Abstract
Background
Individuals with generalized social anxiety disorder (GSAD) exhibit exaggerated amygdala reactivity to aversive social stimuli. These findings could be explained by microstructural abnormalities in white matter (WM) tracts that connect the amygdala and prefrontal cortex, which is known to modulate the amygdala’s response to threat. The goal of this study was to investigate brain frontal WM abnormalities by using diffusion tensor imaging (DTI) in patients with social anxiety disorder.
Method
A Turboprop DTI sequence was used to acquire diffusion tensor images in thirty patients with GSAD and thirty matched healthy controls. Fractional anisotropy, an index of axonal organization, within WM was quantified in individual subjects and an automated voxel-based, whole-brain method was used to analyze group differences.
Results
Compared to healthy controls, patients had significantly lower fractional anisotropy localized to the right uncinate fasciculus WM near the orbitofrontal cortex. There were no areas of higher fractional anisotropy in patients than controls.
Conclusions
These findings point to an abnormality in the uncinate fasciculus, the major WM tract connecting the frontal cortex to the amygdala and other limbic temporal regions, in GSAD which could underlie the aberrant amygdala-prefrontal interactions resulting in dysfunctional social threat processing in this illness.
Introduction
Generalized social anxiety disorder (GSAD), also known as social phobia, is a common, chronic disorder that typically originates prior to adolescence and foretells significant functional impairment and psychiatric comorbidity (1). Individuals with GSAD exhibit exaggerated responses in the amygdala to social fear and anxiety provocation (2-5). Converging evidence from animal and human studies show that regulation of the amygdala and its reactivity to threat is mediated by intact functional interactions with discrete regions of the prefrontal cortex, specifically the orbital prefrontal cortex (OFC) (6,7), and recent work suggests that aberrant amygdala activation and social anxiety symptoms are related to ineffective prefrontal response during emotion regulation (8). Examination of white matter (WM) tracts that structurally connect the amygdala with prefrontal structures such as the orbital prefrontal cortex (OFC) (9) may elucidate the abnormal mechanisms that underlie the functional relationships between and within these brain regions.
Diffusion tensor imaging (DTI), a recently developed magnetic resonance imaging technique, allows the opportunity to examine the integrity of WM microstructure, and thus serves as an important tool for mapping anatomical connectivity in humans (10). DTI measures the directionality and coherence of water diffusion as reflected by the degree of anisotropy, which represents an estimate of axonal organization in the brain (11). To date, the use of DTI to examine WM tracts in phobia-related anxiety disorders has been limited.
The primary aim of this study was to use state-of-the-science Turboprop DTI (12) to examine alterations in fractional anisotropy (FA) with WM tracts in the prefrontal cortex, particularly the OFC, which has been shown in neuroanatomical studies to be reciprocally and densely connected to the amygdala (9), in subjects with GSAD. Given that reduced FA is associated with compromised axonal structure and/or organization (11), we hypothesized that relative to healthy control (HC) subjects, individuals with GSAD would exhibit lower FA in WM tracts that lead from and to the OFC.
Method
Subjects
Sixty subjects (30 with GSAD and 30 age-, gender-, education-matched HC) participated in this study. Demographic and clinical characteristics of the subjects are presented in Table 1. GSAD diagnosis was established using the Structured Clinical Interview for DSM-IV (SCID) with additional probes from the Social Phobia Interview (13) conducted by trained, masters-level clinical assessors, and the self-administered Liebowitz Social Anxiety Scale (LSAS) (14). None of the GSAD subjects had a current/recent depressive episode or alcohol/substance abuse (within 12 months of study entry), or another anxiety disorder that was more clinically salient or preceded GSAD. Eleven GSAD subjects had some form of past history (> 12 months) of substance abuse or dependence, all of whom were in full remission at the time of study entry – five had past alcohol dependence (one of whom with hallucinogen abuse and another with cannabis abuse); five had past alcohol abuse (two of whom had cannabis abuse/dependence); and one had past opiate dependence. Subjects were excluded if they had a history of obsessive-compulsive disorder, post-traumatic stress disorder, bipolar disorder, psychotic disorder, mental retardation, or developmental disorders. Healthy controls had no history of a psychiatric disorder verified by SCID-NP. None of the subjects had a history of a major medical or neurological illness. All subjects were right-handed (based on responses to inquiry about which dominant hand was used to write, make button presses on a mouse, throw a ball, etc.) and free for psychoactive medications (for at least 8 weeks) at the time of study entry, except for one GSAD subject who was taking buproprion. All subjects provided written informed consent, and the study was approved by the local university hospital institutional review board.
Table 1.
Demographic and Clinical Characteristics of Patients and Control Subjects
| GSAD (N=30) | HC (N=30) a | |||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | t | p | |
| Age (years) | 27.20 | 7.80 | 29.90 | 8.13 | 1.31 | 0.194 |
| Education (years) | 15.67 | 1.58 | 15.76 | 1.45 | 0.23 | 0.822 |
| Liebowitz Social Anxiety Scale | 76.70 | 17.31 | 13.38 | 11.32 | 14.68 | <0.0001 |
| Spielberger State Anxiety | 41.17 | 9.85 | 26.40 | 5.56 | 6.08 | <0.0001 |
| Spielberger Trait Anxiety | 48.80 | 9.11 | 28.60 | 5.25 | 8.96 | <0.0001 |
| Beck Depression Inventory | 10.70 | 6.51 | 1.84 | 2.37 | 6.45 | <0.0001 |
| N | % | N | % | χ2 | p | |
| Gender | 1.71 | 0.190 | ||||
| Male | 15 | 50.00 | 10 | 33.33 | ||
| Female | 15 | 50.00 | 20 | 66.67 | ||
| Race | 0.36 | 0.838 | ||||
| Caucasian | 24 | 80.00 | 23 | 76.67 | ||
| African American | 5 | 16.67 | 5 | 16.67 | ||
| Asian | 1 | 3.33 | 2 | 6.67 | ||
Education data was missing from 5 HC subjects, and clinical symptomatology data are not available from 10 HC subjects. GSAD, Generalized Social Anxiety Disorder; HC, Healthy Control
MRI Protocol
Subjects were scanned with Turboprop DTI (12,15) on a 3T GE MRI scanner (General Electric, Waukesha, WI) using the following parameters: TR (repetition time)=5000msec, TE=94msec, 8 spin-echoes per TR/blade, 5 k-space lines acquired per spin-echo (40 lines per blade), 128 samples per line, 16 k-space blades per image, field-of-view=24cm×24cm, 36 contiguous axial slices, slice thickness=3mm, 256×256 final image matrix. Diffusion-weighted images with b=900sec/mm2 images were acquired in each slice for a set of 12 diffusion directions uniformly distributed in 3-dimensional space; two b=0 sec/mm2 images were also acquired in each slice. Turboprop DTI has been shown to be relatively immune to susceptibility-related artifacts, image warping due to eddy currents, and motion-related distortions relative to other DTI techniques (12,15). High-resolution T1-weighted anatomical data were obtained using the 3D magnetization-prepared rapid acquisition gradient-echo (MP-RAGE) sequence and the following parameters: TE=3.2msec, TR=8msec, preparation/inversion time [TI]=725msec, flip angle 6°, field-of-view 24cm×24cm, 124 sagittal slices, 1.5mm slice thickness, 192×256 image matrix reconstructed to 256×256.
DTI Image Processing and Analysis
Details for image processing and analysis have been described elsewhere (16). In brief, the diffusion tensor model was fit to each voxel to create FA images for each subject. The Brain Extraction Tool of the software package FSL (Oxford Center for fMRI of the Brain, Oxford, UK) was applied on all b=0sec/mm2 volumes to remove the skull and noise outside of the brain. The resulting binary brain masks were then applied on the FA maps. The FA images from all subjects were smoothed using Gaussian kernels with full width at half maximum (FWHM) of 9mm and normalized to an FA template using nonlinear registration (Statistical Parametric Mapping, SPM5, Wellcome Department of Imaging and Neuroscience, London, UK). The estimated spatial transformations were then applied on the original FA maps prior to smoothing; in other words, we used un-smoothed FA maps to conduct the subsequent voxel-based analysis. Voxel-based group comparisons of FA values between the HC and GSAD subjects were performed using a General Linear Model covariate analysis, with age included in the model as a covariate (17). Requisite significance was set, a priori, to detect clusters exceeding volume larger than 100 mm3 in which uncorrected group differences across a whole-brain voxel-wise search exceeded a threshold of p < 0.01, as an attempt to balance type I and II error rates and correct for spatial correlation in the FA data. We set a less conservative significance threshold because we had an a priori hypothesis about group differences in white matter FA within tracts that connect the amygdala to the prefrontal cortex (specifically the OFC). However, given that this is the first DTI study in an anxiety disorder other than OCD, we report all group differences that surpassed this exploratory threshold in order to obviate bias and to generate new hypotheses about other areas of white matter pathology. Moreover, this threshold is similar to other published whole-brain voxel-based analyses of FA between psychiatric and control groups in which frontal WM abnormalities were predicted (18-22). Significant cluster(s) were overlaid on averaged FA maps and diffusion anisotropy color maps, and then localized using a published MRI atlas of human white matter (23). The voxel-based analysis was repeated for normalization based on FA maps smoothed with FWHM=5mm. The mean FA averaged across all voxels within the entire cluster(s) with significant differences in FA between patients and controls were estimated and extracted for each subject, in order to: 1) show individual variability with a scatterplot; and 2) calculate Cohen d effect size to guide future studies. In addition to clarify group differences in FA, measures of diffusivity such as trace, primary, secondary and tertiary eigenvalues were also extracted from significant cluster(s) to examine group differences.
Results
Relative to healthy controls, subjects with GSAD exhibited significantly lower FA nearby the OFC localized (by Talairach coordinates, [x=right, y=anterior, z=superior]) to right uncinate fasciculus (UF) ([18, 18, -15], 383 voxels; t = 2.88, p = 0.003, uncorrected) (Figure 1A). No other areas of reduced white matter FA were identified in the GSAD group. Extracted FA values (Mean ± S.D.) from the UF cluster are shown in Figure 1B to show individual variability (HC: 0.32 ± 0.02 vs. GSAD: 0.31 ± 0.02; t58 = 2.41, p=0.02, Cohen d = 0.63) (Figure 1B). Although, the trace, secondary, and tertiary eigenvalues from the UF cluster of the GSAD group were slightly increased compared to the HC group, no significant differences were detected in the trace (HC: 0.00244±0.00015 mm2/sec vs. GSAD: 0.0025±0.00013 mm2/sec; t58 = 1.68, p>0.05), primary (HC: 0.00114±0.00008 mm2/sec vs. GSAD: 0.00115±0.00005 mm2/sec; t58 = 0.53, p>0.05), secondary (HC: 0.000766±0.00006 mm2/sec vs. GSAD: 0.000784±0.00006 mm2/sec; t58 = 1.15, p>0.05), and tertiary (HC: 0.000555±0.00007 mm2/sec vs. GSAD: 0.000583±0.00007 mm2/sec; t58 = 1.46, p>0.05) eigenvalues from the UF cluster. These finding suggest that an increase in radial diffusivity is the most likely cause for the reduction in FA. There were no areas with significantly higher FA in the GSAD group than the HC group. Of note, similar results presented above were obtained when the voxel-based analysis was repeated for normalization based on FA maps smoothed with FWHM=5mm.
Figure 1.
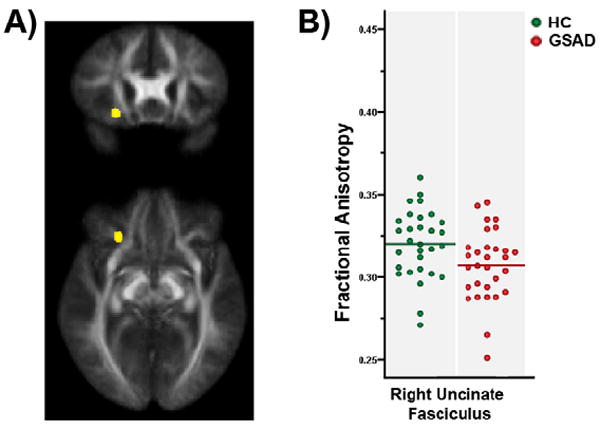
Lower Fractional Anistropy in Right Uncinate Fasciculus in Patients with Generalized Social Anxiety Disorder Than in Healthy Controls. A) Significant group difference (HC > GSAD) in FA values localized to the right uncinate fasciculus; cluster as rendered on a coronal and axial slice of the average FA map from all subjects; B) Scatterplot of individual subjects showing the mean fractional anistropy values across all voxels within the UF cluster for Generalized Social Anxiety Disorder (GSAD) and Healthy Control (HC) subjects. Horizontal lines represent group means.
Discussion
In this Turboprop DTI study, as predicted, we observed lower prefrontal fractional anisotropy localized to right uncinate fasciculus (UF) white matter in the area adjacent to the orbitofrontal cortex in individuals GSAD compared to matched healthy controls. Anatomically, the UF is the major WM fiber tract that connects the inferofrontal and anterotemporal cortices, and it travels over the lateral nuclei of the amygdala terminating in the OFC (Brodmann area 11-12) and subcallosal area (Brodmann area 25) (9). Thus, lower FA within this tract suggests aberrant fronto-amygdala structural connectivity in GSAD. Given that subcallosal frontal regions, such as the OFC, serve important roles in top-down regulation of amygdala reactivity to control negative affect (i.e., anxiety) and mediate threat perception (6), this UF white matter tract is suggested to play a key role in emotional responding (24). This is consistent with evidence from functional neuroimaging studies implicating aberrant reactivity in amygdala to social threat and scrutiny in GSAD (2-4), and thus abnormalities in UF which carries the most prominent fibers between these two regions may explain why differential reactivity exists. This finding is consistent with recent functional neuroimaging studies have implicated the OFC in social anxiety disorder (25,26). Moreover, the Cohen d (0.63) suggests a small to medium size effect, and is consistent with other studies of uncinate fasciculus abnormalities in bipolar disorder (27) and of frontal white matter in schizophrenia (28).
There is no prior study of WM integrity as measured by DTI in social phobia to use for comparison, however, recent DTI studies have shown that reduced uncinate fasciculus FA is associated with lower levels of extraversion and fewer friends (29) and higher levels of suspiciousness and interpersonal difficulties (30) in schizotypal personality disorder. Moreover, an association between uncinate fasciculus FA and impulsivity and aggression in schizophrenia has previously been observed (31,32). Therefore, reductions in FA within the uncinate fasciculus may not be specific to social anxiety disorder, given similar findings are observed in bipolar disorder and schizophrenia (27,28,33). Therefore, we speculate that altered FA in this white matter tract that connects amygdala to OFC, both of which also have been similarly implicated across these disorders, may reflect a common phenotype such as affect dysregulation and/or impaired social interactions.
The reduction in FA was due to an increase in secondary and tertiary eigenvalues which could be attributed to any or all of the following processes: 1) decrease in axonal density; 2) decrease in axonal myelination; 3) abnormal axonal membranes; and/or 4) reorganization of axons at a macroscopic level (11). Moreover, our findings of UF tract abnormalities do not allow inferences about directionality; DTI cannot distinguish unidirectional or bi-directional loci of abnormality. Nevertheless, the difference in GSAD subjects in orbitofrontal white matter integrity may help explain the observed abnormalities in OFC and/or amygdala function in GSAD. Interestingly, recent studies have demonstrated a correlation between fMRI BOLD activation measures (as indexed by blood oxygenation) or electrophysiological changes (as indexed by event-related potentials) and fractional anisotropy (34-36), suggesting that the observed hyper-reactivity in amygdala and/or OFC or aberrant ‘functional’ connectivity of these regions may be related to FA measures in WM tracts that connect these regions. Future studies are needed to directly investigate this interpretation.
It should be noted that the observed finding of group differences in UF nearby the OFC would not have survived correction for multiple comparisons, raising the concern for Type I error. As noted above, this is less likely given that OFC dysfunction has been demonstrated in GSAD and that a cluster size (>100 mm3) was set. Correction for multiple comparisons across all voxels of the whole-brain search volume using Gaussian random field theory has been argued as undesirable because it necessitates the use of smoothed data which results in loss of anatomical information (31,37), and other conservative approaches such as permutation testing and false discovery rate analyses substantially reduce statistical power. Because we conducted a whole-brain voxel-wise analysis, the problem of multiple comparisons and an increased risk of a type I error remain and it should be noted that we were only able to detect differences in FA maps using a liberal threshold, and this does reduce the strength of the conclusions of the study and prompts caution in interpreting these preliminary results. The observation that the size of the current sample of unmedicated GSAD patients without current/active depression and substance abuse (n=30) yielded a relatively focal WM finding of small to moderate effect size may help guide future studies.
In summary, based on findings of altered fractional anisotropy as measured by diffusion tensor imaging, the current study presents preliminary evidence of a white matter abnormality within the uncinate fasciculus, the most prominent fiber tract linking the amygdala and orbitofrontal cortex, in individuals with GSAD. This finding may reflect or explain the aberrant patterns of amygdala, frontal, or their interactions in response to social threat in this disorder, and prompt further studies on white matter pathology in disorders associated with social anxiety and interpersonal difficulties.
Acknowledgments
This work was supported in part by National Institute of Mental Health grant K23MH076198 to KLP and the Brain Research Foundation Seed Grant to KLP. We acknowledge the assistance of Rose McCarron and the staff of the Clinical Neuroscience Research Unit (CNPRU) of the Department of Psychiatry for subject recruitment and clinical assessment.
Footnotes
Financial Disclosures The authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stein MB, Stein DJ. Social anxiety disorder. Lancet. 2008;371:1115–1125. doi: 10.1016/S0140-6736(08)60488-2. [DOI] [PubMed] [Google Scholar]
- 2.Tillfors M, Furmark T, Marteinsdottir I, Fredrikson M. Cerebral blood flow during anticipation of public speaking in social phobia: a PET study. Biol Psychiatry. 2002;52:1113–1119. doi: 10.1016/s0006-3223(02)01396-3. [DOI] [PubMed] [Google Scholar]
- 3.Stein MB, Goldin PR, Sareen J, Zorrilla LT, Brown GG. Increased amygdala activation to angry and contemptuous faces in generalized social phobia. Arch Gen Psychiatry. 2002;59:1027–1034. doi: 10.1001/archpsyc.59.11.1027. [DOI] [PubMed] [Google Scholar]
- 4.Phan KL, Fitzgerald DA, Nathan PJ, Tancer ME. Association between Amygdala Hyperactivity to Harsh Faces and Severity of Social Anxiety in Generalized Social Phobia. Biological Psychiatry. 2006;59:424–429. doi: 10.1016/j.biopsych.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 5.Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry. 2007;164:1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cogn Sci. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 7.Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- 8.Goldin PR, Hakimi S, Manber T, Canli T, Gross JJ. Neural bases of social anxiety disorder: emotional reactivity and cognitive regulation during social and physical threat. Arch Gen Psychiatry. doi: 10.1001/archgenpsychiatry.2008.525. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghashghaei HT, Hilgetag CC, Barbas H. Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. Neuroimage. 2007;34:905–923. doi: 10.1016/j.neuroimage.2006.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lim KO, Helpern JA. Neuropsychiatric applications of DTI - a review. NMR Biomed. 2002;15:587–593. doi: 10.1002/nbm.789. [DOI] [PubMed] [Google Scholar]
- 11.Mori S, Zhang J. Principles of diffusion tensor imaging and its applications to basic neuroscience research. Neuron. 2006;51:527–539. doi: 10.1016/j.neuron.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 12.Arfanakis K, Gui M, Lazar M. White matter tractography by means of Turboprop diffusion tensor imaging. Ann N Y Acad Sci. 2005;1064:78–87. doi: 10.1196/annals.1340.014. [DOI] [PubMed] [Google Scholar]
- 13.Stein MB, Chartier MJ, Hazen AL, Kozak MV, Tancer ME, Lander S, et al. A direct-interview family study of generalized social phobia. Am J Psychiatry. 1998;155:90–97. doi: 10.1176/ajp.155.1.90. [DOI] [PubMed] [Google Scholar]
- 14.Fresco DM, Coles ME, Heimberg RG, Liebowitz MR, Hami S, Stein MB, et al. The Liebowitz Social Anxiety Scale: a comparison of the psychometric properties of self-report and clinician-administered formats. Psychol Med. 2001;31:1025–1035. doi: 10.1017/s0033291701004056. [DOI] [PubMed] [Google Scholar]
- 15.Pipe JG, Zwart N. Turboprop: improved PROPELLER imaging. Magn Reson Med. 2006;55:380–385. doi: 10.1002/mrm.20768. [DOI] [PubMed] [Google Scholar]
- 16.Arfanakis K, Gui M, Tamhane AA, Carew JD. Investigating the Medial Temporal Lobe in Alzheimer’s Disease and Mild Cognitive Impairment, with Turboprop Diffusion Tensor Imaging, MRI-volumetry, and T 2-relaxometry. Brain Imaging and Behavior. 2007;1:11–21. [Google Scholar]
- 17.Sullivan EV, Adalsteinsson E, Hedehus M, Ju C, Moseley M, Lim KO, et al. Equivalent disruption of regional white matter microstructure in ageing healthy men and women. Neuroreport. 2001;12:99–104. doi: 10.1097/00001756-200101220-00027. [DOI] [PubMed] [Google Scholar]
- 18.Nakamae T, Narumoto J, Shibata K, Matsumoto R, Kitabayashi Y, Yoshida T, et al. Alteration of fractional anisotropy and apparent diffusion coefficient in obsessive-compulsive disorder: a diffusion tensor imaging study. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1221–1226. doi: 10.1016/j.pnpbp.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 19.Wang F, Kalmar JH, Edmiston E, Chepenik LG, Bhagwagar Z, Spencer L, et al. Abnormal corpus callosum integrity in bipolar disorder: a diffusion tensor imaging study. Biol Psychiatry. 2008;64:730–733. doi: 10.1016/j.biopsych.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murphy CF, Gunning-Dixon FM, Hoptman MJ, Lim KO, Ardekani B, Shields JK, et al. White-matter integrity predicts stroop performance in patients with geriatric depression. Biol Psychiatry. 2007;61:1007–1010. doi: 10.1016/j.biopsych.2006.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ashtari M, Kumra S, Bhaskar SL, Clarke T, Thaden E, Cervellione KL, et al. Attention-deficit/hyperactivity disorder: a preliminary diffusion tensor imaging study. Biol Psychiatry. 2005;57:448–455. doi: 10.1016/j.biopsych.2004.11.047. [DOI] [PubMed] [Google Scholar]
- 22.Hubl D, Koenig T, Strik W, Federspiel A, Kreis R, Boesch C, et al. Pathways that make voices: white matter changes in auditory hallucinations. Arch Gen Psychiatry. 2004;61:658–668. doi: 10.1001/archpsyc.61.7.658. [DOI] [PubMed] [Google Scholar]
- 23.Mori S, Wakana S, Nagae-Poetscher LM, Van Zijl PC. MRI Atlas of Human White Matter. Amsterdam, The Netherlands: Elsevier B.B; 2005. [Google Scholar]
- 24.Ghashghaei HT, Barbas H. Pathways for emotion: interactions of prefrontal and anterior temporal pathways in the amygdala of the rhesus monkey. Neuroscience. 2002;115:1261–1279. doi: 10.1016/s0306-4522(02)00446-3. [DOI] [PubMed] [Google Scholar]
- 25.Evans KC, Simon NM, Dougherty DD, Hoge EA, Worthington JJ, Chow C, et al. A PET study of tiagabine treatment implicates ventral medial prefrontal cortex in generalized social anxiety disorder. Neuropsychopharmacology. 2009;34:390–398. doi: 10.1038/npp.2008.69. [DOI] [PubMed] [Google Scholar]
- 26.Quadflieg S, Mohr A, Mentzel HJ, Miltner WH, Straube T. Modulation of the neural network involved in the processing of anger prosody: the role of task-relevance and social phobia. Biol Psychol. 2008;78:129–137. doi: 10.1016/j.biopsycho.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 27.Versace A, Almeida JR, Hassel S, Walsh ND, Novelli M, Klein CR, et al. Elevated left and reduced right orbitomedial prefrontal fractional anisotropy in adults with bipolar disorder revealed by tract-based spatial statistics. Arch Gen Psychiatry. 2008;65:1041–1052. doi: 10.1001/archpsyc.65.9.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kanaan RA, Kim JS, Kaufmann WE, Pearlson GD, Barker GJ, McGuire PK. Diffusion tensor imaging in schizophrenia. Biol Psychiatry. 2005;58:921–929. doi: 10.1016/j.biopsych.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 29.Gurrera RJ, Nakamura M, Kubicki M, Dickey CC, Niznikiewicz MA, Voglmaier MM, et al. The uncinate fasciculus and extraversion in schizotypal personality disorder: a diffusion tensor imaging study. Schizophr Res. 2007;90:360–362. doi: 10.1016/j.schres.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakamura M, McCarley RW, Kubicki M, Dickey CC, Niznikiewicz MA, Voglmaier MM, et al. Fronto-temporal disconnectivity in schizotypal personality disorder: a diffusion tensor imaging study. Biol Psychiatry. 2005;58:468–478. doi: 10.1016/j.biopsych.2005.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoptman MJ, Ardekani BA, Butler PD, Nierenberg J, Javitt DC, Lim KO. DTI and impulsivity in schizophrenia: a first voxelwise correlational analysis. Neuroreport. 2004;15:2467–2470. doi: 10.1097/00001756-200411150-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoptman MJ, Volavka J, Johnson G, Weiss E, Bilder RM, Lim KO. Frontal white matter microstructure, aggression, and impulsivity in men with schizophrenia: a preliminary study. Biol Psychiatry. 2002;52:9–14. doi: 10.1016/s0006-3223(02)01311-2. [DOI] [PubMed] [Google Scholar]
- 33.Kyriakopoulos M, Bargiotas T, Barker GJ, Frangou S. Diffusion tensor imaging in schizophrenia. Eur Psychiatry. 2008;23:255–273. doi: 10.1016/j.eurpsy.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 34.Schlosser RG, Nenadic I, Wagner G, Gullmar D, von Consbruch K, Kohler S, et al. White matter abnormalities and brain activation in schizophrenia: a combined DTI and fMRI study. Schizophr Res. 2007;89:1–11. doi: 10.1016/j.schres.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 35.Forstmann BU, Jahfari S, Scholte HS, Wolfensteller U, van den Wildenberg WP, Ridderinkhof KR. Function and structure of the right inferior frontal cortex predict individual differences in response inhibition: a model-based approach. J Neurosci. 2008;28:9790–9796. doi: 10.1523/JNEUROSCI.1465-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Westlye LT, Walhovd KB, Bjornerud A, Due-Tonnessen P, Fjell AM. Error-related negativity is mediated by fractional anisotropy in the posterior cingulate gyrus--a study combining diffusion tensor imaging and electrophysiology in healthy adults. Cereb Cortex. 2009;19:293–304. doi: 10.1093/cercor/bhn084. [DOI] [PubMed] [Google Scholar]
- 37.Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]


