Abstract
It is well established that genetic deletion or pharmacological inhibition of the CB1 receptor disrupts extinction learning in aversive conditioning tasks, but not in appetitive tasks. Consistent with these findings is that genetic deletion or pharmacological inhibition of fatty acid amide hydrolase (FAAH), the primary catabolic enzyme of the endogenous cannabinoid anandamide (AEA), accelerates acquisition as well as extinction in aversive conditioning tasks. However, it is unknown whether FAAH blockade will affect acquisition in an appetitive conditioning task. Therefore, in the present study, we assessed FAAH (−/−) and (+/+) mice in appetitive and aversive Barnes maze conditioning procedures. Here we report that FAAH (−/−) mice displayed accelerated acquisition rates in an aversively-motivated, but not in the appetitively-motivated, Barnes maze task. The CB1 receptor antagonist, rimonabant attenuated enhanced acquisition in the aversive procedure, consistent with the idea that elevated AEA levels mediate this apparent nootropic effect. These findings support the hypothesis that stimulation of the endocannabinoid system enhances learned behavior in aversive, but not appetitive, conditioning paradigms.
Constituents of the endocannabinoid system, including the cannabinoid receptor 1 (CB1), the endocannabinoids 2-arachyldonyl glycerol (2-AG) and anandamide (AEA), and enzymes responsible for the biosynthesis and degradation of endocannabinoids (Ahn, McKinney, and Cravatt, 2008) are present throughout the brain, including regions involved in learning and memory. It is established beyond a doubt that cannabinoid receptor agonists impair short term memory (Lichtman, Varvel, and Martin, 2002). Paradoxically, genetic deletion or pharmacological inhibition of fatty acid amide hydrolase (FAAH), the predominant catabolic enzyme of AEA, accelerates acquisition in aversive conditioning tasks. FAAH (−/−) mice or wild type mice treated with the reversible FAAH inhibitor OL-135 displayed facilitated acquisition and extinction learning in a fixed platform Morris water maze task (Varvel, Wise, Niyuhire, Cravatt, and Lichtman, 2007). Likewise, the irreversible FAAH inhibitor, URB597, enhanced memory acquisition in a rat passive avoidance task (Mazzola, Medalie, Scherma, Panlilio, Solinas, Tanda, Drago, Cadet, Goldberg, and Yasar, 2009). Additionally, AM404, an inhibitor of the endocannabinoid uptake and degradation, facilitated extinction learning in conditioned freezing tasks (Chhatwal, Davis, Maguschak, and Ressler, 2005; Pamplona, Prediger, Pandolfo, and Takahashi, 2006). Thus, elevating endogenous cannabinoid levels by blocking degradation produces qualitatively different effects on learning and memory compared to administration of exogenous cannabinoids that interfere with mnemonic processes.
Several studies have shown that CB1 (−/−) mice as well as mice treated with cannabinoid receptor antagonists display impaired extinction learning in aversively motivated tasks, including conditioned freezing, passive avoidance, and Morris water maze paradigms (Chhatwal et al., 2005; Marsicano, Wotjak, Azad, Bisogno, Rammes, Cascio, Hermann, Tang, Hofmann, Zieglgansberger, Di Marzo, and Lutz, 2002; Niyuhire, Varvel, Thorpe, Stokes, Wiley, and Lichtman, 2007; Pamplona et al., 2006; Suzuki, Josselyn, Frankland, Masushige, Silva, and Kida, 2004; Varvel, Anum, and Lichtman, 2005). However, cannabinoid receptor antagonists do not disrupt extinction learning of appetitively motivated operant tasks (Holter, Kallnik, Wurst, Marsicano, Lutz, and Wotjak, 2005; Niyuhire et al., 2007; Ward, Walker, and Dykstra, 2007). Recently, we reported that the CB1 receptor antagonist rimonabant disrupts extinction learning in an aversive, but not in an appetitive, Barnes maze conditioning task (Harloe, Thorpe, and Lichtman, 2008). Therefore, the primary objective of the present study was to determine whether FAAH (−/−) and (+/+) mice display distinct phenotypes in these different versions of the Barnes maze tasks. In addition, we used the selective CB1 receptor antagonist, rimonabant, to examine underlying receptor mechanisms of action that might account for performance differences between the two genotypes.
FAAH (−/−) and (+/+) mice, born in the Virginia Commonwealth University knockout colony and derived from breeders backcrossed onto a C57Bl/6J background for 13 generations, served as subjects(Lichtman, Shelton, Advani, and Cravatt, 2004). The mice were singly housed in a temperature-controlled (20−22° C) environment, with a 12-h light/dark cycle and ad libitum access to food, and in the aversive condition, water. All experiments have been approved by the Institutional Animal Care and Use Committee at Virginia Commonwealth University.
The Barnes maze (Hamilton-Kinder, Poway, CA) consisted of a round board (122 cm diameter) fabricated from PVC with 40 holes (2.54 cm diameter) surrounding the perimeter of the maze. The maze was divided into six zones, each containing a possible location for the goal box (19.5 cm × 5.5 cm). Contextual cues were attached to white curtains that encircled the maze. A circular starting tube (7.62 cm. diameter) was placed in the center of the maze to ensure that all subjects began each trial from the same location. A digital camera (Panasonic BP-330) connected to AnyMaze software (Stoelting, Wood Dale, IL) recorded trials. The maze and goal box were wiped with an ammonia based cleaner (Whistle; Johnson Diversey Inc., Sturtevant, WI) after each trial.
We employed previously established procedures to train mice in appetitively-motivated and aversively-motivated Barnes maze conditioning tasks (Harloe et al., 2008). These tasks allowed us to focus on the qualitative nature of the reinforcer in tasks that required identical motor responses (i.e., searching for and entering the goal box). In the aversive procedure, the mice entered the goal box to escape bright lights (two 500 watt halogen bulbs producing 3150 lux) and air turbulence generated from two 60 cm wide fans (Holmes, Milford, MA). In the appetitive conditioning procedure, mice were water deprived for 22 h and gained access to drinking water in the goal box. After completion of each acquisition session, subjects were allowed access to water for 2 h in their home cages, which occurred at approximately the same time each day. Water was selected as the reinforcer in the appetitive task because disruption of CB1 receptor signaling reduces operant responding for the intake of palatable food (Holter et al., 2005; Ward et al., 2007), but does not affect water consumption (Arnone, Maruani, Chaperon, Thiebot, Poncelet, Soubrie, and Le Fur, 1997). The maze was illuminated with fluorescent lighting that produced 410 lux in the appetitive procedure.
Following shaping in the Barnes maze, each mouse was given four acquisition trials per day for ten days. Trials were separated by a 30 s ITI during which time the mouse was returned to its home cage. A trial ended when either three min had elapsed or the subject entered the goal box. If a mouse failed to enter the goal box, it was placed in the center of the maze and gently led to the goal box by the investigator, where it remained for 30 s before being returned to its home cage for the 30 s ITI.
In the first two experiments, acquisition rates in FAAH (−/−) and (+/+) mice in the aversive and appetitive conditioning tasks were determined, respectively. In the third experiment, we examined whether differences between the genotypes were mediated by a CB1 receptor mechanism of action. Vehicle or rimonabant (1 mg/kg, i.p.; National Institute on Drug Abuse, Rockville, MD) was administered to subjects 30 min before each acquisition session. This dose does not affect Barnes maze acquisition rates (Harloe et al., 2008).
Dependent measures included distance traveled, latency to enter the hidden goal box, time spent immobile, and running speed [distance traveled/(latency to enter – time immobile)]. Each measure was analyzed using two-way repeated measures analysis of variance (ANOVA), with day as the within subject variable and genotype or treatment condition as the between subject variable. The Tukey post-hoc test was used to analyze differences between genotypes or treatment conditions and days. Differences were considered significant at the p < 0.05 level.
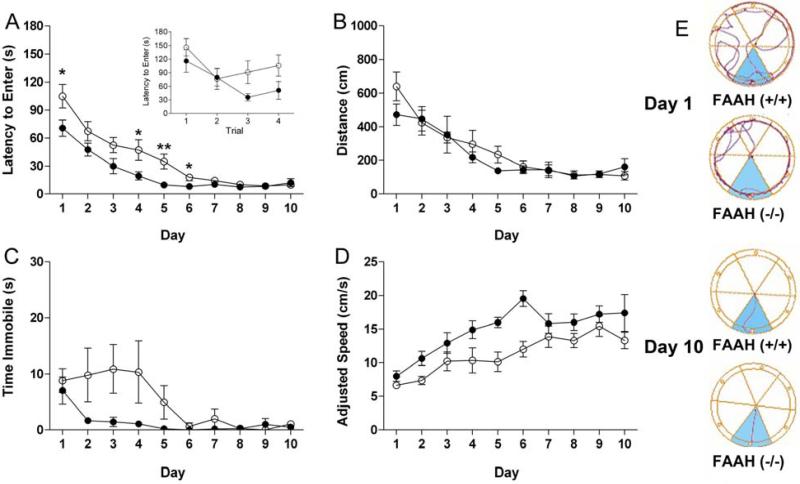
As can be seen in Figure 1, both genotypes acquired the aversive Barnes maze task. Significant main effects of day were found for latency to enter (Figure 1A; F(9,126) = 35.2, p < 0.0001), distance traveled (Figure 1B; F(9,126) = 18.7, p < 0.0001), time immobile (Figure 1C; F(9,126) = 3.5, p < 0.001), and running speed (Figure 1D; F(9,126) = 13.4, p < 0.0001). However, performance was enhanced in FAAH (−/−) mice compared to FAAH (+/+) mice, as revealed by a significant genotype by day interaction for latency to enter (F(9,126) = 2.1, p < 0.05). FAAH (−/−) mice entered the hidden goal box significantly more quickly than FAAH (+/+) mice on days 1, 4, 5, and 6 (p < 0.05 for each). Although FAAH (−/−) mice had quicker latencies than FAAH (+/+) mice on day 1, both genotypes had identical latencies to enter the hidden goal box on trial 1 (Figure 1A inset). FAAH (−/−) mice spent significantly less time immobile (F(1,126) = 11.7, p < 0.01) and had significantly faster running speeds (F(1,126) = 9.7, p < 0.01) than FAAH (+/+) mice.
Figure 1.
FAAH (−/−) mice show enhanced performance during acquisition of an aversive Barnes maze procedure. (○) represents FAAH (+/+) mice and (•) represents FAAH (−/−) mice. Panel A. FAAH (−/−) mice exhibited decreases in average latency to enter the escape box. FAAH (−/−) mice exhibit enhanced acquisition within the first acquisition day but do not differ from FAAH (+/+) mice in trial 1 (inset). Panel B. No differences between genotypes were observed for distance (cm) traveled. Panel C. FAAH (−/−) mice spent significantly less time (s) immobile than FAAH (+/+) mice. Panel D. Running speed was quicker in FAAH (−/−) mice than in FAAH (+/+) mice. Panel E. Representative traces from FAAH (−/−) and (+/+) mice on the first trial on day 1 and the last trial on day 10. * p < 0.05 and ** p < 0.01 indicates a significant difference from FAAH (+/+) mice. The data for each acquisition session are represented as the average of four daily trials ± SEM. N = 8 mice/group.
FAAH (−/−) and (+/+) mice displayed nearly identical performance during acquisition in the appetitive Barnes maze task. Significant effects of day were found for latency to enter (Figure 2A; F(9,162) = 41.3, p < 0.0001), distance traveled (Figure 2B; F(9,162) = 17.9, p < 0.0001), time immobile (Figure 2C; F(9,162) = 12.5, p < 0.0001), and running speed (Figure 2D; F(9,162) = 10.4, p < 0.0001). In contrast to the aversive paradigm, genotype differences were not found for latency to enter in the appetitive paradigm (p = 0.15). Also, no genotype differences were not found for distance traveled (p = 0.40) or running speed (p = 0.70), though FAAH (+/+) mice spent less time immobile than FAAH (−/−) mice (F(1,162) = 7.6, p < 0.05).
Figure 2.
FAAH (−/−) and (+/+) mice show equivalent performance during acquisition in an appetitive Barnes maze task. (○) represents FAAH (+/+) mice and (•) represents FAAH (−/−) mice. Panel A. No genotype effects were observed for latency (s) to enter the goal box. Panel B. No genotype effects were observed for distance (cm) traveled. Panel C. FAAH (−/−) mice spent more time immobile than FAAH (+/+) mice. Panel D. No differences were found between the genotypes for running speed. Panel E. Representative traces from FAAH (−/−) and (+/+) mice on the first trial of day 1 and on the last trial of day 10. The data for each acquisition session are represented as the average of four daily trials ± SEM. N = 10 mice/group.
In the final experiment, we examined whether the enhanced performance of FAAH (−/−) during acquisition in the aversive Barnes maze task was mediated through a CB1 receptor mechanism of action. FAAH (−/−) mice were administered either rimonabant (1 mg/kg) or vehicle before each acquisition session. In addition, a vehicle-injected group of FAAH (+/+) mice served as controls. We have previously demonstrated that rimonabant does not alter acquisition in either appetitive or aversive Barnes maze tasks (Harloe et al., 2008). As shown in Figure 3, all three groups acquired the aversive Barnes maze task, with significant main effects of conditioning day for latency to enter (Figure 3A; F(9,333) = 105, p < 0.0001), distance traveled (Figure 3B; F(9,333) = 61.6, p < 0.0001), time immobile (Figure 3C; F(9,333) = 10.7, p < 0.0001), and running speed (Figure 3 D; F(9,333) = 26.0, p < 0.0001). Importantly, significant genotype by training day interactions were found for latency to enter the hidden box (F(18,333) = 1.7, p < 0.05) and time immobile (F(18,333) = 2.0, p < 0.01). FAAH (−/−) mice treated with vehicle exhibited a decrease in latency to enter the hidden box (p < 0.05) and time immobile (p < 0.01) on day 2 as compared to rimonabant-treated FAAH (−/−) mice and vehicle-treated FAAH (+/+) mice. Thus, rimonabant attenuated the improved acquisition of FAAH (−/−) mice in the aversive Barnes maze task. No genotype differences were found for either distance traveled (p = 0.21) or running speed (p = 0.25).
Figure 3.
The FAAH (−/−) phenotypic enhancement of acquisition in the aversive Barnes maze task is mediated through a CB1 receptor mechanism of action. Rimonabant (1 mg/kg) or vehicle was administered 30 min before each acquisition session. (○) represents vehicle-treat FAAH (+/+) mice, (•) represents vehicle-treated FAAH (−/−) mice, and (▼) represents rimonabant-treated FAAH (−/−) mice. Panel A. Vehicle-treated FAAH (−/−) mice displayed short latencies to enter the goal box than the other groups. Rimonabant blocked this effect. Panel B. No genotype or treatment effects were observed for distance (cm) traveled. Panel C. Vehicle-treated FAAH (−/−) mice spent less time immobile than each of the other two groups. This effect was blocked by rimonabant. Panel D. No genotype or treatment effects were observed for running speed. Panel E. Representative traces from each group on the first trial of day 1 and on the last trial of day 10. * p < 0.05 and **p < 0.01 indicates a significant difference between vehicle-treated FAAH (−/−) mice and vehicle-treated FAAH (+/+) or rimonabant-treated FAAH (−/−) mice. All data are represented as the average of four daily trials ± SEM. N = 13−14 mice/condition.
In the present study FAAH (−/−) mice exhibited enhanced acquisition learning in an aversive, but not in an appetitive, Barnes maze task. During early acquisition sessions (i.e. sessions 1−2) in the aversive paradigm, FAAH (−/−) mice entered the hidden goal box more quickly than FAAH (+/+) mice. Rimonabant normalized acquisition learning in FAAH (−/−) mice in the aversive paradigm, indicating a CB1 receptor mechanism of action. These findings support the hypothesis that stimulating endogenous cannabinoid signaling by blocking FAAH enhances acquisition of aversively reinforced spatial memory tasks (Varvel et al., 2007). Moreover, as the behavioral demands (i.e. locating and entering the goal box) were identical between the appetitive and aversive conditioning procedures, to our knowledge, this is the first report to illustrate that enhancement of acquisition following FAAH deletion is dependent on reinforcement conditions.
Given that there were no genotype differences in the distance traveled to enter the goal box, the improved performance of the FAAH (−/−) mice is most likely due to their decreased immobility time compared to the FAAH (+/+) mice. In particular, both genotypes located the correct hole that contained the hidden goal box with similar latencies; however, the wild type mice paused before entering the hidden goal box, which resulted in an increased latency to enter. Although running speed may also contribute to the genotype difference observed here, there were no differences in running speed in Experiment 3. Neither genetic nor pharmacological approaches to block FAAH appear to affect locomotor behavior (Cippitelli, Bilbao, Gorriti, Navarro, Massi, Piomelli, Ciccocioppo, and Rodriguez de Fonseca, 2007; Cravatt, Demarest, Patricelli, Bracey, Giang, Martin, and Lichtman, 2001; Moreira, Kaiser, Monory, and Lutz, 2008). Conversely, in the fixed platform Morris water maze paradigm (Varvel et al., 2007), a memory paradigm that utilizes aversive reinforcement, FAAH (−/−) mice displayed enhanced acquisition and increased swim speed compared to FAAH (+/+) mice. Thus, aversive reinforcement may reveal genotype differences in motor behavior as well as acquisition of spatial memory.
The enhancement of acquisition presented here may reflect alterations in behavioral flexibility and/or emotionality. Consistent with the idea that activation of the endocannabinoid system is linked to behavioral flexibility, FAAH (−/−) mice display accelerated extinction learning (Varvel et al., 2007), while CB1 (−/−) mice exhibit preservative behavior that interferes with extinction learning (Varvel et al., 2005) in the Morris water maze. Thus, it is conceivable that increased behavioral flexibility led to the enhanced acquisition of the FAAH (−/−) mice in either the aversive Barnes maze task in the present study or the Morris water maze task (Varvel et al., 2007), though behavioral flexibility was not explicitly examined.
The endocannabinoid system has also been demonstrated to modulate emotionality. Pharmacological and genetic attenuation of endocannabinoid signaling have been shown to produce anxiogenic-like behavior (Haller, Bakos, Szirmay, Ledent, and Freund, 2002; Patel and Hillard, 2006; Rodgers, Evans, and Murphy, 2005; Scherma, Medalie, Fratta, Vadivel, Makriyannis, Piomelli, Mikics, Haller, Yasar, Tanda, and Goldberg, 2008), whereas pharmacological inhibitors of FAAH can produce anxiolytic-like responses (Bortolato, Campolongo, Mangieri, Scattoni, Frau, Trezza, La Rana, Russo, Calignano, Gessa, Cuomo, and Piomelli, 2006; Kathuria, Gaetani, Fegley, Valino, Duranti, Tontini, Mor, Tarzia, La Rana, Calignano, Giustino, Tattoli, Palmery, Cuomo, and Piomelli, 2003; Naidu, Varvel, Ahn, Cravatt, Martin, and Lichtman, 2007; Patel and Hillard, 2006; Scherma et al., 2008). Importantly, FAAH inhibition does not affect anxiety under mildly stressful conditions, but can produce anxiolytic-like responses in particularly aversive or stressful conditions (Haller et al., 2009; Naidu et al., 2007; Patel and Hillard, 2006). The accelerated rate of acquisition of FAAH (−/−) mice in the aversive Barnes maze task may be due to the fact that they spent significantly less time immobile than FAAH (+/+) mice. As endogenous cannabinoids are presumed to be released in response to aversive stimuli (Hohmann, Suplita, Bolton, Neely, Fegley, Mangieri, Krey, Walker, Holmes, Crystal, Duranti, Tontini, Mor, Tarzia, and Piomelli, 2005; Marsicano et al., 2002) and blockade of FAAH has been shown to elicit anxiolytic-like activity when the magnitude of stress is increased (Haller et al., 2009; Naidu et al., 2007; Patel and Hillard, 2006), it is plausible that the conditions associated with the appetitive paradigm are insufficient to increase endogenous cannabinoid signaling. Rimonabant normalized acquisition learning in FAAH (−/−) mice as well as increased the duration of time spent immobile in FAAH (−/−) mice to levels indistinguishable from vehicle-treated FAAH (+/+) mice, indicating that the CB1 receptor plays a necessary role in this phenotype.
In conclusion, the results of the present study suggest that stimulation of the endocannabinoid system via FAAH blockade accelerates acquisition under aversive conditions because of a phenotypic reduction in anxiogenic-like behavior. This explanation does not extend to appetitive conditioning procedures in which FAAH (−/−) and FAAH (+/+) mice demonstrated similar acquisition rates, though FAAH (−/−) mice actually spent more time immobile than FAAH (+/+) mice. Taken together, these results support the hypothesis that blocking endocannabinoid degradation enables animals to cope effectively during stressful conditions, as manifested by enhanced acquisition in the Morris water maze (Varvel et al., 2007), passive avoidance (Mazzola et al., 2009), and aversive Barnes maze tasks.
Acknowledgements
This work was supported by NIDA grants R01DA015683, R01DA15197, P01DA017259, P01DA009789, P50DA005274, and T23DA07027.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahn K, McKinney MK, Cravatt BF. Enzymatic pathways that regulate endocannabinoid signaling in the nervous system. Chem Rev. 2008;108:1687–1707. doi: 10.1021/cr0782067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnone M, Maruani J, Chaperon F, Thiebot MH, Poncelet M, Soubrie P, Le Fur G. Selective inhibition of sucrose and ethanol intake by SR 141716, an antagonist of central cannabinoid (CB1) receptors. Psychopharmacology (Berl) 1997;132:104–106. doi: 10.1007/s002130050326. [DOI] [PubMed] [Google Scholar]
- Bortolato M, Campolongo P, Mangieri RA, Scattoni ML, Frau R, Trezza V, La Rana G, Russo R, Calignano A, Gessa GL, Cuomo V, Piomelli D. Anxiolytic-like properties of the anandamide transport inhibitor AM404. Neuropsychopharmacology. 2006;31:2652–2659. doi: 10.1038/sj.npp.1301061. [DOI] [PubMed] [Google Scholar]
- Chhatwal JP, Davis M, Maguschak KA, Ressler KJ. Enhancing cannabinoid neurotransmission augments the extinction of conditioned fear. Neuropsychopharmacology. 2005;30:516–524. doi: 10.1038/sj.npp.1300655. [DOI] [PubMed] [Google Scholar]
- Cippitelli A, Bilbao A, Gorriti MA, Navarro M, Massi M, Piomelli D, Ciccocioppo R, Rodriguez de Fonseca F. The anandamide transport inhibitor AM404 reduces ethanol self-administration. Eur J Neurosci. 2007;26:476–486. doi: 10.1111/j.1460-9568.2007.05665.x. [DOI] [PubMed] [Google Scholar]
- Cravatt BF, Demarest K, Patricelli MP, Bracey MH, Giang DK, Martin BR, Lichtman AH. Supersensitivity to anandamide and enhanced endogenous cannabinoid signaling in mice lacking fatty acid amide hydrolase. Proc Natl Acad Sci U S A. 2001;98:9371–9376. doi: 10.1073/pnas.161191698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller J, Bakos N, Szirmay M, Ledent C, Freund TF. The effects of genetic and pharmacological blockade of the CB1 cannabinoid receptor on anxiety. Eur J Neurosci. 2002;16:1395–1398. doi: 10.1046/j.1460-9568.2002.02192.x. [DOI] [PubMed] [Google Scholar]
- Harloe JP, Thorpe AJ, Lichtman AH. Differential endocannabinoid regulation of extinction in appetitive and aversive Barnes maze tasks. Learn Mem. 2008;15:806–809. doi: 10.1101/lm.1113008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohmann AG, Suplita RL, Bolton NM, Neely MH, Fegley D, Mangieri R, Krey JF, Walker JM, Holmes PV, Crystal JD, Duranti A, Tontini A, Mor M, Tarzia G, Piomelli D. An endocannabinoid mechanism for stress-induced analgesia. Nature. 2005;435:1108–1112. doi: 10.1038/nature03658. [DOI] [PubMed] [Google Scholar]
- Holter SM, Kallnik M, Wurst W, Marsicano G, Lutz B, Wotjak CT. Cannabinoid CB1 receptor is dispensable for memory extinction in an appetitively-motivated learning task. Eur J Pharmacol. 2005;510:69–74. doi: 10.1016/j.ejphar.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Kathuria S, Gaetani S, Fegley D, Valino F, Duranti A, Tontini A, Mor M, Tarzia G, La Rana G, Calignano A, Giustino A, Tattoli M, Palmery M, Cuomo V, Piomelli D. Modulation of anxiety through blockade of anandamide hydrolysis. Nat Med. 2003;9:76–81. doi: 10.1038/nm803. [DOI] [PubMed] [Google Scholar]
- Lichtman AH, Shelton CC, Advani T, Cravatt BF. Mice lacking fatty acid amide hydrolase exhibit a cannabinoid receptor-mediated phenotypic hypoalgesia. Pain. 2004;109:319–327. doi: 10.1016/j.pain.2004.01.022. [DOI] [PubMed] [Google Scholar]
- Lichtman AH, Varvel SA, Martin BR. Endocannabinoids in cognition and dependence. Prostaglandins Leukot Essent Fatty Acids. 2002;66:269–285. doi: 10.1054/plef.2001.0351. [DOI] [PubMed] [Google Scholar]
- Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, Hermann H, Tang J, Hofmann C, Zieglgansberger W, Di Marzo V, Lutz B. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418:530–534. doi: 10.1038/nature00839. [DOI] [PubMed] [Google Scholar]
- Mazzola C, Medalie J, Scherma M, Panlilio L, Solinas M, Tanda G, Drago F, Cadet J, Goldberg SR, Yasar S. Fatty acid amide hydrolase (FAAH) inhibition enhances memory acquisition through activation of PPAR-alpha nuclear receptors. Learning and Memory. 2009 doi: 10.1101/lm.1145209. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira FA, Kaiser N, Monory K, Lutz B. Reduced anxiety-like behaviour induced by genetic and pharmacological inhibition of the endocannabinoid-degrading enzyme fatty acid amide hydrolase (FAAH) is mediated by CB1 receptors. Neuropharmacology. 2008;54:141–150. doi: 10.1016/j.neuropharm.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Naidu PS, Varvel SA, Ahn K, Cravatt BF, Martin BR, Lichtman AH. Evaluation of fatty acid amide hydrolase inhibition in murine models of emotionality. Psychopharmacology (Berl) 2007;192:61–70. doi: 10.1007/s00213-006-0689-4. [DOI] [PubMed] [Google Scholar]
- Niyuhire F, Varvel SA, Thorpe AJ, Stokes RJ, Wiley JL, Lichtman AH. The disruptive effects of the CB1 receptor antagonist rimonabant on extinction learning in mice are task-specific. Psychopharmacology (Berl) 2007;191:223–231. doi: 10.1007/s00213-006-0650-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pamplona FA, Prediger RD, Pandolfo P, Takahashi RN. The cannabinoid receptor agonist WIN 55,212−2 facilitates the extinction of contextual fear memory and spatial memory in rats. Psychopharmacology (Berl) 2006;188:641–649. doi: 10.1007/s00213-006-0514-0. [DOI] [PubMed] [Google Scholar]
- Patel S, Hillard CJ. Pharmacological evaluation of cannabinoid receptor ligands in a mouse model of anxiety: further evidence for an anxiolytic role for endogenous cannabinoid signaling. J Pharmacol Exp Ther. 2006;318:304–311. doi: 10.1124/jpet.106.101287. [DOI] [PubMed] [Google Scholar]
- Rodgers RJ, Evans PM, Murphy A. Anxiogenic profile of AM-251, a selective cannabinoid CB1 receptor antagonist, in plus-maze-naive and plus-maze-experienced mice. Behav Pharmacol. 2005;16:405–413. doi: 10.1097/00008877-200509000-00013. [DOI] [PubMed] [Google Scholar]
- Scherma M, Medalie J, Fratta W, Vadivel SK, Makriyannis A, Piomelli D, Mikics E, Haller J, Yasar S, Tanda G, Goldberg SR. The endogenous cannabinoid anandamide has effects on motivation and anxiety that are revealed by fatty acid amide hydrolase (FAAH) inhibition. Neuropharmacology. 2008;54:129–140. doi: 10.1016/j.neuropharm.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Josselyn SA, Frankland PW, Masushige S, Silva AJ, Kida S. Memory reconsolidation and extinction have distinct temporal and biochemical signatures. J Neurosci. 2004;24:4787–4795. doi: 10.1523/JNEUROSCI.5491-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varvel SA, Anum EA, Lichtman AH. Disruption of CB(1) receptor signaling impairs extinction of spatial memory in mice. Psychopharmacology (Berl) 2005;179:863–872. doi: 10.1007/s00213-004-2121-2. [DOI] [PubMed] [Google Scholar]
- Varvel SA, Wise LE, Niyuhire F, Cravatt BF, Lichtman AH. Inhibition of fatty-acid amide hydrolase accelerates acquisition and extinction rates in a spatial memory task. Neuropsychopharmacology. 2007;32:1032–1041. doi: 10.1038/sj.npp.1301224. [DOI] [PubMed] [Google Scholar]
- Ward SJ, Walker EA, Dykstra LA. Effect of cannabinoid CB1 receptor antagonist SR141716A and CB1 receptor knockout on cue-induced reinstatement of Ensure and corn-oil seeking in mice. Neuropsychopharmacology. 2007;32:2592–2600. doi: 10.1038/sj.npp.1301384. [DOI] [PubMed] [Google Scholar]