Abstract
Emerging evidence suggests that differences between microdialysis- and voltammetry-based estimates of extracellular dopamine in the brain might originate in the different penetration injury associated with each technique. To address this issue in a direct fashion, microdialysis probes and voltammetric microelectrodes were implanted in the rat striatum for 1, 4, or 24 hrs. Tissues were perfused with a suspension of fluorescently labeled nanobeads to assess blood vessels near the implant. Tissue sections (30 μm) were labeled with antibodies for PECAM, an endothelial cell marker, or GFAP, a glial marker. In non-implanted control tissue, blood vessels were reliably double-labeled with nanobeads and antiPECAM. Tissue near microdialysis probe tracks exhibited ischemia in the form of PECAM immunoreactive blood vessels devoid of nanobeads. Ischemia was most apparent after the 4-hr implants. Probe tracks were surrounded by endothelial cell debris, which appeared as a diffuse halo of PECAM immunoreactivity. The halo intensity decreased with implant duration, indicative of an active wound-healing process. Consistent with this, after 24-hr implants, the probe tracks were surrounded by hyperplasic and hypertrophic glia and glial processes were extending towards, and engulfing, the track. Carbon fiber microelectrodes produced a diffuse disruption of nanobead labeling but no focal disruption of blood vessels, no PECAM immunoreactive halo, and no glial activation. These findings illuminate the differences between the extent and nature of the penetration injuries associated with microdialysis and voltammetry.
Keywords: Microdialysis, voltammetry, penetration injury, ischemia, GFAP
1. Introduction
Central dopamine (DA) systems are unique in their ability to participate in a broad constellation of brain functions, ranging from motor control to cognition (Cropley et al., 2006; Groto et al. 2007; Ogura et al., 2005; Salgado-Pineda et al., 2005; Williams and Castner, 2006). Consequently, dysfunction of central DA systems contributes to multiple disorders, including Parkinson’s disease (Cookson, 2005), substance abuse (Koob and Bloom, 1988), and attention deficit hyperactivity disorder (Martinez-Leon, 2006). Presumably due to inputs that DA systems receive from cortical structures, the role of DAergic dysfunction following cortical injury has become a topic of recent inquiry (Wagner et al., 2005), especially in light of the growing use of DA-related pharmacotherapy after traumatic brain injury (Warden et al., 2006). The role of DA in normal and pathological brain function has motivated intense effort to measure extracellular DA concentrations in living animals (Kawagoe et al., 1993; Smith and Justice, 1994; DiChiara et al., 1996; Jones et al., 1998, 1999; Garris et al., 1999; Kulagina et al., 2001; Borland and Michael, 2004; Wightman et al., 2007). The goal of such measurements is to quantify resting, or basal, DA levels and the amplitude, direction, and duration of DA concentration changes. The broader objective is to understand the correlation between DA levels in the extracellular space (ECS) and animal behaviors.
Microdialysis and voltammetry are among the tools used to measure ECS DA. However, evidence of quantitative and qualitative disparities between the outcomes of ECS DA measurements by these techniques confounds the stated objective (Borland et al., 2005; Bungay et al., 2003; Kulagina et al., 2001; Qian et al., 1999). Microdialysis and voltammetry each involve the penetration of living brain tissue with physical objects, probes and microelectrodes, respectively, resulting in penetration injury (Benveniste and Diemer, 1987; Benveniste et al., 1987; Clapp-Lilly et al., 1999; Dykstra et al., 1992; Groothuis et al., 1998; Holson et al., 1996; Kadota et al., 1994; Major et al., 1990; Morgan et al., 1996; Zhou et al., 2001). Since the severity and extent of such injury is related to the size of the penetrator (Shain et al., 2003), and since microdialysis probes are at least 10,000 times larger than carbon fiber voltammetric microelectrodes (volume:volume) (Yang et al., 2007), it is necessary to consider the possibility that differences between the penetration injuries contribute to the differences between the ECS DA results of microdialysis and voltammetry. Probe tracks are visible with the unaided eye in tissue sections prepared after microdialysis, whereas viewing the tracks of carbon fiber voltammetric microelectrodes requires electron microscopy (David et al., 1998; Peters et al., 2004).
Acute 4-hr implantations of microdialysis probes in the rat striatum interrupt perfusion of the surrounding tissue, suggesting that ischemia is likely a contributing factor in the penetration injury (Mitala et al., 2008). However, microdialysis sampling is more conventionally initiated 24 hr after probe implantation (Santiago and Westerink, 1990; Timmerman and Westerink, 1997; Westerink et al., 1987, 1988). In the present study, therefore, we evaluate the vascular effects of the probes 1, 4, and 24 hr after implantation.
We also examine the vascular effects of carbon fiber microelectrodes, as we wish to understand the origins of differences between ECS DA measurements by microdialysis and voltammetry. There is an especially urgent need to examine the effects of carbon fiber microelectrodes on blood vessel perfusion, as we noticed several atypical red blood cells during our previous ultrastructural study of carbon fiber implantation sites (Peters et al., 2004). Red blood cells are usually removed during tissue perfusion and are not normally observed in EM studies of non-implanted tissues.
The present study included immuno-labeling of tissue slices for glial fibrillary acidic protein (GFAP), a well-known marker for glia (Bignami et al., 1980; Eng and DeArmond, 1982), to assess glial responses to tissue penetrations. Glial activation is a well-established component of the brain tissue injury response (Turner et al., 1999; Szarowski et al., 2003; Biran et al., 2005).
2. Materials and Methods
2.1. Reagents
Bovine serum albumin (BSA), paraformaldehyde, and Triton-X 100 were used as received from Sigma (St. Louis; MO). Sucrose and BSA were dissolved in phosphate buffered saline (PBS: 155 mM NaCl, 100 mM phosphate, pH 7.4). 2-methylbutane was obtained from Alfa Aesar (Ward Hill, MA). Perfusion liquids included 2% paraformaldehyde (154 mM sodium phosphate dibasic, 46mM sodium phosphate monobasic), pH adjusted to 7.3 with NaOH or phosphoric acid; filtered (Iso-Disc TM filters, N-25-2 Nylon 25 mm × 0.2 μm, SUPELCO, Bellefonte; PA) in 0.1 M phosphate buffer and a 0.1% solution of yellow-green fluorescent microspheres as received (0.1 μm diameter, FluoSpheres® carboxylate-modified polystyrene microsphere suspensions-2% solids in water plus 2 mM sodium azide, Molecular Probes, Inc.; Eugene, OR) in PBS. Sucrose was obtained from Fisher (Fisher Scientific; Pittsburgh, PA). Probes were perfused with artificial cerebrospinal fluid (aCSF:144 mM Na+, 1.2 mM Ca2+, 2.7 mM K+, 152 mM Cl−, 1.0 mM Mg2+, and 2.0 M PO43− adjusted to pH 7.4 with NaOH). Gelvatol was made as follows: 20 g polyvinyl alcohol (Sigma; St. Louis, MO) dissolved in 80 ml of 0.14 M NaCl solution buffered with 0.01 M KH2PO4-Na2HPO4 at pH 7.2 and stirred for 16 hrs. 40 ml of glycerol (Sigma; St. Louis, MO) was added and stirred for another 16 hrs. This solution was centrifuged at 12000 rpm, and the supernatant was decanted (pH~7). 1, 4-diazabicyclo [2.2.2] octane (DABCO, 100 mg/ml, Sigma; St. Louis, MO) was added. Mouse anti-rat PECAM [CD31] was obtained from Chemicon (Temecula, CA) and mouse anti-rat GFAP cocktail was obtained from BD Biosciences Pharmingen (San Diego, CA). Goat anti-mouse IgG, CY3 was obtained from Jackson Immunoresearch (West Grove, PA). Hoechst stain was obtained from Sigma (St. Louis, MO). All solutions were prepared with ultrapure water (NANOPure; Barnstead; Dubuque, IA).
2.2. Animals and surgical procedures
All procedures involving animals were carried out with the approval of the Institutional Animal Care and Use Committee of the University of Pittsburgh. Male Sprague-Dawley rats (250–375g, Hilltop; Scottsdale, PA) were anesthetized with isoflurane (2% by volume) (Halocarbon Products Corporation; North Augusta, SC) and wrapped in a homoeothermic blanket (EKEG Electronics; Vancouver, BC, Canada) to maintain body temperature at 37°C. The rats were placed in a stereotaxic frame (David Kopf Instruments; Tujunga, CA). For acute studies (1 and 4 hr implants) the animals remained anesthetized and in the stereotax throughout the experiment. For 24-hr studies, rats were anesthetized during an initial surgical procedure to position a guide cannula (MD-2251, Bioanalytical Systems Inc.; West Lafayette, IN) over the striatum. The tip of the cannula was lowered 1 mm beneath the surface of the brain and contained a stylus that was removed at the start of experiments. The cannula was held in place with dental cement and stainless steel bone screws (MD1300 and MF5182, respectively, Bioanalytical Systems, West Lafayette, IN). The animals were given at least 3 days to recover from the cannulation. Animals recovering at least 80% of their pre-surgical body weight were briefly anesthetized a second time with isoflurane for insertion of the microdialysis probe or microelectrode though the guide cannula. Once the device was implanted, anesthesia was removed and the rats were placed in a Raturn chamber (Bioanalytical Systems, West Lafayette, IN) for the duration of the 24 hr implantation period.
2.3. Microdialysis procedures
Vertical concentric microdialysis probes (280 μm o.d., 4 mm in length) were constructed with hollow fiber dialysis membrane (Spectra-Por RC Hollow Fiber; MWCO: 13,000, 160 μm i.d., Spectrum Laboratories, Inc.; Rancho Dominguez, CA) and fused silica outlet lines (150 μm o.d., 75 μm i.d., Polymicro Technologies; Phoenix, AZ) as described elsewhere (Mitala et al., 2008). Microdialysis probes were lowered to a final position of 2.5 mm anterior to bregma, 2.5 mm lateral from midline and 7.0 mm below dura (Pellegrino et al. 1979) over a 30 minute period. During 1- and 4-hr implants the probe was held in place by means of a stereotaxic carrier arm. For 24-hr studies the rat was briefly anesthetized and returned to the stereotax. The probe was lowered through the cannula with a stereotaxic carrier arm and securely attached to the hub of the guide cannula with 5-min epoxy. The probes were perfused throughout all implants with aCSF at 0.586 μL/min. Flow was confirmed by visual inspection of droplets forming at the outlet line but samples were not collected for analysis.
2.4. Voltammetry procedures
Carbon fiber microcylinder electrodes were constructed as described previously (Borland and Michael, 2004). A single 7-μm diameter carbon fiber (Thornell Carbon Fiber; T300, Amoco Performance Products, Inc. Greenville, SC) was sealed with epoxy into a borosilicate glass capillary and trimmed to a length of 400 μm. The electrodes were lowered to a final position of 2.5 mm anterior to bregma, 2.5 mm lateral from midline and 4.0 mm below dura (Pellegrino et al. 1979) over a 30-min period. During 1- and 4-hr implantations, the microelectrodes were mounted on a carrier arm of the stereotax. For 24-hr studies, the microelectrodes were mounted in a micromanipulator (constructed in the machine shop of the Department of Chemistry, University of North Carolina at Chapel Hill, NC) constructed so as to mate with and lock into the previously implanted guide cannula (Rebec et al., 1993; Garris et al., 1997). Fast scan cyclic voltammetry was performed continuously throughout all implants. The voltage scans were performed at 10 Hz. Each scan began at 0 V vs Ag/AgCl and ramped to 1 V, −0.5 V, and back to 0 V at 300 V/s. Electrical stimulation of the medial forebrain bundle (MFB) was performed during the 1- and 4-hr implants. Evoked release was recorded during these studies but the results, which were unremarkable, are not reported here.
2.5. Tissue fixation and processing
Rats underwent intracardial perfusion at the termination of the implants. Perfusion was performed before extraction of microdialysis probes but after extraction of the microelectrodes. Microelectrodes were extracted before infusion to enable post-calibration to confirm the viability of the electrode. Rats were perfused with 160 ml of PBS, followed by 160 ml of 2% paraformaldehyde, and 50 ml of 0.1% solution of yellow-green fluorescent microspheres. Brains were removed from the skull, submerged in 2% paraformaldehyde for 2 hr, placed in 30% sucrose overnight for cryo-protection, frozen by dipping in liquid nitrogen-cooled 2-methylbutane, stored at −80°C until sliced, and mounted to an object holder with tissue freezing medium (Triangle Biomedical Sciences; Durham, NC) and Quick Freeze® (Electron Microscopy Sciences; Hatfield, PA). For microdialysis probes, 30-μm slices were counted to determine when the depth from the top of the brain corresponded to the probe track at the level of the active dialysis membrane. DIC images confirmed that the regions of interest contained the probe tracks. For carbon fiber implants, the region of interest was identified by the methodology described by Peters et al. (2004). Briefly, the track of the tapered electrode barrel was followed from the surface of the brain to the deepest evident trace of the track at the level of light microscopy. The region of interest was taken to be 200 μm (i.e. half the length of the carbon fiber) below the deepest observable point of the track formed by the electrode barrel. Where possible the serial sections were aligned by the use of anatomical landmarks, such as large blood vessels and axon bundles. Peters et al. (2004) used this approach to locate the carbon fiber implantation site by electron microscopy. Where such anatomical landmarks were not available, measurements were taken from the boarders of the slice. The regions of interest were 1 mm square, so it was not difficult to identify the trajectory of the carbon fiber on this scale of resolution. Tissue sections (30 μm) were taken from the region of interest, mounted onto glass slides, and stored at −20°C until antibody labeling.
2.6. Immunofluorescence protocol
Brain slices were rehydrated with two washes of 1xPBS (10x PBS, Fischer Scientific; Pittsburgh, PA, diluted with ultrapure water), treated with 0.1% Triton-X 100 in 1x PBS for 15 min, washed three times with 0.5% BSA, soaked for 45 min in 2% BSA, and washed five times with 0.5% BSA. Prepared slices were soaked in 100 μL of 0.5% as-received primary antibody (anti-PECAM or anti-GFAP) in 0.5% BSA for 1 hr, washed five times with 0.5% BSA, and soaked for 1hr in 100 μL of 0.1% as-received labeled secondary antibody (IgG, CY3) in 0.5% BSA. The slices were washed five times with 0.5% BSA, five times with 1x PBS, soaked in Hoechst stain (diluted in de-ionized water for a final concentration of 1 mg/100 ml) for 30 s, and washed three times with 1x PBS. The slices were covered with a gelvatol-treated coverslip and stored overnight in the dark at 4°C. On the following day the slices were imaged using a confocal microscope (Olympus Fluoview 1000 Confocal Microscope, Olympus; Melville, NY) or a fluorescence microscope (Olympus BX61, Olympus; Melville, NY).
2.7. Fluorescence microscopy
Double-labeled blood vessels were imaged with fluorescence microscopy using a 10X or 20X objective and the appropriate filter (Chroma Technology; Rockingham, VT). Nanobead fluorescence was excited with 488 nm light from a multi-line argon laser, CY3 fluorescence was excited at 543 nm with a helium-neon laser, and Hoechst stain fluorescence was excited at 405 nm with a diode laser. Images were quantitatively analyzed with Metamorph/Fluor Imaging Systems software (Universal Imaging Corporation; Molecular Devices). Background subtraction was based on threshold values. We used the software to determine the co-localization of PECAM and nanobead fluorescence and the intensity of GFAP images. PECAM/nanobead co-localization and GFAP intensities were normalized with respect to non-implanted control tissue in the contralateral hemisphere of each tissue section. Normalized results are expressed as mean ± standard deviation: normalized co-localization results were analyzed by two-way ANOVA and Tukey pair-wise comparisons; co-localization results pooled across implant duration and normalized GFAP intensities were analyzed with the t-test.
3. Results
3.1 Microscopy of double-labeled blood vessels in non-implanted tissue
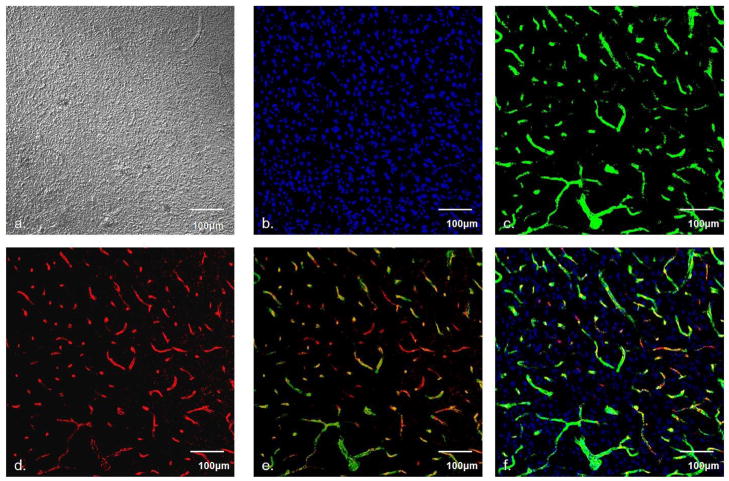
As a control experiment, we performed light and fluorescence microscopy on 30-μm slices of non-implanted striatal tissue from rats (Fig 1). Differential interference contrast (DIC) and DAPI fluorescence images are unremarkable, with a uniform distribution of features (Fig 1a and 1b, respectively). Fluorescent nanobeads (Fig 1c) and PECAM immunoreactivity (Fig 1d) exhibit good registration in overlaid images (Fig 1d and 1e), confirming that our protocol reliably double-labels the blood vessels.
Fig. 1.
Microscopy of striatal tissues. a) DIC image of healthy tissue, b) DAPI labeled nuclei, c) nanobead fluorescence, d) PECAM immunoreactivity, e) overlay of c and d, f) overlay of b, c, and d. Scale bar is 100μm.
3.2. Fluorescence microscopy of microdialysis probe tracks
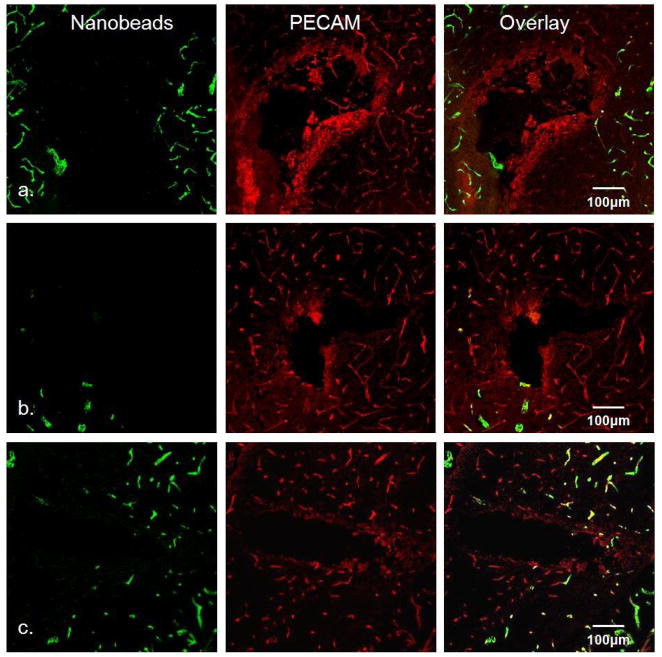
We performed fluorescence microscopy on 30-μm tissue sections of the rat striatum prepared after 1, 4, and 24-hr microdialysis probe implantations (Fig 2). The horizontal slices were oriented perpendicular to the axis of the probes, so the images contain a cross-sectional view of the tracks at the level of the dialysis membrane. In all cases, PECAM immunoreactive blood vessels devoid of nanobeads are clearly evident near the probe tracks (the qualitative images in Fig 2 are representative: a quantitative summary is presented in Fig 5, below). The least amount of nanobead labeling occurred after the 4-hr implant (Fig 2b). Whereas nanobead labeling is reduced after 24 hrs (Fig 2c) compared to 4 hrs, disruption is still evident after 24 hrs. Thus, microdialysis probes cause striatal ischemia for the duration of the 24-hr implants.
Fig. 2.
Fluorescence microscopy of microdialysis probe tracks. Row a) microdialysis probe tracks after 1-hr implant, b) after 4-hr implant, and c) after 24-hr implant. Left column is the green channel (nanobead fluorescence), center column is the red channel (PECAM immunoreactivity), and right column is the overlay of the red and green channels. Scale bar is 100μm.
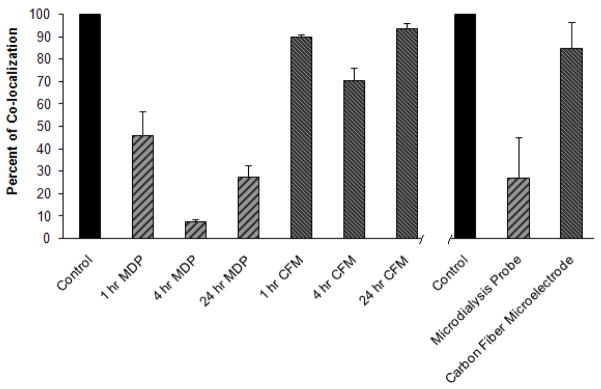
Fig. 5.
Normalized co-localization of PECAM immunoreactivity and nanobead fluorescence. Control (100%) values were determined in non-implanted (contralateral) striatum. Left part of the plot reports values for each device for each implant duration. Both implant type (p<0.0001) and implant duration (p<0.001) were significant factors (two-way ANOVA, see text for details). Right part (right side of the axis break) of the plot reports values for each device pooled across implant duration. Both the pooled values were significantly different from 100% (t-test: probes p<0.0001, microelectrodes p<0.05). (CFM=carbon fiber microelectrode, MDP=microdialysis probe).
The probe tracks are surrounded by a halo of diffuse PECAM immunoreactivity that is most intense after 1-hr implants (Fig 2a). The intensity of the halo diminishes over time and is almost absent by 24 hrs, suggesting an on-going active wound healing response.
3.3. Images of carbon fiber electrode tracks
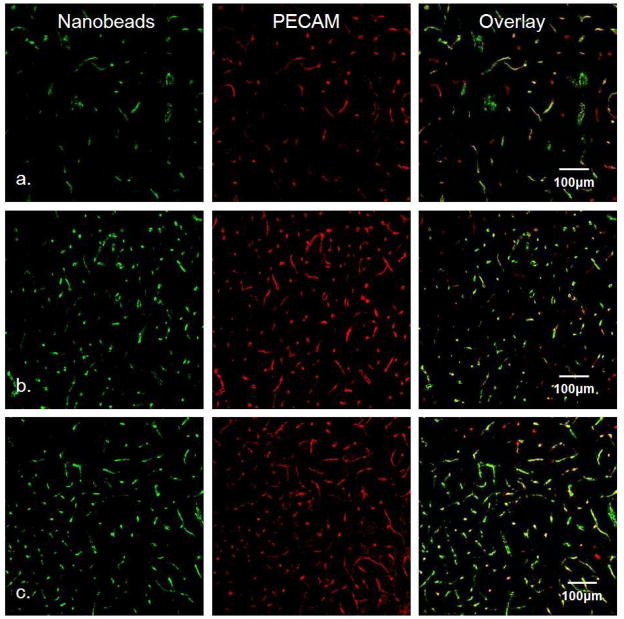
We examined PECAM immunoreactivity and nanobead labeling in striatal slices prepared 1, 4, and 24-hrs after implantation of carbon fiber microelectrodes (Fig 3). There is no evidence of PECAM immunoreactive blood vessels devoid of nanobeads and there is no evidence of a PECAM immunoreactive halo. This is consistent with our previous findings that microelectrodes leave no track visible to light microscopy (Peters et al. 2004). Thus, the penetration injury associated with the fiber microelectrodes does not produce ischemia or endothelial cell debris.
Fig. 3.
Fluorescence microscopy of carbon fiber microelectrode tracks. Row a) microelectrode tracks after 1-hr implant, b) after 4-hr implant, and c) after 24-hr implant. Left column is the green channel (nanobead fluorescence), center column is the red channel (PECAM immunoreactivity), and right column is the overlay of the red and green channels. Scale bar is 100μm.
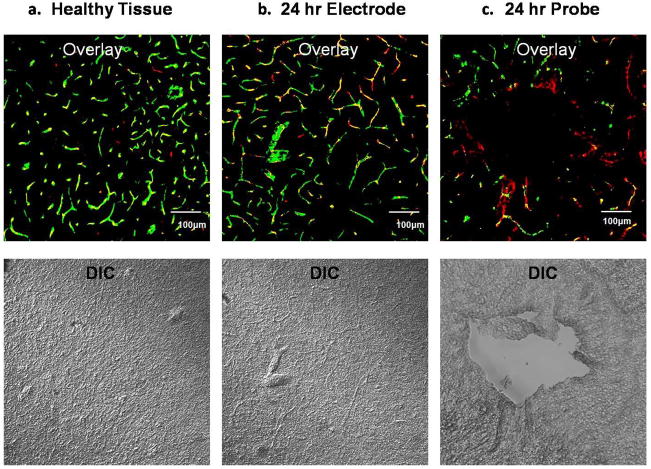
3.4. Comparative fluorescent and DIC images
We compared the DIC and fluorescent images of non-implanted, electrode-implanted, and probe-implanted striatal slices (Fig 4). DIC images of non-implanted (Fig 4a) and electrode-implanted slices (Fig 4b) are uniform in appearance but larger blood vessels are visible that match labeled vessels in the fluorescence image. The DIC image of the probe-implanted slice (Fig 4c) reveals an actual hole at the same location that appears dark in the fluorescence image, confirming that the features in the fluorescence images correspond to the probe track.
Fig. 4.
Comparing fluorescence (top) and DIC (bottom): a) non-implanted healthy tissue, b) after 24-hr microelectrode implant, and c) after 24-hr probe implant. Scale bar is 100 μm.
3.5. Co-localization of PECAM and nanobead fluorescence
Normalized co-localization of PECAM immunoreactivity and the nanobead fluorescence (red and green channels, respectively) was determined with software at each implant duration and type (data collected in n=12 rats; Fig 5). Co-localization in the probe track images was decreased after all implant durations: 45.5 ± 10.8% after 1 hr, 7.6 ± 0.8% after 4 hrs, and 27.3 ± 5.4% after 24 hrs. In contrast, co-localization in microelectrode track images was decreased slightly: 89.8 ± 7.6% after 1 hr, 70.4 ± 5.6% after 4 hrs, and 93.7 ± 1.1% after 24 hrs. According to two-way ANOVA, both implant type and duration were significant factors (implant type: F(df 1,6)=253.8, p<0.0001; implant duration: F(df 2,6)=22.8, p<0.001). There was a significant difference between the co-localization in the probe and microelectrode images (Tukey post-hoc comparison of the means, p<0.0001). Co-localization values were pooled across the three time points as these were relatively homogeneous. The pooled values for the probes and microelectrodes, 26.9 ± 17.9% and 84.6 ± 11.9% respectively (mean ± s.d., n=6), were both significantly different from the control value of 100% (t-test: probes p<0.0001, microelectrodes p<0.05).
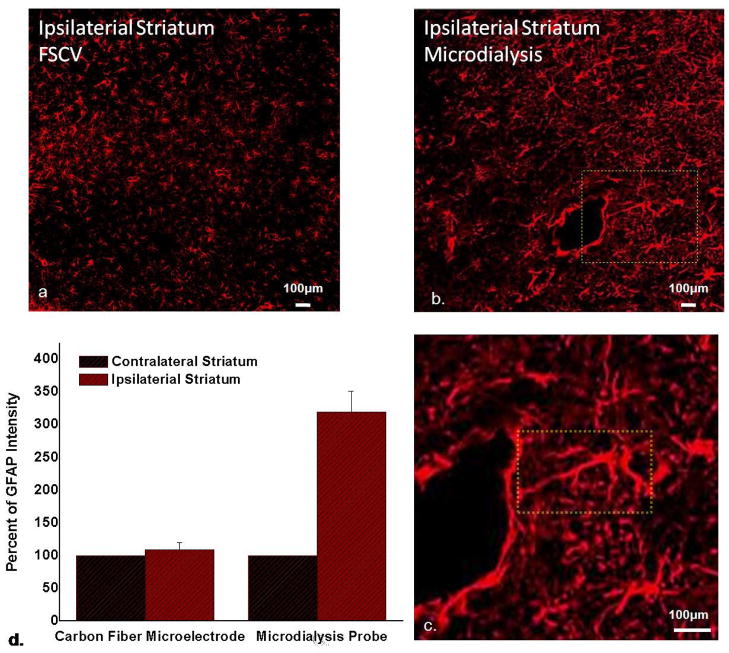
3.6. GFAP immunoreactivity after 24-hr implants
GFAP immunoreactivity reveals the size, general shape, and distribution of glia (Figure 6). The glial content of microelectrode-implanted striatal tissue (Figure 6a) is indistinguishable from that of non-implanted tissue (not shown). Microdialysis probe tracks are surrounded by glia exhibiting marked hyperplasia and hypertrophia (Figure 6b). A closer view (Figure 6c) shows that the track is encircled around most of its circumference (~75%) by a barrier of GFAP immunoreactive elements, revealing that glial encapsulation and isolation of the probe is underway 24 hrs after implantation. The field of view in Figure 6c prominently displays a glial cell extending a process in excess of 300 μm in a linear fashion from the cell body towards the probe track. No glia exhibiting such a shape or size were found in the images of microelectrode- or non-implanted tissue.
Fig. 6.
GFAP immunoreactivity a) after 24-hr microelectrode implant and b) after 24-hr probe implant, c) enlargement of the area in the yellow box in b showing a glial cell extending a process ~300 μm towards the track (scale bar in a–c is 100 μm), d) normalized GFAP intensity (mean±s.d. n=3); GFAP intensity in the probe images is significantly elevated compared to 100% (t-test, p<0.005); GFAP intensity was not significantly elevated in the microelectrode images.
The GFAP images were quantified with the Metamorph software (Fig 6d). GFAP immunoreactivity increased 219.8 ± 31.5 % (n=3) in the probe images and 9.2 ± 11.0% (n=3) in the microelectrode images. The GFAP intensity in the probe images was significantly increased compared to the control value of 100% (t-test, p<0.005). GFAP intensity in microelectrode images was not significantly elevated.
4. Discussion
This study confirms the existence of differences between the penetration injury associated with microdialysis probes and carbon fiber microelectrodes. After 1-, 4-, and 24-hr implantations, microdialysis probe tracks are surrounded by PECAM immunoreactive blood vessels devoid of fluorescently labeled nanobeads. The tracks are surrounded also by a diffuse halo of PECAM immunoreactivity, which fades over time during the 24-hr post-implantation interval. After 24-hr implantations, microdialysis probe tracks are surrounded by hyperplasic and hypertrophic glia and the tracks are in the initial stages of becoming engulfed by glial processes. In one case we noticed a GFAP immunoreactive cell extending a distance of 300 μm along a direct linear route towards the probe track, indicative of a chemotaxic mode of communication between the injury site and nearby glia. The activation of glia, a wound-healing response (Turner et al., 1999; Szarowski et al., 2003; Biran et al., 2005), might explain the fading of the PECAM-immunoreactive halo.
In contrast, carbon fiber microelectrodes produced no halo, track-localized disruption of nanobead labeling, or signs of glial activation. Quantitative evaluation of the microelectrode track images with software (Metamorph) revealed a decreased co-localization of the nanobead label and PECAM immunoreactivity. This was a consistent but relatively small effect that was significant after the 4-hr implants but not the 1- and 24-hr implants. However, the region of interest chosen for evaluation was not tightly confined to the immediate vicinity of the microelectrode track, because the precise location of the track is not evident. Thus, the decreased co-localization is diffusely distributed in the images. It is therefore possible that the effect is not a consequence of tissue penetration by the fiber itself but is instead a more global event, possibly associated with penetration of the cortex overlying the striatal site by the larger barrel of the tapered glass capillary tube or, in the case of 24-hr implantations, the guide cannula. Indeed, a recent report describes hemispheric metabolic disruption following intracranial cannulation (Schiffer et al., 2006).
During a previous ultrastructural examination of striatal tissue slices by electron microscopy (Peters et al., 2004), we noticed several atypical red blood cells trapped adjacent to microelectrode tracks, i.e. red blood cells that were not removed by the perfusion. This is a sign that the microelectrodes do cause vascular disruption, but at levels below the detection limit of the dual-labeling strategy employed for the present study.
The findings of the present study are consistent with an absence of reports in the literature to suggest that the time after implantation of carbon fiber microelectrodes is a critical experimental variable in voltammetry protocols. Usual practice is to implant an electrode and wait for the voltammetric baseline to stabilize before results are collected (Garris et al., 1994; Cragg et al., 1997; Gonon, 1997; Dickinson et al., 1999; Peters and Michael, 2000). Beyond this baseline stabilization, the time after implantation is not described as key experimental variable. In contrast, time after implantation is widely discussed in the microdialysis literature (DeBoer and Abercrombie, 1996; Santiago et al., 1990). Often, procedures specify a 24-hr interval between implantation and data collection on the grounds that there are substantial changes in the quantitative and qualitative nature of DA measures over this interval. In particular, that a 24-hr interval is required to establish full tetrodotoxin-sensitivity of basal dialysate DA levels is widely interpreted as a sign that the properties of the tissues surrounding microdialysis probes undergo changes in the 24-hr post-implantation interval (Westerink et al., 1988). While this interpretation is completely consistent with our findings, we clearly demonstrate herein that the tissue does not return to its pre-implantation, i.e. normal or healthy, state.
Rather, at 24-hr post-implantation signs of ischemic disruption, while reduced compared to 4-hr post-implantation, remain clearly evident in the form of PECAM immunoreactive vessels devoid of fluorescently-labeled nanobeads. The diffuse halo of PECAM immunoreactivity, a sign of endothelial cell debris, also is substantially reduced after 24 hrs, although this may be due to the activated glia that is engulfing the probe.
During the present study we did not collect neurochemical data with the implanted probes and microelectrodes because we have done so previously (Borland et al. 2004 and 2005; Lu et al., 1998; Kulagina et al., 2001; Mitala et al., 2008; Peters et al., 1998; Yang and Michael, 2007). In some prior studies, we placed carbon fiber microelectrodes in the tissue surrounding microdialysis probes (Yang et al., 1998; Borland et al., 2005; Yang and Michael, 2007). We found clear differences between the voltammetric results that depended on the distance of separation between the microelectrode and the probe. When the microelectrode is 1 mm from the probe, voltammetric results are similar to those obtained in the absence of probes. When the microelectrode is ~200 μm from the probe, the amplitude of evoked DA release is decreased by 90%. When the microelectrode is in contact with the outer surface of the probe, evoked DA responses are abolished. These results confirm that the probe depresses evoked DA release in the nearby tissues. However, these prior studies were performed after 4-hr and 16-hr implantations, which are shorter than the recommended 24 hrs, due to the use of an anesthetized preparation to simplify simultaneous implantations of multiple devices. A potential concern with those studies, therefore, is that the implantation time is shorter than the widely recommended 24 hr. Our results, however, clearly show that tissues have not returned to their normal state after 24-hr implants.
Thus, together with our previous studies of voltammetry-near-probes (Lu et al., 1998; Yang et al., 1998; Borland et al. 2005; Yang and Michael, 2007), the present study supports the conclusion that microdialysis probes sample injured tissue even when procedures include a 24-hr post-implantation interval. Presumably, therefore, dialysate DA is derived from those DA terminals that survive the penetration injury. The exact extent of the impact of penetration injury on DA terminals is not known. Voltammetry-near-probes reveals an extreme disruption of evoked DA release, which is consistent with the findings in the present study that the probe itself is surrounded by glial processes. Voltammetry-near-probes also reveals disruptions of pharmacologically induced changes in ECS DA (Borland et al., 2005). But, whether these disruptions are due to an outright loss of DA terminals or a functional disturbance of the surviving DA terminals remains to be determined: a combination of these effects seems likely. Indeed, the theoretical considerations of Bungay et al. (2003) are consistent with the idea that DA terminals near the probes are damaged in such a fashion that their capacity for DA release is compromised to a greater extent than their capacity for DA uptake. Combined with evidence that microdialysis extraction and recovery ratios are affected by DA uptake kinetics (Smith and Justice, 1994; Yang et al., 1998; Bungay et al., 2003), this suggests that DA terminals surviving the penetration injury are functionally disturbed.
Moreover, the present findings suggest that a glial-derived diffusion barrier further explains our previous conclusion that the in vivo microdialysis recovery of DA is lower than expected based on in vitro calibration. Since it is removed when the probe is withdrawn from tissues, the diffusion barrier is not evident during post-in vivo probe calibrations. The glial-derived diffusion barrier is not ‘absolute’ after 24-hr implantations as DA extraction and recovery values are also affected by DA uptake rates. DA uptake is mediated by the dopamine transporter, which is not expressed by glial cells. Nevertheless, a glial-derived diffusion barrier had not been incorporated into any prior theoretical assessments of the quantitative aspects of in vivo microdialysis that are relied upon to justify microdialysis data interpretation.
The contrast between the penetration injuries associated with microdialysis and voltammetry provide a reasonable explanation for those instances where the two techniques report different in vivo DA concentrations. In our hands, for example, voltammetry reports substantially higher DA concentrations than microdialysis (Qian et al., 1999; Kulagina et al., 2001; Borland et al., 2005). The idea that the microelectrodes, by virtue of the smaller penetration injury they cause, get closer to DA terminals provides a simple explanation for the higher voltammetry-based concentration values. The importance of a smaller distance between the electrode and nearby DA terminals has a two-fold origin. First, once DA is released to the extracellular space, the distance it can diffuse is highly constrained by an avid uptake mechanism. Second, if each DA terminal is viewed as a small point-source of DA, then DA undergoes dilution as it diffuses from the terminal into an ever expanding volume of extracellular space (Cragg and Rice, 1997). The two effects combine to create a ‘diffusion sphere’ of DA around each DA terminal. Positioning the electrode closer to DA terminals enables the detection of DA within the diffusion sphere, i.e. before the DA concentration decreases due to the actions of DA uptake and dilution. In the present study, we found no additional evidence of microelectrode-induced ischemia, endothelial-cell debris, or glial activation. So, it appears reasonable to conclude that penetration injury associated with carbon fibers is confined to that which we reported by electron microscopy (Peters et al., 2004). According to EM, the penetration injury surrounding microelectrode tracks is limited to ca 3 μm, which is smaller than existing estimates of the distances DA diffuses from terminals. Thus, we conclude that the greater ability of microelectrodes to enter DA diffusion spheres explains why they report higher DA concentrations.
So, the present study offers an explanation for the differences between the outcome of in vivo DA measurements based on microdialysis and voltammetry, which has been our objective. On the other hand, it is important to acknowledge, even emphasize, that our findings do little if anything to confirm the accuracy of in vivo DA concentrations as measured by voltammetry. An issue that plagues both microdialysis and voltammetry is the absence of in vivo calibration, due for the main part to the absence of bona fide primary standards for such calibration. In both microdialysis and voltammetry, DA concentrations are conventionally determined by post-calibration after the probes and electrodes are extracted from tissues. However, we are increasingly inclined to view the term ‘post-calibration’ as a non-sequitur since it is not possible to confirm that post-in vivo sensitivity data are relevant to in vivo measurements. We consider that ‘post-normalization’ would be a more appropriate term for the use of post-in vivo sensitivity data. Nevertheless, the issue of the uncertain accuracy of post-calibration does not substantially alter the conclusion that penetration injuries differentially affect both the quantitative and qualitative in vivo DA concentrations reported by microdialysis and voltammetry.
Acknowledgments
This work was supported by the National Institute of Health, grant # MH075989. We thank Simon Watkins, the University of Pittsburgh’s Center for Biologic Imaging, Christina M. Mitala and Keith F. Moquin.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Benveniste H, Diemer NH. Cellular reactions to implantation of a microdialysis tube in the rat hippocampus. Acta Neuropathologica. 1987;74:234–38. doi: 10.1007/BF00688186. [DOI] [PubMed] [Google Scholar]
- Benveniste H, Drejer J, Schousboe A, Diemer NH. Regional cerebral glucose phosphorylation and blood flow after insertion of a microdialysis fiber through the dorsal hippocampus in the rat. J Neurochem. 1987;49:729–34. doi: 10.1111/j.1471-4159.1987.tb00954.x. [DOI] [PubMed] [Google Scholar]
- Bignami A, Dahl D, Reuger DC. Glial fibrillary acidic protein (GFAP) in normal neural cells and in pathological conditions. In: Federoff S, editor. Advances in cellular neurobiology. Orlando: Academic Press; 1980. pp. 285–310. [Google Scholar]
- Biran R, Martin DC, Tresco PA. Neuronal cell loss accompanies the brain tissue response to chronically implanted silicon microelectrode arrays. Exp Neurol. 2005;195:115–26. doi: 10.1016/j.expneurol.2005.04.020. [DOI] [PubMed] [Google Scholar]
- Borland LM, Michael AC. Voltammetric study of the control of striatal dopamine release by glutamate. J Neurochem. 2004;91:220–9. doi: 10.1111/j.1471-4159.2004.02708.x. [DOI] [PubMed] [Google Scholar]
- Borland LM, Shi G, Yang H, Michael AC. Voltammetric study of extracellular dopamine near microdialysis probes acutely implanted in the striatum of the anesthetized rat. J Neurosci Methods. 2005;146:149–58. doi: 10.1016/j.jneumeth.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Bungay PM, Newton-Vinson P, Isele W, Garris PA, Justice JB., Jr Microdialysis of dopamine interpreted with quantitative model incorporation probe implantation trauma. J Neurochem. 2003;86:932–46. doi: 10.1046/j.1471-4159.2003.01904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapp-Lilly KL, Roberts RC, Duffy LK, Irons KP, Hu Y, Drew KL. An ultrastructural analysis of tissue surrounding a microdialysis probe. J Neurosci Methods. 1999;90:129–42. doi: 10.1016/s0165-0270(99)00064-3. [DOI] [PubMed] [Google Scholar]
- Cookson MR. The biochemistry of Parkinson’s disease. Ann Rev Biochem. 2005;74:29–52. doi: 10.1146/annurev.biochem.74.082803.133400. [DOI] [PubMed] [Google Scholar]
- Cragg SJ, Rice ME, Greenfield SJ. Heterogeneity of electrically evoked dopamine release and reuptake in substantia nigra, ventral tegmental area, and striatum. J Neurophysiol. 1997;77:862–73. doi: 10.1152/jn.1997.77.2.863. [DOI] [PubMed] [Google Scholar]
- Cropley VL, Fujita M, Innis RB, Nathan PJ. Molecular imaging of the dopaminergic system and its association with human cognition. Biol Psychiatry. 2006;59:898–907. doi: 10.1016/j.biopsych.2006.03.004. [DOI] [PubMed] [Google Scholar]
- David DJ, Zahniser NR, Hoffer BJ, Gerhardt GA. In vivo electrochemical studies of dopamine clearance in subregions of rat nucleus accumbens: differential properties of the core and shell. Exp Neurology. 1998;153:277–86. doi: 10.1006/exnr.1998.6898. [DOI] [PubMed] [Google Scholar]
- DeBoer P, Abercrombie ED. Physiological release of striatal acetylcholine in vivo: modulation by D1 and D2 dopamine receptor subtypes. J Pharm Exp Ther. 1996;277:775–83. [PubMed] [Google Scholar]
- Dickinson SD, Sabeti J, Larson GA, Giardina K, Rubinstein M, Kelley MA, Grandy DK, Low MJ, Gerhardt GA, Zahniser NR. Dopamine D2 receptor-deficient mice exhibit decreased dopamine transporter function but no changes in dopamine release in dorsal striatum. J Neurochem. 1999;72:148–56. doi: 10.1046/j.1471-4159.1999.0720148.x. [DOI] [PubMed] [Google Scholar]
- DiChiara G, Tanda G, Carboni E. Estimation of in-vivo neurotransmitter release by brain microdialysis: the issue of validity. Behav Pharmacol. 1996;7:640–57. [PubMed] [Google Scholar]
- Dyskstra KH, Hsiao JK, Morrison PF, Bungay PM, Mefford IN, Scully MM, Dedrick RL. Quantitative examination of tissue concentration profiles associated with microdialysis. J Neurochem. 1992;58:931–40. doi: 10.1111/j.1471-4159.1992.tb09346.x. [DOI] [PubMed] [Google Scholar]
- Eng LF, DeArmond SJ. Immunohistochemical studies of astrocytes in normal development and disease. In: Federoff S, editor. Advances in cellular neurobiology. Academic Press; Orlando: 1982. pp. 145–71. [Google Scholar]
- Garris PA, Christensen JRC, Rebec GV, Wightman RM. Real-time measurement of electrically evoked extracellular dopamine in the striatum of freely moving rats. J Neurochem. 1997;68:152–61. doi: 10.1046/j.1471-4159.1997.68010152.x. [DOI] [PubMed] [Google Scholar]
- Garris PA, Ciolkowski EL, Wightman RM. Heterogeneity of evoked dopamine overflow within the striatal and striatoamygdaloid regions. Neurosci. 1994;59:417–27. doi: 10.1016/0306-4522(94)90606-8. [DOI] [PubMed] [Google Scholar]
- Garris PA, Kilpatrick M, Bunin MA, Michael D, Walker QD, Wightman RM. Dissociation of dopamine release in the nucleus accumbens from intracranial self-stimulation. Nature. 1999;398:67–9. doi: 10.1038/18019. [DOI] [PubMed] [Google Scholar]
- Gonon F. Prolonged and extrasynaptic excitatory action of dopamine mediated by D1 receptors in the rat striatum in vivo. J Neurosci. 1997;17:5972–78. doi: 10.1523/JNEUROSCI.17-15-05972.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groothius DR, Ward S, Schlageter KE, Itskovish AC, Schwerin SC, Allen CV, Dills C, Levy RM. Changes in blood-brain barrier permeability associated with insertion of brain cannulas and microdialysis probes. Brain Res. 1998;803:218–30. doi: 10.1016/s0006-8993(98)00572-1. [DOI] [PubMed] [Google Scholar]
- Groto Y, Otani S, Grace AA. The Yin and Yang of dopamine release: a new perspective. Neuropharm. 2007;53:583–7. doi: 10.1016/j.neuropharm.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holson RR, Bowyer JF, Clausing P, Gough B. Methamphetamine-stimulated striatal dopamine release declines rapidly over time following microdialysis probe insertion. Brain Res. 1996;739:301–7. doi: 10.1016/s0006-8993(96)00837-2. [DOI] [PubMed] [Google Scholar]
- Jones SR, Gainetdinov RR, Caron MG. Application of microdialysis and voltammetry to assess dopamine functions in genetically altered mice: correlation with locomotor activity. Psychopharm. 1999;147:30–2. doi: 10.1007/s002130051137. [DOI] [PubMed] [Google Scholar]
- Jones SR, Gainetdinov RR, Jaber M, Giros B, Wightman RM, Caron MG. Profound neuronal plasticity in response to inactivation of the dopamine transporter. Proc Natl Acad Sci USA. 1998;95:4029–34. doi: 10.1073/pnas.95.7.4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadota E, Nonaka K, Karasuno M, Nishi K, Nakamura Y, Namikawa K, Okazaki YY, Teramura K, Hashimoto S. Experimental quantitative evaluation of transvascular removal of unnecessary substances in brain edema fluid. Acta Neurochir Suppl. 1994;60(Suppl):162–4. doi: 10.1007/978-3-7091-9334-1_43. [DOI] [PubMed] [Google Scholar]
- Kawagoe KT, Zimmerman JB, Wightman RM. Principles of voltammetry and microelectrode surface states. J Neurosci Methods. 1993;48:225–40. doi: 10.1016/0165-0270(93)90094-8. [DOI] [PubMed] [Google Scholar]
- Koob GF, Bloom FE. Cellular and molecular mechanisms of drug dependence. Science. 1988;242:715–23. doi: 10.1126/science.2903550. [DOI] [PubMed] [Google Scholar]
- Kulagina NV, Zigmond MJ, Michael AC. Glutamate regulates the spontaneous and evoked release of dopamine in the rat striatum. Neuroscience. 2001;102:121–8. doi: 10.1016/s0306-4522(00)00480-2. [DOI] [PubMed] [Google Scholar]
- Lu Y, Peters JL, Michael AC. Direct comparison of the response of voltammetry and microdialysis to electrically evoked release of striatal dopamine. J Neurochem. 1998;70:584–93. doi: 10.1046/j.1471-4159.1998.70020584.x. [DOI] [PubMed] [Google Scholar]
- Major O, Shdanova T, Duffek L, Nagy Z. Continuous monitoring of blood-brain barrier opening to 51Cr-EDTA by microdialysis after probe injury. Acta Neurochir Suppl. 1990;51:46–8. doi: 10.1007/978-3-7091-9115-6_16. [DOI] [PubMed] [Google Scholar]
- Martinez-Leon NC. Psychopathology of attention deficit/hyperactivity disorder (AD/HD) Int J Clin Health Psychol. 2006;6:379–99. [Google Scholar]
- Mitala CM, Wang Y, Borland LM, Jung M, Shand S, Watkins S, Weber SG, Michael AC. Impact of microdialysis probes on vasculature and dopamine in the rat striatum: A combined fluorescence and voltammetry study. J Neurosci Methods. 2008;174:177–85. doi: 10.1016/j.jneumeth.2008.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan ME, Singhal D, Anderson BD. Quantitative assessment of blood-brain barrier damage during microdialysis. J Pharm Exp Ther. 1996;277:1167–76. [PubMed] [Google Scholar]
- Ogura T, Ogata M, Akita H, Jitsuki S, Akiba L, Noda K, Hoka S, Saji M. Impaired acquisition of skilled behavior in rotarod task by moderate depletion of striatal dopamine in a pre-symptomatic stage model of Parkinson’s disease. Neurosci Res. 2005;51:299–308. doi: 10.1016/j.neures.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Pellegrino LJ, Pellegrino AS, Cushman AJ. A stereotaxic atlas of the rat brain. 2. New York: Plenum Press; 1979. [Google Scholar]
- Peters JL, Michael AC. Modeling voltammetry and microdialysis of striatal extracellular dopamine: the impact of dopamine uptake on extraction and recovery ratios. J Neurochem. 1998;70:594–603. doi: 10.1046/j.1471-4159.1998.70020594.x. [DOI] [PubMed] [Google Scholar]
- Peters JL, Michael AC. Changes in the kinetics of dopamine release and uptake have differential effects on the spatial distribution of extracellular dopamine concentration in the rat striatum. J Neurochem. 2000;74:1563–73. doi: 10.1046/j.1471-4159.2000.0741563.x. [DOI] [PubMed] [Google Scholar]
- Peters JL, Miner LH, Michael AC, Sesack SR. Ultrastructure at carbon fiber microelectrode implantation sites after acute voltammetric measurements in the striatum of anesthetized rats. J Neurosci Methods. 2004;137:9–23. doi: 10.1016/j.jneumeth.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Qian J, Wu Y, Yang H, Michael AC. An integrated decoupler for capillary electrophoresis with electrochemical detection: application to analysis of brain microdialysate. Anal Chem. 1999;71:4486–92. doi: 10.1021/ac990338f. [DOI] [PubMed] [Google Scholar]
- Rebec GV, Langley PE, Pierce CR, Wang Z, Heidenrich BA. A simple micromanipulator for mulipe uses in freely moving rats: electrophysiology, voltammtery and simultaneous intracerebral infusions. J Neurosci Methods. 1993;47:53–9. doi: 10.1016/0165-0270(93)90021-i. [DOI] [PubMed] [Google Scholar]
- Salgado-Pineda P, Delaveau P, Blin O, Nieoullon A. Dopaminergic contribution to the regulation of emotional perception. Clinical Neuropharm. 2005;28:228–37. doi: 10.1097/01.wnf.0000185824.57690.f0. [DOI] [PubMed] [Google Scholar]
- Santiago M, Westerink BHC. Characterization of the in vivo release of dopamine as recorded by different types of intracerebral microdialysis probes. Nauyn-Schmied. Arch Pharmacol. 1990;342:407–14. doi: 10.1007/BF00169457. [DOI] [PubMed] [Google Scholar]
- Schiffer WK, Mirrione MM, Biegon A, Alexoff DL, Patel V, Dewey SL. Serial microPET measures of the metabolic reaction to microdialysis probe implants. J Neurosci Methods. 2006;115:272–84. doi: 10.1016/j.jneumeth.2006.01.027. [DOI] [PubMed] [Google Scholar]
- Shain W, Spataro L, Dilgen J, Haverstick K, Retterer S, Isaacson M, Saltsman M, Turner JN. Controlling cellular reactive responses around neural prosthetic devices using peripheral and local intervention strategies. IEEE Trans Neural Syst Rehabil Eng. 2003;11:186–8. doi: 10.1109/TNSRE.2003.814800. [DOI] [PubMed] [Google Scholar]
- Smith AD, Justice JB. The effect of inhibition of synthesis, release, metabolism and uptake on the microdialysis extraction fraction of dopamine. J Neurosci Methods. 1994;54:75–82. doi: 10.1016/0165-0270(94)90161-9. [DOI] [PubMed] [Google Scholar]
- Szarowski DH, Anderson MD, Retterer S, Spence AJ, Isaacson M, Craighead HG, Turner JN, Shain W. Brain responses to mico-machined silicon devices. Brain Res. 2003;983:23–35. doi: 10.1016/s0006-8993(03)03023-3. [DOI] [PubMed] [Google Scholar]
- Timmerman W, Westerink BHC. Brian microdialysis of GABA and glutamate: what does it signify? Synapse. 1997;27:242–61. doi: 10.1002/(SICI)1098-2396(199711)27:3<242::AID-SYN9>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Turner JN, Shain W, Szarowski DN, Andersen M, Martins S, Isaacson M, Craighead HG. Cerebral astrocyte response to micromachined silicon implants. Exp Neurol. 1999;156:33–49. doi: 10.1006/exnr.1998.6983. [DOI] [PubMed] [Google Scholar]
- Wagner AK, Sokoloski JE, Ren D, Chen X, Khan AS, Zafonte RD, Michael AC, Dixon CE. Controlled cortical impact injury affects dopaminergic transmission in the rat striatum. J Neurochem. 2005;95:457–65. doi: 10.1111/j.1471-4159.2005.03382.x. [DOI] [PubMed] [Google Scholar]
- Warden DL, Gordon B, McAllister TW, Silver JM, Barth JT, Bruns J, Drake A, Gentry T, Katz DI, Kraus J, Labbate LA, Ryan LM, Sparling MB, Walters B, Whyte J, Zapata A, Zitnay G. Guidelines for the pharmacologic treatment of neurobehavioral sequelae of traumatic brain injury. J Neurotrauma. 2006;23:1468–1501. doi: 10.1089/neu.2006.23.1468. [DOI] [PubMed] [Google Scholar]
- Westerink BHC, deVries JB. Characterization of in vivo dopamine release as determined by brain microdialysis after acute and subchronic implantations: methodological aspects. J Neurochem. 1988;51:683–7. doi: 10.1111/j.1471-4159.1988.tb01798.x. [DOI] [PubMed] [Google Scholar]
- Westerink BHC, Tuntler J, Damsma G, Rollema H, de Vries JB. The use of tetrodotoxin for the characterization of drug-enhanced dopamine release in conscious rats studied by brain dialysis. Naunyn-Schemiederg’s Arch Pharmacol. 1987;336:502–7. doi: 10.1007/BF00169306. [DOI] [PubMed] [Google Scholar]
- Wightman RM, Heien MLAV, Wassum KM, Sombers LA, Aragona BJ, Khan AS, Ariansen JL, Cheer JL, Phillips PE, Carelli RM. Dopamine release is heterogeneous within microenvironments of the rat nucleus accumbens. Eur J Neurosci. 2007;26:2046–54. doi: 10.1111/j.1460-9568.2007.05772.x. [DOI] [PubMed] [Google Scholar]
- Williams GV, Castner SA. Under the curve: critical issues for elucidating D1 receptor function in working memory. Neurosci. 2006;139:263–76. doi: 10.1016/j.neuroscience.2005.09.028. [DOI] [PubMed] [Google Scholar]
- Yang H, Michael AC. In vivo fast-scan cyclic voltammetry of dopamine near microdialysis probes. In: Michael AC, Borland LM, editors. Electrochemical methods for neuroscience. CRC Press; Boca Raton, FL: 2007. pp. 489–501. [PubMed] [Google Scholar]
- Yang H, Peters JL, Michael AC. Coupled effects of mass transfer and uptake kinetics on in vivo microdialysis of dopamine. J Neurochem. 1998;71:684–92. doi: 10.1046/j.1471-4159.1998.71020684.x. [DOI] [PubMed] [Google Scholar]
- Zhou F, Shu X, Castellani RJ, Stimmelmayr R, Perry G, Smith MA, Drew KL. Hibernation, a model of neuroprotection. Am J Pathol. 2001;158:2145–51. doi: 10.1016/S0002-9440(10)64686-X. [DOI] [PMC free article] [PubMed] [Google Scholar]