Abstract
Objective
Circadian mechanisms underlie the physiology of mammals as an adaptation to the earth’s rotation on its axis. Highly conserved core circadian regulatory proteins (CCRP) maintain an oscillatory expression profile in the central and peripheral tissues. The CCRP include both a positive and negative arm as well as downstream transcriptional regulators. Recent studies in murine models have determined that the mRNAs encoding the CCRP are present in multiple adipose tissue depots and exhibit a robust oscillatory expression profile. The current study set out to examine the expression of CCRP mRNAs in human subcutaneous adipose tissues.
Design
Retrospective analysis of total RNA isolated from subcutaneous adipose tissue.
Subjects
150 healthy female and male lean (BMI < 25), overweight (BMI between 25 and 29.99) or obese (BMI >30) subjects of varied ethnic backgrounds undergoing elective liposuction or surgical procedures.
Results
The expression of the CCRP mRNAs displayed a significant correlation between each other and mRNAs representative of adipogenic biomarkers. Hierarchical cluster analyses of mRNAs isolated from the cohort of female Caucasian subjects (n = 116) identified three major clusters based on expression of downstream CCRP mRNAs. The mRNAs encoding D site of albumin promoter binding protein (DBP), E4 promoter binding protein 4 (E4BP4), PPARγ Co-Activator 1β (PGC-1β), and Rev-erb α were negatively correlated with BMI in a lean cluster (n = 66), positively correlated with BMI in a younger overweight/obese cluster (n = 19), and not significantly correlated with BMI in an older, overweight/obese cluster (n = 31).
Conclusions
These data confirm and extend findings that link the CCRP and circadian mechanisms to the risk of obesity.
Keywords: Adipose tissue, BMAL1, Circadian, Clock, Cryptochrome, Double Binding Protein (DBP), E4BP4, Period, Peroxisome Proliferator Activated Receptor γ Co-Activator-1 (PGC-1), Rev-Erbα
Introduction
The earth’s rotation around the sun has impacted physiology in all organisms. As a consequence, there has evolved a highly conserved set of genes encoding core circadian regulatory proteins (CCRP(1, 2). Two of these proteins, brain muscle Arnt-like-1 (Bmal1) and circadian locomotor output cycles kaput (Clock) or its homolog, neural PAS domain protein 2 (Npas2), serve as the positive arm of the circadian pathway (1, 2). They heterodimerize and bind to conserved E-box cis elements in the promoters of downstream targets (1, 2). Among their many actions, Bmal1-Clock or Bmal1-Npas2 heterodimers activate transcription of their own negative regulators, cryptochrome (Cry) and period (Per), which heterodimerize and repress transcription of Bmal1 through a feedback mechanism (1, 2). This results in an oscillatory anti-phase expression profile between the positive and negative arms of the pathway that repeats every 24 hour cycle (1, 2). Additional downstream genes regulated by Bmal1-Clock include the transcription factors D site of albumin promoter binding protein (DBP), E4 promoter binding protein 4 (E4BP4), and the nuclear hormone receptor family members Rev-erb α and β and retinoid-like orphan receptor (ROR) α and γ (1, 2). For purposes of this manuscript, the following genes are encompassed by the term “CCRP”: (1) The positive arm genes encoding Bmal1, Clock, and Npas2; (2) The negative arm genes encoding Cry and Per isoforms; (3) The downstream targets DBP, E4BP4, Rev-erb and ROR isoforms. Furthermore, the genes encoding glycogen synthase kinase 3 β, responsible for the phosphorylation and proteasomal targeting of Per, and PPARγ2 Coactivator-1 (PGC1), which has been found to modulate circadian rhythms (3, 4), are included under the term “CCRP”.
The CCRP have been linked to obesity, metabolism, and sleep disorders. Murine models homozygous for a mutated version of Clock displayed hyperphagia, hypertriglyceridemia, glucose intolerance, and a propensity towards obesity and the metabolic syndrome (5). Exposure to high fat diets has been observed to attenuate, phase advance, and/or disrupt the circadian rhythm in rodent models (6) (7, 8). Both Bmal1 and Clock have been linked functionally to glucose metabolism in murine models (9). Recent genotyping studies have identified Clock gene polymorphisms that are associated with an increased risk of obesity and metabolic syndrome in human population studies (10–13). Furthermore, murine strains with the Clock mutation or deficient in Bmal1, DBP, Npas2, or Per2 display abnormal sleep/wake cycles when deprived of photic stimuli as an entrainer (14, 15) (16–19). Consistent with this, polymorphisms in the human Clock and Period 3 gene are associated with sleep /wake preferences or disturbances (20–23). The importance of the CCRP genes in regulating sleep is of particular relevance. Pioneering clinical and epidemiological research by Van Cauter and others has demonstrated a direct association between human sleep patterns, glucose metabolism, and risk of obesity (24–30). Reduced length of sleep has been correlated with increased incidence of obesity at various points in human development (31–35).
While the CCRP genes play a critical role within the body’s master circadian clock located within the suprachiasmatic nucleus of the brain (1), the genes have been detected in multiple peripheral tissues(36, 37). Their expression profile displays some degree of autonomy since circadian oscillations persist for several days in organs isolated from Per2 promoter/luciferase reporter transgenic mice in the absence of systemic synchronizing stimuli (37). The mRNAs encoding the CCRP have been found in multiple murine adipose depots and display a robust circadian oscillation in vivo (38–40). Similarly, Bmal1, Cry1, and Per2 have been detected in both subcutaneous and visceral adipose tissue obtained from eight morbidly obese human males (41). In subcutaneous adipose tissue, these CCRP genes correlated with each other as well as parameters associated with the metabolic syndrome (41). Furthermore, in vitro studies have demonstrated that both mRNAs encoding CCRPs (42) and glucocorticoid-metabolic enzymes (43) can display an oscillatory expression profile in primary adipose-derived stem cell cultures and tissue explants, respectively. Based on these findings, the current study set out to examine the expression of a panel of CCRP mRNAs in subcutaneous adipose tissue obtained from healthy human subjects undergoing elective surgeries. The expression level of individual CCRP mRNAs has been analyzed relative to each other and to adipocyte-associated biomarkers and evaluated in the context of donor age and body mass index.
Materials, Subjects, and Methods
Human Subjects
All procedures and collections were performed under a research protocol reviewed and approved by the Pennington Biomedical Research Center Institutional Review Board in accordance with Helsinki Principles and HIPAA requirements. All research subjects provided their informed written consent prior to their surgery allowing the use of their tissue specimens for research purposes. Subcutaneous adipose tissue samples were obtained from otherwise healthy patients undergoing elective tumescent liposuction or bariatric surgery under the care of collaborating surgeons (see Acknowledgements). All subjects (n = 150) were healthy without a history of metabolic disease and displayed the following demographics: (1) mean age of 41.9 ± 10.7 years; (2) mean BMI of 26.9 ± 4.9; (3) ethnicity 81% Caucasian, 11% African American, 3% Asian, 3% Hispanic, 2% Not recorded; female 93%, male 7%. The majority of specimens contained subcutaneous adipose tissue from one or more of the following locations: abdomen (54%), flank (36%), lower extremity (29%), hip (15%), upper extremity (9%), breast/chest (5%), gluteus (1%). Samples were harvested between the hours of 8 AM to 2 PM, transferred to the research laboratory, and frozen at −80° C until processed for RNA recovery.
Quantitative Real Time PCR (qRT-PCR)
Total RNA was purified from human lipoaspirate tissues obtained from 151 subjects using TriReagent (Molecular Research Center) according to the manufacturer’s specifications. Approximately 2µg of total RNA was reverse transcribed using Moloney Murine Leukemia Virus Reverse Transcriptase (MMLV-RT; Promega), with Oligo dT at 42°C for 1 hour in a 20µL reaction. Primers for genes of interest were identified using Primer Express software (Applied Biosystems) as previously described (42, 44). A complete list of primers used in these studies is listed in Supplement Table 1. qRT-PCR was performed on diluted cDNA samples with SYBR® Green PCR Master Mix (Applied Biosystems) using the 7900 Real Time PCR system (Applied Biosystems) under universal cycling conditions (95°C for 10 min; 40 cycles of 95°C for 15 sec; then 60°C for 1 min). All results were normalized relative to the expression of β-Actin, β2 Microglobulin, Cyclophilin B, and 18S RNA as expression controls.
Cluster Analysis
Without any assumption regarding the distributions of the raw data, we used hierarchical clustering method to segment the study population into N clusters on the basis of the samples’ age and BMI data (45). Four of 151 samples are automatically excluded from the procedure due the missing data. R-square value of each clustering is used as index to determine the number of clusters N. Within each cluster, we conducted the spearman’s rank correlation analysis to measure the relationship among the circadian genes. All analysis is performed in SAS v9.1 or JMP (SAS, Cary, NC). All values are expressed as the mean ± S.D.
Results
CCRP mRNA Expression and Correlation with Adipocyte Biomarkers
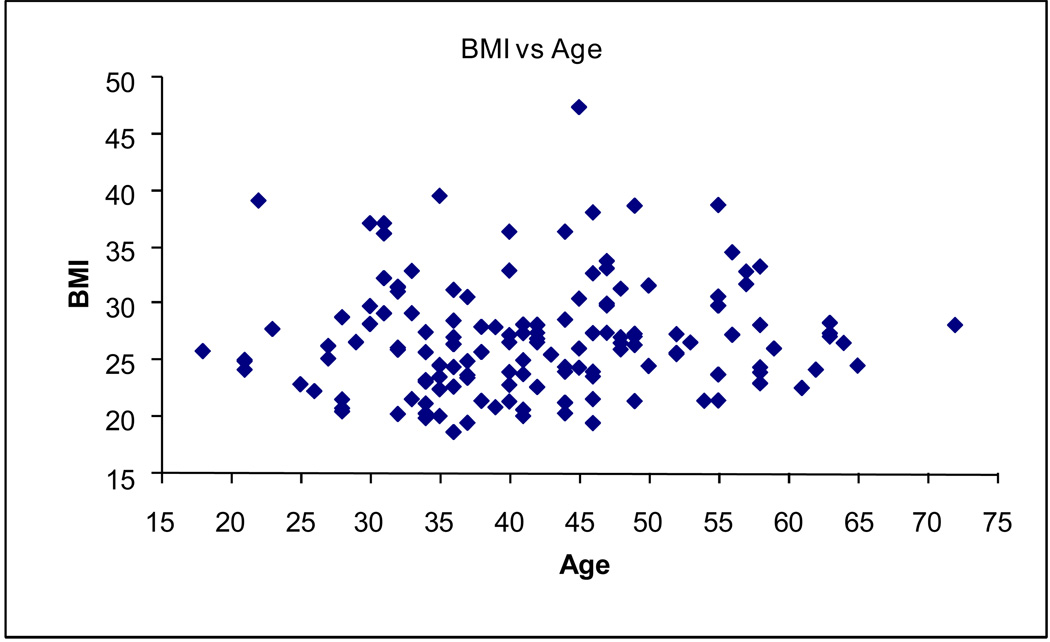
Subcutaneous adipose tissue was obtained from subjects undergoing elective surgery. Total mRNA was extracted from 151 male and female donors with a mean age of 41.7 ± 10.6 years (range 18 to 72) and a mean body mass index (BMI) of 27.0 ± 4.9 (range 18.7 to 47.4) (Figure 1). The CCRP genes Bmal1, Clock, Cry1, DBP, E4BP4, Npas2, Rev-erbα, and Rev-erbβ were detected in adipose tissue. In the total population, the Cry1, DBP, Rev-erbα, Rev-erbβ, and RORα mRNAs displayed a highly significant positive correlation with each other while Npas2 correlated negatively with all genes except Bmal1, E4BP4, and RORα, where no significant correlation was observed (Figure 2). In contrast, the mRNAs encoding Clock, E4BP4, and RORα displayed a less significant correlation with each other and the related CCRP genes. With the exception of RORα, Bmal1 failed to display a significant correlation with any other CCRP mRNA. The correlation among the CCRP mRNAs in the subset of female Caucasian donors (n = 116) displayed a nearly identical pattern (Figure 3). In both groups, the majority of CCRP mRNAs positively correlated with all adipocyte biomarkers (aP2, C/EBPα, LPL, PPARγ2, PGC1β) with the exception of PGC1α, where a negative correlation was observed.
Figure 1. Scatter Plot of Body Mass Index (BMI) versus Age.
The study cohort (n = 150) BMI (Y-axis) was evaluated relative to age (X-axis).
Figure 2. Direct Correlation Between CCRP and Adipogenic Genes in All Subjects.

The correlation individual genes with each other and with age or BMI was determined in the entire study cohort (n = 150). Statistical significance based on t-test is displayed in varying shades of gray and black. Gene abbreviations are defined in Supplement Table 1.
Figure 3. Direct Correlation Between CCRP and Adipogenic Genes in Female Caucasian Subjects.
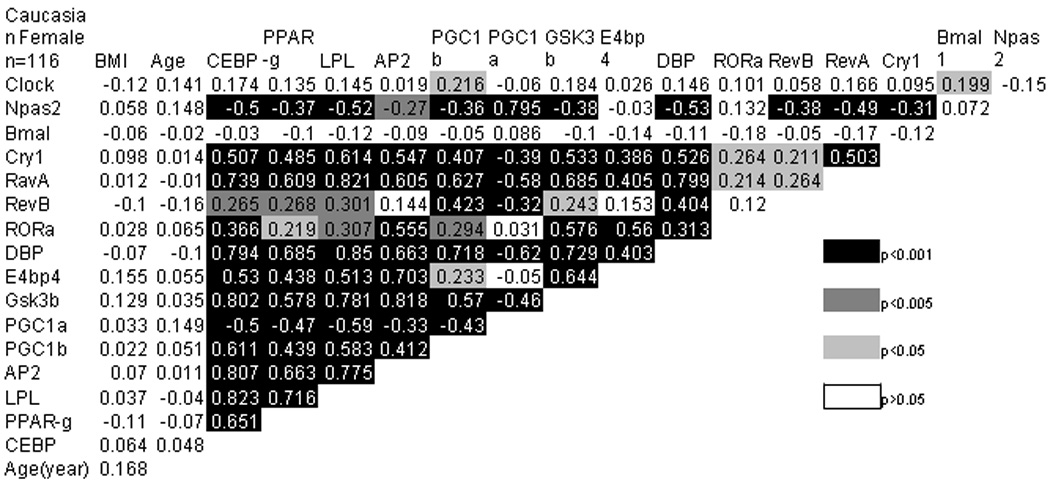
The correlation individual genes with each other and with age or BMI was determined in the subset of Caucasian female subjects (n = 116). Statistical significance based on t-test is displayed in varying shades of gray and black. Gene abbreviations are defined in Supplement Table 1.
CCRP mRNA Correlation with Age and BMI
The individual CCRP and adipocyte biomarker mRNAs were correlated relative to each other in sectors of the total population subdivided based on BMI (Supplemental Table 1). Similar but not identical correlations among the CCRP and adipocyte mRNAs were observed in lean subjects (n = 56, BMI < 25), overweight (n = 59, 25< BMI < 30), and obese (n = 32, BMI > 30). To determine if a correlation exists between gene expression relative to age and BMI, the subset of Caucasian female donors (n = 116) were subjected to hierarchical cluster analysis (Table 1). Three individual clusters were identified. Cluster 1 included 31 older (55.7 year) overweight/obese (BMI 29.1) individuals, Cluster 2 included 66 younger (40 year) lean (24.1 BMI) individuals, and Cluster 3 included 19 younger (30.5 year) overweight/obese (29.9 BMI). While age displayed a significant negative correlation with BMI in the older, overweight/obese subjects (Cluster 1), no significant correlation was noted for CCRP or adipocyte biomarker mRNAs relative to BMI. In contrast, age displayed a significant positive correlation with BMI in the leaner individuals (Cluster 2) and a trend, but not significance, towards this pattern in the younger, overweight/obese individuals (Cluster 3). A subset of CCRP genes (DBP, PGC1β, Rev-erb α) and adipocyte biomarker (PPARγ2) displayed a significant (p < 0.05) or near significant (p < 0.1) negative correlation with BMI in the lean Cluster 2 (Table 2). Furthermore, in Cluster 2, a near significant positive (0.416, p = 0.0965) and negative (−0.434, p = 0.0718) correlation was observed for DBP and Npas2, respectively, relative to age. In contrast, the CCRP (DBP, E4BP4, PGC1β, Rev-erbα) mRNAs displayed a significant or near significant positive correlation with BMI in the younger, overweight/obese Cluster 3.
Table 1.
Age and BMI Dependence of Selected Human Adipose Tissue CCRP mRNAs in Caucasian Female Subjects
| Cluster 1 (n = 31) Overweight/Obese, Older |
Cluster 2 (n = 66) Lean, Younger |
Cluster 3 (n = 19) Overweight/Obese, Younger |
||||
|---|---|---|---|---|---|---|
| Age (± S.D.) | 55.7 ± 6.7 | 40.0 ± 6.1 | 30.5 ± 4.9 | |||
| BMI (± S.D.) | 29.1 ± 4.8 | 24.1 ± 2.8 | 29.9 ± 3.8 | |||
| Gene or Age | BMI Correlation | P Value | BMI Correlation | P Value | BMI Correlation | P Value |
| Age | −0.469 | 0.0078 | 0.378 | 0.0018 | 0.351 | 0.141 |
| DBP | −0.008 | 0.9678 | −0.256 | 0.0447 | 0.485 | 0.0483 |
| Rev-Erbα | −0.114 | 0.5961 | −0.255 | 0.0649 | 0.588 | 0.0165 |
| E4BP4 | −0.111 | 0.5540 | −0.071 | 0.570 | 0.416 | 0.0766 |
| PGC1b | 0.358 | 0.1325 | −.0512 | 0.0763 | 0.583 | 0.0992 |
| PPARγ2 | −0.259 | 0.1662 | −0.278 | 0.0231 | 0.226 | 0.3515 |
Grey color: p value < 0.05.
Discussion
There has been a growing appreciation in the literature of the role circadian mechanisms play in adipose tissue biology, metabolism, and obesity (40, 46–48). The current study documents the expression of multiple CCRP mRNAs in human subcutaneous adipose tissue. The results confirm findings in rodent models (38–40) and confirm and extend the existing literature relating to CCRP gene expression in human adipose tissues (41). It is of note that mRNA expression profiles of Bmal1 and Npas2 display either an absent or negative correlation with the other CCRP mRNAs. This may be explained by the fact that both Bmal1 and Npas2, as components of the CCRP positive arm, are predicted to be out of phase with Cry, Per, and elements of the negative CCRP arm. The expression of the downstream components of the CCRP (DBP, E4BP4, Rev-erb, ROR) in positive correlation with Cry1 may reflect some degree of temporal synchrony in their profiles. This is consistent with observations in murine adipose depots, where the acrophase of Bmal1 and Npas2 were out of phase with Cry, Per, DBP, and Rev-erb mRNAs (39). Because the current study was performed with human adipose tissue obtained from individual donors at only one time point, the conclusions supported by this data are limited relative to in vivo murine studies (39). Further studies involving serial adipose tissue biopsies from a single donor will be necessary to define the relative expression profiles of the human CCRP mRNAs. Likewise, it remains to be determined if the human adipose tissue depot site (subcutaneous vs. visceral) significantly modulates the CCRP mRNA temporal expression profiles as previous studies have suggested (41)
The current results indicate that the expression of a subset of CCRP genes is directly correlated to both subject age and BMI. The CCRP mRNA subset positively correlated with BMI in young, overweight/obese subjects (Cluster 3). This association was absent in an older cluster of nearly identical BMI (Cluster 3). This suggests that advancing age may attenuate or inhibit circadian mechanisms in adipose depots. In contrast, this subset of CCRP mRNAs negatively correlated with BMI in relatively young, lean subjects (Cluster 2). This non-linear correlation of CCRP mRNA expression levels with BMI in the younger individuals remains puzzling. It is well known that increased BMI alters the cellular composition of adipose tissue, reducing the frequency of adipose stem cells or pre-adipocytes while increasing the numbers of macrophages and large volume adipocytes. It is feasible that altered numbers of distinct cell lineages in the adipose tissue itself contributed to the current findings; however, this remains speculative and the subject merits further investigation.
This subset of CCRP genes (DBP, E4BP4, Rev-erb α) are downstream of both the positive and negative arms of the core circadian oscillator where they act as transcriptional regulators, often with a repressor function. The E4BP4 factor, also known as nuclear factor IL-3 (NFIL-3), has been associated with the regulation of osteoblast differentiation (49, 50). This has potential implications in light of the multi-lineage differentiation potential of adipose-derived stem cells and the inverse relationship between the adipocyte and osteoblast pathways (51). The regulation of Rev-erb proteins has been linked to diabetes, metabolic syndrome, and obesity (47, 48). Recently, the Rev-erb α ligand has been identified as the iron containing heme moiety (52–55) which has long been known to enhance adipocyte differentiation in vitro (56). While PGC-1 was first described in the context of PPARγ function in adipose tissue and muscle (57), it has been identified additionally as a component of the circadian mechanism. Mice deficient in either PGC-1α or PGC-1β both display abnormalities in their circadian metabolic activities (3, 4). The significance of the divergent correlation between the subset of CCRP mRNAs in the younger lean and overweight/obese cohorts. Thus, expression of at least four CCRP transcripts correlate with age and/or BMI in human subcutaneous adipose tissue.
In summary, human subcutaneous adipose tissue contained detectable levels of a subset of CCRP mRNAs. At least 4 of the downstream CCRP mRNAs were expressed as a function of subject age and BMI. While these CCRP mRNAs exhibited a negative correlation with BMI in lean, younger subjects, the correlation was positive in obese, younger subjects; however, in older, obese subjects, any significant correlation between CCRP mRNA levels and age was absent.
Supplementary Material
Acknowledgements
The authors thank Elizabeth Clubb, M.D., Thomas Guillot, M.D., James Wade, M.D., their office staff, and patients for donation of lipoaspirate tissues; Laura Dallam for administrative assistance. This work was partially supported by a CNRU Center Grant # 1P30 DK072476 entitled “Nutritional Programming: Environmental and Molecular Interactions” sponsored by NIDDK (G.Y., S.R.S., J.M.G.) and the Pennington Biomedical Research Foundation (X.W., J.M.G.).
References
- 1.Lowrey PL, Takahashi JS. Mammalian circadian biology: elucidating genome-wide levels of temporal organization. Annu Rev Genomics Hum Genet. 2004;5:407–441. doi: 10.1146/annurev.genom.5.061903.175925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hirota T, Fukada Y. Resetting mechanism of central and peripheral circadian clocks in mammals. Zoolog Sci. 2004;21:359–368. doi: 10.2108/zsj.21.359. [DOI] [PubMed] [Google Scholar]
- 3.Liu C, Li S, Liu T, Borjigin J, Lin JD. Transcriptional coactivator PGC-1alpha integrates the mammalian clock and energy metabolism. Nature. 2007;447:477–481. doi: 10.1038/nature05767. [DOI] [PubMed] [Google Scholar]
- 4.Sonoda J, Mehl IR, Chong LW, Nofsinger RR, Evans RM. PGC-1beta controls mitochondrial metabolism to modulate circadian activity, adaptive thermogenesis, and hepatic steatosis. Proc Natl Acad Sci U S A. 2007;104:5223–5228. doi: 10.1073/pnas.0611623104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E, et al. Obesity and metabolic syndrome in circadian Clock mutant mice. Science. 2005;308:1043–1045. doi: 10.1126/science.1108750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kohsaka A, Laposky AD, Ramsey KM, Estrada C, Joshu C, Kobayashi Y, et al. High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab. 2007;6:414–421. doi: 10.1016/j.cmet.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 7.Barnea M, Madar Z, Froy O. High-fat diet delays and fasting advances the circadian expression of adiponectin signaling components in mouse liver. Endocrinology. 2009;150:161–168. doi: 10.1210/en.2008-0944. [DOI] [PubMed] [Google Scholar]
- 8.Kaneko K, Yamada T, Tsukita S, Takahashi K, Ishigaki Y, Oka Y, et al. Obesity alters circadian expressions of molecular clock genes in the brainstem. Brain Res. 2009;1263:58–68. doi: 10.1016/j.brainres.2008.12.071. [DOI] [PubMed] [Google Scholar]
- 9.Rudic RD, McNamara P, Curtis AM, Boston RC, Panda S, Hogenesch JB, et al. BMAL1 and CLOCK, two essential components of the circadian clock, are involved in glucose homeostasis. PLoS Biol. 2004;2:e377. doi: 10.1371/journal.pbio.0020377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scott EM, Carter AM, Grant PJ. Association between polymorphisms in the Clock gene, obesity and the metabolic syndrome in man. Int J Obes (Lond) 2008;32:658–662. doi: 10.1038/sj.ijo.0803778. [DOI] [PubMed] [Google Scholar]
- 11.Prasai MJ, George JT, Scott EM. Molecular clocks, type 2 diabetes and cardiovascular disease. Diab Vasc Dis Res. 2008;5:89–95. doi: 10.3132/dvdr.2008.015. [DOI] [PubMed] [Google Scholar]
- 12.Monteleone P, Tortorella A, Docimo L, Maldonato MN, Canestrelli B, De Luca L, et al. Investigation of 3111T/C polymorphism of the CLOCK gene in obese individuals with or without binge eating disorder: association with higher body mass index. Neurosci Lett. 2008;435:30–33. doi: 10.1016/j.neulet.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 13.Sookoian S, Gemma C, Gianotti TF, Burgueno A, Castano G, Pirola CJ. Genetic variants of Clock transcription factor are associated with individual susceptibility to obesity. Am J Clin Nutr. 2008;87:1606–1615. doi: 10.1093/ajcn/87.6.1606. [DOI] [PubMed] [Google Scholar]
- 14.King DP, Zhao Y, Sangoram AM, Wilsbacher LD, Tanaka M, Antoch MP, et al. Positional cloning of the mouse circadian clock gene. Cell. 1997;89:641–653. doi: 10.1016/s0092-8674(00)80245-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Antoch MP, Song EJ, Chang AM, Vitaterna MH, Zhao Y, Wilsbacher LD, et al. Functional identification of the mouse circadian Clock gene by transgenic BAC rescue. Cell. 1997;89:665–667. doi: 10.1016/s0092-8674(00)80246-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dudley CA, Erbel-Sieler C, Estill SJ, Reick M, Franken P, Pitts S, et al. Altered patterns of sleep and behavioral adaptability in NPAS2-deficient mice. Science. 2003;301:379–383. doi: 10.1126/science.1082795. [DOI] [PubMed] [Google Scholar]
- 17.Franken P, Lopez-Molina L, Marcacci L, Schibler U, Tafti M. The transcription factor DBP affects circadian sleep consolidation and rhythmic EEG activity. J Neurosci. 2000;20:617–625. doi: 10.1523/JNEUROSCI.20-02-00617.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laposky A, Easton A, Dugovic C, Walisser J, Bradfield C, Turek F. Deletion of the mammalian circadian clock gene BMAL1/Mop3 alters baseline sleep architecture and the response to sleep deprivation. Sleep. 2005;28:395–409. doi: 10.1093/sleep/28.4.395. [DOI] [PubMed] [Google Scholar]
- 19.Zheng B, Larkin DW, Albrecht U, Sun ZS, Sage M, Eichele G, et al. The mPer2 gene encodes a functional component of the mammalian circadian clock. Nature. 1999;400:169–173. doi: 10.1038/22118. [DOI] [PubMed] [Google Scholar]
- 20.Ebisawa T, Uchiyama M, Kajimura N, Mishima K, Kamei Y, Katoh M, et al. Association of structural polymorphisms in the human period3 gene with delayed sleep phase syndrome. EMBO Rep. 2001;2:342–346. doi: 10.1093/embo-reports/kve070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Viola AU, Archer SN, James LM, Groeger JA, Lo JC, Skene DJ, et al. PER3 polymorphism predicts sleep structure and waking performance. Curr Biol. 2007;17:613–618. doi: 10.1016/j.cub.2007.01.073. [DOI] [PubMed] [Google Scholar]
- 22.Viola AU, James LM, Archer SN, Dijk DJ. PER3 polymorphism and cardiac autonomic control: effects of sleep debt and circadian phase. Am J Physiol Heart Circ Physiol. 2008;295:H2156–H2163. doi: 10.1152/ajpheart.00662.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mishima K, Tozawa T, Satoh K, Saitoh H, Mishima Y. The 3111T/C polymorphism of hClock is associated with evening preference and delayed sleep timing in a Japanese population sample. Am J Med Genet B Neuropsychiatr Genet. 2005;133B:101–104. doi: 10.1002/ajmg.b.30110. [DOI] [PubMed] [Google Scholar]
- 24.Knutson KL, Spiegel K, Penev P, Van Cauter E. The metabolic consequences of sleep deprivation. Sleep Med Rev. 2007;11:163–178. doi: 10.1016/j.smrv.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spiegel K, Knutson K, Leproult R, Tasali E, Van Cauter E. Sleep loss: a novel risk factor for insulin resistance and Type 2 diabetes. J Appl Physiol. 2005;99:2008–2019. doi: 10.1152/japplphysiol.00660.2005. [DOI] [PubMed] [Google Scholar]
- 26.Spiegel K, Tasali E, Penev P, Van Cauter E. Brief communication: Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med. 2004;141:846–850. doi: 10.7326/0003-4819-141-11-200412070-00008. [DOI] [PubMed] [Google Scholar]
- 27.Van Cauter EV, Polonsky KS, Blackman JD, Roland D, Sturis J, Byrne MM, et al. Abnormal temporal patterns of glucose tolerance in obesity: relationship to sleep-related growth hormone secretion and circadian cortisol rhythmicity. J Clin Endocrinol Metab. 1994;79:1797–1805. doi: 10.1210/jcem.79.6.7989487. [DOI] [PubMed] [Google Scholar]
- 28.Van Cauter E, Polonsky KS, Scheen AJ. Roles of circadian rhythmicity and sleep in human glucose regulation. Endocr Rev. 1997;18:716–738. doi: 10.1210/edrv.18.5.0317. [DOI] [PubMed] [Google Scholar]
- 29.Chaput JP, Despres JP, Bouchard C, Tremblay A. Short sleep duration is associated with reduced leptin levels and increased adiposity: Results from the Quebec family study. Obesity (Silver Spring) 2007;15:253–261. doi: 10.1038/oby.2007.512. [DOI] [PubMed] [Google Scholar]
- 30.Chaput JP, Despres JP, Bouchard C, Tremblay A. The association between sleep duration and weight gain in adults: a 6-year prospective study from the Quebec Family Study. Sleep. 2008;31:517–523. doi: 10.1093/sleep/31.4.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moreno CR, Louzada FM, Teixeira LR, Borges F, Lorenzi-Filho G. Short sleep is associated with obesity among truck drivers. Chronobiol Int. 2006;23:1295–1303. doi: 10.1080/07420520601089521. [DOI] [PubMed] [Google Scholar]
- 32.Gunderson EP, Rifas-Shiman SL, Oken E, Rich-Edwards JW, Kleinman KP, Taveras EM, et al. Association of fewer hours of sleep at 6 months postpartum with substantial weight retention at 1 year postpartum. Am J Epidemiol. 2008;167:178–187. doi: 10.1093/aje/kwm298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taveras EM, Rifas-Shiman SL, Oken E, Gunderson EP, Gillman MW. Short sleep duration in infancy and risk of childhood overweight. Arch Pediatr Adolesc Med. 2008;162:305–311. doi: 10.1001/archpedi.162.4.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patel SR, Blackwell T, Redline S, Ancoli-Israel S, Cauley JA, Hillier TA, et al. The association between sleep duration and obesity in older adults. Int J Obes (Lond) 2008;32:1825–1834. doi: 10.1038/ijo.2008.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patel SR, Hu FB. Short sleep duration and weight gain: a systematic review. Obesity (Silver Spring) 2008;16:643–653. doi: 10.1038/oby.2007.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wilsbacher LD, Yamazaki S, Herzog ED, Song EJ, Radcliffe LA, Abe M, et al. Photic and circadian expression of luciferase in mPeriod1-luc transgenic mice invivo. Proc Natl Acad Sci U S A. 2002;99:489–494. doi: 10.1073/pnas.012248599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yoo SH, Yamazaki S, Lowrey PL, Shimomura K, Ko CH, Buhr ED, et al. PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci U S A. 2004;101:5339–5346. doi: 10.1073/pnas.0308709101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ando H, Yanagihara H, Hayashi Y, Obi Y, Tsuruoka S, Takamura T, et al. Rhythmic mRNA Expression of Clock Genes and Adipocytokines in Mouse Visceral Adipose Tissue. Endocrinology. 2005 doi: 10.1210/en.2005-0771. [DOI] [PubMed] [Google Scholar]
- 39.Zvonic S, Ptitsyn AA, Conrad SA, Scott LK, Floyd ZE, Kilroy G, et al. Characterization of peripheral circadian clocks in adipose tissues. Diabetes. 2006;55:962–970. doi: 10.2337/diabetes.55.04.06.db05-0873. [DOI] [PubMed] [Google Scholar]
- 40.Bray MS, Young ME. Circadian rhythms in the development of obesity: potential role for the circadian clock within the adipocyte. Obes Rev. 2007;8:169–181. doi: 10.1111/j.1467-789X.2006.00277.x. [DOI] [PubMed] [Google Scholar]
- 41.Gomez-Abellan P, Hernandez-Morante JJ, Lujan JA, Madrid JA, Garaulet M. Clock genes are implicated in the human metabolic syndrome. Int J Obes (Lond) 2008;32:121–128. doi: 10.1038/sj.ijo.0803689. [DOI] [PubMed] [Google Scholar]
- 42.Wu X, Zvonic S, Floyd ZE, Kilroy G, Goh BC, Hernandez TL, et al. Induction of circadian gene expression in human subcutaneous adipose-derived stem cells. Obesity (Silver Spring) 2007;15:2560–2570. doi: 10.1038/oby.2007.308. [DOI] [PubMed] [Google Scholar]
- 43.Hernandez-Morante JJ, Gomez-Santos C, Milagro F, Campion J, Martinez JA, Zamora S, et al. Expression of cortisol metabolism-related genes shows circadian rhythmic patterns in human adipose tissue. Int J Obes (Lond) 2009 doi: 10.1038/ijo.2009.4. [DOI] [PubMed] [Google Scholar]
- 44.Wu X, Yu G, Parks H, Hebert T, Goh BC, Dietrich MA, et al. Circadian mechanisms in murine and human bone marrow mesenchymal stem cells following dexamethasone exposure. Bone. 2008;42:861–870. doi: 10.1016/j.bone.2007.12.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.B. Everitt SL, Leese M. Cluster Analysis. 4th edn. Hodder Arnold Publication; 2001. [Google Scholar]
- 46.Zvonic SFZ, Mynatt RL, Gimble JM. Circadian Rhythms and the Regulation of Metabolic Tissue Function and Energy Homeostasis. Obesity. 2006 doi: 10.1038/oby.2007.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Duez H, Staels B. Rev-erb alpha gives a time cue to metabolism. FEBS Lett. 2008;582:19–25. doi: 10.1016/j.febslet.2007.08.032. [DOI] [PubMed] [Google Scholar]
- 48.Duez H, Staels B. The nuclear receptors Rev-erbs and RORs integrate circadian rhythms and metabolism. Diab Vasc Dis Res. 2008;5:82–88. doi: 10.3132/dvdr.2008.0014. [DOI] [PubMed] [Google Scholar]
- 49.Ozkurt IC, Pirih FQ, Tetradis S. Parathyroid hormone induces E4bp4 messenger ribonucleic acid expression primarily through cyclic adenosine 3′,5′-monophosphate signaling in osteoblasts. Endocrinology. 2004;145:3696–3703. doi: 10.1210/en.2003-1436. [DOI] [PubMed] [Google Scholar]
- 50.Ozkurt IC, Tetradis S. Parathyroid hormone-induced E4BP4/NFIL3 down-regulates transcription in osteoblasts. J Biol Chem. 2003;278:26803–26809. doi: 10.1074/jbc.M212652200. [DOI] [PubMed] [Google Scholar]
- 51.Gimble JM, Zvonic S, Floyd ZE, Kassem M, Nuttall ME. Playing with bone and fat. J Cell Biochem. 2006;98:251–266. doi: 10.1002/jcb.20777. [DOI] [PubMed] [Google Scholar]
- 52.Yin L, Wu N, Curtin JC, Qatanani M, Szwergold NR, Reid RA, et al. Rev-erbalpha, a heme sensor that coordinates metabolic and circadian pathways. Science. 2007;318:1786–1789. doi: 10.1126/science.1150179. [DOI] [PubMed] [Google Scholar]
- 53.Burris TP. Nuclear Hormone Receptors for Heme: REV-ERB{alpha} and REV-ERB{beta} Are Ligand-Regulated Components of the Mammalian Clock. Mol Endocrinol. 2008 doi: 10.1210/me.2007-0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Raghuram S, Stayrook KR, Huang P, Rogers PM, Nosie AK, McClure DB, et al. Identification of heme as the ligand for the orphan nuclear receptors REV-ERBalpha and REV-ERBbeta. Nat Struct Mol Biol. 2007;14:1207–1213. doi: 10.1038/nsmb1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rogers PM, Ying L, Burris TP. Relationship between circadian oscillations of Rev-erbalpha expression and intracellular levels of its ligand, heme. Biochem Biophys Res Commun. 2008;368:955–958. doi: 10.1016/j.bbrc.2008.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen JJ, London IM. Hemin enhances the differentiation of mouse 3T3 cells to adipocytes. Cell. 1981;26:117–122. doi: 10.1016/0092-8674(81)90039-8. [DOI] [PubMed] [Google Scholar]
- 57.Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 1999;98:115–124. doi: 10.1016/S0092-8674(00)80611-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.