Abstract
Inhibition of phosphodiesterase-4 (PDE4), an enzyme that catalyzes the hydrolysis of cyclic AMP (cAMP), increases phosphorylation of cAMP-response element binding protein (pCREB) and hippocampal neurogenesis, and produces antidepressant-like effects on behavior; however, causal links among these have not been established. In the present study, chronic administration of rolipram produced antidepressant- and anxiolytic-like effects on behavior in mice. It also increased cAMP and pCREB levels in the hippocampus and prefrontal cortex, but increased Sox2, a marker for mitotic progenitor cells, only in the hippocampus. Chronic rolipram treatment also increased hippocampal neurogenesis, as evidenced by increased bromodeoxyuridine (BrdU)-positive cells in the hippocampal dentate gyrus. Methylazoxymethanol (MAM), which is toxic to proliferating cells, reversed rolipram-induced increases in BrdU-positive cells and pCREB in the hippocampus and partially blocked its behavioral effects. Approximately 84% of BrdU-positive cells became newborn neurons, 93% of which co-expressed pCREB; these proportions were not altered by rolipram or MAM, either alone or in combination. Finally, three weeks following the end of MAM treatment, when neurogenesis was no longer inhibited, rolipram again increased hippocampal pCREB, with its antidepressant- and anxiolytic-like effects resumed. Overall, the present results suggest that rolipram produces its effects on behavior in a manner that at least partially depends on its neurogenic action in the hippocampus, targeting mitotic progenitor cells rather than newborn or mature neurons; cAMP/CREB signaling in hippocampal newborn neurons is critical for neurogenesis and contributes to the behavioral effects of rolipram.
Keywords: phosphodiesterase-4 (PDE4), neurogenesis, CREB, hippocampus, behavior, mice
INTRODUCTION
Hippocampal neurogenesis is the process of generating new neurons in the hippocampal dentate gyrus (Duman et al. 2001; Emsley et al. 2005; Gage 2000; Gould et al. 1999; Van Praag et al. 2002). Specifically, progenitor cells residing in the subgranular zone (SGZ) of the dentate gyrus proliferate to form postmitotic daughter cells, which migrate into the granular cell layer and mature into neurons and other cell types. Neurogenesis is not only an important source of new neurons in the adult mammalian brain, but also an extremely dynamic process that can be up- or down-regulated by a variety of pharmacological, environmental, and endocrinogical factors (Bruel-Jungerman et al. 2007; Dranovsky and Hen 2006; Duman et al. 2001; Eisch et al. 2000; Schmidt and Duman 2007). Hippocampal neurogenesis has been shown to be required for antidepressant-like behavior in some circumstances (Santarelli et al. 2003); however, some behavioral effects of certain antidepressants appear to be neurogenesis-independent (Holick et al. 2008).
PDE4, an enzyme that catalyzes the hydrolysis of cAMP and plays a critical role in the control of its intracellular levels, has been implicated in antidepressant activity and memory. Administration of rolipram, a prototypic, selective PDE4 inhibitor, increases cAMP signaling and produces a variety of behavioral actions, including antidepressant-like (O'Donnell and Frith 1999; Zhang et al. 2002, 2006) and memory-enhancing effects in rodents (Barad et al. 1998; Zhang et al. 2000, 2004, 2005), which are associated with increased pCREB in the hippocampus (Blendy 2006; Monti et al. 2006; Sairanen et al. 2007). Chronic, but not acute, treatment with rolipram also increases hippocampal neurogenesis, as evidenced by increased cell proliferation and survival of newborn neurons in the dentate gyrus (Nakagawa et al. 2002a, b; Sasaki et al. 2007); this effect is accompanied by activation of CREB (Fujioka et al. 2004; Nakagawa et al. 2002b; Nibuya et al. 1996; Zhu et al. 2004). However, it has been reported that mice deficient in CREB or expressing dominant negative CREB display antidepressant-like effects on behavior and increased hippocampal neurogenesis (Gur et al. 2007; Newton et al. 2002). Further studies need to be done to clarify this issue.
Compared to the role of PDE4 in antidepressant activity and memory, little is known about that in anxiety. It has been reported in a preliminary study that acute treatment with rolipram produces an anxiolytic-like effect on behavior in rats (Silvestre et al. 1999a). However, anxiogenic-like behavior induced by PDE4 inhibitors including rolipram has been reported (Heaslip and Evans 1995; Imaizumi et al. 1994). Given that mice deficient in CREB display an anxiety-like effect on behavior (Gur et al. 2007), rolipram may produce an anxiolytic-like effect.
Using tests sensitive to antidepressant and anxiolytic drugs, we determined the behavioral effects of chronic treatment with rolipram. We also examined hippocampal neurogenesis and pCREB expression. In addition, we assessed whether increased neurogenesis was required for the behavioral effects of rolipram by co-administrating MAM, an anti-mitotic DNA methylating agent that has been shown to reduce hippocampal neurogenesis (Johnston and Coyle 1979; Shors et al. 2001).
MATERIALS AND METHODS
Animals
Male ICR mice (Harlan, Indianapolis, IN), initially weighing 21−23 g, were housed in a temperature-controlled room with a 12-h light-dark cycle (lights on from 6:00−18:00). Animals had access to food and water ad libitum. All experiments were carried out according to the “NIH Guide for the Care and Use of Laboratory Animals” (NIH Publications No. 80−23, revised 1996). The procedures were approved by the Animal Care and Use Committee of West Virginia University Health Sciences Center.
Drugs
Rolipram, fluoxetine, diazepam, BrdU (Sigma-Aldrich, St. Louis, MO), and MAM (Midwest Research Institute, Kansas City, MO) were dissolved in saline (for BrdU and MAM) or saline containing 5% dimethyl sulfoxide (DMSO). Injections were i.p. except for MAM (s.c.) at a volume of 10 ml/kg body weight.
For testing the effects of chronic drug treatment on behavior, rolipram (0.31, 0.62, 1.25 mg/kg), fluoxetine (10 mg/kg), diazepam (1 mg/kg), or vehicle was given once per day for 17−23 d. All the behavioral tests were performed 1 h after drug injections following the treatment schedules (Figure 1).
Figure 1.
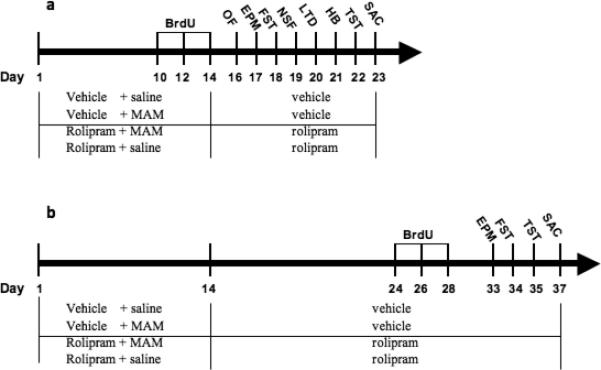
Schedules of drug treatments. (a) Treatment schedule for blockade of neurogenesis by MAM. BrdU or saline was injected (i.p.) on days 10, 12, and 14 (d10, 12, 14; arrowheads). Behavioral tests were carried out on days 16−22 (1 h after the injection of rolipram or vehicle). (b) Treatment schedule for recovery from MAM-induced inhibition of neurogenesis. BrdU was injected on days 24, 26 and 28 (d24, 26, and 28, i.e., 10−14 d after MAM treatment termination). Behavioral tests were carried out on days 33−35. MAM or saline (s.c.) and rolipram or its vehicle (saline containing 5% DMSO; i.p.) were injected once per day for 14 d (i.e., days 1−14), after which only rolipram or vehicle was continued until the end of the tests (d23 or d37) when the animals were sacrificed.
Behavioral experiments
1. Tail-suspension test (TST)
Each mouse was suspended 40 cm above the floor using adhesive tape placed approximately 1 cm from the tip of the tail. The duration of immobility was recorded for 6 min. Mice were considered immobile only when they hung motionless.
2. Forced-swim test (FST)
Mice were placed individually in a plastic cylinder (45 cm high × 20 cm diameter) filled with water (23−24°C; 28 cm in depth), allowing for free swimming. The duration of immobility, which was defined as floating in an upright position without additional movement other than that necessary for the animal to keep its head above the water, was recorded during the last 4 min of the 6-min test period.
3. Elevated plus-maze test
The mouse was placed in the center of the maze (40 cm above the floor) facing an enclosed arm. The number of entries into (with all four paws) and time spent in both open and enclosed arms (30 × 5 cm and 30 × 5 × 15 cm high, respectively) were recorded for 5 min as described previously (Masood et al. 2008). The percentages of entries and time spent in open arms were calculated as open-arm entries and time divided by total arm entries and total time, respectively.
4. Light-dark transition test
Each mouse was placed in the dark compartment (15 × 23 cm) of the light-dark chamber. The latency to cross through an opening (8 × 6 cm) into the light compartment (30 × 23 cm; illuminated with a 60-W bulb positioned 50 cm above), the time spent on the light side, and transitions (i.e., the number of crossings from the dark to light compartments) were recorded for 5 min as described previously (Zhang et al. 2008).
5. Holeboard test
Mice were placed individually in the center of an open Plexiglas box (40 × 40 × 30 cm), which had four holes (3 cm in diameter, 2 cm in depth) in the floor. The number of head-dips and the time spent in head-dipping were recorded for 5 min.
Neurogenesis blockade by MAM
This was performed as described previously (Bruel-Jungerman et al. 2005; Shors et al. 2001) with some modifications. Mice were treated once daily with: 1) vehicle (5% DMSO) + saline, 2) vehicle + MAM (5 mg/kg; chosen based on our preliminary study), 3) rolipram (1.25 mg/kg) + saline, or 4) rolipram + MAM. MAM treatment was terminated after 14 d while rolipram continued to be administered throughout behavioral testing and until animals were sacrificed. For evaluation of neurogenesis, BrdU (100 mg⁄kg) was administered to 4−5 mice in each group on days 10, 12, and 14 (Figure 1a). Beginning from day 16, mice were tested for locomotor activity and anxiolytic- and antidepressant-like behaviors. On day 23, 1 h after the last rolipram injection, the mice were anesthetized with pentobarbital (100 mg/kg) and perfused transcardially with 4% paraformaldehyde. The brains were post-fixed with cold paraformaldehyde overnight and dehydrated with 30% sucrose before coronal sections (30 μm) using a freezing microtome. The remaining mice (4−5 mice/group) were decapitated and the hippocampi and prefrontal cortices dissected and stored at −80°C for immunoblotting analysis or cAMP assay.
Recovery of neurogenesis following MAM inhibition
The treatment assignment and strategy were the same as described above. BrdU was given (also to one-half of the mice in each group) on days 24, 26, and 28 (i.e., 10−14 d after termination of MAM treatment; Figure 1b). Behavioral tests were performed on days 33−35. On day 37, BrdU-treated mice were sacrificed for neurogenesis analysis whereas the remaining mice were used for immunoblotting analysis.
Cyclic AMP assay
The samples extracted by RIPA lysis buffer were diluted with 0.1 N HCl to a final protein concentration of 1 mg/ml. Cyclic AMP levels were determined by ELISA (Assay Designs, Ann Arbor, MI).
Immunoblotting analyses
Brain tissues were sonicated in RIPA lysis buffer (Upstate, Temecula, CA) containing protease and phosphatase inhibitors (Pierce Biotechnology, Rockford, IL) and centrifuged at 16,000×g for 30 min. Samples (75 μg protein each) were separated using SDS-PAGE before transferring to nitrocellulose membranes, which were then incubated with rabbit anti-pCREB (Ser133), anti-CREB, anti-Sox2, or anti-β-actin antibodies (1:1000; Chemicon, Temecula, CA) at 4°C overnight except for the anti-β-actin antibody, which was incubated for 60 min. The membranes were then incubated with Alexa Fluor 700 conjugated goat anti-rabbit antibody (1:20000; Invitrogen, Eugene, OR) for 30 min. The detection and quantification of specific bands were carried out using a fluorescence scanner (Odyssey Infrared Imaging System, LI-COR Biotechnology, Lincoln, NE). For band stripping, the membranes were incubated with stripping buffer (Chemicon, Temecula, CA) for 15 min.
Immunohistochemical analyses
This was performed as described previously (Nakagawa et al. 2002a,b; Fujioka et al. 2004) with minor modifications. For immunofluorescent staining of co-expression of pCREB and calbindin, a selective marker of mature neurons (Encinas et al. 2006), free-floating sections were incubated in 0.01 M PBS containing 0.2% Triton X-100 and 5% normal goat serum (PBS-plus) for 60 min followed by primary antibodies rabbit anti-pCREB IgG (1:200; Chemicon, Temecula, CA) and mouse anti-calbindin IgG (1:50; GeneTex, San Antonio, TX) at 4°C for 1 d. The sections were incubated with anti-mouse rhodamine Red-X (1:200) and anti-rabbit Cy5 (1:400; Jackson ImmunoResearch, West Grove, PA) in PBS for 2 h before mounting on slides using Vectashield (Vector Laboratories, Burlingame, CA).
For immunofluorescent staining of BrdU and its co-localization, free-floating brain sections were incubated in 2×SSC/50% formamide at 65°C for 2 h, followed by 2 N HCl at 37°C for 30 min and 0.1 M boric acid (pH 8.5) for 10 min before blocking with PBS-plus for 60 min. The sections were then incubated at 4°C for 1 d with rat anti-BrdU IgG, mouse anti-polysialic acid-neural cell adhesion molecule (PSA-NCAM, an immature neuron marker; Nakagawa et al. 2002a, b) IgM, rabbit anti-pCREB IgG (1:200), mouse anti-neuron-specific nuclear protein (NeuN) IgG (1:1000; Chemicon, Temecula, CA), or rabbit anti-S100β IgG (1:100; GeneTex, San Antonio, TX), followed by anti-rat FITC (1:200), anti-mouse rhodamine Red-X (1:200), and anti-rabbit Cy5 (1:400; Jackson ImmunoResearch, West Grove, PA) for 2 h before mounting with Vectashield (Vector Laboratories, Burlingame, CA). Fluorescence analyses were performed using confocal laser microscopy (Zeiss LSM510, Thornwood, NY). To determine the percentage of newborn neurons labeled by PSA-NCAM in BrdU-labeled cells and examine pCREB in the newborn cells (i.e., cells labeled with both BrdU and PSA-NCAM), at least fifty BrdU-positive cells in the dentate gyrus were randomly identified from each animal. The number of cells in each category for each animal was determined.
BrdU-positive cells were counted using a modified stereological protocol (Gould et al. 1999; Eisch et al. 2000; Nakagawa et al. 2002a, b; West et al. 1991; Malberg et al. 2000). In brief, every ninth section throughout the entire hippocampus was processed for BrdU immunohistochemistry. All BrdU-labeled cells in the granular cell layer and hilus were counted through a 60× objective lens in order to distinguish individual cells and multiplied by ten, recording as the total number of labeled cells in the dentate gyrus.
Statistical analyses
Data shown represent means ± S.E.M; they were analyzed using one-way analysis of variance (ANOVA) followed by Bonferroni's Multiple Comparison Tests. R2 values were calculated using linear regression.
RESULTS
Anxiolytic-like effects of rolipram
To determine whether rolipram affected anxiety-associated behavior, we examined the performance of mice repeatedly treated with different doses of rolipram in tests sensitive to anxiolytic drugs. In the elevated plus-maze test, a well-established paradigm to detect both anxiolytic- and axiogenic-like behavior, rolipram (0.31−1.25 mg/kg for 17 d) increased the percentages of both entries (F4,36 = 3.04; p < 0.05) into and time (F4,35 = 3.69; p = 0.01) spent in the open arms; it was significant at the dose of 1.25 mg/kg for the former and at all the doses for the latter, compared to the vehicle control (p < 0.05; Figure 2a). A similar effect was produced by the proven anxiolytic diazepam at 1 mg/kg for 17 d (p < 0.05 and p < 0.01 for the percentages of entries and time, respectively). Neither rolipram nor diazepam, at the doses used, altered the total arm entries or the total time spent in arm exploration (Table 1). In the light-dark transition test, another paradigm widely used for anxiolytic testing, rolipram (0.31−1.25 mg/kg for 20 d) decreased the latency to enter the light compartment (F4,36 = 4.41; p < 0.01) and increased the duration on the light side (F4,36 = 6.24; p < 0.001) in a dose-dependent manner; it was significant at the dose of 1.25 mg/kg (p < 0.05; Figure 2b). Again, diazepam produced similar effects (p < 0.05). The effects of rolipram were further confirmed using the holeboard test. Similar to diazepam, the same doses of rolipram administered for 21 d increased the number of head-dips (F4,35 = 3.58; p = 0.01) and the time spent in head-dipping (F4,35 = 3.72; p = 0.01) in a dose-dependent manner (Fig 2c). The effects of rolipram were statistically significant at the dose of 1.25 mg/kg relative to the vehicle control (p < 0.05 for head-dips and p < 0.01 for the head-dipping time). These results suggest anxiolytic-like, behavioral effects of repeated rolipram treatment.
Figure 2.
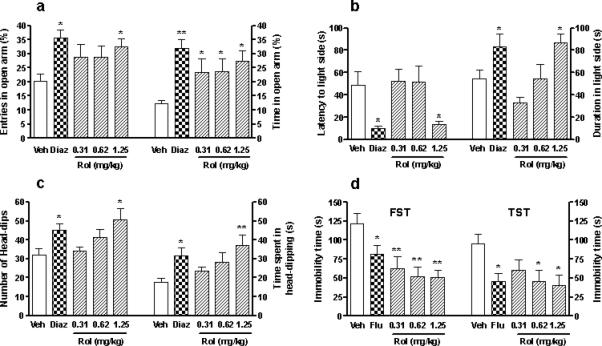
Effects of rolipram on anxiolytic- and antidepressant-like behavior in mice. Treatment with rolipram (Rol) or diazepam (Diaz) increased percentages of entries into and the time spent in the open arms in the elevated plus-maze test (a), decreased latency to enter the light compartment and increased duration on the light side in the light-dark transition test (b), and increased head-dips and the time spent in head-dipping in the holeboard test (c). These suggest anxiolytic-like effects of the drug tretament on behavior. (d) Treatment with rolipram or fluoxetine (Flu) decreased immobility in the forced-swim and tail-suspension tests (FST and TST, respectively), which is indicative of antidepressant-like behavior. Rolipram (0.31−1.25 mg/kg), diazepam (1 mg/kg), fluoxetine (10 mg/kg), or vehicle (Veh) was administered (i.p.) once a day for 17 (a), 20 (b), 21 (c), or 18 d for the FST or 22 d for the TST (d). Tests were performed 1 h after the injection of drugs or Veh on each testing day. Values shown are means ± S.E.M of 8−9 mice per group. * p < 0.05, ** p < 0.01 vs Veh.
Table 1.
Effects of chronic rolipram treatment on total arm entries and exploration time in the elevated plus-maze test in mice
| Treatment and doses (mg/kg) | Total arm activity |
||
|---|---|---|---|
| Entries | Time (s) | ||
| Vehicle | 15.9 ± 2.3 | 234.3 ± 7.8 | |
| Diazepam | 1 | 16.0 ± 0.9 | 222.8 ± 9.1 |
| Rolipram | 0.31 | 15.9 ± 1.8 | 236.0 ± 4.2 |
| 0.62 | 15.6 ± 1.3 | 228.3 ± 9.2 | |
| 1.25 | 16.9 ± 1.1 | 226.1 ± 8.4 | |
Values shown represent means ± S.E.M of 8−9 mice per group. Rolipram, diazepam, or vehicle was injected i.p. once a day for 17 d; the test was performed 1 h after the last injection. Neither diazepam nor rolipram at the doses used altered the total arm entries (p > 0.05) or the total time (p > 0.05) spent in arm exploration.
Antidepressant-like effect of rolipram
Previous studies have shown that rolipram (0.5 mg/kg for 8 d) produces antidepressant-like behavior in the FST and TST in a mixed background (C57 × 129) of mice and NIH Swiss mice (Zhang et al. 2002, 2008). Given that antidepressant-like behavior has strain differences (Lucki et al. 2001), it was necessary to determine whether rolipram produced an antidepressant-like effect in ICR mice using the current treatment strategy. Mice treated with rolipram or the proven antidepressant fluoxetine, a selective inhibitor of serotonin reuptake, were tested for duration of immobility in the FST and TST. Chronic treatment with fluoxetine (10 mg/kg) decreased immobility of mice in both tests (p < 0.05; Figure 2d). Similar effects were produced by rolipram at doses of 0.31−1.25 mg/kg for 18 d (FST) or 22 d (TST) in a dose-dependent manner (F4,35 = 5.36; p < 0.01 for FST; F4,36 = 2.74; p < 0.05 for TST). Specifically, rolipram decreased immobility at all the doses in the FST (p < 0.01) and at the doses of 0.62 and 1.25 mg/kg in the TST (p < 0.05), suggesting an antidepressant-like effect of rolipram on behavior under the present conditions.
Effects of rolipram on cAMP, pCREB and Sox2
The cAMP/CREB pathway plays an important role in the mediation of antidepressant activity (D'Sa and Duman 2002). Fluoxetine (10 mg/kg for 23 d) increased cAMP levels (Figure 3a) and pCREB expression (Figure 3c, d) in both the hippocampus (p < 0.05) and the prefrontal cortex (p < 0.01 for cAMP and p < 0.05 for pCREB), without altering CREB levels. Similarly, chronic treatment with rolipram (0.62−1.25 mg/kg for 23 d; doses chosen based on the behavioral results) also increased cAMP and pCREB in the hippocampus (F3,15 = 3.37; p < 0.05 and F3,12 = 5.55; p = 0.01, respectively) and prefrontal cortex (F3,13 = 6.27; p < 0.01 and F3,12 = 5.21; p < 0.05, respectively); it was significant at the dose of 1.25 mg/kg. By contrast, rolipram did not alter CREB levels in either brain region (F3,12 = 0.32; p = 0.81 for hippocampus and F3,12 = 0.43; p = 0.73 for prefrontal cortex; Figure 3c, d). The effects of rolipram on cAMP and pCREB paralleled its anxiolytic- and antidepressant-like effects, suggesting that the cAMP/CREB cascade in the brain is importantly involved in the mediation of the behavioral effects of rolipram.
Figure 3.
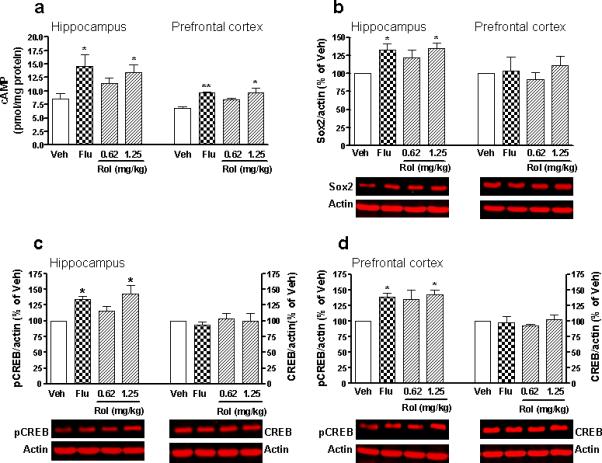
Effects of rolipram (Rol) on levels of cAMP, pCREB, CREB, and Sox2 in the mouse brain. (a) Rolipram and fluoxetine (Flu) increased cAMP levels in the hippocampus and prefrontal cortex. (b) Rolipram and fluoxetine increased Sox2 levels in the hippocampus, but not the prefrontal cortex. (c, d) Rolipram and fluoxetine increased levels of pCREB, but not CREB, in the hippocampus (c) and prefrontal cortex (d). Lower panels (b-d) are representative immunoblots of the measures detected by Western blotting; upper panels are quantification of Sox2, pCREB, or CREB. Rolipram (0.62 and 1.25 mg/kg), fluoxetine (10 mg/kg), or vehicle (Veh) was administered (i.p.) once a day for 23 d; 1 h after the last drug injection, mice were decapitated and hippocampi and prefrontal cortices dissected for cAMP assay or immunoblotting analyses. Values shown are means ± S.E.M of 4−5 mice per group; the immunoblotting data (b-d) are expressed as percentages of corresponding optical density (normalized to β-actin) in the vehicle control samples. * p < 0.05, ** p < 0.01 vs Veh.
To determine the effects of drug treatment on neural progenitors, we examined the expression of Sox2, a transcription factor and an in vivo marker for mitotic neural progenitor cells (Encinas et al. 2006; Episkopou 2005; Graham et al. 2003; Masui et al. 2007; Bani-Yaghoub et al. 2006), in the brain. Fluoxetine increased Sox2 levels in the hippocampus (p < 0.05), but not the prefrontal cortex (Figure 3b). Similarly, chronic treatment with rolipram (0.62−1.25 mg/kg for 23 d) increased the Sox2 levels only in the hippocampus (F3,12 = 4.45; p < 0.05 in contrast to F3,12 = 0.40; p > 0.05 in the prefrontal cortex). These results suggest that rolipram increases progenitor cell proliferation in the hippocampus; the effect appears to be region-specific.
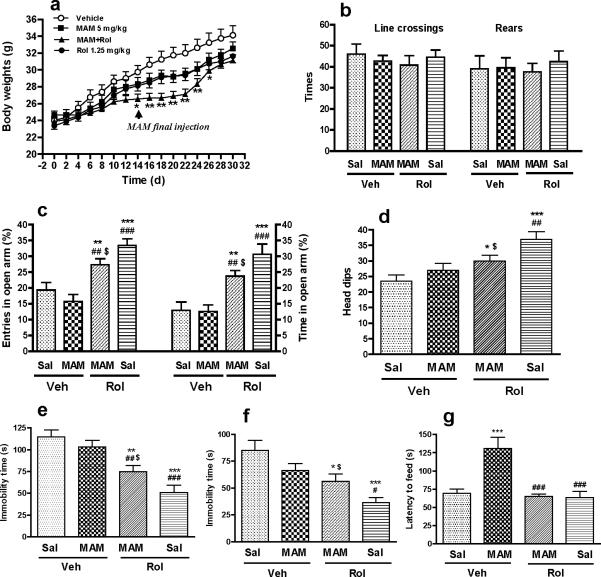
Effects of MAM alone or in combination with rolipram on body weights and locomotor activity
The DNA methylating agent MAM is used for inhibiting neurogenesis (Shors et al. 2001; Bruel-Jungerman et al. 2005). To evaluate the potential, toxic effect of MAM, body weights of mice were monitored throughout the behavioral tests. Fourteen days after continuous administration of MAM (5 mg/kg) or rolipram (1.25 mg/kg), the body weights tended to be decreased, but were not statistically different from those in the vehicle controls. Combination of both drugs led to a slower gain of body weights compared to the vehicle controls; this was significant during days 14−26 (Figure 4a). Beginning from day 28 (i.e., 14 d after termination of MAM), the loss of the body weights recovered to levels that were no longer significant relative to the corresponding control. MAM alone at higher doses (7.5 and 15 mg/kg) significantly reduced the gain of body weights or led to animal deaths (data not shown).
Figure 4.
Effects of MAM and/or rolipram (Rol) on body weights and behaviors. (a) Changes in body weights of mice treated with MAM and rolipram alone or in combination. While the drugs used alone did not significantly alter the body weights, combination of both drugs led to a slower gain of body weights during days 14−26, compared to the corresponding controls. (b) Effects of MAM and/or rolipram on locomotor activity in the open-field test. None of the treatments altered locomotor activity, as assessed by line crossings and rears in the open-field test 2 d after termination of MAM treatment (d16). (c, d) Attenuation by MAM of the anxiolytic-like effects of rolipram on behavior. Rolipram-induced increases in the percentages of entries into and time spent in open arms in the elevated plus-maze test (c) and the number of head-dips in the holeboard test (d) were attenuated by co-administration of MAM. (e, f) Attenuation by MAM of the antidepressant-like effects of rolipram on behavior. Rolipram-induced decreases in immobility in the forced-swim (e) and tail-suspension (f) tests were attenuated by co-administration of MAM. Rolipram (1.25 mg/kg) or vehicle (Veh) was injected (i.p.) once a day for 30 (a), 16 (b), 17 (c), 21 (d), 18 (e), or 22 (f) days. MAM (5 mg/kg) or saline (Sal) was co-administered (s.c.) with rolipram or vehicle for the first 14 d, after which MAM treatment was terminated. The tests were performed 1 h after the daily administration of rolipram or vehicle. Values shown are means ± S.E.M of 8−18 mice per group. * p < 0.05, ** p < 0.01, *** p < 0.001 vs control (Sal + Veh); # p < 0.05, ## p < 0.01, ### p < 0.001 vs MAM + Veh; $ p < 0.05 vs Rol + Sal.
To determine general motor inhibition potentially induced by the drug treatment, locomotor activity of mice treated with MAM and rolipram alone or in combination was examined in the open-field test. The test was carried out twice by splitting the mice (16−18 per group) into two groups, which were tested on days 16 and 19, i.e., 2 and 5 d, respectively, after the last injection of MAM. During this period, the combination of MAM and rolipram led to the slowest gain of body weights (Figure 4a). However, none of the treatments, regardless of using the drugs alone or in combination, altered locomotor activity, as evidenced by unchanged crossings and rears in the open-field test after drug administration (Day 16: F3,27 = 0.7; p > 0.05 for crossings and F3,27 = 0.16; p < 0.05 for rears; Figure 4b; Day19: F3,28 = 0.37; p > 0.05 for crossings and F3,28 = 0.08; p > 0.05 for rears; data not shown). These results suggest that chronic treatment with MAM and/or rolipram at the doses used does not affect the general health nor decrease overall motor activity in mice; rolipram (1.25 mg/kg) administered 1 h prior to the test does not produce sedative effect in the chronic treatment strategy in mice.
Attenuation by MAM of the anxiolytic- and antidepressant-like effects of rolipram on behavior
Repeated treatment with rolipram (1.25 mg/kg plus saline for 17 d) produced anxiolytic-like effects in the elevated-plus maze test, as evidenced by increased percentages of entries into and time spent in the open arms compared to the corresponding control (vehicle + saline; p < 0.001; Figure 4c). The effects of rolipram were attenuated by MAM (5 mg/kg for 14 d; time%: F3,61 = 13.24; p < 0.001; entries%: F3,61 =13.11; p < 0.001); post hoc comparison revealed significant decreases compared to rolipram alone (p < 0.05). MAM alone had no effect. None of the treatments altered the total arm entries (F3,61 = 1.24; p > 0.05) or the total time (F3,61 = 0.19; p > 0.05) in arm exploration (Table 2). Similarly, the anxiolytic-like effect of rolipram in the holeboard test also was attenuated by MAM (F3,61 = 6.69; p < 0.001; Figure 4d). In the FST and TST, MAM alone did not decrease immobility significantly. However, it partially, but significantly, blocked rolipram-induced antidepressant-like effects, i.e., decreased immobility in the FST (F3,61 = 13.33; p < 0.001; Figure 4e) and TST (F3,61 = 8.00; p < 0.001; Figure 4f).
Table 2.
Effects of MAM and/or rolipram on total arm entries and exploration time in the elevate plus-maze test in mice
| Treatments and doses (mg/kg) | Total arm activity |
|
|---|---|---|
| Entries | Time (s) | |
| Vehicle + Saline | 15.6 ± 1.2 | 239.8 ± 5.4 |
| Vehicle + MAM | 16.1 ± 0.9 | 234.0 ± 5.5 |
| Rolipram + MAM | 18.4 ± 1.1 | 237.2 ± 5.5 |
| Rolipram + Saline | 17.3 ± 1.3 | 238.3 ± 6.0 |
Values shown represent means ± S.E.M of 16−18 mice per group. MAM (5 mg/kg, s.c.), saline, rolipram (1.25 mg/kg, i.p.), or vehicle was given once a day for 14 d, after which saline and MAM were terminated while vehicle and rolipram were continued until 1 h prior to the test on d17. None of the treatments altered the total arm entries (p > 0.05) or the total time (p > 0.05) of arm exploration.
Effect of MAM on hippocampal neurogenesis in mice treated with rolipram
To determine the effects of MAM and/or rolipram on hippocampal neurogenesis, mice were sacrificed 13 d after the beginning of BrdU labeling. Cells labeled with BrdU were counted per bilateral, entire hippocampal dentate gyri. BrdU-positive cells were predominantly localized in the subgranular layer and, to a much less extent, in the hilus (Figure 5a). Rolipram (1.25 mg/kg for 23 d) increased, whereas MAM (5 mg/kg for 14 d) decreased the number of BrdU-positive cells in the dentate gyrus (F3,13 = 11.02; p < 0.001; Figure 5a, b). The rolipram-induced increase in BrdU-positive cells was reversed by co-administration of MAM (p < 0.05).
Figure 5.
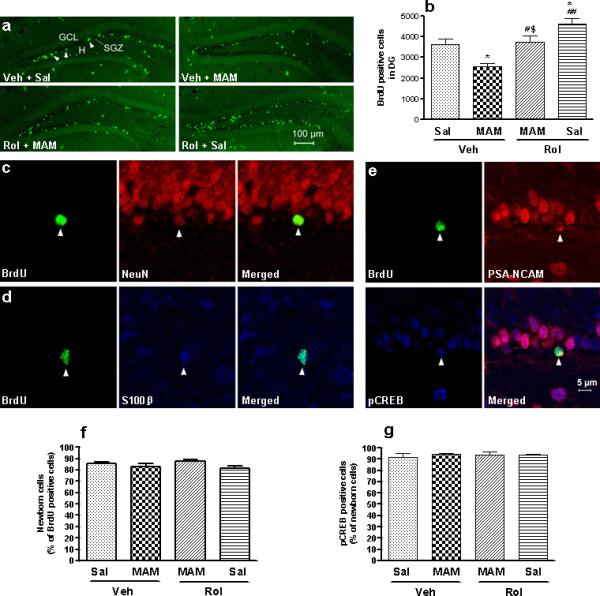
Effects of MAM and/or rolipram (Rol) on BrdU-, PSA-NCAM-, and pCREB-labeled cells in the hippocampal dentate gyrus in mice. (a) Confocal micrographs of BrdU-labeled cells (green) in the dentate gyrus from mice repeatedly treated with vehicle (Veh), MAM, rolipram, or MAM + rolipram. The majority of the BrdU-labeled cells were located in the subgranular zone (SGZ, indicated by arrow). (b) Quantification of BrdU-positive cells following drug treatments. Rolipram increased, while MAM decreased, BrdU-positive cells in the SGZ; the effect of rolipram was reversed by MAM. (c and d) Phenotype of BrdU-positive cells in the dentate gyrus. Confocal micrographs of cells double-labeled for BrdU (green; left panels) and NeuN (red; middle-upper) or S100β (blue; middle-lower). The proportions of neuronal and glial cells (71.4% and 18.5%, respectively) were not altered by any of the treatments. (e) Co-localization of PSA-NCAM and pCREB in developing BrdU-positive cells. Representative confocal micrographs of cells triple-labeled for BrdU, PSA-NCAM (red), and pCREB (blue) in the dentate gyrus. (f) Effects of MAM and/or rolipram on PSA-NCAM-labeled cells, which constituted 84.2% of BrdU-positive cells. (g) Effects of MAM and/or rolipram on pCREB-labeled cells, which constituted 93.4% of PSA-NCAM- and BrdU-positive cells. These percentages were not altered by MAM or rolipram alone or in combination. BrdU (100 mg/kg) was injected (i.p.) once a day on days 10, 12, and 14 of rolipram treatment. Mice were perfused and brain sections were processed 9 d after the last of the three BrdU injections. Values shown are means ± S.E.M of 4−5 mice per group. * p < 0.05 vs Veh; # p < 0.05, ## p < 0.01 vs MAM + Veh; $ p < 0.05 vs Rol + Sal.
To examine the phenotypes of BrdU-positive cells in the dentate gyrus, BrdU staining was carried out 13 d after the first BrdU injection, during which time newborn cells develop differentiated phenotypes (Kempermann et al. 2003); double-labeling for BrdU and NeuN (a neuronal marker; Mullen et al. 1992), or S100β (a glial marker; Boyes et al. 1986) was performed. Confocal microscopy showed that BrdU-positive cells co-localized with NeuN- or S100β-labeled cells (Figure 5c, d), which consisted of 71% and 19% of BrdU-positive cells, respectively. Neither MAM nor rolipram altered the proportions of cells maturing into neurons or glia.
Newborn neurons started to be involved in learning and memory processes when they are approximately 1−2 w of age (Shors, et al. 2001; Bruel-Jungerman et al. 2005); they express PSA-NCAM approximately 2 w before maturing into granule neuronal cells (Encinas et al. 2006). To determine the proportion of newborn neurons among BrdU-positive cells and that of neurons expressing pCREB among newborn neurons, triple staining for BrdU, PSA-NCAM, and pCREB was performed 13 d after the first injection of BrdU. Approximately 84% of BrdU-labeled cells were newborn neurons, as evidenced by co-localization with PSA-NCAM (Figure 5e, f); almost all of the BrdU-labeled newborn neurons (93%) co-expressed pCREB (Figure 5e, g). These proportions were not affected by any of the treatments (F3,12 = 1.67; p > 0.05; Figure 5f and F3,12 = 0.25; p > 0.05; Figure 5g), indicating that pCREB plays an important role in survival of newborn neurons, which appear not to be critical for the initial action of rolipram.
Effects of MAM on pCREB levels in the hippocampus and prefrontal cortex of mice treated with rolipram
To determine the role of cAMP/CREB signaling in the effects of MAM and/or rolipram, we examined the expression of pCREB and CREB in the hippocampus and prefrontal cortex in mice repeatedly treated with either each drug alone or their combination. One-way ANOVA revealed significant changes in levels of pCREB among the treatment groups in the hippocampus (F3,12 = 14.71; p < 0.001; Figure 6a) and prefrontal cortex (F3,12 = 15.76; p < 0.001; Figure 6b). Compared to the control (vehicle + saline), rolipram (1.25 mg/kg for 23 d) increased pCREB levels in both the hippocampus (p < 0.01) and the prefrontal cortex (p < 0.001), whereas MAM (5 mg/kg for 14 d) decreased pCREB only in the hippocampus (p < 0.05). In addition, MAM reversed the rolipram-induced increase in pCREB in the hippocampus (p < 0.01; Figure 6a), but not prefrontal cortex (Figure 6b). Expression of CREB was not altered by any of the treatments (data not shown).
Figure 6.
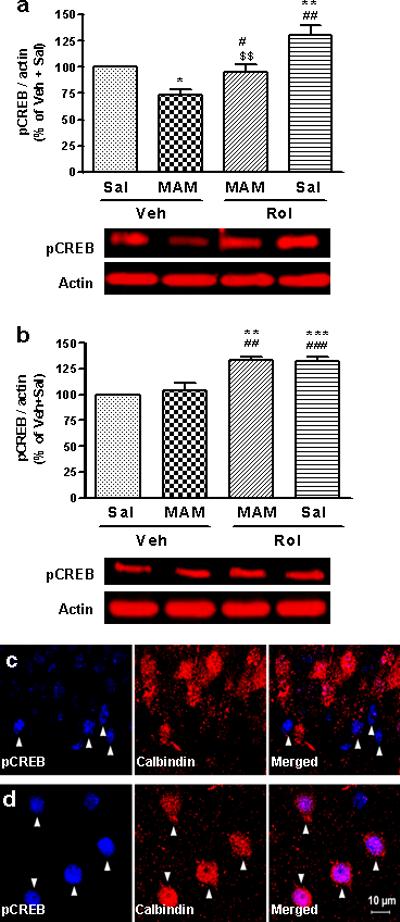
Effects of MAM and/or rolipram (Rol) on pCREB expression and pCREB- and calbindin-labeled cells in the mouse hippocampus and prefrontal cortex. (a) Drug-induced changes in pCREB in the hippocampus; (b) drug-induced changes in pCREB in the prefrontal cortex. Lower panels are representative immunoblots of pCREB detected by Western blotting; upper panels are quantification of pCREB. MAM (5 mg/kg, s.c., 14 d) decreased pCREB in the hippocampus, but not the prefrontal cortex. By contrast, rolipram (1.25 mg/kg, i.p., 23 d) increased pCREB in both brain regions; the effect of rolipram was reversed by MAM only in the hippocampus. (c and d) Confocal micrographs of cells double-labeled for pCREB (blue; left panels) and calbindin (red; middle panels). Mature neurons (labeled by calbindin) in the dentate gyrus (c) did not show pCREB immunostaining; by contrast, calbindin-labeled neurons in the prefrontal cortex (d) all displayed pCREB immunostaining (right panels, indicated by arrows). Values shown are means ± S.E.M of 4 mice per group. * p < 0.05, ** p < 0.01, *** p < 0.001 vs Veh; # p < 0.05, ## p < 0.01, ### p < 0.001 vs MAM + Veh; $$ p < 0.01 vs Rol + Sal.
To explore the potential relationship between the changes in hippocampal neurogenesis and pCREB, the R2 values were calculated using the same treatment groups (i.e., MAM, MAM + Rol, and Rol). It was found that changes in pCREB levels in the hippocampus were highly correlated with those in BrdU-positive cells (R2 = 0.95). Interestingly, while mature neurons labeled by calbindin did not express pCREB in the hippocampal dentate gyrus (Figure 6c), almost all the mature neurons in the prefrontal cortex co-expressed pCREB (Figure 6d). These results suggest that activation of CREB in the newborn neurons in the hippocampus is important in the mediation of hippocampal neurogenesis.
Recovery from MAM-induced changes in hippocampal neurogenesis, pCREB expression, and behavior
To verify the relationship among neurogenesis, pCREB, and behavioral alterations, the effects of MAM and rolipram alone or in combination on these measures were examined 19−23 d and BrdU was injected 10−14d after termination of MAM treatment (Figure 1b); this interval allowed a recovery of neurogenesis from MAM-induced inhibition (Figure 7a, b vs Figure 5a, b). Twenty-three days after the last MAM injection, one-way ANOVA revealed overall significant changes in BrdU-positive cells among treatments (F3,13 = 10.96; p < 0.001). Repeated treatment with rolipram (1.25 mg/kg for 37 d) increased BrdU-positive cells in the dentate gyrus (p < 0.01) and MAM-treated mice no longer displayed a decrease in BrdU-positive cells, compared to the control (vehicle + saline); reversal by MAM of the rolipram-induced increase in BrdU-positive cells (Figure 5a, b) was not observed after the termination of MAM treatment (Figure 7a, b), suggesting a recovery of hippocampal neurogenesis. In addition, expression of pCREB in the hippocampus also was recovered from MAM inhibition in a similar pattern (F3,12 = 6.46; p < 0.01; Figure 7c), i.e., rolipram increased pCREB, which was not changed after the termination of MAM. The rolipram-induced increase in pCREB in the prefrontal cortex was still not changed under this treatment condition (Figure 7d). Expression of CREB was not changed by any of the treatments.
Figure 7.
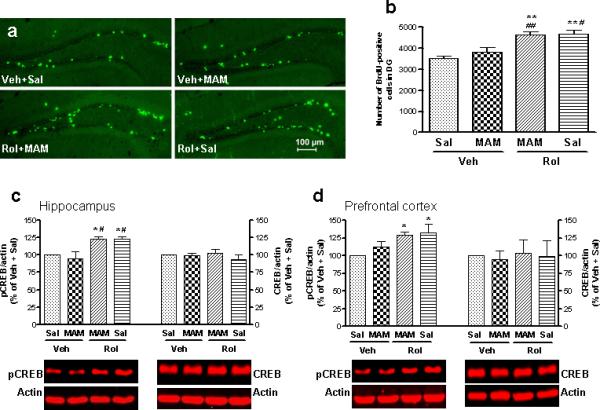
Effects of termination of MAM treatment on rolipram-induced changes in hippocampal neurogenesis and expression of pCREB and CREB in the mouse hippocampus and prefrontal cortex. (a) Confocal micrographs of BrdU-labeled cells (green) in the dentate gyrus from mice, which had been repeatedly treated with vehicle (Veh), MAM, rolipram (Rol), MAM + rolipram, with a 3-week washout of MAM. (b) Quantification of BrdU-positive cells following the drug treatments. Rolipram-induced increases in BrdU-positive cells were no longer inhibited by MAM 3 weeks after termination of its treatment. (c and d) Rolipram-induced increases in pCREB in the hippocampus (c) and prefrontal cortex (d) were not changed after termination of MAM treatment; the treatments did not alter expression of CREB. Lower panels are representative immunoblots of pCREB or CREB detected by Western blotting; upper panels are quantification of pCREB and CREB. Rolipram (1.25 mg/kg) was given (i.p.) for 37 d and MAM (5 mg/kg) was co-administered (s.c.) with rolipram or vehicle for the first 14 d. BrdU (100 mg/kg) was injected (i.p.) once per day on days 24, 26, and 28. Mice were perfused or sacrificed 1 h after the final injection of rolipram or vehicle on day 37. Values shown are means ± S.E.M of 4−5 mice per group. * p < 0.05, ** p < 0.01 vs Veh + Sal; # p < 0.05, ## p < 0.01 vs MAM + Veh.
With the recovery from MAM-induced inhibition of neurogenesis and pCREB expression, rolipram's anxiolytic- and antidepressant-like effects on behavior were no longer attenuated by MAM, including the effects in the elevated plus-maze (entries%: F3,30 = 4.97; p < 0.01 and time%: F3,30 = 8.33; p = 0.001; Figure 8a), FST (F3,30 = 4.22; p = 0.01), and TST (F3,30 = 4.29; p = 0.01; Figure 8b). Total arm activity was not changed in the elevated plus-maze test (Table 3).
Figure 8.
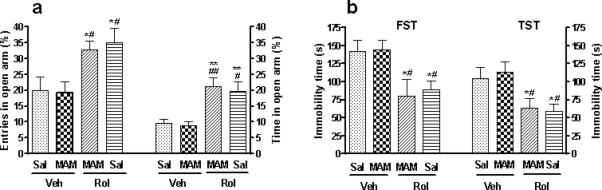
Effects of termination of MAM treatment on rolipram-induced anxiolytic- and antidepressant-like behavior in mice. (a) Rolipram-induced increases in the percentages of entries into and time spent in open arms in the elevated plus-maze were not altered after termination of MAM treatment. (b) Rolipram-induced decreases in immobility in the FST and TST were not changed after termination of MAM. Rolipram (1.25 mg/kg) was given (i.p.) for 33 d before the tests were carried out 1 h after the daily drug injection on days 33 (a), 34 (FST), or 35 (TST). MAM (5 mg/kg) was co-administered (s.c.) with rolipram or vehicle for the first 14 d. Values shown are means ± S.E.M of 8−9 mice per group. * p < 0.05, ** p < 0.01 vs Veh + Sal; # p < 0.05, ## p < 0.01 vs MAM + Veh.
Table 3.
Total arm entries and exploration time in the test of recovery of rolipram-induced anxiolytic-like behavior from MAM inhibition in the elevated plus-maze test in mice
| Treatments and doses (mg/kg) | Total arm activity |
|
|---|---|---|
| Entries | Time (s) | |
| Vehicle + Saline | 15.2 ± 3.1 | 247.8 ± 11.6 |
| Vehicle + MAM | 15.0 ± 1.2 | 250.3 ± 13.4 |
| Rolipram + MAM | 16.4 ± 1.7 | 242.9 ± 8.5 |
| Rolipram + Saline | 14.3 ± 1.1 | 251.5 ± 7.6 |
Values shown represent means ± S.E.M of 8−9 mice per group. MAM (5 mg/kg, s.c.), saline, rolipram (1.25 mg/kg, i.p.), or vehicle was given once a day for 14 d, after which saline and MAM were terminated while vehicle and rolipram were continued until 1 h prior to the test on d33.
DISCUSSION
Chronic treatment with rolipram produced antidepressant- and anxiolytic-like effects on behavior in mice. It also increased neurogenesis and levels of cAMP, pCREB, and Sox2 in the hippocampus. The effects of rolipram on hippocampal neurogenesis and pCREB were completely blocked and those on behavior markedly attenuated by co-administration of MAM, which methylates DNA and inhibits neurogenesis (Shors et al. 2001). The behavioral effects of rolipram were restored following the recovery from MAM-induced decreases in BrdU-positive cells and pCREB in the hippocampus. Overall, changes in hippocampal pCREB were highly correlated with neurogenesis and associated with antidepressant- and anxiolytic-like effects on behavior.
Hippocampal neurogenesis and anxiolytic- and antidepressant-like behavior
It has been shown that cAMP/CREB signaling positively regulates anxiety-like behavior (Pandey et al. 2005; Wand 2005). As a critical controller of this signaling pathway, PDE4 was anticipated to play a role in this process. We found that chronic treatment with rolipram produced anxiolytic-like effects on behavior; this is supported by an earlier study showing that acute treatment with rolipram produces an anxiolytic-like effect (Silvestre et al. 1999a), although opposite results were reported in some other studies (Heaslip and Evans 1995; Imaizumi et al. 1994). The discrepancy may be at least partially due to the sedative effect of PDE4 inhibitors administered acutely (Griebel et al. 1991; Silvestre et al. 1999b), since sedation may be interpreted inappropriately as an anxiogenic-like effect in certain tests (Weiss et al. 1998). However, this was not the case in the present study. Although acute administration of rolipram (1 mg/kg) produces a sedative effect (Silvestre et al. 1999b; Zhang and O'Donnell 2000), repeated treatment with rolipram at 1.25 mg/kg did not alter locomotor activity in the open-field test nor change the total arm exploration in the elevated-plus maze test 1 h post-treatment, when anxiolytic behavior was assessed. The results indicate that, after repeated administration, animals may produce tolerance to the sedative effect of rolipram while sustain sensitivity to its anxiolytic-like action. The behavioral effects produced by chronic rolipram treatment were consistent among different tests sensitive to the proven anxiolytic diazepam. These results are in agreement with the down-regulation of PDE4 induced by diazepam (Cherry et al. 2001) and nicotine (Polesskaya et al. 2007); the latter also exerts anxiolytic- and antidepressant-like effects (Biala and Budzynska 2006; Semba et al. 1998).
Consistent with our previous studies (Zhang et al. 2002, 2006), chronic administration of rolipram also produced antidepressant-like effects on FST and TST behavior. In addition, it increased hippocampal neurogenesis. A causal relationship between anxiolytic/antidepressant and neurogenic effects was indicated by results from co-administration of MAM with rolipram. Inhibition of neurogenesis by MAM attenuated rolipram-induced antidepressant- and anxiolytic-like effects on behavior. The effect of MAM was not due to general toxicity, since MAM at 5 mg/kg decreased neither body weight nor locomotor activity. MAM at higher doses (7.5 and 15 mg/kg) significantly decreased the gain of body weights and even led to animal deaths (data not shown).
When hippocampal neurogenesis recovered to control levels approximately 3 w after termination of MAM treatment, the behavioral effects of rolipram were restored. These results support the contribution of neurogenesis to the antidepressant- and anxiolytic-like effects of rolipram, which is consistent with the requirement of hippocampal neurogenesis for the behavioral effects of certain antidepressants (Santarelli et al. 2003).
Mice treated with combined MAM and rolipram displayed a significantly slower gain of body weights relative to vehicle-treated controls. While the reason for this is not clear, it was likely a physiological change since the animals had normal behavior in terms of general motor activity in the open-field test and total arm exploration in the elevated-plus maze test.
It was noted that, while MAM significantly decreased hippocampal neurogenesis, it did not produce behavioral effects opposite to those of rolipram and only partially blocked the anxiolytic- and antidepressant-like effects of rolipram. These appear to be consistent with the findings that a decrease in neurogenesis is not necessary for the development of depression (Drew and Hen 2007; Reif et al. 2006; Vollmayr et al. 2003). Several reasons may account for this. First, other brain regions, such as the prefrontal cortex and amygdala that do not exhibit adult neurogenesis, also may contribute to the behavioral effects associated with PDE4-mediated cAMP/CREB signaling (Banasr and Duman 2007). This is supported by up-regulation of PDE4 in the frontal cortex induced by learned helplessness, an animal model of depression (Itoh et al. 2003). Second, neurogenesis-independent mechanisms may be involved in the behavioral effects of rolipram; these may include rolipram-induced up-regulation of brain-derived neurotrophic factor (BDNF; Nibuya et al. 1996) and increases in dendritic branching (Fujioka et al. 2004), which also are involved in the regulation of anxiety- and depression-associated behavior (Fujimaki et al. 2000; Vyas et al. 2002). The other possibility is strain specificity. For instance, the antidepressant-like effect of chronic fluoxetine treatment is dependent on its neurogenic effect in 129/Sv mice (Santarelli et al. 2003), but not in BALB/cJ mice (Holick et al. 2008; Huang et al. 2008).
Rolipram also produces a potent anti-inflammatory effect (Zhu et al. 2001). However, this appears not to be involved in the interaction with MAM in terms of behavioral and neurogenic effects, given that MAM does not induce inflammation, which is usually accompanied with the irradiation-induced decrease in neurogenesis (Monje et al. 2002).
Role of hippocampal progenitor cells in rolipram's actions
Adult neurogenesis is characterized by DNA synthesis during the S phase of mitosis of dividing progenitor cells. In the mitotic phase of hippocampal progenitors, quiescent neural progenitors (QNPs) generate amplifying neural progenitors (ANPs) through asymmetric divisions (Encinas et al. 2006). Fluoxetine increases the rate of symmetric divisions of ANPs and subsequently increases newborn neurons in the dentate gyrus (Encinas et al. 2006). However, the type of cells that rolipram may target in the neuronal differentiation cascade has not been investigated. Similar to fluoxetine, rolipram, administered repeatedly at doses that increased pCREB and produced behavioral effects, increased expression of Sox2, a marker of neural progenitor cells (Episkopou 2005; Graham et al. 2003), in the hippocampus. By contrast, neither drug altered Sox2 expression in the prefrontal cortex, suggesting that rolipram increases neural progenitor cells in a brain region-specific fashion.
Rolipram-induced proliferation of progenitor cells appears to be dominated by ANPs. First, Sox2 is only expressed in QNPs and ANPs and BrdU mainly labels ANPs (Encinas et al. 2006). Second, chronic rolipram treatment increases cell proliferation, as evidenced by increased BrdU-labeled cells in the dentate gyrus 2 h after the BrdU injection (Nakagawa et al. 2002b); it increased Sox2 only in the hippocampus. Third, the unaltered proportion of newborn neurons in BrdU-positive cells or pCREB-expressing neurons in newborn neurons indicates that rolipram likely does not directly target the post-mitotic phase of progenitor cells. Thus, rolipram increases neurogenesis likely via originally targeting ANPs in the SGZ. This is supported by the unaltered phenotype of BrdU-positive cells after rolipram administration in the present and previous studies (Malberg et al. 2000; Nakagawa et al. 2002b).
Relationship among cAMP/CREB, neurogenesis, and behavior
The effect of rolipram on pCREB was blocked by MAM in a brain region-specific manner, in that it was observed in the hippocampus but not the prefrontal cortex; the blockade disappeared 3 w after termination of MAM treatment. The changes in pCREB followed the same pattern by those of BrdU-positive cells in the dentate gyrus; these effects were highly correlated. This is supported by previous studies showing that pCREB is restricted to the dentate gyrus in adult mice (Nakagawa et al. 2002a; Thome et al. 2000). By contrast, pCREB in newborn neurons in the dentate gyrus only partially contributed to the behavioral effects of rolipram.
It was interesting that almost all the BrdU-labeled, newborn neurons expressed pCREB, whereas mature neurons in the dentate gyrus labeled by calbindin, a selective marker of mature neurons (Encinas et al. 2006), did not express pCREB. This appears to be supported by previous findings that NeuN-labeled cells (primarily mature neurons) express very low pCREB in the dentate gyrus (Sasaki et al. 2007). By contrast, almost all mature neurons in the prefrontal cortex expressed pCREB, indicating that rolipram differentially affects CREB phosphorylation in the two brain regions. This and the different blocking effects of MAM on rolipram-induced increases in pCREB in the two regions support the brain region-specific profile of CREB function (Carlezon et al. 2005).
In conclusion, the antidepressant- and anxiolytic-like effects of rolipram on behavior are accompanied with increased pCREB and neurogenesis in the hippocampus. Inhibition of neurogenesis with MAM also attenuated the effect of rolipram on both pCREB and behavior. Thus, cAMP/CREB signaling in the hippocampus appears to be critical for hippocampal neurogenesis, which is involved in the mediation of behavioral effects of chronic administration of rolipram; this process likely is mediated via targeting on mitotic progenitor cells in the dentate gyrus. The combined antidepressant- and anxiolytic-like effects of rolipram could benefit in the treatment of comorbid disorders of anxiety and depression, which are thought to share some common genetics (Kendler et al. 1992; Roy et al. 1995).
ACKNOWLEDGEMENTS
This work was supported by research grants from NARSAD and NIA (AG031687 to HTZ) and the NIMH (MH051175, MH040697 to JMO). The authors thank Dr. Albert S. Berrebi for his advice and support and Mr. Dennis Cole, Mr. Jeffrey B. Altemus, and Dr. Karen H. Martin for their technical assistance.
Footnotes
DISCLOSURE/CONFLICT OF INTEREST
Han-Ting Zhang and James M. O'Donnell have received financial support for their research from Memory Pharmaceuticals, Lundbeck Pharmaceuticals, and Wyeth Pharmaceuticals. James M. O'Donnell is on the Scientific Advisory Board of Fission Pharmaceuticals (unpaid). The other authors do not have financial interests to disclose.
REFERENCES
- Bani-Yaghoub M, Tremblay RG, Lei JX, Zhang D, Zurakowski B, Sandhu JK, Smith B, Ribecco-Lutkiewicz M, Kennedy J, Walker PR, Sikorska M. Role of Sox2 in the development of the mouse neocortex. Dev Biol. 2006;295:52–66. doi: 10.1016/j.ydbio.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Banasr M, Duman RS. Regulation of neurogenesis and gliogenesis by stress and antidepressant treatment. CNS Neurol Disord Drug Targets. 2007;6:311–320. doi: 10.2174/187152707783220929. [DOI] [PubMed] [Google Scholar]
- Barad M, Bourtchouladze R, Winder DG, Golan H, Kandel E. Rolipram, a type IV-specific phosphodiesterase inhibitor, facilitates the establishment of long-lasting long-term potentiation and improves memory. Proc Natl Acad Sci USA. 1998;95:15020–15025. doi: 10.1073/pnas.95.25.15020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biala G, Budzynska B. Effects of acute and chronic nicotine on elevated plus maze in mice: involvement of calcium channels. Life Sci. 2006;79:81–88. doi: 10.1016/j.lfs.2005.12.043. [DOI] [PubMed] [Google Scholar]
- Blendy JA. The role of CREB in depression and antidepressant treatment. Biol Psychiatry. 2006;59:1144–1150. doi: 10.1016/j.biopsych.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Boyes B, Kim SU, Lee V, Sung SC. Immunohistochemical colocalization of S-100b and the glial fibrillary acidic protein in rat brain. Neuroscience. 1986;17:857–865. doi: 10.1016/0306-4522(86)90050-3. [DOI] [PubMed] [Google Scholar]
- Bruel-Jungerman E, Laroche S, Rampon C. New neurons in the dentate gyrus are involved in the expression of enhanced long-term memory following environmental enrichment. Eur J Neurosci. 2005;21:513–521. doi: 10.1111/j.1460-9568.2005.03875.x. [DOI] [PubMed] [Google Scholar]
- Bruel-Jungerman E, Rampon C, Laroche S. Adult hippocampal neurogenesis, synaptic plasticity and memory: facts and hypotheses. Rev Neurosci. 2007;18:93–114. doi: 10.1515/revneuro.2007.18.2.93. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Duman RS, Nestler EJ. The many faces of CREB. Trends Neurosci. 2005;28:436–445. doi: 10.1016/j.tins.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Cherry JA, Thompson BE, Pho V. Diazepam and rolipram differentially inhibit cyclic AMP-specific phosphodiesterases PDE4A1 and PDE4B3 in the mouse. Biochim Biophys Acta. 2001;1518:27–35. doi: 10.1016/s0167-4781(01)00164-6. [DOI] [PubMed] [Google Scholar]
- Dranovsky A, Hen R. Hippocampal neurogenesis: regulation by stress and antidepressants. Biol Psychiatry. 2006;59:1136–1143. doi: 10.1016/j.biopsych.2006.03.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew MR, Hen R. Adult hippocampal neurogenesis as target for the treatment of depression. CNS Neurol Disord Drug Targets. 2007;6:205–218. doi: 10.2174/187152707780619353. [DOI] [PubMed] [Google Scholar]
- Duman R, Malberg J, Nakagawa S. Regulation of adult neurogenesis by psychotropic drugs and stress. J Pharmacol Exp Ther. 2001;299:401–407. [PubMed] [Google Scholar]
- D'Sa C, Duman RS. Antidepressants and neuroplasticity. Bipolar Disorders. 2002;4:183–194. doi: 10.1034/j.1399-5618.2002.01203.x. [DOI] [PubMed] [Google Scholar]
- Eisch A, Barrot M, Schad CA, Self DW, Nestler EJ. Opiates inhibit neurogenesis in the adult rat hippocampus. Proc Natl Acad Sci USA. 2000;97:7579–7584. doi: 10.1073/pnas.120552597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emsley JG, Mitchell BD, Kempermann G, Macklis JD. Adult neurogenesis and repair of the adult CNS with neural progenitors, precursors, and stem cells. Prog Neurobiol. 2005;75:321–341. doi: 10.1016/j.pneurobio.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Encinas JM, Vaahtokari A, Enikolopov G. Fluoxetine targets early progenitor cells in the adult brain. Proc Natl Acad Sci USA. 2006;103:8233–8238. doi: 10.1073/pnas.0601992103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Episkopou V. SOX2 functions in adult neural stem cells. Trends Neurosci. 2005;28:219–221. doi: 10.1016/j.tins.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Fujioka T, Fujioka A, Duman RS. Activation of cAMP signaling facilitates the morphological maturation of newborn neurons in adult hippocampus. J Neurosci. 2004;24:319–328. doi: 10.1523/JNEUROSCI.1065.03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimaki K, Morinobu S, Duman RS. Administration of a cAMP phosphodiesterase 4 inhibitor enhances antidepressant-induction of BDNF mRNA in rat hippocampus. Neuropsychopharmacology. 2000;22:42–51. doi: 10.1016/S0893-133X(99)00084-6. [DOI] [PubMed] [Google Scholar]
- Gage F. Mammalian neural stem cells. Science. 2000;287:1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- Gould E, Beylin A, Tanapat P, Reeves A, Shors TJ. Learning enhances adult neurogenesis in the hippocampal formation. Nat Neurosci. 1999;2:260–265. doi: 10.1038/6365. [DOI] [PubMed] [Google Scholar]
- Graham V, Khudyakov J, Ellis P, Pevny L. SOX2 functions to maintain neural progenitor identity. Neuron. 2003;39:749–765. doi: 10.1016/s0896-6273(03)00497-5. [DOI] [PubMed] [Google Scholar]
- Griebel G, Misslin R, Vogel E, Bourguignon JJ. Behavioral effects of rolipram and structurally related compounds in mice: behavioral sedation of cAMP phosphodiesterase inhibitors. Pharmacol Biochem Behav. 1991;39:321–323. doi: 10.1016/0091-3057(91)90186-6. [DOI] [PubMed] [Google Scholar]
- Gur TL, Conti AC, Holden J, Bechtholt AJ, Hill TE, Lucki I, Malberg JE, Blendy JA. cAMP response element-binding protein deficiency allows for increased neurogenesis and a rapid onset of antidepressant response. J Neurosci. 2007;27:7860–7868. doi: 10.1523/JNEUROSCI.2051-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaslip RJ, Evans DY. Emetic, central nervous system, and pulmonary activities of rolipram in the dog. Eur J Pharmacol. 1995;286:281–290. doi: 10.1016/0014-2999(95)00457-2. [DOI] [PubMed] [Google Scholar]
- Holick KA, Lee DC, Hen R, Dulawa SC. Behavioral effects of chronic fluoxetine in BALB/cJ mice do not require adult hippocampal neurogenesis or the serotonin 1A receptor. Neuropsychopharmacology. 2008;33:406–417. doi: 10.1038/sj.npp.1301399. [DOI] [PubMed] [Google Scholar]
- Huang GJ, Bannerman D, Flint J. Chronic fluoxetine treatment alters behavior, but not adult hippocampal neurogenesis, in BALB/cJ mice. Mol Psychiatry. 2008;13:119–121. doi: 10.1038/sj.mp.4002104. [DOI] [PubMed] [Google Scholar]
- Imaizumi M, Miyazaki S, Onodera K. Effects of a non-xanthine adenosine antagonist, CGS 15943, and a phosphodiesterase inhibitor, Ro 20−1724, in a light/dark test in mice. Methods Find Exp Clin Pharmacol. 1994;16:717–721. [PubMed] [Google Scholar]
- Itoh T, Abe K, Tokumura M, Horiuchi M, Inoue O, Ibii N. Different regulation of adenylyl cyclase and rolipram-sensitive phosphodiesterase activity on the frontal cortex and hippocampus in learned helplessness rats. Brain Res. 2003;991:142–149. doi: 10.1016/j.brainres.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Johnston MV, Coyle JT. Histological and neurochemical effects of fetal treatment with methylazoxymethanol on rat neocortex in adulthood. Brain Res. 1979;170:135–155. doi: 10.1016/0006-8993(79)90946-6. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Gast D, Kronenberg G, Yamaguchi M, Gage FH. Early determination and long-term persistence of adult-generated new neurons in the hippocampus of mice. Development. 2003;130:391–399. doi: 10.1242/dev.00203. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Neale MC, Kessler RC, Heath AC, Eaves LJ. Major depression and generalized anxiety disorder. Same genes, (partly) different environments? Arch Gen Psychiatry. 1992;49:716–722. doi: 10.1001/archpsyc.1992.01820090044008. [DOI] [PubMed] [Google Scholar]
- Lucki I, Dalvi A, Mayorga AJ. Sensitivity to the effects of pharmacologically selective antidepressants in different strains of mice. Psychopharmacology (Berl) 2001;155:315–322. doi: 10.1007/s002130100694. [DOI] [PubMed] [Google Scholar]
- Malberg J, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult hippocampus. J Neurosci. 2000;20:9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masood A, Nadeem A, Mustafa SJ, O'Donnell JM. Reversal of oxidative stress-induced anxiety by inhibition of phosphodiesterase-2 in mice. J Pharmacol Exp Ther. 2008;326:369–379. doi: 10.1124/jpet.108.137208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masui S, Nakatake Y, Toyooka Y, Shimosato D, Yagi R, Takahashi K, Okochi H, Okuda A, Matoba R, Sharov AA, Ko MS, Niwa H. Pluripotency governed by Sox2 via regulation of Oct3/4 expression in mouse embryonic stem cells. Nat Cell Biol. 2007;9:625–635. doi: 10.1038/ncb1589. [DOI] [PubMed] [Google Scholar]
- Monje ML, Mizumatsu S, Fike JR, Palmer TD. Irradiation induces neural precursor-cell dysfunction. Nat Med. 2002;8:955–962. doi: 10.1038/nm749. [DOI] [PubMed] [Google Scholar]
- Monti B, Berteotti C, Contestabile A. Subchronic rolipram delivery activates hippocampal CREB and arc, enhances retention and slows down extinction of conditioned fear. Neuropsychopharmacology. 2006;31:278–286. doi: 10.1038/sj.npp.1300813. [DOI] [PubMed] [Google Scholar]
- Mullen R, Buck CR, Smith AM. NeuN, a neuronal specific nuclear protein in vertebrates. Development. 1992;116:201–211. doi: 10.1242/dev.116.1.201. [DOI] [PubMed] [Google Scholar]
- Nakagawa S, Kim JE, Lee R, Chen J, Fujioka T, Malberg J, Tsuji S, Duman RS. Localization of phosphorylated cAMP response element binding protein in immature neurons of adult hippocampus. J Neurosci. 2002a;22:9868–9876. doi: 10.1523/JNEUROSCI.22-22-09868.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa S, Kim JE, Lee R, Malberg JE, Chen J, Steffen C, Zhang YJ, Nestler EJ, Duman RS. Regulation of neurogenesis in adult mouse hippocampus by cAMP and the cAMP response element-binding protein. J Neurosci. 2002b;22:3673–3682. doi: 10.1523/JNEUROSCI.22-09-03673.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton SS, Thome J, Wallace TL, Shirayama Y, Schlesinger L, Sakai N, Chen J, Neve R, Nestler EJ, Duman RS. Inhibition of cAMP response element-binding protein or dynorphin in the nucleus accumbens produces an antidepressant-like effect. J Neurosci. 2002;22:10883–10890. doi: 10.1523/JNEUROSCI.22-24-10883.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nibuya M, Nestler EJ, Duman RS. Chronic antidepressant administration increases the expression of cAMP response element binding protein (CREB) in rat hippocampus. J Neurosci. 1996;16:2365–2372. doi: 10.1523/JNEUROSCI.16-07-02365.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell JM, Frith S. Behavioral effects of family-selective inhibitors of cyclic nucleotide phosphodiesterases. Pharmacol Biochem Behav. 1999;63:185–192. doi: 10.1016/s0091-3057(98)00267-6. [DOI] [PubMed] [Google Scholar]
- Pandey SC, Zhang H, Roy A, Xu T. Deficits in amygdaloid cAMP-responsive element-binding protein signaling play a role in genetic predisposition to anxiety and alcoholism. J Clin Invest. 2005;115:2762–2773. doi: 10.1172/JCI24381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polesskaya OO, Smith RF, Fryxell KJ. Chronic nicotine doses down-regulate PDE4 isoforms that are targets of antidepressants in adolescent female rats. Biol Psychiatry. 2007;61:56–64. doi: 10.1016/j.biopsych.2006.03.038. [DOI] [PubMed] [Google Scholar]
- Reif A, Fritzen S, Finger M, Strobel A, Lauer M, Schmitt A, Lesch KP. Neural stem cell proliferation is decreased in schizophrenia, but not in depression. Mol Psychiatry. 2006;11:514–522. doi: 10.1038/sj.mp.4001791. [DOI] [PubMed] [Google Scholar]
- Roy MA, Neale MC, Pedersen NL, Mathé AA, Kendler KS. A twin study of generalized anxiety disorder and major depression. Psychol Med. 1995;25:1037–1049. doi: 10.1017/s0033291700037533. [DOI] [PubMed] [Google Scholar]
- Sairanen M, O'Leary OF, Knuuttila JE, Castrén E. Chronic antidepressant treatment selectively increases expression of plasticity-related proteins in the hippocampus and medial prefrontal cortex of the rat. Neuroscience. 2007;144:368–374. doi: 10.1016/j.neuroscience.2006.08.069. [DOI] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, Hen R. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Kitagawa K, Omura-Matsuoka E, Todo K, Terasaki Y, Sugiura S, Hatazawa J, Yagita Y, Hori M. The phosphodiesterase inhibitor rolipram promotes survival of newborn hippocampal neurons after ischemia. Stroke. 2007;38:1597–1605. doi: 10.1161/STROKEAHA.106.476754. [DOI] [PubMed] [Google Scholar]
- Schmidt HD, Duman RS. The role of neurotrophic factors in adult hippocampal neurogenesis, antidepressant treatments and animal models of depressive-like behavior. Behav Pharmacol. 2007;18:391–418. doi: 10.1097/FBP.0b013e3282ee2aa8. [DOI] [PubMed] [Google Scholar]
- Semba J, Mataki C, Yamada S, Nankai M, Toru M. Antidepressant-like effects of chronic nicotine on learned helplessness paradigm in rats. Biol Psychiatry. 1998;43:389–391. doi: 10.1016/s0006-3223(97)00477-0. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Miesegaes G, Beylin A, Zhao MR, Rydel T, Gould E. Neurogenesis in the adult is involved in the formation of trace memories. Nature. 2001;410:372–376. doi: 10.1038/35066584. [DOI] [PubMed] [Google Scholar]
- Silvestre JS, Fernandez AG, Palacios JM. Effects of rolipram on the elevated plus-maze test in rats: a preliminary study. J Psychopharmacol. 1999a;13:274–277. doi: 10.1177/026988119901300309. [DOI] [PubMed] [Google Scholar]
- Silvestre JS, Fernandez AG, Palacios JM. Preliminary evidence for an involvement of the cholinergic system in the sedative effects of rolipram in rats. Pharmacol Biochem Behav. 1999b;64:1–5. doi: 10.1016/s0091-3057(98)00243-3. [DOI] [PubMed] [Google Scholar]
- Thome J, Sakai N, Shin K, Steffen C, Zhang YJ, Impey S, Storm D, Duman RS. cAMP response element- mediated gene transcription is upregulated by chronic antidepressant treatment. J Neurosci. 2000;20:4030–4036. doi: 10.1523/JNEUROSCI.20-11-04030.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollmayr B, Simonis C, Weber S, Gass P, Henn F. Reduced cell proliferation in the dentate gyrus is not correlated with the development of learned helplessness. Biol Psychiatry. 2003;54:1035–1040. doi: 10.1016/s0006-3223(03)00527-4. [DOI] [PubMed] [Google Scholar]
- Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci. 2002;22:6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wand G. The anxious amygdala: CREB signaling and predisposition to anxiety and alcoholism. J Clin Invest. 2005;115:2697–2699. doi: 10.1172/JCI26436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss SM, Wadsworth G, Fletcher A, Dourish CT. Utility of ethological analysis to overcome locomotor confounds in elevated maze models of anxiety. Neurosci Biobehav Rev. 1998;23:265–271. doi: 10.1016/s0149-7634(98)00027-x. [DOI] [PubMed] [Google Scholar]
- West M, Slomianka L, Gundersen H. Unbiased stereological estimation of the total number of neurons in the subdivisions of the rat hippocampus using the optical fractionator. Anat Rec. 1991;231:482–497. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- Zhang HT, O'Donnell JM. Effects of rolipram on scopolamine-induced impairment of working and reference memory in the radial-arm maze tests in rats. Psychopharmacology (Berl) 2000;150:311–316. doi: 10.1007/s002130000414. [DOI] [PubMed] [Google Scholar]
- Zhang HT, Crissman AM, Dorairaj NR, Chandler LJ, O'Donnell JM. Inhibition of cyclic AMP phosphodiesterase (PDE4) reverses memory deficits associated with NMDA receptor antagonism. Neuropsychopharmacology. 2000;23:198–204. doi: 10.1016/S0893-133X(00)00108-1. [DOI] [PubMed] [Google Scholar]
- Zhang HT, Huang Y, Jin SL, Frith SA, Suvarna N, Conti M, O'Donnell JM. Antidepressant-like profile and reduced sensitivity to rolipram in mice deficient in the PDE4D phosphodiesterase enzyme. Neuropsychopharmacology. 2002;27:587–595. doi: 10.1016/S0893-133X(02)00344-5. [DOI] [PubMed] [Google Scholar]
- Zhang HT, Zhao Y, Huang Y, Dorairaj NR, Chandler LJ, O'Donnell JM. Inhibition of the phosphodiesterase 4 (PDE4) enzyme reverses memory deficits produced by infusion of the MEK inhibitor U0126 into the CA1 subregion of the rat hippocampus. Neuropsychopharmacology. 2004;29:1432–1439. doi: 10.1038/sj.npp.1300440. [DOI] [PubMed] [Google Scholar]
- Zhang HT, Huang Y, Suvarna NU, Deng C, Crissman AM, Hopper AT, De Vivo M, Rose GM, O'Donnell JM. Effects of the novel PDE4 inhibitors MEM1018 and MEM1091 on memory in the radial-arm maze and inhibitory avoidance tests in rats. Psychopharmacology (Berl) 2005;179:613–619. doi: 10.1007/s00213-004-2085-2. [DOI] [PubMed] [Google Scholar]
- Zhang HT, Zhao Y, Huang Y, Deng C, Hopper AT, De Vivo M, Rose GM, O'Donnell JM. Antidepressant-like effects of PDE4 inhibitors mediated by the high-affinity rolipram binding state (HARBS) of the phosphodiesterase-4 enzyme (PDE4) in rats. Psychopharmacology (Berl) 2006;186:209–217. doi: 10.1007/s00213-006-0369-4. [DOI] [PubMed] [Google Scholar]
- Zhang HT, Huang Y, Masood A, Stolinski LR, Li Y, Zhang L, Dlaboga D, Jin SL, Conti M, O'Donnell JM. Anxiogenic-like behavioral phenotype of mice deficient in phosphodiesterase 4B (PDE4B). Neuropsychopharmacology. 2008;33:1611–1623. doi: 10.1038/sj.npp.1301537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Mix E, Winblad B. The antidepressant and antiinflammatory effects of rolipram in the central nervous system. CNS Drug Rev. 2001;7:387–398. doi: 10.1111/j.1527-3458.2001.tb00206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu DY, Lau L, Liu SH, Wei JS, Lu YM. Activation of cAMP-response-element-binding protein (CREB) after focal cerebral ischemia stimulates neurogenesis in the adult dentate gyrus. Proc Natl Acad Sci USA. 2004;101:9453–9457. doi: 10.1073/pnas.0401063101. [DOI] [PMC free article] [PubMed] [Google Scholar]