Abstract
Cancer metastasis is a leading cause of cancer morbidity and mortality. More needs to be learned about mechanisms that control this process. In particular, the role of chemokine receptors in metastasis remains controversial. Here, using a highly metastatic breast cancer (4T1) model, we demonstrate that lung metastasis is a feature of only a proportion of the tumor cells that express CCR4. Moreover, the primary tumor growing in mammary pads activates remotely the expression of TARC/CCL17 and MDC/CCL22 in the lungs. These chemokines acting through CCR4 attract both tumor and immune cells. However, CCR4 mediated chemotaxis was not sufficient to produce metastasis, as tumor cells in the lung were efficiently eliminated by NK cells. Lung metastasis required CCR4+ Tregs which directly killed NK cells utilizing beta-galactoside-binding protein. Thus, strategies that abrogate any part of this process should improve the outcome through activation of effector cells and prevention of tumor cell migration. We confirm this prediction by killing CCR4+ cells through delivery of TARC-fused toxins or depleting Tregs and preventing lung metastasis.
Keywords: Breast cancer, Tregs, NK cell regulation, CCR4, CCL17, βGBP
Introduction
The formation of metastatic colonies is a non-random process that starts early, during the growth of the primary tumor. In analogy with immune cells, tumor dissemination is presumed to require chemokine receptors. To date, expression of chemokine receptors were associated with cancer metastases, such as CXCR4, CCR7 and CCR10 in breast cancer (1), and CCR6, CCR7, CXCR5 and CX3CR1 in pancreatic, gastric, prostate and NSCLC cancers (2). Among them, CXCR4 appears to be a major metastasis-regulating receptor (see review by Balkwill (3)) that utilizes the lymph node trafficking network of hematopoietic progenitors and endothelial cells (1;4). Despite this, a direct role for chemokine receptors in mediating metastasis has not been defined, as the expression of chemokine receptors on the surface of tumors is rarely shown and difficult to confirm. Even for a widely used metastasis model, such as mouse mammary 4T1 carcinoma, the role of chemokine receptors has not been confirmed. The 4T1 tumor mimics human breast cancer in several ways; it readily and spontaneously metastasizes to lung, lymph nodes, liver, bone and other sites after implantation into the mammary pad of immune competent BALB/C mice (5). Metastasis of 4T1 tumor was mostly associated with a tumor-induced suppressive microenvironment and myeloid-derived suppressive cells (Gr1+ CD11b+ MDSCs) (6-8). It appears that 4T1 tumor activates and expands these cells through production of GM-CSF (8;9) to impair antitumor T cell function directly (8) or indirectly through the development of Tregs (10). Tregs are a specialized subset of CD4+ T cells that control peripheral tolerance to self- and allo-antigens (11). CCR4+ Tregs have been shown to infiltrate human tumors and suppress antitumor activity of tumor-infiltrating effector T cells (1−215). Consequently, Tregs are presumed to support metastasis through inhibition of antitumor T cell immune responses.
Chemokines TARC/CCL17 and MDC/CCL22 control homing of CCR4-expressing immune cells into lungs and skin to elicit protective pulmonary responses to pathogens. They recruit Th2 type CD4+ T cells (16), regulatory T cells (Tregs) (17;18); and some IL-2-activated NK cells, iNKT cells, and B cells (19-21). An aberrant expression of TARC or MDC is often associated with allergic diseases causing massive infiltration of Th2 type CD4+ T cells (16;22). Interestingly, we have observed that lungs of mice with 4T1 tumor growing in mammary glands secreted significant amounts of TARC. This has led us to hypothesize that 4T1 cells may express CCR4 and migrate to lungs the same way as CCR4+ immune cells. Utilizing various tools, including a TARC-fused toxin (that we developed) which specifically kills CCR4+ cells (17;23), we demonstrate that CCR4 is expressed on a proportion of tumor cells that chemotax to TARC and metastasize to lung. However, despite this inherent capacity of the tumor to metastasize, lung metastasis required additional help from CCR4+ Tregs. We demonstrate that Tregs neutralize anti-metastatic effect of NK cells by killing them utilizing beta-galactoside-binding protein (βGBP), an immunomodulatory protein which is also expressed by CCR4+ Tregs (Baatar et al., unpublished data and ((24)) and shown to induce death of activated T cells (25-27).
Materials and Methods
Chemicals and reagents
were from Sigma (St. Louis, MO), unless specified otherwise. The following antibodies were used: anti-mouse pan-NK-FITC, anti-mouse CD27-PE, anti-mouse Ly-6G and Ly-6C (Gr-1)-FITC, anti-mouse CD11b-PE, and anti-mouse F4/80-APC (Caltag); anti-mouse CD4-FITC, anti-mouse CD25-PE and anti-mouse Foxp3-APC (eBioscience); rabbit anti-mouse CCR4 Ab (Capralogics); Alexa647-conjugated donkey anti-rabbit IgG pAb (Invitrogen). Anti-human Gal-1 (AF1152 and BAF1152), anti-CD8-PE Abs, and CD3+ T cell enrichment columns were from R&D Systems Inc. (Minneapolis, MN). RPMI 1640 medium, fetal bovine serum, Dynabeads® for CD8, CD25, CD4 isolation and DETACHaBEAD® reagent, carboxyfluorescein diacetate succinimidyl ester (CFSE), and ProLong anti-fade reagent were purchased from Invitrogen Corp. (Carlsbad, CA). Cells were blocked with Fc block (anti-CD16/32; BD Biosciences). CD25+CD4+ cells were negatively selected using a mouse T cell CD4 subset column kit (R&D Systems), which was followed with anti-mouse CD25-PE antibody and anti-PE Ab-conjugated microbeads (Miltenyi Biotec Inc); and NK cells were isolated using the NK cell isolation kit (Miltenyi Biotec Inc.) following manufacturer's instructions. Cell purity was >90%, as assessed by flow cytometry. Human βGBP was previously described (27;28) and was a generous gift of Dr. Livio Mallucci (King's College London, London, UK). Production of TARC-PE38, CCL27-PE38 and SLC-PE38, chemokines fused with a truncated form of Pseudomonas exotoxin PE38. was reported elsewhere (17;23). Control non-toxic TARC-Ag protein consisted of TARC fused with tumor antigen OFA. The proteins were expressed and purified from BL21 (DE3) E. coli (Stratagene, La Jolla, CA) with > 90% purity.
Cell lines, mice and in vivo manipulations
The 4T1 mouse mammary carcinoma cells, human breast cancer MCF-7 and MDA-231, human CCRF-CEM (CEM, CCL-19), and MOLT-4 (CRL-1582) were from the American Type Culture Collection, Rockville, MD); 4T1.2 is a single cell subclone of 4T1 cells (5). All experiments were performed using 4−8 weeks old female BALB/C mice and BALB/C background IL-10 KO and NOD/SCID (NOD.CB17-Prkdcscid/J; H-2d) (The Jackson Laboratory, Bar Harbor, ME) in pathogen-free environment at the National Institute on Aging Animal Facility, Baltimore, MD. Animal care was provided in accordance with the procedures outlined in the Guide for the Care and Use of Laboratory Animals (NIH Publication No. 86−23, 1985). Mice were challenged s.c. into the fourth mammary gland (pad) with 1 × 104 of tumor cells. After 28 days of tumor challenge, lungs were analyzed for metastasis by ex vivo injecting India ink through the trachea, which was destained in Fekete's solution to count tumor nodules. NK cells or Tregs were depleted injecting i.v. 50 μL anti – asialo GM1 antiserum (Wako, Osaka, Japan) or 500 μg i.p. anti – mouse CD25 Ab or control IgG (PC61, BioXcell, West Lebanon, NH) at −1, 3, 10, and 18 days of tumor challenge, respectively. TARC-PE38 and other toxins (5μg) were injected intratumorally or via the tail vein at days 3, 7 and 10 post tumor challenge. BALB/C or IL-10 KO mice 107 splenocytes, or 106 Tregs alone or mixed with 106 non-Tregs (as a source of IL-2), or 2×106 non-Tregs) were i.v. injected at days −3, 2 and 7 post tumor challenge to restore metastasis in NOD/SCID mice.
Detection of chemokine expression in lungs
TARC and MDC were tested by ELISA with anti-mouse CCL17 or CCL22 Abs (R&D, Minneapolis USA, #MAB529 and #MAB4391, respectively) in lung extracts or broncheoalveolar lavage (BAL) washed with 1ml PBS. Lung cells were isolated by digestion with 1 mg/ml collagenase (Sigma). Paraffin blocks of lung metastasis from three different patients with breast cancer and lung tissues of two healthy humans were from the Department of Pathology, University of Leipzig, Germany. Lungs of naïve BALB/C mice (i.p. injected 5 days with 0.5 ml of serum-free tumor conditioned medium ) or at day 28 post tumor challenge were paraffin embedded. Immunohistochemistry staining was performed as reported elsewhere (29) using the following reagents: Human/mouse TARC Ab (sc-12271, Santa Cruz Biotechnology Inc., Santa Cruz, CA); antigen unmasking solution (H-3300), horse serum (S-2000), Avidin-Biotin blocking kit (SP2001), goat IgG (I-5000), biotinilated anti-goat IgG (BA-9500), Strepatavidin-peroxidase and DAB Plus Substrate System (Thermo Scientific, Fremont, CA). All slides were also counterstained with hematoxylin.
In vitro manipulations
Chemotaxis of tumor cells and Tregs were assessed in a 48-well microchemotaxis chamber (NeuroProbe) with a 10-μm polycarbonate filter (Osmonics) coated with rat tail collagen type I (BD Bioscience) or using 24-well tissue transwell plate (5-μm pore size, Costar; Corning Life Science, Acton, MA) as previously described (30). Results were expressed as mean Chemotactic Index (CI, number of migrated cells to chemoattractant/number of migrated cells to control media) ± SEM of three wells. Tumor cell viability was assessed after 48−72 hours culture using cell proliferation reagent WST-1 (Roche Applied Science). To test Treg-mediated apoptosis of NK cells, CFSE stained NK cells were cultured for 16 hours with titrated amounts of either Tregs, or non-Tregs, or βGBP in U-bottom 96-well plates in RPMI with 10% FBS and 1000 U/ml human IL-2. PI and annexin-V-Fluor Staining kit (Roche Applied Science) was used assess apoptosis.
Statistical Analysis
The results are presented as the mean of triplicates ± SD of at least three experiments. Differences were tested using Student's t test and a 2 sided p-value less than 0.05 was considered statistically significant. Data on lymphocyte subsets and breast cancer stage at study entry were collected during IRB-approved breast cancer therapy trials conducted at the National Cancer Institute from 1993 − 2007; patients provided informed consent to research sample collection. The absolute number of NK, CD4+ and CD8+ T cells/mm3 was calculated based upon the percentage of these cell in the lymphocyte gate, the percentage of lymphocytes (defined as CD45bright, CD14−, SSClow) and the white blood cell counts. Anonymized data were analyzed using Mann-Whitney non-parametric unpaired test to compare lymphocyte subsets from patients at Stages II-IV at study entry.
Results
Primary tumor induces production of TARC and MDC in the lungs
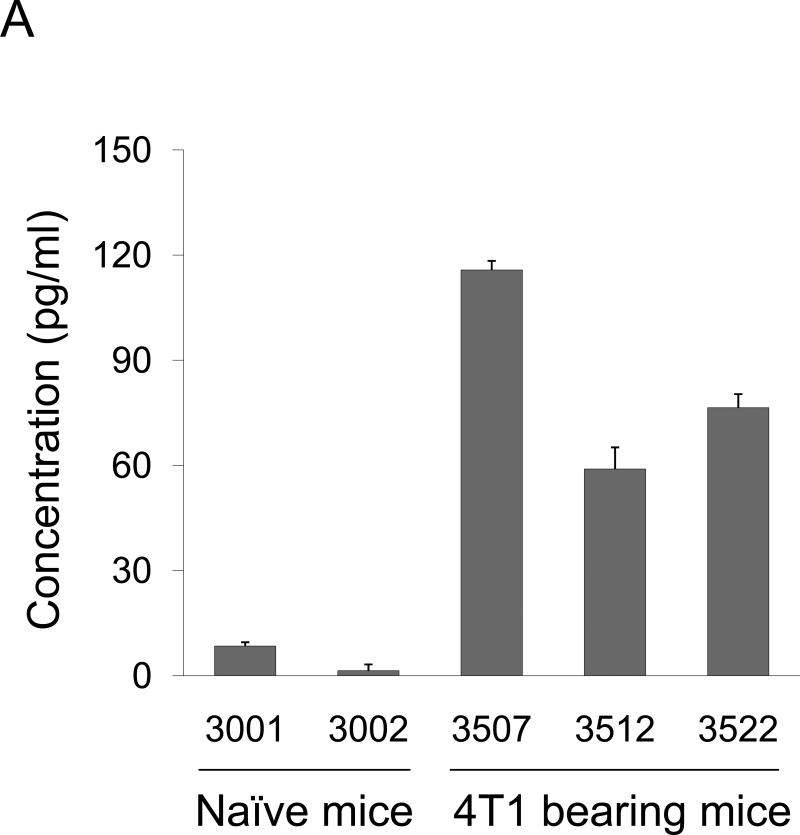
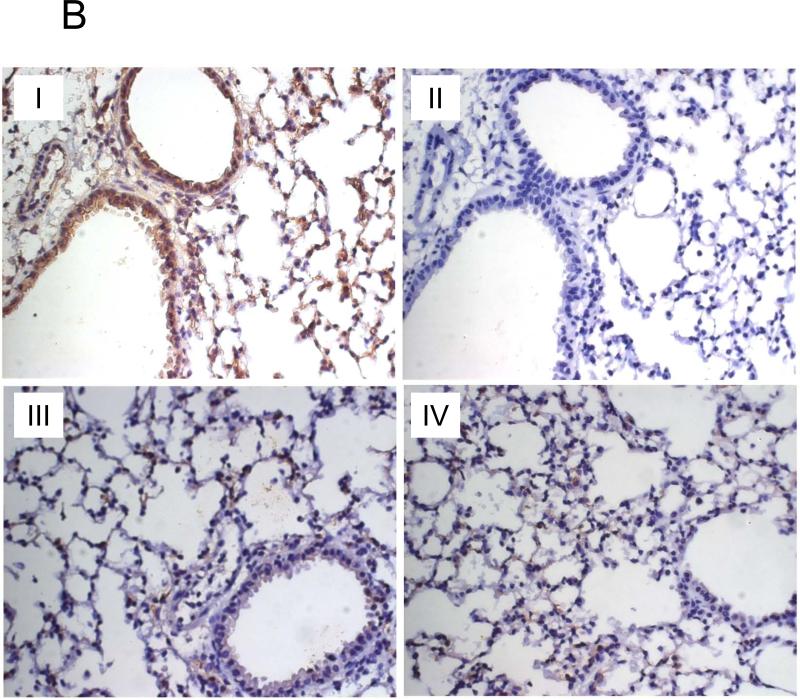
We have detected significant amounts of TARC in the broncheoalveolar lavage (BAL) of mice that were implanted with 4T1 cells in the mammary pad (Fig.1A). Since 4T1 and its derivative 4T1.2 cells readily metastasize to lungs (5), this raises an interesting possibility that the primary tumor activated CCR4 ligand (TARC or MDC) production in the lungs to facilitate recruitment of CCR4+ cells. To test this hypothesis, lungs of naïve BALB/C mice were stained for TARC expression after i.p. injections of serum-free conditioned medium from 4T1 cells (tumor CM) or control medium. TARC was significantly up regulated in lungs of mice that were treated with tumor CM (panel I), but not control medium (panel III) or PBS (panel IV, Fig.1B). It was predominantly expressed by epithelial and stromal cells (panel I, Fig.1B), but not by infiltrating immune cells (Suppl.Fig.1). Expression of MDC was also significantly up-regulated in the lung cells of tumor CM-treated mice, it was mostly produced by non-immune cells (Suppl.Fig.2A). In concordance, MDC was also produced from ex vivo cultured mouse primary fibroblasts when they were treated with tumor CM, but not control medium (Suppl.Fig.2B). Thus, the primary tumor growing in the mammary gland can activate lungs to produce chemokine ligands for CCR4.
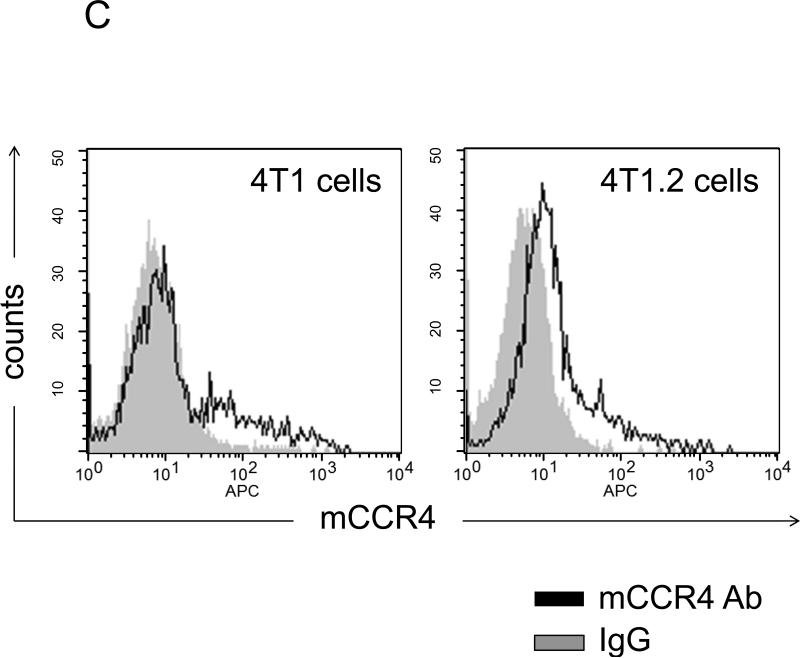
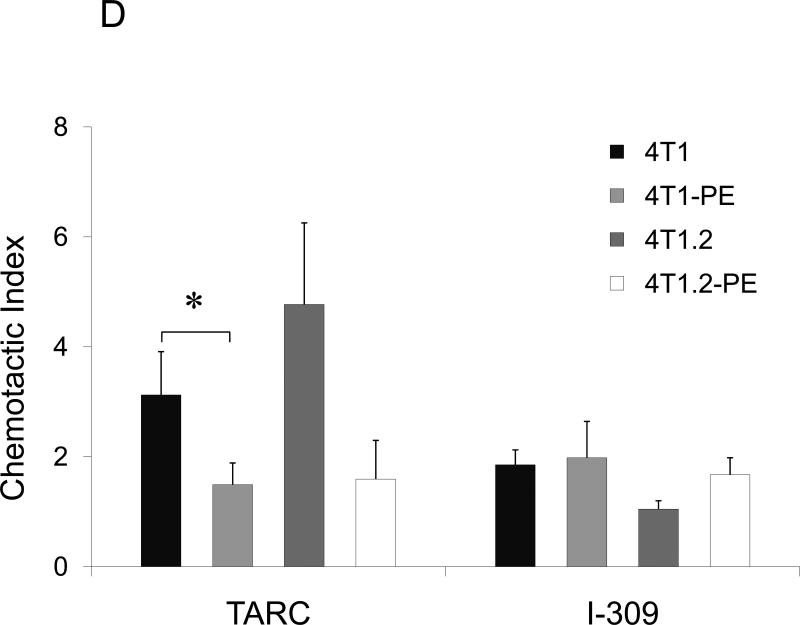
FIGURE 1. Phenotypic characterization of murine breast 4T1 tumor cells.
(A) Expression of TARC (pg/ml ± SEM of triplicate tests, Y-axis) in BAL of individual naïve mice (#3001 and 3002) and tumor-bearing mice (#3507, 3512, and 3522) was assessed by ELISA. To prepare BAL, lungs were washed with 1 ml PBS. (B) Tumor CM, but not control medium (III), induces TARC expression in lungs of mice (I). Lungs were formalin fixed and paraffin embedded at day six after daily single i.p. injections with 0.5 ml tumor CM (I and II), or control medium (III), or PBS (IV). A representative staining result with anti-TARC Ab (I, III and IV) or control isotype-matched Ab (II) from three mice per group experiment is shown. (C) CCR4 on the surface of 4T1 tumor cells was stained with anti-CCR4 Ab (black line) and control Ab (grey line). (D) Unlike 4T1-PE and 4T1.2-PE cells, CCR4+4T1 tumor cells migrate to TARC (100 ng/ml), but not I-309 (100 ng/ml). Y– axis, Chemotactic Index ± SEM of triplicate experiment. *P<0.05.
CCR4 is also expressed on a fraction of breast cancer cells that migrate to TARC
Next, we have tested whether 4T1 tumor cells (the name that will be used to collectively describe 4T1 and 4T1.2 cells) express CCR4. Indeed, as corroborated by RT/PCR assay (Suppl.Fig.3A,B), a low level of CCR4 was detected on the surface of 4T1 tumor cells (Fig.1C). The receptor expression was functional, as 4T1 tumor cells chemotaxed to TARC (Fig.1D). Similar low but significant CCR4 expression (11%) was also detected on the surface of human breast cancer MCF7 cells (Suppl.Fig.3C). Thus, CCR4 might be expressed only on a proportion of 4T1 tumor cells. To test this possibility, the cells were treated with TARC-PE38 (TARC fusion with a truncated toxin PE38 (17;23)), a formulation that kills CCR4+ cells without adverse effects on CCR4− cells (Suppl.Fig.4A). Significant cell death was induced by the treatment with TARCPE38 (Suppl.Fig.4B), but not with control toxins (SLC-PE38 and CCL27-PE38 which target CCR7 and CCR10, respectively, Suppl.Fig.4B). However, TARC-PE38 treatment also generated resistant 4T1 and 4T1.2 cells (designated 4T1-PE and 4T1.2-PE, respectively, Suppl.Fig.4C) that could not chemotax to TARC (Fig.1D). In conclusion, a proportion of 4T1 tumor cells express functionally active CCR4 and chemotax to TARC.
CCR4+ 4T1 cells metastasize to lung
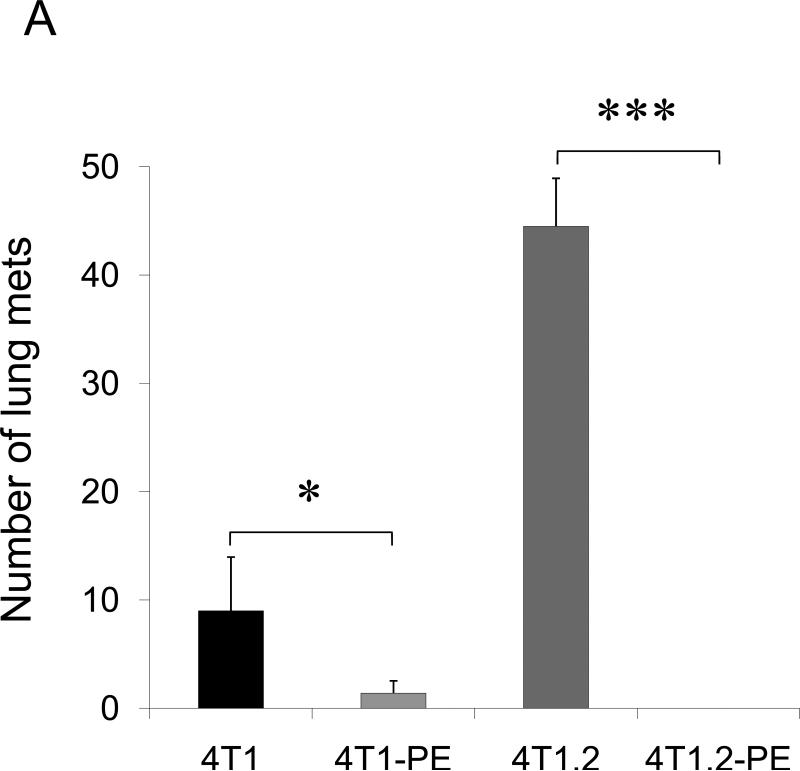
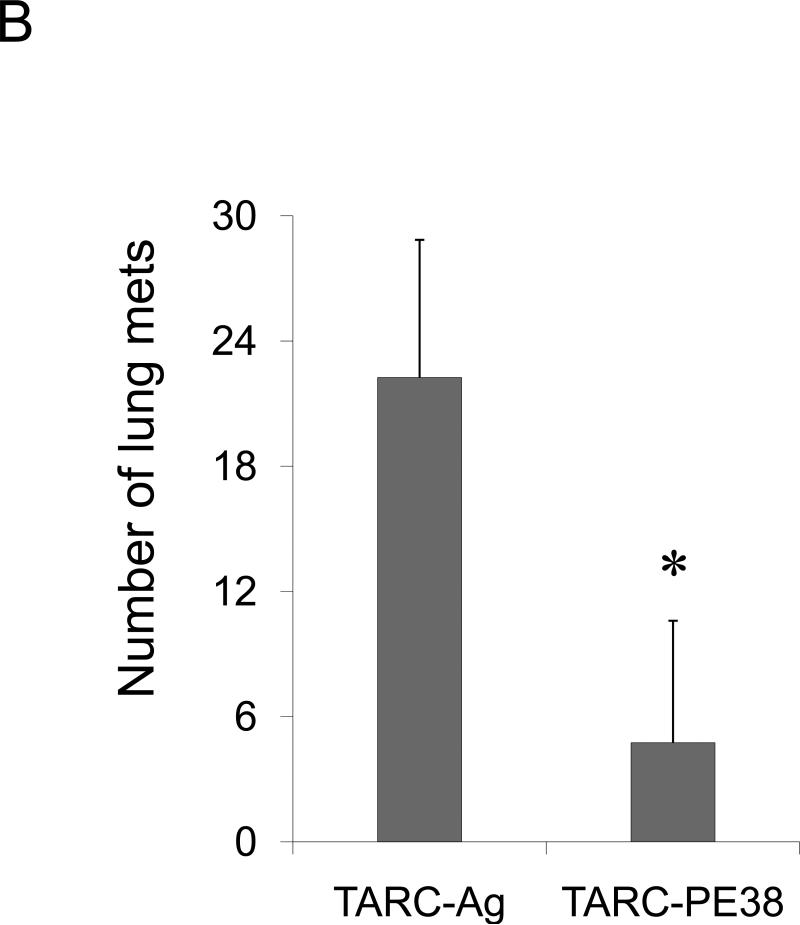
To test the role of CCR4+ tumor cells in lung metastasis, 4T1 tumor cells and 4T1-PE tumor cells (named to collectively describe 4T1-PE and 4T1.2-PE cells) were transplanted in the mammary pad of syngeneic BALB/C mice. Mice with 4T1 tumor cells succumbed to significant lung metastasis (Fig.2A and Suppl.Fig.5) and started dying by four weeks after tumor transplantation (data not shown). In contrast, almost no lung metastases were detected in mice challenged with 4T1-PE tumor cells, indicating that the loss of the CCR4+ subset abrogates lung metastasis. Specifically, 4T1.2-PE challenged mice were completely free of lung metastasis (Fig.2A and Suppl.Fig.5). This is not due to in vitro selection-introduced artifacts of 4T1-PE cells, as lung metastasis of parental 4T1 tumors was also significantly reduced by in vivo intratumoral treatments with TARC-PE38 (P<0.01, Fig.2B). In contrast, treatments with control TARC-Ag (a non-toxic formulation that also signals through CCR4 (17)) failed to reduce lung metastasis (Fig.2B). In conclusion, the lung metastases are caused by CCR4+ 4T1 tumor cells.
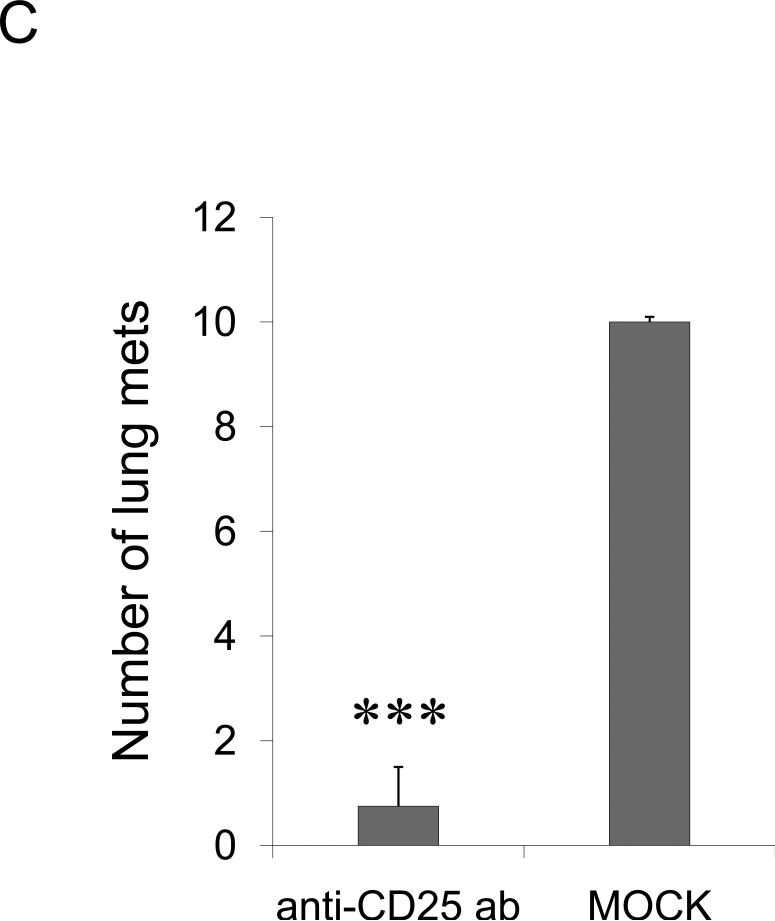
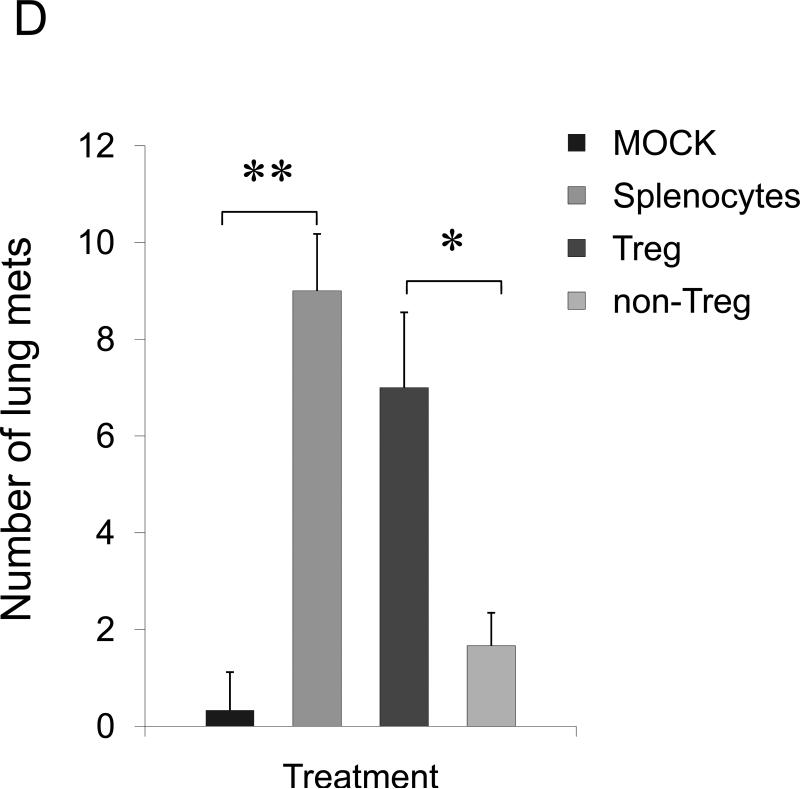
FIGURE 2. The role of CCR4+ cells in lung metastasis.
Shown, mean lung metastasis foci ± SEM of four-five per group mice experiments of: (A) BALB/C mice that werechallenged with 4T1, 4T1.2, 4T1-PE and 4T1.2-PE cells; (B) 4T1.2 tumor-bearing BALB/C mice that were intratumorally treated with 10 μg of TARC-PE38 or control TARC-Ag; (C) 4T1.2 tumor-bearing BALB/C mice that were depleted of Tregs using anti-CD25 Ab. Control mice were treated with isotype-matched antibody (mock); and (D) 4T1.2 tumor-bearing NOD/SCID mice that were transferred with splenocytes or Tregs from naïve BALB/C mice, or injected with PBS (Mock). All experiments were reproduced at least three times. *P<0.05; **P<0.01; *** P<0.001.
Tregs are required for lung metastasis
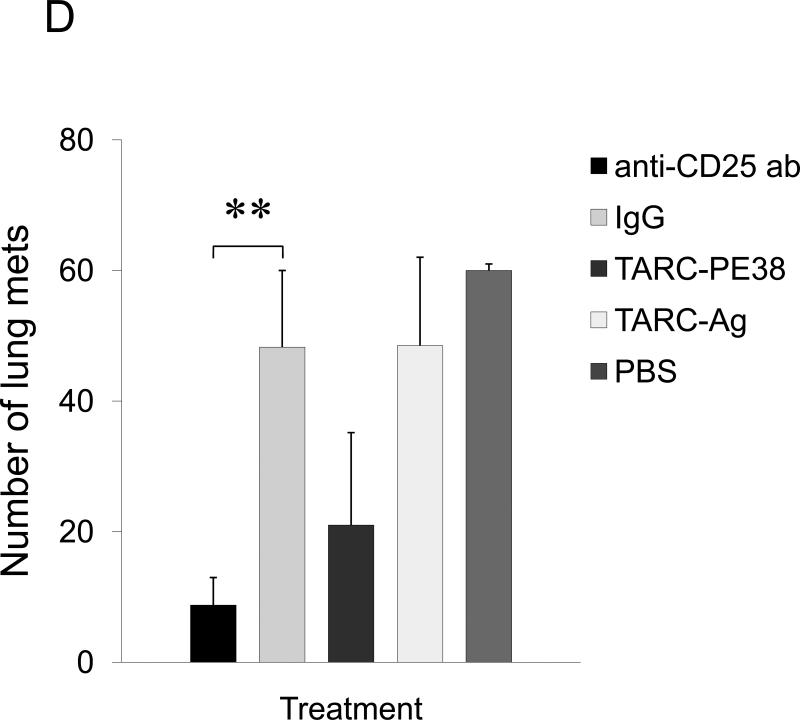
Tregs also express CCR4 and infiltrate lungs and tumors producing TARC/MDC (13;18;31). Thus, they could be also recruited in the lungs of 4T1-bearing mice to protect metastasis through suppression of antitumor responses. To test this possibility, 4T1.2 tumor-bearing BALB/C mice were depleted of Tregs by systemic treatment with PC61 antibody. The mice treated with PC61, but not with isotype-matched control Ab (mock), were almost completely free of lung metastasis (anti-CD25 Ab, Fig.2C). Thus, although some effector T cells could be also depleted, the data indicate that Tregs were required for lung metastasis of 4T1 tumor cells. To confirm this, we have transplanted 4T1.2 cells into T and B cell-deficient NOD/SCID mice. In these mice the 4T1.2 tumor growth was not affected at the primary challenge site (mammary pad, data not shown), yet the tumor cells were unable to metastasize. The lack of metastasis is not due to the inability of the tumor to activate lungs, as their BALs had readily detectable TARC and MDC (Suppl.Fig.6). In contrast, lung metastasis was restored in NOD/SCID mice after transfer of splenocytes from naïve BALB/C mice (Fig.2D). Similarly, lung metastases were also restored after transfer of Tregs (CD25+CD4+) either alone or in combination with non-Tregs (CD25−CD4+, as source of IL-2) (Fig.2D). The transfer of the same number of CD25−CD4+ T cells did not restore metastasis and only sporadically yielded a few metastatic foci (non-Tregs, Fig.2D).
Lungs of tumor-bearing mice (data not shown) or mice i.p. injected with tumor CM, but not control medium, contained significantly enhanced amounts of Foxp3+Tregs (P>0.05, Fig.3A). Similarly, in the lungs of tumor-bearing NOD/SCID mice, their numbers were increased and maintained for four weeks after only transfer of Tregs and splenocytes (Fig.3B). The Tregs were predominantly CCR4+ (Fig.3C) and chemotaxed to TARC (Fig.3D and Suppl.Fig.7) and to lung extracts from the mice injected with tumor CM utilizing TARC (as the chemotaxis was completely abolished when TARC was present in the upper wells of the chemotaxis chamber, p<0.001, Fig.3D). Thus, as reported for inflamed lungs (13;18;31), tumor may also facilitate lung recruitment of CCR4+Tregs to support lung metastasis. In support, the pretreatment of BALB/C splenocytes or Tregs with TARC-PE38, but not control TARC-Ag, prior adoptive transfer almost completely abolished the ability of BALB/C splenocytes (Fig.4A) or Tregs (Suppl.Fig.8) to restore lung metastasis in NOD/SCID mice.
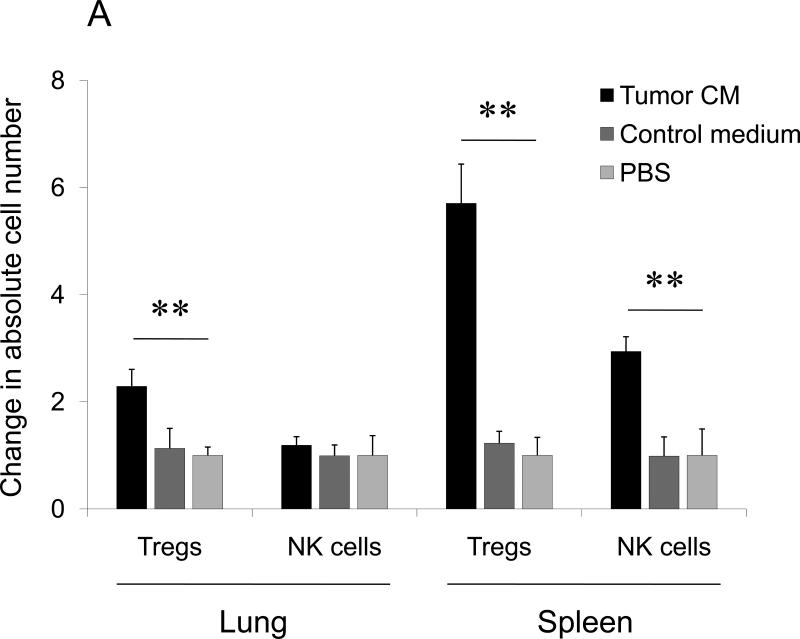
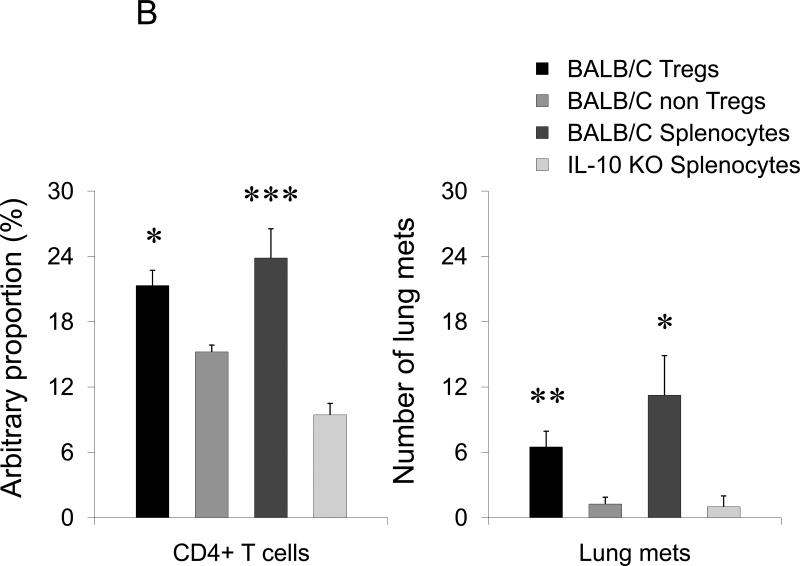
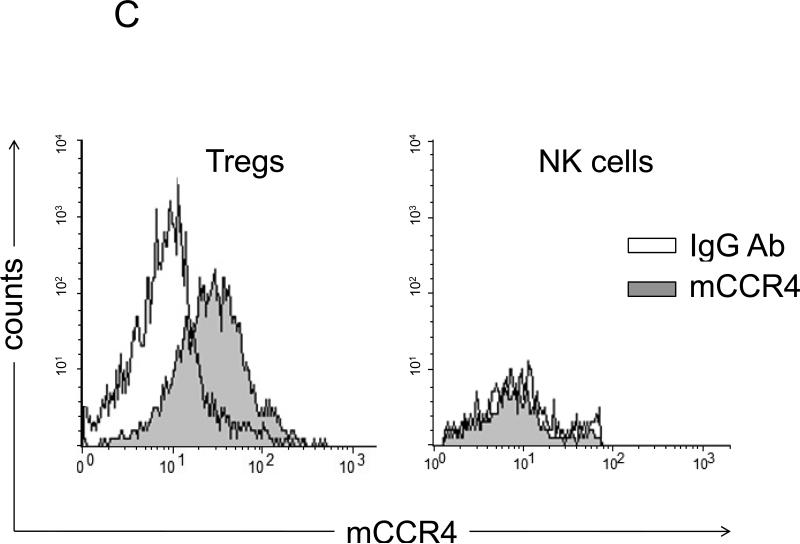
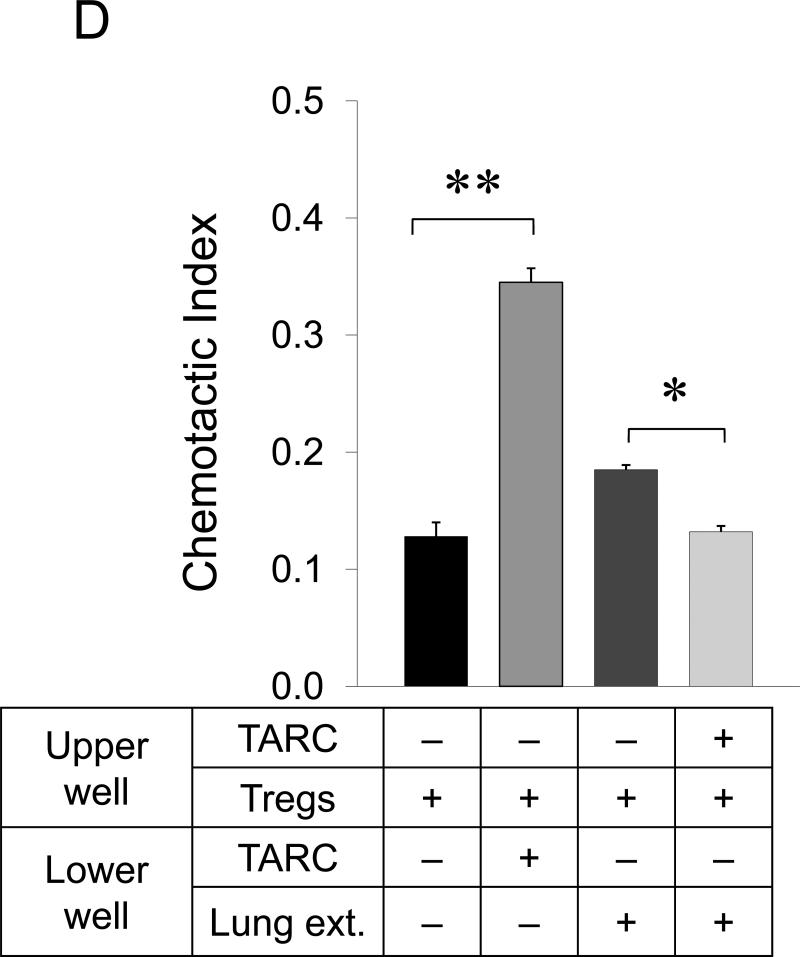
FIGURE 3. Tumor induces lung recruitment of CCR4+ Tregs.
(A) FoxP3+ cells (Tregs), but not NK cells, are significantly increased in the lungs of mice i.p. treated with tumor CM (as in Fig.1B). In contrast, both populations were increased in spleens of tumor CM –treated mice. Y- axis, change in absolute numbers of cells compared with PBS-treated group ±SEM of three mice per group experiment repeated twice. (B) Tregs presence in the lungs of NOD/SCID mice after transfer with BALB/C Tregs or splenocytes, or non-Tregs (Non-Tregs) or splenocytes from IL-10 deficient mice (IL-10 KO) at day 28 post tumor challenge. Shown, mean proportion ±SEM of CD4+ cells (left, Y-axis); and mean of lung metastasis ±SEM (right, Y-axis) of four mice per group experiment. (C) CCR4 expression on Tregs (left panel) and NK cells (right panel) is shown after staining with anti-CCR4 Ab (grey) and control Ab (open area), respectively. (D) Tregs shown in Fig.2D chemotax to TARC (100 ng/ml). They also migrate to extracts from lungs (Lung ext.) of mice treated with tumor CM (as in Fig.1B) utilizing CCR4. TARC was added in upper wells of chemotactic chamber to abrogate chemotaxis. Y– axis, Chemotactic Index ± SEM of triplicate experiment repeated three times. *P<0.05; **P<0.01; *** P<0.001.
FIGURE 4. The role of NK cells in lung metastasis.
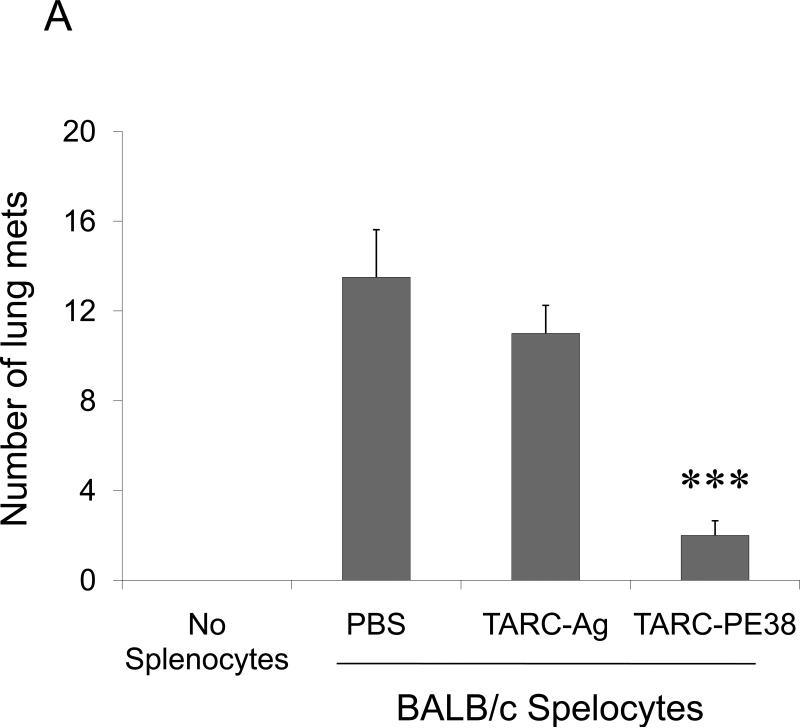
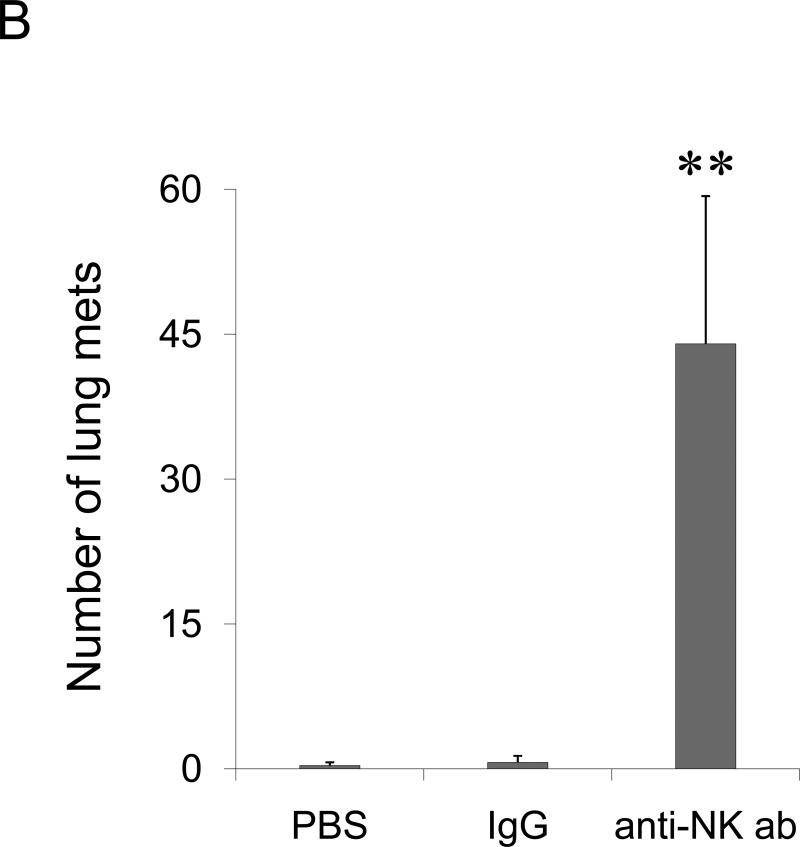
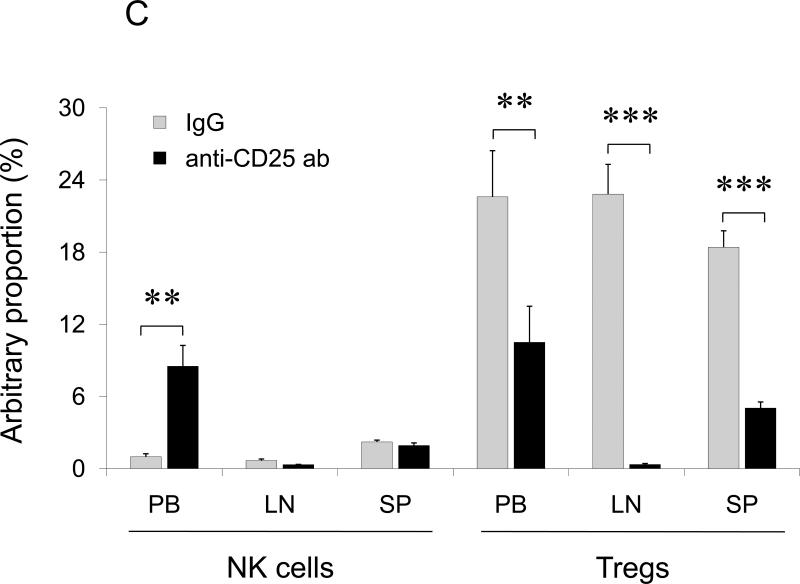
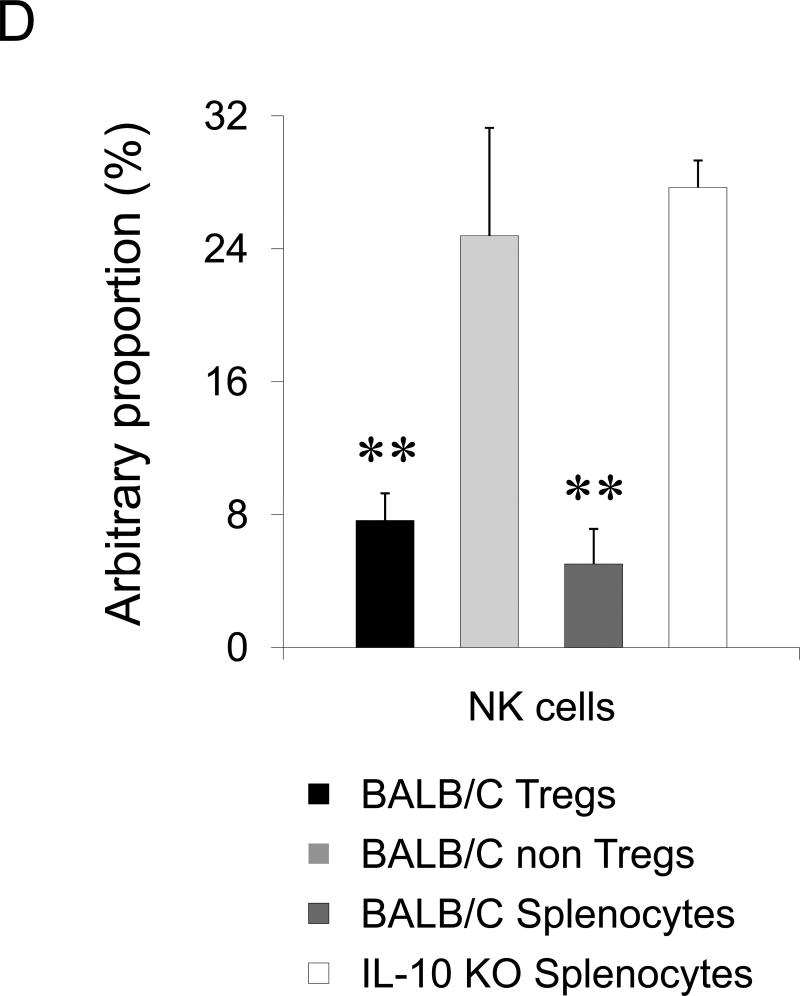
(A) BALB/C splenocytes pretreated with TARC-Ag or mock (PBS) restore lung metastasis of 4T1.2 tumor cells in NOD/SCID mice, which is abrogated by one hour TARC-PE38 pre-treatment of the splenocytes prior the adoptive transfer (TARC-PE38). Control mice (No Splenocytes) were only challenged with 4T1.2 tumor. Shown, mean of lung metastasis ±SEM of five mice per group experiment repeated three times. (B) NK cells protect lungs of NOD/SCID mice from metastasis of 4T1.2 tumor cells. Tumor-bearing mice were i.v. treated with anti-asialo GM1. Control mice were injected with rabbit sera (IgG) or with PBS. Shown, mean of lung metastasis ±SEM of four mice per group experiment reproduced twice. (C) The numbers of NK cells and Tregs inversely correlate in tumor – bearing BALB/C mice. Tregs were depleted as in Fig.2c. Shown, mean proportion ±SEM (%) of Tregs and NK cels in peripheral blood (PB), lymph nodes (LN) and spleens (SP) of four mice per group experiment. (D) The transfer of BALB/C Tregs or BALB/C splenocytes significantly reduces NK cell numbers in PB of tumor – bearing NOD/SCID mice. Control mice were transferred with BALB/C non-Tregs or splenocytes from IL-10 deficient mice (IL-10 KO splenocytes). Cells were analyzed by FACS after staining for Pan-NK, CD27 and CD25High CD4+ markers. *P<0.05; **P<0.01; ***P<0.001.
Tregs promote lung metastasis by regulating antitumor NK cells
Tregs primarily regulate T cell responses and promote escape from immune surveillance (13;14;32). However, 4T1 tumors failed to metastasize in mice with impaired T cells (NOD/SCID mice) in the absence of Tregs; and depletion of CD4+ and CD8+ T cells did not affect lung metastasis of 4T1 tumors in BALB/C mice (Suppl.Fig.9), indicating that lung metastasis was controlled by other immune cells. Since NK cells can exhibit anti-metastatic activity (33) and be controlled by Tregs (34), the inability of 4T1 cells to metastasize in NOD/SCID mice might be due to the loss of Tregs. Indeed, depletion of NK cells alone rendered NOD/SCID mice susceptible to metastasis drastically enhancing 4T1.2 tumor foci in the lungs (Fig.4B). Since the transfer of Tregs in NOD/SCID mice (Fig.2D, 4B) or depletion of Tregs in BALB/C mice (Fig.2C) restored or abolished lung metastasis of 4T1 tumors, respectively, these data taken together indicate that Tregs regulated NK cells to support lung metastasis. In fact, their numbers were inversely correlated in peripheral blood (PB) of tumor-bearing mice; and the PC61 Ab-mediated depletion of Tregs resulted in a significant increase of NK cell numbers (Fig.4C). The NK cell numbers were significantly reduced in PB of NOD/SCID mice that succumbed to lung metastasis due to the adoptive transfer BALB/C Tregs or splenocytes, (Fig.4D). In control mice treated with control Ab (IgG, Fig.4C) or transfused with non-Tregs (Fig.4D), the NK cell numbers were not changed. Tregs, but not NK cells, were also increased in the lungs of tumor CM- treated mice (Fig.3A).
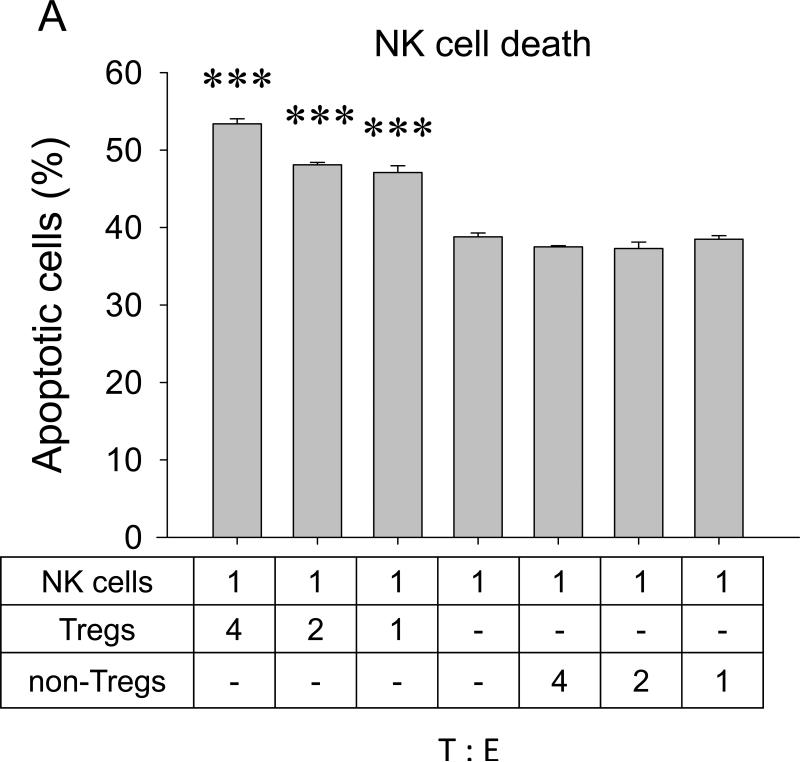
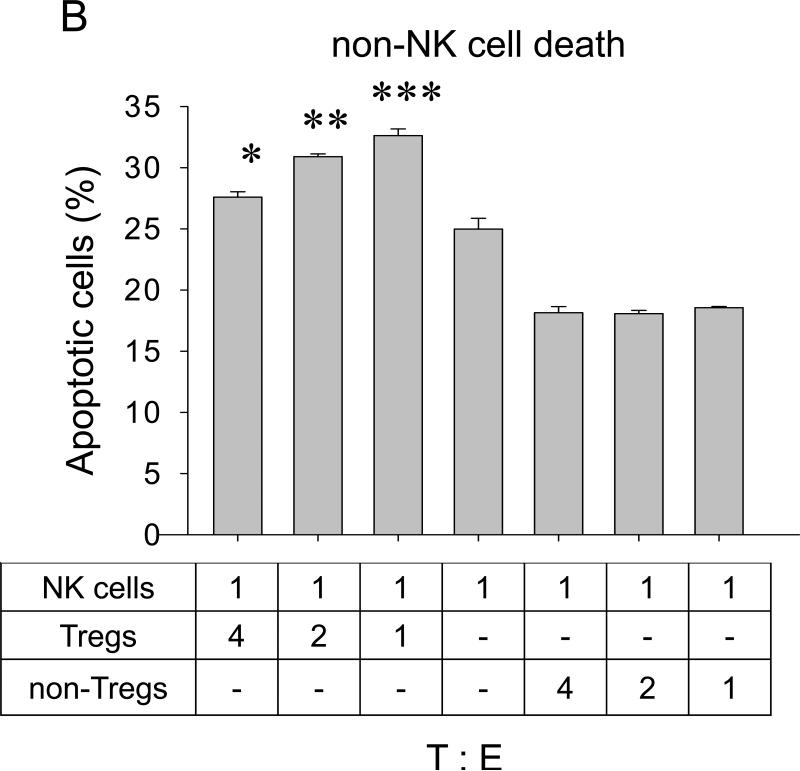
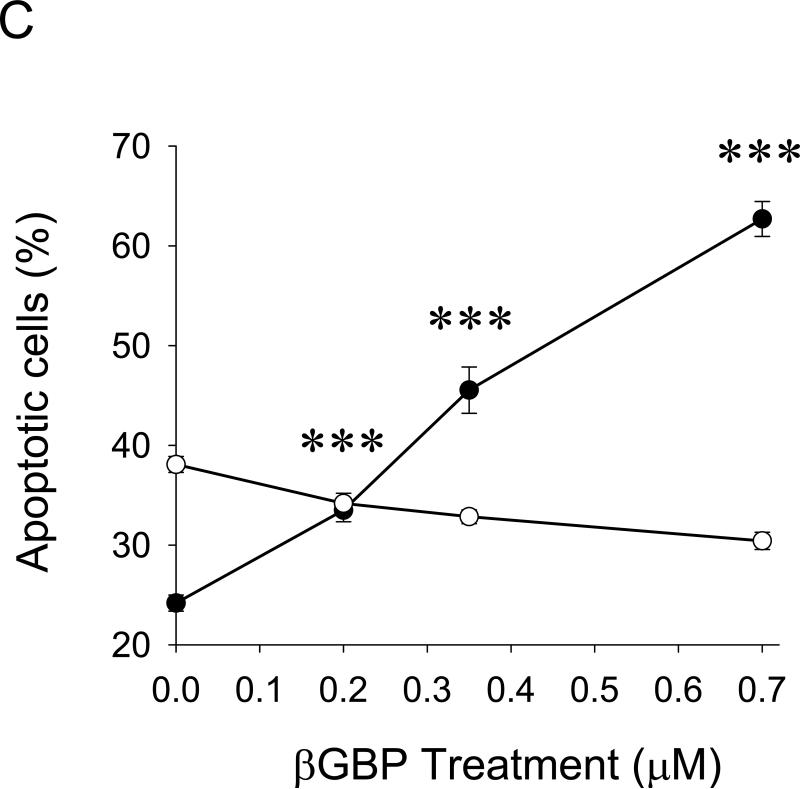
The inverse correlation between Tregs and NK cell numbers also suggests that Tregs may directly affect viability of NK cells. Indeed, Tregs induced significant NK apoptosis when they were co – cultured in vitro (Fig.5A). However, to do this efficiently, the proportion of Tregs had to be at least four- fold higher (4:1, Fig.5A), as they were by themselves killed by NK cells if mixed at lower doses (1:1, Fig.5b). This cytotoxic interaction between Tregs and NK cells is specific, as no reciprocal killing was detected when non-Tregs were co-cultured with NK cells (Fig.5A,B). Although the purpose of the current study was not to elucidate the mechanisms of this killing, Tregs appear to utilize a previously unknown pathway involving the β-galactoside binding protein (βGBP). Freshly isolated Tregs, but not non-Tregs, abundantly secreted βGBP (data not shown); and micromole amounts of recombinant βGBP induced significant apoptosis of NK cells, but not non-activated T cells (Fig.5C). Of note: These amounts are several logs below the “natural” cytotoxic doses of βGBP (35).
FIGURE 5. Tregs can directly kill NK cells.
Purified Tregs or non-Tregs were incubated with CFSE – labeled NK cells at indicated ratio prior staining with PI. Shown, proportion (%) of apoptotic NK cells (PI+CFSE+ ±SEM, A) and Tregs (PI+CFSE− ±SEM, B) of triplicate experiments. (C) Effects of βGBP on NK cells (filled circles) and T cells (open circles). Proportion of apoptotic cells (%, PI+ ±SEM of triplicate experiment) is shown. Experiments were repeated at least three times. (D) Lung metastasis can be controlled by targeting CCR4+ cells. 4T1.2 tumor-bearing mice were i.v. treated with TARC-PE38 or TARC-Ag, or control TARC-Ag or mock (PBS). Shown, mean lung metastasis ±SEM. Experiments were reproduced three times. * P<0.05; **P<0.01; ***P<0.001.
The CCR4 – targeting strategies can efficiently control lung metastasis
Taken together, these data suggest that TARC recruits CCR4+ tumor cells together with CCR4+ Tregs to protect them from NK cells. Therefore, we hypothesized that lung metastasis may be controlled by strategies that target CCR4. To test this, 4T1 tumor- bearing BALB/C mice were injected intravenously with 5μg TARC-PE38 a total of three times. In parallel, separate groups of mice were injected with 5 μg control TARC-Ag, or toxin that targeted CCR10 (CCL27-PE38), or with 500 μg of either anti-CD25 Ab or control IgG. While control treatments, such as CCL27-PE38 (Suppl.Fig.10), or TARC-Ag and control IgG did not affect lung metastasis (Fig.5D), lungs of the mice injected with TARC-PE38 or anti-CD25 Ab had significantly less metastatic foci (Fig.5D), indicating that the strategies that eliminate CCR4- expressing cells can reduce lung metastasis as efficiently as antibody- mediated depletion of Tregs.
Discussion
The role of chemokine receptors in cancer metastasis remains undefined. Even in the highly metastatic murine 4T1 breast cancer model, the role of chemokines and chemokine receptors has been thought to be indirect and associated with recruitment of other cells (36). Here, we demonstrate that 4T1 tumor is actually heterogeneous, and lung metastasis is only mediated by CCR4+ tumor cells. CCR4 has never been linked with breast cancer or lung metastasis; and the fact that a small but significant proportion of human breast cancer MCF7 cells also expressed CCR4 indicates that this was not an isolated case. Nevertheless, CCR4 expression alone was not sufficient in the absence of an active participation of host immune cells. 4T1 tumor is known to expand Gr1+ MDSCs through production of GM-CSF, IL-1β, IL-6 and TGFβ (6;7;37), which directly or indirectly impair immune responses and support survival and metastasis of 4T1 tumor cells (8;10). However, MDSCs may not be primary contributors of lung metastasis, since they were comparably well expanded in NOD/SCID mice that did not support tumor metastasis; and the depletion of Tregs also reduced the proportion of Gr1+ and GR1Int/F4/80+ cells in tumor- bearing BALB/C mice (data not shown). Our data suggest that CCR4+Tregs facilitate lung metastasis of 4T1 tumors. Although the methods used here could also affect non-Treg T cells, we do not think that non-Treg cells helped lung metastasis. First, the depletion of CD4+ or CD8+ T cells did not affect lung metastasis in BALB//C mice. Second, the transfer of non-Treg T cells did not restore lung metastasis in non-permissive NOD/SCID mice. In contrast, only depletion of Tregs could abrogate lung metastasis of 4T1 tumors in BALB/C mice. Moreover, the inability of 4T1 tumors to metastasize in NOD/SCID mice was reversed and lung metastasis ability was restored by adoptive transfer of Tregs. This restoration failed if Tregs were pretreated with TARCPE38 before the transfer that specifically killed CCR4+ cells. These results are in concordance with our previous report that Tregs are efficient suppressors of T cell responses (17).
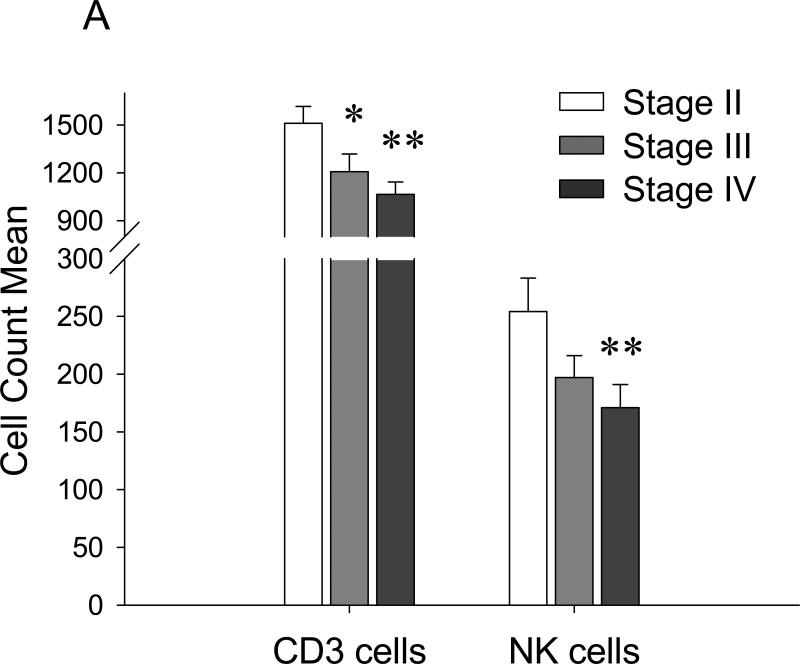
Tregs adversely affect NK cell- mediated cancer therapy (34) presumably by suppressing NK cell activity (38) or NK cell maturation (39), or through inhibition of NK cell and DC cross- talk (40). In concordance, our data indicate that Treg can also kill NK cells to protect lung metastasis. To do this, they utilize βGBP, a lectin- type immunomodulatory protein which was shown to be abundantly expressed by Tregs (24), and specifically by CCR4+Tregs (Baatar et al., unpublished data). At present, we can only speculate that the Treg-mediated killing of NK cells has probably occurred in the lungs. Unlike PB and lungs, no inverse correlation between Treg and NK cell numbers were detected in the secondary lymphoid organs of tumor CM-treated or tumor-bearing mice (Fig.3A,4C). Since 4T1 tumor closely reflects human metastatic breast cancer, it is tempting to speculate that Tregs may also regulate NK cells facilitating human cancer progression, particularly in advanced disease when metastasis is prevalent. In support, using a retrospective analysis of lymphocyte subsets in 73 previously untreated patients with breast cancer, we have found that NK and T cell levels were significantly reduced in patients with metastatic Stage IV disease as compared to those with non-metastatic Stage II tumors (Mann-Whitney non-parametric comparison for NK cells, p< 0.004, Fig.6A). Similar reduced NK cell counts and increased proportion of Tregs were also observed by others in PB of cancer patients with the advanced stages of head and neck squamous cell carcinoma (41). The decrease in numbers of tumor- infiltrating CD8+ and CD56+ cells was also associated with a unfavorable clinical course of patients with Hodgkin's disease and colorectal cancer (42;43). These observations could presumably be explained either by the ability of Tregs to control the generation of mature NK cells (39), or by our finding that Tregs were in fact able to kill NK cells.
FIGURE 6. NK cells are reduced in PB of breast cancer patients with the advanced/metastatic disease.
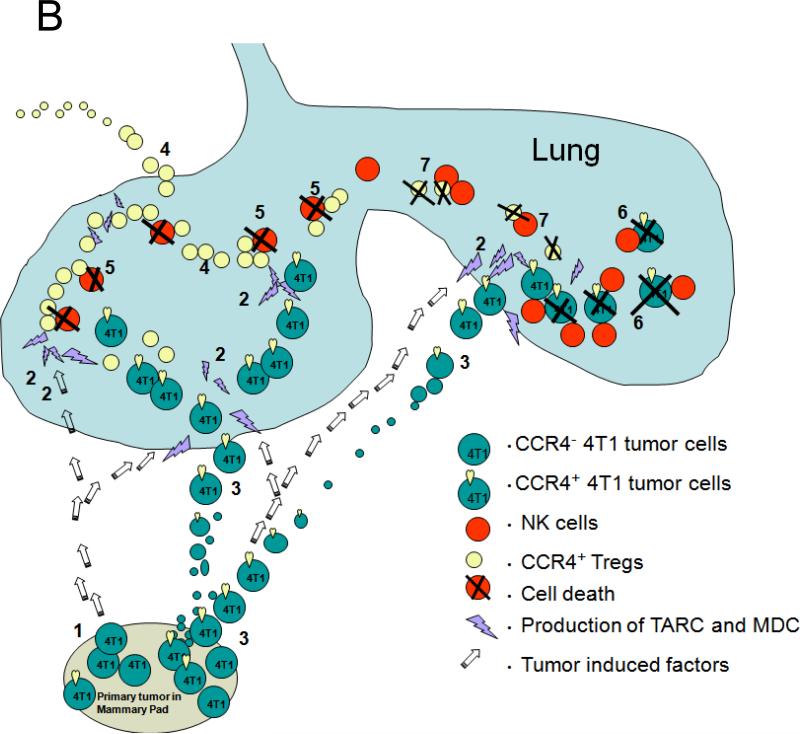
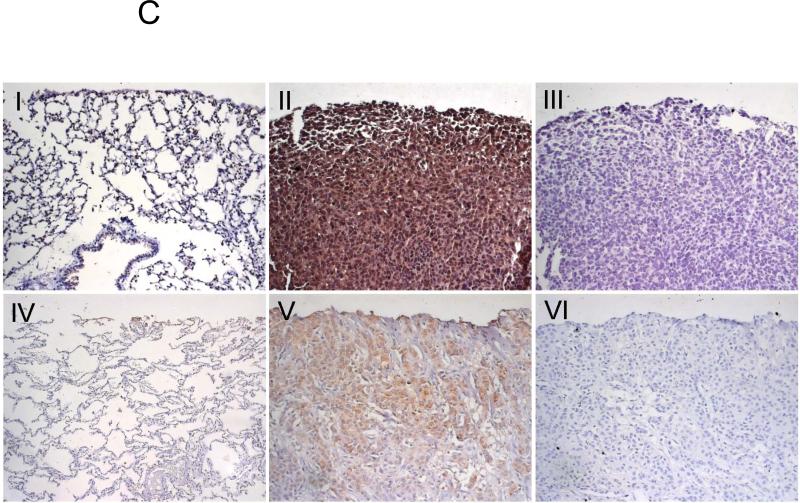
(A). The data are from nonparametric Mann Whitney analysis of previously untreated 73 breast cancer patients. Shown, T cell (CD3+) and NK cell counts in PB of patients with stage II and with the advanced disease (stage III and IV). (B) Schema that summarizes the finding. Primary breast cancer is heterogeneous, it consists of at least CCR4+ and CCR4− cells (1). TARC is induced in lungs by the primary tumor growing in mammary pad (open arrows, 2). This allows CCR4+ tumors to leave the primary site (3) and metastasize to lungs (2). However, the cells are efficiently killed by NK cells (6), unless they migrate together with their protectors – CCR4+ Tregs (4). Tregs inactivate NK cells using βGBP (5). However, Tregs can be also killed by NK cells (7), if they are present at lower numbers. (C) Immunohistochemistry staining of mouse (I-III) and human (IV-VI) lungs with 4T1 tumors (II and III) and human breast cancer (V and VI). Shown, control Ab (III and VI) or anti-TARC Ab (II and V) stainings of tumor-bearing lungs or normal tumor-free lungs (I and IV) of mice and human, respectively.
Overall, our data (summarized in Fig.6B) demonstrate that lung metastasis of breast cancer 4T1 cells is an active multistep process: on one hand, it requires CCR4 expression on the tumor cells to home into lungs that are producing TARC or MDC. In fact, the primary tumor growing in the mammary gland actively prepares its metastasis target site though induction of TARC and MDC expression in lung. Although the mechanism of this remote induction of chemokine production remains unknown, lungs could be activated by a wide range tumor-produced soluble factors that induce the mobilization and activation of MDSCs, such as TNFα, IL-6, and GM-CSF (6;7;37). However, even in the absence of this, TARC/MDC may be produced during pulmonary inflammation and various allergic diseases (22;44), which would periodically provide chemotactic stimuli for tumors. Despite this, lung metastasis can not be established without the active participation of immune cells. As CCR4+Tregs that infiltrate inflamed lungs (22), we define CCR4+Tregs as the required subset that infiltrate inflamed lungs. In support, FoxP3+ cell count was significantly increased in the lungs of tumor-bearing mice or mice i.p. injected with tumor CM. In addition, their long- term presence (at least four weeks) in the lungs of NOD/SCID mice was only detected in the mice that restored lung metastasis after adoptive transfer BALB/C Tregs, but not non-Tregs. Importantly, both the presence of Tregs and the support of metastasis in NOD/SCID mice were abolished, if CCR4+Tregs were killed by pretreatment with TARC-PE38 before transfer.
TARC/MDC expression is often correlated with a poor disease prognosis for cancer patients (1;45). In concordance, both murine and human breast cancer expressed TARC, as shown by immunohistochemistry staining of lungs with metastatic 4T1 tumor (panel II) and several (3/3) patients with breast cancer (panel V, Fig.6C). Although the biological meaning of this remains unknown, it is tempting to speculate that, as in human ovarian cancer (13), this is to recruit CCR4+Tregs and escape from immune surveillance. Taken together, our data suggest that CCR4 is also an important metastasis-associated receptor. Thus, strategies that target CCR4 (utilizing TARC-PE38 treatment), or Tregs (using the antibody – mediated depletion of Tregs) would be expected to have significant benefit in the control of lung metastasis, as they would shift responses towards protective antitumor innate and adaptive immune responses. The restoration of NK activity is often a sign of better cumulative survival outcome in cancer patients (46;47).
Supplementary Material
Supplemental Figure 1. TARC and CD11b/c expression in lungs of 4T1 challenged mice. Confocal imaging picture of mouse tumor-bearing lung tissues stained for TARC Ab (green) and CD11b/c (red). The data indicate that TARC is not expressed by CD11b/c stained cells. Panels B and C show staining for TARC alone and CD11b/c alone, respectively. Panel D is light microscopy image, Panel E is overlay of B, C and D (same as panel A, in higher magnification). Panel F is for control antibody staining (red blood cells show some low level auto fluorescence). Results of confocal study: TARC was predominantly expressed in metastatic lungs and possibly by tumor stromal cells. There was a significant infiltration of tumor with CD11b/c positive cells which did not express significant amounts of TARC. A few infiltrating T cells (CD3+) and B cells (CD45R+) were also negative for TARC (data not shown).
Antibodies and reagents and experimental procedure used for confocal staining of mouse lungs: Anti-TARC Ab (N20, sc-12271) was from Santa Cruz Biotechnology Inc., Santa Cruz, CA; anti-CD11b/c (ab53187), anti-CD3ε (ab49943), and anti-CD45R (ab64100) Abs were from Abcam Inc., Cambridge, MA. Antigen unmasking solution (H-3300), normal horse serum (S-2000), Avidin-Biotin blocking kit (SP2001), goat IgG (I-5000), fluorescein Avidin DCS (A2011), biotinilated anti-goat IgG (BA-9500), and vectashield Mounting medium (H-1400) were from Vector Laboratories, Inc., Burlingame, CA. Alexa 555 conjugated anti-rat and anti-rabbit IgGs were from Invitrogen Corp., Carlsbad, CA.
Formalin fixed and paraffin embedded lung tissue sections were deparafffinazed and rehydrated. Antigen retrieval was performed by boiling for 25 min in antigen unmasking solution. Sections were blocked with with avidin-biotin blocking kit followed by 5% normal horse serum and were incubated with goat anti-Tarc Ab (2 ug/ml) diluted in 5% normal horse serum o/n at 4C. Biotinilated anti-goat IgG produced in horse was used at 3 ug/ml for 1 hours at 37C followed by fluorescein avidin DCS (5 ug/ml) for 30 min at RT. After washings, sections were blocked with 5% normal donkey serum for 1 hour at 37C and incubated with either anti-CD11b/c (1:100), CD3ε (1:200), or CD45R (1:100) Abs for 1 hour at 37C. Thereafter, section were incubated with corresponding donkey secondary Abs conjugated to Alexa 555 for 30 min at 37C and mounted using Vectashield mounting medium. Images were acquired with a x40 objective on an Axiovert 200 microscope (Carl Zeiss Vision) and using Axiovison software (Carl Zeiss Vision).
Supplemental Figure 2. (A) CD11b− cells, but not CD11b+ immune cells, constitutively produce MDC. It can be further augmented by in vivo treatment with tumor CM (tumor CM, mice # 1−3), but not with control medium (Medium, mice # 4 and 5) or PBS (Naïve, mouse # 6). Mice were i.p. injected with media as in Fig.1B and lung CD11b+ and CD11b− cells were separated by magnetic sorting and overnight ex vivo cultured before testing for production of MDC by ELISA. Note, TARC levels were below sensitivity of the assay, but only mouse #2 CD11b− expressed about 80 pg/ml TARC. (B) Cultured mouse primary fibroblasts (C57, MLE12) produce MDC upon treatment with tumor CM. In contrast, immortalized fibroblast cell line NIH3T3 failed to produce MDC. Shown, mean ± SD (pg/ml) of triplicates of secreted MDC from cells after overnight treatment with tumor CM (Tumor CM) or control medium (Medium).
Supplemental Figure 3. CCR4 is expressed in murine breast 4T1 tumor cells. (A) Schema of CCR4 – specific primer locations on cDNA of murine CCR4 (NM_009916) and (B) results of RT/PCR are shown. Several sets of specific primers were tested to circumvent non-specific amplification. The coding region of CCR4 is in a single exon (rectangle). Total RNA was isolated combining Trizol reagent (Invitrogen) and a RNeasy kit (Qiagen Inc., Valencia, CA); and cDNA was made using Superscript II RT with a hexamer random primer at 37°C for 30 min and amplified using 2U Taq DNA polymerase (New England Biolabs Inc., Beverly, MA): 35 cycles of 94°C for 1 min, 60°C for 1 min, and 72°C for 1 min. Murine CCR4 cDNA fragments were PCR amplified using any combination of pairs of primers (see Suppl. Fig.1), such as forward primers: PRmCCR4−6 (ATTCTGTTGTGGTTCTGGTCCT), or PRmCCR4-R1 (AGGTCTGTGCAAGATCGTTTCATGG), or PRmCCR4−2 (GGCCTCTTGTTCAGCACTTG); and reverse primers: PRmCCR4-R6 (GCAGTGTTGCAGAGTCCTAATG), or PRmCCR4-R4 (CCGTACAACGTGGTGCTTTT), or PRmCCR4−1 (ATCCTGAAGGACTTCAAGCTCCA), or PRmCCR4-R8 (CACCTGCAGGGTGAGAAGCCATCTTGCCAT). Control RT/PCR was performed using murine glyceraldehyde-3-phosphate dehydrogenase, PRuGAPDH-1, 5’-TGTGGAAGGGCTCATGACCACAGTCCAT-3’ and PRuGAPDH-R1, 5’-GCCTGCTTCACCACCTTCTTGATG-3’. (C) CCR4 is also expressed on a small but significant proportion of human breast cancer MCF-7, but not MDA-231. The cells were stained with anti-CCR4 Ab or control isotype-matched Ab (not shown) and tested by FACS. Numbers indicate percentage of cells in the corresponding quadrant.
Supplemental Figure 4. (A) TARC-PE38 specifically kills purified CCR4-expressing CCRF-CEM (CEM), but not CCR4-negative MOLT-4 cells. The viability of cells treated with TARC-Ag or PBS was not affected (not shown). Cell death was evaluated using WST assay after 2 days of treatment. Shown, triplicate mean percentage of viable cells compared with untreated cells ± SD from three independent experiments. X-axis, treatment dose of TARC-PE38 (μg/ml). *P<0.01 is for comparisons with control treatments. (B) TARC-PE38 induces death of 4T1 and 4T1.2 tumor cells. In contrast, toxins that target CCR7 and CCR10 (SLC-PE38 and CCL27, respectively) or control TARC-Ag do not induce cell death of 4T1 tumor cells. Shown as percentage of viable cells to untreated cells evaluated by WST assay after 2 days of treatment. X-axis, treatment dose of TARC-PE38 (μg/ml). Representative data (mean ± SD of triplicates) from four independent experiments. X-axis, treatment dose (μg/ml). (C) 4T1-PE and 4T1.2-PE cells have stable phenotype, as they retain TARC-PE38 resistance even after several months of cultivation. Viability was assessed after treatment with titrated amounts of TARC-PE38 (μg/ml, X-axis). Control TARC-Ag or irrelevant chemotoxins did not kill any of the cells (data not shown).
Supplemental Figure 5. CCR4 – expressing tumors (4T1 and 4T1.2 tumors), but not TARC-PE38 -resistant tumors (4T1wt-PE and 4T1.2-PE), metastasize to lung. Metastatic nodules (arrows) are readily visible in lungs of BALB/C mice after challenge with 4T1 and 4T1.2 cells. Mice were euthanized at day 28 after tumor challenge with 4T1, 4T1.2, 4T1wt-PE and 4T1.2-PE tumors.
Supplemental Figure 6. TARC and MDC are produced in lungs of 4T1 tumor – bearing NOD/SCID mice. BAL of individual tumor free naïve mice (Naïve) and 4T1.2 tumor – bearing mice (4T1.2 bearer) was assessed for TARC and MDC expression by ELISA. Average amount of secreted chemokines (pg/ml) of three individual lungs ± SEM is shown on Y-axis.
Supplemental Figure 7. The majority of cells that migrated to TARC are FoxP3+. The cells from lower wells of the chemotaxis chamber (migrated cells in Fig.3D) were stained for CD4 (Y-axis) and FoxP3 (X-axis) and analyzed by by FACS. Numbers indicate percentage of cells in the corresponding quadrant. Data are reproduced two times.
Supplemental Figure 8. CCR4+ Tregs, but not non-Tregs facilitate lung metastasis of 4T1 tumors. Splenic Tregs and non Tregs, which were isolated from naïve BALB/C mice, were pretreated with TARC-PE38 for 1 hour. Then, the cells were washed and adoptively transferred into NOD/SCID mice challenged with 4T1.2 cells. Control tumor – bearing NOD/SCID mice that did not receive cells (PBS) did not metastasize to lung. Mean of lung metastasis ±SEM of four per group experiment, which was reproduced twice.
Supplemental Figure 9. Effector non-Treg T cells do not affect lung metastasis. 4T1.2 tumor-bearing four per group BALB/C mice were i.p. treated with 400 μg anti-CD4 mAb (FGK 1.5), or anti-CD8 mAb (FGK 2.43), or control Ab at 3, 7, 12 and 15 days post tumor challenge. The depletion of CD4+ and CD8+ cells was >98% at the time of sacrifice (day 28) as assessed by FACS. Shown, mean lung metastasis ±SEM at day 28 post tumor challenge of four mice per group experiment. Experiments were reproduced two times.
Supplemental Figure 10. Lung metastasis can be controlled by targeting CCR4+ cells. 4T1.2 tumor-bearing mice were i.v. treated daily 5μg/ml for 5 days with TARCPE38, or CCL27-PE38, or mock (PBS). Shown, mean lung metastasis ±SEM at day 28 post tumor challenge of four mice per group experiment. Experiments were reproduced two times. *P <0.05.
Acknowledgements
We are grateful to Dr. Dan Longo (NIA/NIH) for helpful comments and suggestions, and Ana Lustig (NIA/NIH) for critical reading of the manuscript, Drs. Dennis Taub, Ashani Weerarartna (NIA/NIH) and Fred E. Indig (NIA) for help with immunohistochemistry and confocal microscopy imaging; and Dr. Lars-Christian Horn (University of Leipzig) for providing the human lung tissue slides. This research was supported by the Intramural Research Program of the National Institute on Aging, NIH; and the authors do not have any conflict of interest.
Reference
- 1.Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–6. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 2.Rubie C, Frick VO, Wagner M, Rau B, Weber C, Kruse B, et al. Enhanced expression and clinical significance of CC-chemokine MIP-3 alpha in hepatocellular carcinoma. Scand J Immunol. 2006;63:468–77. doi: 10.1111/j.1365-3083.2006.001766.x. [DOI] [PubMed] [Google Scholar]
- 3.Balkwill F. Cancer and the chemokine network. Nat Rev Cancer. 2004;4:540–50. doi: 10.1038/nrc1388. [DOI] [PubMed] [Google Scholar]
- 4.Gunther K, Leier J, Henning G, Dimmler A, Weissbach R, Hohenberger W, et al. Prediction of lymph node metastasis in colorectal carcinoma by expressionof chemokine receptor CCR7. Int J Cancer. 2005;116:726–33. doi: 10.1002/ijc.21123. [DOI] [PubMed] [Google Scholar]
- 5.Lelekakis M, Moseley JM, Martin TJ, Hards D, Williams E, Ho P, et al. A novel orthotopic model of breast cancer metastasis to bone. Clin Exp Metastasis. 1999;17:163–70. doi: 10.1023/a:1006689719505. [DOI] [PubMed] [Google Scholar]
- 6.DuPre SA, Redelman D, Hunter KW., Jr. The mouse mammary carcinoma 4T1: characterization of the cellular landscape of primary tumours and metastatic tumour foci. Int J Exp Pathol. 2007;88:351–60. doi: 10.1111/j.1365-2613.2007.00539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Danna EA, Sinha P, Gilbert M, Clements VK, Pulaski BA, Ostrand-Rosenberg S. Surgical removal of primary tumor reverses tumor-induced immunosuppression despite the presence of metastatic disease. Cancer Res. 2004;64:2205–11. doi: 10.1158/0008-5472.can-03-2646. [DOI] [PubMed] [Google Scholar]
- 8.Serafini P, De SC, Marigo I, Cingarlini S, Dolcetti L, Gallina G, et al. Derangement of immune responses by myeloid suppressor cells. Cancer Immunol Immunother. 2004;53:64–72. doi: 10.1007/s00262-003-0443-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DuPre' SA, Hunter KW., Jr. Murine mammary carcinoma 4T1 induces a leukemoid reaction with splenomegaly: association with tumor-derived growth factors. Exp Mol Pathol. 2007;82:12–24. doi: 10.1016/j.yexmp.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 10.Huang B, Pan PY, Li Q, Sato AI, Levy DE, Bromberg J, et al. Gr-1+CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res. 2006;66:1123–31. doi: 10.1158/0008-5472.CAN-05-1299. [DOI] [PubMed] [Google Scholar]
- 11.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–64. [PubMed] [Google Scholar]
- 12.Ishida T, Utsunomiya A, Iida S, Inagaki H, Takatsuka Y, Kusumoto S, et al. Clinical significance of CCR4 expression in adult T-cell leukemia/lymphoma: its close association with skin involvement and unfavorable outcome. Clin Cancer Res. 2003;9:3625–34. [PubMed] [Google Scholar]
- 13.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–9. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 14.Woo EY, Yeh H, Chu CS, Schlienger K, Carroll RG, Riley JL, et al. Cutting edge: Regulatory T cells from lung cancer patients directly inhibit autologous T cell proliferation. J Immunol. 2002;168:4272–6. doi: 10.4049/jimmunol.168.9.4272. [DOI] [PubMed] [Google Scholar]
- 15.Beyer M, Kochanek M, Darabi K, Popov A, Jensen M, Endl E, et al. Reduced frequencies and suppressive function of CD4+CD25hi regulatory T cells in patients with chronic lymphocytic leukemia after therapy with fludarabine. Blood. 2005;106:2018–25. doi: 10.1182/blood-2005-02-0642. [DOI] [PubMed] [Google Scholar]
- 16.Lloyd CM, Rankin SM. Chemokines in allergic airway disease. Curr Opin Pharmacol. 2003;3:443–8. doi: 10.1016/s1471-4892(03)00069-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baatar D, Olkhanud P, Sumitomo K, Taub D, Gress R, Biragyn A. Human Peripheral Blood T Regulatory Cells (Tregs), Functionally Primed CCR4+ Tregs and Unprimed CCR4- Tregs, Regulate Effector T Cells Using FasL. J Immunol. 2007;178:4891–900. doi: 10.4049/jimmunol.178.8.4891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iellem A, Mariani M, Lang R, Recalde H, Panina-Bordignon P, Sinigaglia F, et al. Unique chemotactic response profile and specific expression of chemokine receptors CCR4 and CCR8 by CD4(+)CD25(+) regulatory T cells. J Exp Med. 2001;194:847–53. doi: 10.1084/jem.194.6.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Inngjerdingen M, Damaj B, Maghazachi AA. Human NK cells express CC chemokine receptors 4 and 8 and respond to thymus and activation-regulated chemokine, macrophage-derived chemokine, and I-309. J Immunol. 2000;164:4048–54. doi: 10.4049/jimmunol.164.8.4048. [DOI] [PubMed] [Google Scholar]
- 20.Meyer EH, Wurbel MA, Staton TL, Pichavant M, Kan MJ, Savage PB, et al. iNKT cells require CCR4 to localize to the airways and to induce airway hyperreactivity. J Immunol. 2007;179:4661–71. doi: 10.4049/jimmunol.179.7.4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johansson C, Ahlstedt I, Furubacka S, Johnsson E, Agace WW, Quiding-Jarbrink M. Differential expression of chemokine receptors on human IgA+ and IgG+ B cells. Clin Exp Immunol. 2005;141:279–87. doi: 10.1111/j.1365-2249.2005.02843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jacobsen EA, Ochkur SI, Pero RS, Taranova AG, Protheroe CA, Colbert DC, et al. Allergic pulmonary inflammation in mice is dependent on eosinophil-induced recruitment of effector T cells. J Exp Med. 2008;205:699–710. doi: 10.1084/jem.20071840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baatar D, Olkhanud P, Newton D, Sumitomo K, Biragyn A. CCR4-expressing T cell tumors can be specifically controlled via delivery of toxins to chemokine receptors. J Immunol. 2007;179:1996–2004. doi: 10.4049/jimmunol.179.3.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garin MI, Chu CC, Golshayan D, Cernuda-Morollon E, Wait R, Lechler RI. Galectin-1: a key effector of regulation mediated by CD4+CD25+ T cells. Blood. 2007;109:2058–65. doi: 10.1182/blood-2006-04-016451. [DOI] [PubMed] [Google Scholar]
- 25.Ravatn R, Wells V, Nelson L, Vettori D, Mallucci L, Chin KV. Circumventing multidrug resistance in cancer by beta-galactoside binding protein, an antiproliferative cytokine. Cancer Res. 2005;65:1631–4. doi: 10.1158/0008-5472.CAN-04-1970. [DOI] [PubMed] [Google Scholar]
- 26.Novelli F, Allione A, Wells V, Forni G, Mallucci L. Negative cell cycle control of human T cells by beta-galactoside binding protein (beta GBP): induction of programmed cell death in leukaemic cells. J Cell Physiol. 1999;178:102–8. doi: 10.1002/(SICI)1097-4652(199901)178:1<102::AID-JCP13>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 27.Allione A, Wells V, Forni G, Mallucci L, Novelli F. Beta-galactoside-binding protein (beta GBP) alters the cell cycle, up-regulates expression of the alpha- and beta-chains of the IFN-gamma receptor, and triggers IFN-gamma-mediated apoptosis of activated human T lymphocytes. J Immunol. 1998;161:2114–9. [PubMed] [Google Scholar]
- 28.Wells V, Mallucci L. Identification of an autocrine negative growth factor: mouse beta-galactoside-binding protein is a cytostatic factor and cell growth regulator. Cell. 1991;64:91–7. doi: 10.1016/0092-8674(91)90211-g. [DOI] [PubMed] [Google Scholar]
- 29.Almanzar G, Olkhanud PB, Bodogai M, Dell'Agnola C, Baatar D, Hewitt SM, et al. Sperm-Derived SPANX-B Is a Clinically Relevant Tumor Antigen That Is Expressed in Human Tumors and Readily Recognized by Human CD4+ and CD8+ T Cells. Clin Cancer Res. 2009 doi: 10.1158/1078-0432.CCR-08-1290. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Falk W, Goodwin RHJ, Leonard EJ. A 48-well micro chemotaxis assembly for rapid and accurate measurement of leukocyte migration. J Immunol Methods. 1980;33:239–47. doi: 10.1016/0022-1759(80)90211-2. [DOI] [PubMed] [Google Scholar]
- 31.Saito K, Torii M, Ma N, Tsuchiya T, Wang L, Hori T, et al. Differential regulatory function of resting and preactivated allergen-specific CD4+ CD25+ regulatory T cells in Th2-type airway inflammation. J Immunol. 2008;181:6889–97. doi: 10.4049/jimmunol.181.10.6889. [DOI] [PubMed] [Google Scholar]
- 32.Aandahl EM, Michaelsson J, Moretto WJ, Hecht FM, Nixon DF. Human CD4+ CD25+ regulatory T cells control T-cell responses to human immunodeficiency virus and cytomegalovirus antigens. J Virol. 2004;78:2454–9. doi: 10.1128/JVI.78.5.2454-2459.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zheng LM, Ojcius DM, Garaud F, Roth C, Maxwell E, Li Z, et al. Interleukin-10 inhibits tumor metastasis through an NK cell-dependent mechanism. J Exp Med. 1996;184:579–84. doi: 10.1084/jem.184.2.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smyth MJ, Teng MW, Swann J, Kyparissoudis K, Godfrey DI, Hayakawa Y. CD4+CD25+ T regulatory cells suppress NK cell-mediated immunotherapy of cancer. J Immunol. 2006;176:1582–7. doi: 10.4049/jimmunol.176.3.1582. [DOI] [PubMed] [Google Scholar]
- 35.Camby I, Le MM, Lefranc F, Kiss R. Galectin-1: a small protein with major functions. Glycobiology. 2006;16:137R–57R. doi: 10.1093/glycob/cwl025. [DOI] [PubMed] [Google Scholar]
- 36.van Deventer HW, O'Connor W, Jr., Brickey WJ, Aris RM, Ting JP, Serody JS. C-C chemokine receptor 5 on stromal cells promotes pulmonary metastasis. Cancer Res. 2005;65:3374–9. doi: 10.1158/0008-5472.CAN-04-2616. [DOI] [PubMed] [Google Scholar]
- 37.Sinha P, Clements VK, Ostrand-Rosenberg S. Reduction of myeloid-derived suppressor cells and induction of M1 macrophages facilitate the rejection of established metastatic disease. J Immunol. 2005;174:636–45. doi: 10.4049/jimmunol.174.2.636. [DOI] [PubMed] [Google Scholar]
- 38.Barao I, Hanash AM, Hallett W, Welniak LA, Sun K, Redelman D, et al. Suppression of natural killer cell-mediated bone marrow cell rejection by CD4+CD25+ regulatory T cells. Proc Natl Acad Sci U S A. 2006;103:5460–5. doi: 10.1073/pnas.0509249103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Giroux M, Yurchenko E, St-Pierre J, Piccirillo CA, Perreault C. T regulatory cells control numbers of NK cells and CD8alpha+ immature dendritic cells in the lymph node paracortex. J Immunol. 2007;179:4492–502. doi: 10.4049/jimmunol.179.7.4492. [DOI] [PubMed] [Google Scholar]
- 40.Terme M, Chaput N, Combadiere B, Ma A, Ohteki T, Zitvogel L. Regulatory T cells control dendritic cell/NK cell cross-talk in lymph nodes at the steady state by inhibiting CD4+ self-reactive T cells. J Immunol. 2008;180:4679–86. doi: 10.4049/jimmunol.180.7.4679. [DOI] [PubMed] [Google Scholar]
- 41.Bose A, Chakraborty T, Chakraborty K, Pal S, Baral R. Dysregulation in immune functions is reflected in tumor cell cytotoxicity by peripheral blood mononuclear cells from head and neck squamous cell carcinoma patients. Cancer Immun. 2008;8:10. [PMC free article] [PubMed] [Google Scholar]
- 42.varo-Naranjo T, Lejeune M, Salvado-Usach MT, Bosch-Princep R, Reverter-Branch, Jaen-Martinez J, et al. Tumor-infiltrating cells as a prognostic factor in Hodgkin's lymphoma: a quantitative tissue microarray study in a large retrospective cohort of 267 patients. Leuk Lymphoma. 2005;46:1581–91. doi: 10.1080/10428190500220654. [DOI] [PubMed] [Google Scholar]
- 43.Milasiene V, Stratilatovas E, Norkiene V, Jonusauskaite R. Lymphocyte subsets in peripheral blood as prognostic factors in colorectal cancer. J BUON. 2005;10:261–4. [PubMed] [Google Scholar]
- 44.Gonzalo JA, Pan Y, Lloyd CM, Jia GQ, Yu G, Dussault B, et al. Mouse monocyte-derived chemokine is involved in airway hyperreactivity and lung inflammation. J Immunol. 1999;163:403–11. [PubMed] [Google Scholar]
- 45.White ES, Flaherty KR, Carskadon S, Brant A, Iannettoni MD, Yee J, et al. Macrophage migration inhibitory factor and CXC chemokine expression in non-small cell lung cancer: role in angiogenesis and prognosis. Clin Cancer Res. 2003;9:853–60. [PubMed] [Google Scholar]
- 46.Imai K, Matsuyama S, Miyake S, Suga K, Nakachi K. Natural cytotoxic activity of peripheral-blood lymphocytes and cancer incidence: an 11-year follow-up study of a general population. Lancet. 2000;356:1795–9. doi: 10.1016/S0140-6736(00)03231-1. [DOI] [PubMed] [Google Scholar]
- 47.Ruggeri L, Capanni M, Urbani E, Perruccio K, Shlomchik WD, Tosti A, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295:2097–100. doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. TARC and CD11b/c expression in lungs of 4T1 challenged mice. Confocal imaging picture of mouse tumor-bearing lung tissues stained for TARC Ab (green) and CD11b/c (red). The data indicate that TARC is not expressed by CD11b/c stained cells. Panels B and C show staining for TARC alone and CD11b/c alone, respectively. Panel D is light microscopy image, Panel E is overlay of B, C and D (same as panel A, in higher magnification). Panel F is for control antibody staining (red blood cells show some low level auto fluorescence). Results of confocal study: TARC was predominantly expressed in metastatic lungs and possibly by tumor stromal cells. There was a significant infiltration of tumor with CD11b/c positive cells which did not express significant amounts of TARC. A few infiltrating T cells (CD3+) and B cells (CD45R+) were also negative for TARC (data not shown).
Antibodies and reagents and experimental procedure used for confocal staining of mouse lungs: Anti-TARC Ab (N20, sc-12271) was from Santa Cruz Biotechnology Inc., Santa Cruz, CA; anti-CD11b/c (ab53187), anti-CD3ε (ab49943), and anti-CD45R (ab64100) Abs were from Abcam Inc., Cambridge, MA. Antigen unmasking solution (H-3300), normal horse serum (S-2000), Avidin-Biotin blocking kit (SP2001), goat IgG (I-5000), fluorescein Avidin DCS (A2011), biotinilated anti-goat IgG (BA-9500), and vectashield Mounting medium (H-1400) were from Vector Laboratories, Inc., Burlingame, CA. Alexa 555 conjugated anti-rat and anti-rabbit IgGs were from Invitrogen Corp., Carlsbad, CA.
Formalin fixed and paraffin embedded lung tissue sections were deparafffinazed and rehydrated. Antigen retrieval was performed by boiling for 25 min in antigen unmasking solution. Sections were blocked with with avidin-biotin blocking kit followed by 5% normal horse serum and were incubated with goat anti-Tarc Ab (2 ug/ml) diluted in 5% normal horse serum o/n at 4C. Biotinilated anti-goat IgG produced in horse was used at 3 ug/ml for 1 hours at 37C followed by fluorescein avidin DCS (5 ug/ml) for 30 min at RT. After washings, sections were blocked with 5% normal donkey serum for 1 hour at 37C and incubated with either anti-CD11b/c (1:100), CD3ε (1:200), or CD45R (1:100) Abs for 1 hour at 37C. Thereafter, section were incubated with corresponding donkey secondary Abs conjugated to Alexa 555 for 30 min at 37C and mounted using Vectashield mounting medium. Images were acquired with a x40 objective on an Axiovert 200 microscope (Carl Zeiss Vision) and using Axiovison software (Carl Zeiss Vision).
Supplemental Figure 2. (A) CD11b− cells, but not CD11b+ immune cells, constitutively produce MDC. It can be further augmented by in vivo treatment with tumor CM (tumor CM, mice # 1−3), but not with control medium (Medium, mice # 4 and 5) or PBS (Naïve, mouse # 6). Mice were i.p. injected with media as in Fig.1B and lung CD11b+ and CD11b− cells were separated by magnetic sorting and overnight ex vivo cultured before testing for production of MDC by ELISA. Note, TARC levels were below sensitivity of the assay, but only mouse #2 CD11b− expressed about 80 pg/ml TARC. (B) Cultured mouse primary fibroblasts (C57, MLE12) produce MDC upon treatment with tumor CM. In contrast, immortalized fibroblast cell line NIH3T3 failed to produce MDC. Shown, mean ± SD (pg/ml) of triplicates of secreted MDC from cells after overnight treatment with tumor CM (Tumor CM) or control medium (Medium).
Supplemental Figure 3. CCR4 is expressed in murine breast 4T1 tumor cells. (A) Schema of CCR4 – specific primer locations on cDNA of murine CCR4 (NM_009916) and (B) results of RT/PCR are shown. Several sets of specific primers were tested to circumvent non-specific amplification. The coding region of CCR4 is in a single exon (rectangle). Total RNA was isolated combining Trizol reagent (Invitrogen) and a RNeasy kit (Qiagen Inc., Valencia, CA); and cDNA was made using Superscript II RT with a hexamer random primer at 37°C for 30 min and amplified using 2U Taq DNA polymerase (New England Biolabs Inc., Beverly, MA): 35 cycles of 94°C for 1 min, 60°C for 1 min, and 72°C for 1 min. Murine CCR4 cDNA fragments were PCR amplified using any combination of pairs of primers (see Suppl. Fig.1), such as forward primers: PRmCCR4−6 (ATTCTGTTGTGGTTCTGGTCCT), or PRmCCR4-R1 (AGGTCTGTGCAAGATCGTTTCATGG), or PRmCCR4−2 (GGCCTCTTGTTCAGCACTTG); and reverse primers: PRmCCR4-R6 (GCAGTGTTGCAGAGTCCTAATG), or PRmCCR4-R4 (CCGTACAACGTGGTGCTTTT), or PRmCCR4−1 (ATCCTGAAGGACTTCAAGCTCCA), or PRmCCR4-R8 (CACCTGCAGGGTGAGAAGCCATCTTGCCAT). Control RT/PCR was performed using murine glyceraldehyde-3-phosphate dehydrogenase, PRuGAPDH-1, 5’-TGTGGAAGGGCTCATGACCACAGTCCAT-3’ and PRuGAPDH-R1, 5’-GCCTGCTTCACCACCTTCTTGATG-3’. (C) CCR4 is also expressed on a small but significant proportion of human breast cancer MCF-7, but not MDA-231. The cells were stained with anti-CCR4 Ab or control isotype-matched Ab (not shown) and tested by FACS. Numbers indicate percentage of cells in the corresponding quadrant.
Supplemental Figure 4. (A) TARC-PE38 specifically kills purified CCR4-expressing CCRF-CEM (CEM), but not CCR4-negative MOLT-4 cells. The viability of cells treated with TARC-Ag or PBS was not affected (not shown). Cell death was evaluated using WST assay after 2 days of treatment. Shown, triplicate mean percentage of viable cells compared with untreated cells ± SD from three independent experiments. X-axis, treatment dose of TARC-PE38 (μg/ml). *P<0.01 is for comparisons with control treatments. (B) TARC-PE38 induces death of 4T1 and 4T1.2 tumor cells. In contrast, toxins that target CCR7 and CCR10 (SLC-PE38 and CCL27, respectively) or control TARC-Ag do not induce cell death of 4T1 tumor cells. Shown as percentage of viable cells to untreated cells evaluated by WST assay after 2 days of treatment. X-axis, treatment dose of TARC-PE38 (μg/ml). Representative data (mean ± SD of triplicates) from four independent experiments. X-axis, treatment dose (μg/ml). (C) 4T1-PE and 4T1.2-PE cells have stable phenotype, as they retain TARC-PE38 resistance even after several months of cultivation. Viability was assessed after treatment with titrated amounts of TARC-PE38 (μg/ml, X-axis). Control TARC-Ag or irrelevant chemotoxins did not kill any of the cells (data not shown).
Supplemental Figure 5. CCR4 – expressing tumors (4T1 and 4T1.2 tumors), but not TARC-PE38 -resistant tumors (4T1wt-PE and 4T1.2-PE), metastasize to lung. Metastatic nodules (arrows) are readily visible in lungs of BALB/C mice after challenge with 4T1 and 4T1.2 cells. Mice were euthanized at day 28 after tumor challenge with 4T1, 4T1.2, 4T1wt-PE and 4T1.2-PE tumors.
Supplemental Figure 6. TARC and MDC are produced in lungs of 4T1 tumor – bearing NOD/SCID mice. BAL of individual tumor free naïve mice (Naïve) and 4T1.2 tumor – bearing mice (4T1.2 bearer) was assessed for TARC and MDC expression by ELISA. Average amount of secreted chemokines (pg/ml) of three individual lungs ± SEM is shown on Y-axis.
Supplemental Figure 7. The majority of cells that migrated to TARC are FoxP3+. The cells from lower wells of the chemotaxis chamber (migrated cells in Fig.3D) were stained for CD4 (Y-axis) and FoxP3 (X-axis) and analyzed by by FACS. Numbers indicate percentage of cells in the corresponding quadrant. Data are reproduced two times.
Supplemental Figure 8. CCR4+ Tregs, but not non-Tregs facilitate lung metastasis of 4T1 tumors. Splenic Tregs and non Tregs, which were isolated from naïve BALB/C mice, were pretreated with TARC-PE38 for 1 hour. Then, the cells were washed and adoptively transferred into NOD/SCID mice challenged with 4T1.2 cells. Control tumor – bearing NOD/SCID mice that did not receive cells (PBS) did not metastasize to lung. Mean of lung metastasis ±SEM of four per group experiment, which was reproduced twice.
Supplemental Figure 9. Effector non-Treg T cells do not affect lung metastasis. 4T1.2 tumor-bearing four per group BALB/C mice were i.p. treated with 400 μg anti-CD4 mAb (FGK 1.5), or anti-CD8 mAb (FGK 2.43), or control Ab at 3, 7, 12 and 15 days post tumor challenge. The depletion of CD4+ and CD8+ cells was >98% at the time of sacrifice (day 28) as assessed by FACS. Shown, mean lung metastasis ±SEM at day 28 post tumor challenge of four mice per group experiment. Experiments were reproduced two times.
Supplemental Figure 10. Lung metastasis can be controlled by targeting CCR4+ cells. 4T1.2 tumor-bearing mice were i.v. treated daily 5μg/ml for 5 days with TARCPE38, or CCL27-PE38, or mock (PBS). Shown, mean lung metastasis ±SEM at day 28 post tumor challenge of four mice per group experiment. Experiments were reproduced two times. *P <0.05.