Abstract
Endothelin (ET) acts at selected brain loci to elicit a pressor response and vasopressin (AVP) secretion. The pressor action of centrally acting ET is mediated via enhanced efferent sympathetic nerve activity. ET-induced VP secretion depends upon the ET receptor subtype and the brain region involved. ETAR activation at the subfornical organ (SFO) increases mean arterial pressure and renal sympathetic nerve activity (RSNA) as well as AVP secretion in awake rats. These effects are only partly mediated by glutamatergic receptors in paraventricular nucleus (PVN). Recent data indicate dendritic release of AVP may act as a neurotransmitter. We therefore hypothesized that dendritic release of AVP from magnocellular PVN neurons contributes to the increase in arterial pressure and RSNA due to ETA receptor activation at SFO. Male Sprague Dawley rats equipped with vascular catheters, renal nerve electrodes, and intracerebral cannulae directed into SFO and magnocellular PVN bilaterally were studied 48hr after recovery in the awake state. Hemodynamic and neural parameters were monitored continuously. Microinjection of 5 pmol ET-1 into SFO increased mean arterial pressure by 15.8 ± 4.2 mmHg accompanied by reflex decreases in heart rate and RSNA. Microinjection of 100 ng of the V1a receptor antagonist alone bilaterally into the PVN did not change baseline parameters; however, the pressor response to ET1 was significantly attenuated with mean arterial pressure increasing only by 6.1 ± 3.0 mmHg (P<0.05). Reflex changes in heart rate and RSNA did not change. These findings support the concept that dendritic release of VP from magnocellular neurons within the PVN mediates, at least in part, the pressor response to ETA receptor activation at the SFO.
Keywords: blood pressure, hemodynamics, rat, renal sympathetic nerve activity
INTRODUCTION
The endothelins (ET) are a family of peptides that exert direct potent actions on many peripheral vascular beds. ET peptides are also found within neuronal and glial cells of the central nervous system (1,2) and can act as peptidergic neurotransmitters (3,4). The expression of ET peptides and ET receptors is particularly robust within brain nuclei implicated in regulation of cardiovascular function, sympathetic output, and vasopressin (AVP) secretion (2,5,6). Moreover, ET1 within the peripheral circulation can gain access to discrete brain loci of the circumventricular region devoid of the blood brain barrier, such as the subfornical organ (SFO). The SFO possesses abundant ETA receptors (7,8) and sends projections caudally via the paraventricular nucleus (PVN) to caudal medullary cardiovascular centers that modulate sympathetic output controlling heart rate and systemic arterial pressure. In addition, neural projections from the SFO also extend to the magnocellular region of the PVN as well as the supraoptic nucleus where AVP is synthesized (9). The axons of these vasopressinergic neurons terminate in the posterior pituitary from which AVP is secreted into the circulation.
In normal rats, ET1 injected into the lateral cerebral ventricles or directly into the SFO reproducibly elicits a pressor response (6,10) mediated by enhanced efferent sympathetic activity (10-12). Although early reports attributed the rise in systemic arterial pressure to increased circulating AVP (13,14), the role of AVP has since proven to be more complex. In normotensive intact rats, the pressor response is independent of circulating AVP (10). In sinoaortically denervated rats or in rats with an impaired arterial baroreflex, such as heart failure, this central ET1-induced increase in arterial pressure may be mediated by both sympathetic and peripheral vasopressinergic mechanisms (10,15,21). Moderate to severe heart failure is associated with high plasma ET (16,17) and AVP levels (18). Both ET and AVP have been implicated in the myocardial depression (19), sympathoexcitation (20) and impaired baroreflex function (21) that occur with heart failure. Notably, individuals with heart failure accompanied by high plasma big ET1 or ET1 carry a greater risk of death (16,17) as do those with hyponatremia and elevated plasma AVP (22,23).
Several studies have provided unequivocal evidence that AVP is released not only from the axon terminals within the neurohypophysis but also from dendrites within the supraoptic nucleus and PVN (24,25). Thus far, the consensus is that dendritically released AVP serves to autoregulate the electrical activity of the cells in the microenvironment of the cells of origin (26) which are known to possess V1a vasopressin receptors. Notably, AVP in the extracellular compartment of the supraoptic nucleus is present at concentrations 100-fold greater than found in plasma, has a half-life 10-fold longer and, since it is not released into a classic synaptic cleft, may diffuse more widely within the nucleus (27). Similar conditions exist within the PVN. Thus, it is becoming increasingly clear that not only does AVP released within the magnocellular region of the PVN serve to sustain the phasic activity pattern that is maximally efficient for AVP secretion into the circulation (28-30), but may also influence nearby cells (31) such as the parvocellular neurons which also express vasopressin V1a receptors (32) and have efferent connections to autonomic nuclei that regulate cardiovascular function (33).
The present experiments were designed to test the hypothesis whether the hemodynamic and renal sympathetic nerve responses to ET1 stimulation of the SFO observed in conscious normal rats are, at least in part, modulated by AVP acting upon V1a receptors within the PVN. These studies would lay the groundwork for evaluating the role not only of peripheral AVP, but also central AVP, mechanisms in sympathoexcitatory states such as heart failure.
MATERIALS AND METHODS
Animals
Adult male Sprague Dawley rats weighing approximately 250 g were obtained from Harlan Sprague Dawley, Inc. (Indianapolis, IN). They were housed under controlled conditions (21−23°C; lights on, 06:00−18:00 h) and had free access to water and standard rat chow. The rats were cared for in accordance with the principles of the NIH Guide for the Care and Use of Laboratory Animals (Dept. of Health, Education and Welfare No 86−23). All protocols were reviewed and approved by our Institutional Committee for the Care and Use of Animals as well as the Chief Veterinary Medical Officer of the Department of Veterans Affairs.
Experimental procedures
Four days prior to running the protocols, each rat was anesthetized with ketamine 90 mg/kg and xylazine 10 mg/kg ip and then positioned within a cranial stereotaxic instrument (Kopf, Tujunga, CA). Three stainless steel guide cannulas (Plastics One, Roanoke, VA) were inserted such that the 33-gauge injection cannulas would result in microinjections into the subfornical organ (SFO coordinates to bregma: −0.9 anteroposterior, +1.0 mediolateral, +4.8 dorsoventral at an angle of 11° mediolaterally) and bilateral magnocellular regions of the paraventricular nucleus (PVN coordinates to bregma: [left] −3.1 anteroposterior; +0.6 mediolateral; +7.75 dorsoventral at an angle of 10° posteroanteriorly; [right] −1.5 anteroposterior, +2.6 mediolateral; +7.8 dorsoventral at an angle of 13° mediolaterally). The guide cannulas were affixed with cranioplastic cement and dummy cannulas were inserted to maintain patency until the time of experimentation. After recovering from surgery, each rat was returned to its individual cage.
Two days later, the rats were anesthetized with sodium pentobarbital, 40 mg • kg−1 body weight ip. Arterial and jugular catheters were inserted via a ventral incision into the carotid artery and jugular vein, respectively. The distal ends of the catheters were tunneled subcutaneously and exteriorized at the base of the neck. Catheters were filled with 50 μL sodium heparin, 1000 U/mL to maintain patency.
Renal nerve electrodes were implanted using a retroperitoneal approach to isolate the left renal nerve. The nerve was placed on electrodes constructed of Teflon-coated silver wire (0.0055 in. diameter, A-M Systems, Carlsborg, WA) with the exposed ends wound into single loops. The nerve and electrodes were covered with silicone gel (Kwik-Cast, World Precision Instruments) which was allowed to harden before closure. A ground wire was sewn into the surrounding tissue. The electrodes were likewise tunneled subcutaneously and exteriorized at the base of the neck. After recovery from surgery, each rat was returned to its individual cage.
A minimum of 48 hr was allowed after the last surgery for complete recovery from anesthetic effects prior to running any protocols (34). In all cases, the animals were grooming themselves normally, eating, drinking and displaying normal cage activity. Cannula placement was verified histologically for each rat at the end of the protocols.
Conditioning to the experimental chamber
During the two to three days prior to running the protocols, each rat was acclimated for 120 minutes to the Plexiglas study chamber (Braintree Scientific, Braintree, MA) that restricted its movement but did not restrain it.
Hemodynamic monitoring and renal nerve activity recordings
On the day of the study, the rat was placed into the chamber. The dummy cannula was replaced with the infusion cannula whose internal tip projected 1 mm below the guide cannula. Arterial pressure was measured by connecting the arterial catheter with a pressure transducer (Gould P23 XL) which was coupled to an amplifier (Digi-Med BPA-200). Heart rate and mean arterial pressure (MAP) were derived by data-acquisition software (DasyLab, Biotech Products) using the arterial pressure pulse and averaged over 1-s intervals. Renal nerve activity was amplified (5,000 − 20,000 times) and filtered (100 − 1,000 Hz) with a Grass P511 differential preamplifier and a high-impedance probe (HIP511GB). The probe and animals were located inside a shielded Faraday cage. The amplified and filtered neurogram signal was channeled to an oscilloscope and Grass AM8 audiomonitor for visual and auditory evaluation, respectively. The amplified nerve activity was digitized, rectified, integrated, and averaged over 1-s intervals by the computer data acquisition software (DasyLab, Biotech Products). Background noise was determined at the end of experiment after administration of a bolus dose of the ganglionic blocker, trimethaphan camsylate, 20 mg/kg iv (Hoffman-La Roche). RSNA was defined as the amount of recorded nerve activity after subtraction of background noise. RSNA was normalized using resting nerve activity as the 100% value.
Experimental protocols
All protocols were performed on conscious unrestrained rats. Each rat was subjected to only one protocol on any given day. Protocols were performed in random order.
Verification of V1a receptor (V1aR) antagonism
After an ∼30 min baseline period during which all parameters were permitted to stabilize, the PVN was microinjected bilaterally with 100 ng V1aR antagonist, [1-(β-mercapto-β,β-cyclopentamethyleneproprionic acid),2-(O-methyl) tyrosine] vasopressin (Sigma, St. Louis, MO), dissolved in 250 nl artificial cerebrospinal fluid (aCSF) or with aCSF alone. Four minutes later, the PVN was injected with 100 ng AVP (Sigma, St. Louis, MO) dissolved in 250 nl aCSF.
V1aR antagonism in PVN of the effect of ET1 at SFO
After an ∼30 min baseline period, the PVN was injected bilaterally with either aCSF or the V1aR antagonist as above. Four minutes later, 5 pmol ET1 in 250 nl aCSF was injected into the SFO.
Statistics
All data are presented as the mean ± SE. Comparisons of the effect of ET1 with aCSF vs V1aR antagonist into the PVN in the same animal were made using the paired t-test. A P value less than 0.05 was accepted as significant.
RESULTS
Blockade of exogenous AVP with V1aR antagonist in PVN
As shown in the examples in Figure 1, injection of artificial CSF into the PVN bilaterally did not significantly alter MAP, heart rate or RSNA. Injection of AVP, however, produced a rapid increase in RSNA to a maximum 276 ± 17% baseline (P < 0.001 vs baseline) that peaked at 100−140 sec. Heart rate increased to 513 ± 13 bpm and MAP to 139.2 ± 3.4 mmHg, values significantly higher than baseline 458 ± 10 bpm (P < 0.01) and 128.0 ± 2.1 mmHg (P < 0.05), respectively, and remained elevated for up to 8 minutes. Administration of the V1aR antagonist alone into the PVN did not alter any baseline parameters (MAP 129.1 ± 2.8 mmHg, heart rate 434 ± 11 bpm, and RSNA 103 ± 3% baseline) but completely abolished the responses to exogenous AVP (MAP 128.3 ± 3.2 mmHg; heart rate 426 ± 15 bpm; and RSNA 105 ± 7% baseline).
Fig. 1.
Representative recordings of mean arterial pressure (MAP), heart rate (HR), and renal sympathetic nerve activity (RSNA) after bilateral microinjection of the paraventricular nucleus (PVN) with (A) artificial cerebrospinal fluid (CSF) or (B) V1a receptor antagonist (V1aR antag, 100 ng) followed by arginine vasopressin (AVP, 100 ng) into the PVN. Arrows indicate time of each microinjection.
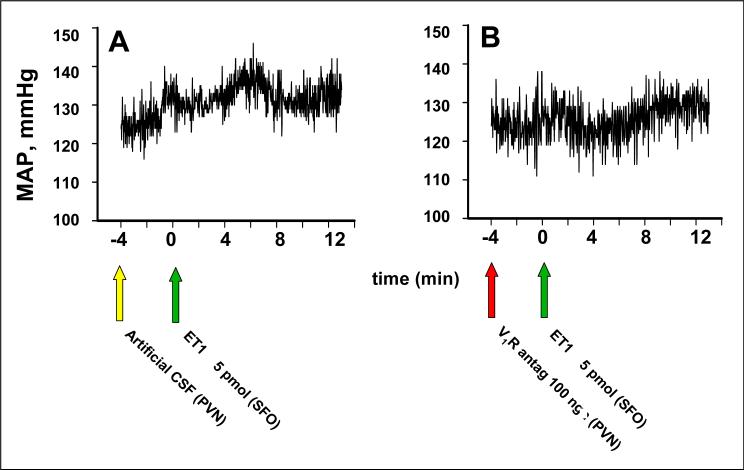
Effect of V1aR antagonist in PVN on stimulation of SFO by ET1
Figure 2 shows the sites of microinjection into PVN and SFO in the five rats whose data are shown in Table 1 and Figures 3 and 4. As in the previous set of experiments, neither artificial CSF nor V1aR antagonist injection into the PVN changed baseline parameters. Injection of ET1 into the SFO resulted in a pressor response that peaked at ∼10 min. V1aR antagonism of the PVN significantly inhibited the rise in MAP associated with ET1 injection into the SFO, but did not prevent the decreases in heart rate and RSNA (Table 1, Fig. 3 and 4).
Fig. 2.
Injection sites in subfornical organ (SFO, ◆) and paraventricular nucleus (PVN, •). (A) Sagittal section through SFO. (B) Coronal section through PVN. DG, dentate gyrus; f, fornix; vhc, ventral hippocampal gyrus; 3V, third ventricle.
Table 1.
Hemodynamic parameters and renal sympathetic nerve activity in response to ET1 administration into the SFO with and without antagonism of V1a receptors in PVN
| MAP mmHg | Heart Rate bpm | RSNA %baseline | |
|---|---|---|---|
| Baseline | 130.6 ± 4.1 | 407 ± 21 | 100 ± 0 |
| 10 min After ET1 (SFO) | 146.4 ± 5.4 | 374 ± 27 | 96.9 ± 15.8 |
| MAP mmHg | Heart Rate bpm | RSNA %baseline | |
|---|---|---|---|
| Baseline | 130.1 ± 4.8 | 403 ± 14 | 100 ± 0 |
| After V1aR antagonism (PVN) | 131.9 ± 5.8 | 403 ± 13 | 91.7 ± 8.2 |
| 10 min After ET1 (SFO) | 138.0 ± 5.6* | 385 ± 15 | 87.1 ± 7.0 |
V1aR antagonist, 10 ng; ET1, 5 pmol.
Values are mean ± SE; n = 5
P < 0.015 vs ET1 without V1aR antagonism
Fig. 3.
Representative recordings of MAP after bilateral microinjection of the paraventricular nucleus (PVN) with (A) artificial cerebrospinal fluid (CSF) or (B) V1a receptor antagonist (V1aR antag, 100 ng) followed by microinjection of 5 pmol ET 1 into the SFO. Arrows indicate time of each microinjection.
Fig. 4.
Changes from baseline in mean MAP, HR, and RSNA after bilateral microinjection of PVN with either artificial CSF or 100 ng V1aR antagonist followed by 5 pmol ET1 into SFO. Values are mean ± SE; n = 5. *P < 0.05 vs artificial CSF.
DISCUSSION
The present studies demonstrate that AVP injected into the PVN of normal rats results in a prompt increase in arterial pressure, heart rate and RSNA that could be completely blocked by prior antagonism of V1a vasopressin receptors. Importantly, the V1a receptor antagonist also significantly decreased the pressor response observed with ET1 administration into the SFO; however, in these normal rats the V1a receptor inhibitor alone did not change any of the baseline parameters.
Evidence for peptide release from the dendrites of magnocellular neurons has existed for over 20 years (24,35,36). Dendritically released AVP is known to act on V1a receptors on the dendrites and soma of magnocellular neurons to inhibit further release of AVP from axon terminals in the posterior pituitary and into the systemic circulation (28,37). In contrast, within the supraoptic nucleus where it has been studied, dendritically released AVP stimulates further release of AVP from the dendrites themselves (38), an action that could be blocked by the V2 receptor inhibitor (30). It has been suggested that this dendritic release may serve to limit the extent of AVP release from the posterior pituitary.
It seems reasonable to infer that vasopressinergic magnocellular neurons within the PVN respond in a similar way to those of the supraoptic nucleus. However, vasopressinergic cells within the PVN project not only to the neurohypophysis, but also to the rostroventrolateral medulla and spinal cord where they influence cardiovascular and sympathetic nerve responses (39-41). Malpas and Coote (42) demonstrated that electrical stimulation of the PVN reproducibly increased RSNA and arterial pressure, and that these responses could be prevented by intrathecal administration of a V1a blocker. These findings support a role for axonal vasopressinergic projections from axons projecting to the spinal cord in sympathetic activation. However, it is not clear if this pathway is required for the sympathetic activation and pressor response observed with central ET1 as the pressor response in Brattleboro rats devoid of central AVP is identical to that of normal Long Evans rats (10).
In contrast to the many studies on the role of dendritically released AVP on the secretion of AVP into the systemic circulation, to date, the extent to which AVP release within the PVN itself may also affect cardiovascular and sympathetic activity has been given little if any attention. Just as application of AVP to the spinal cord increased arterial pressure and the firing rate of preganglionic sympathetic neurons (43), the present findings clearly show that exogenous application of AVP directly into the PVN increased heart rate, arterial pressure and RSNA via V1a receptor activation. It could be argued that the hemodynamic and sympathetic response to exogenous AVP occurs due to ischemia resulting from vasoconstriction of cerebral microvessels in the PVN. That the V1a receptor antagonist had no effect on the baseline parameters would be consistent with such an interpretation. However, in the conscious animal unprovoked by osmotic or volume challenges, the PVN vasopressinergic neurons are relatively inactive (44). Under such circumstances, as in the present experiments, the effect of V1a receptor inhibition on baseline activities would be minimal. In addition, the observation that the V1a receptor inhibitor was also able to block the pressor effect of ET1 injected into the SFO, which is anatomically distinct and distant from the PVN, suggests that the hemodynamic and sympathetic nerve responses cannot be solely attributed to neural ischemia of the PVN by exogenous AVP. Taken together, these observations suggest that AVP can act within the PVN itself to elicit increases in RSNA, arterial pressure and heart rate.
Several investigators have shown that ET1 administered into the lateral cerebral ventricles elicits a pressor response that is mediated by an increase in sympathetic output (10,13,45). In pathophysiological states when plasma ET1 levels are high, ET1 in the systemic circulation, can also gain access to the SFO which lies outside the blood brain barrier and is richly endowed with ET receptors (7,8). The current results confirm previous studies (21,46) showing that injection of ET1 into the SFO evokes an increase in arterial pressure and reflex changes in heart rate and RSNA. Neuronal fibers project from the SFO either anteroventrally to the region surrounding the third ventricle or caudally to synapse within the PVN (9). Direct injection of ET1 into the SFO elicits activation of PVN neurons (3) and induces c-fos expression particularly, but not exclusively, in the magnocellular region of the PVN (47). Electrolytic lesions of the PVN (46) or injection of a non-N-methyl-d-aspartate glutamatergic antagonist into the magnocellular region of the PVN (48) abolishes the pressor response to ET1 at the SFO. Notably, V1a receptor inhibition of the PVN significantly decreased the pressor response to ET1 applied to SFO by ∼60%; however, it did not totally eliminate the increase in arterial pressure. Moreover, the reflex bradycardia and decrease in RSNA were not significantly changed. These observations could be due to the fact that the injections of the V1a receptor blocker were specifically directed to the magnocellular region of the PVN. The present experiments do not exclude the possibility that AVP that is released from dendrites of the magnocellular vasopressinergic neurons and extending toward the third ventricle or from parvocellular neurons activated by the SFO projections may activate V1a receptors outside the volume of diffusion of the acute microinjection of the V1a antagonist. Such a mechanism could potentially explain the reason for the variability in the RSNA responses between different animals after V1a receptor antagonism, such that in some animals the reflex decrease in RSNA was totally blocked and in others there was no change despite the consistent decrease in arterial pressure.
Furthermore, Ludwig (25) and others (38) have definitively shown that dendritic release of AVP does not necessarily parallel its axonal release. Moreover, the regulation of neuropeptide release from the dendrites and soma differs from that at the terminal axon (25,26,31,49,50). Thus, even though plasma AVP does not increase in response to ETA receptor activation of the SFO in normal baroreceptor intact animals such as those in the present studies, somatodendritic release of AVP may still occur from magnocellular neurons within the PVN and diffuse over greater spatial and temporal dimensions via the extracellular fluid to activate neurons projecting to medullary and spinal cardiovascular regulating centers. In conditions of sustained activation of the SFO due to high ambient plasma ET1 levels as in heart failure, intra-PVN AVP may be one mechanism contributing to the sustained and elevated state of sympathoactivation, and may be a target for therapeutic manipulation. Finally, it is important to note that the present findings do not distinguish whether the action of AVP is direct or indirect. For example, AVP may directly stimulate PVN neurones responsible for efferent sympathoexcitation. Alternatively, AVP within the PVN may inhibit an inhibitory interneuron (e.g., GABAergic neuron), which would also result in increased sympathetic efferent activity and elevated systemic pressure.
In summary, the present findings support the model whereby stimulation of ETA receptors within the SFO results in activation of efferent projections to the PVN, particularly the magnocellular region but also very likely the parvocellular region as well. As a result of these inputs into the PVN, AVP secretion from axons of the magnocellular neurons terminating in the posterior pituitary increases. In addition, caudal projections to the ventrolateral medulla and spinal cord result in increased sympathetic activity, arterial pressure and heart rate (46). However, once activated, the vasopressinergic neurons also release AVP from their soma and dendrites into the extracellular fluid within the PVN where this pool of AVP, in turn, can further modulate the pressor response either directly by stimulation of neurons projecting to the cardiovascular regulatory regions or indirectly by inhibition of inhibitory elements within the PVN. Modulation of heart rate and activity of the renal sympathetic nerves may be more variable depending upon the spatial and temporal extent of diffusion of somatodendritically released AVP and the neuronal pathways that are subject to its influence.
Acknowledgements
This work was supported by a Merit Award from the Department of Veterans Affairs and grant HL 07109 from the National Institutes of Health, USA. The authors and their spouses have no conflict of interest to declare relevant to this publication.
REFERENCES
- 1.MacCumber MW, Ross CA, Glaser BM, Snyder SH. Endothelin: visualization of mRNAs by in situ hybridization provides evidence for local action. Proc Natl Acad Sci USA. 1989;86:7285–7289. doi: 10.1073/pnas.86.18.7285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Takahashi K, Ghatei MA, Jones PM, Murphy JK, Lam HC, O'Halloran DJ, Bloom SR. Endothelin in human brain and pituitary gland: presence of immunoreactive endothelin, endothelin messenger ribonucleic acid, and endothelin receptors. J Clin Endo Metab. 1991;72:693–699. doi: 10.1210/jcem-72-3-693. [DOI] [PubMed] [Google Scholar]
- 3.Wall KM, Ferguson AV. Endothelin acts at the subfornical organ to influence the activity of putative vasopressin and oxytocin-secreting neurons. Brain Res. 1992;586:111–116. doi: 10.1016/0006-8993(92)91378-r. [DOI] [PubMed] [Google Scholar]
- 4.Yamamoto S, Inenaga K, Eto S, Yamashita H. Cardiovascular-related peptides influence hypothalamic neurons involved in control of body water homeostasis. Obesity Res Suppl. 1995;5:789S–794S. doi: 10.1002/j.1550-8528.1995.tb00501.x. [DOI] [PubMed] [Google Scholar]
- 5.Yoshizawa T, Shinmi O, Giaid A, Yanagisawa M, Gibson SJ, Kumua S, Uchiyama Y, Polak JM, Masaki T, Kanazawa I. Endothelin: a novel peptide in the posterior pituitary system. Science. 1990;247:462–464. doi: 10.1126/science.2405487. [DOI] [PubMed] [Google Scholar]
- 6.Nakamura S, Naruse M, Naruse K, Shioda S, Nakai Y, Uemura H. Colocalization of immunoreactive endothelin-1 and neurohypophysial hormones in the axons of the neural lobe of the rat pituitary. Endocrinol. 1993;132:530–533. doi: 10.1210/endo.132.2.8425473. [DOI] [PubMed] [Google Scholar]
- 7.Jones CR, Hiley CR, Pelton JR, Mohr M. Autoradiographic visualization of the binding sites for [125I]endothelin in rat and human brain. Neurosci Lett. 1989;97:276–279. doi: 10.1016/0304-3940(89)90610-1. [DOI] [PubMed] [Google Scholar]
- 8.Koseki C, Imai M, Hirata Y, Yanagisawa M, Masaki T. Autoradiographic distribution in rat tissues of binding sites for endothelin: a neuropeptide? Am J Physiol. 1989;256:R858–R866. doi: 10.1152/ajpregu.1989.256.4.R858. [DOI] [PubMed] [Google Scholar]
- 9.Miselis RR. The efferent projections of the subfornical organ of the rat: a circumventricular organ within the neural network subserving water balance. Brain Res. 1981;230:1–23. doi: 10.1016/0006-8993(81)90388-7. [DOI] [PubMed] [Google Scholar]
- 10.Rossi NF, O'Leary DS, Chen H. Mechanisms of centrally administered ET-1-induced increases in systemic arterial pressure and AVP secretion. Am J Physiol. 1997;272:E126–E132. doi: 10.1152/ajpendo.1997.272.1.E126. [DOI] [PubMed] [Google Scholar]
- 11.Kuwaki T, Cao W-H, Kumada M. Endothelin in the brain and its effect on central control of the circulation and other functions. Jap J Physiol. 1994;44:1–18. doi: 10.2170/jjphysiol.44.1. [DOI] [PubMed] [Google Scholar]
- 12.Gulati A, Rebello S, Kumar A. Role of sympathetic nervous system in cardiovascular effects of centrally administered endothelin-1 in rats. Am J Physiol. 1997;273:H1177–H1186. doi: 10.1152/ajpheart.1997.273.3.H1177. [DOI] [PubMed] [Google Scholar]
- 13.Nishimura M, Takahashi J, Masusawa M, Ikegaki I, Nakanishi T, Hirabashi M, Yoshimura M. Chronic intracerebroventricular infusions of endothelin elevate arterial pressure in rats. J Hypertension. 1991;9:71–76. doi: 10.1097/00004872-199101000-00011. [DOI] [PubMed] [Google Scholar]
- 14.Yamamoto T, Kimura T, Ota K, Shoji M, Inoue M, Sato K, Ohta M, Yoshinaga K. Central effects of endothelin 1 on vasopressin and atrial natriuretic peptide release and cardiovascular and renal function in conscious rats. J Cardiovasc Pharmacol. 1991;17:S316–S318. doi: 10.1097/00005344-199100177-00090. [DOI] [PubMed] [Google Scholar]
- 15.Rossi NF, Chen H, Musch TI. Endothelin 1-induced pressor response and vasopressin release in heart failure. J Cardiovasc Pharmacol. 2002;40:80–89. doi: 10.1097/00005344-200207000-00010. [DOI] [PubMed] [Google Scholar]
- 16.Spieker LE, Noll F, Rushchitzka FT, Lusher TF. Endothelin receptor antagonists in congestive heart failure: a new therapeutic principle for the future? J Am Coll Cardiol. 2001;37:1493–1505. doi: 10.1016/s0735-1097(01)01210-4. [DOI] [PubMed] [Google Scholar]
- 17.Spinarova L, Spinar J, Vasků A, Goldbergova M, Ludka O, Toman J, Vitovec J, Tomandalova M, Tomandl J. Big endothelin in chronic heart failure: marker of disease severity or genetic determination. Int J Cardiol. 2004;93:63–68. doi: 10.1016/s0167-5273(03)00112-8. [DOI] [PubMed] [Google Scholar]
- 18.Szatalowicz VL, Arnold PE, Chaimovitz C, Bichet D, Berl T, Schrier RW. Radioimmunoassay of plasma arginine vasopressin in hyponatremic patients with congestive heart failure. N Engl J Med. 1981;305:263–6. doi: 10.1056/NEJM198107303050506. [DOI] [PubMed] [Google Scholar]
- 19.Arai M, Yoguchi A, Iso T, Takahashi T, Mai S, Murata K, Suzuki T. Endothelin-1 and its binding sites are upregulation in pressure overload cardiac hypertrophy. Am J Physiol. 1995;268:H2084–H2091. doi: 10.1152/ajpheart.1995.268.5.H2084. [DOI] [PubMed] [Google Scholar]
- 20.McConnell PI, Olson CE, Patel KP, Blank DU, Olivari MT, Gallagher KP, Quenby-Brown E, Zucker IH. Chronic endothelin blockade in dogs with pacing-induced heart failure: Possible modulation of sympathoexcitation. J Cardiac Failure. 2000;6:56–65. doi: 10.1016/s1071-9164(00)00012-9. [DOI] [PubMed] [Google Scholar]
- 21.Rossi NF, Maliszewska-Scislo M, Chen H. Central endothelin: effects on vasopressin and the arterial baroreflex in doxorubicin heart failure rats. Can J Physiol Pharmacol. 2008;86:343–52. doi: 10.1139/Y08-027. [DOI] [PubMed] [Google Scholar]
- 22.Pacher R, Stanek B, Hulsmann M, Koller-Strametz J, Berger R, Schuller M, Hartter E, Ogris E, Frey B, Heinz G, Maurer G. Prognostic impact of big endothelin–1 plasma concentrations compared with invasive hemodynamic evaluation in severe heart failure. J Am Coll Cardiol. 1996;27:633–641. doi: 10.1016/0735-1097(95)00520-x. [DOI] [PubMed] [Google Scholar]
- 23.Schrier RW, Abraham WT. Hormones and hemodynamics in heart failure. N Engl J Med. 1999;341:577–585. doi: 10.1056/NEJM199908193410806. [DOI] [PubMed] [Google Scholar]
- 24.Pow DV, Morris JF. Dendrites of hypothalamic magnocellular neurons release neurohypophysial peptides by exocytosis. Neuroscience. 1989;32:435–439. doi: 10.1016/0306-4522(89)90091-2. [DOI] [PubMed] [Google Scholar]
- 25.Ludwig M. Dendritic release of vasopressin and oxytocin. J Neuroendocrinol. 1998;10:881–895. doi: 10.1046/j.1365-2826.1998.00279.x. [DOI] [PubMed] [Google Scholar]
- 26.Ludwig M, Pittman QJ. Talking back: dendritic neurotransmitter release. Trends in Neurosci. 2003;26:255–261. doi: 10.1016/S0166-2236(03)00072-9. [DOI] [PubMed] [Google Scholar]
- 27.Ludwig M, Callahan MF, Neumann I, Landgraf R, Morris M. Systemic osmotic stimulation increases vasopressin and oxytocin release within the supraoptic nucleus. J Neuroendocrinol. 1994;6:369–373. doi: 10.1111/j.1365-2826.1994.tb00595.x. [DOI] [PubMed] [Google Scholar]
- 28.Gouzenes L, Desarmenien MG, Hussy N, Richard P, Moos FC. Vasopressin regularizes the phasic firing pattern of rat hypothalamic magnocellular neurons. J Neurosci. 1998;18:1879–1885. doi: 10.1523/JNEUROSCI.18-05-01879.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sabatier N, Richard P, Datanithi G. Activation of multiple intracellular transduction signals by vasopressin in vasopressin-sensitive neurones of the rat supraoptic nucleus. J Physiol [Lond] 1998;513:699–710. doi: 10.1111/j.1469-7793.1998.699ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gillard EF, Coburn CG, de Leon A, Snissarenko EP, Bauce LG, Pittman QJ, Hou B, Curras-Collazo MC. Vasopressin autoreceptors and nitric oxide-dependent glutamate release are required for somatodendritic vasopressin release from rat magnocellular neuroendocrine cells responding to osmotic stimuli. Endocrinol. 2007;148:479–489. doi: 10.1210/en.2006-0995. [DOI] [PubMed] [Google Scholar]
- 31.Ludwig M, Leng G. Dendritic peptide release and peptide-dependent behaviors. Nature Rev Neurosci. 2006;7:126–136. doi: 10.1038/nrn1845. [DOI] [PubMed] [Google Scholar]
- 32.Ostrowski NL, Lolait SJ, Young WS., 3rd Cellular localization of vasopressin V1a receptor messenger ribonucleic acid in adult male rat brain, pineal and brain vasculature. Endocrinol. 1994;135:1511–1528. doi: 10.1210/endo.135.4.7925112. [DOI] [PubMed] [Google Scholar]
- 33.Pyner S, Coote JH. Identification of branching paraventricular neurons of the hypothalamus that project to the rostroventrolateral medulla and spinal cord. Neuroscience. 2000;100:549–556. doi: 10.1016/s0306-4522(00)00283-9. [DOI] [PubMed] [Google Scholar]
- 34.Maliszewska-Scislo M, Scislo T, Rossi NF. Effect of endogenous angiotensin on baroreflexes in conscious streptozotocin diabetic rats. Am J Physiol Heart and Circ. 2003;284:H1601–H1611. doi: 10.1152/ajpheart.00578.2002. [DOI] [PubMed] [Google Scholar]
- 35.Moos F, Freund-Mercier M, Guerne Y, Guerne J, Stoekel M, Richard P. Release of oxytocin and vasopressin by magnocellular nuclei in vitro: specific facilitatory effect of oxytocin on its own release. J Endocrinol. 1984;102:63–72. doi: 10.1677/joe.0.1020063. [DOI] [PubMed] [Google Scholar]
- 36.Mason WT, Hatton GI, Ho YW. Central release of oxytocin, vasopressin and neurophysin by magnocellular neurone depolarization: evidence in slices of guinea pig and rat hypothalamus. Neuroendocrinology. 1986;42:311–322. doi: 10.1159/000124457. [DOI] [PubMed] [Google Scholar]
- 37.Ludwig M, Leng G. Autoinhibition of supraoptic nucleus vasopressin neurons in vivo: a combined retrodialysis/electrophysiological study in rats. Eur J Neurosci. 1997;9:2532–2540. doi: 10.1111/j.1460-9568.1997.tb01682.x. [DOI] [PubMed] [Google Scholar]
- 38.Wotjak CT, Ganster J, Kohl G, Holsboer F, Landgraf R, Engelmann M. Dissociated central and peripheral release of vasopressin, but not oxytocin, in response to repeated swim stress: new insights into the secretory capacities of peptidergic neurons. Neuroscience. 1998;85:1209–1222. doi: 10.1016/s0306-4522(97)00683-0. [DOI] [PubMed] [Google Scholar]
- 39.Swanson LW. Immunohistochemical evidence for a neurohypophysin-containing autonomic pathway arising in the paraventricular nucleus of the hypothalamus. Brain Res. 1977;128:356–363. doi: 10.1016/0006-8993(77)91000-9. [DOI] [PubMed] [Google Scholar]
- 40.Swanson LW, Kuypers HGJM. The paraventricular nucleus of the hypothalamus: cyto-architectonic subdivisions and organization of projections to the pituitary, dorsal vagal complex, and spinal cord as demonstrated by retrograde fluorescence double labelling methods. J Comp Neurol. 1980;194:555–570. doi: 10.1002/cne.901940306. [DOI] [PubMed] [Google Scholar]
- 41.Swanson LW, Sawchenko PE. Hypothalamic integration: organization of the paraventricular and supraoptic nuclei. Annu Rev Neurosci. 1983;6:269–324. doi: 10.1146/annurev.ne.06.030183.001413. [DOI] [PubMed] [Google Scholar]
- 42.Malpas SC, Coote JH. Role of vasopressin in sympathetic response to paraventricular nucleus stimulation in anesthetized rats. Am J Physiol Reg Integr Comp Physiol. 1994;266:R228–R236. doi: 10.1152/ajpregu.1994.266.1.R228. [DOI] [PubMed] [Google Scholar]
- 43.Gilbey MP, Coote JH, Fleetwood-Walker S, Peterson DF. The influence of the paraventriculo-spinal pathway, and oxytocin and vasopressin on sympathetic preganglionic neurons. Brain Res. 1982;251:283–290. doi: 10.1016/0006-8993(82)90745-4. [DOI] [PubMed] [Google Scholar]
- 44.Lovick TA, Coote JH. Electrophysiological properties of paraventriculo-spinal neurons in the rat. Brain Res. 1988;454:123–130. doi: 10.1016/0006-8993(88)90810-4. [DOI] [PubMed] [Google Scholar]
- 45.Takeda K, Nakata T, Takesako T, Itoh H, Hirata M, Kawasaki S, Hayashi J, Ogura M, Sasaki S, Nakagawa M. Sympathetic inhibition and attenuation of spontaneous hypertension by PVN lesions in rats. Brain Res. 1991;543:286–300. doi: 10.1016/0006-8993(91)90040-3. [DOI] [PubMed] [Google Scholar]
- 46.Rossi NF, Chen H. Paraventricular nucleus lesions prevent the endothelin 1-induced increase in arterial pressure and vasopressin. Am J Physiol. 2001;280:E349–E356. doi: 10.1152/ajpendo.2001.280.2.E349. [DOI] [PubMed] [Google Scholar]
- 47.Zhu B, Herbert J. Behavioral, autonomic and endocrine responses associated with c-fos expression in the forebrain and brainstem after intracerebroventricular infusions of endothelins. Neuroscience. 1996;71:1049–1062. doi: 10.1016/0306-4522(95)00512-9. [DOI] [PubMed] [Google Scholar]
- 48.Rossi NF, Chen H. Aminopropionic acid receptors in paraventricular nucleus mediate pressor and vasopressin responses to endothelin 1 in subfornical organ. Exper Biol and Medicine. 2006;231:1075–1080. [PubMed] [Google Scholar]
- 49.Ludwig M, Bull PM, Tobin VA, Sabatier N, Landgraf R, Dayanathi G, Leng G. Regulation of activity dependent dendritic release from rat supraoptic neurons. J Physiol [Lond] 2005;564:515–522. doi: 10.1113/jphysiol.2005.083931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Landgraf R, Neumann ID. Vasopressin and oxytocin release within the brain: a dynamic concept of multiple and variable modes of neuropeptide communication. Frontiers in Neuroendocrinol. 2004;25:150–176. doi: 10.1016/j.yfrne.2004.05.001. [DOI] [PubMed] [Google Scholar]