi was used against a different, nonoverlapping region of GSNOR. Next, we checked whether this response depended on NO. Indeed, the response to GSNOR knockdown was suppressed by treatments that down-regulate an NO signal, either L-NAME pretreatment (48 h) or PTIO treatment (Figure 3B, □). These findings suggest that in the absence of hypoxia there is a low level of NO that would be adequate to mediate signaling except that GSNOR-mediated degradation of GSNO normally increases the threshold of NO required for signaling. Perhaps a higher level of GSNOR might down-regulate signaling in hypoxia.
Figure 3.
GSNO modulates the response to hypoxia. (A) RNAi against GSNOR promotes translocation of GFP-Rel. (A) Top, GFP-Rel cells were cultured without (con) or with dsRNA against GSNOR (GSNOR−) for 5 d before induction of GFP-Rel expression and fixed, and GFP was visualized by fluorescence. Bottom, cells treated as in the top panel were analyzed for GSNOR expression by RT-PCR. RP49 was used as a control for equal input. (B) Top, expression of GFP-Rel was induced (lane 1) or was induced after a 5-d pretreatment with GSNOR dsRNA (lanes 2–4). L-NAME was added for the last 48 h of the preincubation (lane 3); PTIO (0.2 mM) was added at the time of GFP-Rel induction (lane 4). Cells were cotransfected with GFP-Rel and GSNOR (lanes 5 and 7) or empty vector (lane 6), without (lane 5) or with subsequent o/n hypoxia incubation (lanes 6 and 7). Cells were fixed, and the percentage of cells displaying nuclear GFP fluorescence was counted. □, normoxic cells; ■, hypoxic cells. Bottom, localization of GSNOR. Cells were transfected with HA-tagged GSNOR, stained for hemagglutinin (HA, green) and mitochondria (mt, red), using a mitochondria-specific antibody, COXIV, and DNA (Hoechst, blue).
To see whether GSNO breakdown can interfere with HIT, GSNOR was overexpressed and was found to block HIT (Figure 3B, ■). To see where GSNO breakdown takes place, we determined the localization of GSNOR and examined the expression of a C-terminally HA-tagged GSNOR transgene (GSNOR-HA). The HA staining was diffusely distributed, without obvious colocalization with a mitochondrial protein (COXIV). We conclude that GSNOR is extra-mitochondrial and hence that the resulting GSNO breakdown by overexpression of GSNOR does not occur in the mitochondria. These observations suggest an involvement of GSNO in hypoxia-induced signal transduction and that GSNO production or its effect depends on NO.
Involvement of cGMP
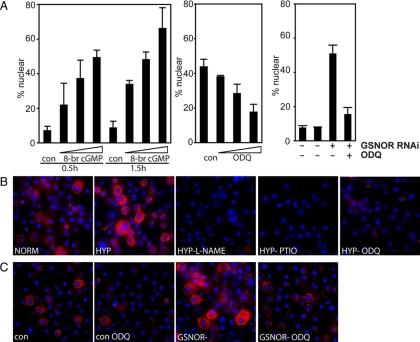
Because soluble guanylyl cyclases (sGCs) are well-recognized targets of NO and are shown to be activated and to generate cGMP in hypoxia (reviewed in Postovit et al., 2005), we assessed if cGMP would be sufficient to promote GFP-Rel translocation and if sGC inhibition would interfere with HIT. Addition of 8Br-cGMP, a cell-permeable cGMP analog, promoted GFP-Rel translocation in normoxic cells (Figure 4A, left), suggesting that cGMP is sufficient for signaling. Addition of the heme-oxidizing sGC inhibitor ODQ suppressed HIT (Figure 4A, middle), suggesting that sGCs mediates at least part of the hypoxic response. Preliminary data with RNAi against sGC in hypoxia supports this conclusion (not shown). The ability of GSNOR RNAi to induce GFP-Rel translocation (Figure 3A) is also inhibited by ODQ (Figure 4A, right), suggesting that GSNO acts through cGMP.
Figure 4.
Hypoxia increases cGMP, a necessary and sufficient signal for GFP-Rel translocation. (A) Left, GFP-Rel cells were either left untreated or treated with 8Br-cGMP (0.1, 0.5, or 1 mM) for the times indicated, fixed and analyzed for GFP localization. Middle, GFP-Rel cells were either left untreated or exposed to sGC inhibitor ODQ (50, 10 or 5 μM) for 30 min before exposure to o/n hypoxia, fixation and analysis of GFP localization. Right, control cells (CON) or cells treated with GSNOR dsRNA for 5 d (GSNOR−) were left untreated o/n or cultured o/n in the presence of ODQ (50 μM) (con ODQ; GSNOR− ODQ, respectively), and GFP-Rel was localized. (B) S2 cells were cultured under normoxia (NORM) or hypoxia (HYP) in the presence of IBMX (0.2 mM) for 2 h before fixation and staining of cGMP (red) and DNA (blue). The effect of L-NAME (48-h pretreatment HYP-L-NAME), PTIO (HYP-PTIO), or ODQ (HYP-ODQ) on hypoxia-induced cGMP staining was determined. (C) Effect of GSNOR RNAi on cGMP levels in the presence or absence of ODQ. cGMP staining of the cells as in B.
To visualize cGMP generation during hypoxia, we examined staining with a cGMP-specific antibody that has been extensively characterized (de Vente et al., 1987). To eliminate cGMP degradation by phosphodiesterases, phosphodiesterase inhibitor IBMX was included. The cGMP staining revealed modest and variable staining of normoxic S2 cells, and this staining was dramatically elevated in hypoxic cells (Figure 4B). Inhibition of NO signaling by adding PTIO or by pretreating (48 h) with L-NAME blocked the cGMP induction, as did ODQ, suggesting that cGMP elevation in hypoxia is dependent on NO and sGC, similarly to HIT.
To determine whether GFP-Rel translocation induced by GSNOR RNAi (Figure 3A) is accompanied by sGC-dependent cGMP elevation, as suggested by Figure 4A, we tested whether GSNOR RNAi enhanced cGMP staining. Indeed, GSNOR RNAi promoted cGMP staining, and this enhancement was inhibited by ODQ (Figure 4C). These findings indicate that NO mediates induction of cGMP levels in response to hypoxia, and further suggest that GSNO might act as secondary mediator of this effect.
Responses Downstream of cGMP Include Calcium Mobilization via cGMP-dependent Channels
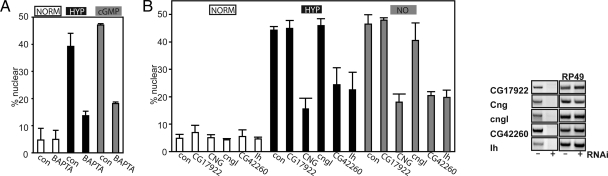
Recently, we examined GFP-Rel translocation following treatment of S2 cells with an NO donor and demonstrated that calcium acts downstream of NO. Cytoplasmic calcium elevation is also sufficient to promote GFP-Rel translocation (Dijkers and O'Farrell, 2007). We expected that calcium would similarly mediate the NO-dependent response to hypoxia. We tested involvement of calcium in HIT and whether it acted downstream of cGMP generation. To this purpose, we preincubated cells with a cell-permeable Ca2+ chelator (BAPTA-AM) and subsequently subjected them to either hypoxia or treatment with 8-Br-cGMP. Ca2+ scavenging suppressed HIT, as well as 8-Br-cGMP–induced GFP-Rel translocation (Figure 5A). These data show that hypoxia-induced GFP-Rel translocation requires calcium and that this occurs downstream of cGMP.
Figure 5.
Hypoxia promotes calcium mobilization via cGMP-gated channels. (A) GFP-Rel cells were untreated (con) or treated with Ca2+ scavenger BAPTA-AM (25 μM), for 30 min before exposure to hypoxia (o/n, 1% O2) or addition of 8-Br-cGMP (1 mM, 1.5 h), and GFP localization was determined. (B) Calcium mobilization is mediated via cGMP-gated channels. Left, analysis of RNAi-mediated knockdown of the cGMP-gated channels as in the left panel on GFP-Rel localization in normoxia or hypoxia (1% O2, o/n). Right, efficacy of dsRNA-mediated knockdown. S2 cells were pretreated with dsRNA against different cGMP-gated channels (CG17922, CNG, cngl, CG42260, or Ih) for 4 d and mRNA expression was measured by RT PCR. RP49 was used as a control for equal input. Left, analysis of RNAi-mediated knockdown of the cGMP-gated channels as in the left panel on GFP-Rel localization in normoxia or hypoxia (1% O2, o/n).
A study of oxygen-influenced behavior in Caenorhabditis elegans identified an sGC, GCY-35, and two downstream cGMP-dependent channels, Tax-2 and -4, as components in an oxygen-sensing signal transduction pathway. Drosophila has several cGMP-dependent channels homologous to Tax-2 and -4: CG17922, Cng (CG7779), cgnl (CG9176), CG42260, and Ih (CG8585). We tested putative involvement of these channels in HIT by RNAi. We targeted each of five candidate cGMP-dependent channels with two nonoverlapping dsRNAs that effectively knocked down the levels of the cognate RNA (Figure 5B, right) and tested the effect on GFP-Rel translocation induced by hypoxia or by NO. Knockdown of Cng (CG7779), CG42260, or Ih (CG8585) attenuated the induced translocation in response to either inducing condition. This suggests that cGMP generation can promote calcium mobilization through the activation of cGMP-dependent channels and that this calcium mobilization occurs downstream of NO signaling.
DISCUSSION
The complexity of an organism's response to hypoxia confounds investigations of NO signal generation and transduction. Here, we describe a cell-based model of hypoxia-induced NO-mediated signaling that is amenable to a surrogate genetic approach using RNAi as well as expression of NO-modulating enzymes. Our data demonstrate the importance for mitochondria for NO signaling during hypoxia and implicate GSNO as a signaling intermediate to promote cGMP generation and subsequent calcium mobilization (Figure 6).
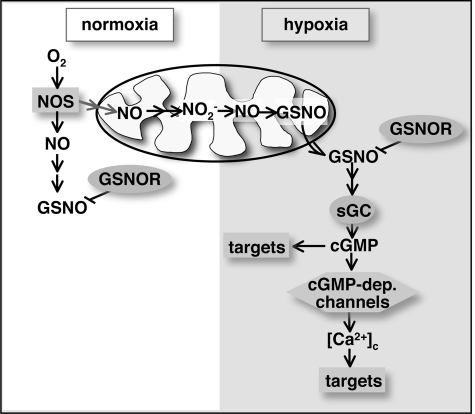
Figure 6.
Model for hypoxia-induced signaling. During normoxia (white area), activity of NOS presumably generates a pool of NO, possibly nitrite, which is stored in the mitochondria. During shortfalls of oxygen (hypoxia, shaded area), this pool gets converted back to NO. NO is present in the cytoplasm as GSNO. In the cytoplasm, generation of cGMP through sGC subsequently activates cGMP-dependent channels, resulting in mobilization of calcium through these channels. The two most novel steps in this proposed pathway are the production of NO by mitochondrial reduction and the activation of sGC by GSNO. The reductive production of NO by the mitochondrial has been documented in other contexts (Kozlov et al., 1999; Nohl et al., 2000). The activation of sGC by GSNO is suggested by our data and a recently recognized diversity in the mechanisms regulating sGCs leads us to suggest an activating input by transnitrosylation (see Discussion). Activation of this signaling pathway may allow adaptation to conditions of hypoxia. The signaling pathway as delineated in this article was defined by our data using S2 cells.
Means of NO Generation and NO Intermediates
NO donors induce GFP-Rel nuclear translocation (Dijkers and O'Farrell, 2007), and induction of translocation by knockdown of GSNOR suggests that GSNO is also an inducer. Reciprocally, the response to hypoxia is blocked by the NO scavenger PTIO, by prolonged treatment of L-NAME, expression of the enzymatic NO scavenger mtNOR, or by scavenging of NO-derived GSNO by GSNOR expression. Thus, NO signaling is sufficient to induce GFP-Rel translocation and is necessary in the context of the hypoxia response.
The fast-acting NOS inhibitor L-NAME only suppressed HIT if added well in advance (2 days; Figure 1B, not shown) before inducing hypoxia. Thus, NOS, which requires oxygen for activity (Rengasamy and Johns, 1991), acts before hypoxia to satisfy a precondition needed for later response to hypoxia. Because the half-life of NO in the presence of oxygen and sulfhydryls is only seconds, accumulated NO would decay after brief inhibition of NOS. Apparently, NOS activity yields a more stable product. Although this stable product could in theory be due to some unknown activity of NOS, more likely it is an NO derivative such as nitrite (NO2−), which results from rapid oxidation of NO (Ignarro et al., 1993), or an SNO. After prolonged L-NAME exposure even these more stable products would decay.
A number of reports suggest that mitochondria can reduce NO2− in hypoxia and thereby convert it to NO (Walters and Taylor, 1965; Kozlov et al., 1999; Castello et al., 2006; Feelisch et al., 2008). If this were the means of generating NO in our experimental setting, we would expect the NO to be produced within the mitochondria. Indeed, we find that targeting of the NO-eliminating enzyme NOR to the mitochondria inhibited HIT, whereas there was no effect with cytoplasmic NOR. We conclude that mitochondrial NO contributes to hypoxia signaling. To influence GFP-Rel, the mitochondrial signal needs to be transduced to the cytoplasm.
NO-induced Signaling in the Cytoplasm: Putative Role of S-Nitrosothiols
The failure of cytoplasmic NOR to block HIT suggests that if mitochondrial NO is transferred to the cytoplasm, it must be very rapidly converted to a form that is resistant to NOR. Our findings that GSNOR RNAi induced nuclear translocation of GFP-Rel, whereas overexpression of cytoplasmic GSNOR inhibited HIT (Figure 3, A and B) suggest GSNO as an alternative form of the signal in the cytoplasm. This is consistent with nitrosylation of GSH in conjunction with transfer of NO to the cytoplasm. GSNO could transduce the signal by transnitrosylating target proteins (Liu et al., 2001), and GSNOR would down-regulate signaling by removing GSNO (Jensen et al., 1998). This possibility is consistent with recent findings arguing that GSNO is a hypoxia-mimetic signal (Palmer et al., 2007; Lima et al., 2009).
Although the specificity of GSNOR clearly implicates GSNO levels as an important modulator of signaling, it should be noted that GSNO can breakdown to release NO, as well as transfer the NO adduct to other sulfhydryls (Bryan et al., 2004). Accordingly, although the level of cytoplasmic signal might be coupled to GSNO levels, the direct mediator of the signal might not be GSNO itself.
Signaling through cGMP
The most studied target of NO is sGC, and its product cGMP has a well-established role in vasodilation. Our data show that cGMP is induced by hypoxia, that a cGMP analog can induce GFP-Rel translocation, and that a sGC inhibitor (ODQ) inhibits HIT (Figure 4). We conclude that cGMP is an essential signaling intermediate in this hypoxia-signaling pathway.
Interestingly, GSNOR RNAi resulted in cGMP generation, which can be blocked by ODQ (Figure 4, A and C). This is the first time that GSNO signaling has been linked to the activation of sGC. We currently do not know if this means that sGC can be activated by transnitrosylation through GSNO or if GSNO is ultimately converted to NO to activate sGC, a route that is not supported by our data with cytoplasmic NOR. Activation of sGC through nitrosylation has been reported (Ignarro et al., 1980), although nitrosylation of sGC has also been associated with subsequent inhibition (Sayed et al., 2007).
It is now realized that the family of sGCs exhibit diverse modes of regulation. Recently, atypical soluble guanylyl cyclases in Drosophila have been shown to be activated by anoxia, independently of NO, as well as being activated by NO (Morton, 2004; Vermehren et al., 2006). In contrast, C. elegans, which does not possess NOS activity, has a member of the sGC family that is activated by O2 and mediates a behavioral response to hyperoxia (Gray et al., 2004). Our findings that 1) sGC inhibitor ODQ inhibited the effect of either GSNOR RNAi (Figure 4A) or SNAP treatment (not shown), and that 2) hypoxia-induced cGMP generation was ablated by either L-NAME pretreatment or PTIO treatment (Figure 4B) suggest NO/GSNO-dependent activation of sGC during hypoxia. However, preliminary evidence using RNAi suggested redundant contributions from the classical and the atypical sGCs to HIT (not shown). Given the diverse regulatory inputs into this family of sGCs it seems plausible that GSNO can act as activator of cGMP production, as suggested by our data.
Previous work in our lab suggested that treatment with an NO donor results in calcium mobilization (Dijkers and O'Farrell, 2007). HIT was blocked by scavenging calcium (Figure 5A). Similarly, the effect with a cGMP analog was blocked, indicating that cGMP can promote calcium mobilization. Calcium mobilization by cGMP can occur through cGMP-gated channels. In Caenorhabditis elegans, the cGMP-gated channels Tax-2 and -4 are involved in behavioral responses to hyperoxia downstream of sGC (Gray et al., 2004).
Drosophila larvae display a strong behavioral response to hypoxia (Wingrove and O'Farrell, 1999) that is dependent on NO, as well as the cGMP target PKG, but a role for cGMP-dependent channels therein has not been established. Interestingly, we did find a role for several of these channels in HIT (Figure 5B), suggesting that NO generated in hypoxia promotes calcium mobilization through cGMP-dependent channels. The observation that RNAi against several of these channels interfered with HIT may be explained by the fact that they form dimers and thus function together: in C. elegans, mutation of either Tax-2 or -4 yielded a similar phenotype as did the double mutant (Coburn and Bargmann, 1996). It would be of interest to see whether cGMP-dependent channels are involved in the Drosophila behavioral response to hypoxia.
In summary, we provide a system to dissect NO signaling during hypoxia, which originates in the mitochondria and is transduced to the cytoplasm (Figure 6), promoting cGMP generation and subsequent calcium mobilization. The common involvement of NO signaling in responses to hypoxia and findings that GSNO is hypoxia-mimetic suggest that the pathway described here may be used widely.
ACKNOWLEDGMENTS
We thank Steve DeLuca, Soo Jung Lee, and Marc McCleland for reading the manuscript and Rita Teodoro, who initially developed NOR as a tool for studying NO function in vivo. P.F.D. was supported by Human Frontiers of Science Program and the work was supported by National Institutes of Health Grant AI60102 to P.H.O'F.
Abbreviations used:
- HIT
hypoxia-induced translocation.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E09-05-0362) on July 22, 2009.
REFERENCES
- Armknecht S., Boutros M., Kiger A., Nybakken K., Mathey-Prevot B., Perrimon N. High-throughput RNA interference screens in Drosophila tissue culture cells. Methods Enzymol. 2005;392:55–73. doi: 10.1016/S0076-6879(04)92004-6. [DOI] [PubMed] [Google Scholar]
- Bryan N. S., Rassaf T., Maloney R. E., Rodriguez C. M., Saijo F., Rodriguez J. R., Feelisch M. Cellular targets and mechanisms of nitros(yl)ation: an insight into their nature and kinetics in vivo. Proc. Natl. Acad. Sci. USA. 2004;101:4308–4313. doi: 10.1073/pnas.0306706101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castello P. R., David P. S., McClure T., Crook Z., Poyton R. O. Mitochondrial cytochrome oxidase produces nitric oxide under hypoxic conditions: implications for oxygen sensing and hypoxic signaling in eukaryotes. Cell Metab. 2006;3:277–287. doi: 10.1016/j.cmet.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Clemens J. C., Worby C. A., Simonson-Leff N., Muda M., Maehama T., Hemmings B. A., Dixon J. E. Use of double-stranded RNA interference in Drosophila cell lines to dissect signal transduction pathways. Proc. Natl. Acad. Sci. USA. 2000;97:6499–6503. doi: 10.1073/pnas.110149597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coburn C. M., Bargmann C. I. A putative cyclic nucleotide-gated channel is required for sensory development and function in C. elegans. Neuron. 1996;17:695–706. doi: 10.1016/s0896-6273(00)80201-9. [DOI] [PubMed] [Google Scholar]
- de Vente J., Steinbusch H. W., Schipper J. A new approach to immunocytochemistry of 3′,5′-cyclic guanosine monophosphate: preparation, specificity, and initial application of a new antiserum against formaldehyde-fixed 3′,5′-cyclic guanosine monophosphate. Neuroscience. 1987;22:361–373. doi: 10.1016/0306-4522(87)90226-0. [DOI] [PubMed] [Google Scholar]
- Dijkers P. F., O'Farrell P. H. Drosophila calcineurin promotes induction of innate immune responses. Curr. Biol. 2007;17:2087–2093. doi: 10.1016/j.cub.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feelisch M., Fernandez B. O., Bryan N. S., Garcia-Saura M. F., Bauer S., Whitlock D. R., Ford P. C., Janero D. R., Rodriguez J., Ashrafian H. Tissue processing of nitrite in hypoxia: an intricate interplay of nitric oxide-generating and -scavenging systems. J. Biol. Chem. 2008;283:33927–33934. doi: 10.1074/jbc.M806654200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foe V. E., Alberts B. M. Reversible chromosome condensation induced in Drosophila embryos by anoxia: visualization of interphase nuclear organization. J. Cell Biol. 1985;100:1623–1636. doi: 10.1083/jcb.100.5.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley E., O'Farrell P. H. Nitric oxide contributes to induction of innate immune responses to gram-negative bacteria in Drosophila. Genes Dev. 2003;17:115–125. doi: 10.1101/gad.1018503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley E., O'Farrell P. H. Functional dissection of an innate immune response by a genome-wide RNAi screen. PLoS Biol. 2004;2:E203. doi: 10.1371/journal.pbio.0020203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster M. W., McMahon T. J., Stamler J. S. S-nitrosylation in health and disease. Trends Mol. Med. 2003;9:160–168. doi: 10.1016/s1471-4914(03)00028-5. [DOI] [PubMed] [Google Scholar]
- Fulop V., Moir J. W., Ferguson S. J., Hajdu J. The anatomy of a bifunctional enzyme: structural basis for reduction of oxygen to water and synthesis of nitric oxide by cytochrome cd1. Cell. 1995;81:369–377. doi: 10.1016/0092-8674(95)90390-9. [DOI] [PubMed] [Google Scholar]
- Gray J., Karow D., Lu H., Chang A., Chang J., Ellis R., Marletta M., Bargmann C. Oxygen sensation and social feeding mediated by a C. elegans guanylate cyclase homologue. Nature. 2004;430:317–322. doi: 10.1038/nature02714. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J., Edwards J. C., Gruetter D. Y., Barry B. K., Gruetter C. A. Possible involvement of S-nitrosothiols in the activation of guanylate cyclase by nitroso compounds. FEBS Lett. 1980;110:275–278. doi: 10.1016/0014-5793(80)80091-3. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J., Fukuto J. M., Griscavage J. M., Rogers N. E., Byrns R. E. Oxidation of nitric oxide in aqueous solution to nitrite but not nitrate: comparison with enzymatically formed nitric oxide from L-arginine. Proc. Natl. Acad. Sci. USA. 1993;90:8103–8107. doi: 10.1073/pnas.90.17.8103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isbell T., et al. SNO-hemoglobin is not essential for red blood cell–dependent hypoxic vasodilation. Nat. Med. 2008;14:773–777. doi: 10.1038/nm1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen D. E., Belka G. K., Du Bois G. C. S-Nitrosoglutathione is a substrate for rat alcohol dehydrogenase class III isoenzyme. Biochem. J. 1998;331(Pt 2):659–668. doi: 10.1042/bj3310659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim-Shapiro D. B., Schechter A. N., Gladwin M. Unraveling the reactions of nitric oxide, nitrite, and hemoglobin in physiology and therapeutics. Arterioscler. Thromb. Vasc. Biol. 2006;26:697–705. doi: 10.1161/01.ATV.0000204350.44226.9a. [DOI] [PubMed] [Google Scholar]
- Kozlov A. V., Staniek K., Nohl H. Nitrite reductase activity is a novel function of mammalian mitochondria. FEBS Lett. 1999;454:127–130. doi: 10.1016/s0014-5793(99)00788-7. [DOI] [PubMed] [Google Scholar]
- Krumenacker J. S., Hanafy K. A., Murad F. Regulation of nitric oxide and soluble guanylyl cyclase. Brain Res. Bull. 2004;62:505–515. doi: 10.1016/S0361-9230(03)00102-3. [DOI] [PubMed] [Google Scholar]
- Lavista-Llanos S., Centanin L., Irisarri M., Russo D. M., Gleadle J. M., Bocca S. N., Muzzopappa M., Ratcliffe P. J., Wappner P. Control of the hypoxic response in Drosophila melanogaster by the basic helix-loop-helix PAS protein similar. Mol. Cell. Biol. 2002;22:6842–6853. doi: 10.1128/MCB.22.19.6842-6853.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. J., Feldman R., O'Farrell P. H. An RNA interference screen identifies a novel regulator of target of rapamycin that mediates hypoxia suppression of translation in Drosophila S2 cells. Mol. Biol. Cell. 2008;19:4051–4061. doi: 10.1091/mbc.E08-03-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leone A. M., Palmer R. M., Knowles R. G., Francis P. L., Ashton D. S., Moncada S. Constitutive and inducible nitric oxide synthases incorporate molecular oxygen into both nitric oxide and citrulline. J. Biol. Chem. 1991;266:23790–23795. [PubMed] [Google Scholar]
- Lima B., et al. Endogenous S-nitrosothiols protect against myocardial injury. Proc. Natl. Acad. Sci. USA. 2009;106:6297–6302. doi: 10.1073/pnas.0901043106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton A. J., Johnson M. A., Macdonald T., Lieberman M. W., Gozal D., Gaston B. S-nitrosothiols signal the ventilatory response to hypoxia. Nature. 2001;413:171–174. doi: 10.1038/35093117. [DOI] [PubMed] [Google Scholar]
- Lipton S. A. Physiology. Nitric oxide and respiration. Nature. 2001;413:118–119. 121. doi: 10.1038/35093186. [DOI] [PubMed] [Google Scholar]
- Liu L., Hausladen A., Zeng M., Que L., Heitman J., Stamler J. S. A metabolic enzyme for S-nitrosothiol conserved from bacteria to humans. Nature. 2001;410:490–494. doi: 10.1038/35068596. [DOI] [PubMed] [Google Scholar]
- Liu L., et al. Essential roles of S-nitrosothiols in vascular homeostasis and endotoxic shock. Cell. 2004;116:617–628. doi: 10.1016/s0092-8674(04)00131-x. [DOI] [PubMed] [Google Scholar]
- Lundberg J., Weitzberg E., Gladwin M. The nitrate–nitrite–nitric oxide pathway in physiology and therapeutics. Nat. Rev. Drug Discov. 2008;7:156–167. doi: 10.1038/nrd2466. [DOI] [PubMed] [Google Scholar]
- Millar T. M., Stevens C. R., Benjamin N., Eisenthal R., Harrison R., Blake D. R. Xanthine oxidoreductase catalyses the reduction of nitrates and nitrite to nitric oxide under hypoxic conditions. FEBS Lett. 1998;427:225–228. doi: 10.1016/s0014-5793(98)00430-x. [DOI] [PubMed] [Google Scholar]
- Moncada S., Higgs E. A. The discovery of nitric oxide and its role in vascular biology. Br. J. Pharmacol. 2006;147(Suppl 1):S193–S201. doi: 10.1038/sj.bjp.0706458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton D. B. Atypical soluble guanylyl cyclases in Drosophila can function as molecular oxygen sensors. J. Biol. Chem. 2004;279:50651–50653. doi: 10.1074/jbc.C400461200. [DOI] [PubMed] [Google Scholar]
- Nakahara K., Tanimoto T., Hatano K., Usuda K., Shoun H. Cytochrome P-450 55A1 (P-450dNIR) acts as nitric oxide reductase employing NADH as the direct electron donor. J. Biol. Chem. 1993;268:8350–8355. [PubMed] [Google Scholar]
- Nohl H., Staniek K., Sobhian B., Bahrami S., Redl H., Kozlov A. V. Mitochondria recycle nitrite back to the bioregulator nitric monoxide. Acta Biochim. Pol. 2000;47:913–921. [PubMed] [Google Scholar]
- Okamoto N., Tsuruta K., Imai Y., Tomura D., Shoun H. Fungal P450nor: expression in Escherichia coli and site-directed mutageneses at the putative distal region. Arch. Biochem. Biophys. 1997;337:338–344. doi: 10.1006/abbi.1996.9786. [DOI] [PubMed] [Google Scholar]
- Palmer L. A., Doctor A., Chhabra P., Sheram M. L., Laubach V. E., Karlinsey M. Z., Forbes M. S., Macdonald T., Gaston B. S-nitrosothiols signal hypoxia-mimetic vascular pathology. J. Clin. Invest. 2007;117:2592–2601. doi: 10.1172/JCI29444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postovit L. M., Sullivan R., Adams M. A., Graham C. H. Nitric oxide signalling and cellular adaptations to changes in oxygenation. Toxicology. 2005;208:235–248. doi: 10.1016/j.tox.2004.11.022. [DOI] [PubMed] [Google Scholar]
- Rengasamy A., Johns R. A. Characterization of endothelium-derived relaxing factor/nitric oxide synthase from bovine cerebellum and mechanism of modulation by high and low oxygen tensions. J. Pharmacol. Exp. Ther. 1991;259:310–316. [PubMed] [Google Scholar]
- Sayed N., Baskaran P., Ma X., van den Akker F., Beuve A. Desensitization of soluble guanylyl cyclase, the NO receptor, by S-nitrosylation. Proc. Natl. Acad. Sci. USA. 2007;104:12312–12317. doi: 10.1073/pnas.0703944104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal S. Regulation of blood flow in the microcirculation. UMIC. 2005;12:33–45. doi: 10.1080/10739680590895028. [DOI] [PubMed] [Google Scholar]
- Stamler J. S., Lamas S., Fang F. C. Nitrosylation. the prototypic redox-based signaling mechanism. Cell. 2001;106:675–683. doi: 10.1016/s0092-8674(01)00495-0. [DOI] [PubMed] [Google Scholar]
- Steffen M., Sarkela T. M., Gybina A. A., Steele T. W., Trasseth N. J., Kuehl D., Giulivi C. Metabolism of S-nitrosoglutathione in intact mitochondria. Biochem. J. 2001;356:395–402. doi: 10.1042/0264-6021:3560395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teodoro R. O., O'Farrell P. H. Nitric oxide-induced suspended animation promotes survival during hypoxia. EMBO J. 2003;22:580–587. doi: 10.1093/emboj/cdg070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari Z., Sun D., Huang A., Kaley G., Koller A. Role of endothelial [Ca2+]i in activation of eNOS in pressurized arterioles by agonists and wall shear stress. Am. J. Physiol. Heart Circ. Physiol. 2001;281:H606–H612. doi: 10.1152/ajpheart.2001.281.2.H606. [DOI] [PubMed] [Google Scholar]
- Vermehren A., Langlais K. K., Morton D. B. Oxygen-sensitive guanylyl cyclases in insects and their potential roles in oxygen detection and in feeding behaviors. J. Insect. Physiol. 2006;52:340–348. doi: 10.1016/j.jinsphys.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Walters C. L., Taylor A. M. The reduction of nitrite by skeletal-muscle mitochondria. Biochim. Biophys. Acta. 1965;96:522–524. doi: 10.1016/0005-2787(65)90570-8. [DOI] [PubMed] [Google Scholar]
- Wingrove J. A., O'Farrell P. H. Nitric oxide contributes to behavioral, cellular, and developmental responses to low oxygen in Drosophila. Cell. 1999;98:105–114. doi: 10.1016/S0092-8674(00)80610-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D., Xue J., Lai J. C., Schork N. J., White K. P., Haddad G. G. Mechanisms underlying hypoxia tolerance in Drosophila melanogaster: hairy as a metabolic switch. PLoS Genet. 2008;4:e1000221. doi: 10.1371/journal.pgen.1000221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweier J. L., Wang P., Samouilov A., Kuppusamy P. Enzyme-independent formation of nitric oxide in biological tissues. Nat. Med. 1995;1:804–809. doi: 10.1038/nm0895-804. [DOI] [PubMed] [Google Scholar]