Abstract
Evidence is emerging that the intestinal microbiota is intrinsically linked with overall health, including cancer risk. Moreover, its composition is not fixed, but can be influenced by several dietary components. Dietary modifiers, including the consumption of live bacteria (probiotics), nondigestible or limited digestible food constituents such as oligosaccharides (prebiotics) and polyphenols, or both (synbiotics), are recognized modifiers of the numbers and types of microbes and have been reported to reduce colon cancer risk experimentally. Microorganisms also have the ability to generate bioactive compounds from food components. Examples include equol from isoflavones, enterodiol and enterolactone from lignans, and urolithins from ellagic acid, which have also been demonstrated to retard experimentally induced cancers. The gastrointestinal microbiota can also influence both sides of the energy balance equation; namely, as a factor influencing energy utilization from the diet and as a factor that influences host genes that regulate energy expenditure and storage. Because of the link between obesity and cancer incidence and mortality, this complex relationship deserves greater attention. Thus, a complex interrelationship exists between the intestinal microbiota and colon cancer risk which can be modified by dietary components and eating behaviors.
Keywords: prebiotics, probiotics, microbiota, colon cancer
Microbes and Colon Cancer
The adult human gut is estimated to contain 100 trillion microbial organisms, collectively referred to as the microbiota [1,2]. The human microbiota is known to be dominated by strict anaerobes including Bacteriodes, Eubacterium, Bifidobacterium, Fusobacterium, Peptostreptococcus, and Atopobium [3]. Facultive anaerobes occur in numbers approximately 1000-fold lower and include lactobacilli, enterococci, streptococci and Enterobacteriaceae [4]. More than 500 different bacterial species may be present in the normal commensal microbiota, although the exact number and the variability among individuals remains an area of investigation [5]. Advances in defining the quality, quantity, and physiologic activity of the intestinal microbiota have occurred as a result of the conversion from culture-based techniques to metagenomics, an emerging field in which the power of genomic analysis (the analysis of the entire DNA in an organism) is applied to entire communities of microbes. A benefit of this approach is elimination of isolating and culturing individual microbial species. One limitation is that stool and mucosal community populations differ [6,7]. Thus, the analysis of the bacteria in the stool probably does not always reflect that in early parts of the gastrointestinal tract.
A complex dynamic relationship between the host and the gastrointestinal bacteria occurs shortly after birth [8]. The microbiota diversifies as a function of age to form an intestinal microbiota that is unique for each individual [8]. Several findings suggest that themicrobial cohort remains relatively constant once adulthood is reached; however, the composition of the resident biota may alter as a result of environmental factors such as diet and antibiotic usage [9].
The colonic microflora has been suggested to have a critical role in setting the tone for a healthy bowel including the risk for developing colorectal cancer [10]. Key physiological functions that might be related to cancer risk include control of epithelial cell proliferation and differentiation, production of essential nutrients and/or bioactive food components, prevention of overgrowth of pathogenic organisms, and stimulation of intestinal immunity [11].
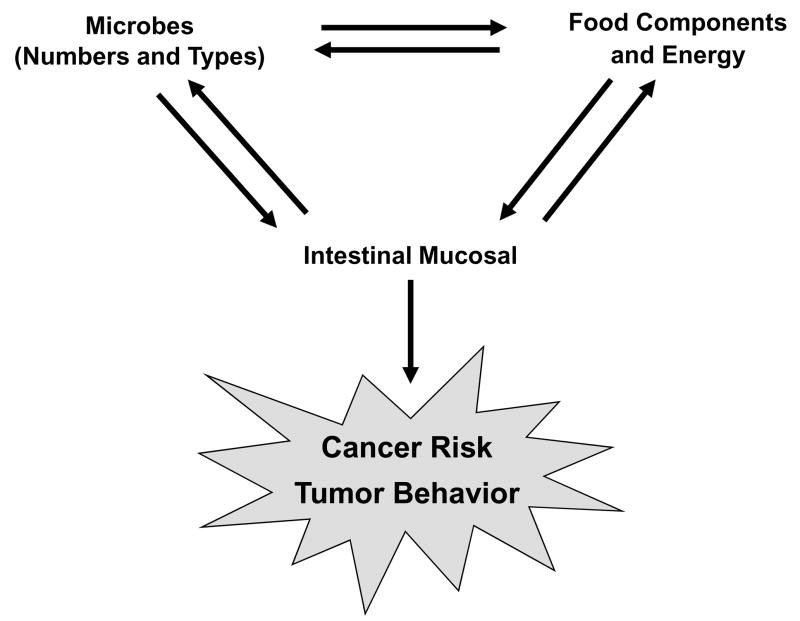
Thus, microbes may influence multiple processes associated with a change in cancer risk. This review provides an overview of the interrelationship of this association as influenced by dietary exposures (Figure 1).
Figure 1.
A dynamic relationship exits among the gastrointestinal microbiota, the intake and metabolism of dietary bioactive food components and energy, and the intestinal mucosal cells. Both the numbers and types of microbes and dietary factors can influence colon cancer risk and tumor behavior. Genomics within the microbes and the mucosal cells can influence the direction and/or magnitude of this relationship.
Yin-Yang and Microbes
The microflora within the large intestine is provided an opportunity to ferment a range of dietary substances that are not completely digested and absorbed in the small intestine. The two main types of anaerobic fermentation that are carried out in the gastrointestinal tract are carbohydrate and proteolytic [12]. A yin-yang occurs since the main end products of carbohydrate metabolism are thought to be positive while those associated with proteins may be negative. Carbohydrate fermentation produces microbially generated short chain fatty acids (butyrate, acetate and propionate) which can be further metabolized by mammalian cells for energy [12]. In contrast, end products of proteolytic fermentation including phenolic compounds, amines, ammonia, N-nitroso compounds and indoles, can be toxic to the host [12].
Specific strains of bacteria have been implicated in the pathogenesis of cancer, including Streptococcus bovis, Bacteriodes, Clostridia, and Helicobacter pylori [13–16]. Conversely, some strains of bacteria, including Lactobacillus acidophilus and Bifidobacterium longum, have been shown to inhibit carcinogen-induced colon tumor development [17,18]. Thus, a balance between “detrimental” and “beneficial” bacteria has implications in setting the stage for cancer. Shifting the proportion of microbes has been reported to influence carcinogen bioactivation and thus cancer risk. It is increasingly apparent that dietary components can significantly modify this balance.
Interactions between microbes and the genetics of cells lining the intestinal mucosa may also dictate the overall response. Thus, animal models should provide an important tool for characterizing the role of bacteria in understanding the diet-cancer paradigm. There are a number of genetically engineered models of intestinal cancer, such as interleukin-10 and Muc2 knockout mice [19,20], and TCRβ and p53 double knockout mice [21], which when exposed to germ-free conditions appear normal, but when intestinal bacterial colonization is promoted, spontaneously develop intestinal inflammation which is followed by tumors. These studies also suggest that bacterial modulation of intestinal inflammation may be one mechanism whereby the gut microflora may contribute to colorectal carcinogenesis.
Diet influences the amount and strains of gastrointestinal microorganisms
A. Prebiotics
A prebiotic is a nondigestible food ingredient whose beneficial effects on the host result from the selective stimulation of growth and/or activity of the gut microbiota, particularly lactobacilli and bifidobacteria [22]. Most of the attention in this area has been aimed at nondigestible oligosaccharides [23]. Common prebiotics include inulin, other oligosaccharides, lactulose and resistant starch [22]. Dietary fiber has also been shown to convey a prebiotic response [22].
Inulin occurs naturally in several foods such as leek, asparagus, chicory, Jerusalem artichoke, garlic, artichoke, onion, wheat, banana, oats and soybeans [23]. However, these may not be biologically significant sources because Manning and Gibson [10] estimate that an individual would need to consume 4–8 g/day of fructooligosaccharide to significantly (about one log10 value) elevate bifidobacteria in the human gut. A functional food approach has been utilized to add inulin to more frequently consumed products, such as cereals, biscuits, infant foods, yogurts breads and drinks, at concentrations at which a prebiotic effect may occur [23]. There are also a number of dietary supplements which contain fructooligosachharides, primarily inulin, that are commercially available.
In a double-blind, placebo-controlled, cross-over trial, consuming 30 grams isomalt (a mixture of the polyols 1-O-∀-D—glucopyranolsyl-D-manitol and 6-O-∀-D-glucopyranosul-D-sorbitol) per day for 4-weeks led to a 65% increase in the proportion of bifidobacteria and a 47% increase in total bifidobacteria cell counts compared to feeding sucrose [24]. In another study in which 12 volunteers ingested 10 g inulin/day for 16 days in comparison to a control period without any supplement intake, Bifidobacterium adolescentis showed the strongest response, increasing from 0.89 to 3.9% of the total microbiota [25].
B. Probiotics
In contrast to prebiotics, probiotics are provided in processed foods or in dietary supplements as live bacteria. Yogurt is the most common probiotic-carrying food; however, cheese, fermented and unfermented milks, juices, smoothies, cereal, nutrition bars, and infant/toddler formula all are vehicles for probiotic delivery. The main probiotic supplements on the market utilize lactobacilli, streptococci and bifidobacteria, which are normal constituents of the human gastrointestinal microflora. However, studies are also investigating potential probiotic roles of other microbes such as yeast (Saccharomyces boulardii), which are not normally found in the gastrointestinal tract [26,27]. Probiotic microorganisms do not act exclusively in the large intestine by affecting the intestinal flora but also affect other organs, either by modulating immunological parameters, intestinal permeability and bacterial translocation, or by providing bioactive metabolites [28].
A number of studies with a variety of probiotic strains have been conducted to determine the extent to which probiotics colonize the gastrointestinal tract. These studies have been reviewed by Cothesy et al. [29] and reveal that ingested strains do not become established members of the normal microbiota but may persist only during periods of dosing or for relatively short periods afterwards. Undeniably, greater attention is needed about the most beneficial probiotics and their optimal quantity and exposure duration needed for health promotion.
The combination of a probiotic with a prebiotic to support its viability and activity has been termed a synbiotic [30]. Evidence suggests that synbiotics may be efficacious in altering the composition of the microbiota. For example, the synbiotic combination of a specific oligofructose-enriched inulin (SYN1) and Lactobacillus rhammnosus GG and Bifdobacterium lactis Bb12 for 12 weeks caused a 16% and 18% increase in the numbers of Lactobacillus and Bifidobacterium, respectively, and a 31% decrease in the numbers of Clostridium perfringens [31]. Recent in vitro studies have demonstrated that synbiotics were more effective than prebiotics or probiotics in modulating the gut microflora [32]. These findings need to be documented in well controlled human intervention studies.
The gut microbiota may mediate the effects of diet as a modifier of colon cancer risk. An increase in the number of bifidobacteria and/or lactobacilli resulting from the use of probiotics, prebiotics or synbiotics has been demonstrated to protect against chemically induced colonic DNA damage in animal models [33]. Interestingly, several strains of lactobacilli and bifidobacteria were effective in protecting rats from this DNA damage, as measured by the Comet assay [34]. Rowland et al [18] reported that in rats inoculated with human flora and fed a diet containing lactulose compared to those fed a diet containing a comparable amount of sucrose that their colonoctyes had less DNA damage following oral treatment with dimethylhydrazine [18]. More recently, another plausible mechanism has surfaced in the synbiotic combination of resistant starch, Lactobacillus acidophilus and Bifidobacterium lactis [34]. These investigations identified enhanced apoptosis of carcinogen-damaged cells in rat colon by the combination treatment [34]. In contrast, the probiotics provided no protection when a low resistant starch diet was fed and the resistant starch had no protective response in the absence of the probiotic [35].
In addition to a potential role in the prevention of cancer, probiotics have also been suggested to enhance the immune system and inhibit the growth of existing tumors [36]. For example, probiotics containing lactic acid bacteria increased the survival rate of mice injected with tumor cells which correlated with an increase in cellular immunity as reflected an increase in the number of total T cells, NK cells and MHC class II+ cells, and CD4-CD8+ T cells [37]. Moreover, peptidoglycan from a lactobacillus species produced a dose-dependent reduction in the growth of CT26 colon cancer cells in mice via increased apoptosis but had no effect on apoptosis of these cells in vitro, suggesting that the in vivo anti-tumorigenic effect may have been mediated by an immune response [38].
C. Combined Response
Providing prebiotics, probiotics, or a combination is known to inhibit aberrant crypt foci (ACF), a preneoplastic lesion for colon cancer. For example, rats fed a high-fat and low-fiber diet supplemented daily with the probiotic B. polyfermenticus (3 × 108 cfu/1.3g) had a 50% reduction in ACF formation compared to rats fed the control diet [39]. Similarly, several studies have found that adding a relative large amount of inulin (10%) to a diet reduced ACF [40–42]. Synbiotics may be particularly efficacious for reducing colonic preneoplastic lesions based on studies by Rowland et al. [40]. They found that the combination of inulin and Bifidobacterium longum decreased ACF formation by 80% whereas inulin alone decreased ACF by 41% and Bifidobacterium longum alone decreased ACF by 26%. Studies in experimental animals have also suggested that prebiotics are protective against tumor development. For example, fruco-oligossacharides reduced the occurrence of colon tumors in Min mice, a genetic model of human colon cancer [43].
Probiotics and synbiotics have also been found to be efficacious against risk factors for colon cancer in humans. A four-year study of 398 subjects found that Lactobacillus casei decreased the recurrence of atypical colonic polyps [44]. A human clinical trial was recently conducted to examine the effect of a synbiotic product containing the probiotic strains Lactobacillus rhamnosus GG and Bifidobacterium lactis Bb12 and the prebiotic inulin or a placebo (maltodextrose) on biomarkers of colon cancer risk in 37 colon cancer patients and 43 polypectimized patients [31,45]. The synbiotic treatment of polyp patients was most effective in reducing DNA damage, coloncyte cell proliferation and fecal water genotoxicity (used as a biomarker for colon cancer risk) [46]. Synbiotic consumption prevented an increased secretion of interleukin-2 by peripheral blood mononuclear cells and increased the production of interferon in the cancer patients [31]. These results suggest that synbiotics can reduce multiple factors associated with colon cancer risk in humans.
D. Other Dietary Modifiers
Several dietary components, other than complex carbohydrates, may modulate the microbiome. When bacteria are cultured with various polyphenols that occur in tea the growth of certain pathogenic bacteria such as Clostridum perfringens and Bacteroides was significantly repressed, while commensal anaerobes like Bifidobacterium and Lactobacillus were affected less [46]. Interestingly adding bacterial metabolites of the tea polyphenols was found to lead to a similar response. To date several polyphenols (caffeic acid, catechnin, epicatechin, coumaric acid, phloridzin, rutin, naringenin, daidzein, genistein and quercetin) have been demonstrated to inhibit the growth and adhesion of bacterial pathogens to human Caco-2 cells, and to enhance the proliferation and adhesion of a probiotic, L. rhamnosus [47]. Providing wine polyphenols (57 mg/kg body weight by gavage for 10 days) resulted in predominantly fecal Bacteroides, Lactobacillus and Bifidobacterium in rats compared to the controls which had predominantly Bacteroides, Clostridium and Propionibacterium [48]. It remains to be determined whether wine consumption or consumption of other polyphenols results in a similar effect in humans.
A host of food constituents have been reported to have bactericidial properties [49]. Among the plants that killed H. pylori, turmeric was the most efficient, but ginger, chili, black caraway, oregano and licorice were also bactericidial. It remains unclear if these agents have physiological importance in modulating the number and types of microorganisms in the gastrointestinal tract following traditional exposures.
Diet can also influence cancer risk by modifying microbial metabolism
Bacterial transformation of dietary components and other chemicals in the intestinal lumen are associated with the production of carcinogenic agents and may therefore be another mechanism whereby the gut microflora may influence cancer risk. Microbial enzymes including nitroreductases, azoreductases, hydrolases and β-glucuronidase, can convert inactive compounds to active metabolites which may exert adverse effects. For example, β-glucoronidase hydrolyzes glucuronic acid conjugates of heterocyclic amines (carcinogens formed in food during cooking), to form reactive metabolites which can damage the colonic mucosal cells [50].
Evidence has revealed the potential of probiotics, prebiotics and synbiotics to reduce toxic metabolite production in the gut. In a study using a synbiotic mix of Bifidobacterium longum and dietary inulin (5% w/w), human fecal associated rats fed the active diets had 55% lower fecal β-glucoronidase activity and 30% lower ammonia concentrations than the control rats [37]. Furthermore, the synbiotic mix was more efficacious than either probiotic or prebiotic alone [37]. Mice fed yogurt had reduced β-glucoronidase and nitroreductase activities [51]. Similarly, in 36 humans fed lactulose twice daily (2 × 10 g/day) for 4 weeks, there was a significant reduction in fecal azoreductase, 7α-dehydroxylase, β-glucoronidase, nitroreductase and urease activities, as well as a reduction in fecal concentrations of cresol, indole, phenol and skatol compared to when they were fed a placebo [52].
Some polyphenol containing dietary components may also influence bacterial metabolizing enzymes and thus influence overall cancer risk. For example, resveratrol supplementation (8 mg/kg body weight/day, intragastrically) significantly reduced activities of fecal and host colonic mucosal enzymes, such as β-glucoronidase, β-glucosidase, β-galactosidase, mucinase, and nitroreductase activities (21%, 45%, 37%, 41% and 26% respectively) compared to control animals [53]. The reduced bacterial enzyme activity was associated with a significant reduction in colonic tumor incidence in the resveratrol fed compared to control rats [53].
The mechanism(s) accounting for these food related alteration in bacterial and host enzymes are not currently known. While these observations are intriguing, it remains to be determined if these changes are a result of modifications of enzymatic activity within a subpopulation of microorganisms or a change in the proportion of specific bacteria. Regardless, they are another mechanism whereby dietary components can interact with the microbiota to influence colon cancer risk.
Bacteria can influence cancer risk by modifying metabolism of dietary components
Bacteria may also generate new metabolites, which are more biologically active, from dietary components (Table 1). For example, short chain fatty acids, which are formed from the bacterial fermentation of indigestible carbohydrates, are nutrients and growth signals for the intestinal epithelium and may play a role in colon cancer prevention [5]. Butyrate is the most widely studied of these short chain fatty acids and the preferred energy source of colonocytes. In normal colonocytes, butyrate prevents apoptosis and subsequent mucosal atrophy [54,55]. In contrast, in colon carcinoma cells, butyrate has been shown to stimulate differentiation, inhibit cell proliferation, induce apoptosis and inhibit angiogenesis [56–58]. Additionally, butyrate protects human colon cells from DNA damage [59]. At a molecular level, butyrate has been shown to affect gene expression via the phosphorylation and acetylation of histone proteins, particularly H3 and H4 [60]. Hyperacetylation of histones disrupts ionic interactions with the adjacent DNA backbone, creating less densely packed chromatin, or euchromatin, and allows transcription factors to activate specific genes.
Table 1.
Bacterial metabolites from dietary components with cancer preventive properties
| Dietary Component | Food Sources | Bacterial Metabolite | References |
|---|---|---|---|
| Fiber | Grains/grain products | Butyrate | 54–63 |
| Linoleic acid | Vegetable oils | Conjugated linoleic acid | 64–68 |
| Daidzein | Soy | Equol | 69–80 |
| Secoisolariciresinol | Flaxseed, sesame | Enterolactone, Enterodiol | 81–88 |
| Isoxanthohumol | Hops/hop-derived products such as beer | 8-Prenylnaringenin | 89–92 |
| Ellagic acid | Strawberries, raspberries, walnuts, pomegranates | Urolithins A and B | 93–95 |
Human and animal studies of butyrate production and cancer risk are difficult to perform. This difficulty stems from dietary butyrate being fully absorbed in the small intestine; whereas colonic butyrate is endogenously produced by bacterial fermentation of luminal carbohydrates [61,62]. Nevertheless, animal studies have shown that the production of short chain fatty acids correlates with bacterial modulation of colonocyte proliferation, differentiation and apoptosis [61]. Furthermore, luminal delivery of butyrate at high concentrations appears to reduce aberrant crypt formation by 45% compared to untreated rats [62]. In humans, the relationship between luminal butyrate exposure and colorectal cancer risk has only been examined indirectly in case-control studies, by measuring fecal butyrate concentrations. Unfortunately this may not accurately reflect colonic butyrate exposure [63]. Future studies are needed which focus on understanding how different types of dietary fiber influence colonic butyrate production, the influence of age and stage of the cancer process as a variables, and better ways to assess luminal butyrate exposure.
In addition to butyrate, bacteria are also involved in the formation of another group of beneficial fatty acids; namely conjugated linoleic acids (CLA). These are a group of isomers of linoleic acid possessing anti-inflammatory and cancer protective properties [64]. Several studies have investigated the conversion of linoleic acid to CLA when incubated with various strains of lactobacilli and bifidobacteria [65,66]. A combination of probiotic bacteria has been shown to convert linoleic acid to CLA, decreasing cancer cell viability and inducing apoptosis [64]. One isomer, 9t,11t-CLA, inhibits the development of carcinogen-induced ACF in rat [67] and polyp number in Min mice [68].
One of the most abundant isoflavones in soy, daidzein, is differentially metabolized to equol and O-desmethylangolensin (DMA) by gut microflora in humans [69]. Recent investigations suggest a consortium of bacteria may be involved in equol production [70], and the bacteria responsible for equol production differ from the bacteria responsible for DMA production. Equol and DMA have been detected in a variety of body fluids, including blood, urine, feces, prostatic fluid and breast tissue [71,72]. Equol and DMA have been shown to bind to human estrogen receptors α and β with a greater affinity than the parent compound, daidzein [73,74]. Furthermore, in studies that have assessed estrogen receptor-dependent transcription of β-galactosidase in transfected yeast assays, equol induced transcription to a greater extent than daidzein, in yeast carrying estrogen receptor α or β [75]. Therefore, because equol mediates many of its biological effects by binding to the estrogen receptors, in vitro studies suggest that equol is more biologically active than daidzein.
The capacity to form equol, which is present in approximately 30–40% of humans, is positively correlated with an abundance of sulfate-reducing bacteria and negatively with Clostrium coccoides-Eubacterium rectale counts [76]. Furthermore, individuals with a higher PUFA and alcohol intake were more likely to be strong equol producers [76]. An individual’s ability to produce equol appears to be relatively stable over time. A 2-month intervention with a synbiotic capsule containing a total of 109colony-forming units of Lactobacillus acidophilus and Bifidobacterium longum and 10–15 mg fructooligosaccharidedid not significantly alter equol production or plasma hormone concentrations in premenopausal women [77] or in men [78]. Similarly, equol excretion was not altered after soy protein or wheat bran consumption [79,80]. Data such as this suggests that equol production is quite consistent in most individuals and the primary determinant is the occurrence of selected microbes. Why these exist in some individuals and not in other remains to be resolved.
Besides daidzein, other plant components can be metabolized by intestinal bacteria to cancer protective compounds. For example, plant lignans can be converted to the mammalian lignans, enterodiol and enterolactone by the intestinal microbiota. In contrast to the bacterial production of equol, which only occurs in about one-third of the population, the conversion of secoisolarciresinol to enteroldiol and enterolactone occurs in most individuals [81]. Eleven bacterial species involved in the metabolism of secoisolariciresinol diglucoside have been isolated from human feces or obtained from bacterial culture collections [82]. Flaxseed is the richest source of lignan precursors in the typical human diet [83]. However, the total plant lignan concentration in sesame seed (2180 μmol/100 g) was higher than in flaxseed (820 μmol/100 g) [84]. In vitro fermentation with human fecal inoculums demonstrate that sesamin can be converted to lignans suggesting that sesame seed may also be a rich dietary source in humans [84]. Gut microbial metabolites of plant lignans may also have beneficial effects against colon cancer. Elevated plasma concentrations of enterolignans, in particular, enterodiol, were associated with a significant reduction in colorectal adenoma risk in a case control study [85]. Enterolactone has been reported to induce apoptosis and inhibit growth of Colo201 human colon cancer cells in culture and following transplantation into athymic mice [86]. Similarly, SW480 cell growth is inhibited in a dose- and time-dependent manner by enterolactone and enterodiol [87]. Feeding the lignans matairesinol and secoisolariciresinol to Min mice, increased plasma concentrations of enterolactone and enterodiol but did not inhibit intestinal tumorigenesis [88]. In contrast, secoisolariciresinol diglycoside concentrations from wheat bran from four selected wheat cultivars correlated with the cancer protective effects in Min mice, suggesting that secoisolariciresinol diglycoside may contribute to the cancer preventive effects of wheat bran [88]. The reasons for these inconsistencies are unclear but warrant additional examination.
Prenylfavonoids including xanthohumol, isoxanthohumol and 8-prenylnaringenin (8-PN), are found in hops and hop-derived products such as beers [89]. 8-PN is formed by bacterial metabolism of isoxanthohumol and is one of the most potent phytoestrogens [90]. In contrast, 8-PN is less efficacious than xanthohumol in inhibiting growth of colon cancer cell lines [91]. Recently, it was shown that intestinal 8-PN production only occurs in one-third of humans, and it is clear that substantial interindividual differences exist in the production of this active metabolite, which may be associated with differences in health benefits [90]. Brunelli et al. [92] provided evidence that 8-PN inhibits epidermal growth factor-induced MCF-7 breast cancer cell proliferation by targeting phosphatidylinositol-3-OH kinase activity.
Ellagic acid, a polyphenol which is present in many foods including strawberries, raspberries, walnuts and pomegranates, has been reported to show a multitude of biological properties including antioxidant and cancer protective activitities [93]. Ellagic acid is metabolized by human colonic microflora to yield urolithins A and B [94]. These urolthins have been shown to exert both estrogenic and antiestrogenic activities. Both urolithins A and B showed estrogenic activity in a dose-dependent manner even at high concentrations (40 microM), without antiproliferative or toxic effects towards MCF-7 breast cancer cells. They also exhibit antiestrogenic activity by antagonizing the growth promoting effect of estradiol in a dose-dependent manner [94]. Similar to equol, the production of urolithins has been hypothesized to depend on the microflora. Large interindividual variability in production has been reported and the reason remains poorly understood [94]. The bacteria responsible for the production of urolithins remain to be characterized. The variability was demonstrated in a human supplementation study: when 10 volunteers consumed 25 g fresh strawberries, excretion of urolithin B derivatives ranged from 0.05 to 6.3% [95]. When they consumed 35 g of walnuts, the excretion ranged from 1.2 to 81%. Consuming 300 ml of oak-aged red wine caused a range of excretion from 1.8 to 7.4% [95]. The potential biological effects for this cancer protective dietary compound may also be different among individuals depending on their microflora.
Metabolism by gut microflora may also influence tissue exposure to higher-molecular-weight polyphenols including proanthocyanidins or oxidized polymeric polyphenols, which are poorly absorbed in the proximal part of the gastrointestinal tract. These polyphenols are abundant in wine, tea, chocolate and many fruits [96]. A major fraction of the polyphenols present in the plasma and excreted in urine of rats fed red wine polyphenols are aromatic acid metabolites formed in the gut [97]. Incubating an anothocyanin extract from Cabernet Sauvignon grapes with the contents of the large intestine of pigs, after 6 hours results in a loss of the parent compound but the generation of three identifiable metabolites [98]. It is possible that these metabolites offer the protective effect against colon cancer, such as decreased carcinogen-induced aberrant crypt formation, colonic cell proliferation and oxidative DNA damage, which have been attributed to anthocyanin consumption [99]
The dynamic relationship between obesity and the gut microbiota: another link to cancer?
Obesity has been linked with both cancer incidence and mortality [100]. Recent evidence suggests that the gut microbiota affects nutrient acquisition and energy regulation; it further suggests that obese and lean people have a different microbiota [101–105]. Investigators have used genetic sequencing to identify the different strains of bacteria in the gut of 12 obese individuals and compared them with five lean volunteers [103]. Obese individuals had more Firmicutes and nearly 90% less Bacteroidetes than the lean individuals. Furthermore, when obese volunteers consumed a low-fat or low-carbohydrate diet for one year and lost as much as 25% of their body weight, the proportion of Firmicutes in their colon dropped and that of the Bacteroidetes rose. However, the levels of the two types of bacteria never reached those of the group that was lean in the beginning [103].
Differences in fecal microbiota of infants (6 and 12 months) have been associated with the risk of being overweight or obese 7 years of age [106]. Children of normal weight had higher Bifidobacterial and lower Staphylococcus aureus concentrations at ages 6 and 12 months than did children who became overweight/obese [106]. These results suggest that differences in the microbiota precede overweight/obesity. Future work is needed to determine whether manipulation of the gut microbial community could be an approach for the treatment and/or prevention of obesity.
Conventionally reared mice have a 40% higher body fat content and 47% higher gonadal fat content than germ-free mice even though they consume less food than their germ-free counterparts [101]. Furthermore, when the distal gut microbiota from the normal mice was than transplanted into the gnotobiotic mice, there was a 60% increase in body fat within 2 weeks without any increase in food consumption or obvious differences in energy expenditure. These results support the hypothesis that the microbiota affects the amount of energy extracted from the diet. Mechanistic studies revealed that the transplanted microbiota not only increased caloric release from dietary plant polysaccharides with glycosidic linkages that the host is ill-equipped to cleave with its own complement of glycoside hydrolases, but also modulates host genes that affect energy deposition in adipocytes including fasting-induced adipocyte factor (Fiaf) [101]. Fiaf is a circulating lipoprotein lipase inhibitor and its suppression is essential for the microbiota-induced deposition of triglycerides in adipocytes. These findings suggest that the composition of the gut microbial community may affect the amount of dietary energy that is extracted [101].
Similar to humans, mice that are genetically obese (ob/ob) have a higher proportion of intestinal Firmicutes and 50% fewer Bacteroidetes than their lean siblings [102]. When germ-free mice were colonized with either the microbiota from obese (ob/ob) or lean (+/+) littermates, the mice given the microbiota from obese mice extracted more calories from their food and had a significantly greater increase in total body fat than in mice colonized with the microbiota from lean mice (mean percent of fat gain, 47% versus 27%; representing a difference of 4 kcal/g or 2% of total calories consumed) [103]. These data suggest that differences in the efficiency of caloric extraction from food may be determined b the microbiota, further suggesting a microbial component in the pathogenesis of obesity.
In contrast to mice with a gut microbiota, germ-free animals are protected against the obesity that develops after consumption of a Western-style, high fat, sugar-rich diet [104]. Their continuously lean phenotype is associated with increased skeletal muscle levels of AMP-activated protein kinase and its downstream targets involved in fatty acid oxidation such as acetyl CoA carboxylase and carnitine-palmitoyl transferase [105]. Moreover, germ-free knockout animals lacking Fiaf are not protected from diet-induced obesity because of reduced expression of genes involved in fatty acid oxidation [105]. These findings suggest that the gut microbiota can influence both sides of the energy balance equation; namely, as a factor that influences energy utilization from the diet and as a factor that affects host genes that regulate how energy is expended and stored [105]. It is not currently known whether the microbiota has a similar effect on energy utilization and gene expression patterns in humans.
Conclusion
A complex interrelationship exists between the intestinal microbiota and colon cancer risk which can be modified by dietary behavior. Not only can eating behaviors modify the numbers and types of microoganisms, but microorganisms can also generate new compounds from food components some of which can be beneficial while others may be harmful. Many of the specific bacteria, as well as microbially generated metabolites, may have a role in cancer risk or development. More in depth studies investigating the interrelationships among intestinal bacteria, diet and cancer risk are desperately needed. Many unanswered issues remain including: a better understanding of how an individual’s genetic background influences their microflora; who might benefit from dietary interventions to alter their indigenous microflora; what are the microbially generated metabolites of bioactive food components; how can these be utilized to better understand their molecular targets/mechanisms for cancer prevention; and, can we identify inter-individual variability in the production of these metabolites? Once answers to these fundamental questions are available, it should be possible to develop specific dietary recommendations for cancer prevention based on modification of the composition or activities of the colon’s commensal microflora.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Savage DC. Microbial ecology of the gastrointestinal tract. Annu Rev Microbiol. 1977;31:107–33. doi: 10.1146/annurev.mi.31.100177.000543. [DOI] [PubMed] [Google Scholar]
- 2.Suau A, Bonnet R, Sutren M, Goddon JJ, Gibson GR, Collins MD, Dore J. Direct analysis of genes encoding 16S rRNA from complex communities reveals many novel molecular species within the human gut. Appl Environ Microbiol. 1999;65:4799–807. doi: 10.1128/aem.65.11.4799-4807.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tiaskalova-Hogenova H, Stepankova R, Hudcovic T, Tuckova L, Cukrowsak B, Lodinova-Zadnikova R, Kozakova H, Rossmann P, Bartova J, Sokol D, Funda DP, Borovska D, Rehakova Z, Sinkora J, Hofman J, Drastich P, Kokesova A. Commensal bacteria (normal microflora), mucosal immunity and chronic inflammatory and autoimmune disease. Inmmunol Lett. 2004;15:97–108. doi: 10.1016/j.imlet.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 4.Rastall RA. Bacteria in the gut: friends and foes and how to alter the balance. J Nutr. 2004;134:2022s–2026s. doi: 10.1093/jn/134.8.2022S. [DOI] [PubMed] [Google Scholar]
- 5.Mai V. Dietary modification of the intestinal microbiota. Nutr Rev. 2004;62:235–242. doi: 10.1301/nr2004.jun235-242. [DOI] [PubMed] [Google Scholar]
- 6.Vaughann EE, Schut F, Heilig HG, Zoetendal EG, de Vos WM, Akkermans AD. A molecular view of the intestinal ecosystem. Curr Issues Intest Microbiol. 2000;1:1–12. [PubMed] [Google Scholar]
- 7.Hope ME, Hold GL, Kain R, El-Omar EM. Sporadic colorectal cancer- role of the commensal microbiota. FEMS Microbiol Lett. 2001;244:1–7. doi: 10.1016/j.femsle.2005.01.029. [DOI] [PubMed] [Google Scholar]
- 8.Salminen S, Isolauri E. Intestinal colonization, microbiota and probiotics. J Pediatr. 2006;149:S115–S120. [Google Scholar]
- 9.Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, Gill SR, Nelson KE, Relman DA. Diversity of the human intestinal microbial flora. Science. 2005;308:1635–1638. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Keefe SJ. Nutrition and colonic health: the critical role of the microbiota. Curr Opin Gastronterol. 2008;24:51–58. doi: 10.1097/MOG.0b013e3282f323f3. [DOI] [PubMed] [Google Scholar]
- 11.Tappenden KA, Deutsch AS. The physiological relevance of the intestinal microbiota- contributions to human health. J Am Coll Nutr. 2007;26:679s–683s. doi: 10.1080/07315724.2007.10719647. [DOI] [PubMed] [Google Scholar]
- 12.Manning TS, Gibson GR. Microbial-gut interactions in health and disease. Prebiotics Best Pract Res Clin Gastroenterol. 2004;18:287–298. doi: 10.1016/j.bpg.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 13.Gold JS, Bayar S, Salem RR. Association of Steptoccocus bovis bacterium with colonic neoplasia and extracolonic malignancy. Arch Surg. 2004;139:760–765. doi: 10.1001/archsurg.139.7.760. [DOI] [PubMed] [Google Scholar]
- 14.Moore WE, Moore LH. Intestinal floras of populations that have a high risk of colon cancer. Appl Environ Microbiol. 1995;61:3202–3207. doi: 10.1128/aem.61.9.3202-3207.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakamura J, Kubota Y, Miyaoka M, Saitoh T, Mizuno F, Benno Y. Comparison of four microbial enzymes in Clostridia and Bacteroides isolated from human feces. Microbiol Immunol. 2002;46:487–490. doi: 10.1111/j.1348-0421.2002.tb02723.x. [DOI] [PubMed] [Google Scholar]
- 16.Peek RM, Blaser MJ. Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nat Rev Cancer. 2002;2:28–37. doi: 10.1038/nrc703. [DOI] [PubMed] [Google Scholar]
- 17.McCintosh GH, Royle PJ, Playne MJ. A probiotic strain of L. acidophilus reduces DMH-induced large intestinal tumors in male Sprague-Dawley rats. Nutr Cancer. 1999;35:153–159. doi: 10.1207/S15327914NC352_9. [DOI] [PubMed] [Google Scholar]
- 18.Rowland IR, Bearne CA, Fischer R, Pool-Zobel BL. The effect of lactulose on DNA damage induced by DMH in the colon of human flora-associated rats. Nutr Cancer. 1996;26(1):37–47. doi: 10.1080/01635589609514461. [DOI] [PubMed] [Google Scholar]
- 19.Berg DJ, Davidson N, Kuhn R, Muller W, Menon S, Holland G, Thompson-Snipes L, Leach MW, Rennick D. Enterocolitis and colon cancer in interleukin-10-deficient mice are associated with aberrant cytokine production and CD4(+) TH-1-like responses. J Clin Invest. 1996;98:1010–1020. doi: 10.1172/JCI118861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang W, Velcich A, Lozonschi I, Liang J, Nicholas C, Zhuang M, Bancroft L, Augenlicht LH. Inactivation of pw1WAF1/cip1 enhances intestinal tumor formation in Muc2-/- mice. Am J Pathol. 2005;166:1239–1246. doi: 10.1016/S0002-9440(10)62342-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kado S, Uchida K, Funabashi H, Iwata S, Nagata Y, Ando M, Onoue M, Matsuoka Y, Ohwaki M, Mortomi M. Intestinal microflora are necessary for development of spontaneous adenocarcinoma of the large intestine in T-cell receptor beta chain and p53 double-knockout mice. Cancer Res. 2001;61:2395–2398. [PubMed] [Google Scholar]
- 22.Lim CC, Ferguson LR, Tannock GW. Dietary fibres as “prebiotics”: implications for colorectal cancer. Mol Nutr Food Res. 2005;49:609–619. doi: 10.1002/mnfr.200500015. [DOI] [PubMed] [Google Scholar]
- 23.Kolinda S, Gibson GR. Prebiotic capcacity of inulin-type fructans. J Nutr. 2007;137:2503s–2506s. doi: 10.1093/jn/137.11.2503S. [DOI] [PubMed] [Google Scholar]
- 24.Gostner A, Blaut M, Schaffer V, Kozianowski G, Theis S, Klingeberg M, Dombrowski Y, Martin D, Ehrhardt S, Taras D, Schwiertz A, Kleessen B, Luhrs H, Schauber J, Dorbath D, Menzel T, Scheppach W. Effect of isomalt consumption on faecal microflora and colonic metabolism in healthy volunteers. Br J Nutr. 2006;95:40–50. doi: 10.1079/bjn20051589. [DOI] [PubMed] [Google Scholar]
- 25.Ramirez-Farias C, Slezak K, Fuller Z, Duncan A, Holtrop G, Louis P. Effect of inulin on the human gut microbiota: stimulation of Bifidobacterium adolescentis and Faecalibacterium prausnitzil Br. J Nutr. 2008;1:1–10. doi: 10.1017/S0007114508019880. [DOI] [PubMed] [Google Scholar]
- 26.Penner R, Fedorak RN, Madsen KL. Probiotics and nutraceuticals: non-medicinal treatments of gastrointestinal diseases. Curr Opin Pharmacol. 2005;5:596–603. doi: 10.1016/j.coph.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 27.NASPGHAN Nutrition Report Committee. Michail S, Sylvester F, Fuchs G, Issenman R. Clinical efficacy of probiotics: review of the evidence with focus on children. J Pediatr Gastroenterol Nutr. 2006;43:550–557. doi: 10.1097/01.mpg.0000239990.35517.bf. [DOI] [PubMed] [Google Scholar]
- 28.de Brese M, Schrezenmeir J. Probiotics, prebiotics, and synbiotics. Adv biochem Engin Biotechnol. 2008;111:1–66. doi: 10.1007/10_2008_097. [DOI] [PubMed] [Google Scholar]
- 29.Corthesy B, Gaskins HR, Mercenier A. Cross-talk between probiotic bacteria and the host immune system. J Nutr. 2007;137:781s–790s. doi: 10.1093/jn/137.3.781S. [DOI] [PubMed] [Google Scholar]
- 30.Gibson GR, Roberfroid MB. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr. 1995;125:1401–1412. doi: 10.1093/jn/125.6.1401. [DOI] [PubMed] [Google Scholar]
- 31.Rafter J, Bennett M, Caderni G, Clune Y, Hughes R, Karlsson PC, Klinder A, O’Riordan M, O’Sullivan GC, Pool-Zobel B, Rechkemmer G, Roller M, Rowland I, Salvadori M, Thijs H, Van Loo J, Watzl B, Collins JK. Dietary synbiotics reduce cancer risk factors in polypectomized and colon cancer patients. Am J Clin Nutr. 2007;85:488–496. doi: 10.1093/ajcn/85.2.488. [DOI] [PubMed] [Google Scholar]
- 32.Saulnier DMA, Gibson GR, Kolida S. In vitro effects of selected synbiotics on the human faecal microbiota composition. FEMS Microbiol Ecol. 2008:1–12. doi: 10.1111/j.1574-6941.2008.00561.x. [DOI] [PubMed] [Google Scholar]
- 33.Tuohy KM, Rouzaud GC, Bruck WM, Gibson GR. Modulation of the human gut microflora towards improved health using prebiotics-assessment of efficacy. Curr Pharm Des. 2005;11:75–90. doi: 10.2174/1381612053382331. [DOI] [PubMed] [Google Scholar]
- 34.Pool-Zobel BL, Neudecker C, Domizlaff I, Ji S, Schillinger U, Rumney C, Moretti M, Vilarini I, Cassellati-Sforzolini R, Rowland I. Lactobacillus- and bifidobacterium-mediated antigenotoxicity in the colon of rats. Nutr Cancer. 1996;26:365–380. doi: 10.1080/01635589609514492. [DOI] [PubMed] [Google Scholar]
- 35.Le Leu RK, Brown IL, Hu Y, Bird AR, Jackson M, Esterman A, Young GP. A synbiotic combination of resistant starch and Bifidobacterium lactis facilitates apoptotic deletion of carcinogen-damaged cells in rat colon. J Nutr. 2005;135:996–1001. doi: 10.1093/jn/135.5.996. [DOI] [PubMed] [Google Scholar]
- 36.Geier MS, Butler RN, Howarth GS. Probiotics, prebiotics and synbiotics: a role in chemoprevention of colon cancer? Cancer Biol Ther. 2006;5:1265–1269. doi: 10.4161/cbt.5.10.3296. [DOI] [PubMed] [Google Scholar]
- 37.Lee JW, Shin JG, Kim EH, Kang HE, Yim IB, Kim JY, Joo HG, Woo HJ. Immunomodulatory and antitumor effects in vivo by the cytoplasmic fraction of Lactabacillus casei and Bifidobacterium longum. J Vet Sci. 2004;5:41–48. [PubMed] [Google Scholar]
- 38.Sun J, Shi YH, Le GW, Ma XY. Distinct immune response induced by peptidoglycan derived from Lactobacillus sp. World J Gastroenterol. 2005;11:6330–6337. doi: 10.3748/wjg.v11.i40.6330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Park E, Jeon G-I, Park J-S, Paik H-D. A probiotic strain of Bacillus polyfermenticus reduces DMHinduced precancerous lesions in F344 male rat. Biol Pharm Bull. 2007;30:569–574. doi: 10.1248/bpb.30.569. [DOI] [PubMed] [Google Scholar]
- 40.Rowland IR, Rumney CJ, Coutts JT, Lievense LC. Effect of Bifidobacterium longum and inulin on gut bacterial metabolism and carcinogen-induced aberrant crypt foci in rats. Carcinogenesis. 1998;19:281–285. doi: 10.1093/carcin/19.2.281. [DOI] [PubMed] [Google Scholar]
- 41.Reddy BS, Hamid R, Rao CV. Effect of dietary oligofructose and inulin on colonic preneoplastic aberrant crypt foci inhibition. Carcinogenesis. 1997;18:1371–1374. doi: 10.1093/carcin/18.7.1371. [DOI] [PubMed] [Google Scholar]
- 42.Rao CV, Chou D, Simi B, Ku H, Reddy BS. Prevention of colonic aberrant crypt foci and modulation of large bowel microbial activity by dietary coffee fiber, inulin and pectin. Carcinogenesis. 1998;19:1815–1819. doi: 10.1093/carcin/19.10.1815. [DOI] [PubMed] [Google Scholar]
- 43.Pierre F, Perrin P, Champ M, Bornet F, Meflah K, Menanteau J. Short-chain fructo-oligosaccharides reduce the occurrence of colon tumors and develop gut-associeated lymphoid tissue in Min mice. Cancer Res. 1997;57:225–228. [PubMed] [Google Scholar]
- 44.Ishikawa H, Akedo I, Otani T, Suzuki T, Nakamura T, takeyama I, Ishiguro S, Miyaoka E, sobue T, Kakizoe T. Randomized trial of dietary fiber and Lactoacillus casei administration for prevention of colorectal tumors. Int J Cancer. 2005;116:762–767. doi: 10.1002/ijc.21115. [DOI] [PubMed] [Google Scholar]
- 45.Pool-Zobel BL. Inulin-type fructans and reduction in colon cancer risk: review of experimental and human data. Br J Nutr. 2005;93:S73–S90. doi: 10.1079/bjn20041349. [DOI] [PubMed] [Google Scholar]
- 46.Lee HC, Jenner AM, Low CS, Lee YK. Effect of tea phenolics and their aromatic fecal bacteria metabolites on intestinal microbiota. Res Microbiol. 2006;157:876–884. doi: 10.1016/j.resmic.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 47.Parkar SG, Stevenson DE, Skinner MA. The potential influence of fruit polyphenols on colonic microflora and human gut health. Int J Food Microbiol. 2008;124:295–298. doi: 10.1016/j.ijfoodmicro.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 48.Dolara P, Luceri C, Fillippl CD, Femia AP, Giovannelli L, Caderni G, Cecchini C, Silvi S, Orpianesi C, Cresci A. Red wine polyphenols influence carcinogenesis, intestinal microflora, oxidative damage and gene expression profiles of colonic mucosa in F344 rats. Mutat Res. 2005;591:237–246. doi: 10.1016/j.mrfmmm.2005.04.022. [DOI] [PubMed] [Google Scholar]
- 49.O’Mahoney R, Al-Khtheeri H, Weerasekera D, Fernando N, Vaira D, Holton B, Basset C. Bactericidal and anti-adhesive properties of culinary and medicinal plants against Helicobacter pylori. World J Gastroenerol. 2005;11:7499–7507. doi: 10.3748/wjg.v11.i47.7499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Humblot C, Lhoste E, Knasmuller S, Gloux K, Bruneau A, Bensaada M, Durao J, Rabot S, Andrieux C, Kassie F. Protective effects of Brussels sprouts, oligosaccharides and fermented milk towards 2-amino-3-methylimidazo[4,5-f]quinoline (IQ)-induced genotoxicity in the human flora associated F344 rat: role of xenobiotic metabolising enzymes and intestinal microflora. J Chromatogr B Analyt Technol Biomed Life Sci. 2004;802:231–237. doi: 10.1016/j.jchromb.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 51.de Moneo de, LeBranc A, Perdigon G. Reduction of beta-glucoronidase and nitroreductase activity by yoghurt in a murine colon cancer model. Biocell. 2005;29:15–24. [PubMed] [Google Scholar]
- 52.Ballongue J, Schumann C, Quignon P. Effects of lactulose and lacitol on colonic microflora and enzymatic activity. Scand J Gastroneterol Suppl. 1997;222:41–44. doi: 10.1080/00365521.1997.11720716. [DOI] [PubMed] [Google Scholar]
- 53.Sengottuvelan M, Nalini N. Dietary supplementation of resveratrol suppresses colonic tumour incidence in 1,2-dimethylhydrazine-treated rats by modulating biotransforming enzymes and aberrant crypt foci development. Br J Nutr. 2006;96:145–153. doi: 10.1079/bjn20061789. [DOI] [PubMed] [Google Scholar]
- 54.Wachtershauser A, Stein J. Rationale for the luminal provision of butyrate in intestinal diseases. Eur J Nutr. 2000;39:164–171. doi: 10.1007/s003940070020. [DOI] [PubMed] [Google Scholar]
- 55.Klampfer L, Huang J, Sasazuki T, Shirasawa S, Augenlicht L. Inhibition of interferon gamma signaling by the short chain fatty acid butyrate. Mol Cancer Res. 2003;1:855–862. [PubMed] [Google Scholar]
- 56.Zoran DL, Barhoumi R, Burghardt RC, Chapkin RS, Lupton JR. Diet and carcinogen alter luminal butyrate concentrations and intracellular pH in isolated rat colonocytes. Nutr Cancer. 1997;27:222–230. doi: 10.1080/01635589709514530. [DOI] [PubMed] [Google Scholar]
- 57.Basson MD, Liu YW, Hanly AM, Emenaker NJ, Shoney SG, Gould Rothberg BE. Identification and comparative analysis of human colonocyte short-chain fatty acid response genes. J Gastrintest Surg. 2000;4:501–512. doi: 10.1016/s1091-255x(00)80093-1. [DOI] [PubMed] [Google Scholar]
- 58.Pryde SE, Duncan SH, Hold GL, Stewart CS, Flint HJ. The microbiology of butyrate formation in the human colon. FEMS Microbiol Lett. 2002;17:133–139. doi: 10.1111/j.1574-6968.2002.tb11467.x. [DOI] [PubMed] [Google Scholar]
- 59.Ebert MN, Klinder A, Peters WH, Schaferhenrich A, Sendt W, Scheele J, Pool-Zobel BL. Expression of glutathione S-transferases (GSTs) in human colon cells and inducibility of GSTMs by butyrate. Carcinogenesis. 2003;10:1637–1644. doi: 10.1093/carcin/bgg122. [DOI] [PubMed] [Google Scholar]
- 60.Myzak MC, Dashwood RH. Histone deacetylases as targets for dietary cancer preventive agents: lessons learned with butyrate, diallyl sulfide and sulforaphane. Curr Drug Targets. 2006;7:443–452. doi: 10.2174/138945006776359467. [DOI] [PubMed] [Google Scholar]
- 61.Deschner EE, Ruperto JF, Lupton JR, Newmark HL. Dietary butyrate (tributyrin) does not enhance AOM-induced colon tumorigenesis. Cancer Lett. 1990;52:79–82. doi: 10.1016/0304-3835(90)90080-h. [DOI] [PubMed] [Google Scholar]
- 62.Valazquez OC, Rombeau JL. Butyrate. Potential role in colon cancer prevention and treatment. Adv Exp Med Biol. 1997;427:169–181. [PubMed] [Google Scholar]
- 63.Frankel WL, Zhang W, Singh A, Klurfeld DM, Don S, Sakata T, Modlin I, Rombeau JL. Mediation of the trophic effects of short chain fatty acids on the rat jejunum and colon. Gastrenterology. 1994;106:375–380. doi: 10.1016/0016-5085(94)90595-9. [DOI] [PubMed] [Google Scholar]
- 64.Wong CS, Sengupta S, Tjandra JJ, Gibson PR. The influence of specific luminal factors on the colonic epithelium: high-dose butyrate and physical changes suppress early carcinogenesis events in rats. Dis Colon Rectum. 2005;48:549–559. doi: 10.1007/s10350-004-0810-x. [DOI] [PubMed] [Google Scholar]
- 65.Sengupta S, Muir JG, Gibson PR. Does butyrate protect from colorectal cancer? J Gastroenterol Hepatol. 2006;21:209–218. doi: 10.1111/j.1440-1746.2006.04213.x. [DOI] [PubMed] [Google Scholar]
- 66.Ewaschuk JB, Walker JW, Diaz H, Madsen KL. Bioproduction of conjugated linoleic acid by probiotic bacteria occurs in vitro and in vivo in mice. J Nutr. 2006;136:1483–1487. doi: 10.1093/jn/136.6.1483. [DOI] [PubMed] [Google Scholar]
- 67.Yasui Y, Suzuki R, Kohno H, Miyamoto S, beppu F, Hosokawa M, Miyashita K, Tanaka T. 9trans, 11trans conjugated linoleic acid inhibits the development of azoxymethane-induced colonic aberrant crypt foci in rats. Nutr Cancer. 2007;59:82–91. doi: 10.1080/01635580701419055. [DOI] [PubMed] [Google Scholar]
- 68.Mandir N, Goodland RA. Conjugated linoleic acids differentially alter polyp number and diameter in the Apc(min+) mouse model of intestinal cancer. Cell Prolif. 2008;41:279–291. doi: 10.1111/j.1365-2184.2008.00524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Atkinson C, Frankenfeld CL, Lampe JW. Gut bacterial metabolism of the soy isoflavone daidzein: exploring the relevance to human health. Exp Biol Med. 2005;230:155–170. doi: 10.1177/153537020523000302. [DOI] [PubMed] [Google Scholar]
- 70.Decroos K, Vanhemmens S, Cattoir S, Boon N, Verstraete W. Isolation and characterization of an equol-producing mixed microbial culture from human faecal sample and its activity under gastrointestinal conditions. Arch Microbiol. 2005;183:45–55. doi: 10.1007/s00203-004-0747-4. [DOI] [PubMed] [Google Scholar]
- 71.Morton MS, Chan PS, Cheng C, Blacklock N, Matos-Ferreira A, Abranches-Monteiro L, Correia R, Lloyd S, Griffiths K. Lignans and isoflavonoids in plasma and prostatic fluid in me: samples from Portugal, Hong Kong; and the United Kingdom. Prostate. 1997;32:122–128. doi: 10.1002/(sici)1097-0045(19970701)32:2<122::aid-pros7>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 72.Maubach J, Bracke ME, Heyerick A, Depypere HT, Serreyn RF, Mareel MM, De Keukeleire D. Quantitation of soy-derived phytoestrogens in human breast tissue and biological fluids by high performance liquid chromatography. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;784:137–144. doi: 10.1016/s1570-0232(02)00789-4. [DOI] [PubMed] [Google Scholar]
- 73.Muthyala RS, Ju YH, Sheng S, Williams LD, Doerge DR, Katzenellenbogen BS, Belferich WG, Katzenellenbogen JA. Equol, a natural estrogenic metabolite from soy isoflavones: convenient preparation and resolution R- and S-equols and their differing binding and biological activity through estrogen receptors alpha and beta. Bioorg Med Chem. 2004;12:1559–1667. doi: 10.1016/j.bmc.2003.11.035. [DOI] [PubMed] [Google Scholar]
- 74.Kinjo J, Tsuschihashi R, Morito K, Hirose T, Aomori T, Nagao T, Okabe H, Nohara T, Masamune Y. Interactions of phytoestrogens with estrogen receptors alpha and beta (III). Estrogenic activities of soy isoflavones aglycones and their metabolites isolated from human urine. Biol Pharm Bull. 2004;27:185–188. doi: 10.1248/bpb.27.185. [DOI] [PubMed] [Google Scholar]
- 75.Moritor K, Hirose T, Kinjo J, Hirakawa T, Okawa M, Nohara T, Ogawa S, Inoue S, Muramatsu M, Masamune Y. Interaction of phytoestrogens with estrogen receptors alphas and beta. Biol Pharm Bull. 2001;24:351–356. doi: 10.1248/bpb.24.351. [DOI] [PubMed] [Google Scholar]
- 76.Bolca S, Possemiers S, Herragat A, Huybrechts I, Heyerick A, de Vriese S, Verbruggen M, Depypere H, De Keukeleire D, Bracke M, De Henauw S, Verstraete W, Van de Wioele T. Microbital and dietary factors are associated with the equol producer phenotype in healthy posmenopausal women. J Nutr. 2007;l132:2242–2246. doi: 10.1093/jn/137.10.2242. [DOI] [PubMed] [Google Scholar]
- 77.Bonorden MJ, Greany KA, Wangen KE, Phipps WR, Feirtag J, Adlercreutz H, Kurzer MS. Consumption of Lactobacillus acidophilus and Bifidobacterium longum do not alter urinary equol exretion and plasma reproductive hormones in premenopausal women. Eur J Clin Nutr. 2004;58:1635–1642. doi: 10.1038/sj.ejcn.1602020. [DOI] [PubMed] [Google Scholar]
- 78.McMullen MH, Hamilton-Reeves JM, Bonorden MJ, Wangen KE, Phipps WR, Feirtag JM, Kurzer MS. Consumption of Lactobacillus acidophilus and Bifidobacterium longum does not alter phytoestrogen metabolism and plasma hormones in men: a pilot study. J Altern Complement Med. 2006;12:887–894. doi: 10.1089/acm.2006.12.887. [DOI] [PubMed] [Google Scholar]
- 79.Lampe JW, Karr SC, Hutchins AM, Slavin JL. Urinary equol excretion with a soy challenge: influence of habitual diet. Proc Soc Exp Biol Med. 1998;217:335–339. doi: 10.3181/00379727-217-44241. [DOI] [PubMed] [Google Scholar]
- 80.Nettleton JA, Greany KA, Thomas W, Wangen KE, Adlecreutz H, Kurzer MS. Plasma phytoestrogens are not altered by probiotic consumption in postmenopausal women with and without a history of breast cancer. J Nutr. 2004;134:1998–2003. doi: 10.1093/jn/134.8.1998. [DOI] [PubMed] [Google Scholar]
- 81.Blaut M, Clavel T. Metabolic diversity of the intestinal microbiota: implications for health and disease. J Nutr. 2007;137:751s–755s. doi: 10.1093/jn/137.3.751S. [DOI] [PubMed] [Google Scholar]
- 82.Clavel T, Henderson G, Engst W, Dore J, Blaut M. Phylogeny of human intestinal bacteria that activate the dietary lignan secoisolariciresinal diglucoside. FEMS Microbiol Ecol. 2006;55:471–478. doi: 10.1111/j.1574-6941.2005.00057.x. [DOI] [PubMed] [Google Scholar]
- 83.Fletcher RJ. Food sources of phyto-estrogens and their precursors in Europe. Br J Nutr. 2003;89:539–543. doi: 10.1079/BJN2002795. [DOI] [PubMed] [Google Scholar]
- 84.Liu Z, Saarinen NM, Thompson LU. Sesamin is one of the major precursors of mammalian lignans in same seed (Sesamum indicum) as observed in vitro in rats. J Nutr. 2006;136:906–912. doi: 10.1093/jn/136.4.906. [DOI] [PubMed] [Google Scholar]
- 85.Kuijsten A, Arts IC, Hollman PC, van’t Veer P, Kampman E. Plasma enterolignans are associated with lower colorectal adenoma risk. Cancer Epidemiol Biomarkers Prev. 2006;15:1132–1136. doi: 10.1158/1055-9965.EPI-05-0991. [DOI] [PubMed] [Google Scholar]
- 86.Danbara N, Yuri T, Tsujita-Kyutoku M, Tsukamoto R, Uehara N, Tsubura A. Enterolactone induces apoptosis and inhibits growth of Colo 201 human colon cancer cells both in vitro and in vivo. Anticancer Res. 2005;25:2269–2276. [PubMed] [Google Scholar]
- 87.Qu H, Madi RL, Takemota DJ, Baybutt RC, Wang W. Lignans are involved in the antitumor activity of wheat bran in colon cancer SW480 cells. J Nutr. 2005;135:598–602. doi: 10.1093/jn/135.3.598. [DOI] [PubMed] [Google Scholar]
- 88.Pajari AM, Smeds AI, Oikarinen SI, Ecklund PC, Sjoholm RE, Mutanene M. The plant lignans matairesinol and seoisolariciresinol administered to Min mice do not protect against intestinal tumor formation. Cancer Lett. 2006;233:309–314. doi: 10.1016/j.canlet.2005.03.061. [DOI] [PubMed] [Google Scholar]
- 89.Bolca S, Possemiers S, Maervoet V, Huybrechts I, Heyerick A, Vervarcke S, Depypere H, De Keukeleir D, Bracke M, De Henauw S, Verstraete W, Van de Wiele T. Microbial and dietary factors associated with the 8-prenylnaringenin producer phenotype: a dietary intervention trial with fifty healthy post-menopausal Caucasian women. Br J Nutr. 2007;1:10. doi: 10.1017/S0007114507749243. [DOI] [PubMed] [Google Scholar]
- 90.Possemiers S, Bolca S, Eekhaut E, Hepypere H, Verstraete W. Metabolism of isoflavones, lignans and prenylflavonoids by intestinal bacteria: producer phenotyping and relation with intestinal community. Fems Microbiol Ecol. 2007:1–12. doi: 10.1111/j.1574-6941.2007.00330.x. [DOI] [PubMed] [Google Scholar]
- 91.Lee SH, Kim HJ, Lee JS, Lee IS, Kang BY. Inhibition of topoisomerase I activity and efflux drug transporters’ expression by xanthohumol from hops. Arch Pharm Res. 2007;11:1435–9. doi: 10.1007/BF02977368. [DOI] [PubMed] [Google Scholar]
- 92.Brunelli E, Pinton G, Chianale F, Graziani A, Appendino G, Moro L. *-Prenylnaringenin inhbits epidermal growth factor-induced MCF-7 breast cancer cell proliferation by targeting phosphatidylinositol-3-OH kinase activity. J Steroid Biochem Mol Biol. 2008 doi: 10.1016/j.jsbmb.2008.11.013. (Epub) [DOI] [PubMed] [Google Scholar]
- 93.Losso JN, Bansode RR, Trappey A, Bawadi HA, Truax R. In vitro anti-proliferative activities of ellagic acid. J Nutr Biochem. 2004;15:672–678. doi: 10.1016/j.jnutbio.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 94.Larrosa M, Gonzalez-Sarrias A, Garcia-Conesa MT, Tomas-Barberan FA, Espin JC. Urolithins, ellagic acid-derived metabolites produced by human colonic microflora, exhibit estrogenic and antiestrogenic activities. J Agric Food Chem. 2006;54:1611–1620. doi: 10.1021/jf0527403. [DOI] [PubMed] [Google Scholar]
- 95.Cerda B, Tomas-Barberan FA, Espin JC. Metabolism of antioxidant and chemopreventive ellagitannins from strawberries, raspberries, walnuts, and oak-aged wine in humans: identification of biomarkers an individual variability. J Agric Food Chem. 2005;53:227–235. doi: 10.1021/jf049144d. [DOI] [PubMed] [Google Scholar]
- 96.Sntos-Buelga C, Scalbert A. Proanthocyanidins and tannin-like compounds: nature, occurrence, dietary intake and effects on nutrition and health. J Sci Food Agric. 2000;80:1094–1117. [Google Scholar]
- 97.Gonthier MP, Cheynier V, Donovan JL, Manach C, Morand C, Mila I, Lapierre C, Remesy C, Scalbert A. Microbial aromatic acid metabolites formed in the gut account for a major fraction of the polyphenols excreted in urine of rats fed red wine polyphenols. J Nutr. 2003;133:461–467. doi: 10.1093/jn/133.2.461. [DOI] [PubMed] [Google Scholar]
- 98.Forester SC, Waterhouse AL. Identification of Cabernet Sauvignon anthocyanin gut microflora metabolites. J Agric Food Chem. 2008;56:9299–9304. doi: 10.1021/jf801309n. [DOI] [PubMed] [Google Scholar]
- 99.Lala G, Malik M, Zhao C, He J, Kwon Y, Giusti MM, Magnuson BA. Anothocanin-rich extracts inhibits multiple biomarkers of colon cancer in rats. Nutr. 2006;54:84–93. doi: 10.1207/s15327914nc5401_10. [DOI] [PubMed] [Google Scholar]
- 100.Calle EE, Rodriquez C, Walker-Thurmond K, Thun MJ. Overweight, obesity and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 101.Backhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, Semenkovich CF, Gordon JI. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci USA. 2004;101:15718–15723. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ley RE, Backhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad Sci USA. 2005;102:11070–11075. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 104.Bajzer M, Seeley RJ. Physiology: obesity and gut flora. Nature. 2006;444:1009–1010. doi: 10.1038/4441009a. [DOI] [PubMed] [Google Scholar]
- 105.Backhed F, Manchester JK, Semenkovich CF, Gordon JI. Mechanisms underlying the resistance of diet-induced obesity in germ-free mice. Proc Natl Acad Sci USA. 2007;104:979–984. doi: 10.1073/pnas.0605374104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kalliomaki M, Collado MC, Salminen S, Isolauri E. Early differences in fecal microbiota composition in children may predict overweight. Am J Clin Nutr. 2008;87:534–538. doi: 10.1093/ajcn/87.3.534. [DOI] [PubMed] [Google Scholar]