Abstract
Macaque monkeys are used in many research applications, including cerebrovascular investigations. However, detailed catalogs of the relevant vascular anatomy are scarce. We present our experience with macaque vessel patterns as determined by digital subtraction angiography of 34 different monkeys. METHODS AND MATERIALS: We retrospectively analyzed digital subtraction angiograms obtained during experimental internal carotid artery catheterization and subsequent injection of 1-methyl 4-phenyl 1,2,3,6-tetrahydropyridine (MPTP). Results were catalogued according to vascular distribution and variants observed. RESULTS: Macaque monkeys have a bovine aortic arch. The carotid vessels generally bifurcate, but are occasionally observed to divide into three vessels. The external carotid gives rise primarily to two trunks: an occipital branch and a common vessel that subsequently gives off the lingual, facial, and superior thyroid arteries. The internal maxillary artery may be present as a terminal branch of the external carotid or as a branch of the occipital artery. The internal carotid artery is similar in course to that of the human. The anterior circle of Willis was intact in all monkeys in our study. Its primary difference from that of the human is the union of the bilateral anterior cerebral arteries as a single (azygous) median vessel. CONCLUSIONS: Macaque cervical carotid and circle of Willis arterial anatomy differs from humans in a couple of specific patterns. Knowledge of these differences and similarities between human and macaque anatomy is important in developing endovascular macaque models of human diseases, such as ischemic stroke.
Keywords: Angiography, arteries, macaque
INTRODUCTION
Various species of macaque monkeys are used to provide animal models of human disease. Many of these models either directly involve the cerebrovasculature for studies of stroke or indirectly use these vessels to selectively deliver drugs to the brain. However, data on the vascular anatomical variations in these monkeys are limited, with few comparisons between the vessels of macaques and man.
We undertook this study in order to identify normal variations within the macaque monkey's vasculature and compare these to known patterns within humans. These data will be valuable to investigators who employ macaque monkeys for animal models of disease that either directly affect cerebrovasculature or use the cervical and cerebral arteries to deliver agents to the brain.
MATERIALS AND METHODS
As part of an Animal Research Committee-approved study of an animal model of dystonia and Parkinson disease, macaque (Macaca nemestrina, Macaca fascicularis, and Macaca mulatta) monkeys underwent unilateral intra-arterial injection of 1-methyl 4-phenyl 1,2,3,6-tetrahydropyridine (MPTP) in the internal carotid artery (Tabbal et al., 2006). Digital subtraction angiograms were obtained prior to the infusion of MPTP to confirm proper placement of the catheter. All monkeys were initially anesthetized with ketamine, intubated, and anesthesia maintained with inhalation of isoflurane. The inguinal region was shaved and then sterilely prepared and draped. Access to the common femoral artery was obtained via percutaneous puncture or direct visualization after a skin incision. A 0.018 inch guidewire was inserted through a 20 gauge introducer, and then a 3 French catheter was advanced over the wire through the femoral artery to the internal carotid under fluoroscopic guidance. Bony landmarks were generally used, but angiographic runs were sometimes employed when there was difficultly catheterizing the desired vessel.
A portable digital subtraction unit (OEC Diasonics Series 9800, Salt Lake City, Utah), consisting of a C-arm fluoroscope, a digital image processor and storage unit, and a video monitor, was used in all cases. This unit allows routine fluoroscopy and real-time DSA. The distance between the X-ray source and the image intensifier was fixed at 36.07 inches. A tri-mode image intensifier allowed the use of three field sizes (9, 6, and 4 inches) for the purposes of magnification. Fluoroscopy was performed during selective catheterization without magnification or the use of a boost mode. The range of technique factors during fluoroscopy was 66 kVp and 0.2 to 5.0 mA. All DSA runs were performed in the boost mode with magnification. The range of technique factors during DSA in the boost mode was 69 kVp and 100 to 300 mAs. Field size used during DSA was typically 4 inches. The frame rate selected for the arteriogram was four per second. Conray-60 (lothalamate Meglumine Injection USP 60%, Covidien, Inc., Mansfield, MA) was injected by hand for all DSA acquisitions. Typical volumes of injection were 1 to 2 cc's injected over 1 to 2 seconds.
Thirty-seven angiograms were obtained from 34 distinct Macaca nemestrina (n = 2), Macaca fascicularis (n = 31), and Macaca mulatta (n = 1) monkeys over a period of six years; each angiogram contained multiple runs from a variety of angles. The large majority of monkeys included in the study were Macaca fascicularis and exceptions are explicitly noted in the results below. In general, a near-lateral projection was obtained by turning the supine animals' head to the left and the radiographic tube was kept in an anterio-posterior projection in relation to the table. The angiographic studies were digitally recorded and archived. The archived studies were saved in an unsubtracted format. A digital subtraction algorithm was applied to the data. Subtracted images were subsequently retrospectively reviewed and catalogued according to visible vessels and their respective courses.
Three-dimensional reference views were created with Anim8or v0.95 (Silicon Valley Software, www.anim8or.com) and rotated to the corresponding DSA projection. For head and neck runs, these included the skull structures. These images were included with the DSA figures in this manuscript to help orient the readers.
RESULTS
The angiograms obtained in this study were performed to guide or confirm catheterization of the internal carotid artery, prior to the injection of a neurotoxic compound. Thus, most of the runs analyzed were directed at the distribution of this artery and its intracranial course. However, other runs recorded in a variety of arterial distributions were also cataloged.
Aortic Arch and Great Vessels
Although arch images were recorded for only four separate M. fascicularis monkeys, we catheterized this region in all animals. The configuration of the great vessels appears to be very similar in almost all monkeys, with the two large branches arising from the arch: the brachiocephalic trunk (innominate artery) and the left subclavian artery.
The brachiocephalic trunk is the larger of the branches of the aorta, arising from the convexity of the aortic arch and in turn giving rise to both the right and left common carotid and right subclavian arteries. A typical example of this structural configuration is depicted in Figure 1. Proximal branches of the right thyrocervical trunk were seen in the monkeys in whom we had obtained arch images. The internal thoracic artery was seen in one monkey.
Fig. 1.
The brachiocephalic trunk (1) injection in macaque monkeys appears to give rise to the left common carotid (2), right common carotid (3), and right subclavian (4) arteries. Three-dimensional reference view on right.
Common Carotid Artery and Carotid Bifurcation
A contrast run with injection into the common carotid artery was performed in 11 individual monkeys, including 2 M. nemestrina. In addition, there were several subjects in which contrast injected from the catheter within the internal carotid refluxed into the region of the bifurcation, permitting visualization of the common carotid distribution in a total of 26 angiograms from 25 individual monkeys. The common carotid arteries course superiorly and appear to bifurcate into the internal and external carotid arteries at the level of C3–C4. The external carotid tends to branch anteriorly, with the internal carotid following a more vertical trajectory posteriorly.
In 2 monkeys, there appeared to be a common carotid “trifurcation,” with occipital, internal, and external carotid arteries branching simultaneously at the level of C2. Figure 2 shows examples of carotid branching.
Fig. 2.
(a) Reference view; (b) right carotid artery injection demonstrating the more typical scenario of the common carotid artery dividing into internal and external branches; (c) right carotid artery injection shows a carotid “trifurcation” configuration.
External Carotid Artery
The external carotid artery was selectively catheterized in nine individual monkeys; one monkey was catheterized twice, yielding a total of 10 external carotid angiograms. As noted above, however, reflux of contrast from the internal carotid artery occasionally allowed visualization of the proximal portion of the external carotid. Thus, when these angiograms are considered in combination with those of the common carotid, portions of the external carotid artery were visualized in a total of 26 monkeys.
In macaques, there appears to be variability in the course and branching patterns of the external carotid. The first branch of this artery is typically a common trunk which then divides into the lingual and facial arteries. In six of the monkeys, the superior thyroid artery was visualized, and arose from this common trunk as well. The second branch appears to be the occipital artery, which courses posteriorly to the occiput. The ascending pharyngeal artery was not convincingly demonstrated in any specimen.
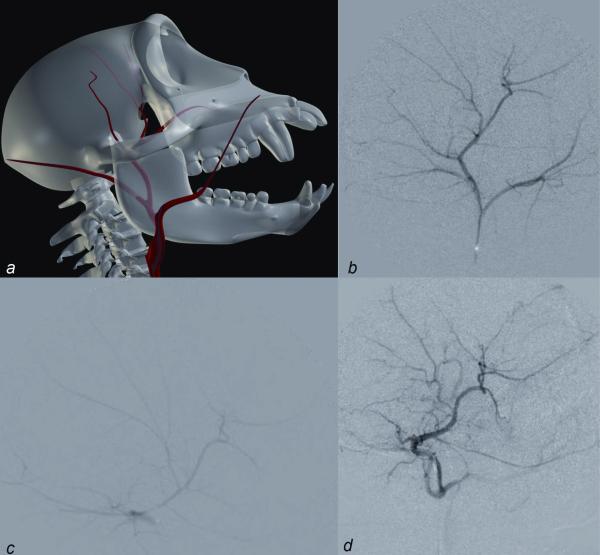
The internal maxillary artery was visualized, to varying degrees, in 10 separate monkeys. In the majority of cases, this vessel originates from the external carotid, but in 2 monkeys the vessel arose from the occipital artery. The diverse branches of this vessel are seen to a variable extent, but those that are visualized, based on their vicinity and the distribution described in humans, may correspond to the deep auricular, anterior tympanic, inferior alveolar, middle meningeal, posterior superior alveolar, and greater (descending) palatine arteries. Figure 3 highlights selected images of the external carotid and its branches.
Fig. 3.
(a) Three-dimensional reference image; (b, c) the internal maxillary artery and its branches; (d) a variant in which the occipital artery gives off the internal maxillary artery.
Internal Carotid Artery
The internal carotid artery (ICA) was seen in a total of 26 monkeys, including 2 M. nemestrina and 1 M. mulatta. In all but 1 case, the right carotid artery was injected. This vessel exhibits minimal variability within the macaque monkeys in our study. The most proximal portion of the artery demonstrates a slight dilatation at the carotid bulb, but subsequently maintains homogeneous caliber until its most distal segments. Shortly after entering the cranial cavity, the artery curves anteromedially, and a second bend later in its course creates a posteromedial curvature.
At the genu, the internal carotid gives rise to the ophthalmic artery, which in turn courses superiorly towards the orbit. In 17 monkeys, including 1 M. mulatta, an ophthalmic artery was reliably identified. A choroidal blush may be appreciated in some instances as the artery provides vascular supply to the retina.. Of the 17 visualized ophthalmic arteries, 11 had a supraorbital arterial branch. This vessel courses anterosuperiorly, following a more cephalad course than in the human, and wraps around the superior margin of the orbital rim. In 3 monkeys, further branches corresponding to anterior and posterior ethmoidal arteries, as well as the lacrimal artery, were observed on anteroposterior projections. Figure 4 shows the course of the ICA and ophthalmic artery typically encountered.
Fig. 4.
(a,c) are reference views depicting the most commonly used projections for ideal visualization of the internal carotid artery; (b) demonstrates the course of the ICA and its branches, with the black arrow denoting the choroidal blush of the retinal vessels; (d) an anteroposterior view of the ICA and coincidentally imaged vessels; arrowheads denote the anterior and posterior ethmoidal arteries.
Circle of Willis
Convincing evidence of a complete circle of Willis was observed in the large majority of angiograms performed. The continuous anastamosis is formed by the posterior, vertebrobasilar circulation and the vessels of the anterior circulation. The ICAs arise bilaterally as noted above, and divide into the anterior and middle cerebral arteries (ACA and MCA, respectively). The posterior elements are formed by the basilar artery, which begins with the union of the vertebral arteries, and the posterior cerebral arteries (PCA), which represent the terminal bifurcation of the basilar artery. The anterior and posterior components are joined by the posterior communicating arteries (PCOM). An example of the typical macaque circle of Willis is depicted in Figure 5.
Fig. 5.

The circle of Willis as seen from an inferior projection. Elements of the posterior and anterior circulation are well-visualized.
Anterior Cerebral Artery
The ACA arises as one of the terminal branch points of the ICA. The proximal portion, by convention termed the A1 segment, travels horizontally and medially. In macaques, the ACA is azygous, a single vessel formed by the union of the left and right A1 segments. This second segment travels vertically and posteriorly to supply the interhemispheric fissure. Callosomarginal branches were reliably demonstrated in 5 monkeys.
We did not definitively observe an anterior communicating artery (ACOM) in any monkeys in spite of rigorous efforts to locate one. In one macaque, we were unable to visualize an ACA despite careful examination of a variety of AP and lateral projections and rigorous subjection of the original angiogram to a variety of subtraction methods.
Middle Cerebral Artery
The MCA represents the other terminal branch of the ICA, and appears to be larger in size than its counterpart, the ACA. It curves posterolaterally and gives rise to a multitude of smaller, penetrating branches. AP views capturing the course of the MCA were obtained in 15 monkeys. In this projection, a proximal genu can be appreciated, corresponding to the approximate location of the insular cortex, followed by two subsequent right angle turns corresponding to the sylvian fissure. Cortical branches appear to then spread over the surface of the brain. The MCA appears to be well preserved in its anatomy and general course, and no significant variations were encountered in the monkeys under study. Examples of the typical MCA are displayed in Figure 7.
Fig. 7.
(a) Reference view showing head position of the following two images; (b) and (c) represent early and late phase angiograms of the MCA, respectively. The arrowhead indicates the posterior communicating artery, and the black arrow the PCA. (d) An AP view demonstrating the course of the MCA.
Posterior Cerebral Artery
The posterior communicating arteries (PCOM) connect the anterior circulation to the posterior cerebral arteries (PCA). The ipsilateral PCA was visualized in 21 of the 26 individual monkeys after internal carotid injection. In all 21, the vessel was briefly opacified during the injection and then washed out from basilar artery inflow. Retrograde filling of the basilar artery was present in several animals. The PCA arises as a terminal bifurcation of the basilar artery and arcs laterally while curving posteriorly to supply the occipital lobes of the brain. It may be artificially divided into P1 and P2 segments by the PCOM. The P2 segment gives rise to several branches whose full courses cannot be completely appreciated in any of the angiograms of the PCA. Figure 7(b) illustrates both the PCOM and the PCA in the most commonly encountered scenario. Amongst the monkeys in our study, there were no significant variations in the distribution of this artery.
Vertebrobasilar Circulation
Data regarding the posterior circulation are limited in our study. Vertebral artery angiography was performed in only one monkey. This artery originates from the subclavian artery on either side. The first segment of the vertebral artery is entirely extraosseous, but exhibits a slight kink as it enters the transverse foramina of the cervical vertebrae at approximately the level of C6. It courses superiorly within these confines before entering the cranial cavity, where the artery anastomoses with the corresponding contralateral vessel to form the basilar artery. In this case, contrast injected within the posterior circulation was seen to first fill the PCA, and subsequently the anterior circulation via the PCOM. The vertebral artery was appreciated as a background finding in another angiogram as well. Figure 8 demonstrates our imaging of this vessel.
Fig. 8.
(a) Reference view depicting head positioning in the following images; (b) shows catheterization of the right vertebral artery (indicated by arrowhead), with contrast subsequently filling the anterior circulation; (c) shows a monkey in whom the vertebral artery (indicated by arrowhead) was seen as a background finding.
DISCUSSION
The anatomy of the aortic arch in macaque monkeys appears to correlate well with the configuration typically termed the “bovine aortic arch.” In this pattern, as described by Layton et al. (2006), the left common carotid shares its origin with the innominate artery. This variant is considered the norm in canines and felines (Dome et al., 1996), and is seen in approximately 27% of humans (Osborn, 1999).
The bifurcation of the common carotid artery appears analogous in structure to that of the human. The similarity is notable since this site is commonly affected by atherosclerotic changes. Recent studies reinforce the intuitive concept that the geometry of this site strongly influence relevant hemodynamics of the region (Lee et al., 2008; Xue et al. 2008). Coupled with corroborating data from Beere et al. (1992) that suggest the configuration of experimentally induced plaques in macaque carotids is similar to that found in humans, our data lend further credence to the variety of atherosclerotic models employing macaque monkeys. However, our angiographic analysis of this region suggests that some monkeys may in fact have a carotid “trifurcation,” whereby the two trunks of the external carotid (see below) and the internal carotid branch simultaneously. There has been a case report by Gürbüz et al. (2001) of at least one human carotid trifurcation encountered during neck dissection, but this is by no means a commonly encountered variant. Thus, it may be worthwhile for researchers studying lesions involving this anatomical site to confirm that the region contains the expected anatomy.
As in humans, macaques seem to exhibit a great deal of variability in the branching patterns of the external carotid artery. In essence, two main trunks branch almost immediately at the proximal portion of this artery. The first branch, which we have termed the “linguofacial trunk,” gives rise to arteries whose courses correspond to the lingual and facial arteries. In humans, this conformation is present in 10–20% of individuals (Midy et al., 1996; Hayashi et al., 2006). We hypothesize that this prominent difference is likely the result of the differences in the facial structure of the respective species. This configuration may contribute to higher flow to the distal portions of the snout, which is, comparatively, of greater importance to the macaque than to the human.
The second branch we have termed the “occipital trunk,” as it forms the basis for the artery of the same name. The internal maxillary artery, which supplies a variety of deep facial structures, derives from the occipital branch in the minority of cases and represents a continuation of the external carotid in the majority. In either event, it likely represents a terminal branch of the ECA, just as it does in humans (Osborn, 1999).
Our findings further suggest that the superior thyroid artery is a minor tributary of the linguofacial trunk. The aforementioned vessels represent the major branches of the external carotid artery visible in most of our angiograms. The ascending pharyngeal artery was not convincingly observed in any specimen, which is likely the result of its small caliber proving insufficient for adequate visualization of contrast. There were not, to our knowledge, any visible correlates to the transverse facial, superficial temporal, or posterior auricular arteries. This may be a result of the differences in facial anatomy between man and macaque.
In humans, the internal carotid artery is conventionally divided into seven parts along its course, designated C1–C7: the cervical, petrous, lacerum, cavernous, clinoid, ophthalmic, and communicating segments, with partitions reflecting juxtaposed anatomical structures (Obsorn, 1999). These divisions can be applied to the macaque's ICA as well, whose chief dissimilarity with the counterpart vessel in humans is its lesser degree of tortuosity. The carotid bulb in macaques does not appear as pronounced as that seen in humans. In other respects, the vessels seem largely similar, and the macaque ICA can likely be considered an adequate surrogate for that of the human.
The circle of Willis appears intact in the majority of monkeys studied. Grossly, the structure corresponds well to that of the human, with the absence of an anterior communicating artery being the obvious distinction. Previous dissection studies of macaque monkeys by Kassel and Langfitt (1965) and Kapoor et al. (2003) closely related to those in our study have yielded similar results. As noted earlier, we encountered one monkey in which we could not find, in spite of rigorous efforts, an ACA. It is possible that the introduction of the catheter into the internal carotid artery caused a vasospastic response and prevented distal diffusion of the contrast media into the ACA, but it is also possible that this vessel was hypoplastic or absent. The remainder of the cerebrovascular circulation appears to mirror that of humans rather closely. Although Coceani and Gloor (1966) have suggested that in certain cases, closely related macaque monkeys may derive their posterior cerebral arteries from the anterior (rather than posterior) circulation, we did not note such examples in our study.
It has been noted by Fukuda and del Zoppo (2003) that humans are uniquely affected by stroke, and there has been long-standing interest in developing animal models of stroke for experimentation involving novel neuroprotective compounds as well as for understanding the pathophysiological underpinnings of this disease process. Our findings suggest strong anatomical correlation between monkey and human, and indicate that the macaque would likely be a highly suitable candidate for such proposed testing.
Early methods of inducing focal cerebral ischemia proposed by Watanabe et al. (1997) and Bremer et al. (1975) were rather invasive; more recently, a variety of less intrusive, catheter-based approaches have been developed, including balloon occlusion (Gao et al., 2006), autologous blood clot injection (Kito et al., 2006), microcatheter occlusion (de Crespigny et al., 2001; de Crespigny et al., 2005), and plastic bead microembolization (Brassel et al., 1989). Alternative procedures lend themselves to assessment of reperfusion following acute occlusion (Maeda et al., 2005). Techniques used by Macdonald et al. (1995) can lend themselves to study of penetrating branches in addition to larger caliber vessels. Similar models could serve as a platform for hypothermia-oriented therapy.
Additionally, our work may be of interest to researchers whose work involves intracarotid injection of neuroactive compounds. Neuroprotective agents have failed in human trials, and assembling studies for acute ischemic protection presents a significant challenge (Diener et al., 2008; Sacchetti. 2008). In part, this may be due to the exquisite sensitivity of neural tissue and the delay in presentation in human strokes, thereby limiting the therapeutic window - a condition not necessarily imposed upon animal models. Recent work by Nakagawa et al. (2006) with intracarotid injection of granulocyte macrophage colony-stimulating factor (GM-CSF) and related compounds has shown promising results in delayed angiogenic formation, after focal ischemic insult in rodent brains has already occurred. Studies by Hossmann and Buschmann (2005) with this compound have also yielded information on the patterns of collateralization of vasculature. We posit that the macaque monkey would be an ideal specimen for exploring the possible development of collaterals with this drug, with possible predictive value for humans.
Our angiographic analysis of macaque arterial anatomy has identified typical patterns and common variants in these monkeys. These data may have a variety of applications in research settings involving similar animals. Our findings corroborate the homology of human and macaque cervical and circle of Willis arterial anatomy, with the notable exception of the azygous ACA. We propose that the similarity in distribution of most intracranial vessels suggests that focal ischemic models makes extrapolation of such data to humans. Additionally, analogous vessel geometry implies similarity in the hemodynamic milieu, which is of some importance in considering pathologies in which flow patterns are thought to contribute to pathogenesis, such as atherosclerosis or cerebral aneurysm. Some researchers may find these data of use during intra-arterial injections by increasing predictability, as well as potential explanations for otherwise anomalous findings in the course such experiments.
Fig. 6.
The anterior cerebral artery in lateral (a) and AP (b) projections. The black arrow denotes the origin of the callosomarginal artery in each. Note the absence of an anterior communicating artery and azygous nature of the ACA in (b).
Acknowledgments
This work has been supported by National Institute of Neurological Disease and Stroke grants NS39821, NS058714, NS41509, the American Parkinson's Disease Association (APDA) Advanced Research Center at Washington University, the Greater St. Louis Chapter of the APDA, the Barnes-Jewish Hospital Foundation (Jack Buck Fund for PD Research and the Elliot H. Stein Family Fund), and the McDonnell Center for Higher Brain Function and the Murphy Fund.
Grant Sponsor: National Institute of Neurological Disorders and Stroke
Grant Numbers: NS39821, NS058714, NS41509
Footnotes
Significance: We present anatomical variations in the cerebrovasculature of macaque monkeys.
This material has not been published elsewhere; nor is it under consideration for publication elsewhere.
Qualified Referees: Kanchan Kapoor, Department of Anatomy Government Medical College Sector 32, Chandigarh PIN-160030, INDIA Telephone: +91-172-2690338 Fax: +91-172-2702254 kapoorkanchan@rediffmail.com
Field of interest: Anatomy [prior experience in animal dissections of cerebrovasculature]
R. Loch Macdonald, MD, Ph.D, FRCSC, FACS University of Toronto 2 Queen Street E. Suite 1005 Toronto, ON M5C 3G7 Telephone: 416 864-5452 Fax: 416 864-5634 macdonaldlo@smh.toronto.on.ca
Field of interest: Neuroradiology [prior experience in cerebrovascular experimentation involving macaque monkeys]
Kennith F. Layton, Department of Radiology Baylor University Medical Center 3500 Gaston Ave, Dallas, TX 75246 klayton@americanrad.com Phone: 214 826-8822 Fax: 214 826-9792
Field of interest: Neuroradiology
Literature Cited
- Beere PA, Glagov S, Zarins CK. Experimental atherosclerosis at the carotid bifurcation of the cynomolgus monkey. Localization, compensatory enlargement, and the sparing effect of lowered heart rate. Arterioscler Thromb. 1992;12:1245–53. doi: 10.1161/01.atv.12.11.1245. [DOI] [PubMed] [Google Scholar]
- Brassel F, Dettmers C, Nierhaus A, Hartmann A, Solymosi L. An intravascular technique to occlude the middle cerebral artery in baboons. Neuroradiology. 1989;31:418–24. doi: 10.1007/BF00343867. [DOI] [PubMed] [Google Scholar]
- Bremer AM, Watanabe O, Bourke RS. Artificial embolization of the middle cerebral artery in primates. Description of an experimental model with extracranial technique. Stroke. 1975;6:387–90. doi: 10.1161/01.str.6.4.387. [DOI] [PubMed] [Google Scholar]
- Coceani F, Gloor P. The distribution of the internal carotid circulation in the brain of the macaque monkey (Macaca mulatta) J Comparative Neurol. 1966;128:419–429. [Google Scholar]
- Diener HC, Lees KR, Lyden P, Grotta J, Davalos A, Davis SM, Shuaib A, Ashwood T, Wasiewski W, Alderfer V, Hårdemark HG, Rodichok L, SAINT I and II Investigators NXY-059 for the treatment of acute stroke: pooled analysis of the SAINT I and II Trials. Stroke. 2008;39:1751–8. doi: 10.1161/STROKEAHA.107.503334. [DOI] [PubMed] [Google Scholar]
- de Crespigny AJ, D'Arceuil HE, Maynard K, Cocoros N, McAuliffe D, Putman CM, Budzik RF, Norbash A, Hamberg L, Hunter G, Gonzalez RG. A New Model for Acute Stroke Therapy. Proc Intl Soc Mag Reson Med. 2001;9:354. [Google Scholar]
- de Crespigny AJ, D'Arceuil HE, Maynard KI, He J, McAuliffe D, Norbash A, Sehgal PK, Hamberg L, Hunter G, Budzik RF, Putman CM, Gonzalez RG. Acute studies of a new primate model of reversible middle cerebral artery occlusion. J Stroke Cerebrovasc Dis. 2005;14:80–7. doi: 10.1016/j.jstrokecerebrovasdis.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Dome SH, Goody PC, Strickland NC, et al. The Dog and Cat. Volume 3. Mosby-Year Book; St Louis: 1996. Color Atlas of Veterinary Anatomy. [Google Scholar]
- Fukuda S, del Zoppo GJ. Models of focal cerebral ischemia in the nonhuman primate. ILAR J. 2003;44:96–104. doi: 10.1093/ilar.44.2.96. [DOI] [PubMed] [Google Scholar]
- Gao H, Liu Y, Lu S, Xiang B, Wang C. A reversible middle cerebral artery occlusion model using intraluminal balloon technique in monkeys. J Stroke Cerebrovasc Dis. 2006;15:202–8. doi: 10.1016/j.jstrokecerebrovasdis.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Gürbüz J, Cavdar S, Ozdoğmuş O. Trifurcation of the left common carotid artery: a case report. Clin Anat. 2001;14:58–61. doi: 10.1002/1098-2353(200101)14:1<58::AID-CA1011>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Hayashi N, Hori E, Ohtani Y, Ohtani O, Kuwayama N, Endo S. Surgical anatomy of the cervical carotid artery for carotid endarterectomy. Neurol Med Chir (Tokyo) 2005;45:25–9. doi: 10.2176/nmc.45.25. discussion 30. [DOI] [PubMed] [Google Scholar]
- Hossmann KA, Buschmann IR. Granulocyte-macrophage colony-stimulating factor as an arteriogenic factor in the treatment of ischaemic stroke. Expert Opin Biol Ther. 2005;5:1547–56. doi: 10.1517/14712598.5.12.1547. [DOI] [PubMed] [Google Scholar]
- Kapoor K, Kak VK, Singh B. Morphology and comparative anatomy of circulus arteriosus cerebri in mammals. Anat Histol Embryol. 2003;32:347–55. doi: 10.1111/j.1439-0264.2003.00492.x. [DOI] [PubMed] [Google Scholar]
- Kassel NF, Langfitt TW. Variations in the circle of Willis in Macaca mulatta. Anat Rec. 1965;152:257–63. doi: 10.1002/ar.1091520305. [DOI] [PubMed] [Google Scholar]
- Kito G, Nishimura A, Susumu T, Nagata R, Kuge Y, Yokota C, Minematsu K. Experimental thromboembolic stroke in cynomolgus monkey. J Neurosci Methods. 2001;30(105):45–53. doi: 10.1016/s0165-0270(00)00351-4. [DOI] [PubMed] [Google Scholar]
- Layton KF, Kallmes DF, Cloft HJ, Lindell EP, Cox VS. Bovine Aortic Arch Variant in Humans: Clarification of a Common Misnomer. Am J Neuroradiology. 2006;27:1541–1542. [PMC free article] [PubMed] [Google Scholar]
- Lee SW, Antiga L, Spence JD, Steinman DA. Geometry of the carotid bifurcation predicts its exposure to disturbed flow. Stroke. 2008;39:2341–7. doi: 10.1161/STROKEAHA.107.510644. [DOI] [PubMed] [Google Scholar]
- Macdonald RL, Kowalczuk A, Johns L. Emboli enter penetrating arteries of monkey brain in relation to their size. Stroke. 1995;26:1247–50. doi: 10.1161/01.str.26.7.1247. discussion 1250-1. [DOI] [PubMed] [Google Scholar]
- Maeda M, Takamatsu H, Furuichi Y, Noda A, Awaga Y, Tatsumi M, Yamamoto M, Ichise R, Nishimura S, Matsuoka N. Characterization of a novel thrombotic middle cerebral artery occlusion model in monkeys that exhibits progressive hypoperfusion and robust cortical infarction. J Neurosci Methods. 2005;146:106–15. doi: 10.1016/j.jneumeth.2005.01.019. [DOI] [PubMed] [Google Scholar]
- Midy D, Mauruc B, Vergnes P, Caliot P. A contribution to the study of the facial artery, its branches and anastomoses; application to the anatomic vascular bases of facial flaps. Surg Radiol Anat. 1986;8:99–107. doi: 10.1007/BF02421376. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Suga S, Kawase T, Toda M. Intracarotid injection of granulocyte-macrophage colony-stimulating factor induces neuroprotection in a rat transient middle cerebral artery occlusion model. Brain Res. 2006;1089:179–85. doi: 10.1016/j.brainres.2006.03.059. [DOI] [PubMed] [Google Scholar]
- Osborn AG. Diagnostic Cerebral Angiography. 2nd ed. Lippincott Williams & Wilkins; Philadelphia, Pa: 1999. The aortic arch and great vessels; pp. 3–29. [Google Scholar]
- Sacchetti ML. Is it time to definitely abandon neuroprotection in acute ischemic stroke? Stroke. 2008;39:1659–60. doi: 10.1161/STROKEAHA.107.505024. [DOI] [PubMed] [Google Scholar]
- Tabbal SD, Mink JW, Antenor JA, Carl JL, Moerlein SM, Perlmutter JS. 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced acute transient dystonia in monkeys associated with low striatal dopamine. Neuroscience. 2006;141:1281–7. doi: 10.1016/j.neuroscience.2006.04.072. [DOI] [PubMed] [Google Scholar]
- Watanabe O, Bremer AM, West CR. Experimental regional cerebral ischemia in the middle cerebral artery territory in primates. Part 1: Angio-anatomy and description of an experimental model with selective embolization of the internal carotid artery bifurcation. Stroke. 1977;8:61–70. doi: 10.1161/01.str.8.1.61. [DOI] [PubMed] [Google Scholar]
- Xue YJ, Gao PY, Duan Q, Lin Y, Dai CB. Preliminary study of hemodynamic distribution in patient-specific stenotic carotid bifurcation by image-based computational fluid dynamics. Acta Radiol. 2008;49:558–65. doi: 10.1080/02841850801918548. [DOI] [PubMed] [Google Scholar]