Abstract
Autism is a complex neurodevelopmental disorder of unknown etiology. While the past decade has witnessed a proliferation of neuroimaging studies of autism, theoretical approaches for understanding systems-level brain abnormalities remain poorly developed. We propose a novel anterior insula-based systems-level model for investigating the neural basis of autism, synthesizing recent advances in brain network functional connectivity with converging evidence from neuroimaging studies in autism. The anterior insula is involved in interoceptive, affective and empathic processes, and emerging evidence suggests it is part of a “salience network” integrating external sensory stimuli with internal states. Network analysis indicates that the anterior insula is uniquely positioned as a hub mediating interactions between large-scale networks involved in externally- and internally-oriented cognitive processing. A recent meta-analysis identifies the anterior insula as a consistent locus of hypoactivity in autism. We suggest that dysfunctional anterior insula connectivity plays an important role in autism. Critical examination of these abnormalities from a systems neuroscience perspective should be a priority for further research on the neurobiology of autism.
Keywords: connectivity, empathy, autism spectrum disorders, network, von Economoneuron, fMRI
Introduction
Autism spectrum disorders (ASD) are developmental disorders characterized by social impairments, restricted interests, and repetitive and stereotyped behaviors, with an estimated incidence of 1:150 ("Prevalence of autism spectrum disorders--autism and developmental disabilities monitoring network, 14 sites, United States, 2002", 2007). An overwhelming number of theoretical accounts of autism have been offered, with relatively few attempts at synthesis across studies (Waterhouse, 2008). Despite considerable efforts to delineate precise brain functional and structural differences between individuals with ASD and typically developing individuals (Sokol & Edwards-Brown, 2004), very little is known regarding differences in large-scale brain network interactions that underlie the cognitive and behavioral symptoms of ASD. The field of autism research has been largely dominated by theories positing malfunction of individual brain regions in ASD, such as the amygdala (Adolphs, Sears, & Piven, 2001; Baron-Cohen et al., 2000), superior temporal sulcus (STS) (Pelphrey & Carter, 2008), or fusiform gyrus (Schultz, 2005). At the other extreme, there are claims that ASD is a distributed disorder characterized by widespread abnormalities throughout the brain (Muller, 2007). Recent conceptualizations of the brain basis of ASD have taken a systems-level approach, and proposed that ASD may be explained by abnormalities in the mirror neuron system (Oberman & Ramachandran, 2007; Williams, Whiten, Suddendorf, & Perrett, 2001), the default-mode network (Kennedy & Courchesne, 2008; Kennedy, Redcay, & Courchesne, 2006), or both (Iacoboni, 2006). These conceptualizations, however, have primarily focused on specific brain systems and have largely ignored the critical interactions between multiple distinct brain systems, which may be important for understanding the neurobiology of a complex neurodevelopmental disorder such as ASD.
Recent work in systems neuroscience has characterized several canonical brain networks that are identifiable in both the resting (Damoiseaux et al., 2006; Seeley et al., 2007) and the active brain (Toro, Fox, & Paus, 2008). Conceptualizing the brain as comprised of multiple, distinct, and interacting networks provides a new framework for understanding the complex symptomatology of ASD. Here we suggest that analysis of large-scale brain networks will provide a parsimonious account of the recent neuroimaging literature on ASD, and that the anterior insula (AI) is a brain region of particular interest in understanding this disorder. We discuss the rationale behind our approach, taking into account recent advances in the study of brain networks.
Brain under-connectivity in ASD
One of the earliest and most prominent theories of brain abnormalities underlying ASD is that the disorder is one of connectivity (Frith, 2004; Geschwind & Levitt, 2007). In postmortem anatomical studies, Courchesne’s group observed that the brains of individuals with ASD showed hyper-connectivity within frontal lobe regions, and decreased long-range connectivity and reciprocal interactions with other cortical regions. His team proposed that excessive, disorganized, and inadequately selective connectivity within the frontal lobes leads to poorly synchronized connectivity between frontal cortex and other brain systems (Courchesne & Pierce, 2005b). Even before the widespread use of fMRI to study brain connectivity, correlations between regional cerebral metabolic rates for glucose determined by PET were used to provide a measure of functional associations between regions. Horwitz and colleagues demonstrated two decades ago that individuals with ASD showed reduced correlations between the insula and fronto-parietal regions (Horwitz, Rumsey, Grady, & Rapoport, 1988). Strong evidence for functional and structural under-connectivity in the autistic brain is available from studies utilizing a variety of methods (see (Hughes, 2007) for review).
Increasing evidence for abnormal brain connectivity in autism comes from studies using functional connectivity measures (Just, Cherkassky, Keller, Kana, & Minshew, 2007; Kana, Keller, Cherkassky, Minshew, & Just, 2006). One study found reduced functional connectivity between primary visual cortex and the right inferior frontal gyrus in individuals with autism compared to controls (Villalobos, Mizuno, Dahl, Kemmotsu, & Muller, 2005), and another has shown decreased functional connectivity between frontal regions and the fusiform gyrus during a working memory task involving faces (Koshino et al., 2008). Another group has recently demonstrated abnormal functional connectivity in the limbic system during face processing in individuals with autism (Kleinhans et al., 2008). These findings support the hypothesis that under-connectivity between specific brain regions is a characteristic feature of ASD. To date, however, few studies have examined functional connectivity within and between key large-scale canonical brain networks in autism (Cherkassky, Kana, Keller, & Just, 2006; Kennedy & Courchesne, 2008). The majority of published studies to date have examined connectivity of specific individual brain regions, without a broader theoretically driven systems-level approach.
We propose that a systems-level approach is critical for understanding the neurobiology of autism, and that the anterior insula is a key node in coordinating brain network interactions, due to its unique anatomy, location, function, and connectivity. The examination of this structure is an important yet neglected area of research in autism.
Functions and connectivity of anterior insula
The insular cortex, located deep within the lateral sulcus of the brain, is traditionally considered to be paralimbic (Mesulam & Mufson, 1982) or “limbic integration cortex” (Augustine, 1996). This characterization stems in large part from the patterns of structural connectivity of this region, which has efferent projections to the amygdala, lateral orbital cortex, olfactory cortex, anterior cingulate cortex (ACC), and STS, and receives input from orbitofrontal, olfactory cortex, ACC and STS (Mesulam & Mufson, 1982; Mufson & Mesulam, 1982). The insula is a multifaceted brain region, participating in visceral sensory and somatic sensory roles, autonomic regulation of the gastrointestinal tract and heart, as well as a functioning as a motor association area (Augustine, 1996). While the posterior portion of the insula is thought to be more involved with representing stimulus intensities, the AI (Fig. 1) appears to track the feelings and perceptions associated with bodily states. Craig and colleagues have shown that while posterior insula activation correlates with actual changes in thermal intensity, right AI activation tracks perceived thermal intensity (Craig, Chen, Bandy, & Reiman, 2000). It has also been suggested that interoception, or the sense of the physiological condition of the entire body, constitutes the basis for subjective evaluation of one’s condition, and is implemented in the right AI (Craig, 2002). Craig has recently further hypothesized that the AI contains the anatomical substrate for the evolved capacity of humans to be aware of themselves, others, and the environment (Craig, 2009).
Figure 1. Right Anterior Insula.
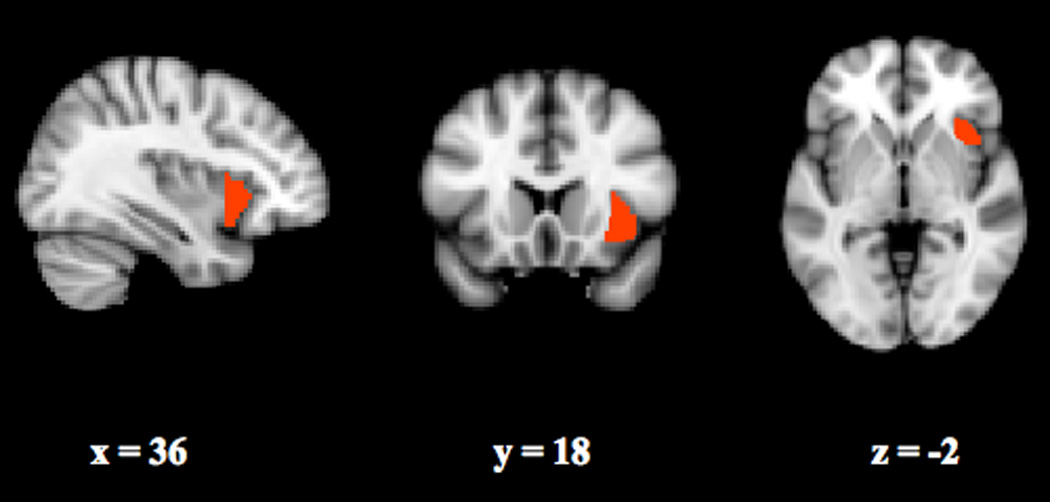
The anterior insula is located within the lateral sulcus of the brain.
The insula has long been thought to play a role in the experience of emotion derived from information about bodily states. Pure autonomic failure (PAF) is an idiopathic disorder in which peripheral denervation disrupts autonomic responses. Critchley and colleagues (Critchley, Mathias, & Dolan, 2001) used PET to demonstrate that patients with PAF show reduced activation in the right insula during performance of “stressor” tasks (e.g. mental arithmetic) compared to controls. These patients also exhibited subtle impairments in emotional responses, and identified with statements such as “I can no longer feel sad” and “I have lost my ability to feel emotional”. This data is in line with the theory that signals from the autonomic nervous system shape emotional experience (Damasio, 1996), and that the insula is a key brain region involved in this process. Critchley and colleagues have also reported that activity in the right AI predicts participants’ accuracy in a task requiring detection of one’s own heartbeat. Furthermore, they report that gray matter volume in the AI correlates with interoceptive accuracy and subjective ratings of visceral awareness. The final link comes from the finding that in these subjects, emotional experience correlated with interoceptive accuracy (Critchley, Wiens, Rotshtein, Ohman, & Dolan, 2004). This study therefore provides evidence for the claim that there are strong links between the right AI, perception of one’s own bodily state, and the experience of emotion. In a study of smokers with brain damage involving the insula, it was found that these smokers found it easier to quit smoking than smokers with brain damage involving other areas, demonstrating the key role for the insula in representation of conscious bodily urges (Naqvi, Rudrauf, Damasio, & Bechara, 2007). A recent PET study corroborates this finding, identifying the insula as a region involved in the control and suppression of natural urges, in this case blinking (Lerner et al., 2009).
Functional neuroimaging studies have shown that the right AI is active across a wide variety of paradigms involving the subjective awareness of feelings, including studies of anger, disgust, judgments of trustworthiness, and sexual arousal (see Craig, 2002 for review). A recent study examining the pathophysiology of auditory verbal hallucinations found that during times when patients with schizophrenia reported experiencing hallucinations, increased activation of the right insula was observed (Sommer et al., 2008). The AI has also been implicated in empathy, or the “capacity to understand emotions of others by sharing their affective states” (Singer, 2006). An intriguing study by Singer and colleagues showed that while the posterior insula was activated when subjects received painful stimulation, anterior insula and ACC was activated both when the subject received pain and when the subject witnessed a loved one receiving pain. Activation in the AI was positively correlated with individual’s scores on an empathy scale. The results of this experiment led the authors to conclude that AI activation reflects emotional experience that may constitute the neural basis of our understanding of the feelings of others (empathy) and ourselves (Singer et al., 2004). Reduced empathic and emotional responsiveness is considered a hallmark of ASD, and is part of the core symptomatology of the disorder (Baron-Cohen & Wheelwright, 2004). A recent study used alexithymia and empathy scales to assess emotional awareness of the self and others, respectively, in individuals with high functioning autism and typically developing adults. The authors report that difficulties in emotional awareness are related to hypoactivity in the AI of both autistic individuals and controls. The poorer the awareness of one’s own and other’s emotions, the weaker the activity in the AI (Silani et al., 2008). Another recent functional neuroimaging study found that individuals with borderline personality disorder showed different anterior insula responses than healthy controls during an economic exchange game designed to assess perception of social gestures (King-Casas et al., 2008). Taken together, these studies suggest an important role for the AI in both self- and other-related social and affective processes.
The right and left AI are often co-activated, a finding which is not surprising considering that strong interhemispheric coupling between homologous regions in each hemisphere is often observed (Stark et al., 2008). However, right AI activation predominates in the majority of studies, though some notable instances of selective left AI activation in healthy individuals have also been documented. During positive emotional and affiliative moments, such as the experience of maternal and romantic love (Bartels & Zeki, 2004) and viewing pleased facial expressions (Jabbi, Swart, & Keysers, 2007), greater left than right AI activation is seen. Stimuli activating the right AI are typically arousing, and this asymmetry of activation has previously been attributed to relationships between cortical asymmetry and asymmetric autonomic innervation of the heart (Craig, 2009). Further work is needed to address the issue of how the left and right AI work in an integrated manner, and how their functions differ.
Spindle neurons: unique location and function
The AI is among the few brain regions containing a special class of neurons thought to be unique to higher primates, known as Von Economo or “spindle” neurons. These neurons have been found in humans, bonobos, chimpanzees, gorillas, and orangutans, but in no other primate species examined (Nimchinsky et al., 1999). Spindle neurons are large projection neurons with a distinctive morphology, and are thought to be a relatively recent phylogenetic specialization (Allman, Hakeem, & Watson, 2002). These cells appear in small numbers around the 35th week of gestation. At birth only about 15% of postnatal numbers are present, and adult numbers are typically attained by 4 years of age. While spindle cells are located in layer 5, which is typically an output layer, it is not known where they ultimately project (Allman, Watson, Tetreault, & Hakeem, 2005). It is speculated that the function of these cells is to rapidly relay to other parts of the brain a signal derived from information processed within the AI. Interestingly, spindle neurons are 30% more numerous in the right hemisphere than the left (Allman, Watson, Tetreault, & Hakeem, 2005).
It has previously been suggested that abnormal development of spindle neurons may cause the social disabilities characteristic of ASD (Frith, 2001; Mundy, 2003). Allman and colleagues proposed that the large size of these neurons may enable them to relay fast intuitive assessments of complex social situations and that they are likely involved in social emotions, bonding, and intuitive responses involving uncertainty. This group was one of the first to propose that abnormal development of spindle neurons can lead to difficulty in evaluating social situations, a hallmark of ASD (Allman, Watson, Tetreault, & Hakeem, 2005). It is noteworthy that vulnerability of spindle neurons is also thought to play a role in frontotemporal dementia (FTD), a disorder involving disruptions to the anterior cingulate, orbitofrontal and insula regions, and is associated with abnormalities in social interactions, emotion recognition, and empathy (Seeley, Crawford, Zhou, Miller, & Greicius, 2009; Viskontas, Possin, & Miller, 2007). FTD has been associated with severe and selective spindle neuron loss, including a 74% reduction in spindle neurons compared with control subjects (Seeley et al., 2006). Thus, these neurons have been linked to abnormal social functioning in more than one disorder.
While the hypothesis that spindle neuron abnormality or deficiency is responsible for the social deficits in autism is intriguing, empirical support for this theory is lacking. In the only study to date, Kennedy and colleagues showed that there is no difference in spindle neuron number between autistic and normal brains (Kennedy, Semendeferi, & Courchesne, 2007). However, it is possible that while individuals with ASD do not differ from controls in overall spindle cell number, the short- and long-range connectivity of these neurons is disrupted. Courchesne hypothesized that the protracted developmental time course of spindle neurons may make them particularly susceptible to early developmental derailment in the autistic brain (Courchesne & Pierce, 2005a). No study has yet attempted to understand the connectivity of the region containing spindle neurons in the brains of individuals with ASD. We hypothesize that it is connectivity, rather than cell number, that will prove to be the critical factor in understanding potential spindle neuron dysfunction in ASD.
Anterior insula as a network hub: Role in switching between brain networks
Recent work using resting-state fMRI suggests that the human brain is intrinsically organized into distinct functional networks (Damoiseaux et al., 2006; Greicius, Krasnow, Reiss, & Menon, 2003). Resting-state functional connectivity enables the characterization of large-scale networks without contamination from cognitive tasks (Fox & Raichle, 2007; Greicius, Supekar, Menon, & Dougherty, 2009; Uddin, Kelly, Biswal, Castellanos, & Milham, 2009; Vincent et al., 2006). This framework has identified at least three canonical networks: 1) a central-executive network (CEN) comprised of the dorsolateral prefrontal cortex (DLPFC) and posterior parietal cortex (PPC); 2) the default-mode network (DMN) including the ventromedial prefrontal cortex (VMPFC) and posterior cingulate cortex (PCC); and 3) a salience network (SN) with key nodes in the AI and ACC (Fox, Corbetta, Snyder, Vincent, & Raichle, 2006; Seeley et al., 2007). The ACC has previously been shown to exhibit diminished responses in individuals with ASD during an interpersonal exchange game requiring reciprocal social interaction (Chiu et al., 2008), and hypoactivity in the ACC has been linked to deficits in response shifting and executive functioning (Shafritz, Dichter, Baranek, & Belger, 2008). While the ACC and AI are often co-activated (Craig, 2009), the specific roles of the AI and ACC in ASD have not been established.
Evidence from brain network analyses suggests that the anterior insula can be considered as part of a “salience network” which serves to integrate sensory data with visceral, autonomic, and hedonic information. Seeley and colleagues used region-of-interest (ROI) and independent component analyses (ICA) of resting state fMRI data to demonstrate the existence of this independent brain network comprised of the anterior insula, dorsal ACC, along with subcortical structures including the amygdala, substantia nigra/ventral tegmental area, and thalamus (Seeley et al., 2007). They propose that the function of this salience network is to identify the most homeostatically relevant among several internal and extrapersonal stimuli in order to guide behavior. The right AI has also recently been demonstrated to aid in the coordination and evaluation of task performance across behavioral tasks with varying perceptual and response demands (Eckert et al., 2008).
A recent study used Granger causality analyses to examine the directionality of influence of specific network nodes on other brain regions. Granger causality analyses (GCA) enable the detection of causal interactions between brain regions by assessing the extent to which signal changes in one brain region can predict signal changes in another brain region (Goebel, Roebroeck, Kim, & Formisano, 2003). Sridharan and colleagues showed, across three independent datasets, that the right AI plays a critical and causal role in switching between two other networks (the CEN and the DMN) known to demonstrate competitive interactions during cognitive information processing (Sridharan, Levitin, & Menon, 2008). This study shows that the right AI is involved in switching between brain networks across task paradigms and stimulus modalities, and thus acts as a “causal outflow hub” coordinating two large-scale networks important for mediating attention to the external (central-executive) and internal (default-mode) worlds. This study is the first, to our knowledge, to demonstrate that right AI activity temporally precedes activity in these two other extensively characterized networks. It is suggested that the right AI, part of the previously described salience network, enables task-related information processing by initiating appropriate transient control signals to the networks mediating attentional, working memory, and higher order cognitive processes while disengaging the default-mode network (Sridharan, Levitin, & Menon, 2008). This new understanding of the right AI as a critical node for initiating network switching provides key insight into the potential for profound deficits in cognitive functioning should AI integrity or connectivity be compromised. Indeed, AI hyperactivity has been implicated in anxiety disorders, suggesting that when the salience network goes into over-drive, pathology subsequently results (Paulus & Stein, 2006; Stein, Simmons, Feinstein, & Paulus, 2007). Individuals scoring high on the trait neuroticism, defined as the tendency to experience negative emotional states, demonstrate greater right AI activation during decision-making, even when the outcome of the decision is certain (Feinstein, Stein, & Paulus, 2006). It seems that an appropriate level of AI activity is necessary to provide an alert signal to initiate brain responses to salient stimuli, but this signal can be over-active, in the case of anxiety, or under-active, as may be the case in ASD (Silani, 2008). In sum, the AI appears to be uniquely positioned to detect changes in bodily states and initiate motivated behaviors, which are key to interpersonal and social processes.
Anterior Insula in ASD
The AI is a region that is critically involved in operations critical to social processing. While previous theories of ASD have focused on hypoactivity in regions such as the fusiform gyrus, superior temporal sulcus, or amygdala, the role of the AI is often overlooked. However, in a recent comprehensive meta-analysis of functional neuroimaging studies of social processing in ASD, Di Martino and colleagues demonstrated that across a of group of 24 studies examining various aspects of social processing ranging from face processing to theory of mind, one of the regions consistently showing significant hypoactivity in ASD was the right anterior insula (Di Martino et al., 2009). This meta-analysis was not driven by current theories of autism, and thus provides an unbiased survey of the current literature. The identification of the AI as a region of consistent hypoactivity in ASD represents the first critical step in designing future experiments to more clearly elucidate the specific functional abnormalities within the insula that may contribute to the behavioral and cognitive symptoms of ASD.
Critically, of the studies reviewed, those reporting hypoactivity of the AI in autism utilized tasks commonly employed to assess social abilities, including viewing emotional facial expressions (Hubl et al., 2003) and incongruent eye gaze (Dichter & Belger, 2007). Emotional awareness tasks (Silani, 2008) and other tasks involving facial processing (Di Martino et al., 2009) were also associated with hypoactivity of the AI in ASD.
Brain networks in ASD: Synthesis and future directions
The study of brain connectivity, while previously only accessible by post-mortem examination of brain tissue, has been aided greatly in recent years by the development of novel methods to analyze fMRI data. Indeed, such studies now constitute quite a large percentage of the neuroimaging literature on ASD. To date, there have been reports of evidence for reduced functional connectivity between regions critical for social processing in ASD, among several others, as previously reviewed. However, functional and structural connectivity of the AI in ASD is still poorly understood, and most theoretical approaches to understanding the disorder have ignored this brain structure.
As we have discussed, the AI serves an integral function with respect to representing and evaluating salient stimuli, and is uniquely positioned as a hub mediating interactions between large-scale brain networks involved in attentional and self-directed processes. Just as the insula has previously been shown to mediate interactions between internal bodily states (interoception/autonomic nervous system) and the outward expression of emotion, it seems to be uniquely positioned as a hub mediating interactions between systems dedicated to externally-oriented attention (ECN) and internally-oriented cognitive processing (DMN). The right AI region has recently been shown to demonstrate hypoactivity in individuals with ASD, across a wide variety of social cognitive task paradigms (Di Martino et al., 2009). We suspect that this hypoactivity may be due to a disconnect between the anterior insula and the sensory and limbic structures that project to it, leading to a reduction in “salience detection” and subsequent mobilization of attentional resources necessary for guiding appropriate social behavior (Fig. 2). If in the typically developing brain, the AI functions to integrate inputs from multiple sources to initiate switches between the DMN and the CEN, we propose that in ASD this critical system is impaired, leading to the social dysfunctions characteristic of the disorder. Individuals with ASD demonstrate a lack of motivation for orienting to social cues (Charman et al., 1998), which may be due to the fact that they do not find such stimuli to be rewarding (Dawson, Meltzoff, Osterling, Rinaldi, & Brown, 1998). This may explain the hypoactivity in the AI, which seems to be specific to studies of social cognition, and not other non-social processes such as cognitive control and working memory (Di Martino et al., 2009).
Figure 2. Model of AI Function.
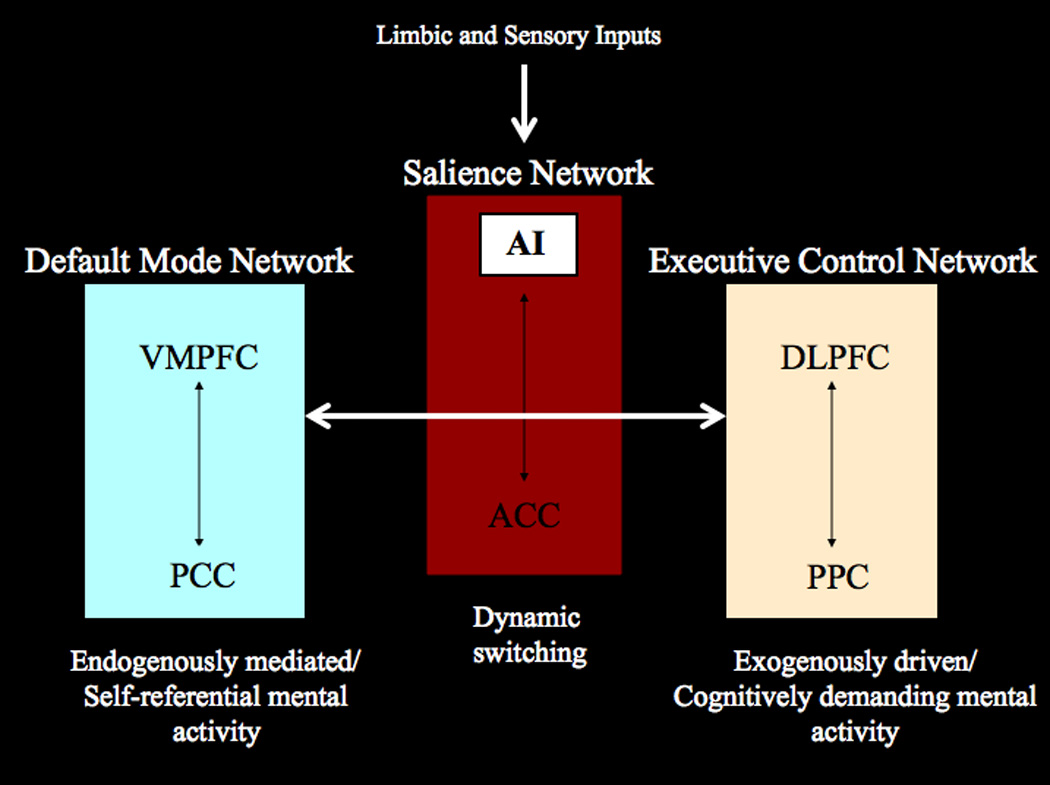
The anterior insula is part of a salience network which serves to initiate dynamic switches between the DMN and CEN. In our model of AI dysfunction in autism, limbic and sensory inputs are inadequately processed by the AI during social cognition, leading to disruption of the AI’s role in coordination of these large-scale brain networks.
Thus, we conclude that integrity, function, and connectivity of the AI in ASD warrant further investigation. A systems neuroscience approach taking into account advances in network analysis of brain function, as reviewed above, should be a priority for future studies aimed at understanding the neurobiological basis of ASD. We suggest that the field of autism research may benefit from future research efforts targeting the following questions: 1) What is the nature of functional and structural AI deficits in ASD? 2) Do functional deficits arise primarily from weak inputs to the AI, or inefficient network switching mechanisms involving the AI? 3) How is the development of functional and structural connectivity of the AI disrupted in individuals with ASD? 4) In what context is activity within right or left AI asymmetrically compromised in individuals with ASD? 5) Can training to attend to social stimuli “normalize” activity within the AI and associated networks? We believe that future research targeting these questions will reveal important insights into the systems-level brain abnormalities underlying ASD and provide a novel theoretical framework for subsequent empirical work in the field.
Acknowledgments
We thank Kaustubh Supekar for critical comments and suggestions. This research was supported by a fellowship from the Children’s Health Research Program at the Lucille Packard Children’s Hospital to L.Q.U. (Tashia and John Morgridge Endowed Postdoctoral Fellow) and grants from the National Institutes of Health (NS058899, HD047520, HD059205), and the National Science Foundation (BCS/DRL 0449927) to V.M.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adolphs R, Sears L, Piven J. Abnormal processing of social information from faces in autism. J Cogn Neurosci. 2001;13(2):232–240. doi: 10.1162/089892901564289. [DOI] [PubMed] [Google Scholar]
- Allman J, Hakeem A, Watson K. Two phylogenetic specializations in the human brain. Neuroscientist. 2002;8(4):335–346. doi: 10.1177/107385840200800409. [DOI] [PubMed] [Google Scholar]
- Allman JM, Watson KK, Tetreault NA, Hakeem AY. Intuition and autism: a possible role for Von Economo neurons. Trends Cogn Sci. 2005;9(8):367–373. doi: 10.1016/j.tics.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Augustine JR. Circuitry and functional aspects of the insular lobe in primates including humans. Brain Res Brain Res Rev. 1996;22(3):229–244. doi: 10.1016/s0165-0173(96)00011-2. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Ring HA, Bullmore ET, Wheelwright S, Ashwin C, Williams SC. The amygdala theory of autism. Neurosci Biobehav Rev. 2000;24(3):355–364. doi: 10.1016/s0149-7634(00)00011-7. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Wheelwright S. The empathy quotient: an investigation of adults with Asperger syndrome or high functioning autism, and normal sex differences. J Autism Dev Disord. 2004;34(2):163–175. doi: 10.1023/b:jadd.0000022607.19833.00. [DOI] [PubMed] [Google Scholar]
- Bartels A, Zeki S. The neural correlates of maternal and romantic love. Neuroimage. 2004;21(3):1155–1166. doi: 10.1016/j.neuroimage.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Charman T, Swettenham J, Baron-Cohen S, Cox A, Baird G, Drew A. An experimental investigation of social-cognitive abilities in infants with autism: clinical implications. Infant Mental Health. 1998;19(2):260–275. [Google Scholar]
- Cherkassky VL, Kana RK, Keller TA, Just MA. Functional connectivity in a baseline resting-state network in autism. Neuroreport. 2006;17(16):1687–1690. doi: 10.1097/01.wnr.0000239956.45448.4c. [DOI] [PubMed] [Google Scholar]
- Chiu PH, Kayali MA, Kishida KT, Tomlin D, Klinger LG, Klinger MR, et al. Self responses along cingulate cortex reveal quantitative neural phenotype for high-functioning autism. Neuron. 2008;57(3):463–473. doi: 10.1016/j.neuron.2007.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchesne E, Pierce K. Brain overgrowth in autism during a critical time in development: implications for frontal pyramidal neuron and interneuron development and connectivity. Int J Dev Neurosci. 2005a;23(2–3):153–170. doi: 10.1016/j.ijdevneu.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Pierce K. Why the frontal cortex in autism might be talking only to itself: local over-connectivity but long-distance disconnection. Curr Opin Neurobiol. 2005b;15(2):225–230. doi: 10.1016/j.conb.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3(8):655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel - now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10(1):59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Craig AD, Chen K, Bandy D, Reiman EM. Thermosensory activation of insular cortex. Nat Neurosci. 2000;3(2):184–190. doi: 10.1038/72131. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Mathias CJ, Dolan RJ. Neuroanatomical basis for first-and second-order representations of bodily states. Nat Neurosci. 2001;4(2):207–212. doi: 10.1038/84048. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nat Neurosci. 2004;7(2):189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Damasio AR. The somatic marker hypothesis and the possible functions of the prefrontal cortex. Philos Trans R Soc Lond B Biol Sci. 1996;351(1346):1413–1420. doi: 10.1098/rstb.1996.0125. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Rombouts SA, Barkhof F, Scheltens P, Stam CJ, Smith SM, et al. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci U S A. 2006;103(37):13848–13853. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson G, Meltzoff AN, Osterling J, Rinaldi J, Brown E. Children with autism fail to orient to naturally occurring social stimuli. J Autism Dev Disord. 1998;28(6):479–485. doi: 10.1023/a:1026043926488. [DOI] [PubMed] [Google Scholar]
- Di Martino A, Ross K, Uddin LQ, Sklar AB, Castellanos FX, Milham MP. Functional Brain Correlates of Social and Nonsocial Processes in Autism Spectrum Disorders: An Activation Likelihood Estimation Meta-Analysis. Biol Psychiatry. 2009;65(1):63–74. doi: 10.1016/j.biopsych.2008.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter GS, Belger A. Social stimuli interfere with cognitive control in autism. Neuroimage. 2007;35(3):1219–1230. doi: 10.1016/j.neuroimage.2006.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert MA, Menon V, Walczak A, Ahlstrom J, Denslow S, Horwitz A, et al. At the heart of the ventral attention system: The right anterior insula. Hum Brain Mapp. 2008 doi: 10.1002/hbm.20688. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinstein JS, Stein MB, Paulus MP. Anterior insula reactivity during certain decisions is associated with neuroticism. Soc Cogn Affect Neurosci. 2006;1(2):136–142. doi: 10.1093/scan/nsl016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Corbetta M, Snyder AZ, Vincent JL, Raichle ME. Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc Natl Acad Sci U S A. 2006;103(26):10046–10051. doi: 10.1073/pnas.0604187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8(9):700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Frith C. Is autism a disconnection disorder? Lancet Neurol. 2004;3(10):577. doi: 10.1016/S1474-4422(04)00875-0. [DOI] [PubMed] [Google Scholar]
- Frith U. Mind blindness and the brain in autism. Neuron. 2001;32(6):969–979. doi: 10.1016/s0896-6273(01)00552-9. [DOI] [PubMed] [Google Scholar]
- Geschwind DH, Levitt P. Autism spectrum disorders: developmental disconnection syndromes. Curr Opin Neurobiol. 2007;17(1):103–111. doi: 10.1016/j.conb.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Goebel R, Roebroeck A, Kim DS, Formisano E. Investigating directed cortical interactions in time-resolved fMRI data using vector autoregressive modeling and Granger causality mapping. Magn Reson Imaging. 2003;21(10):1251–1261. doi: 10.1016/j.mri.2003.08.026. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 2003;100(1):253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Supekar K, Menon V, Dougherty RF. Resting-State Functional Connectivity Reflects Structural Connectivity in the Default Mode Network. Cereb Cortex. 2009;19(1):72–78. doi: 10.1093/cercor/bhn059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz B, Rumsey JM, Grady CL, Rapoport SI. The cerebral metabolic landscape in autism. Intercorrelations of regional glucose utilization. Arch Neurol. 1988;45(7):749–755. doi: 10.1001/archneur.1988.00520310055018. [DOI] [PubMed] [Google Scholar]
- Hubl D, Bolte S, Feineis-Matthews S, Lanfermann H, Federspiel A, Strik W, et al. Functional imbalance of visual pathways indicates alternative face processing strategies in autism. Neurology. 2003;61(9):1232–1237. doi: 10.1212/01.wnl.0000091862.22033.1a. [DOI] [PubMed] [Google Scholar]
- Hughes JR. Autism: the first firm finding = underconnectivity? Epilepsy Behav. 2007;11(1):20–24. doi: 10.1016/j.yebeh.2007.03.010. [DOI] [PubMed] [Google Scholar]
- Iacoboni M. Failure to deactivate in autism: the co-constitution of self and other. Trends Cogn Sci. 2006;10(10):431–433. doi: 10.1016/j.tics.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Jabbi M, Swart M, Keysers C. Empathy for positive and negative emotions in the gustatory cortex. Neuroimage. 2007;34(4):1744–1753. doi: 10.1016/j.neuroimage.2006.10.032. [DOI] [PubMed] [Google Scholar]
- Just MA, Cherkassky VL, Keller TA, Kana RK, Minshew NJ. Functional and anatomical cortical underconnectivity in autism: evidence from an FMRI study of an executive function task and corpus callosum morphometry. Cereb ortex. 2007;17(4):951–961. doi: 10.1093/cercor/bhl006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kana RK, Keller TA, Cherkassky VL, Minshew NJ, Just MA. Sentence comprehension in autism: thinking in pictures with decreased functional connectivity. Brain. 2006;129(Pt 9):2484–2493. doi: 10.1093/brain/awl164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy DP, Courchesne E. The intrinsic functional organization of the brain is altered in autism. Neuroimage. 2008;39(4):1877–1885. doi: 10.1016/j.neuroimage.2007.10.052. [DOI] [PubMed] [Google Scholar]
- Kennedy DP, Redcay E, Courchesne E. Failing to deactivate: resting functional abnormalities in autism. Proc Natl Acad Sci U S A. 2006;103(21):8275–8280. doi: 10.1073/pnas.0600674103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy DP, Semendeferi K, Courchesne E. No reduction of spindle neuron number in frontoinsular cortex in autism. Brain Cogn. 2007;64(2):124–129. doi: 10.1016/j.bandc.2007.01.007. [DOI] [PubMed] [Google Scholar]
- King-Casas B, Sharp C, Lomax-Bream L, Lohrenz T, Fonagy P, Montague PR. The rupture and repair of cooperation in borderline personality disorder. Science. 2008;321(5890):806–810. doi: 10.1126/science.1156902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinhans NM, Richards T, Sterling L, Stegbauer KC, Mahurin R, Johnson LC, et al. Abnormal functional connectivity in autism spectrum disorders during face processing. Brain. 2008;131(Pt 4):1000–1012. doi: 10.1093/brain/awm334. [DOI] [PubMed] [Google Scholar]
- Koshino H, Kana RK, Keller TA, Cherkassky VL, Minshew NJ, Just MA. fMRI investigation of working memory for faces in autism: visual coding and underconnectivity with frontal areas. Cereb Cortex. 2008;18(2):289–300. doi: 10.1093/cercor/bhm054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner A, Bagic A, Hanakawa T, Boudreau EA, Pagan F, Mari Z, et al. Involvement of Insula and Cingulate Cortices in Control and Suppression of Natural Urges. Cereb Cortex. 2009;19(1):218–223. doi: 10.1093/cercor/bhn074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ. Insula of the old world monkey. III: Efferent cortical output and comments on function. J Comp Neurol. 1982;212(1):38–52. doi: 10.1002/cne.902120104. [DOI] [PubMed] [Google Scholar]
- Mufson EJ, Mesulam MM. Insula of the old world monkey. II: Afferent cortical input and comments on the claustrum. J Comp Neurol. 1982;212(1):23–37. doi: 10.1002/cne.902120103. [DOI] [PubMed] [Google Scholar]
- Muller RA. The study of autism as a distributed disorder. Ment Retard Dev Disabil Res Rev. 2007;13(1):85–95. doi: 10.1002/mrdd.20141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy P. Annotation: the neural basis of social impairments in autism: the role of the dorsal medial-frontal cortex and anterior cingulate system. J Child Psychol Psychiatry. 2003;44(6):793–809. doi: 10.1111/1469-7610.00165. [DOI] [PubMed] [Google Scholar]
- Naqvi NH, Rudrauf D, Damasio H, Bechara A. Damage to the insula disrupts addiction to cigarette smoking. Science. 2007;315(5811):531–534. doi: 10.1126/science.1135926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimchinsky EA, Gilissen E, Allman JM, Perl DP, Erwin JM, Hof PR. A neuronal morphologic type unique to humans and great apes. Proc Natl Acad Sci U S A. 1999;96(9):5268–5273. doi: 10.1073/pnas.96.9.5268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberman LM, Ramachandran VS. The simulating social mind: the role of the mirror neuron system and simulation in the social and communicative deficits of autism spectrum disorders. Psychol Bull. 2007;133(2):310–327. doi: 10.1037/0033-2909.133.2.310. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Stein MB. An insular view of anxiety. Biol Psychiatry. 2006;60(4):383–387. doi: 10.1016/j.biopsych.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Carter EJ. Brain mechanisms for social perception: lessons from autism and typical development. Ann N Y Acad Sci. 2008;1145:283–299. doi: 10.1196/annals.1416.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prevalence of autism spectrum disorders--autism and developmental disabilities monitoring network, 14 sites, United States, 2002. MMWR Surveill Summ. 2007;56(1):12–28. [PubMed] [Google Scholar]
- Schultz RT. Developmental deficits in social perception in autism: the role of the amygdala and fusiform face area. Int J Dev Neurosci. 2005;23(2–3):125–141. doi: 10.1016/j.ijdevneu.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Carlin DA, Allman JM, Macedo MN, Bush C, Miller BL, et al. Early frontotemporal dementia targets neurons unique to apes and humans. Ann Neurol. 2006;60(6):660–667. doi: 10.1002/ana.21055. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Crawford RK, Zhou J, Miller BL, Greicius MD. Neurodegenerative diseases target large-scale human brain networks. Neuron. 2009;62(1):42–52. doi: 10.1016/j.neuron.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27(9):2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafritz KM, Dichter GS, Baranek GT, Belger A. The neural circuitry mediating shifts in behavioral response and cognitive set in autism. Biol Psychiatry. 2008;63(10):974–980. doi: 10.1016/j.biopsych.2007.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silani G, Bird G, Brindley R, Singer T, Frith C, Frith U. Levels of emotional awareness and autism: an fMRI study. Soc Neurosci. 2008;3(2):97–112. doi: 10.1080/17470910701577020. [DOI] [PubMed] [Google Scholar]
- Singer T. The neuronal basis and ontogeny of empathy and mind reading: Review of literature and implications for future research. Neurosci Biobehav Rev. 2006;30(6):855–863. doi: 10.1016/j.neubiorev.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Singer T, Seymour B, O'Doherty J, Kaube H, Dolan RJ, Frith CD. Empathy for pain involves the affective but not sensory components of pain. Science. 2004;303(5661):1157–1162. doi: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- Sokol DK, Edwards-Brown M. Neuroimaging in autistic spectrum disorder (ASD) J Neuroimaging. 2004;14(1):8–15. [PubMed] [Google Scholar]
- Sommer IE, Diederen KM, Blom JD, Willems A, Kushan L, Slotema K, et al. Auditory verbal hallucinations predominantly activate the right inferior frontal area. Brain. 2008;131(Pt 12):3169–3177. doi: 10.1093/brain/awn251. [DOI] [PubMed] [Google Scholar]
- Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci U S A. 2008;105(34):12569–12574. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark DE, Margulies DS, Shehzad ZE, Reiss P, Kelly AM, Uddin LQ, et al. Regional variation in interhemispheric coordination of intrinsic hemodynamic fluctuations. J Neurosci. 2008;28(51):13754–13764. doi: 10.1523/JNEUROSCI.4544-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MB, Simmons AN, Feinstein JS, Paulus MP. Increased amygdala and insula activation during emotion processing in anxiety-prone subjects. Am J Psychiatry. 2007;164(2):318–327. doi: 10.1176/ajp.2007.164.2.318. [DOI] [PubMed] [Google Scholar]
- Toro R, Fox PT, Paus T. Functional coactivation map of the human brain. Cereb Cortex. 2008;18(11):2553–2559. doi: 10.1093/cercor/bhn014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Kelly AMC, Biswal BB, Xavier Castellanos F, Milham MP. Functional connectivity of default mode network components: Correlation, anticorrelation, and causality. Hum Brain Mapp. 2009;30(2):625–637. doi: 10.1002/hbm.20531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalobos ME, Mizuno A, Dahl BC, Kemmotsu N, Muller RA. Reduced functional connectivity between V1 and inferior frontal cortex associated with visuomotor performance in autism. Neuroimage. 2005;25(3):916–925. doi: 10.1016/j.neuroimage.2004.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Snyder AZ, Fox MD, Shannon BJ, Andrews JR, Raichle ME, et al. Coherent spontaneous activity identifies a hippocampal-parietal memory network. J Neurophysiol. 2006;96(6):3517–3531. doi: 10.1152/jn.00048.2006. [DOI] [PubMed] [Google Scholar]
- Viskontas IV, Possin KL, Miller BL. Symptoms of frontotemporal dementia provide insights into orbitofrontal cortex function and social behavior. Ann N Y Acad Sci. 2007;1121:528–545. doi: 10.1196/annals.1401.025. [DOI] [PubMed] [Google Scholar]
- Waterhouse L. Autism overflows: increasing prevalence and proliferating theories. Neuropsychol Rev. 2008;18(4):273–286. doi: 10.1007/s11065-008-9074-x. [DOI] [PubMed] [Google Scholar]
- Williams JH, Whiten A, Suddendorf T, Perrett DI. Imitation, mirror neurons and autism. Neurosci Biobehav Rev. 2001;25(4):287–295. doi: 10.1016/s0149-7634(01)00014-8. [DOI] [PubMed] [Google Scholar]


