Abstract
Context
While the nature of common fears changes over development, we do not know whether genetic effects on fear-proneness are developmentally stable or developmentally dynamic.
Objective
To determine the temporal pattern of genetic and environmental effects on the level of intensity of common fears.
Design
Prospective, 4-wave longitudinal twin study. Structural modeling was performed with Mx.
Setting
General community.
Participants
Two thousand four hundred ninety twins and their parents from the Swedish Twin Study of Child and Adolescent Development.
Main Outcome Measure
The level of parent- and/or self-reported fears obtained at ages 8 to 9, 13 to 14, 16 to 17, and 19 to 20 years.
Results
Thirteen questionnaire items formed 3 distinct fear factors: situational, animal, and blood/injury. For all 3 fears, the best-fit model revealed developmentally dynamic effects and, in particular, evidence for both genetic attenuation and innovation. That is, genetic factors influencing fear intensity at age 8 to 9 years decline substantially in importance over time. Furthermore, new sets of genetic risk factors impacting fear intensity “come on line” in early adolescence, late adolescence, and early adulthood. As the twins aged, the influence of the shared environment declined and unique environment increased. No sex effects were found for situational fears while for animal and blood/injury fears, genetic factors in males and females were correlated but not identical. Shared environmental factors were both more important and more stable for animal fears than for situational or blood/injury fears.
Conclusions
Genetic effects on fear are developmentally dynamic from middle childhood to young adulthood. As children age, familial-environmental influences on fears decline in importance.
When considering the role of individual differences in the etiology of excessive fears and phobias, emphasis is typically given to traits that are conceptualized as being temporally stable, such as behavioral inhibition, autonomic lability, neuroticism, cognitive bias (for threatening stimuli), and disgust sensitivity.1–4 However, fears demonstrate a dynamic progression through development3—what Marks5 has termed an ontogenetic parade. Furthermore, epidemiological investigations show consistent differences in the ages at onset of individual phobia subtypes.6,7 Realistic etiologic models for excessive fears and phobias will require an understanding of the developmentally dynamic processes that underlie risk.
Cross-sectional studies in children,8–10 adolescents,11 and adults6,12–15 consistently show genetic contributions to excessive fears and phobias. While developmentally dynamic genetic effects have been demonstrated for a range of phenotypes, including antisocial behavior,16,17 cognitive abilities,18 plasma lipids,19 and weight,20,21 to our knowledge, no study to date has examined fears in a longitudinal and genetically informative sample to elucidate the patterns of temporal stability or change in genetic influences. Therefore, in this report, we examine the development of genetic and environmental risk factors for situational, animal, and blood/injury fears in a population-based cohort of Swedish twins. Because the twins were assessed 4 times between the ages of 8 and 20 years, they all passed through puberty, a developmental period of particular interest given prior evidence for the impact of gonadal hormones on fear mechanisms.22–24
The primary goal of the study was to discriminate between 2 hypotheses about the developmental pattern of genetic risk factors for fears. The “developmentally stable” hypothesis predicts that a single set of genetic risk factors impacts the level of fears at age 8 years and these same genes constitute the only genetic influences on fear-proneness throughout development. By contrast, the “developmentally dynamic” hypothesis predicts that genetic effects on fear-proneness will vary over time. This variability might be manifested through genetic innovation, in which new genes become active that previously were without effect on fear intensity, or genetic attenuation, in which genes that impact at one developmental age decline in influence at later periods.
These analyses have 2 additional goals: (1) To determine the nature and stability of shared and unique environmental influences on fears from childhood to young adulthood. (2) To explore whether the magnitude or nature of the genetic and environmental risk factors for fears differs in males and females.
METHODS
SAMPLE
The sample was obtained from the population-based Swedish Twin Registry, which contains information on all twins born in Sweden since 1886.25 As detailed elsewhere, this sample—called the Swedish Twin Study of Child and Adolescent Development (or T-CHAD)—began with all twin pairs born in Sweden between May 1985 and December 1986 where both twins were alive and residing in Sweden in 1994.26 To date, this sample has been assessed 4 times for their level of fears: at the age of 8 to 9 years by a mailed questionnaire to parents (n=1109 or 75% response); age 13 to 14 years with a mailed questionnaire to parents (n=1063, 73%) and children (n=2263, 78%); age 16 to 17 years with a mailed questionnaire to parents (n=1067, 74%) and children (n=2369 children, 82%); and age 19 to 20 years with a questionnaire solely to the twins (n=1705, 59%). Each of the questionnaires was approved by the Ethics Committee of the Karolinska Institute, Stockholm, Sweden. No specific informed consent was required as response to the questionnaire is seen in Sweden as constituting consent.
ZYGOSITY DETERMINATION
Zygosity determination was based on well-validated questions to both twins and parents chosen from a discriminant analysis of 106 same-sex pairs from this sample that had their zygosity determined by typing 16 polymorphic DNA markers.26 Those with uncertain scores were classified as zygosity unknown.
MEASURES
Using a questionnaire developed by Fredrikson et al,27 the children were asked to rate their own fear intensity and the parents were asked to rate each child’s fear intensity for the specific objects or situations on a scale ranging from 0 (no fear) to 10 (maximal fear). Social fears were not added until the assessment at age 13 to 14 years and so are not included herein. Because the initial scale contained only 2 items to assess animal fears (snakes and spiders), 3 additional items (rats, dogs, and wasps) were added from those most commonly listed in an open-ended question to respondents. These items were added for the age 13 to 14 years and all subsequent assessments. The exact wording (in English translation) of the items used in these analyses is seen in Table 1. The present study is a follow-up of a previous report from the assessment at age 8 to 9 years.8
Table 1.
| Factor Loadings |
|||
|---|---|---|---|
| Fear-Producing Stimulus | Situational | Animal | Blood/Injury |
| Enclosed places | 0.81 | 0.03 | −0.13 |
| Heights | 0.74 | −0.01 | −0.02 |
| Dark | 0.71 | 0.09 | −0.01 |
| Flying | 0.69 | −0.11 | 0.18 |
| Lightning | 0.57 | 0.02 | 0.19 |
| Rats | 0.03 | 0.80 | −0.03 |
| Snakes | 0.06 | 0.79 | −0.09 |
| Spiders | 0.02 | 0.75 | 0.07 |
| Wasps | 0.04 | 0.42 | 0.29 |
| Dogs | −0.13 | 0.35 | 0.21 |
| Dentists | −0.01 | −0.03 | 0.81 |
| Injections | 0.02 | 0.08 | 0.78 |
| Injuries and blood | 0.28 | 0.04 | 0.51 |
Bold loadings (≥ + 0.30) indicate the factor to which the individual fear item was assigned.
DATA ANALYSIS
For these analyses of fears, we began with 2717 twin individuals, of whom 28 had missing data and 199 had unknown zygosity. The remaining 2490 individuals came from 1237 complete pairs and 16 single twins. Of these pairs, 246 were female monozygotic (MZ); 184, female dizygotic (DZ); 243, male MZ; 177, male DZ; and 387, opposite-sex DZ pairs.
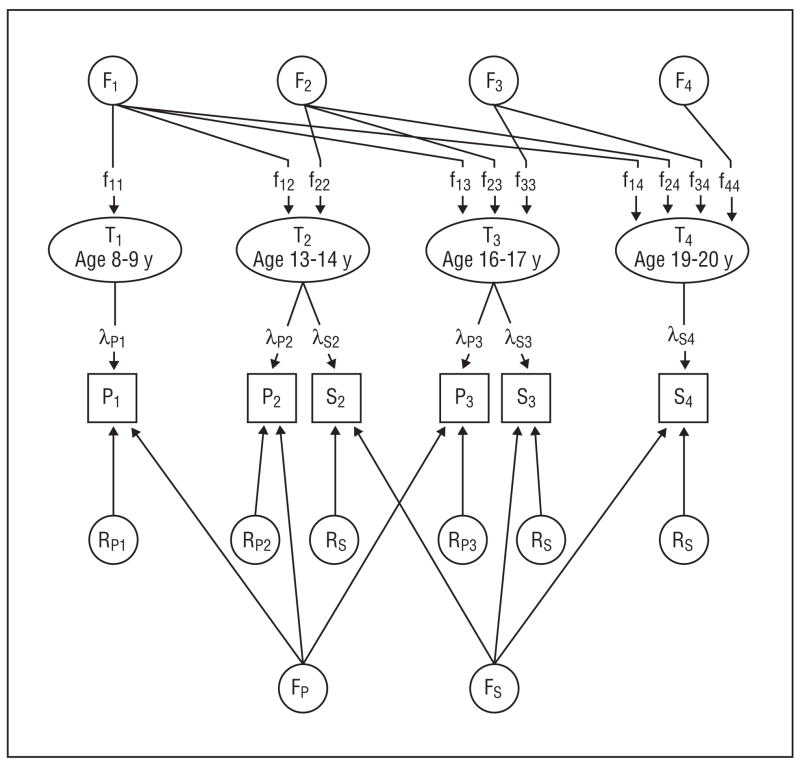
The model used in these analyses is presented in Figure 1, which illustrates for a single individual the sources of liability for only additive genetic effects. The model has 4 major features. First, it contains 4 latent fear scores (T1–T4) that reflect the “true” level of fear at ages 8 to 9, 13 to 14, 16 to 17, and 19 to 20 years, herein called, for simplicity, times 1, 2, 3, and 4. Second, these latent variables are indexed by ratings of fear either by parental report (P) or self-report (S). Both reports are available for times 2 and 3, while only the parent report is available for time 1 and only self-report at time 4. The degree to which the parent- and self-reported fear ratings index the latent fear level is reflected by the paths λP and λS. Third, the genetic and environmental influences on the latent fear scores at times 1, 2, 3, and 4 are modeled as a Cholesky decomposition. Taking genes as an example, this developmentally informative approach divides up genetic risk into 4 factors (F1–F4). The first (F1) begins in childhood (age 8–9 years) and is continuously active over the entire developmental period. The strength of its effect at each of the 4 ages is reflected, respectively, in the path coefficients f11, f12, f13, and f14. The second factor begins in early adolescence (age 13–14 years) and impacts times 2, 3, and 4 via paths f22, f23, and f24. The third factor starts in late adolescence (age 16–17 years) and impacts times 3 and 4 via paths f33 and f34. The fourth and final factor acts only at time 4, young adulthood (age 19–20 years), via path f44. The “developmentally stable” hypothesis predicts that all of the genetic liability to fears will be captured by the first factor with no evidence for genetic innovation later in development. By contrast, the “developmentally dynamic” hypothesis predicts such innovation—that new genetic variation that impacts fear levels will be seen in early and late adolescence and young adulthood. Furthermore, consistent with the “developmentally dynamic” hypothesis would be evidence for genetic attenuation—that the impact of the first and perhaps second genetic factors acting early in development decline over time. Fourth, the model contains 2 reporter-specific common factors, one each for parent and self, as well as rater- and time-specific residuals. To identify the f44 path, it was necessary to constrain the 3 self-report–specific loadings to equality.
Figure 1.
The model used in these analyses presented for 1 source of liability, such as additive genetic effects. The model contains 4 latent fear scores (T1–T4) reflecting the true level of fear at time 1 (age 8–9 years), time 2 (age 13–14 years), time 3 (age 16–17 years), and time 4 (age 19–20 years). These latent variables are indexed by ratings of fear by parental report (P) (available for times 1–3) and by self-report (S) (available for times 2–4). The degree to which the parent- and self-reported fear ratings index the latent fear level is reflected by the paths λP and λS. The genetic and environmental influences on the latent fear scores are modeled as a Cholesky decomposition. See the text of this article for more details. F indicates the 4 genetic risk factors; f, the path from the genetic factors to the latent fear scores at each of the 4 ages; R, residual effects.
Our analyses focus on the latent measures of fear that were reported by both parents and children because these measures are most likely to be valid given that they reflect both the subjective and objective manifestations of these fears. The reporter-specific factors—reported by one but not the other rater—are required in the model but are of less interest to us and are not focused on herein.
Estimates of heritability and shared environmental effects for fears estimated from this model are not directly comparable with those obtained from standard twin models and would be expected to be higher. In standard twin models, errors of measurement contribute to and are confounded with individual-specific environment, thereby reducing estimates of heritability or shared environment. However, through the use of multiple raters, this model unconfounds the true individual-specific environment that impacts the latent fear scores (T1–T4) from the errors of measurement that contribute to the rater-specific effects (P1–P3 and S2–S4).
For each factor, we examined both qualitative and quantitative sex effects. Qualitative sex effects arise when genetic factors that influence a trait are not entirely the same in males and females and are measured by the genetic correlation rg. rg can vary from zero (ie, entirely distinct sets of genes in the 2 sexes) to unity (ie, identical genetic factors impacting males and females). Quantitative sex effects arise when the same genetic factors impact to different degrees in males and females.
Analyses were performed using the Mx software package.29 Levels of fear were treated as a continuous variable. Because the sum scores for the items identified by factor analyses had a skewed distribution, we did a square root transformation followed by standardization separately for each sex, age, and zygosity group.
Given the complexity of these models, aside from evaluating sex effects, we did not attempt further simplification. For evaluating fits, we used the Bayesian Information Criterion (BIC),30 which performs well with complex models.31 The lower the BIC value, the better the balance of explanatory power and parsimony.
To determine the factor structure of the fear items prior to our genetic analyses, we conducted an oblique Promax factor analysis,28 identifying the number of factors using a traditional eigenvalue criterion.
RESULTS
FACTOR ANALYSIS
Starting, arbitrarily, with self-report data at age 16 to 17 years, our Promax rotation28 (Table 1) produced 3 readily interpretable factors: situational (fears of closed places, heights, flying, dark, and lightening), animal (fears of rats, snakes, spiders, wasps, and dogs) and blood/injury (fears of dentists, injections, and blood). Only fear of dogs loaded less than +0.40 on its expected factor. This factor structure was very similar to that seen with the other time-rater combinations at which the full list of fears was assessed: parents, age 13 to 14 years; self, ages 13 to 14 years; parents, age 16 to 17 years; and self, age 19 to 20 years. The mean (SD) of the 10 congruency coefficients (a measure of factorial similarity)32 between these 5 occasions of measurement for each of the factors were, respectively, situational, 0.979 (0.009); animal, 0.981 (0.008); and blood/injury 0.972 (0.197).
MEAN FEAR INTENSITIES
Table 2 presents mean (SE) levels of reported fear intensity as a function of the type of fear, the sex of the twin, the informant (self vs parent), and age. Four patterns are of interest. First, with only one exception (animal fear by parental report in females increased slightly from ages 8–9 to 13–14 years), across both sexes and both informants and all fears, mean fear levels declined with age. Second, across both informants, all ages, and all fear factors, females had greater fear intensity than males. Third, at both ages where we had reports from parent and child, for both sexes, and across all fear factors, self-report fear intensity exceeded parent-reported fear intensity. Fourth, fear intensities were generally greater for animal than for situational or blood/injury stimuli.
Table 2.
Mean (SE) per Item Rating of Fear Intensity as a Function of Type of Fear, Sex, Informant, and Age
| Mean (SE) |
||||||
|---|---|---|---|---|---|---|
| Situational |
Animal |
Blood/Injury |
||||
| Male | Female | Male | Female | Male | Female | |
| Age 8–9 y | ||||||
| Parent report | 1.43 (0.05) | 1.68 (0.06) | 1.23 (0.06) | 2.33 (0.80) | 1.44 (0.06) | 1.68 (0.06) |
| Age 13–14 y | ||||||
| Parent report | 0.94 (0.04) | 1.52 (0.05) | 1.18 (0.04) | 2.36 (0.05) | 1.03 (0.04) | 1.60 (0.06) |
| Self-report | 1.45 (0.05) | 2.55 (0.06) | 1.83 (0.05) | 3.06 (0.05) | 1.17 (0.05) | 2.19 (0.06) |
| Age 16–17 y | ||||||
| Parent report | 0.61 (0.03) | 1.12 (0.04) | 0.97 (0.04) | 2.08 (0.05) | 0.67 (0.03) | 1.15 (0.05) |
| Self-report | 1.06 (0.04) | 2.21 (0.05) | 1.57 (0.04) | 2.85 (0.05) | 0.78 (0.04) | 1.70 (0.05) |
| Age 19–20 y | ||||||
| Self-report | 0.90 (0.04) | 2.02 (0.05) | 1.43 (0.05) | 2.42 (0.05) | 0.72 (0.04) | 1.35 (0.05) |
TWIN ANALYSIS
Situational Fears
The correlation matrix across raters and across time for situational fears, seen in Table 3, has 3 noteworthy patterns. First, correlations within time between parents and self-ratings are moderately high (about +0.50). Second, correlations within rater across time (eg, self, age 13–14 years to self, age 16–17 years of +0.61) are somewhat higher than cross-rater cross-time (eg, parent, age 13–14 years to self, age 16–17 years of +0.40). Third, cross-time correlations tend to decline as the interval between ratings increase. eTable 1 (available at http://www.archgenpsychiatry.com) depicts the twin correlations for situational fears within and across time for the 5 zygosity groups (female MZ, female DZ, male MZ, male DZ, and male-female DZ) separately for parental and self-rating.
Table 3.
Pearson Correlations Between Raters and Across Time for a Standardized Measure of Situational Feara
| Age, y | 8–9 | 13–14 | 13–14 | 16–17 | 16–17 | 19–20 | ||
|---|---|---|---|---|---|---|---|---|
| Age, y | Rater | Rater | Parent | Parent | Self | Parent | Self | Self |
| 8–9 | Parent | - | +0.37 | +0.23 | +0.34 | +0.18 | +0.17 | |
| 13–14 | Parent | - | +0.53 | +0.51 | +0.40 | +0.40 | ||
| 13–14 | Self | - | +0.40 | +0.61 | +0.59 | |||
| 16–17 | Parent | - | +0.46 | +0.40 | ||||
| 16–17 | Self | - | +0.67 | |||||
| 19–20 | Self | - | ||||||
All correlations significant at P<.001.
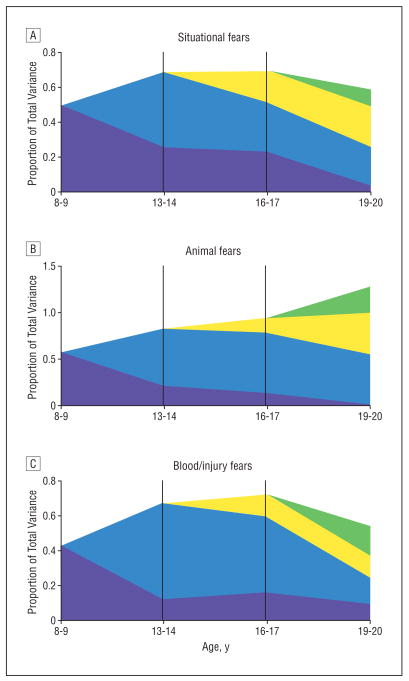
For situational fears, the best BIC value was obtained for a model with no qualitative or quantitative sex effects (Table 4). Parameter estimates for this best-fit model are seen in Table 5 and results for the genetic factors illustrated in Figure 2A, where purple represents the first genetic factor starting at age 8 to 9 years; blue, the second genetic factor starting at age 13 to 14 years; yellow, the third genetic factor starting at age 16 to 17 years; and green, the fourth genetic factor acting only at age 19 to 20 years. Seven results are noteworthy. First, genetic factors play a strong role in influencing the situational fears as indexed by self-ratings and parent ratings. Heritability was estimated at 50%, 68%, 69%, and 59% across the 4 periods. Second, consistent with the predictions of the “developmentally dynamic” hypothesis, we find substantial evidence for genetic innovation as seen in the loadings on genetic factors 2, 3, and 4. That is, in addition to the temporally stable genetic influences that begin at age 8 to 9 years, as illustrated in Figure 2A, the model demonstrated substantial new genetic influences on situational fears that emerged at ages 13 to 14, 16 to 17, and 19 to 20 years. Stable genetic influences on situational fears constitute a minority of the total genetic effect after age 8 to 9 years. Third, we also see evidence for genetic attenuation. In particular, the first genetic factor accounts for a total of 50% of the phenotypic variance in the intensity of situational fears at age 8 to 9 years but declines steeply in influence and by age 19 to 20 years accounts for only 4% of phenotypic variance. Fourth, except at age 8 to 9 years, shared environmental effects on the liability to situational fears were relatively modest accounting for 41%, 5%, 4%, and 12% of the variance across the 4 periods. Unlike the genetic factors, shared environmental influences on situational fears had modest temporal stability. Fifth, unique environmental factors accounted for 9%, 26%, 26%, and 29% of the variance in situational fears as reported by both parent and child. These unique environmental effects had somewhat greater temporal stability than did the shared environmental effects. Sixth, at the 2 points for which the estimates were meaningful (because we had reports from both parent and child), the λP path was somewhat higher than the λS path, suggesting that parental ratings were a better index of fears than self-report. Seventh, parameter estimates for the parent- and self-report factors for situational fears (and other examined fears) are seen in eTable 2 (available at http://www.archgenpsychiatry.com). More consistent reporter-specific genetic factors were seen for self-ratings than for parent ratings.
Table 4.
BIC Scores for Qualitative and Quantitative Sex Effects for Animal, Blood/Injury, and Situational Fearsa
|
ra Variable, Sexes Equal |
ra Variable, Sexes Unequal |
||||
|---|---|---|---|---|---|
| Fear | BIC Score, ra=1.00, Sexes Equal | ra | BIC Score | ra | BIC Score |
| Animal | −27 099.9 | +0.34 | −27 111.1b | +0.34 | −26 950.1 |
| Blood/injury | −26 881.1 | +0.50 | −26 888.5b | +0.50 | −26 776.9 |
| Situational | −25 852.4b | +0.86 | −25 849.5 | +1.00 | −25 724.4 |
Abbreviations: BIC, Bayesian Information Criteria; ra, genetic correlation, which assesses the degree of qualitative sex effects.
Sexes equal or unequal, that is, whether the genetic and environmental parameter estimates are constrained to equality in males and females, assess quantitative sex effects.
Best-fit model by BIC criterion.30
Table 5.
Parameter Estimates for the Best-Fit Model for Situational Fearsa
| λ Path |
Residual |
Genetic Factors |
Shared Environmental Factors |
Unique Environmental Factor |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Factor (Age, y) | P | S | P | S | Total a2, % | 1 | 2 | 3 | 4 | Total c2, % | 1 | 2 | 3 | 4 | Total e2, % | 1 | 2 | 3 | 4 |
| 1 (8–9) | 0.93 | NA | .32 | NA | 50 | 0.71 | … | … | … | 41 | 0.64 | … | … | … | 9 | 0.30 | … | … | … |
| 2 (13–14) | 0.87 | 0.58 | 0.02 | 0.54 | 68 | 0.50 | 0.66 | … | … | 5 | 0.01 | 0.23 | … | … | 26 | 0.25 | 0.45 | … | … |
| 3 (16–17) | 0.77 | 0.54 | 0.24 | 0.54 | 69 | 0.48 | 0.53 | 0.43 | … | 4 | −0.03 | −0.02 | 0.21 | … | 26 | 0.09 | 0.36 | 0.35 | … |
| 4 (19–20) | NA | 0.60 | NA | 0.54 | 59 | 0.19 | 0.48 | 0.48 | 0.30 | 12 | 0.11 | 0.32 | 0.10 | −0.01 | 29 | 0.35 | 0.28 | 0.13 | 0.28 |
Abbreviations: a2, heritability or the proportion of variance in fear scores resulting from genetic factors; c2, the proportion of variance in fear scores resulting from shared environmental factors; e2, the proportion of variance in fear scores resulting from unique environmental factors; NA, not applicable; P, parent report; S, self-report.
Results for the “upper part” of the model only (Figure 1), which includes fear symptoms shared by parent and self-reporters. Parameter estimates constrained to equality across the sexes and the value of rg set to 1.0. Parameter estimates for the lower part of the model are available in eTable 2 (http://www.archgenpsychiatry.com). The λ path, depicted in Figure 1, connects the latent liability to fears to the report level of fears from parent report (P) and self-report (S).
Figure 2.
The proportion of total variance in fears accounted for by genetic factors through development. The y-axis represents the total phenotypic variance so the sum of all the factors equals the total heritability. Purple represents the first genetic factor starting at age 8 to 9 years. Blue represents the second genetic factor starting at age 13 to 14 years. Yellow represents the third genetic factor starting at age 16 to 17 years and green represents the fourth genetic factor acting only at age 19 to 20 years. A, Results for situational fears, the exact parameter estimates of which are seen in Table 5. B, Results for animal fears, the exact parameter estimates of which are seen in Table 6. C, Results for blood/injury fears, the exact parameter estimates of which are seen in Table 7.
Other Fears
The correlation matrices across raters and across time for animal and blood/injury fears are seen in eTable 3 and eTable 4 (available at http://www.archgenpsychiatry.com) and are very similar to that seen for situational fears (Table 2). eTable 5 and eTable 6 (available at http://www.archgenpsychiatry.com) depict the twin correlations for animal and blood/injury fears within and across time for the 5 zygosity groups separately for parental and self-ratings. As seen in Table 4, we did not find evidence for quantitative sex effects for any of the other fear dimensions. However, for animal and blood/injury fears, the best-fit model contained qualitative sex effects, with estimates of rg of +0.34 and +0.50, respectively.
Parameter estimates for the best-fit models for animal and blood/injury fears are seen in Table 6 and Table 7 and Figure 2B and C. The results for animal and blood/injury fears resembled those found for situational fears in strongly supporting the “developmentally dynamic” rather than the “developmentally stable” hypothesis for genetic effects. More specifically, our modeling results provided evidence for both genetic innovation and genetic attenuation for the intensity of animal and blood/injury fears. As with situational fears, the role of shared environment generally declined with increasing age for both animal and blood/injury fears.
Table 6.
Parameter Estimates for the Best-Fit Model for Animal Fearsa
| λ Path |
Residual |
Genetic Factors |
Shared Environmental Factors |
Unique Environmental Factor |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Factor (Age, y) | P | S | P | S | Total a2, % | 1 | 2 | 3 | 4 | Total c2, % | 1 | 2 | 3 | 4 | Total e2, % | 1 | 2 | 3 | 4 |
| 1 (8–9) | 0.90 | NA | 0 | NA | 34 | 0.58 | … | … | … | 62 | 0.79 | … | … | … | 4 | 0.21 | … | … | … |
| 2 (13–14) | 0.92 | 0.55 | 0.27 | 0.54 | 44 | 0.28 | 0.60 | … | … | 42 | 0.37 | 0.53 | … | … | 15 | 0.32 | 0.22 | … | … |
| 3 (16–17) | 0.84 | 0.51 | 0.26 | 0.54 | 45 | 0.14 | 0.64 | 0.15 | … | 38 | 0.47 | 0.27 | 0.30 | … | 17 | 0.35 | −0.13 | 0.16 | … |
| 4 (19–20) | NA | 0.61 | NA | 0.54 | 56 | 0.01 | 0.53 | 0.45 | 0.27 | 19 | 0.40 | 0.16 | 0 | 0 | 25 | 0.40 | −0.08 | −0.23 | 0.18 |
Abbreviations: See Table 5.
Results for the “upper part” of the model only (Figure 1), which includes fear symptoms shared by parent and self-reporters. Parameter estimates constrained to equality across the sexes and the value of rg set to 0.34. Parameter estimates for the lower part of the model are available in eTable 2 (http://www.archgenpsychiatry.com). The λ path, depicted in Figure 1, connects the latent liability to fears to the report level of fears from parent report (P) and self-report (S).
Table 7.
Parameter Estimates for the Best-Fit Model for Blood/Injury Fearsa
| λ Path |
Residual |
Genetic Factors |
Shared Environmental Factors |
Unique Environmental Factor |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Factor (Age, y) | P | S | P | S | Total a2, % | 1 | 2 | 3 | 4 | Total c2, % | 1 | 2 | 3 | 4 | Total, e2, % | 1 | 2 | 3 | 4 |
| 1 (8–9) | 0.93 | NA | 0.15 | NA | 43 | 0.66 | … | … | … | 36 | 0.60 | … | … | … | 21 | 0.46 | … | … | … |
| 2 (13–14) | 0.74 | 0.68 | 0.40 | 0.58 | 67 | 0.35 | 0.74 | … | … | 18 | 0.16 | 0.39 | … | … | 15 | 0.13 | 0.37 | … | … |
| 3 (16–17) | 0.71 | 0.62 | 0.29 | 0.58 | 72 | 0.41 | 0.66 | 0.36 | … | 01 | 0.02 | 0.12 | −0.01 | … | 26 | 0.04 | 0.31 | 0.40 | … |
| 4 (19–20) | NA | 0.62 | NA | 0.58 | 54 | 0.31 | 0.39 | 0.36 | 0.41 | 16 | 0.03 | 0.39 | 0.05 | 0 | 30 | 0.15 | 0.12 | 0.52 | 0 |
Abbreviations: See Table 5.
Results for the “upper part” of the model only (Figure 1), which includes fear symptoms shared by parent and self-reporters. Parameter estimates constrained to equality across the sexes and the value of rg set to 0.5. Parameter estimates for the lower part of the model are available in eTable 2 (http://www.archgenpsychiatry.com). The λ path, depicted in Figure 1, connects the latent liability to fears to the report level of fears from parent report (P) and self-report (S).
COMMENT
MAJOR FINDINGS
The major goal of this report was to discriminate between a “developmentally stable” and a “developmentally dynamic” hypothesis of genetic risk for the intensity of common fears from childhood to young adulthood. For all 3 fears examined, our model results unequivocally supported the “developmentally dynamic” hypothesis. For situational, animal, and blood/injury fears, our parameter estimates showed evidence for 2 dynamic processes: genetic innovation and attenuation. We identified 1 set of genetic risk factors that act in childhood and have a steep decline in influence with age. Furthermore, we see evidence for new sets of genetic risk factors “coming on line” in early adolescence, late adolescence, and early adulthood.
This article had 2 subsidiary goals, the first of which was to clarify the developmental impact of environmental factors on fears. For all 3 fears, as the twins aged, the influence of the shared environment declined and unique environment increased. This is an expected pattern given that adolescence is a time of declining influence of the home environment as individuals spend less time with family and progressively make their own world, spending more time with friends.33
Shared environmental influences on fears could result from 2 previously articulated mechanisms of fear acquisition.34–36 Most likely they arise because twins learn to fear particular stimuli from parental instruction or through social learning by exposure to parental fears. Twins also could have been correlated for exposure to fear-inducing experiences (eg, rats in the basement or a particularly unsympathetic dentist). Shared environmental influences for both situational and blood/injury fears were not very stable over time. By contrast, animal fears demonstrated an enduring and substantial effect of a single set of familial-environmental factors from age 8 to 9 years through young adulthood. Unfortunately, our data provide no insight into why shared environmental experiences would have a more transitory effect on situational and blood/injury than on animal fears.
Across all 4 ages of assessment, the shared environment was substantially more important for animal than for situational or blood/injury fears. Perhaps twins were more exposed to parental fear reactions or warnings about dangerous animals than about situational or blood/injury stimuli. Alternatively, as they were growing up, twins might have been particularly correlated in their exposure to rats, snakes, spiders, and wasps in their home or neighborhood.
Individual-specific environmental influences on fears were generally more temporally stable than familial-environmental effects. This pattern would be predicted if fears arising from individual exposure to frightening stimuli had a more enduring impact on fear levels than did instruction or observation of parental fears.
The second subsidiary goal of these analyses was to clarify sex differences in the genetic and environmental risk factors for fears in childhood and adolescence. Consistent with earlier results with this fear scale,27 mean levels of fear intensity were higher in females than in males across all 3 fear factors, both raters, and all 4 ages. While we found strong evidence for qualitative sex effects for animal and blood/injury but not for situational fears, we found little support for quantitative genetic effects for any of the fear dimensions examined. Consistent with evidence in rodents for the impact of gonadal hormones on fear conditioning,22,24 sex appears to be an important modifier of genetic risk factors in humans for animal and blood/injury fears. By contrast, our results suggest that after controlling for mean differences in fears between the sexes, the magnitude and developmental progression of genetic and environmental effects on fears were similar in males and females.
RELATIONSHIP WITH PRIOR STUDIES
Our findings can be best appreciated when put in the context of prior genetically informative studies of fears and phobias. Our results are consistent with prior investigations in toddlers,37 children,8–10 adolescents,11 and adults6,12–15 in suggesting genetic effects on fears and phobias. The heritability estimates obtained in this sample are comparable with those found in several other studies of children and adolescents10,37,38 but generally higher than those reported for fears and phobias in adults.12,15,39,40 Evidence of shared environmental influences on fears have been found in some12,37 but not all studies of fears and phobias.15,39,40 Our results would suggest that family-environmental influences should be much more likely detected for fears and phobias in younger samples, although the literature is not entirely consistent in this regard. One prior study specifically examined for sex effects on situational, blood/injury, and animal fears and phobias in adult twins from the Virginia twin register.40 No quantitative sex effects were found for any of these fears/phobias while qualitative sex effects were found for situational and blood/injury but not for animal fear/phobias.
LIMITATIONS
These results should be considered in the context of 6 potentially important methodological limitations. First, since we had no measures of fear from subjects younger than 8 years, we could not examine the development of those specific fears (eg, of strangers) that emerge and typically disappear in early childhood. Second, our list of fears was abbreviated at age 8 to 9 years, especially for animal fears. We reran our analyses of animal fears using only the 2 items (snakes and spiders) available at all ages. The pattern of results was very similar to that seen in Table 6 with one exception—the genetic loading on the fears in young adulthood were largely shared with those seen at early periods rather than specific to that age.
Third, prior studies suggest a hierarchical structure of fears,41 and twin studies in both adults6,14,15 and children8 have suggested that genetic risk factors for individual fears and phobias are substantially intercorrelated. Therefore, the ideal approach to this data would have been a multivariate model that would examine genetic and environmental factors common to all 3 fears as well as fear-specific factors. However, given the problems of developing, fitting, and interpreting such a complex model, herein, we chose the simpler approach of examining the fears one at a time.
Fourth, our analyses examined the full range of the intensity of fear in response to phobic stimuli, from none to maximal. Our results are therefore not necessarily the same as those that would be obtained if we examined only the extreme high scorers, many of whom might have met criteria for a clinical phobia.
Fifth, some of our findings might have arisen artifactually because we had only parental reports at age 8 to 9 years and only self-reports at age 19 to 20 years. To examine this question, we developed an alternative model that did not combine parent and twin ratings but instead contained the same 4 genetic and environmental factors seen in Figure 1 fitted directly to our 6 measures of fear: parental report at ages 8 to 9, 13 to 14, and 16 to 17 years and self-report at ages 13 to 14, 16 to 17, and 19 to 20 years. Reassuringly, this less elegant model showed all the major trends reported earlier (results available on request). For example, the sharp decline in shared environmental effects could result from our reliance solely on parental report for fears at age 8 to 9 years compared with the later measures, which all included self-report data. However, looking only at parental reports, we see exactly the same trends. For example, for situational fears, shared environment in parental ratings accounts for 30% of variance at age 8 to 9 years, declining to 9% at age 13 to 14 years and 5% at age 16 to 17 years. We also saw strong support for our “developmentally dynamic” hypothesis using this model. For example, for situational fears, the second genetic factor had robust effects on both parental ratings (with loadings at ages 13–14 and 16–17 years of 0.32 and 0.39, respectively) and self-ratings (with loadings of 0.50, 0.52, and 0.46 at ages 13–14, 16–17, and 19–20 years, respectively). When we examine only parental reports or only twin reports, we still have strong evidence for developmentally dynamic genetic effects on fears.
Sixth, it was not feasible to examine jointly developmental changes in levels of fear reported by both parent and child and those specific to parental or child report. Our analyses focused on the former because of their greater validity. As seen in eTable 1, significant genetic effects were found that were unique to individual reporters. These genetic effects tended to be higher for child than parental report and higher for animal than for blood/injury or situational fears.
CONCLUSIONS
Most approaches to psychiatric genetics and especially those involving gene finding assume a static genome, one in which the impact of individual genetic variants on risk is stable over time and through periods of development. This assumption may be appropriate for some phenotypes over certain periods, such as major depression in adulthood.42 That this is not uniformly the case is demonstrated by prior studies of a range of behavioral and physiological phenotypes.16–21
For some phenotypes, the genome is likely to be particularly dynamic during childhood and adolescence. It cannot therefore be assumed that genes that influence a trait during one age period will be entirely the same as those that impact the same trait in a later developmental phase. To capture the temporal variation in gene action underlying some phenotypes, gene-finding studies will need to use longitudinal designs.
Our study provides no direct insight into the nature of the dynamic changes in the genetic influences on fear. However, because we studied subjects from ages 8 to 20 years, we of necessity captured the impact of their pubertal transition on genetic and environmental risk factors for fear. This may be of significance because both animal22,24 and human studies23 suggest that fear mechanisms can be altered by the hormonal changes occurring during puberty. Future work will need to clarify whether the genetic effects on these developmental changes in fear intensity can be best understood at the level of mental processes, such as changes in cognitive biases or disgust sensitivity,2 and/or at the level of neurobiology, for example, altered functioning of brain fear circuitry in structures such as the amygdala and medial prefrontal cortex.43,44
What evolutionary forces might be responsible for the developmental complexity of genetic risk factors for fears? Genetic influences on fear-proneness may have arisen through selection during evolution because moderate levels of innate fears for dangerous stimuli such as snakes, spiders, heights, and blood have been more adaptive than either no fear or cripplingly high levels of fear.45,46 In such a situation—where intermediate levels of a trait are maximally fit—an evolutionary process termed stabilizing selection47 occurs, which produces substantial additive genetic variation.47,48 However, the adaptiveness of specific fears is probably age dependent. Stimuli that are particularly hazardous for a child are likely to differ from those that pose danger to a late adolescent. If this is the case, selective forces over evolutionary time are likely to sculpt a temporally dynamic set of genetic risk factors with expression tied to developmental stage.
Supplementary Material
Kendler KS, Gardner CO, Annas P, Neale MC, Eaves LJ, Lichtenstein P. A Longitudinal twin study of fears from middle childhood to early adulthood: evidence for a developmentally dynamic genome. Arch Gen Psychiatry. 2008;65(4):421–429.
eTable 1. Twin Correlations for Situational Fears
eTable 2. Shared Residual Rater-Specific Parameter Estimates From the Best-Fit Models for Situational, Animal, and Blood/Injury Fears
eTable 3. Pearson Correlations Between Raters and Across Time for a Standardized Measure of Animal Fear
eTable 4. Pearson Correlations Between Raters and Across Time for a Standardized Measure of Blood/Injury Fear
eTable 5. Twin Correlations for Animal Fears
eTable 6. Twin Correlations for Blood/Injury Fears
Acknowledgments
Funding/Support: This study was supported in part by National Institutes of Health grants MH-068643 and MH-65322, the Swedish Council for Working Life and Social Research (project 2004-0383), and the Swedish Research Council (project 2004-1415).
Footnotes
Additional Information: eTables 1–6 are available at http://www.archgenpsychiatry.com.
Financial Disclosure: None reported.
References
- 1.Muris P, Merckelbach H. The etiology of childhood specific phobia: a multifactorial model. In: Vasey MW, Dadds MR, editors. The Developmental Psychopathology of Anxiety. Oxford, NY: Oxford University Press; 2001. pp. 355–385. [Google Scholar]
- 2.Armfield JM. Cognitive vulnerability: a model of the etiology of fear. Clin Psychol Rev. 2006;26(6):746–768. doi: 10.1016/j.cpr.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 3.Silverman WK, Moreno J. Specific phobia. Child Adolesc Psychiatr Clin N Am. 2005;14(4):819–843. doi: 10.1016/j.chc.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 4.Fyer AJ. Current approaches to etiology and pathophysiology of specific phobia. Biol Psychiatry. 1998;44(12):1295–1304. doi: 10.1016/s0006-3223(98)00274-1. [DOI] [PubMed] [Google Scholar]
- 5.Marks IM. Fears, Phobias, and Rituals. New York, NY: Oxford University Press; 1987. [Google Scholar]
- 6.Kendler KS, Neale MC, Kessler RC, Heath AC, Eaves LJ. The genetic epidemiology of phobias in women: the interrelationship of agoraphobia, social phobia, situational phobia, and simple phobia. Arch Gen Psychiatry. 1992;49(4):273–281. doi: 10.1001/archpsyc.1992.01820040025003. [DOI] [PubMed] [Google Scholar]
- 7.Becker ES, Rinck M, Turke V, Kause P, Goodwin R, Neumer S, Margraf J. Epidemiology of specific phobia subtypes: findings from the Dresden Mental Health Study. Eur Psychiatry. 2007;22(2):69–74. doi: 10.1016/j.eurpsy.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 8.Lichtenstein P, Annas P. Heritability and prevalence of specific fears and phobias in childhood. J Child Psychol Psychiatry. 2000;41(7):927–937. [PubMed] [Google Scholar]
- 9.Rose RJ, Ditto WB. A developmental-genetic analysis of common fears from early adolescence to early adulthood. Child Dev. 1983;54(2):361–368. [PubMed] [Google Scholar]
- 10.Stevenson J, Batten N, Cherner M. Fears and fearfulness in children and adolescents: a genetic analysis of twin data. J Child Psychol Psychiatry. 1992;33(6):977–985. doi: 10.1111/j.1469-7610.1992.tb00919.x. [DOI] [PubMed] [Google Scholar]
- 11.Nelson EC, Grant JD, Bucholz KK, Glowinski A, Madden PAF, Reich W, Heath AC. Social phobia in a population-based female adolescent twin sample: comorbidity and associated suicide-related symptoms. Psychol Med. 2000;30 (4):797–804. doi: 10.1017/s0033291799002275. [DOI] [PubMed] [Google Scholar]
- 12.Phillips K, Fulker DW, Rose RJ. Path analysis of seven fear factors in adult twin and sibling pairs and their parents. Genet Epidemiol. 1987;4(5):345–355. doi: 10.1002/gepi.1370040504. [DOI] [PubMed] [Google Scholar]
- 13.Torgersen S. The nature and origin of common phobic fears. Br J Psychiatry. 1979;134:343–351. doi: 10.1192/bjp.134.4.343. [DOI] [PubMed] [Google Scholar]
- 14.Kendler KS, Myers J, Prescott CA, Neale MC. The genetic epidemiology of irrational fears and phobias in men. Arch Gen Psychiatry. 2001;58(3):257–265. doi: 10.1001/archpsyc.58.3.257. [DOI] [PubMed] [Google Scholar]
- 15.Sundet JM, Skre I, Okkenhaug JJ, Tambs K. Genetic and environmental causes of the interrelationships between self-reported fears: a study of a non-clinical sample of Norwegian identical twins and their families. Scand J Psychol. 2003;44(2):97–106. doi: 10.1111/1467-9450.00326. [DOI] [PubMed] [Google Scholar]
- 16.Jacobson KC, Prescott CA, Kendler KS. Sex differences in the genetic and environmental influences on the development of antisocial behavior. Dev Psychopathol. 2002;14(2):395–416. doi: 10.1017/s0954579402002110. [DOI] [PubMed] [Google Scholar]
- 17.Silberg JL, Rutter M, Tracy K, Maes HH, Eaves L. Etiological heterogeneity in the development of antisocial behavior: the Virginia Twin Study of Adolescent Behavioral Development and the Young Adult Follow-Up. Psychol Med. 2007;37(8):1193–1202. doi: 10.1017/S0033291707000293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cardon LR, Fulker DW, DeFries JC, Plomin R. Continuity and change in general cognitive ability from 1 to 7 years of age. Dev Psychol. 1992;28(1):64–73. [Google Scholar]
- 19.Middelberg RP, Martin NG, Whitfield JB. Longitudinal genetic analysis of plasma lipids. Twin Res Hum Genet. 2006;9(4):550–557. doi: 10.1375/183242706778024946. [DOI] [PubMed] [Google Scholar]
- 20.Fischbein S, Molenaar PCM, Boomsma DI. Simultaneous genetic-analysis of longitudinal means and covariance structure using the simplex model—application to repeatedly measured weight in a sample of 164 female twins. Acta Genet Med Gemellol (Roma) 1990;39(2):165–172. doi: 10.1017/s0001566000005390. [DOI] [PubMed] [Google Scholar]
- 21.Fabsitz RR, Carmelli D, Hewitt JK. Evidence for independent genetic influences on obesity in middle age. Int J Obes Relat Metab Disord. 1992;16(9):657–666. [PubMed] [Google Scholar]
- 22.Koshibu K, Levitt P. Gene×environment effects: stress and memory dysfunctions caused by stress and gonadal factor irregularities during puberty in control and TGF-alpha hypomorphic mice. Neuropsychopharmacology. 2008;33 (3):557–565. doi: 10.1038/sj.npp.1301436. [DOI] [PubMed] [Google Scholar]
- 23.Killgore WD, Oki M, Yurgelun-Todd DA. Sex-specific developmental changes in amygdala responses to affective faces. Neuroreport. 2001;12(2):427–433. doi: 10.1097/00001756-200102120-00047. [DOI] [PubMed] [Google Scholar]
- 24.Toufexis DJ, Myers KM, Davis M. The effect of gonadal hormones and gender on anxiety and emotional learning. Horm Behav. 2006;50(4):539–549. doi: 10.1016/j.yhbeh.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 25.Lichtenstein P, de Faire U, Floderus B, Svartengren M, Svedberg P, Pedersen NL. The Swedish Twin Registry: a unique resource for clinical, epidemiological and genetic studies. J Intern Med. 2002;252(3):184–205. doi: 10.1046/j.1365-2796.2002.01032.x. [DOI] [PubMed] [Google Scholar]
- 26.Lichtenstein P, Tuvblad C, Larsson H, Carlstrom E. The Swedish Twin study of CHild and Adolescent Development: the TCHAD-Study. Twin Res Hum Genet. 2007;10(1):67–73. doi: 10.1375/twin.10.1.67. [DOI] [PubMed] [Google Scholar]
- 27.Fredrikson M, Annas P, Fischer H, Wik G. Gender and age differences in the prevalence of specific fears and phobias. Behav Res Ther. 1996;34(1):33–39. doi: 10.1016/0005-7967(95)00048-3. [DOI] [PubMed] [Google Scholar]
- 28.SAS Institute Inc. SAS OnlineDoc Version 9.1.3. Cary, NC: SAS Institute Inc; 2002–2005. NC State University SCDoS, editor. 2005. Cary, NC. [Google Scholar]
- 29.Neale MC, Boker SM, Xie G, Maes HH. Mx: Statistical Modeling. 6. Richmond: Virginia Commonwealth University Medical School; 2003. [Google Scholar]
- 30.Schwarz G. Estimating the dimension of a model. Ann Stat. 1978;6:461–464. [Google Scholar]
- 31.Markon KE, Krueger RF. An empirical comparison of information-theoretic selection criteria for multivariate behavior genetic models. Behav Genet. 2004;34(6):593–610. doi: 10.1007/s10519-004-5587-0. [DOI] [PubMed] [Google Scholar]
- 32.Derogatis LR, Serio JC, Cleary PA. An empirical comparison of three indices of factorial similarity. Psychol Rep. 1972;30:791–804. [Google Scholar]
- 33.Larson R, Richards MH. Daily companionship in late childhood and early adolescence—changing developmental contexts. Child Dev. 1991;62(2):284–300. doi: 10.1111/j.1467-8624.1991.tb01531.x. [DOI] [PubMed] [Google Scholar]
- 34.Rachman S. The conditioning theory of fear-acquisition: a critical examination. Behav Res Ther. 1977;15(5):375–387. doi: 10.1016/0005-7967(77)90041-9. [DOI] [PubMed] [Google Scholar]
- 35.Ost LG. Ways of acquiring phobias and outcome of behavioral treatments. Behav Res Ther. 1985;23(6):683–689. doi: 10.1016/0005-7967(85)90066-x. [DOI] [PubMed] [Google Scholar]
- 36.Kendler KS, Myers J, Prescott CA. The etiology of phobias: an evaluation of the stress-diathesis model. Arch Gen Psychiatry. 2002;59(3):242–248. doi: 10.1001/archpsyc.59.3.242. [DOI] [PubMed] [Google Scholar]
- 37.Eley TC, Bolton D, O’Connor TG, Perrin S, Smith P, Plomin R. A twin study of anxiety-related behaviours in pre-school children. J Child Psychol Psychiatry. 2003;44(7):945–960. doi: 10.1111/1469-7610.00179. [DOI] [PubMed] [Google Scholar]
- 38.Bolton D, Eley TC, O’Connor TG, Perrin S, Rabe-Hesketh S, Rijsdijk F, Smith P. Prevalence and genetic and environmental influences on anxiety disorders in 6-year-old twins. Psychol Med. 2006;36(3):335–344. doi: 10.1017/S0033291705006537. [DOI] [PubMed] [Google Scholar]
- 39.Hettema JM, Neale MC, Kendler KS. A review and meta-analysis of the genetic epidemiology of anxiety disorders. Am J Psychiatry. 2001;158(10):1568–1578. doi: 10.1176/appi.ajp.158.10.1568. [DOI] [PubMed] [Google Scholar]
- 40.Kendler KS, Jacobson KC, Myers J, Prescott CA. Sex differences in genetic and environmental risk factors for irrational fears and phobias. Psychol Med. 2002;32(2):209–217. doi: 10.1017/s003329170100513x. [DOI] [PubMed] [Google Scholar]
- 41.Taylor S. The hierarchic structure of fears. Behav Res Ther. 1998;36(2):205–214. doi: 10.1016/s0005-7967(98)00012-6. [DOI] [PubMed] [Google Scholar]
- 42.Kendler KS, Neale MC, Kessler RC, Heath AC, Eaves LJ. A longitudinal twin study of 1-year prevalence of major depression in women. Arch Gen Psychiatry. 1993;50(11):843–852. doi: 10.1001/archpsyc.1993.01820230009001. [DOI] [PubMed] [Google Scholar]
- 43.Pezze MA, Feldon J. Mesolimbic dopaminergic pathways in fear conditioning. Prog Neurobiol. 2004;74(5):301–320. doi: 10.1016/j.pneurobio.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 44.Cannistraro PA, Rauch SL. Neural circuitry of anxiety: evidence from structural and functional neuroimaging studies. Psychopharmacol Bull. 2003;37(4):8–25. [PubMed] [Google Scholar]
- 45.Marks I, Nesse RM. Fear and fitness: an evolutionary analysis of anxiety disorders. Ethol Sociobiol. 1994;15:247–261. [Google Scholar]
- 46.Seligman MEP. Phobias and preparedness. Behav Ther. 1971;2:307–320. [Google Scholar]
- 47.Hartl DL. Principles of Population Genetics. Sunderland, MA: Sinauer; 1980. [Google Scholar]
- 48.Lande R. Natural selection and random genetic drift in phenotypic evolution. Evolution Int J Org Evolution. 1976;30(2):314–334. doi: 10.1111/j.1558-5646.1976.tb00911.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Kendler KS, Gardner CO, Annas P, Neale MC, Eaves LJ, Lichtenstein P. A Longitudinal twin study of fears from middle childhood to early adulthood: evidence for a developmentally dynamic genome. Arch Gen Psychiatry. 2008;65(4):421–429.
eTable 1. Twin Correlations for Situational Fears
eTable 2. Shared Residual Rater-Specific Parameter Estimates From the Best-Fit Models for Situational, Animal, and Blood/Injury Fears
eTable 3. Pearson Correlations Between Raters and Across Time for a Standardized Measure of Animal Fear
eTable 4. Pearson Correlations Between Raters and Across Time for a Standardized Measure of Blood/Injury Fear
eTable 5. Twin Correlations for Animal Fears
eTable 6. Twin Correlations for Blood/Injury Fears