Summary
Prenatal ethanol (E) exposure programs the fetal hypothalamic-pituitary-adrenal (HPA) and -gonadal (HPG) axes such that E rats show HPA hyperresponsiveness to stressors and altered HPG and reproductive function in adulthood. Importantly, prenatal ethanol may differentially alter stress responsiveness in adult male and female offspring compared to their control counterparts. To test the hypothesis that alterations in HPA activity in E males are mediated, at least in part, by ethanol-induced changes in the capacity of testosterone to regulate HPA activity, we explored dose-related effects of testosterone on HPA and HPG function in adult male offspring from prenatal E, pair-fed (PF) and ad libitum-fed control (C) dams. Our data suggest that E males show changes in both HPA and HPG regulation, as well as altered sensitivity to the inhibitory effects of testosterone. While gonadectomy (GDX) reduced weight gain in all animals, low testosterone replacement restored body weights in PF and C but not E males. Further, sensitivity of the thymus and adrenal to circulating testosterone was reduced in E rats. In addition, stress-induced corticosterone (CORT) levels were increased in PF and C but not E males following GDX, and while low dose testosterone replacement restored CORT levels for PF and C, high testosterone levels were needed to normalize CORT levels for E males. A negative correlation between pre-stress testosterone and post-stress CORT levels in C but not in E and PF males further supports the finding of reduced sensitivity to testosterone. Importantly, testosterone appeared to have reduced effects on central corticotrophin releasing hormone (CRH) pathways in E, but greater effects on central arginine vasopressin (AVP) pathways in E and/or PF compared to C males. Testosterone also had less of an inhibitory effect on stress-induced luteinizing hormone increases in E than in PF and C males following GDX. In addition, androgen receptor mRNA levels in the medial preoptic nucleus and the principal nucleus of posterior bed nucleus of the stria terminalis were lower in E and PF compared to C males under intact conditions.
Together, these data support our previous work suggesting altered sensitivity to testosterone in E males. Furthermore, differential effects of testosterone on the complex balance between central CRH and central AVP pathways may play a role in the HPA alterations observed. That some findings were similar in E and PF males suggests that nutritional effects of diet may have played a role in mediating at least some of the changes seen in E animals.
Keywords: prenatal ethanol, testosterone, stress, pituitary-adrenal, pituitary-gonadal
1. Introduction
Fetal Alcohol Spectrum Disorder (FASD) refers to the wide range of abnormalities or deficits that result from prental alcohol exposure (Hoyme et al., 2005). The effects range from physical and physiological abnormalities to altered cognitive and behavioral function, compromising an individual’s ability to adapt to his/her environment. Work in our lab has focused on the effects of prenatal ethanol exposure on responses to stress and hypothalamic-pituitary-adrenal (HPA) regulation in adult offspring. Both clinical and animal studies have shown that ethanol exposure in utero results in HPA hyperactivity (Jacobson and Jacobson, 1999; Lee et al., 2000; Ramsay et al., 1996; Taylor et al., 1984; Weinberg, 1989). Importantly, however, while HPA hyperresponsiveness is a robust phenomenon, differential effects of prenatal ethanol exposure may be observed in male and female offspring, depending on the nature and intensity of the stressor, and the time course and hormonal endpoint examined (Lee and Rivier, 1996; Taylor et al., 1983; Taylor et al., 1988; Weinberg, 1988; 1992; Weinberg et al., 2008). These findings raise the possibility that ethanol-induced alterations in the gonadal hormones and/or in HPA-hypothalamic-pituitary-gonadal (HPG) interactions may play a role in mediating prenatal ethanol effects on HPA activity in adulthood.
Effects of the gonadal hormones on HPA function have been demonstrated at different levels of the axis. In general, estradiol activates, and androgens inhibit HPA activity. For example, androgens inhibit corticotrophin releasing hormone (CRH) expression (Bingaman et al., 1994), and gonadectomy (GDX) of adult male rats increases both adrenocorticotropin (ACTH) and CORT responses to physical and psychological stressors (Handa et al., 1994), an effect reversed by replacement with testosterone or the non-aromatizable androgen, 5α-dihydrotestosteone (5α-DHT) (Handa et al., 1994; Viau et al., 2003). GDX rats also show greater stress-induced Fos expression and higher arginine vasopressin (AVP) hnRNA levels than intact males, both of which are negatively correlated with plasma testosterone levels (Viau et al., 2003).
HPA responses are driven by neurons located in the medial parvocellular subdivision of the paraventricular nucleus (mpd PVN) of the hypothalamus. The mpd PVN contains both CRH- and AVP-expressing neurosecretory neurons, and is the final pathway that integrates multiple excitatory and inhibitory inputs from other brain areas regulating the HPA axis, Mapping studies have deomonstrated that androgen receptors (ARs) are not localized in mpd PVN, but restricted to the parvocellular ventral division (mpv PVN) and the periventricular and dorsal parvocellular areas of the PVN (Bingham et al., 2006; Shughrue et al., 1997; Simerly et al., 1990; Zhou et al., 1994), suggesting that androgens act upstream from the PVN to regulate HPA output. Candidate brain areas for androgenic effects are the medial preoptic area (MPOA) [ARs are densest in the medial preoptic nucleus (MPN) of the MPOA], bed nucleus of the stria terminalis (BNST), amygdala, and hippocampus, regions that all contain high densities of ARs (Bingham et al., 2006; Kerr et al., 1995; Shughrue et al., 1997; Simerly et al., 1990; Williamson and Viau, 2007; Zhou et al., 1994). Furthermore, the anterior and posterior divisions of the BNST contain CRH- and AVP-projecting cells that terminate in the PVN (Champagne et al., 1998; Moga and Saper, 1994), and relay information to the PVN from the central and medial nuclei of the amygdala (Dong et al., 2001a; Dong et al., 2001b; Prewitt and Herman, 1998). The amygdala is activated during stress primarily by ascending catecholaminergic neurons in the brainstem or by emotional stressors, and the activation of these neurons leads to anxiety, fear, and stress system activation (Davis, 1992).
In a previous study, we found that intact E rats show increased ACTH but blunted testosterone and luteinizing hormone (LH) responses to restraint stress, and no stress-induced elevation in AVP mRNA levels compared to PF and/or control rats. Importantly, GDX significantly increased ACTH responses to stress in control but not E and PF males, eliminated differences among groups in plasma ACTH and AVP mRNA levels, and altered LH and gonadotropin-releasing hormone (GnRH) responses in E males. These findings indicate that central regulation of both the HPA and HPG axes are altered by prenatal ethanol exposure, with normal testicular influences on HPA function markedly reduced in E males (Lan et al., 2006).
To explore more directly the effects of testosterone on HPA regulation and responsiveness, the present study examined HPA and HPG activity in male rats under intact (sham GDX) conditions, and following GDX, with or without testosterone replacement at low or high concentrations. Our experimental questions were: 1) Do E males differ from controls in adrenal and gonadal hormone levels under intact conditions, following GDX and following GDX with low or high testosterone replacement? 2) Do differences between E and control animals with different circulating testosterone levels occur under both basal and stress conditions? 3) Do central measures of HPA and HPG activity differ in E compared to control males under different circulating testosterone levels? and 4) Is activity of central testosterone-sensitive pathways that regulate CRH and AVP neurosecretory neurons altered in E compared to controls males? We tested the hypothesis that the differential alterations in HPA activity observed in E males compared to their control counterparts are mediated, at least in part, by ethanol induced changes in the capacity of testosterone to regulate HPA activity.
2. Materials and methods
2.1. Animals and Breeding
Male (275–300 g, n = 18) and female (230–275 g, n = 46) Sprague-Dawley rats were obtained from Charles River Laboratories (St. Constant, PQ, Canada). Rats were group-housed by sex until breeding and maintained on a 12:12 hr light/dark cycle (lights on at 06:00 hr), with controlled temperature (21–22 °C), and ad libitum access to standard lab chow and water. Animals were bred one to two weeks following arrival. All animal use and care procedures were in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals, and were approved by the University of British Columbia Animal Care Committee.
2.2. Diets and Feeding
On gestation day (G) 1, females were singly housed and randomly assigned to one of three treatment groups: 1) Ethanol (E), liquid ethanol diet (36 % ethanol-derived calories) and water, ad libitum (n = 16); 2) Pair-fed (PF), liquid control diet with maltose-dextrin isocalorically substituted for ethanol, and intake matched to the amount consumed by an E partner (g/kg body weight/gestation d), and water ad libitum (n = 15); 3) Control (C): standard lab chow and water, ad libitum (n = 15). E females were gradually introduced to the ethanol diet by providing a mixture of ethanol and control diet over 3 days: 1/3 ethanol : 2/3 control diet on G 1, 2/3 ethanol : 1/3 control diet on G 2, and 100% ethanol diet on G 3. The liquid diets (Dyets Inc., Bethlehem, PA) were formulated in our lab to provide adequate nutrition to pregnant rats regardless of ethanol intake. E and PF dams were provided with fresh liquid diet daily within 1.5 hr prior to lights off, and the previous night’s bottle was weighed to determine the amount consumed by each animal. Experimental diets were continued through G 21, and beginning on G 22, animals were provided ad libitum access to standard lab chow and water, which they received throughout lactation. Pregnant dams were handled only on G 1, G 7, G 14 and G 21 for cage changing and weighing. On postnatal day 1 (PN 1), pups were weighed and litters were randomly culled to 10 (5 males and 5 females when possible). If necessary, pups from the same prenatal treatment group born on the same day were fostered into a litter to maintain the litter size. We did not observe alcohol withdrawal symptoms (eg. reduced food intake, anxiety-like behaviours, tremors) in our E dams during the lactation period.
Litters remained with their natural mothers until weaning. Previous research indicates that cross-fostering is not a necessary control in our animal model, as ethanol-induced alterations in mother-pup interactions appear to result primarily from direct effects of ethanol on the pup rather than through alterations in maternal behavior. For example, pups prenatally exposed to ethanol show selective changes in isolation-induced ultrasonic vocalizations that may result in impaired maternal-infant communication (Barron and Gilbertson, 2005; Kehoe and Shoemaker, 1991; Marino et al., 2002; Vorhees, 1989), and a decreased ability to elicit retrieval behavior from both control and ethanol-exposed dams (Ness and Franchina, 1990). Dams and pups were weighed on PN 1, PN 8, PN 15 and PN 22. On PN 22, pups were weaned, and group-housed by litter and sex. Animals were weighed once a week until testing.
2.3. Gonadectomy (GDX) and Testosterone Replacement
Male offspring (53–58 d of age) from the 3 prenatal groups were randomly assigned to Sham GDX (Intact, INT), GDX or GDX with low (GDX-L) or high (GDX-H) testosterone replacement conditions. Testes were removed under halothane anaesthesia via a longitudinal scrotal incision. Sham surgery was done by making an incision and then suturing the scrotum, without touching the testes. Testosteronewas provided by implantation of silastic capsules (2.5 cm length; 0.062 i.d., 0.125 o.d.) packed with crystalline testosterone (Sigma-Aldrich, Saint Louis MO, USA, T-1500) designed to provide circulating testosterone concentrations approximating low (~ 1 ng/ml) and high (4 ~ 6 ng/ml) physiological basal levels. Surgery was done at 0900–1200 hr each day, and animals were weighed prior to surgery and again 1 d before testing.
2.4 Restraint Stress, Sampling and Tissue Collection
Testing occurred 14 d after surgery. Rats were placed into a polyvinyl chloride restraint tube for a 30 min period. The restraint tubes were 5.5 × 20 cm (inner diameter × length) for rats weighing less than 380 g and 7.5 × 20 cm (inner diameter × length) for rats weighing more than 380 g at testing. Plastic caps with holes for ventilation and the tail were secured at both ends of the tube with tape. Rats (n = 6–8 per prenatal group, surgical treatment and time) were decapitated either 0 or 30 min after the onset of a 30 min restraint stress and trunk blood were collected. For the animals subjected to 30 min stress, 200 μl blood was collected from the tail immediately following entry into the restraint tube to determine pre-stress testosterone levels. Brains were rapidly removed and immediately frozen on dry ice, then stored at − 80 °C until sectioning. All sampling was done during the circadian trough between 0930 – 1200 hr when HPA levels and activity are at the nadir.
2.5. Radioimmunoassays (RIA)
Blood samples were centrifuged at 3200 rpm for 10 min at 0 °C. Serum was transferred into 600 μl Eppendorf tubes and stored at −80 °C until assayed.
Corticosterone
Total corticosterone (bound plus free) levels were measured using an RIA kit from MP Biomedicals (Solon, OH) with [125I] corticosterone as tracer. The antiserum cross-reacts 100 % for corticosterone, 0.34 % for deoxycorticosterone, 0.10 % for testosterone, 0.10 % for cortisol, but not cross-reacts for progesterone and estrogens (< 0.01%). The minimum detectable corticosterone concentration was 0.63 μg/dl and the intra- and inter-assay coefficients of variation were 1.55 % and 4.26 % respectively.
Testosterone
Testosterone levels were measured using an adaptation of the testosterone RIA kit of MP Biomedicals (Solon, OH) with [125I] testosterone as the tracer and all reagent volumes halved. The testosterone antibody (solid phase) cross-reacts slightly with 5α-DHT (3.4 %), 5α-androstane-3β, 17β-diol (2.2 %) and 11-oxotestosterone (2 %) but does not cross-react with progesterone, estrogen, or the glucocorticoids (all < 0.01 %). 25 μl of each plasma sample were used to determine testosterone concentrations. The minimum detectable testosterone concentration was 0.1 ng/ml and the intra- and inter-assay coefficients of variation were 4.6 % and 7.5 % respectively.
Luteinizing Hormone (LH)
LH levels were measured by RIA in the laboratory of Dr. A.F. Parlow, NIDDK, National Hormone and Peptide Programe (Harbor-UCLA Medical Centre, California, USA). The cross-reactivity with other pituitary hormones was negligible. The minimum detectable LH concentration of the assay was 0.1 ng/ml. The intra- and inter-assay coefficients of variation were less than 10% (Attademo et al., 2004).
2.6. In situ Hybridization
Brain Preparation
Brains were sectioned into five one-in-five series of 30 μm coronal sections from the nucleus of the vertical limb of the diagonal band (VDB, Bregma 0.48 mm) to the medial nucleus of amygdala (MeA, Bregma −3.36 mm) (Paxinos and Watson, 2005) of the hypothalamus. Frozen sections were thaw-mounted onto precleaned colorfrost/plus microscope slides (Fisher Scientific, AB, CA) and stored at −80 °C.
Oligonucleotide Probes and Labeling
The oligonucleotide probes used to measure CRH and AVP mRNA in the PVN were described previously (Lan et al., 2006). Sense oligos for CRH and AVP mRNA were used as negative controls. Probes were 3′ tail labeled with 35S-dATP (Amersham Biosciences, NJ, USA) using terminal deoxytransferase (New England Biolabs Inc., ON, Canada) as per supplier protocol. Probes were purified using Roche DNA G-25 Sephadex Columns (Roche Scientific, IN., USA). 1 M dithiothreitol (DTT) was added to prevent oxidation.
Hybridization with Oligonucleotides
Sections for hybridization with oligonucleotide probes were prehybridized as previously described (Lan et al., 2006). Briefly sections were thawed, fixed in formalin, followed by 1 × PBS (10 min) twice, 0.1M TEA+ 0.25 % acetic anhydride (10 min), 2 × SSC (5 min), dehydrated through a graded series of ethanol, chloroform (5 min) followed by 100 % ethanol, and air-dried.50 % hybridization buffer (Lan et al., 2006) and the probe at a hybridization activity of 1.03 × 105 cpm/section for CRH and1.17 × 105 cpm/section for AVP were applied and covered with hybrislips (Sigma-Aldrich Canada Ltd., ON, Canada). Sections were incubated overnight at 40 °C in 50 % formamide humidified containers. The following morning, the hybrislips were removed and post hybridization washes comprised of a series of decreasing salt concentrations in SSC, along with washes at 45 °C and 50 % formamide as previously described (Lan et al., 2006). Sections were dehydrated in 70 % ethanol (5 min) and air dried overnight. Sections for CRH and AVP mRNA were exposed to Kodak BioMax MR film (Eastman Kodak Co., NY, USA). All slides were dipped in Kodak NTB2 autoradiography emulsion (Eastman Kodak Co.) diluted 1:1 (Deionized H2O) and exposed for 20 d for CRH in the PVN, 101 d for CRH in the central nucleus of amygdala (CeA), 94 d for CRH in the anterior division of the BNST (aBNST); 24 hr for AVP in the PVN, 16 d for AVP in the medial nucleus of amygdala (MeA) and posterior division of the BNST (pBNST) in desiccated, light tight boxes at 4 °C. Slides were developed with Kodak D-19 developer at 14 °C and fixed with Kodak Polymax T fixer at 14 °C, then counterstained with Cresyl Violet. Coverslips were mounted with Permount (Fisher Scientific Ltd., ON, Canada).
Ribonucleotide Probes and Labeling
Ribonucleotide probes were used to measure MR (Cullinan et al., 1995), GR (Cullinan et al., 1995), and AR (Chang and Kokontis, 1988; Simerly et al., 1990) mRNA (probes are identical to the probes used in reference paper). Probes were labeled with 35S-UTP (Amersham Biosciences, NJ, USA) using Promega Riboprobe System (Promega Corp., Madison, WI, USA). All probes were purified using Roche RNA G-50 Sephadex Columns (Roche Scientific, IN., USA). 1 M DTT was added to prevent oxidation.
Hybridization with Ribonucleotides
Thawed sections were fixed in formalin (30 min), and then prehybridized as follows; 1 × PBS (10 min) twice, Proteinase K (100 μg/L, at 37 °C for 9 min) DEPC-treated deionized water (5 min), 0.1M triethanolamine-hydrochloride −0.9 % NaCl + 0.25 % acetic anhydride (10 min), 2 × SSC (5 min, twice), dehydrated through a graded series of ethanol, chloroform (5 min) followed by 100 % ethanol, and air-dried. Hybridization buffer (75 % formamide, 3 × SSC, 1 × Denhardt’s solution, 200 μg/ml yeast tRNA, 50 mM sodium phosphate buffer (pH 7.4), 10 % dextran sulphate, 10 mM DTT) was applied (probe activity for MR: 2.8 × 105 cpm/section; GR: 3.2 ×105 cpm/section; AR: 2.7 × 105 cpm/section) and covered with hybrislips. Sections were incubated overnight at 55 °C in 75 % formamide humidified containers. Hybrislips were removed and slides were washed in 2 × SSC (20 min) twice, RNAse A solution (50ug/L, 37 °C, 90 min), 2 × SSC/0.01 M DTT (10 min), 1 × SSC (15 min), 0.5 × SSC (15 min), 0.1 × SSC/0.01 M DTT (60 °C, 20 min), and 0.1 × SSC (5 min). Sections were dehydrated through a graded series of ethanol and air dried overnight. Sections were exposed to Kodak BioMax MR film for 6 d, 3 d, and 18 d for the GR, MR and AR probe, respectively. AR slides were then dipped in autoradiography emulsion, exposed for 124 d, and then developed, stained and coverslipped as described above for CRH and AVP
2.7. Densitometric Analysis
In situ signals were visualized with a Q-imaging monochrome 12-bit camera attached to a Zeiss Axioskop 2 motorized plus microscope. Images were captured using Northern Elite 6.0v (Empix Imaging Inc., Mississauga, ON, Canada) and semiquantitative densitometric analyses were performed using Image J 1.33v software (National Institutes of Health, Bethesda, MD). The mean optical density (OD) of hybridization signal, corrected by background subtraction, was taken at 150 μm intervals under dark-field illumination. Semiquantitative densitometric analysis for CRH and AVP in the mpd PVN was described in an earlier study (Lan et al., 2006). Semiquantitative densitometric analysis of CRH mRNA in the aBNST and CeA, AVP mRNA in the pBNST and the MeA, AR mRNA in the MPN, principal nucleus of pBNST and the MeA was assisted by redirected sampling of dark-field autoradiographic images aligned to corresponding Nissl stained sections. CRH mRNA expression within the aBNST were measured within two cell groups, the oval and fusiform BNST nuclei, at the decussation of the anterior commissure (Bregma −0.12 mm ~ −0.48 mm) (Paxinos and Watson, 2005). The oval nucleus of the aBNST was traced by outlining a fixed rectangle (Width = 0.45; Height = 0.87; scale 300 pixels/inch). The fusiform nucleus of the aBNST was traced by free-hand around the CRH mRNA signal. The CeA was traced by outlining a fixed oval (Width = 0.45; Height = 0.71; scale 300 pixels/inch). The MeA was traced by outlining a fixed triangle (length: 1.65, 0.60, and 1.36; angle: 280.0, 48.5, 120.0; scale 300 pixels/inch for the left side. length: 1.45, 0.55, and 1.60; angle: 232.5, 316.0, 72.6; scale 300 pixels/inch for the right side). The MPN was traced by outlining a fixed square (0.33 × 0.33, scale 300 pixels/inch). The principal nucleus of pBNST was traced by free-hand around the AR signal. Background signal was measured over a region immediately lateral to each side of the area of interest. The corrected grey levels from both sides of two to three sections of each region were averaged to obtain a mean corrected grey level of the four to six measurements for each animal.
The majority of AVP mRNA signals were found in the transverse and intrafascicular nuclei of the pBNST (Bregma −0.6 mm to −0.84 mm) (Paxinos and Watson, 2005). Because AVP mRNA is widely distributed in the pBNST, signal was measured by outlining neurons containing labeled AVP mRNA and using Image J to analyze the pixel area and the mean grey value of each signal cluster. Total grey value from both sides of the two sections of the pBNST was calculated by pixel area × mean grey value of each cluster and then summing the measured grey values for each brain.
In the dorsal hippocampus, a representative section, in which the two arms of the dentate gyrus (DG) were equal in length (Bregma −3.00 mm) (Paxinos and Watson, 2005), was chosen for analysis of MR and GR mRNA levels. Measurements were taken from the subfields CA1, CA2, CA3 and DG from two sections for each animal. Background signal was measured from the molecular layer of each side of the hippocampus. The corrected grey levels from both sides of each subfield were averaged to obtain a mean corrected grey level of the four measurements for each animal. 2.8.
Statistical Analyses
Developmental and pup body weight data were analyzed using repeated measures ANOVA and Newman-Keuls post hoc tests. The hormone data were analyzed using 3-way between-factors ANOVA with the factors of prenatal group (E, PF, C), surgical condition (INT, GDX, GDX-L, and GDX-H) and time (0 and 30 min). For serum LH levels, 2-way ANOVAs within each surgical treatment group were performed. As adult body weight, organ weight, and brain mRNA levels of neuropeptides and receptors were measured under basal conditions (0 min) only, these data were analyzed using two-way between-factors ANOVA (prenatal group and surgical treatment). Significant main effects and interactions were further analyzed using Fisher’s LSD for factors with only 3 levels, and pairwise comparisons with a Šidák correction for factors with > 3 levels. To test the a priori hypothesis that testosterone mediates the effect of prenatal ethanol exposure on HPA and HPG responsiveness, planned comparisons using a Šidák correction were carried out for all comparisons. Because the Šidák correction accurately controls for familywise α, an overall F test is not required to be significant in order to explore a priori differences between factors using planned comparisons (Cardinal and Aitken, 2006; Myers and Well, 2003). Statistical significance was set at P < 0.05. P values between 0.05 and 0.10 were considered a statistical trend.
To determine appropriate Ns per group, we ran a power analysis (Faul et al., 2007) using the data of Viau et al (Viau et al., 2003) on whose study the present one was modeled. Based on the effect size calculated, we required a total sample size of 78 to be able to detect 2-way or 3-way interactions. Our total sample size is 166, well above this level. We also ran a post hoc test to compute the actual power we had in our present study, and determined that Power (1−β err prob) = 0.673041. Although this power is lower than that typically needed in behavioral studies, we believe that the large effect size coupled with the relatively small experimental error in our biological study justifies use of this lower power, and that our study does indeed have enough statistical power to detect a true effect.
3. Results
3.1 Adult body and organ weights
Body weights
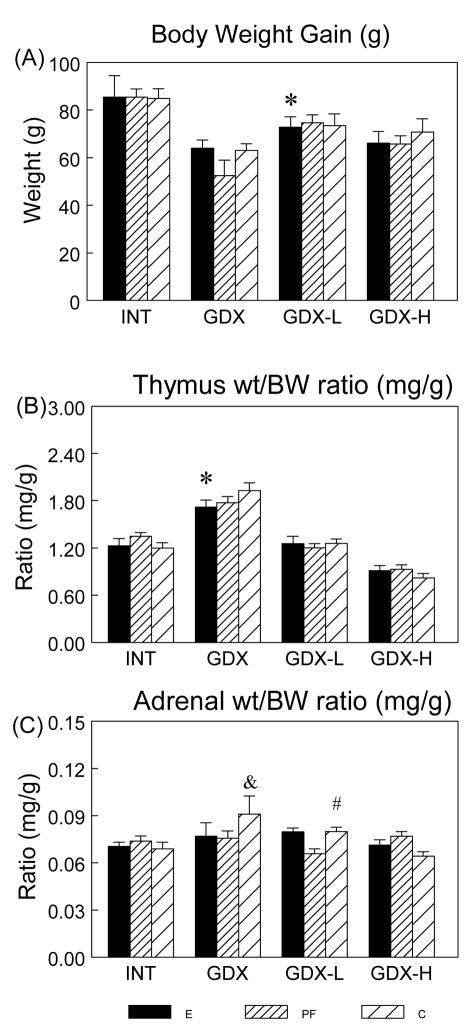
Analysis of adult body weights prior to surgery indicated a significant main effect of prenatal group (F(2,154) = 4.0; P < 0.05); E and PF had lower weights than C males (P < 0.05). Analysis of weight gain over the 2 wks between surgery and testing (Figure 1A) indicated significant effects of surgical condition (F(3,154) = 12.839; P < 0.001). Overall, INT males gained the most, and GDX and GDX-H males gained the least, weight (INT > GDX = GDX-H, Ps < 0.05; INT > GDX-L, P < 0.07; GDX-L > GDX, P < 0.05). Furthermore, low testosterone replacement restored body weights in PF and C, but not in E males (GDX-L < INT in E, P < 0.05).
Figure 1.
Body weight (BW) gain (g) (Panel A) and organ wt/BW ratio (mg/g) (Panels B, C) (mean ± SEM, n = 14 per group) in E, PF and C males from intact, GDX, GDX-L and GDX-H surgical conditions. BW gain: overall INT > GDX = GDX-H, Ps < 0.05; GDX-L > GDX, P < 0.05; * GDX-L < INT in E males only, P < 0.05. Thymus wt/BW ratio: * E < C, P < 0.05 in GDX rats. Adrenal wt/BW ratio: & GDX > INT = GDX-H, Ps < 0.005; # GDX-L > GDX-H, P = 0.05 in C males.
Thymus Weight
A significant effect of surgical condition (F(3,154) = 78.5; P < 0.001) (Figure 1B) indicated that GDX rats had the highest, and GDX-H rats had the lowest, thymus weight (corrected for body weight, i.e., thymus wt/BW ratio) among the 4 surgical conditions (GDX > all, GDX-H < all, Ps < 0.001). In addition, planned comparisons revealed that GDX E showed a lower thymus wt/BW ratio than GDX C males (P < 0.05). Low testosterone replacement was sufficient to restore thymus wt/BW ratios to those of intact animals for all prenatal groups.
Adrenal Weight
Analysis of adrenal weight corrected for body weight (adrenal wt/BW ratio) indicated a significant effect of surgical condition (F(3,149) = 4.05; P < 0.01) and a trend for a group X condition interaction (F(6,149) = 2.087; P < 0.06) (Figure 1C). Adrenal/BW ratios were higher in GDX C males compared to C males in all other surgical conditions, whereas surgical condition did not differentially affect adrenal weights in E and PF males (C males: GDX > INT = GDX-H, Ps < 0.01; GDX > GDX-L, P < 0.09; GDX-L > GDX-H, P = 0.05).
3.2 HPA data
Serum CORT Levels
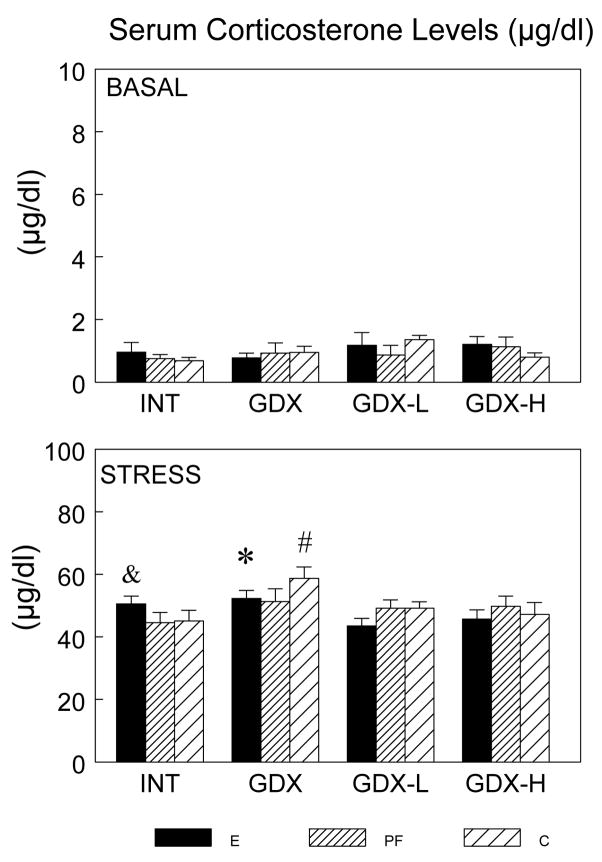
As expected, a main effect of time (F(1,126) = 1985.33; P < 0.001) (Figure 2) indicated that CORT increased significantly over basal (0 min) levels at 30 min (P < 0.001). There was trend for a main effect of surgical condition (F(3,126) = 2.452; P < 0.07) and a significant condition X time interaction (F(3, 126) = 2.621, P = 0.05). Prenatal treatment had no effect on basal hormone levels, but differentially altered the stress-induced CORT response depending on surgical condition. For E males, CORT levels at 30 min were similar in intact and GDX conditions, whereas GDX increased CORT levels for PF and C males (PF: GDX > INT, P < 0.06; C: GDX > INT = GDX-L = GDX-H, P < 0.01). In addition, replacement with the low concentration of testosterone restored CORT levels for PF and C males, whereas high testosterone levels were needed to restore CORT levels for GDX E males to those of intact E males. Indeed, low testosterone replacement was actually somewhat suppressive to CORT in E males (E : INT > GDX-L, P = 0.05; GDX > GDX-L = GDX-H, Ps < 0.05).
Figure 2.
Serum CORT (μg/dl) levels (mean ± SEM, n = 6–8 per group) in E, PF and C males from intact, GDX, GDX-L and GDX-H surgical conditions at 0 and 30 min after stress onset. Overall, 30 min > 0 min, Ps < 0.001; E males: * GDX > GDX-L = GDX-H, Ps < 0.05; & INT > GDX-L, P = 0.05; C males: # GDX > all, Ps < 0.01; PF males: GDX > INT, P < 0.06.
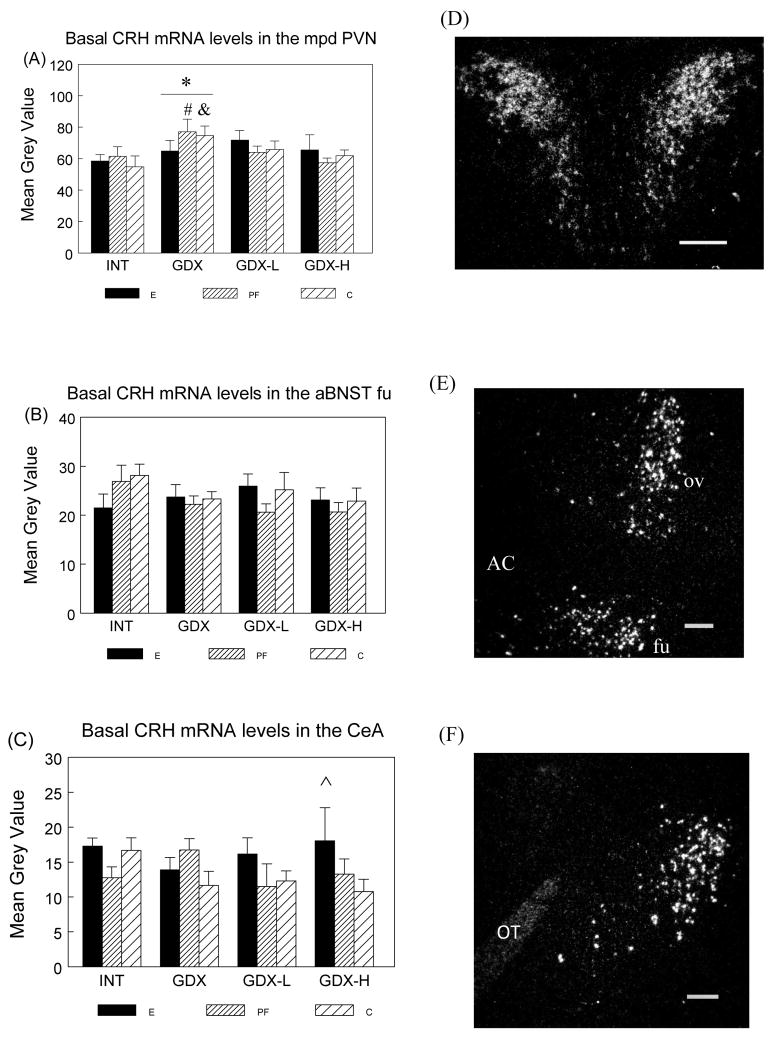
Basal CRH mRNA in the mpd PVN, aBNST and CeA
Two-way ANOVA indicated a significant effect of surgical condition (F(3,58) = 3.009; P < 0.05) on basal CRH mRNA levels in the mpd PVN. Overall, GDX increased basal CRH mRNA levels whereas testosterone replacement restored CRH mRNA levels to those in intact animals (GDX > INT, P < 0.05) (Figure 3A). Furthermore, planned comparisons revealed that basal CRH mRNA levels changed across surgical condition only in PF and C males (PF: GDX > INT, P < 0.09; GDX > GDX-H, P < 0.05; C: GDX > INT, P < 0.05), whereas E males did not differ in CRH mRNA levels across surgical conditions.
Figure 3.
Basal CRH mRNA levels (mean ± SEM, n = 6 per group) in the mpd PVN (Panel A), fusiform nucleus of the aBNST (aBNST fu) (Panel B) and CeA (Panel C) in E, PF and C males from intact, GDX, GDX-L and GDX-H surgical conditions and representative dark-field photomicrographs of nuclear emulsion-dipped sections demonstrating CRH mRNA patterns in the mpd PVN (Panel D), oval and fusiform nucleus of the aBNST (Panel E) and CeA (Panel F). Scale bar = 0.2 mm. AC: anterior commisure; ov: oval nucleus of the aBNST; fu: fusiform nucleus of the aBNST; OT: optic tract.
mpd PVN: Overall * GDX > INT, P < 0.05; PF males: # GDX > GDX-H, P < 0.05; GDX > INT, P < 0.09; C males: &: GDX > INT, P < 0.05.
aBNST fu: E < C in intact males, P < 0.06.
CeA: ^ E > C in GDX-H males, P < 0.05.
There were no significant main effects or interactions for basal CRH mRNA levels in the aBNST or MeA. However, planned comparisons revealed that INT E had marginally lower CRH mRNA levels than INT C males in the fusiform nucleus of the aBNST (P < 0.06) (Figure 3B). Furthermore, following high dose testosterone replacement, E males had significantly higher CRH mRNA in the CeA than C males (P < 0.05) (Figure 3C).
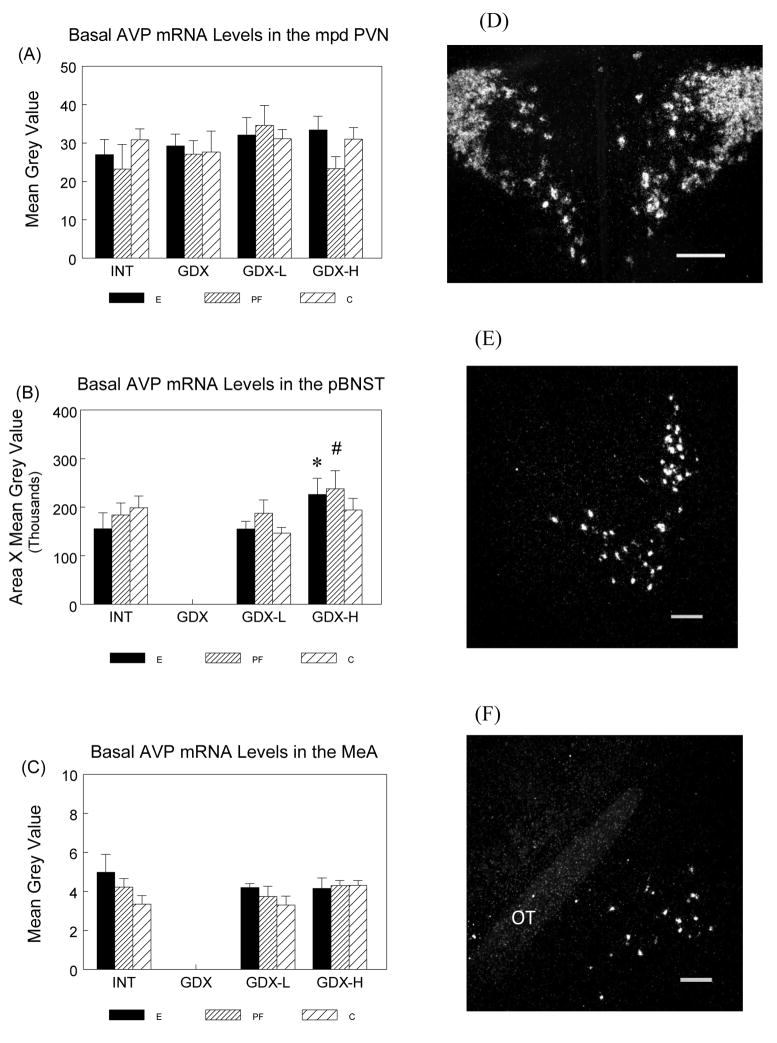
Basal AVP mRNA in the mpd PVN, aBNST and MeA
There were no significant effects of prenatal group or surgical condition on basal AVP mRNA levels in the PVN (Figure 4A) or the MeA (Figure 4C). However, a significant main effect of surgical condition (F(2,40) = 3.194; P = 0.05) for AVP mRNA levels in the pBNST (Figure 4B), followed by planned comparisons, indicated high testosterone replacement increased AVP mRNA levels in the pBNST for E and PF but not C males (E males: GDX-H > INT, P = 0.05; GDX-H > GDX-L, P < 0.05; PF males: GDX-H > INT, P < 0.05). INT E males also had marginally higher AVP mRNA in the MeA than INT C males (P < 0.06) (Figure 4C). The AVP mRNA signals in the pBNST and the MeA were undetectable in GDX animals.
Figure 4.
Basal AVP mRNA levels (mean ± SEM, n = 6 per group) in the mpd PVN (Panel A), pBNST (Panel B) and MeA (Panel C) in E, PF and C males from intact, GDX, GDX-L and GDX-H surgical conditions and representative dark-field photomicrographs of nuclear emulsion-dipped sections demonstrating AVP mRNA patterns in the mpd PVN (Panel D), pBNST (Panel E) and MeA (Panel F). Scale bar = 0.2 mm. OT: optic tract.
mpd PVN: There were no significant effects of prenatal ethanol or surgical treatment on AVP mRNA levels in the mpd PVN.
pBNST: E males: * GDX-H > GDX-L, P < 0.05. PF males: # GDX-H > INT, P < 0.05.
The AVP mRNA signals in the pBNST were undetectable in GDX animals.
MeA: E > C in INT males, P < 0.06. The AVP mRNA signals in the pBNST were undetectable in GDX animals.
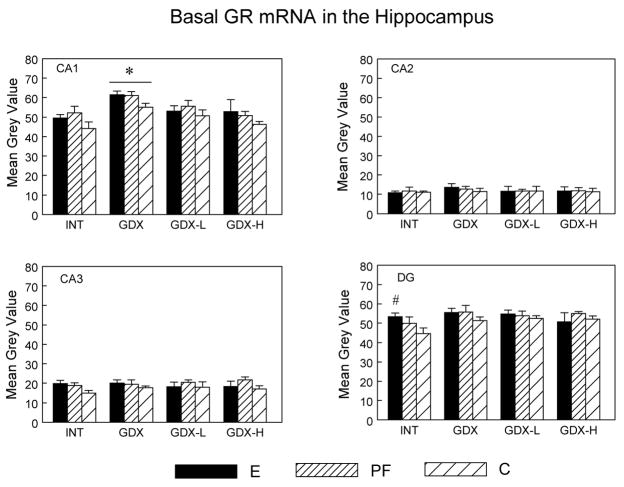
Basal MR and GR mRNA levels in the Hippocampus
Effects of surgical condition for MR mRNA levels (Ps < 0.05) in the CA1, CA2 and CA3 subfields reflected the fact that MR mRNA levels were higher in GDX compared to GDX-L and GDX-H animals (Ps < 0.05) (data not shown). Thus testosterone replacement normalized MR mRNA levels to those of intact animals, and prenatal treatment did not alter this effect. There were no differences in MR mRNA among the three prenatal treatment groups.
By contrast, analysis of GR mRNA levels indicated main effects of prenatal group (F(2,53) = 3.756; P < 0.05) and surgical condition (F(3,53) = 6.35; P < 0.001) in the CA1 subfield (Figure 5). GDX males had higher GR mRNA levels than INT and GDX-H males (Ps < 0.01). Importantly, E and PF males had higher CA1 GR mRNA levels overall compared to C males (E > C, P < 0.08; PF > C, P < 0.05), and under intact conditions, E males had higher GR mRNA levels than C males in both the CA3 (P < 0.08) and DG (P < 0.05).
Figure 5.
Basal GR mRNA levels (mean ± SEM, n = 6 per group) in the CA1, CA2, CA3 and DG subregions of the dorsal hippocampus in E, PF and C males from intact, GDX, GDX-L and GDX-H surgical conditions. CA1: Overall, E > C, P < 0.08; PF > C, P < 0.05; * GDX > INT = GDX-H, Ps < 0.01; E > C in INT males in DG (# P < 0.05) and in the CA3 (P < 0.08).
3.3 HPG data
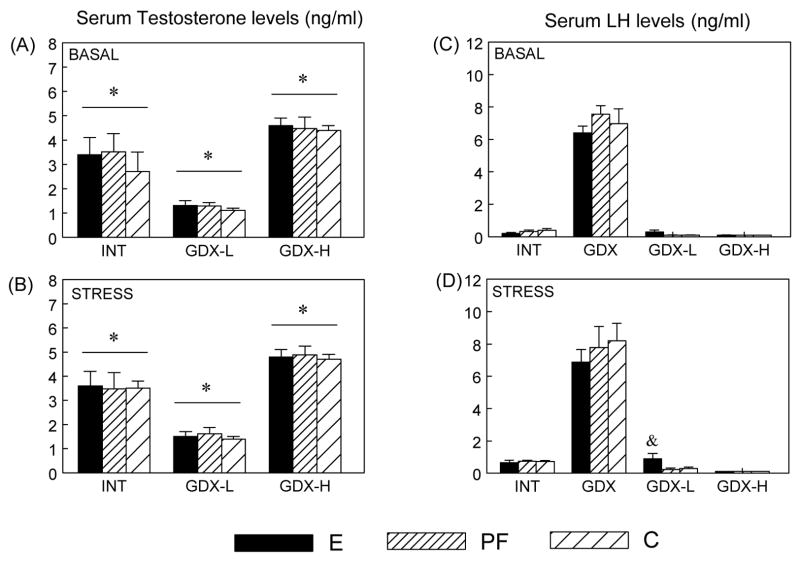
Serum Testosterone Levels
As expected, serum testosterone levels in GDX rats were in the undetectable range, and the main effect of surgical condition (F(2,103) = 78.072; P < 0.001) indicated that overall, GDX-H > INT > GDX-L, Ps < 0.001 (Figure 6A, B).
Figure 6.
Serum testosterone (ng/ml) (Panels A, B) and LH (ng/ml) (Panels C, D) levels levels (mean ± SEM, n = 6 - 8 per group) in E, PF and C males from intact, GDX, GDX-L and GDX-H surgical conditions at 0 and 30 min after stress onset. Testosterone: * GDX-H > INT > GDX-L, Ps < 0.001. LH: & E > PF = C in GDX-L conditions, Ps < 0.05
Serum LH Levels
A 3-way ANOVA revealed main effects of surgical condition (F(3,135) = 345.697; P < 0.001) and time (F(1,139) = 3.290; P < 0.07), indicating that, not surprisingly, GDX animals had higher LH levels compared to animals in the other 3 surgical conditions (Ps < 0.001) (Figure 6C, D). As such, separate 2-way ANOVAs within each surgical condition were conducted. LH levels did not change following stress in GDX or GDX-H animals. By contrast, INT males in all prenatal groups showed a significant stress-induced increase in LH levels (P < 0.05) [main effect of time (F(1,35) = 29.116; P < 0.001)], whereas in the GDX-L condition, effects of prenatal group (F(2,35) = 3.613; P < 0.05) and time (F(1,35) = 4.064; P = 0.05) indicated that only E males showed an LH increase following 30 min stress, resulting in higher LH levels than PF and C males (Ps < 0.05).
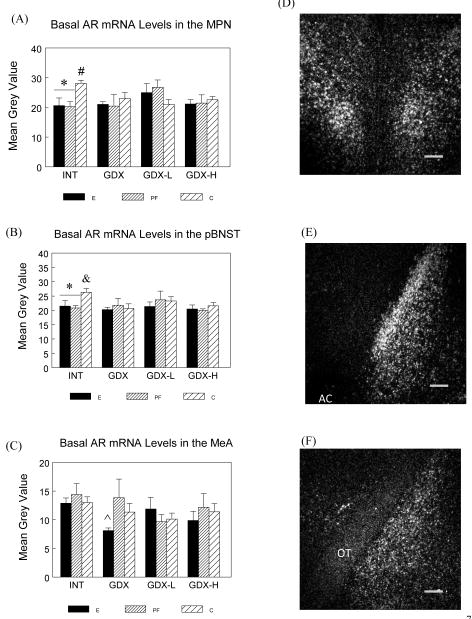
Basal AR mRNA in the MPN, pBNST, MeA and hippocampus
Analyses revealed that E and PF males showed no changes in AR mRNA levels in either the MPN (Figure 7A) or the principal nucleus of pBNST (Figure 7B) across surgical conditions, whereas C males had higher AR mRNA levels in the INT condition compared to the other surgical conditions (Planned comparisons: MPN: INT > all, Ps < 0.05; pBNST: INT > GDX, P < 0.05; INT > GDX-H, P < 0.06). Furthermore, under intact conditions, E and PF animals had lower AR mRNA expression in both the MPN and the principal nucleus of pBNST than C males (Ps < 0.05). Similarly, in the MeA (Figure 7C), E but not PF and C animals showed a trend for decreased AR mRNA levels in the GDX relative to the intact condition (GDX E < INT E, P < 0.08), whereas PF and C males did not differ in AR mRNA levels across surgical conditions. Furthermore, GDX E had lower AR mRNA levels than GDX PF males (P < 0.05). There were no effects of prenatal treatment or surgical condition on AR mRNA levels in any hippocampal subfields (data not shown).
Figure 7.
Basal AR mRNA levels (mean ± SEM, n = 6 per group) in the MPN (Panel A), principal nucleus of pBNST (Panel B) and MeA (Panel C) in E, PF and C males from intact, GDX, GDX-L and GDX-H surgical conditions and representative dark-field photomicrographs of nuclear emulsion-dipped sections demonstrating AR mRNA patterns in the MPN (Panel D), principal nucleus of pBNST (Panel E) and MeA (Panel F). Scale bar = 0.2 mm. AC: anterior commissure; OT: optic tract.
MPN: * E = PF < C in INT males, Ps < 0.05; # C: INT > all, Ps < 0.05.
pBNST: * E = PF < C in INT males, Ps < 0.05; & C: INT > GDX, P < 0.05;.
MeA: E: INT > GDX, P < 0.09; whereas PF and C males did not differ in AR mRNA levels across surgical conditions. ^ E < PF in GDX males, P < 0.05.
3.4 Correlations between pre-stress testosterone and post-stress CORT levels
In intact animals, analysis revealed negative correlations between pre-stress testosterone and post-stress CORT levels in C (r2 = 0.6982, P < 0.05) but not E and PF males.
4. Discussion
The present findings support and extend those of our previous work (Lan et al., 2006), providing strong evidence that regulation of both the HPA and HPG axes is altered by prenatal ethanol exposure, and that E males show altered sensitivity to testosterone. Importantly, examination of central CRH and AVP expression profiles demonstrates a complex balance of effects; that is, testosterone appears to have a reduced effect on central CRH pathways, but an increased effect on central AVP pathways in E compared to PF and/or C animals.
4.1. Ethanol effects on body weight, organ weight, and peripheral hormone levels
Overall, INT males gained the most weight and GDX males gained the least weight, consistent with previous data from our lab (Lan et al., 2006) and others (Wade, 1976) showing that GDX decreases food intake and weight gain. Importantly, low dose testosterone replacement was sufficient to restore body weights in PF and C but not E males, and high dose testosterone replacement was not better than low dose replacement (if anything, was slightly worse) in restoring body weights in E males. This was not entirely surprising in view of previous studies showing that the decrease in weight gain and food intake following GDX can be reversed by testosterone treatment, but that effects of testosterone are dose-dependent. For example, low concentrations of testosterone restored metabolism in GDX rats to normal, whereas higher doses of testosterone decreased weight gain and food intake (Gentry and Wade, 1976). The effect of low dose testosterone on weight gain is due to its anabolic action on lean body mass, whereas high dose testosterone causes fat loss, mediated by aromatization to estradiol (Dallman et al., 2002). These findings on body weight regulation support our hypothesis that E males have decreased sensitivity to testosterone.
All males had increased thymus wt/BW ratios following GDX, consistent with previous reports (Oner and Ozan, 2002; Viau et al., 1999). Androgens exert considerable effects on the size and composition of the thymus. Removal of androgens by castration results in thymic enlargement even in old rats, and androgen replacement reverses this effect (Greenstein et al., 1986). Importantly, we found that GDX E males had a lower thymus/BW ratio than GDX C males, and that GDX increased adrenal wt/BW ratio in C but not E and PF males, indicating that the capacity of circulating testosterone to regulate the thymus and adrenal is reduced in E and PF rats.
In line with the above results, we found that prenatal treatment differentially altered the stress CORT response depending on surgical condition. CORT levels were similar in intact and GDX E males, whereas GDX increased CORT levels for PF and C males, indicating that E males were less sensitive to the effects of androgen removal than controls. In addition, low testosterone replacement restored CORT levels for PF and C males, whereas high testosterone levels were needed to restore CORT levels for E males. Indeed, low testosterone replacement was actually somewhat suppressive to CORT in E males. The finding that a negative correlation exists between pre-stress testosterone and post-stress CORT levels in C but not in E and PF males further demonstrates the reduced adrenal sensitivity to testosterone in E and PF males. It is possible that both peripheral (directly on adrenal gland) and central (on the hypothalamus) mechanisms could contribute to the reduced capacity of testosterone to modulate CORT activity.
As expected, testosterone replacement at low and high basal concentrations resulted in low (~1 ng/ml) and high (4–6 ng/ml) physiological testosterone levels in all males, but there were no stress effects on testosterone levels. In addition, GDX animals had higher LH levels than animals in the other three surgical treatment groups due to the absence of testosterone feedback. Furthermore, consistent with previous reports that acute stress elicits a small and transient increase in plasma LH levels in male rats (Briski and Sylvester, 1987a; b; Krulich et al., 1974), intact males from all prenatal treatment groups showed a significant LH increase following 30 min restraint stress. Importantly, E but not PF and C males in the GDX-L condition showed an LH increase following 30 min stress, suggesting that testosterone had less of an inhibitory effect on stress-induced LH increases in E than in PF and C males.
4.3 Ethanol effects on central CRH and AVP expression profiles
CRH and AVP are expressed widely throughout the brain, and send projections to the PVN. It is reported that 90.5% and 91.2% of AVP-immunoreactive (ir) neurons in the BNST and MeA, respectively, contain ARs, suggesting that androgens may influence AVP expression by acting directly on these neurons (Zhou et al., 1994). Importantly, as noted, neither ARs nor estrogen receptors are present on the neurosecretory neurons of the PVN that project to the median eminence (Bingham et al., 2006; Simerly et al., 1990; Williamson et al., 2005; Williamson and Viau, 2007; Zhou et al., 1994), suggesting that testosterone likely has its effects on HPA output by acting on neurons upstream from the PVN.
In contrast to our previous study (Lan et al., 2006), the present data demonstrate that basal CRH mRNA levels the mpd PVN were increased by GDX in PF and C but not E males. Methodological differences between the two studies may have contributed to these contrasting results. For example, a greater slice thickness (30 μm vs 14 μm) in the present study might have included more CRH neurons in the analysis. Naturally occurring variability in testosterone levels could also play a role in the differential CRH mRNA expression observed. Previous reports showed that GDX increases the number of CRH-ir neurons in the hypothalamus (Bingaman et al., 1994) as well as restraint-induced Fos-ir, CRH hnRNA, and AVP hnRNA expression in the mpd PVN (Viau et al., 2003). On the other hand, Yukananov and colleagues counted the number of silver grains per cell and found no changes in basal CRH mRNA in the mpd PVN following GDX (Yukhananov and Handa, 1997). Similarly, it was shown that GDX had no effect on adrenalectomy (ADX)-induced increases in CRH mRNA levels in the PVN in brains collected at 60 min after the onset of a 30 min restraint stress (Viau et al., 1999) or under basal conditions (Viau et al., 2001). These studies together with our data suggest that the experimental conditions, methods of analysis and endpoint measured may all influence findings on the effects of GDX on CRH expression in the PVN.
Importantly, changes in neuropeptide expression in PVN-projecting neurons can also influence mpd PVN neuroscretory neurons. We found that following high testosterone replacement, E males had significantly higher CRH mRNA in the CeA than C males. It has been shown that CRH mRNA levels in the CeA vary negatively with testosterone, but only in the presence of CORT (Viau et al., 2001). Thus higher CRH mRNA levels in the CeA in E rats might reflect a reduced testosterone effect on CRH mRNA expression in this region. Our finding that basal CRH mRNA levels were increased by GDX in PF and C but not E males in the mpd PVN demonstrates the inhibitory role of testosterone on CRH mRNA expression on control animals, most likely due to its effects on neurons upstream from the PVN, and suggests that prenatal ethanol exposure reduces the capacity for testosterone to regulate CRH mRNA expression in central CRH pathways.
In contrast, testosterone appeared to have enhanced effects on central AVP pathways in E and/or PF compared to C males. Consistent with previous findings of androgen-dependent influences on AVP mRNA in the pBNST and MeA (De Vries et al., 1994; Miller et al., 1992; Viau et al., 2001), we found that AVP mRNA expression in the pBNST and MeA were totally abolished by GDX. Importantly, there were no significant effects of prenatal or surgical treatment on basal AVP mRNA levels in the mpd PVN, consistent with the finding that GDX has no effect on basal AVP mRNA levels (Viau et al., 2001). However, like CRH, testosterone likely exerts its effects on the AVP upstream from the mpd PVN (Viau et al., 2001). In the present study, both E and PF males showed higher AVP mRNA levels in the pBNST following high testosterone replacement compared to either intact or low testosterone conditions. In addition, inspection of Figure 4C suggests a greater effect of low testosterone replacement on E compared to C males, although the differences were not statistically significant. These findings suggest enhanced AVP responsiveness to testosterone upstream from the PVN in E and/or PF compared to control animals.
Importantly, while higher pBNST AVP mRNA levels in E males may suggest an enhanced inhibitory effect on PVN neurons, we also found decreased AR mRNA levels in the MPN and the principal nucleus of the pBNST (decribed below). If the reduced AR mRNA levels reflect a downregulation in AR, this may counteract the increased inhibitory effect of AVP on these PVN-projecting neurons.
4.4 Ethanol effects on AR mRNA expression in the PVN-projecting neurons
The present study represents the first report of prenatal ethanol effects on AR mRNA levels in the brain. We found that INT C males had higher AR mRNA levels in the MPN than C males in the other surgical conditions, and higher AR mRNA levels in the principal nucleus of pBNST compared to C males in the GDX and GDX-H conditions. Importantly, AR mRNA expression was decreased in INT E and PF males in the MPN and principal nucleus of pBNST compared to INT C males. Furthermore, in the MeA, AR mRNA levels were differentially altered by surgical condition in E and PF but not C males. That is, INT E males had higher AR mRNA levels than GDX E males, and INT PF males had higher AR mRNA levels than GDX-L PF males; by contrast C males did not differ in AR mRNA levels across surgical treatments. Findings from previous studies suggest a unique biphasic regulatory pattern of AR mRNA changes following GDX that also depend on the time and tissue examined. AR mRNA levels were increased in the MPOA and BNST (Handa et al., 1996) but decreased in the hippocampus (Kerr et al., 1995) 4 days after GDX, whereas at 7–8 weeks following GDX, AR mRNA levels were decreased in the MPOA and BNST (Handa et al., 1996) but increased in the hippocampus (Burgess and Handa, 1993). On the other hand, AR-ir was decreased in the MPOA and BNST after both short- and long-term GDX (Handa et al., 1996). Steady state AR mRNA levels may reflect AR synthesis and thus the rate of synthesis may change with time following GDX to compensate for the receptor turnover rate (Handa et al., 1996). If so, the finding of AR downregultion in E and PF males in the MPN and principal nucleus of pBNST, which both exert tonic inhibition to the neurosecretory neurons in the mpd PVN, supports our suggestion that E and PF males are less sensitive to the inhibitory effects of testosterone than C males. It is possible that AR downregulation represents a counteracting mechanism that attenuates the increased inhibitory effects of central AVP circuits on the mpd PVN, thus contributing to the HPA hyperresponsiveness observed in E males.
4.5 Ethanol effects on MR and GR expression in the dorsal hippocampus
The hippocampus is a major negative feedback site of the HPA axis. Hippocampal MRs are important in control of threshold or sensitivity of the stress system, and are thought to mediate primarily tonic inhibition of HPA activity (De Kloet et al., 1998) as well as regulation of HPA activity during mild stressors (Pace and Spencer, 2005). In contrast, GRs primarily mediate feedback inhibition in response to stress-induced CORT elevations as well as tonic inhibition at the circadian peak (De Kloet et al., 1998; Sapolsky et al., 2000). Sex differences in hippocampal CORT-binding and GR mRNA and protein levels suggest a role for gonadal hormone in glucocorticoid receptor expression (Ahima et al., 1992; Bohn et al., 1994; Turner and Weaver, 1985). The hippocampus contains high levels of AR, MR and GR, especially in CA1 pyramidal cells, which form the major signal output of the hippocampal trisynaptic circuit (Bingham et al., 2006; Herman et al., 1989; Kerr et al., 1995; Shughrue et al., 1997; Simerly et al., 1990), indicating that AR may synergize or antagonize with other receptors in regulating hippocampal functions.
In the present study, as expected, GDX increased MR and GR mRNA levels, consistent with findings showing that androgens can exert a suppressive effect on the transcriptions of both receptors (Turner, 1997). Handa’s group also found that DHT decreased GR mRNA levels in CA1, as well as prevented the increase in GR mRNA levels following ADX (Kerr et al., 1996). Since GR is an autologously regulated gene, they proposed that DHT binding to AR is substituiting for CORT feedback inhibition at the level of the hormone responsive element of the GR gene. Hence DHT normalizes the GR upregulation that occurs with ADX, and downregulates GR when given to an intact animal. While we found no differences in MR mRNA among the three prenatal treatment groups, overall, E and PF males had higher GR mRNA levels compared to C males, and under intact conditions, E males had higher GR mRNA levels than C males. Thus testosterone binding to AR may have been less effective in substituting for GR feedback inhibition in E and/or PF compared to C males. These data suggest that prenatal ethanol exposure may differentially regulate basal hippocampal GR expression in adult males, an effect that may reflect increased basal HPA tone, and that may be, at least partially, nutritionally mediated.
4.6. Nutritional effects of prenatal ethanol exposure
In this study, we found specific effects of ethanol on body and thymus weights, CORT and LH responses, CRH expression in the PVN and CeA, and GR expression in the hippocampus. On the other hand, some effects of ethanol, including adrenal sensitivity to testosterone, AVP expression in the pBNST, and AR expression in the MPN and pBNST, appear to be, at least partially, nutritionally mediated, as effects were similar in E and PF offspring. Ethanol-derived calories replace the calories supplied by food, which has associated nutrients, and thus consumption of ethanol-containing diets results in suboptimal nutrition. Pair-feeding is typically employed as a standard control for the nutritional effects of ethanol intake. However, PF dams experience mild stress due to the hunger that accompanies consuming less than they would eat ad libitum. Thus, pair-feeding is a treatment in itself, and there is a mild prenatal stress component superimposed on the nutritional aspect of reduced food intake in the pair-feeding paradigm. Prenatal stress has been shown to affect the hormonal and behavioural development of offspring (Koehl et al., 1999; Kofman, 2002; Matthews, 2002; Seckl, 2004), and can program the fetal HPA axis such that development and stress responsiveness are altered in adulthood. Moreover, maternal undernutrition can in itself program fetal HPA activity (Lesage et al., 2001). Thus, as suggested previously (Glavas et al., 2007), it is possible that similar outcomes in E and PF offspring may be mediated by different mechanisms (ethanol vs stress) rather than occurring along a continuum of effects on the same pathway.
5. Summary and Conclusions
In summary, examination of dose-related effects of testosterone unmasked alterations in HPA activity in E males that relate specifically to testosterone status, as well as central changes in both HPA and HPG regulation that are specific to prenatal ethanol exposure. We propose that although basal HPA hormone levels and CRH/AVP mRNA expression in the mpd PVN appear unchanged compared to those in control males, prenatal ethanol exposure significantly alters upstream CRH and AVP pathways that regulate the neurosecretory neurons in the mpd PVN, which might set the stage for the HPA hyperresponsiveness that is observed. The enhanced effects of testosterone on central AVP inhibitory pathways may be a compensatory mechanism that accounts for the normal basal HPA activity in E males. However, in the face of challenge by stressors, this mechanism may be insufficient to maintain normal HPA regulation.
Table 1.
Maternal body weights (g) during gestation (days 1 and 21) and lactation (day 1); Gestation Length
| Gestation Day 1 | Gestation Day 21 | Lactation Day 1 | Gestation Length | |
|---|---|---|---|---|
| E | 262.6 ± 2.2 | 355.6 ± 3.9* | 285.5 ± 4.5^ | 22.0 ± 0.00† |
| PF | 262.1 ± 1.8 | 357.1 ± 4.5* | 291.9 ± 3.9^ | 21.73 ± 0.12 |
| C | 258.8 ± 2.8 | 405.2 ± 5.4 | 312.5 ± 5.8 | 21.83 ± 0.11 |
Values represent the mean ± SEM of 12–16 rats per group.
P < 0.001 compared to C;
P < 0.01 compared to C;
P < 0.05 compared to PF
Table 2.
Body weights (g) of male and female offspring at birth (postnatal day 1, PN 1) and weaning (PN 22), and of male offspring prior to surgery and testing.
| Males | Females | |||||
|---|---|---|---|---|---|---|
| PN 1 | PN 22 | PN 55~58 (pre-surgery) | PN 69~72 (pre-testing) | PN 1 | PN 22 | |
| E | 6.1 ± 0.1* | 46.7 ± 1.0* | 317.4± 9.7* | 402.9± 10.5 | 5.8 ± 0.1* | 46.5 ± 0.9# |
| PF | 6.0 ± 0.2* | 49.1 ± 1.6^ | 311.1 ± 4.5* | 396.5± 5.6 | 5.4 ± 0.2* | 48.5 ± 1.6 |
| C | 6.7 ± 0.2 | 53.9 ± 1.7 | 330.1 ± 5.4 | 415.0±5.6 | 6.3 ± 0.1 | 51.0 ± 1.2 |
Values represent the mean ± SEM of the average male or female pup body weight per litter in 12–16 litters per group.
P < 0.01 compared to C;
P = 0.058 compared to C;
P < 0.05 compared to C.
Acknowledgments
A portion of these data has been presented orally at the International Society for Developmental Psychobiology (ISDP) 39th Annual Meeting, 2006, Atlanta, supported by Sandra G. Wiener Award from ISDP to NL. We thank Stephanie Westendorp, Paxton Pach, Teri Herbert, Yun Han and Wayne K. Yu for their technical assistance on the experiments.
Role of funding source
This research was supported by NIH/NIAAA AA07789 to JW and VV, grants from the Canadian Institute for Advanced Research and the UBC Human Early Learning Partnership to JW, an NSERC Canada Graduate Scholarship to NL, Fellowships from IMPART (CIHR Strategic Training Initiative in Health Research) and the Michael Smith Foundation for Health Research to KH. The funding agencies have no further role in study design, in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Conflict of interest
None declared.
Contributors
Ni Lan, Victor Viau and Joanne Weinberg contributed to the design of the study. Ni Lan, Linda Ellis wrote the protocol and did the animal experiments and in situ hybridization. Kim Hellemans did the statistical analysis and photographed CRH and AVP mRNA signals in the PVN. Ni Lan did the hormone assay and brain slicing, photographed the rest of the in situ slides and analyzed the in situ images, managed the literature searches, and wrote the first draft of the manuscript. Victor Viau and Joanne Weinberg assisted in editing and revising the manuscript. All authors contributed to and have approved the final manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahima RS, Lawson AN, Osei SY, Harlan RE. Sexual dimorphism in regulation of type II corticosteroid receptor immunoreactivity in the rat hippocampus. Endocrinology. 1992;131:1409–1416. doi: 10.1210/endo.131.3.1505471. [DOI] [PubMed] [Google Scholar]
- Attademo AM, Sanchez-Borzone M, Lasaga M, Celis ME. Intracerebroventricular injection of neuropeptide EI increases serum LH in male and female rats. Peptides. 2004;25:1995–1999. doi: 10.1016/j.peptides.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Barron S, Gilbertson R. Neonatal ethanol exposure but not neonatal cocaine selectively reduces specific isolation-induced vocalization waveforms in rats. Behav Genet. 2005;35:93–102. doi: 10.1007/s10519-004-0859-2. [DOI] [PubMed] [Google Scholar]
- Bingaman EW, Magnuson DJ, Gray TS, Handa RJ. Androgen inhibits the increases in hypothalamic corticotropin-releasing hormone (CRH) and CRH-immunoreactivity following gonadectomy. Neuroendocrinology. 1994;59:228–234. doi: 10.1159/000126663. [DOI] [PubMed] [Google Scholar]
- Bingham B, Williamson M, Viau V. Androgen and estrogen receptor-beta distribution within spinal-projecting and neurosecretory neurons in the paraventricular nucleus of the male rat. J Comp Neurol. 2006;499:911–923. doi: 10.1002/cne.21151. [DOI] [PubMed] [Google Scholar]
- Bohn MC, Dean D, Hussain S, Giuliano R. Development of mRNAs for glucocorticoid and mineralocorticoid receptors in rat hippocampus. Brain Res Dev Brain Res. 1994;77:157–162. doi: 10.1016/0165-3806(94)90192-9. [DOI] [PubMed] [Google Scholar]
- Briski KP, Sylvester PW. Effects of repetitive daily acute stress on pituitary LH and prolactin release during exposure to the same stressor or a second novel stress. Psychoneuroendocrinology. 1987a;12:429–437. doi: 10.1016/0306-4530(87)90077-1. [DOI] [PubMed] [Google Scholar]
- Briski KP, Sylvester PW. Effects of sequential acute stress exposure on stress-induced pituitary luteinizing hormone and prolactin secretion. Life Sci. 1987b;41:1249–1255. doi: 10.1016/0024-3205(87)90203-7. [DOI] [PubMed] [Google Scholar]
- Burgess LH, Handa RJ. Hormonal regulation of androgen receptor mRNA in the brain and anterior pituitary gland of the male rat. Brain Res Mol Brain Res. 1993;19:31–38. doi: 10.1016/0169-328x(93)90145-f. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Aitken aMRF. ANOVA for the Behavioural Sciences Researcher. Lawrence Erbaum Associates; London, UK: 2006. [Google Scholar]
- Champagne D, Beaulieu J, Drolet G. CRFergic innervation of the paraventricular nucleus of the rat hypothalamus: a tract-tracing study. J Neuroendocrinol. 1998;10:119–131. doi: 10.1046/j.1365-2826.1998.00179.x. [DOI] [PubMed] [Google Scholar]
- Chang C, Kokontis J. Identification of a new member of the steroid receptor superfamily by cloning and sequence analysis. Biochem Biophys Res Commun. 1988;155:971–977. doi: 10.1016/s0006-291x(88)80591-6. [DOI] [PubMed] [Google Scholar]
- Cullinan WE, Herman JP, Battaglia DF, Akil H, Watson SJ. Pattern and time course of immediate early gene expression in rat brain following acute stress. Neuroscience. 1995;64:477–505. doi: 10.1016/0306-4522(94)00355-9. [DOI] [PubMed] [Google Scholar]
- Dallman MF, Viau V, Bhatnagar S, Gomez F, Laugero K, Bell ME. Corticotropin-Releasing Factor, Corticosteroids, Stress, and Sugar: Energy Balance, the Brain, and Behavior. In: PFAFF DW, ARNOLD AP, ETGEN AM, FAHRBACH SE, RUBIN RT, editors. Hormones, Brain and Behavior. Vol. 1. Academic Press; New York: 2002. pp. 571–631. [Google Scholar]
- Davis M. The role of the amygdala in fear and anxiety. Annu Rev Neurosci. 1992;15:353–375. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- De Kloet ER, Vreugdenhil E, Oitzl MS, Joels M. Brain corticosteroid receptor balance in health and disease. Endocr Rev. 1998;19:269–301. doi: 10.1210/edrv.19.3.0331. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Wang Z, Bullock NA, Numan S. Sex differences in the effects of testosterone and its metabolites on vasopressin messenger RNA levels in the bed nucleus of the stria terminalis of rats. J Neurosci. 1994;14:1789–1794. doi: 10.1523/JNEUROSCI.14-03-01789.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong HW, Petrovich GD, Swanson LW. Topography of projections from amygdala to bed nuclei of the stria terminalis. Brain Res Brain Res Rev. 2001a;38:192–246. doi: 10.1016/s0165-0173(01)00079-0. [DOI] [PubMed] [Google Scholar]
- Dong HW, Petrovich GD, Watts AG, Swanson LW. Basic organization of projections from the oval and fusiform nuclei of the bed nuclei of the stria terminalis in adult rat brain. J Comp Neurol. 2001b;436:430–455. doi: 10.1002/cne.1079. [DOI] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- Gentry RT, Wade GN. Androgenic control of food intake and body weight in male rats. J Comp Physiol Psychol. 1976;90:18–25. doi: 10.1037/h0077264. [DOI] [PubMed] [Google Scholar]
- Glavas MM, Ellis L, Yu WK, Weinberg J. Effects of prenatal ethanol exposure on basal limbic-hypothalamic-pituitary-adrenal regulation: role of corticosterone. Alcohol Clin Exp Res. 2007;31:1598–1610. doi: 10.1111/j.1530-0277.2007.00460.x. [DOI] [PubMed] [Google Scholar]
- Greenstein BD, Fitzpatrick FT, Adcock IM, Kendall MD, Wheeler MJ. Reappearance of the thymus in old rats after orchidectomy: inhibition of regeneration by testosterone. J Endocrinol. 1986;110:417–422. doi: 10.1677/joe.0.1100417. [DOI] [PubMed] [Google Scholar]
- Handa RJ, Kerr JE, DonCarlos LL, McGivern RF, Hejna G. Hormonal regulation of androgen receptor messenger RNA in the medial preoptic area of the male rat. Brain Res Mol Brain Res. 1996;39:57–67. doi: 10.1016/0169-328x(95)00353-t. [DOI] [PubMed] [Google Scholar]
- Handa RJ, Nunley KM, Lorens SA, Louie JP, McGivern RF, Bollnow MR. Androgen regulation of adrenocorticotropin and corticosterone secretion in the male rat following novelty and foot shock stressors. Physiol Behav. 1994;55:117–124. doi: 10.1016/0031-9384(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Herman JP, Patel PD, Akil H, Watson SJ. Localization and regulation of glucocorticoid and mineralocorticoid receptor messenger RNAs in the hippocampal formation of the rat. Mol Endocrinol. 1989;3:1886–1894. doi: 10.1210/mend-3-11-1886. [DOI] [PubMed] [Google Scholar]
- Hoyme HE, May PA, Kalberg WO, Kodituwakku P, Gossage JP, Trujillo PM, Buckley DG, Miller JH, Aragon AS, Khaole N, Viljoen DL, Jones KL, Robinson LK. A practical clinical approach to diagnosis of fetal alcohol spectrum disorders: clarification of the 1996 institute of medicine criteria. Pediatrics. 2005;115:39–47. doi: 10.1542/peds.2004-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson JL, Jacobson SW. Drinking moderately and pregnancy. Effects on child development. Alcohol Res Health. 1999;23:25–30. [PMC free article] [PubMed] [Google Scholar]
- Kehoe P, Shoemaker W. Opioid-dependent behaviors in infant rats: effects of prenatal exposure to ethanol. Pharmacol Biochem Behav. 1991;39:389–394. doi: 10.1016/0091-3057(91)90197-a. [DOI] [PubMed] [Google Scholar]
- Kerr JE, Allore RJ, Beck SG, Handa RJ. Distribution and hormonal regulation of androgen receptor (AR) and AR messenger ribonucleic acid in the rat hippocampus. Endocrinology. 1995;136:3213–3221. doi: 10.1210/endo.136.8.7628354. [DOI] [PubMed] [Google Scholar]
- Kerr JE, Beck SG, Handa RJ. Androgens modulate glucocorticoid receptor mRNA, but not mineralocorticoid receptor mRNA levels, in the rat hippocampus. J Neuroendocrinol. 1996;8:439–447. doi: 10.1046/j.1365-2826.1996.04735.x. [DOI] [PubMed] [Google Scholar]
- Koehl M, Darnaudery M, Dulluc J, Van Reeth O, Le Moal M, Maccari S. Prenatal stress alters circadian activity of hypothalamo-pituitary-adrenal axis and hippocampal corticosteroid receptors in adult rats of both gender. J Neurobiol. 1999;40:302–315. [PubMed] [Google Scholar]
- Kofman O. The role of prenatal stress in the etiology of developmental behavioural disorders. Neurosci Biobehav Rev. 2002;26:457–470. doi: 10.1016/s0149-7634(02)00015-5. [DOI] [PubMed] [Google Scholar]
- Krulich L, Hefco E, Illner P, Read CB. The effects of acute stress on the secretion of LH, FSH, prolactin and GH in the normal male rat, with comments on their statistical evaluation. Neuroendocrinology. 1974;16:293–311. doi: 10.1159/000122576. [DOI] [PubMed] [Google Scholar]
- Lan N, Yamashita F, Halpert AG, Ellis L, Yu WK, Viau V, Weinberg J. Prenatal ethanol exposure alters the effects of gonadectomy on hypothalamic-pituitary-adrenal activity in male rats. J Neuroendocrinol. 2006;18:672–684. doi: 10.1111/j.1365-2826.2006.01462.x. [DOI] [PubMed] [Google Scholar]
- Lee S, Rivier C. Gender differences in the effect of prenatal alcohol exposure on the hypothalamic-pituitary-adrenal axis response to immune signals. Psychoneuroendocrinology. 1996;21:145–155. doi: 10.1016/0306-4530(95)00038-0. [DOI] [PubMed] [Google Scholar]
- Lee S, Schmidt D, Tilders F, Rivier C. Increased activity of the hypothalamic-pituitary-adrenal axis of rats exposed to alcohol in utero: role of altered pituitary and hypothalamic function. Mol Cell Neurosci. 2000;16:515–528. doi: 10.1006/mcne.2000.0890. [DOI] [PubMed] [Google Scholar]
- Lesage J, Blondeau B, Grino M, Breant B, Dupouy JP. Maternal undernutrition during late gestation induces fetal overexposure to glucocorticoids and intrauterine growth retardation, and disturbs the hypothalamo-pituitary adrenal axis in the newborn rat. Endocrinology. 2001;142:1692–1702. doi: 10.1210/endo.142.5.8139. [DOI] [PubMed] [Google Scholar]
- Marino MD, Cronise K, Lugo JN, Jr, Kelly SJ. Ultrasonic vocalizations and maternal-infant interactions in a rat model of fetal alcohol syndrome. Dev Psychobiol. 2002;41:341–351. doi: 10.1002/dev.10077. [DOI] [PubMed] [Google Scholar]
- Matthews SG. Early programming of the hypothalamo-pituitary-adrenal axis. Trends Endocrinol Metab. 2002;13:373–380. doi: 10.1016/s1043-2760(02)00690-2. [DOI] [PubMed] [Google Scholar]
- Miller MA, DeVries GJ, al-Shamma HA, Dorsa DM. Decline of vasopressin immunoreactivity and mRNA levels in the bed nucleus of the stria terminalis following castration. J Neurosci. 1992;12:2881–2887. doi: 10.1523/JNEUROSCI.12-08-02881.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moga MM, Saper CB. Neuropeptide-immunoreactive neurons projecting to the paraventricular hypothalamic nucleus in the rat. J Comp Neurol. 1994;346:137–150. doi: 10.1002/cne.903460110. [DOI] [PubMed] [Google Scholar]
- Myers JL, Well AD. Research Design and Statistical Analysis. Lawrence Erlbaum Associates; Mahwah, New Jersey: 2003. [Google Scholar]
- Ness JW, Franchina JJ. Effects of prenatal alcohol exposure on rat pups’ ability to elicit retrieval behavior from dams. Dev Psychobiol. 1990;23:85–99. doi: 10.1002/dev.420230109. [DOI] [PubMed] [Google Scholar]
- Oner H, Ozan E. Effects of gonadal hormones on thymus gland after bilateral ovariectomy and orchidectomy in rats. Arch Androl. 2002;48:115–126. doi: 10.1080/014850102317267427. [DOI] [PubMed] [Google Scholar]
- Pace TW, Spencer RL. Disruption of mineralocorticoid receptor function increases corticosterone responding to a mild, but not moderate, psychological stressor. Am J Physiol Endocrinol Metab. 2005;288:E1082–1088. doi: 10.1152/ajpendo.00521.2004. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Elsevier; Burlington, MA, USA: 2005. [Google Scholar]
- Prewitt CM, Herman JP. Anatomical interactions between the central amygdaloid nucleus and the hypothalamic paraventricular nucleus of the rat: a dual tract-tracing analysis. J Chem Neuroanat. 1998;15:173–185. doi: 10.1016/s0891-0618(98)00045-3. [DOI] [PubMed] [Google Scholar]
- Ramsay DS, Bendersky MI, Lewis M. Effect of prenatal alcohol and cigarette exposure on two- and six-month-old infants’ adrenocortical reactivity to stress. J Pediatr Psychol. 1996;21:833–840. doi: 10.1093/jpepsy/21.6.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Seckl JR. Prenatal glucocorticoids and long-term programming. Eur J Endocrinol. 2004;151(Suppl 3):U49–62. doi: 10.1530/eje.0.151u049. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. J Comp Neurol. 1997;388:507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Chang C, Muramatsu M, Swanson LW. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study. J Comp Neurol. 1990;294:76–95. doi: 10.1002/cne.902940107. [DOI] [PubMed] [Google Scholar]
- Taylor AN, Branch BJ, Kokka N, Poland RE. Neonatal and long-term neuroendocrine effects of fetal alcohol exposure. Monogr Neural Sci. 1983;9:140–152. doi: 10.1159/000406886. [DOI] [PubMed] [Google Scholar]
- Taylor AN, Branch BJ, Van Zuylen JE, Redei E. Maternal alcohol consumption and stress responsiveness in offspring. Adv Exp Med Biol. 1988;245:311–317. doi: 10.1007/978-1-4899-2064-5_25. [DOI] [PubMed] [Google Scholar]
- Taylor AN, Nelson LR, Branch BJ, Kokka N, Poland RE. Altered stress responsiveness in adult rats exposed to ethanol in utero: neuroendocrine mechanisms. Ciba Found Symp. 1984;105:47–65. doi: 10.1002/9780470720868.ch4. [DOI] [PubMed] [Google Scholar]
- Turner BB. Influence of gonadal steroids on brain corticosteroid receptors: a minireview. Neurochem Res. 1997;22:1375–1385. doi: 10.1023/a:1022023207326. [DOI] [PubMed] [Google Scholar]
- Turner BB, Weaver DA. Sexual dimorphism of glucocorticoid binding in rat brain. Brain Res. 1985;343:16–23. doi: 10.1016/0006-8993(85)91153-9. [DOI] [PubMed] [Google Scholar]
- Viau V, Chu A, Soriano L, Dallman MF. Independent and overlapping effects of corticosterone and testosterone on corticotropin-releasing hormone and arginine vasopressin mRNA expression in the paraventricular nucleus of the hypothalamus and stress-induced adrenocorticotropic hormone release. J Neurosci. 1999;19:6684–6693. doi: 10.1523/JNEUROSCI.19-15-06684.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viau V, Lee P, Sampson J, Wu J. A testicular influence on restraint-induced activation of medial parvocellular neurons in the paraventricular nucleus in the male rat. Endocrinology. 2003;144:3067–3075. doi: 10.1210/en.2003-0064. [DOI] [PubMed] [Google Scholar]
- Viau V, Soriano L, Dallman MF. Androgens alter corticotropin releasing hormone and arginine vasopressin mRNA within forebrain sites known to regulate activity in the hypothalamic-pituitary-adrenal axis. J Neuroendocrinol. 2001;13:442–452. doi: 10.1046/j.1365-2826.2001.00653.x. [DOI] [PubMed] [Google Scholar]
- Vorhees CV. A fostering/crossfostering analysis of the effects of prenatal ethanol exposure in a liquid diet on offspring development and behavior in rats. Neurotoxicol Teratol. 1989;11:115–120. doi: 10.1016/0892-0362(89)90049-4. [DOI] [PubMed] [Google Scholar]
- Wade GN. Sex hormone, regulatory behaviors, and body weight. In: ROSENBLATT JS, HINDE RA, SHAW E, BEER CG, editors. Advances in the Study of Behavior. Academic Press; New York: 1976. pp. 201–279. [Google Scholar]
- Weinberg J. Hyperresponsiveness to stress: differential effects of prenatal ethanol on males and females. Alcohol Clin Exp Res. 1988;12:647–652. doi: 10.1111/j.1530-0277.1988.tb00258.x. [DOI] [PubMed] [Google Scholar]
- Weinberg J. Prenatal ethanol exposure alters adrenocortical development of offspring. Alcohol Clin Exp Res. 1989;13:73–83. doi: 10.1111/j.1530-0277.1989.tb00287.x. [DOI] [PubMed] [Google Scholar]
- Weinberg J. Prenatal ethanol effects: sex differences in offspring stress responsiveness. Alcohol. 1992;9:219–223. doi: 10.1016/0741-8329(92)90057-h. [DOI] [PubMed] [Google Scholar]
- Weinberg J, Sliwowska JH, Lan N, Hellemans KG. Prenatal alcohol exposure: foetal programming, the hypothalamic-pituitary-adrenal axis and sex differences in outcome. J Neuroendocrinol. 2008;20:470–488. doi: 10.1111/j.1365-2826.2008.01669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson M, Bingham B, Viau V. Central organization of androgen-sensitive pathways to the hypothalamic-pituitary-adrenal axis: implications for individual differences in responses to homeostatic threat and predisposition to disease. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:1239–1248. doi: 10.1016/j.pnpbp.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Williamson M, Viau V. Androgen receptor expressing neurons that project to the paraventricular nucleus of the hypothalamus in the male rat. J Comp Neurol. 2007;503:717–740. doi: 10.1002/cne.21411. [DOI] [PubMed] [Google Scholar]
- Yukhananov RY, Handa RJ. Estrogen alters proenkephalin RNAs in the paraventricular nucleus of the hypothalamus following stress. Brain Res. 1997;764:109–116. doi: 10.1016/s0006-8993(97)00432-0. [DOI] [PubMed] [Google Scholar]
- Zhou L, Blaustein JD, De Vries GJ. Distribution of androgen receptor immunoreactivity in vasopressin- and oxytocin-immunoreactive neurons in the male rat brain. Endocrinology. 1994;134:2622–2627. doi: 10.1210/endo.134.6.8194487. [DOI] [PubMed] [Google Scholar]