Abstract
The replication of human immunodeficiency type-1 (HIV-1) is restricted in macaque cells, in part due to host factors that provide intrinsic immunity after entry. Here we show that a rhesus macaque epithelial cell line engineered to express human CD4, sMAGI cells, has at least two post-entry restrictions to HIV-1 replication: one that is dependent on a previously described post-entry restriction factor of macaque cells, TRIM5α, and another that is primarily TRIM5α-independent. The TRIM5α restriction, which was observed with particles that had an HIV-1 core pseudotyped with VSV-G envelope, is saturable and can be completely abrogated by introducing TRIM5α-specific siRNA into the cells. A similar TRIM5α-dependent restriction was observed when sMAGI cells expressing human CCR5 were infected with an R5-HIV-1. In contrast, even when viruses enter sMAGI cells using CD4 and an endogenous rhesus coreceptor at levels sufficient to saturate TRIM5α, they do not productively infect the sMAGI cells. Nor does treatment of sMAGI cells with TRIM5α-specific siRNA relieve this post-entry restriction; this was true whether the HIV-1 core was pseudotyped with SIV envelope or an R5-HIV-1 envelope. Together these data suggest that there is an alternate restriction to the replication of viruses that enter via interaction with CD4 and an endogenous rhesus coreceptor, which we call here Lv3. Thus, these findings suggest that post-entry events are dependent upon the mechanism by which HIV-1 enters the cell.
Introduction
The ability of human immunodeficiency virus type 1 (HIV-1) to replicate in a particular cell is determined by multiple requisite interactions between the virus and that target cell. The first of these is binding of the envelope surface unit to the receptor CD4 and a coreceptor, typically CCR5, which initiates fusion of the viral and cellular membranes. (Reviewed in (Berger, Murphy, and Farber, 1999)). Once inside the cell, HIV-1 must then overcome several countermeasures within the cell that provide anti-retroviral intrinsic immunity. These factors, typified by the recently discovered TRIM5α and APOBEC3G proteins (reviewed in (Zheng and Peterlin, 2005)) are most often cell-type specific, and viruses that are unable to counter these intrinsic immunity factors in a cell are blocked in their replication. Thus, multiple virus-host interactions, both at the plasma membrane and within the cell, contribute to the host cell specificity of HIV-1.
Previously, this lab described a rhesus macaque epithelial cell line expressing human CD4 (sMAGI) that can be productively infected by SIV (Chackerian, Haigwood, and Overbaugh, 1995), but not by HIV-1 (Chackerian et al., 1997). sMAGI cells do not express any of the major HIV-1 or SIV coreceptors (CCR5, CXCR4, GPR15 or CXCR6), suggesting that they express a novel coreceptor that has yet to be identified ((Pohlmann et al., 2004) and Pineda and Overbaugh, unpublished). Despite the absence of a known HIV-1/SIV coreceptor, sMAGI cells permit entry of some HIV-1 strains, as evidenced by the accumulation of cytoplasmic, late reverse transcription intermediates (Chackerian et al., 1997). This post-entry restriction encountered by some HIV-1, typified by the R5 viral clone HIV-1 SF162, was rescued by complementing the HIV-1 envelope with SIV genes as a SHIV chimera. Productive infection was also observed for R5-HIV-1 in sMAGI cells that were engineered to express human CCR5 (sMAGI-CCR5) (Chackerian et al., 1997). Thus, this restriction is influenced both by 5′ sequences in the virus as well as by the nature of the envelope/coreceptor interactions.
Subsequently, two post-entry restrictions that bear some similarities to the HIV-1 restriction observed in sMAGI cells have been described in other primate cells: Lv1 (Cowan et al., 2002) and Lv2 (Marchant et al., 2005; McKnight et al., 2001; Schmitz et al., 2004). While the molecular mechanism responsible for mediating the Lv2 restriction is unclear, the cellular factor responsible for the Lv1 restriction has been identified as TRIM5α (Stremlau et al., 2004). TRIM5α restriction occurs during uncoating and reverse transcription (Bieniasz, 2003; Cowan et al., 2002) and is thought to be the result of accelerated uncoating of capsid in the viral particle (Stremlau et al., 2006). The TRIM5α restriction is saturable: infecting target cells with a viral inoculum above a threshold dose results in breakthrough infection of the target cells. The Lv2 restriction is also dependent on capsid, but it is distinguished from the Trim5α restriction in that the restriction is influenced by the viral envelope protein: some viral envelopes target HIV-1 to an Lv2-restricted compartment, while other viral envelopes shuttle HIV-1 to a compartment in which Lv2 is absent or less potent (Marchant et al., 2005).
Here, we examined the role of TRIM5α in the post-entry restriction to HIV-1 infection in sMAGI cells. We showed that in sMAGI cells engineered to express human CCR5, TRIM5α is an intrinsic early post-entry inhibitor to R5-HIV-1. TRIM5α also caused a restriction to HIV-1 particles pseudotyped with the glycoprotein of Vesicular Stomatitis Virus (VSV-G) in sMAGI cells. However, the post entry restriction to R5-HIV-1 in sMAGI cells lacking human CCR5 was not dependent on TRIM5α. In sMAGI cells, HIV-1 cores pseudotyped with either HIV-1 or SIV envelope remained restricted even at doses that saturate TRIM5α. These data suggest that within this single cell type, there are two mechanisms of restriction to R5-HIV-1.
Results
The restriction HIV-1 SF162 infection in sMAGI cells is not overcome with increasing virus dose or by reducing TRIM5α levels
The post-entry restriction to HIV-1 SF162 and other variants that was previously described in sMAGI cells was observed when testing viruses at a fixed dose that was presumably less than one infectious virus particle per cell (0.25 TCID50 based on infection of permissive human cells per sMAGI cell: (Chackerian et al., 1997)). To test whether the restriction to HIV-1SF162 in rhesus sMAGI cells could be overcome by saturation, we infected rhesus sMAGI cells with increasing doses of particles pseudotyped with HIV-1 SF162 envelope protein (HIV-1/SF162) (Fig. 1A). Little or no productive infection above background variation (typically this variation is 5–10 blue cells depending on the experiment) was observed in rhesus cells infected with HIV-1/SF162 over the greater than four-log range of viral doses tested (Fig. 1A). In contrast, the number of β-galactosidase positive cells increased in direct relation with the increasing doses of HIV-1/SF162 in a permissive human cell line used as a control—human HeLa cells engineered to express human CD4 and human CCR5, MAGI-CCR5 cells (Chackerian et al., 1997)—reaching the upper limit of reliable quantification at an input virus level of approximately 1000ng p24 Gag (corresponding to an MOI of ~0.1; (Fig. 1A)].
Fig. 1.
Infection of sMAGI cells and human MAGI-CCR5 across a range of virus doses. The number of blue, β-galactosidase positive cells was determined using microscopy to count blue cell foci, which has an upper range or reliable quantification at ~1000 blue cells. The X-axis represents increasing infectious dose of virus as defined by Gagp24 (or Gagp27 for SIV) concentration. Error bars represent standard deviation of three replicate infections. The average number of blue cells detected in mock-infected cells was subtracted from each data point. Hollow symbols represent the number of infected rhesus sMAGI cells; black solid symbols represent the number of infected human MAGI-CCR5 cells. A. Infection with HIV-1/SF162. B. Infection with HIV-1/VSV-G. C. Infection with SIV/VSV-G. D. Infections with HIV-1/VSV-G (square symbols) and SIV/VSV-G (circle symbols) in the presence of HIV-1 VLP with a VSV-G envelope (left panel) or HIV-1 VLP lacking envelope (right panel).
The TRIM5α restriction has most extensively been studied with HIV-1 particles pseudotyped with the glycoprotein of the Vesicular Stomatitis Virus (HIV-1/VSV-G); it has also been specifically demonstrated for wild type HIV-1 in some cells (Munk et al., 2002; Stremlau et al., 2004). Given the focus on HIV-1/VSV-G infection in several previous studies, we wanted to specifically examine whether HIV-1/VSV-G was restricted in sMAGI cells and whether the restriction was similar to that of HIV-1/SF162. We observed a restriction to HIV/VSV-G in sMAGI cells at low doses of input virus, but unlike the restriction of HIV-1/SF162, this restriction was abrogated by increasing the dose of virus (Fig. 1B). As expected, the replication of SIV/VSV-G, a SIV core pseudotyped with VSV-G envelope, was not restricted in the sMAGI cells; the infectivity of SIV/VSV-G was similar in rhesus sMAGI and human MAGI-CCR5 cells across all virus doses tested (Fig. 1C). The restriction to HIV-1/VSV-G could be abrogated by adding high doses of virus-like particles pseudotyped with VSV-G envelope, but it was not affected by the addition of virus-like particles lacking envelope (Fig. 1D). Together, these data suggest there is a saturable restriction to HIV-1/VSV-G, but not to SIV/VSV-G, in sMAGI cells, suggesting that this restriction may be due to TRIM5α. In contrast, there is a restriction to HIV-1/SF162 that can not be saturated by increasing the virus dose.
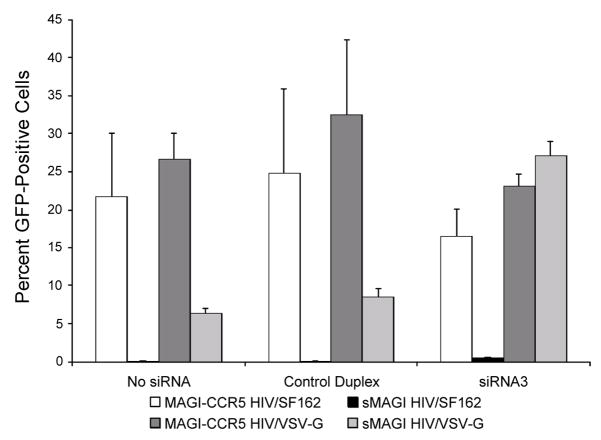
To more directly examine whether there was a role for TRIM5α in the restriction to HIV-1SF162 in sMAGI cells, TRIM5α mRNA concentrations were reduced by transfection with short interfering RNA (siRNA3) that targets rhesus TRIM5α (Stremlau et al., 2004). To increase the upper limit of quantification of infected cells, rhesus sMAGI cells were infected with virus carrying an HIV-1 proviral genome encoding an enhanced green fluorescent protein (eGFP, (Yamashita and Emerman, 2004)), and flow cytometry was used to detect eGFP-positive infected cells. As a positive control for this experiment, we showed that TRIM5α-specific siRNA pre-treatment of sMAGI cells restored the infectivity of the HIV-1/VSV-G control virus to the same levels as permissive human cells (Fig. 2; compare light and dark gray shaded bars in siRNA3). TRIM5α-specific siRNA pre-treatment of sMAGI cells also resulted in an increase in the percentage of eGFP positive sMAGI cells upon exposure to HIV-1/SF162 (from 0.08% to 0.56%, compare black bars in no siRNA versus siRNA3 in Fig. 2)). However, there remained a ~32-fold difference in infectivity of HIV-1/SF162 in rhesus versus permissive human cells (0.56% vs. 18.38%; compare white and black bars in siRNA3), even when TRIM5α levels were reduced to levels that completely abrogated the restriction to HIV-1/VSV-G in the rhesus cells. The differential ability of TRIM5α siRNA to abrogate the restriction of HIV-1/SF162 and HIV-1/VSV-G was also observed at other doses of virus (data not shown). These findings were specific to the siRNA directed to TRIM5α and were not seen with a control siRNA (Fig. 2). The presence or absence of TRIM5α-specific siRNA had no discernable effect on the percentage of HIV-1/VSV-G or HIV-1/SF162 infected human cells, consistent with previous reports suggesting that human TRIM5α does not appreciably restrict HIV-1 ((Stremlau et al., 2004); Fig. 2). Together, these experiments indicate that under the same conditions where HIV-1/VSV-G TRIM5α-restriction is completely abrogated, there remains a significant restriction to HIV-1/SF162 in sMAGI cells.
Fig. 2.
Effect of reducing TRIM5α mRNA levels by small interfering RNA. The results are presented as the percentage of GFP positive cells, as determined by flow cytometry. The siRNA tested in the cells is indicated below the bar graph. Below that is shown the key to the cell/virus combinations tested with each siRNA condition. Rhesus sMAGI cells and human MAGI-CCR5 were transfected with the indicated siRNA at a final concentration of 120nM (See Materials and Methods). Two days after siRNA transfection, cells were infected with HIV-1/SF162 or HIV-1/VSV-G at an MOI of ~1.0, based on titers in permissive human MAGI-CCR5 cells. Error bars represent standard deviation of three replicate infections.
Infection of sMAGI cells expressing human CCR5
Our previous studies suggested that HIV-1SF162 restriction was dependent on the route of viral entry because rhesus sMAGI cells that express human CCR5 (sMAGI-CCR5) were infectable by R5-HIV-1 (Chackerian et al., 1997). Here, we tested whether HIV-1/SF162 had a saturable, TRIM5α- dependent phenotype in these cells. In sMAGI-CCR5 cells, HIV-1/SF162 showed evidence for a restriction at lower doses, but not at higher doses; as expected this abrogation of restriction was not seen with HIV-1/SF162 tested in parallel on sMAGI cells (Fig 3A). In contrast, HIV-1/VSV-G infectivity was similar across all doses in sMAGI-CCR5 and sMAGI cells, approaching the infectivity observed for permissive human cells at the highest dose tested (Fig. 3B). TRIM5α siRNA abrogated the restriction of HIV-1/SF162 in sMAGI-CCR5 cells, as evidence by a ~10 fold increase in positive cells at input virus concentrations of 1–10ng Gagp24 (data not shown). These data suggest that infection of rhesus sMAGI-CCR5 cells with an R5-HIV-1 results in the same saturable, TRIM5α-dependent restriction as infection with HIV-1/VSV-G.
Fig. 3.
Viral infectivity of sMAGI-CCR5 versus sMAGI cells. The percentage of GFP positive cells versus virus dose was determined by flow cytometry. Error bars represent standard deviation of three replicate infections. Open triangles represent the number of infected rhesus sMAGI-CCR5 cells, filled triangles represent the number of infected rhesus sMAGI cells, and black squares represent the number of infected human MAGI-CCR5 cells. (A) Infection with HIV-1/SF162. (B) Infection with HIV-1/VSV-G. The infection in the two panels were performed in parallel.
TRIM5α-mediated restriction occurs before reverse transcription, as evidenced by a lack of HIV-1 DNA reverse transcription intermediates in restricted cells exposed to non-saturating levels of HIV-1 (Cowan et al., 2002). As the restriction to TRIM5α is saturated with increasing amounts of virus, reverse transcriptase intermediates accumulate and productive infection ensues. To further examine whether the restriction to HIV-1/SF162 in sMAGI cells is independent of TRIM5α, we examined whether reverse transcriptase intermediates accumulate in sMAGI cells exposed to HIV-1/SF162 at levels comparable to those seen during permissive infection. The infection was performed across a range of doses, starting at Gagp24 concentration of ~0.1 ng (corresponding to an MOI of ~0.016 based on infection of permissive human cells). We chose this dose because it was around the lowest dose where HIV-1/SF162 infection can first be detected in permissive sMAGI-CCR5 cells (e.g. Fig. 3 and data not shown), which served as a positive control for this experiment. At this lowest virus dose, we detected 25 HIV-1 proviral copies per twenty thousand sMAGI-CCR5 cells plated for infection (Fig. 4). Therefore,≥25 copies per twenty thousand sMAGI-CCR5 cells infected represents the level of proviral copies that correspond to the threshold where productive infection can be detected in these cells. Beginning at a Gagp24concentration of ~2ng/ml for HIV-1/SF162, the copy number of reverse transcription intermediates exceeded this saturation threshold number (25 copies/20,000 cells infected; denoted by a hatched line in Fig. 4) in the non-permissive rhesus sMAGI cells
Fig. 4.
Quantification of HIV-1 SF162 proviral DNA in sMAGI and sMAGI-CCR5. Cells were infected with viruses at the indicated concentration. To minimize signal due to any remaining contaminating DNA in the virus, the background HIV-1 DNA copies observed in AZT/3TC treated cultures, infected at the indicated virus dose, have been subtracted. Error bars represent standard deviation in the HIV-1 pol DNA copy number of two replicate infections. The dashed line highlights the number of proviral DNA per twenty thousand cells in sMAGI-CCR5 cells infected with HIV-1 SF162 at the lowest viral dose at which productively infected sMAGI-CCR5 cells were detected by counting GFP positive cells (not shown, but see Fig. 3). This level of proviral DNA thus represents a threshold level that corresponds to productive infection.
It is worth noting that the HIV-1/SF162 infection of rhesus cells at all doses tested was reduced an average of 100-fold compared to the parallel HIV-1/SF162 infection of sMAGI-CCR5 cells. This reduction in proviral copy number in rhesus cells suggests that compared to HIV-1 SF162 infection of sMAGI-CCR5 cells, HIV-1/SF162 may have reduced entry efficiency in rhesus sMAGI cells expressing only the endogenous rhesus coreceptor. To test this, we performed a fusion experiment comparing the efficiency of fusion of the HIV-1/SF162 envelope in the two cell lines. In this assay, envelope-positive syncytia indicate that the viral envelope of interest has fused with the target cells – a prerequisite for viral entry. We observed an ~5-fold increase in HIV-1/SF162 positive syncytia in sMAGI-CCR5 cells compared to sMAGI cells, and a similar increase when we compared human cells expressing CCR5 (MAGI-CCR5 cells) and sMAGI cells. As expected, a control CXCR4-using envelope, HIV-1SF33, caused a similar level of syncytia whether or not CCR5 was expressed in the human cells (Fig. 5A). These data support the suggestion that the HIV-1/SF162 may have a reduced ability to fuse and enter sMAGI cells expressing only the endogenous rhesus coreceptor compared to cells expressing CCR5.
Fig. 5.
Comparison of the efficiency of fusion of envelope with sMAGI cells compared to cells expressing CCR5. Envelope-positive syncytia scored using an immunofluorescence fusion assay are shown. The viruses tested are indicated at the bottom of each chart and the coding for the cell lines tested is indicated at the very bottom of the figure. (A) Fusion of HIV-1 SF162 and a CXCR4 viral envelope, HIV-1 SF33 with sMAGI, sMAGI-CCR5, MAGI and MAGI-CCR5. (B) Fusion of SIVMneCL8 envelope with sMAGI, sMAGI-CCR5 and MAGI-CCR5. The HIV-1 envelopes shown in panel A were included as controls. Arrows indicate results where the values were above 0, but below 10 syncytia.
Despite the apparent reduction in fusion and entry efficiency of HIV-1/SF162 in sMAGI versus sMAGI-CCR5 cells, the number of proviral copies detected in sMAGI cells infected with HIV-1 SF162 increased with increasing virus dose, reaching nearly one thousand proviral copies per twenty thousand cells infected at the highest dose of virus tested (Fig. 4). Thus, HIV-1 SF162 infection of sMAGI cells resulted in levels of reverse transcription intermediates that are indicative of productive infection of HIV-1 SF162 in sMAGI-CCR5 cells (Fig. 4); in sMAGI cells, there remains a block to HIV-1/SF162 at a stage after reverse transcription even at the highest virus doses (Figs. 1 and 3).
HIV-1 infection of sMAGI cells is not limited by inefficient entry
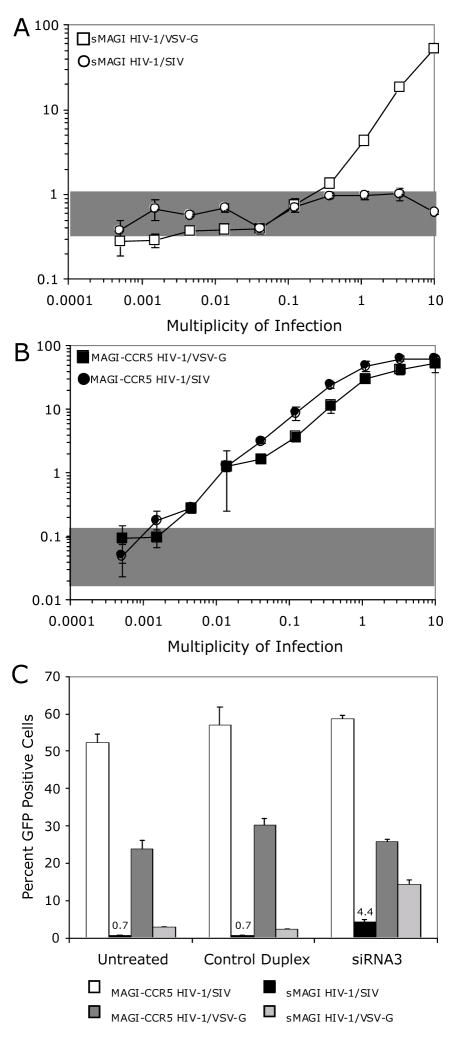
The detection of reverse transcriptase intermediates suggest that the inability of HIV-1/SF162 to saturate TRIM5α in the rhesus sMAGI cells is not likely to be due simply to limited entry of the virus by the endogenous coreceptor. As another approach to rule out this possibility, we infected rhesus sMAGI cells with HIV-1 particles pseudotyped with an envelope protein from SIV (HIV-1/SIV), because the infectivity of SIV is comparable between human MAGI-CCR5 cells and rhesus sMAGI cells (Chackerian, Haigwood, and Overbaugh, 1995; Chackerian et al., 1997). Moreover, we observed that the SIV envelope induced an ~10-fold higher level of envelope-positive syncytia in sMAGI cells compared to human cells expressing CCR5, further demonstrating that there are no restrictions that would be expected to limit fusion and entry of a HIV-1 particle pseudotyped with an SIV envelope in sMAGI cells (Fig. 5B). HIV-1/SIV infection of rhesus cells could not be detected above background even at the highest dose tested, which corresponded to 10 infectious particles (based on infection of permissive human cells) per sMAGI cell; in the same experiment, HIV-1/VSV-G infection was detected at these high virus doses (Fig. 6A). Parallel infection of human cells with HIV-1/SIV, as well as HIV-1/VSV-G, demonstrated that the viruses were equally infectious in human cells (Fig. 6B). Thus, HIV-1 pseudotyped with an SIV envelope protein, which permits efficient entry into sMAGI cells, is unable to saturate the post-entry restriction factor present in sMAGI cells.
Fig. 6.
Infection of rhesus sMAGI and human MAGI-CCR5 cells with HIV-1/SIV. The percentage of GFP positive cells versus virus dose was determined by flow cytometry. Shaded regions represent the percentage of GFP positive cells detected in mock (no virus) treated cells. Error bars represent standard deviation of three replicate infections. The symbols used for various cell/virus combinations tested are shown within each panel. (A) Infection of rhesus sMAGI cells with increasing dose of HIV-1/SIV and HIV-1/VSV-G. (B) Infection of MAGI-CCR5 cells with increasing dose of HIV-1/SIV and HIV-1/VSV-G. (C) Effects ofTRIM5α-specific siRNA-treatment of sMAGI and MAGI-CCR5 cells on infection of HIV-1/SIV. Transfection of siRNA was performed as in Figure 2. In this experiment, we maintained the control HIV-1/VSV-G MOI at 1.0 but increased the MOI of HIV-1/SIV (to 2.0) to increase the likelihood that we might observe a substantial change in the percentage of HIV-1/SIV infected rhesus cells upon siRNA-mediated reduction of TRIM5α mRNA.
To determine if TRIM5α restricts HIV-1/SIV, we infected rhesus sMAGI cells with HIV-1/SIV that had been treated with TRIM5α-specific siRNA (Fig. 6C). TRIM5α-specific siRNA3 pre-treatment of rhesus cells rescued the infection of HIV-1/SIV from ~0.7% infected rhesus cells in the absence of TRIM5α-specific siRNA to ~4.4% infected rhesus cells in the presence of TRIM5α-specific siRNA, siRNA3 (Fig. 6C). However, this is far short of the almost 60% infected cells observed in parallel HIV-1/SIV infections of human cells at this dose of virus. Consistent with the results of the experiment shown in Fig. 2, in this experiment, HIV-1/VSV-G infections of rhesus cells were rescued to levels near those observed in human cells upon pre-treatment with siRNA3. Thus, the restriction to HIV-1/SIV in rhesus sMAGI cells, like the restriction to HIV-1/SF162, is only partially relieved by reduction of TRIM5α, even under conditions where the restriction to HIV-1/VSV-G is abrogated.
Discussion
A rhesus cell line that expresses human CD4 (sMAGI (Chackerian, Haigwood, and Overbaugh, 1995; Chackerian et al., 1997)) and an as yet unidentified endogenous coreceptor, restricts HIV-1 by two distinct mechanisms: one that is dependent on TRIM5α, and one that is primarily independent of TRIM5α. sMAGI cells have a TRIM5α-mediated, saturable restriction to infection by HIV-1 pseudotyped with VSV-G. Additionally, HIV-1 encountered the same TRIM5α-dependent restriction in sMAGI cells that have been engineered to express human CCR5 (sMAGI-CCR5). In contrast, HIV-1 that entered sMAGI cells using an endogenous rhesus coreceptor were restricted after reverse transcription, but by a mechanism that was largely independent of TRIM5α.
There was a very modest positive effect of reducing TRIM5α levels on HIV-1/SF162 infection of sMAGI cells, but even under conditions where the TRIM5α restriction of HIV-1/VSV-G was completely abrogated, HIV-1/SF162 infectivity was ~32-fold lower relative to permissive cells. This suggests that in addition to TRIM5α there may be a second factor that provides intrinsic immunity against HIV-1 infection in rhesus cells.
Infection of sMAGI-CCR5 cells with HIV-1/SF162 led to saturation of endogenous rhesus TRIM5α and resulted in productive infection. However, sMAGI cells lacking CCR5 that were infected with HIV-1/SF162 in parallel remained uninfected over the wide range of doses tested. Analyses of reverse transcription intermediates, which provide a surrogate measure of the extent of viral entry (Zack et al., 1990), suggested that the inability of HIV-1/SF162 to saturate TRIM5α is not simply due to limited entry at higher doses. HIV-1/SF162 reverse transcriptase intermediates reached levels in sMAGI cells that were well above the level at which TRIM5α was saturated in sMAGI-CCR5 cells. The inability to abrogate the restriction of HIV-1/SF162 in sMAGI cells even at virus levels where there is clear evidence for reverse transcriptase intermediates, together with the finding that siRNA knock down of TRIM5α does not render the cells permissive to HIV-1/SF162 infection, strongly suggest that the major post-entry restriction encountered by HIV-1/SF162 in sMAGI cells is distinct from TRIM5α. Here we call the restriction Lv3 for Lentiviral restriction factor 3.
We further examined the potential role of limited entry in the observed phenotype of HIV-1/SF162 by examining infection of particles that had an HIV-1 core, but a different envelope. We chose the SIV envelope because sMAGI cells are highly permissive to infection by SIV (Chackerian, Haigwood, and Overbaugh, 1995) and there is efficient fusion of SIV envelope with sMAGI cells even compared to cells expressing CCR5, suggesting that entry is not limited. HIV-1 pseudotyped with an SIV envelope was also restricted in sMAGI cells over a wide range of doses tested. This suggests that HIV-1 cores that enter via an endogenous coreceptor for SIV also encounter the Lv3 restriction. We do not know if the coreceptor used by HIV-1 SF162 and SIV are the same, but the similarity of the restriction of HIV-1 cores bearing these envelopes suggests that this could be the case. It is possible that HIV-1 infection of sMAGI cells that use CD4 and the endogenous sMAGI coreceptor deposits HIV-1 cores into a different cellular context than HIV-1/VSV-G infection of sMAGI cells in which entry is mediated by endocytosis. In this model, HIV-1 cores that enter sMAGI cells using CD4 and the endogenous coreceptor are shuttled down a pathway in which they do not encounter TRIM5α, but do encounter Lv3. In contrast, HIV-1 cores that enter by VSV-G-mediated endocytosis bypass the Lv3 restriction, but encounter the TRIM5α-dependent restriction.
Previous studies have also provided evidence that the mechanism of HIV-1 entry influences the intracellular compartment into which HIV-1 is deposited and, therefore, the success of post-entry stages of HIV-1 replication. Bukarynskaya et al. demonstrated that disruption of the actin cytoskeleton resulted in an early post-entry restriction of HIV-1 that entered cells using CD4 and CXCR4 via wild type HIV-1 envelope-mediated entry; but, the requirement for an intact actin cytoskeleton was bypassed by HIV-1 that enter cells by endocytosis via VSV-G-mediated entry (Bukrinskaya et al., 1998). Schmidtmayerova et al. showed that T-lymphotropic HIV-1 entered macrophages in a CD4/CXCR4-dependent manner, but were restricted prior to nuclear import, whereas HIV-1 that entered via CD4/CCR5 were not restricted (Schmidtmayerova et al., 1998). Because CCR5 and CXCR4 have been shown to localize to different plasma membrane microdomains (Popik, Alce, and Au, 2002), differences in coreceptor localization may account for the role that envelope-coreceptor interactions play in determining the location, and therefore, the success of post-entry stages of HIV-1 replication.
Lv3 also shares similarities to the intrinsic immunity factor Lv2. Lv2 was first described as a post-entry restriction to HIV-2 infection in macrophages and some human cell lines (e.g. GHOST cells and HeLa-CD4 cells) that is distinct from Lv1/TRIM5α restriction of HIV-1 (McKnight et al., 2001; Schmitz et al., 2004). Recently, Marchant et al. demonstrated that altering the route of entry determined whether HIV-2 encountered Lv2 in restrictive cells (Marchant et al., 2005). Moreover, it appears that some HIV-1 strains also encounter Lv2 (Marchant et al., 2005). Schmitz et al. demonstrated that HIV-2 restricted envelopes direct restricted HIV-2 cores to a non-productive pathway; escape from this non-productive pathway is possible if the restricted envelope is complemented with a non-restricted CA protein (Schmitz et al., 2004). The infection of sMAGI cells that results in the Lv3 restriction can be rescued by complementing HIV-1 SF162 Env-mediated infection with an unrestricted SIV gag-pol(Chackerian et al., 1997). Thus, a salient characteristic shared by Lv2 and Lv3 is the ability of 5′ viral sequences to rescue the post-entry restriction. Another common feature is that Lv2 was bypassed when HIV particles were pseudotyped with VSV-G (Schmitz et al., 2004), as was Lv3 for HIV-1. There are differences, however, between Lv3 and Lv2. Infection of Lv2-restrictive HeLa-CD4 cells is leaky, as low-level productive infection can be detected; in contrast, Lv3-restrictive sMAGI cells cannot be productively infected even at high viral doses. Unlike Lv3, Lv2 is apparently not coreceptor-dependent, as expression of alternate coreceptors in cells does not modulate the Lv2 phenotype (Schmitz et al., 2004). Lv2 and Lv3 are also distinct in being present in different species, and it remains to be seen whether Lv3 is common in other species or even other cells from rhesus monkeys. The relationship of the Lv2 restriction and the Lv3 restrictions remains to be determined, but it is possible they will share some overlapping mechanisms.
The ability of HIV-1 to engage the necessary cellular components as it navigates from the inner leaf of the plasma membrane to the nucleus is, in part, determined by the mechanism of entry. The studies described here, suggest that within a single cell, multiple potential pathways exist, some of which may be non-productive for infection. In addition to TRIM5α, a restriction we have termed Lv3 results in a post-entry restriction to HIV-1 infection in a rhesus epithelial cell line. Lv3 is distinguished by its dependence on the entry pathway of the virus, by the fact that the restriction occurs at least in part after reverse transcription and by the fact that the restriction is not saturated at high doses of virus or by reduction of TRIM5α levels. Further characterization of this early post-entry restriction may provide novel therapeutic targets to combat HIV infection.
Materials and Methods
Cell Lines
The sMAGI cell line (Chackerian et al., 1997) was derived from a rhesus macaque epithelial cell line (CMMT) and was engineered to express human CD4. The sMAGI derivative cell line sMAGI-CCR5 was engineered to express human CCR5. sMAGI and sMAGI-CCR5 cells were cultured in DMEM complete (Dulbecco modified Eagle medium supplemented with 10% fetal bovine serum, 2 mM glutamine, 100 U of penicillin per ml, 100 mg of streptomycin per ml, 0.25 mg of amphotericin B per ml) supplemented with 50 units of hygromycin per ml and 50 μg G418 per ml (and 6 μg puromycin per ml for derivatives). The MAGI-CCR5 (Chackerian et al., 1997) cell line was cultured in DMEM complete supplemented with 100 μg of hygromycin per ml, 50 μg G418 per ml and 1 μg puromycin per ml.
Construction of Envelope Expression Plasmids
Amplicons encoding full-length HIV-1 envelopes were generated by polymerase chain reaction (PCR) amplification of the 3′ hemi-genome of HIV-1 SF162 (Cheng-Mayer et al., 1990) and HIV-1 SF33 (York-Higgins et al., 1990) using the second round primers vpr11 and nef30 described by Long et al. (Long et al., 2002). HIV-1 envelope PCR was performed with Native Pfu DNA Polymerase from Stratagene (La Jolla, CA) according to manufacturer instructions. The reaction was performed using AmpliWax PCR Gem 50 wax beads, using the hot start method (Applied Biosystems, Foster City, CA). The thermocycling conditions were as follows: 94°C for 2 minutes; 25 cycles of 94°C for 45 sec, 55°C for 45 sec, 72°C for 4.5 min; and a final incubation at 72°C for 10 min. HIV-1 envelope (env) PCR amplicons were digested with NotI and MluI and cloned into NotI and MluI restriction enzyme-digested pCI-Neo (Promega, Madison, WI) to generate pCI-Neo-HIV-1SF162env. The sequence of the clone used was verified by nucleotide sequencing of both strands.
Full-length SIVMneCL8 (M32741) envelope (referred to as SIV env for simplicity) was generated by PCR amplification of full-length genome of SIVMneCL8 using SIVenv35 (5′-AAA CAA GAA TTC ATG GGA TCT CTT GGG AAT CAG (6060–6092, position relative to SIVMneCL8)) and SIVenv36 (5′-TTG TAT TGC GGC CGC TCA CAA GAG AGT AAG CTC GAG (8697–8732)). SIV envelope PCR was performed with Native Pfu DNA Polymerase from Stratagene (La Jolla, CA) according to manufacturer instructions. The reaction was performed using AmpliWax PCR Gem 50 wax beads, using the hot start method (Applied Biosystems, Foster City, CA). The thermocycling conditions were as follows: 94°C for 2 minutes; 25 cycles of 94°C for 45 sec, 63°C for 45 sec, 72°C for 4 min; and a final incubation at 72°C for 10 min. The full-length SIVCL8env PCR amplicon was digested with NotI and EcoRI and cloned into NotI and EcoRI restriction enzyme-digested pCI-Neo (Promega, Madison WI) to generate pCI-Neo-SIVMneCL8env. The sequence of the clone was verified by nucleotide sequencing of both strands.
Preparation of pseudotyped virus particles
Virus stocks were prepared by transient transfection of HEK293T cells with the Fugene6 reagent (Roche, Indianapolis, IN) according to manufacturer instructions. Cell-free virus was harvested two days post transfection, filtered through 0.22μm pore size filter (Steriflip Filters, Millipore, Billerica, MA), and frozen at −80°C for long-term storage. HIV-1 envelope pseudotyped viral stocks were prepared by co-transfection of a HIV-1 provirus plasmid lacking the envelope gene (either the clade A Q23Δenv (Long et al., 2002), or the clade B pLAI3ΔenvGFP3 (Yamashita and Emerman, 2004)] and either a lentiviral envelope expression plasmid or the vesicular stomatitis virus G protein (VSV-G) expression plasmid pMD. G (Naldini et al., 1996). When high-titer HIV-1 envelope pseudotyped virus was needed, virus was concentrated ~ten-fold using 100,000 MWCO Amicon Ultra-15 Centrifugal Filter Devices from Millipore (Billerica, MA) by centrifugation at 4000rpm in Beckman Allegra 6KR Centrifuge at 4°C for 15 min followed by two washes using an equal volume of cold PBS prior to freezing at −80°C. HIV-1 VLP were generated by transient transfection of HEK293T cells with a packaging-defective and envelope-deleted HIV-1 proviral genome (Zufferey et al., 1997) in the presence and absence (to generate bald VLP) of pMD. G. HIV-1 Gag p24 concentration was determined by Gag p24 antigen capture ELISA as per manufacturer instructions (Beckman-Coulter, Miami, FL). The titer of the virus stocks were determined using MAGI–CCR5 cells, which express both CCR5 and CXCR4, as well as CD4, and thus are permissive to both R5 and X4 HIV-1, as well as SIV (Chackerian et al., 1997).
Infection assays
For infections, target cells were seeded the day before in 24-well plates: 4×104 MAGI-CCR5 cells per well, 2×104 sMAGI (and derivatives) cells per well. MAGI-CCR5 cells, and sMAGI cells were infected in the presence of DEAE Dextran at 20μg per ml or 15μg per ml, respectively. Infectivity was assessed at two (MAGI-CCR5) or three (sMAGI) days post infection. In cases where cells were infected with pseudotypes of Q23Δenv, cells were fixed and stained for β-galactosidase expression as in Chackerian et al.(Chackerian, Haigwood, and Overbaugh, 1995). In cases where cells were infected with pseudotypes of pLAI3ΔenvGFP3, cells were processed for analysis of GFP expression by flow cytometry using a Becton Dickenson FACS Calibur with a High Throughput Sampler: Cells in 24-well plates were trypsinized (MAGI-CCR5 with 0.25% Trypsin, sMAGI and derivatives with 0.05% Trypsin), suspended in DMEM complete and transferred to a 96-well V-bottom plate. Cells were pelleted by centrifugation at 300×g, then suspended and incubated in 1.2% paraformaldehyde in PBS (USB Corporation, Cleveland, OH) for 5 min at room temperature. Cells were washed twice in PBS and suspended in a final volume of 75μl PBS in preparation for flow cytometry.
RNA interference
Rhesus sMAGI TRIM5α mRNA was reduced using short interfering RNAs (siRNA) that correspond to siRNA3 and the control siRNA as described by Stremlau et al. (Stremlau et al., 2004). One hundred thousand target cells were seeded into each well of a six well plate and transfected with 120nM siRNA by using 10μl of Oligofectamine (Invitrogen, Carlsbad, CA) according to the manufacturer instructions. After 24 hours, cells were re-plated for infection by HIV-1 pseudotype virus as described above.
Quantification of reverse transcription intermediates
To minimize DNA contamination in these experiments, we used replication-competent HIV-1 SF162 that had been passaged for 12 days in human peripheral blood mononuclear cells (PBMCs). PBMC cell-free virus was prepared by centrifugation at 2000rpm in Beckman Allegra 6KR centrifuge for 5 min at room temperature. The HIV-1 SF162 virus preparation was aliquotted and stored at −80°C. As an additional means to reduce DNA contamination, the virus was incubated for 30 min at room temperature with 200 units DNaseI (Invitrogen, Carlsbad, CA) per ml and 10mM MgCl2.
In preparation for infection, 2×104 sMAGI or sMAGI-CCR5 cells were plated into each well of a 24-well dish. The next day, the appropriate control wells were incubated at 37°C for two hours with 50μM AZT (Sigma, St. Louis, MO) and 50μM 3TC (NIH AIDS Research and Reference Reagent Program). The cells were then infected in duplicate, in the presence of DEAE Dextran as described above, with different MOIs of HIV-1 SF162. Four hours post-infection, cells were washed twice in PBS and then lysed in lysis buffer (50 mM KCl, 10 mM Tris-HCl (pH8.3), 2.5 mM MgCl2, 0.1 mg of gelatin per ml, 0.45% Nonidet P-40, and 0.45% Tween 20). Lysates were frozen at −20°C until total DNA was extracted using QiAmp DNA Mini-prep kit according to manufacturer instructions (Qiagen, Valencia, CA). The DNA was eluted with 100μl of water. HIV-1 proviral copies were determined by real time PCR as described in Rousseau et al. (Rousseau et al., 2004) with 10μl of template in a final reaction volume of 50μl. To control for residual DNA in the virus preparations after DNaseI treatment, AZT/3TC infections were performed in the presence of 50μM AZT and 50μM 3TC. The values obtained for the AZT/3TC-treated control were subtracted from the values for untreated infections at each virus dilution tested.
Fusion assay
Cos7 cells were plated at a density of 5×105 cells per 6 cm dish. The next day, the Cos7 cells were transiently transfected with 2μg of various Env expressing plasmids using Fugene6. Within 24 hours of transfection, Cos7 cells were trypsinized, counted and plated at a density of 5×104 cells per well of a two chamber culture slide (Becton-Dickinson, Bedford, MA). sMAGI, sMAGI-CCR5, MAGI and MAGI-CCR5 target cells were added to the Env-expressing Cos7 cells at cell densities of 2×105, 2×105, 5×104 and 5×104 cells per well, respectively. After 48 hours, culture slides were washed twice with cold 1X PBS and incubated at −20°C in 1 ml of 1:1 acetone:methanol for 10 min. Acetone:methanol mixture was aspirated and culture slides were air-dried for 10 min at room temperature. Culture slides were then rehydrated in cold 1X PBS and stored at 4°C until the immunofluorescence assay was performed. Slides were stained for 1 hour at 37°C in 400μl of IFA Diluent (1X PBS, 1% BSA, 5% normal goat serum) containing 1ng per ml IgG1 b12 to examine HIV-1 envelope syncytia (IgG1 b12 was a gift of D. Burton, The Scripps Research Institute, San Diego, CA) or 50ng per ml SIVIG to examine SIV envelope syncytia (SIVIG was a gift of N. Haigwood, SBRI, Seattle, WA). At the end of the incubation, culture slides were washed twice for 10 min at room temperature on a nutator with 2 ml of 1X PBS. Four Hundred μl of IFA Diluent containing FITC-conjugated goat anti-human antibody (ICN, Aurora, OH) was added to each well of the culture slide and the slides were incubated at 37°C for 1 hour. The stained culture slides were washed as before and then air-dried at room temperature for 10 min. Slides were mounted with VectaShield (Vector Laboratories, Burlingame, CA) and stored in the dark at 4°C until examined visually by fluorescence microscopy
Acknowledgments
We thank Stephanie Rainwater for her help with fusion assays and in the final stages of preparing this manuscript, Michael Emerman and Masahiro Yamashita for many helpful discussions and for providing the VSV-G expression plasmid, pMD. G, as well as pLAI3ΔenvGFP3; Nancy Haigwood and Nicole Doria-Rose for sharing their fusion assay protocol. In addition, we thank Michael Emerman, Jaisri Lingappa and Bryce Chackerian for their comments on the manuscript. This work was supported by NIH grant RO1 AI34251 and by a Poncin Scholarship and NIH Training Grant AI07140 to MJP.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Berger EA, Murphy PM, Farber JM. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu Rev Immunol. 1999;17:657–700. doi: 10.1146/annurev.immunol.17.1.657. [DOI] [PubMed] [Google Scholar]
- Bieniasz PD. Restriction factors: a defense against retroviral infection. Trends Microbiol. 2003;11(6):286–91. doi: 10.1016/s0966-842x(03)00123-9. [DOI] [PubMed] [Google Scholar]
- Bukrinskaya A, Brichacek B, Mann A, Stevenson M. Establishment of a functional human immunodeficiency virus type 1 (HIV-1) reverse transcription complex involves the cytoskeleton. J Exp Med. 1998;188(11):2113–25. doi: 10.1084/jem.188.11.2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chackerian B, Haigwood NL, Overbaugh J. Characterization of a CD4-expressing macaque cell line that can detect virus after a single replication cycle and can be infected by diverse simian immunodeficiency virus isolates. Virology. 1995;213:386–394. doi: 10.1006/viro.1995.0011. [DOI] [PubMed] [Google Scholar]
- Chackerian B, Long EM, Luciw PA, Overbaugh J. Human immunodeficiency virus type 1 coreceptors participate in postentry stages in the virus replication cycle and function in simian immunodeficiency virus infection. J Virol. 1997;71(5):3932–3939. doi: 10.1128/jvi.71.5.3932-3939.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng-Mayer C, Quiroga M, Tung JW, Dina D, Levy JA. Viral determinants of human immunodeficiency virus type 1 T-cell or macrophage tropism, cytopathogenicity, and CD4 antigen modulation. J Virol. 1990;64(9):4390–4398. doi: 10.1128/jvi.64.9.4390-4398.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan S, Hatziioannou T, Cunningham T, Muesing MA, Gottlinger HG, Bieniasz PD. Cellular inhibitors with Fv1-like activity restrict human and simian immunodeficiency virus tropism. Proc Natl Acad Sci U S A. 2002;99(18):11914–9. doi: 10.1073/pnas.162299499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long EM, Rainwater SMJ, Lavreys L, Mandaliya K, Overbaugh J. HIV Type 1 Variants Transmitted to Women in Kenya Require the CCR5 Coreceptor for Entry, Regardless of the Genetic Complexity of the Infecting Virus. AIDS Research and Human Retroviruses. 2002;18(8):567–76. doi: 10.1089/088922202753747914. [DOI] [PubMed] [Google Scholar]
- Marchant D, Neil SJ, Aubin K, Schmitz C, McKnight A. An envelope-determined, pH-independent endocytic route of viral entry determines the susceptibility of human immunodeficiency virus type 1 (HIV-1) and HIV-2 to Lv2 restriction. J Virol. 2005;79(15):9410–8. doi: 10.1128/JVI.79.15.9410-9418.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight A, Griffiths DJ, Dittmar M, Clapham P, Thomas E. Characterization of a late entry event in the replication cycle of human immunodeficiency virus type 2. J Virol. 2001;75(15):6914–22. doi: 10.1128/JVI.75.15.6914-6922.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munk C, Brandt SM, Lucero G, Landau NR. A dominant block to HIV-1 replication at reverse transcription in simian cells. Proc Natl Acad Sci U S A. 2002;99(21):13843–8. doi: 10.1073/pnas.212400099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naldini L, Blomer U, Gage FH, Trono D, Verma IM. Efficient transfer, integration, and sustained long-term expression of the transgene in adult rat brains injected with a lentiviral vector. Proc Natl Acad Sci U S A. 1996;93(21):11382–8. doi: 10.1073/pnas.93.21.11382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohlmann S, Davis C, Meister S, Leslie GJ, Otto C, Reeves JD, Puffer BA, Papkalla A, Krumbiegel M, Marzi A, Lorenz S, Munch J, Doms RW, Kirchhoff F. Amino acid 324 in the simian immunodeficiency virus SIVmac V3 loop can confer CD4 independence and modulate the interaction with CCR5 and alternative coreceptors. J Virol. 2004;78(7):3223–32. doi: 10.1128/JVI.78.7.3223-3232.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popik W, Alce TM, Au WC. Human immunodeficiency virus type 1 uses lipid raft-colocalized CD4 and chemokine receptors for productive entry into CD4(+) T cells. J Virol. 2002;76(10):4709–22. doi: 10.1128/JVI.76.10.4709-4722.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseau CM, Nduati RW, Richardson BA, John-Stewart GC, Mbori-Ngacha DA, Kreiss JK, Overbaugh J. Association of levels of HIV-1-infected breast milk cells and risk of mother-to-child transmission. J Infect Dis. 2004;190(10):1880–8. doi: 10.1086/425076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidtmayerova H, Alfano M, Nuovo G, Bukrinsky M. Human immunodeficiency virus type 1 T-lymphotropic strains enter macrophages via a CD4- and CXCR4-mediated pathway: replication is restricted at a postentry level. J Virol. 1998;72(6):4633–42. doi: 10.1128/jvi.72.6.4633-4642.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz C, Marchant D, Neil SJ, Aubin K, Reuter S, Dittmar MT, McKnight A. Lv2, a novel postentry restriction, is mediated by both capsid and envelope. J Virol. 2004;78(4):2006–16. doi: 10.1128/JVI.78.4.2006-2016.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stremlau M, Owens CM, Perron MJ, Kiessling M, Autissier P, Sodroski J. The cytoplasmic body component TRIM5alpha restricts HIV-1 infection in Old World monkeys. Nature. 2004;427(6977):848–53. doi: 10.1038/nature02343. [DOI] [PubMed] [Google Scholar]
- Stremlau M, Perron M, Lee M, Li Y, Song B, Javanbakht H, Diaz-Griffero F, Anderson DJ, Sundquist WI, Sodroski J. Specific recognition and accelerated uncoating of retroviral capsids by the TRIM5alpha restriction factor. Proc Natl Acad Sci U S A. 2006;103(14):5514–9. doi: 10.1073/pnas.0509996103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita M, Emerman M. Capsid is a dominant determinant of retrovirus infectivity in nondividing cells. J Virol. 2004;78(11):5670–8. doi: 10.1128/JVI.78.11.5670-5678.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- York-Higgins D, Cheng-Mayer C, Bauer D, Levy JA, Dina D. Human immunodeficiency virus type 1 cellular host range, replication, and cytopathicity are linked to the envelope region of the viral genome. J Virol. 1990;64(8):4016–4020. doi: 10.1128/jvi.64.8.4016-4020.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zack JA, Arrigo SJ, Weitsman SR, Go AS, Haislip A, Chen ISY. HIV-1 entry into quiescent primary lymphocytes: molecular analysis reveals a labile, latent viral structure. Cell. 1990;61:213–222. doi: 10.1016/0092-8674(90)90802-l. [DOI] [PubMed] [Google Scholar]
- Zheng YH, Peterlin BM. Intracellular Immunity to HIV-1: newly defined battles inside infected cells. Retrovirology. 2005;2(25):1–13. doi: 10.1186/1742-4690-2-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zufferey R, Nagy D, Mandel RJ, Naldini L, Trono D. Multiply attenuated lentiviral vector achieves efficient gene delivery in vivo. Nat Biotechnol. 1997;15(9):871–5. doi: 10.1038/nbt0997-871. [DOI] [PubMed] [Google Scholar]