Abstract
The pupose of this study was to determine the viability of cell-based delivery of brain-derived neurotrophic factor (BDNF) from genetically modified mesenchymal stem cells (MSCs) for neuroprotection of RGC-5 cells. RGC-5 cells were differentiated with the protein kinase inhibitor staurosporine (SS) and exposed to the cellular stressors glutamate or H2O2. As a neuroprotective strategy, these cells were then co-cultured across a membrane insert with mesenchymal stem cells (MSCs) engineered with a lentiviral vector for production of BDNF (BDNF-MSCs). As a positive control, recombinant human BDNF (rhBDNF) was added to stressed RGC-5 cells. After SS differentiation RGC-5s developed neuronal-like morphologies, and a significant increase in the proportion of RGC-5s immunoreactive for TuJ-1 and Brn3a was observed. Differentiated RGC-5s also had prominent TrkB staining, demonstrating expression of the high-affinity BDNF receptor. Treatment of SS differentiated RGC-5s with glutamate or H2O2, produced significant cell death (56.0 ± 7.02 and 48.90 ± 4.58% of control cells, respectively) compared to carrier-solution treated cells. BDNF-delivery from MSCs preserved more RGC-5 cells after treatment with glutamate (80.0 ± 5.40% cells remaining) than control GFP expressing MSCs (GFP-MSCs, 57.29 ± 1.89%, p < 0.01). BDNF-MSCs also protected more RGC-5s after treatment with H2O2 (65.6 ± 3.47%) than GFP-MSCs (46.0 ± 4.20%, p < 0.01). We have shown survival of differentiated RGC-5s is reduced by the cellular stressors glutamate and H2O2. Additionally, our results demonstrate that genetically modified BDNF-producing MSCs can enhance survival of stressed RGC-5 cells and therefore, may be effective vehicles to deliver BDNF to retinal ganglion cells affected by disease.
Keywords: Neuroprotection, Mesenchymal Stem Cells, BDNF, Glaucoma, Ganglion Cells, RGC-5
Introduction
Glaucoma is a progressive, chronic, optic neuropathy that is characterized by retinal ganglion cell (RGC) death, and subsequent loss of visual function. Several studies suggest secondary degeneration of RGCs in optic neuropathies can be mediated, in part, via glutamate excitotoxicity1-4, as well as the presence of excessive reactive oxygen species (ROS), which are capable of inducing DNA damage, the oxidative modification of proteins and cellular dysfunction resulting in cell death5-8. Developing neuroprotective approaches that can preserve cells following insult with these compounds is an important step in developing long-term treatments for optic neuropathies.
Studying optic neuropathies requires reproducible and reliable models9. While these models have proved invaluable for researchers, the cost of animal care, as well as the labor involved in generating the models and carrying out the experiments is prohibitive to high-throughput assays of potential neuroprotective compounds. Thus, developing relevant in vitro assays for evaluating the potential neuroprotective effects of different pharmacological compounds or cell-based strategies is a useful and powerful approach in developing treatment strategies, which can be tested in vivo. The RGC-5s are a transformed retinal ganglion cell line that retain some characteristics of retinal ganglion cells10. The RGC-5s initially proved useful in modeling the apoptotic events of RGCs with respect to serum deprivation11, oxidative stress12, glutamate excitotoxicity13, as well as cell death resulting from hydrostatic pressure14. While the rapid proliferation of these cells facilitates expansion of the cell line, it creates difficulties analyzing neuroprotection experiments, as the presence of mitotic cells confounds the results. Recently, Frassetto et al. demonstrated that transient treatment of RGC-5s with staurosporine (SS) induced them to become highly branched cells, with some electrophysiological and phenotypic characteristics similar to RGCs15. With this advance it is now possible to use RGC-5s as a model for retinal ganglion cell damage assays to evaluate potential neuroprotective factors and delivery systems in a reproducible manner, without the confounding variables that highly mitotic cells would introduce into these types of experiments.
A candidate for neuroprotection of RGCs in glaucomatous eyes is brain-derived neurotrophic factor (BDNF), a 14 kDa protein16 that predominately signals through its high affinity TrkB receptor17. Brain-derived neurotrophic factor has been shown to be essential for correct RGC development18, 19, and survival of RGCs in vitro20, 21. Bolus injections of BDNF have proven neuroprotective for retinal neurons in different disease models, as well as optic nerve axotomy22-28. Additionally, viral mediated transfer of BDNF has been shown to be neuroprotective for RGCs in models of glaucoma29, 30. While these results are encouraging, it is important to address the prolonged delivery of BDNF, since frequent and repeated injections into the eye may not be a viable treatment option for chronic optic neuropathies. Therefore, it is essential to develop a minimally invasive treatment that is capable of prolonged delivery of BDNF to disease-compromised optic nerves.
We have investigated a cell-based delivery strategy to provide BDNF, or other neuroprotective factors, to glaucomatous eyes (Sakaguchi DS, et al. IOVS 2007;48: ARVO E-Abstract 1303). Mesenchymal stem cells (MSCs) are an excellent choice as cellular vehicles to deliver BDNF to damaged retinas. These multipotent cells are easily isolated31, display significant plasticity32 and may provide a source for cells that can be engineered for autologous transplants. Mesenchymal stem cells bypass the ethical concerns typically associated with embryonic, fetal, and neonatal derived cells, and the difficulty associated with deriving adult progenitors from CNS tissues. In addition, MSCs have the ability to survive and migrate when transplanted to CNS tissues33-37, differentiate into neural-like cells in vitro33, 37-39, and display electrophysiological properties consistent with mature neurons40, 41. Furthermore, naïve MSCs have also shown the potential to be neuroprotective when transplanted into models of retinal degeneration42-44. Together, these studies indicate that MSCs are excellent candidates for genetic engineering and autologous transplants into damaged CNS environments.
The purpose of this study was to demonstrate the feasibility of using MSCs to deliver BDNF in an attempt to rescue RGC-5s exposed to different cellular insults. We have demonstrated that BDNF released from MSCs engineered with a lentiviral vector was sufficient to protect RGC-5s from glutamate- or H2O2-mediated cell loss. To substantiate our observations, rhBDNF was added to SS differentiated RGC-5s treated with the cellular stressors and produced similar protection of RGC-5s. Taken together, these results demonstrate RGC-5s can serve as a model of retinal ganglion cells, and that cell-based delivery of neuroprotective factors from engineered MSCs has important clinical implications for treatment of different forms of optic neuropathies.
Materials and Methods
RGC-5 Maintenance and Differentiation
The RGC-5 cells (a generous gift from Dr. N. Agarwal, University of North Texas Health Science Center, Fort Worth, TX) were maintained as an adherent cell line in uncoated T-75 tissue culture flasks. RGC-5s were grown in medium containing DMEM (1 g glucose/ L, Gibco) 10% fetal bovine serum (FBS; SH30071.03, Hyclone, Logan, UT), L-glutamine (4 mM, 25038-081, Gibco, Grand Island, NY) and a penicillin (100 U/mL)/ streptomycin (100 μg/mL) solution (P0781, Sigma, St. Louis, MO). Cells were grown to 80% confluency and were gently lifted from the flask using 2 mL 0.05% Trypsin-EDTA solution (25300-054, Gibco) and placed in a 37°C incubator for 30 seconds. Trypsin was diluted with fresh medium, and RGC-5s were centrifuged at 800g for 5 minutes to pellet the cells. Pellets were re-suspended in fresh medium and split into new flasks at a ratio of 1:10. RGC-5 cultures were fed every other day by supplementing the flask with fresh medium.
RGC-5s were induced to differentiate into highly branched, non-mitotic cells using a protocol developed by Frassetto et al15. Briefly, RGC-5s were cultured as previously described and plated onto glass coverslips coated with an entactin-collagen-laminin substrate (ECL, 10 μg/mL, 08-110, Chemicon, Temecula, CA). Cells were allowed to adhere to the coverslip for 1 hour at 37°C. Cells were subsequently treated with the general kinase inhibitor staurosporine (1 μM, ALX-380-014-C100, Alexis, San Diego, CA) for 1 hour. Following differentiation, medium was changed and RGC-5 cells were grown an additional 3 days and analyzed for expression of neuronal markers using immunocytochemistry, or used immediately for subsequent experiments.
Bromo-deoxyuridine Incorporation Analysis
Control or SS-differentiated RGC-5s were incubated with 5 μM Bromo-deoxyuridine (BrdU) for 6 hours. BrdU containing medium was removed, fresh medium added, and cells were allowed to grow overnight. Cells were then fixed with 4% paraformaldehyde in 0.1 M PO4 buffer (pH 7.4). Following rinses in phosphate-buffered saline (PBS; 137 mM NaCL, 2.68 mM KCl, 10.14 mM Na2HPO4, 1.76 mM KH2PO4, pH 7.2) cells were incubated in 2 N HCl for 15 minutes at 37° C. HCl was neutralized by incubation with 0.1 M sodium borate for 15 minutes at room temperature. BrdU incorporation was detected with an anti-BrdU antibody (1:250, Accurate Chemical, Westbury, NY). The number of BrdU immunolabeled cells was counted in 5 fields on each coverslip at 10× magnification. The percentage of BrdU positive cells was calculated by dividing the total number of BrdU immunoreactive cells by the total number of cells as determined from nuclear counterstaining using 4′, 6′-diamidino-2-phenylindole, dilactate (DAPI, 1 μg/ mL, D3571, Sigma). This analysis was performed from three separate culture sessions.
Rat Mesenchymal Stem Cells
Rat MSCs (Tulane Center for Gene Therapy, New Orleans, LA) were maintained as an adherent cell line in α-MEM medium (12561-049, Invitrogen) containing 20% hybridoma qualified FBS (S11595, Atlanta Biologicals, Norcross, GA), 2 mM L-glutamine, and antibiotic-antimycotic (1%, 15240-096, Invitrogen; 10,000 U/mL penicillin, 10,000 μg/mL streptomycin, 25 ng/mL amphotericin B). Cells were maintained as low-density cultures plated at 75-150 cells/ cm2. When cultures reached 70-80% confluency MSCs were gently lifted from the dish using 0.25% trypsin, 0.1% EDTA solution (Invitrogen), and pelleted at 800g. MSCs were subsequently plated into 150 mm culture dishes at 75-150 cells/ cm2. Cultures were fed by supplementing the dish with fresh medium every other day.
Engineering Stem Cells Ex-Vivo with Lentiviral Vectors
Mesenchymal stem cells were engineered to produce and secrete the neurotrophic factor BDNF using lentiviral vectors. Briefly, MSCs were plated in 6 well plates at a density of 1000-1200 cells per well and allowed to adhere for 12 hours. After adhering to the plate the growth medium was substituted in each well with α-MEM containing 2% FBS and 12 μg/ml sequabrene (152667, Sigma). Two separate lentiviral constructs encoding 1) BDNF (LV-BDNF) and 2) green fluorescent protein (GFP, LV-GFP) were added simultaneously to MSCs at a multiplicity of infection (MOI) of 15 for each construct. A population of control MSCs was engineered with only the LV-GFP vector at an MOI of 30 to match the viral titer of the BDNF/GFP engineered MSCs. Viral particles were removed after 8 hours of exposure, and medium was changed to fresh growth medium. Engineered MSCs were subsequently maintained as previously described.
Neurotrophin Bioactivity Assay
All animal studies were conducted in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research, and had approval of the Iowa State University Institutional Animal Care and Use Committee. Primary explant cultures of rat embryonic day 17 (E17) dorsal root ganglia (DRG) were used to assess the bioactivity of BDNF released from MSCs, as DRG have been shown to elongate neurites in response to BDNF exposure45, 46. Briefly, E17 rat pups were anesthetized by hypothermia, decapitated, rinsed in ice-cold 70% ethanol and subsequently placed in ice-cold DMEM. Lumbar DRG were dissected from isolated spinal cords and pooled in ice-cold L-15 medium (21683-027, Invitrogen). Following dissection, DRG were rinsed with ice-cold L15 and transferred to warm DRG growth medium containing DMEM, L-glutamine, penicillin-streptomycin, and 10% FBS. Three to four DRG explants were transferred in approximately 75 μL of medium onto poly-L-ornithine coated 12 mm coverglass placed into wells of 24 well culture plates. DRG were allowed to adhere for 8 hours, and wells were subsequently flooded with experimental medium. Four different media conditions were tested: 1) MSC growth medium only, 2 and 3) MSC medium conditioned by BDNF-MSCs or GFP-MSCs, or 4) MSC medium containing 100 ng/ mL recombinant human BDNF (rhBDNF, 450-02, Peprotech, Rocky Hill, NJ). Explants were allowed to grow for an additional 48 hours, and subsequently fixed with 4% paraformaldehyde (pH 7.4). To visualize and quantify neurite outgrowth, cultures were stained with an anti-neurofilament antibody (RMO.308, 1:50, Virginia Lee, University of Pennsylvania), as described in the next section. Images of each DRG were captured using a 10× objective, and montage images were prepared. Each montage image was presented to naïve observers in order to judge the extent and density of neurite outgrowth in each condition. Each DRG was scored on a scale from 1-5; with 1 representing little neurite growth in terms of length and density, and 5 representing long, dense neurite arborizations from the DRG. Data from this analysis were pooled and statistical analysis was performed using one-way ANOVA with Dunnet's post-test.
Immunocytochemical Analysis
Cultures were fixed with 4% paraformaldehyde (in 0.1 M PO4 buffer), rinsed in filtered PBS, and incubated in blocking solution containing 5% normal donkey serum (NDS, 017-000-121, Jackson ImmunoResearch, West grove, PA), 0.04% bovine serum albumin (BSA, A9647, Sigma), and 0.04% Triton X-100 to eliminate non-specific antibody labeling. Coverslips were incubated in the primary antibodies: anti-TuJ-1 (1:250, R&D Systems, Minneapolis, MN), anti-Brn3a (1:100, Chemicon), anti-TrkB (1:100, Santa Cruz, Santa Cruz, CA), or anti-BDNF (1:100, Santa Cruz), diluted in blocker overnight at 4°C. Coverslips were then rinsed in PBS containing 0.1% Triton X-100. A donkey anti-mouse Cy-3 conjugated secondary antibody (1:150, 711-175-152, Jackson ImmunoResearch), was applied for 2 hours, and subsequently rinsed in filtered PBS. DAPI (1 μg/mL, Sigma) and Alexa-488 Phalloidin (5 U/mL A-12379, Molecular Probes, Eugene, OR) were diluted in PBS containing 0.04% Triton X-100 and incubated for 30 min at room temperature in order to visualize nuclei and the F-actin cytoskeleton, respectively. Coverslips were subsequently rinsed with PBS and mounted in Vectashield (H-1000, Vector Laboratories, Burlingame, CA) anti-fade mounting media and sealed with fingernail polish. Negative controls were processed in parallel by omission of the primary or secondary antibody, and no signal was detected. Images of cells were captured using a Nikon Microphot fluorescent microscope (Nikon, Melville, NY). Micrographs were captured using a MegaPlus digital camera (Kodak, Rochester, NY) and images prepared using Adobe Photoshop (Ver. 9.0, Adobe, San Jose, CA) and Macromedia Freehand (Ver. 10.0, Macromedia, San Francisco, CA).
Glutamate- and Hydrogen Peroxide-Mediated Cell Death
L-glutamic acid (glutamate, 49449, Sigma), and hydrogen peroxide (H2O2, H325-500, Fisher), were used in these studies to induce cell death in differentiated RGC-5 cells. Staurosporine differentiated RGC-5 cells were exposed to varying concentrations of glutamate (50 μM - 1.4 mM), or hydrogen peroxide (10-300 μM) for 8 hours. Media were replaced with fresh growth media and cells grown for an additional 2 days. Glutamate treated cells, and their respective control cells, were exposed to buthionine sulfoximine (BSO, B2640, Sigma) following SS differentiation until the completion of the experiment to deplete cellular glutathione levels13, in order to make the cells more susceptible to glutamate induced stress. Cells were fixed with 4% paraformaldehyde in 0.1 M PO4 and stained with Alexa-488 Phalloidin (5 U/ mL, Molecular Probes) in order to visualize the F-actin cytoskeleton. A blinded observer imaged 5 regions of each coverslip at pre-defined areas, and quantified the percentage of cells present on each coverslip as compared to control, vehicle treated RGC-5 cells. The concentration at which approximately 50% of RGC-5s were present compared to controls, was used for all subsequent experiments. Each experiment in these series of studies was conducted three times, with at least two replicates per timepoint for each experiment. Results were analyzed statistically using one-way ANOVA with Bonferroni's test for multiple comparisons.
Cell death was verified by staining H2O2 or glutamate treated cells with membrane-impermeable Sytox DNA stain (S7020, Invitrogen), which labels cells undergoing cell death. Briefly, SS treated RGC-5s were subjected to H2O2 or glutamate treatment as previously described. Following treatment, cells were exposed to 15 nM Sytox for 30 minutes at 37°C, fixed with 4% paraformaldehyde, and counterstained with DAPI. Cells were imaged and the proportion of cells labeled with Sytox was determined for H2O2-, glutamate-treated, and control cells. Student's t-test was used to analyze control and experimental conditions.
Co-Culturing Lentiviral Transduced MSCs with Differentiated RGC-5s
Genetically engineered MSCs secreting BDNF, or control MSCs expressing GFP were generated as previously described. Engineered MSCs were plated into Transwell culture inserts with 0.4 μm diameter semi-permeable membranes (07-200-147, Fisher) in 24 well plates at a density of 452 cells/ mm2. RGC-5s were differentiated as previously described in separate dishes on entactin-collagen-laminin (ECL, 10 μg/ mL, Chemicon) coated glass coverslips. Following differentiation, RGC-5s were transferred and grown in the presence of BDNF-MSCs or GFP-MSCs for 24 hours directly below the insert in the lower chamber. Following this initial period, cellular stressors were added to the co-cultures for 8 hours. Media were changed to fresh growth media, and cells were grown for an additional 48 hours in the presence of engineered MSCs. To serve as a positive control, a dose-response analysis of purified rhBDNF (Peprotech) was performed, based on concentrations previously reported to be effective in eliciting responses from cultured RGCs21, 47. The lowest concentration that resulted in significantly more cells than glutamate- or H2O2- treated RGC-5 cells (100 ng/mL) was placed in the medium of some chambers immediately, and remained throughout the course of the insult. Surviving cells were analyzed as previously described for each of three experiments, with at least two replicates per experiment.
Statistical Analysis
All statistical analysis was performed using GraphPad Prism (Ver. 3.0, GraphPad Software, San Diego, CA).
Results
Staurosporine-induced differentiation of RGC-5s
Development of in vitro systems that model RGCs and permit screening of potential neuroprotective compounds is paramount to developing new treatments for glaucoma. Naïve, undifferentiated RGC-5s have elongated, simple morphologies with few processes emanating from the cell body 24-72 hours following plating (Fig. 1 A-C). When RGC-5s were treated with SS they elaborated extensive processes within 24 hours following treatment, and maintained these complex morphologies up to 72 hours in culture (Fig 1. D-F). While SS-treated RGC-5s displayed complex morphologies reminiscent of retinal neurons, we sought to determine if these cells were capable of expressing neuron-specific class III β-tubulin (TuJ-1 antibody labeling), and the RGC specific transcription factor Brn3a, that are expressed by RGCs in vivo. Figure 2 illustrates extensive TuJ-1 immunoreactivity (IR) in the neurites (Fig. 2 A) and cell bodies, whereas RGC-5s treated with carrier solution expressed very low levels of TuJ-1-IR. Differentiated RGC-5s also expressed Brn3a in the cell soma (Fig. 2 B, arrow), while low levels of Brn3a-IR were detected in undifferentiated RGC-5s (Fig. 2 D). The proportion of RGC-5s expressing IR for each antibody was quantified, and it was determined that 70.26 ± 8.37% (±S.E.M.) of SS- differentiated RGC-5s expressed TuJ-1, a significant increase from 13 ± 1.3% of control RGC-5s (Fig. 2 E, p < 0.0001). Furthermore, SS differentiated RGC-5s also displayed a higher incidence of Brn3a immunoreactive cells, with 17.36 ± 5.57% of SS treated RGC-5s expressing Brn3a-IR, compared to just 1.06 ± 0.82% of control RGC-5s (Fig. 2 E, p < 0.05). A BrdU incorporation analysis was used to determine the percentage of mitotic cells in SS-treated vs. untreated populations of RGC-5s. Undifferentiated RGC-5s were a highly mitotic population of cells, with 66.7 ± 1.91% of RGC-5s immunoreactive for anti-BrdU following a 6 hour BrdU pulse (Fig. 2 F). In contrast, SS-differentiated RGC-5s had significantly less BrdU uptake, with only 4.62 ± 0.76% of cells BrdU immunoreactive following differentiation (Fig. 2 F, p < 0.0001). These results demonstrate that SS-differentiated RGC-5s display a decreased proliferative capacity and develop complex morphologies consistent with retinal neurons. In addition, they express neuronal markers consistent with RGCs in vivo.
Figure 1.
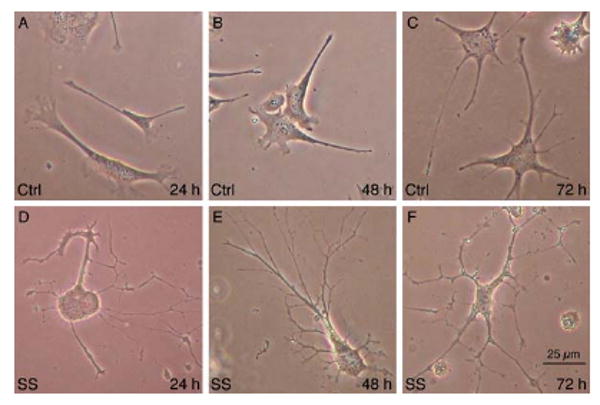
Differentiation of RGC-5s with staurosporine (SS). Control, carrier solution treated RGC-5s at 24 (A), 48 (B), or 72 (C) hours after plating had simple, flat morphologies. In contrast, differentiation of RGC-5s with 1 μM SS for 1 hour induced RGC-5s to become highly branched cells 24 (D), 48 (E), or 72 (F) hours post-treatment.
Figure 2.
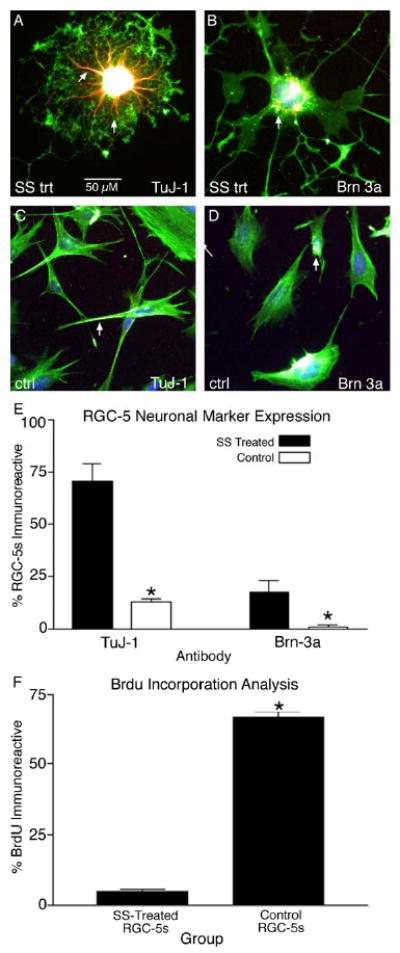
Staurosporine differentiation upregulates neuronal marker expression in RGC-5s. Differentiated RGC-5s were highly immunoreactive for the phenotypic neuronal marker TuJ-1 in the processes (A, arrows) and cell body. In addition, SS differentiated RGC-5s were immunoreactive for the RGC transcription factor Brn3a (B, arrow). In contrast, undifferentiated, control RGC-5s expressed TuJ-1 (C, arrow) and Brn3a (D, arrow) at relatively low levels. Quantification of the number of SS-treated RGC-5s expressing phenotypic markers revealed a significant increase from 13.0 ± 1.3 % (± S.E.M.) in control RGC-5s to 70.26 ± 8.37 % in SS differentiated RGC-5s for TuJ-1 (E, p < 0.0001). This trend was also observed for Brn3a expression patterns, with a significant increase from 1.06 ± 0.82 % of control RGC-5s expressing Brn3a to 17.36 ± 5.57 % of differentiated RGC-5s immunoreactive for Brn3a (E, p < 0.05). Finally, BrdU analysis revealed that only a small proportion of the differentiated RGC-5s incorporated BrdU (4.62 ± 0.76 %), a significant decrease from 66.7 ± 1.91 % of undifferentiated RGC-5s, (F, p < 0.0001).
Engineering MSCs Ex Vivo
Engineering mesenchymal stem cells (MSCs) ex vivo is an important strategy for a cell-based delivery of neurotrophic factors to compromised retinal neurons. Mesenchymal stem cells were engineered to produce and secrete BDNF using lentiviral vectors. Immunocytochemical analysis of engineered MSCs revealed modest IR for BDNF in control MSCs (GFP-MSCs) within the nucleus and cell body (Fig. 3 A, B). In contrast, robust BDNF-IR was observed in the perinuclear region and cell body of LV-BDNF transduced MSCs (BDNF-MSCs), demonstrating the effectiveness of the lentiviral constructs in transducing these cells (Fig. 3 C, D). We examined the cellular localization of the high affinity BDNF receptor, TrkB, in control and SS differentiated RGC-5s to determine if RGC-5s express the receptor necessary for BDNF to elicit a response. Control RGC-5s had diffuse TrkB-IR throughout the entire cell (Fig. 3 E, F). However, upon SS-differentiation RGC-5s displayed intense TrkB-IR in the cell body and base of the processes (Fig. 3 G, H).
Figure 3.
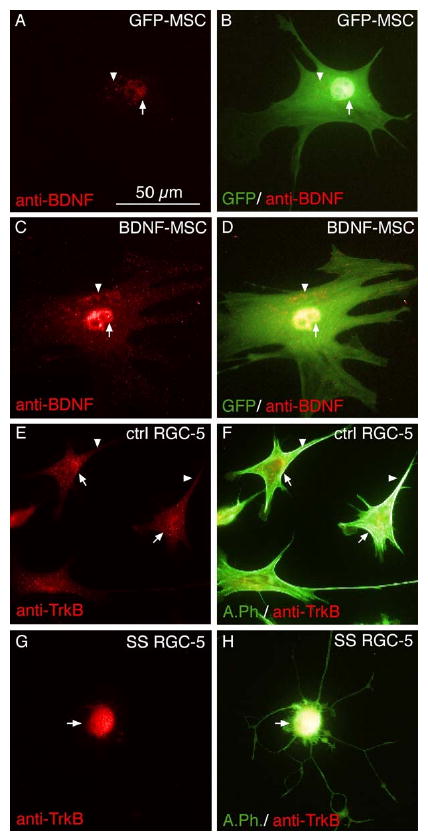
Successful transduction of mesenchymal stem cells (MSCs) using lentiviral vectors. MSCs were transduced with lentiviral vectors encoding green fluorescent protein (GFP-MSC; A, B) or brain-derived neurotrophic factor (BDNF-MSC; C, D). Transduced cells were probed with anti-BDNF antibodies. A low level of BDNF was detected in the peri-nuclear region (A, arrow), and cell body (A, arrowhead) of GFP-MSC controls. This control population of MSCs had high expression of GFP (B). Merged image of GFP and BDNF immunoreactivity (B) demonstrates the cellular localization of BDNF. In contrast to control cells, BDNF-MSCs had increased levels of BDNF-IR in the perinuclear area (C, arrows), in addition to the cell body (C, arrowhead). Merged images also revealed high levels of GFP expression (D), which demonstrates the localization of BDNF-IR in the cell. Control and differentiated RGC-5s were examined to determine if the high affinity BDNF receptor TrkB was present. Undifferentiated RGC-5s had high expression of TrkB in the perinuclear region (E, arrows), and in distal parts of the cell (E, arrowhead). TrkB immunoreactivity (IR), merged with the Alexa-488 phalloidin stained cytoskeleton demonstrates the cellular localization of TrkB (F). Differentiated RGC-5s displayed localized TrkB-IR in the cell body (G, arrow), as compared to undifferentiated RGC-5s. Merged image (H) demonstrates cellular localization of TrkB in differentiated RGC-5s.
To determine whether the secreted BDNF was bioactive, the length and density of neurites emanating from E17 dorsal root ganglion (DRG) cultures were judged by blind observers following treatment with experimental media. Upon treatment with normal MSC growth medium the DRG displayed modest neurite outgrowth (Fig. 4 A, E), which was also observed for DRG exposed to GFP-MSC conditioned media (CM) (Fig. 4 B, E). No significant difference (Fig. 4 E, p > 0.05) was observed in terms of DRG neurite outgrowth scores when comparing the two control conditions, MSC media (1.87 ± 0.27) and GFP-MSC CM (1.70 ± 0.24). In contrast, DRG that were grown in the presence of BDNF-MSC CM (Fig. 4 C) and DRG treated with 100 ng of rhBDNF (Fig. 4 D) displayed extensive neurite outgrowth. Although DRG treated with BDNF-MSC CM (3.02 ± 0.31), or rhBDNF (3.49 ± 0.24) did not differ in the extent of neurite outgrowth (Fig. 4 E, p > 0.05), these two treatment groups displayed significantly more neurite outgrowth than DRG treated with MSC media alone, or GFP-MSC CM (Fig. 4 E, p < 0.05). These results demonstrate that MSCs engineered with a lentiviral BDNF vector produced and released bioactive BDNF that stimulated extensive neurite outgrowth. Furthermore, SS differentiated RGC-5s expressed the necessary receptor to mediate BDNF signaling.
Figure 4.
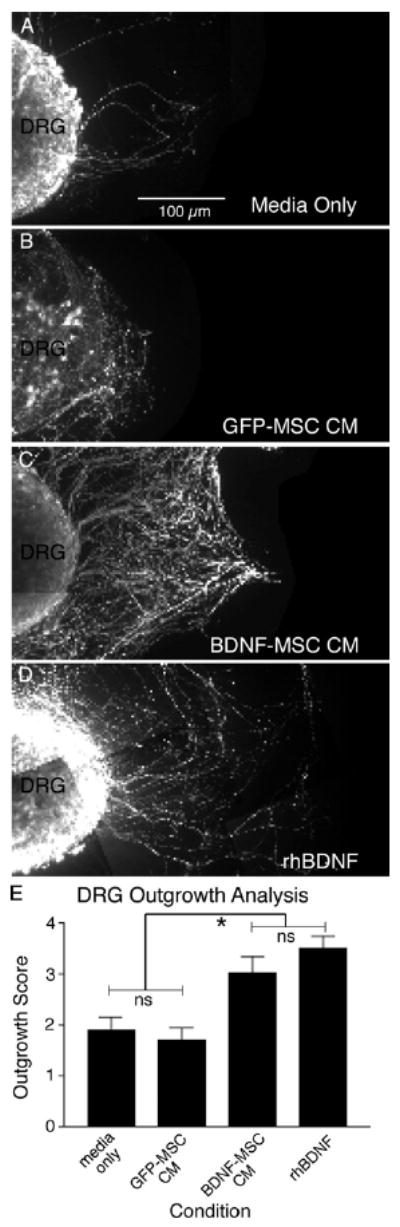
MSCs engineered with a lentiviral-BDNF vector produced bioactive BDNF. Embryonic dorsal root ganglia (DRG) bioassay examining neurite outgrowth and density. When cultured with standard MSC growth media the DRG elaborated few neurites (A). Likewise, GFP-MSC conditioned media (CM) did not induce significant neurite outgrowth (B). However, when DRG were cultured in either BDNF-MSC CM (C), or media containing rhBDNF (D), significant neurite outgrowth was observed. Qualitative analysis using naïve observers (E) revealed no significant difference in the neurite outgrowth scores for DRG grown in media, or GFP-MSC conditioned media (p > 0.05). No significant difference was observed in neurite outgrowth scores for DRG treated with BDNF-MSC CM, or rhBDNF. However, significantly greater neurite outgrowth scores in DRG treated with BDNF-MSC CM or rhBDNF was observed when compared to DRG grown in either GFP-MSC CM or media alone (p < 0.05).
Glutamate- and ROS-induced RGC-5 Death
We sought to determine if differentiated RGC-5s would respond to the cellular stressors glutamate and hydrogen peroxide, which have been implicated in RGC death in glaucomatous eyes. We have shown that glutamate concentrations greater than 800 μM induced significant death of RGC-5, as compared to RGC-5s not exposed to glutamate (Fig. 5 A, p < 0.01). For subsequent experiments 1 mM glutamate was used as only 56.0 ± 7.92% (± S.E.M.) of RGC-5s were present after treatment. In addition, we have shown H2O2 to be a potent cellular stressor, with concentrations greater than 10 μM inducing significant RGC-5 death (Fig. 5 B, p < 0.001). For subsequent experiments 10 μM H2O2 was used as only 48.90 ± 4.58% of RGC-5s were present, as compared to carrier-solution treated cells. In order to determine if BDNF could prevent loss of RGC-5s following treatment with glutamate or H2O2, rhBDNF was applied to cultures throughout the period of stressor insult, and once after treatment. It was determined that rhBDNF concentrations greater than 100 ng/ mL were sufficient to attenuate glutamate mediated RGC-5 loss, protecting 87.77 ± 7.42% of RGC-5s, a significant increase from 60.60 ± 3.13% of RGC-5s present in cultures treated with glutamate only (Fig. 5 C, p < 0.05). Addition of rhBDNF also prevented RGC-5 cell loss due to H2O2 treatment at concentrations greater than 100 ng/ mL. This application of rhBDNF showed 70.0 ± 14.81% of RGC-5s present, with only 48.88 ± 4.58% of cells present in H2O2 treated cultures (Fig. 5 D, p < 0.05). Cell death of RGC-5s was verified by application of Sytox following treatment with glutamate or H2O2. We have shown that 14.27 ± 3.01% of control RGC-5s were labeled with Sytox, while 48.61 ± 7.02 % and 53.99 ± 5.12 % of H2O2 and glutamate treated cells were labeled, respectively (Fig. 5 E, p < 0.0001). Taken together these results demonstrate cell death can be induced in RGC-5s with glutamate and H2O2, factors implicated in secondary degeneration of ganglion cells in glaucomatous eyes. Furthermore, we have demonstrated that application of rhBDNF during the time of cellular insult can attenuate RGC-5 death induced by glutamate and H2O2.
Figure 5.
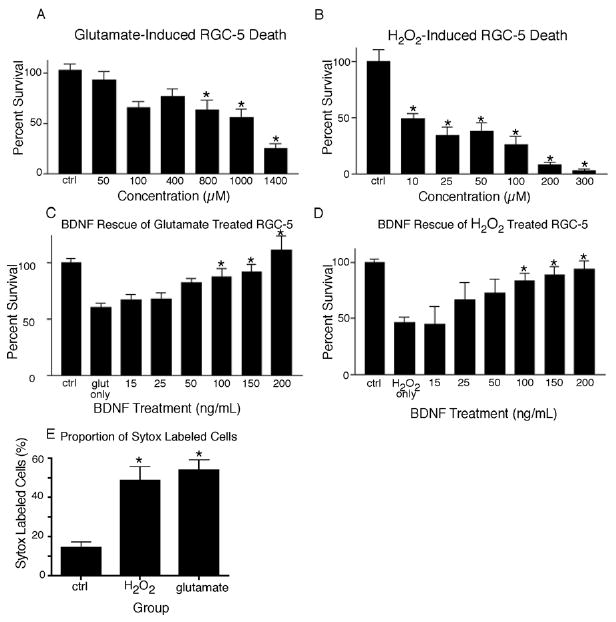
Differentiated RGC-5s are susceptible to glutamate and H2O2 induced cell death that can be prevented with rhBDNF. Significant cell death can be induced in SS differentiated RGC-5s with glutamate treatment at concentrations greater than 800 μM, as compared to vehicle treated, control RGC-5s (A, p < 0.05). H2O2 also proved to be a potent inducer of significant cell death at concentrations of 10 μM and greater (B, p < 0.05). Cell death induced by glutamate (C), or H2O2 (D) could be attenuated by addition of rhBDNF at concentrations of 100 ng/ mL and greater (p < 0.05), as compared to RGC-5s treated with glutamate or H2O2 only. Cell death was confirmed in these experiments by application of Sytox, which labels dying cells. Control RGC-5s had significantly less Sytox labeling than H2O2 or glutamate treated cells (E, p < 0.0001).
Delivery of BDNF to RGC-5s from Genetically Modified MSCs
Finally, we sought to determine if BDNF released from MSCs was able to protect SS-differentiated RGC-5s following treatment with glutamate or H2O2. We have shown that after glutamate treatment 54.0 ± 5.99% (± S.E.M.) of RGC-5s remained, a significant decrease from non-treated RGC-5s (Fig. 6 A-C, p < 0.001). We have also demonstrated significant protection, with 81 ± 3.20% of RGC-5s present after treatment with rhBDNF (Fig. 6 A, p < 0.001). Delivery of BDNF from BDNF-MSC co-cultures also proved very effective in attenuating RGC-5 cell death, with 80 ± 5.40% of RGC-5s present following glutamate treatment (Fig. 6 A, D), which was significantly more than the 57.29 ± 1.89% remaining in RGC-5 co-cultures with MSCs transduced with only the LV-GFP vector (Fig. 6 A, E, p < 0.01). Cellular based rescue experiments were also carried out using H2O2 to induce RGC-5 death. We observed a significant decrease in remaining RGC-5s as compared to control cells when RGC-5s were exposed to H2O2 (Fig. 7 A-C, p < 0.001), as previously shown. Moreover, addition of rhBDNF, as well as co-culturing with BDNF-MSCs produced a protection of RGC-5s, with 68.67 ± 3.33% and 65.6 ± 3.47% of cells remaining, respectively (Fig. 7 A, D). This was significantly greater than the 46.0 ± 4.20% of cells remaining in GFP-MSC co-cultures (Fig. 7 A, E, p < 0.01). Taken together these results demonstrate that MSCs genetically modified for production of BDNF are reliable cellular vehicles for delivering neuroprotective substances to compromised cells.
Figure 6.
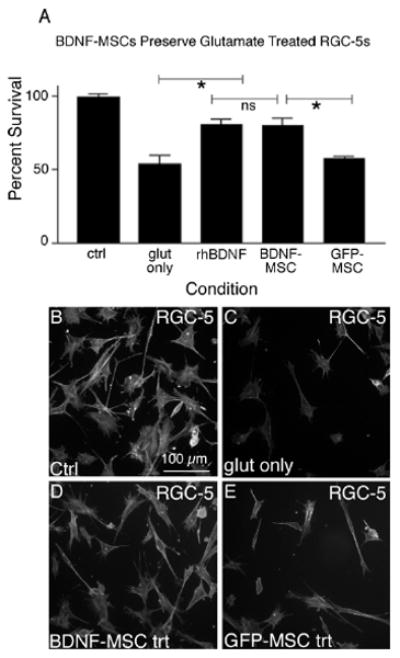
BDNF released from MSCs can protect RGC-5s from glutamate-mediated death. Following treatment with 1 mM glutamate, only 57.29 ± 1.89 % of RGC-5s remained. When glutamate treated RGC-5s were grown in the presence of BDNF-MSCs or rhBDNF significantly more cells were present, compared to cultures not exposed to BDNF (A, p < 0.05). We have demonstrated GFP-MSCs were not able to prevent RGC-5 cell loss following glutamate treatment. Representative images are shown for control RGC-5s (B), RGC-5s exposed to 1 mM glutamate (C), and RGC-5s exposed to 1 mM glutamate and grown in the presence of BDNF-MSCs (D) or GFP-MSCs (E).
Figure 7.
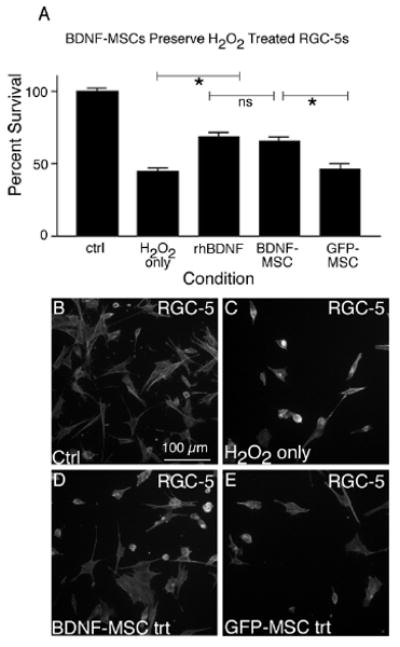
BDNF released from MSCs can protect RGC-5s from H2O2 mediated cell death. Following treatment with 10 μM H2O2, only 44.5 ± 2.2 % of RGC-5s were present, as compared to vehicle-only treated cultures (A). When H2O2 treated RGC-5s were grown in the presence of BDNF-MSCs or rhBDNF, a significantly greater percentage of cells were present, as compared to RGC-5s grown in the presence of GFP-MSCs (p < 0.001). We have demonstrated less RGC-5s present when grown in the presence of BDNF-MSCs, as compared to vehicle and GFP-exposed cells. Representative images are shown for control RGC-5s (B), RGC-5s exposed to 10 μM H2O2 (C), and RGC-5s exposed to H2O2 and grown in the presence of BDNF-MSCs (D) or GFP-MSCs (E).
Discussion
Staurosporine-differentiated RGC-5s develop into highly branched cells that morphologically resemble cultured retinal ganglion cells, consistent with findings presented by Frassetto et al15. Under these differentiation conditions few RGC-5s incorporated BrdU, indicating a significant decrease in cell proliferation. We have shown a morphological differentiation of RGC-5s from simple cells into highly branched, complex cells consistent with mature RGCs. In addition, greater numbers of RGC-5s were immunolabeled with the neuronal markers TuJ-1 and Brn3a following differentiation. We have also shown a shift in the cellular distribution to the cell body for the high affinity BDNF receptor, TrkB. This receptor is essential for correct retinal ganglion cell development18, 19 and survival20, 21, and we propose that the expression of this receptor by RGC-5s indicates these cells are assuming a more mature phenotype following differentiation.
We investigated the potential use of the SS-differentiated RGC-5 cells as an in vitro system to model ganglion cell death accompanying glaucoma. In these studies the cellular stressors glutamate and H2O2, which have been implicated in glaucomatous secondary degeneration of RGCs1-5, 7, 8, induce RGC-5 cell death, as compared to vehicle-treated RGC-5s. Previous experiments using undifferentiated RGC-5s have modeled the apoptotic response of RGC-5s to serum deprivation11, oxidative stress12, glutamate excitotoxicity13, and hydrostatic pressure14. While these earlier experiments have demonstrated that undifferentiated RGC-5s are a useful model for ganglion cells, it is not clear if SS-differentiated RGC-5s would respond in a similar or different fashion. We have demonstrated SS-differentiated RGC-5s can express proteins consistent with differentiated ganglion cells and thus can serve as a non-mitotic model of in vivo RGCs. Furthermore, we have demonstrated a dramatic decrease in numbers of differentiated RGC-5s after treatment with the cellular stressors glutamate and H2O2. In addition to susceptibility to the cellular stressors glutamate and H2O2, RGC-5s have also been shown to express complement proteins48, and have mitochondrial abnormalities49, which are each prevalent in eyes with optic neuropathies50, 51.
In these experiments we have demonstrated that rhBDNF can prevent SS-differentiated RGC-5 loss mediated by glutamate and H2O2. BDNF has been shown to prevent oxidative damage in retinal52, brain53 and auditory54 neurons, which is hypothesized to occur by secondary upregulation of other neuroprotective factors52 or by increasing cellular GSH to combat ROS damage54. BDNF has clearly been linked as a candidate neuroprotectant to eyes with glaucomatous optic neuropathies. Additionally, BDNF has been shown to be neuroprotective in primary cultured RGCs55, and when delivered via engineered astrocytes56. In vivo experiments have demonstrated that transfected Müller glia57 are neuroprotective in models of optic nerve axotomy. Furthermore, intracerebral injections of BDNF-producing lentiviral vectors in excitotoxic models of brain damage were neuroprotective58. We have shown similar neuroprotection in SS-differentiated RGC-5 cells, demonstrating their ability to model RGCs.
BDNF and its receptor TrkB are expressed by ganglion cells in the neural retina59, and BDNF is retrogradely transported to the retina18, 60, 61. It has previously been shown that in glaucomatous eyes the retrograde transport of BDNF from the superior colliculus via the RGC axons is severely diminished62, 63. We hypothesize that prolonged delivery of BDNF to glaucomatous eyes may prevent RGC degeneration, as the loss of trophic support is likely one of the causes of neurodegeneration. The results we have presented here are encouraging, as BDNF delivered from engineered MSCs was able to attenuate the loss of the RGC-5s following glutamate or H2O2 insults. These results are consistent with previous studies that demonstrate lentiviral transduced MSCs are able to survive and express transgenes following transplantation64. While our results suggest this is a feasible approach in the retina, further in vivo experimentation needs to be conducted.
We have demonstrated that MSCs are a useful cellular delivery vehicle capable of providing neuroprotection to compromised RGC-5s by release of BDNF. We feel that MSC-based delivery systems have the potential for use in clinical settings as MSCs are easily engineered, and can be used in an autologous fashion, which makes immune-mediated rejection of the transplanted cells less likely. Additionally, MSCs are easily isolated in large quantities as compared to adult CNS progenitors, and bypass the moral and ethical concerns typically associated with embryonic, fetal, and neonatal stem/progenitor cell isolation. Transplantation of genetically modified MSCs to produce neurotrophic growth factors may be a useful strategy for treatment of different forms of optic neuropathies in the future.
Acknowledgments
The authors would like to acknowledge Drs. Neeraj Agarwal (University of North Texas Health Sciences Center, for the kind gift of the RGC-5s) and Virginia Lee (University of Pennsylvania, for the gift of the anti-neurofilament antibody). We would also like to thank Roxanne Reger and Darwin Prockop (Center For Gene Therapy, Tulane University) for their help and advice with the mesenchymal stem cells. The MSCs employed in this work were provided by the Tulane Center for Gene Therapy through a grant from NCRR of the NIH, Grant # P40RR017447. Finally, we would like to thank members of the Sakaguchi lab for their help with the preparation of this manuscript.
Funding: NIGMS RO1 GM072005-01; The Glaucoma Foundation, New York; Fight for Sight, The Stem Cell Research Fund, Biosciences Alliance Initiative from the State of Iowa, The Miami Project to Cure Paralysis
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- 1.Nickells RT. From ocular hypertension to ganglion cell death: a theoretical sequence of events leading to glaucoma. Canadian Journal of Ophthalmology. 2007;42:278–287. [PubMed] [Google Scholar]
- 2.Casson RJ. Possible role of excitotoxicity in the pathogenesis of glaucoma. Clinical and Experimental Ophthalmology. 2006;34:54–63. doi: 10.1111/j.1442-9071.2006.01146.x. [DOI] [PubMed] [Google Scholar]
- 3.Luo X, Heidinger V, Picaud S, et al. Selective excitotoxic degeneration of adult pig retinal ganglion cells in vitro. Investigative Ophthalmology and Visual Science. 2001;42:1096–1106. [PubMed] [Google Scholar]
- 4.Nucci C, Tartaglione R, Rombola L, Morrone LA, Fazzi E, Bagetta G. Neurochemical evidence to implicate elevated glutamate in the mechanisms of high intraocular pressure (IOP)-induced retinal ganglion cell death in rat. NeuroToxicology. 2005;26:935–941. doi: 10.1016/j.neuro.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 5.Tezel G, Yang Z, Cai J. Proteomic identification of oxidatively modified retinal proteins in a chronic pressure-induced rat model of glaucoma. Investigative Ophthalmology and Visual Science. 2005;46:3177–3187. doi: 10.1167/iovs.05-0208. [DOI] [PubMed] [Google Scholar]
- 6.Tezel G. Oxidative stress in glaucomatous neurodegeneration: Mechanisms and consequences. Progress in Retinal and Eye Research. 2006;25:490–513. doi: 10.1016/j.preteyeres.2006.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Izzotti A, Bagnis A, Sacca SC. The role of oxidative stress in glaucoma. Mutation Research. 2006;612:105–114. doi: 10.1016/j.mrrev.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 8.Ferreira S, Lerner SF, Brunzini R, Evelson PA, Llesuy SF. Oxidative stress markers in aqueous humor of glaucoma patients. American Journal of Ophthalmology. 2004;137:62–69. doi: 10.1016/s0002-9394(03)00788-8. [DOI] [PubMed] [Google Scholar]
- 9.Morrison JC. Elevated intraocular pressue and optic nerve injury models in the rat. Journal of Glaucoma. 2005;14:315–317. doi: 10.1097/01.ijg.0000169410.09258.bf. [DOI] [PubMed] [Google Scholar]
- 10.Krishnamoorthy RR, Agarwal P, Prasanna G, et al. Characterization of a transformed rat retinal ganglion cell line. Molecular Brain Research. 2001;86:1–12. doi: 10.1016/s0169-328x(00)00224-2. [DOI] [PubMed] [Google Scholar]
- 11.Charles I, Khalyfa A, Kumar DM, et al. Serum deprivation induces apoptotic cell death of transformed rat retinal ganglion cells via mitochondrial signaling pathways. Investigative Ophthalmology and Visual Science. 2005;46:1330–1338. doi: 10.1167/iovs.04-0363. [DOI] [PubMed] [Google Scholar]
- 12.Maher P, Hanneken A. The molecular basis of oxidative stress-induced cell death in an immortalized retinal ganglion cell line. Investigative Ophthalmology and Visual Science. 2005;46:749–757. doi: 10.1167/iovs.04-0883. [DOI] [PubMed] [Google Scholar]
- 13.Aoun P, Simpkins JW, Agarwal N. Role of PPAR-gamma ligands in neuroprotection agains glutamate-induced cytoxicity in retinal ganglion cells. Investigative Ophthalmology and Visual Science. 2003;44:2999–3004. doi: 10.1167/iovs.02-1060. [DOI] [PubMed] [Google Scholar]
- 14.Agar A, Li S, Agarwal N, Coroneo MT, Hill MA. Retinal ganglion cell line apoptosis induced by hydrostatic pressure. Brain Research. 2006;1086:191–200. doi: 10.1016/j.brainres.2006.02.061. [DOI] [PubMed] [Google Scholar]
- 15.Frassetto LJ, Schlieve CR, Lieven CJ, et al. Kinase-dependant differentiation of a retinal ganglion cell precursor. Investigative Ophthalmology and Visual Science. 2006;47:427–438. doi: 10.1167/iovs.05-0340. [DOI] [PubMed] [Google Scholar]
- 16.Mowla SJ, Farhadi HF, Pareek S, et al. Biosynthesis and Post-translational processing of the precursor to brain-derived neurotrophic factor. Journal of Biological Chemistry. 2001;276:12660–12666. doi: 10.1074/jbc.M008104200. [DOI] [PubMed] [Google Scholar]
- 17.Klein R, Parada LF, Coulier F, Barbacid M. trkB, a novel tyrosine protein kinase receptor expressed during mouse neural development. EMBO Journal. 1989;8:3701–3709. doi: 10.1002/j.1460-2075.1989.tb08545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma YT, Hsieh T, Forbes ME, Johnson JE, Frost DO. BDNF injected into the superior colliculus reduces developmental retinal ganglion cell death. Journal of Neuroscience. 1998;18:2097–2107. doi: 10.1523/JNEUROSCI.18-06-02097.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cohen-Cory S, Escandon E, Fraser SE. The cellular patterns of BDNF and trkB expression suggest multiple roles for BDNF during Xenopus visual system development. Developmental Biology. 1996;179:102–115. doi: 10.1006/dbio.1996.0244. [DOI] [PubMed] [Google Scholar]
- 20.Johnson JE, Barde YA, Schwab M, Thoenen H. Brain-derived neurotrophic factor supports the survival of cultured rat retinal ganglion cells. Journal of Neuroscience. 1986;6:3031–3038. doi: 10.1523/JNEUROSCI.06-10-03031.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meyer-Franke A, Kaplan MR, Pfreiger FW, Barres BA. Characterization of the signaling interactions that promote the survival and growth of developing retinal ganglion cells in culture. Neuron. 1995;15:805–819. doi: 10.1016/0896-6273(95)90172-8. [DOI] [PubMed] [Google Scholar]
- 22.Mansour-Robaey S, Clarke DB, Wang YC, Bray GM, Aguayo AJ. Effects of ocular injury and administration of brain-derived neurotrophic factor on survival and regrowth of axotomized retinal ganglion cells. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:1632–1636. doi: 10.1073/pnas.91.5.1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ko ML, Hu DN, Ritch R, Sharma SC. The combined effect of brain-derived neurotrophic factor and a free radical scavenger in experimental glaucoma. Investigative Ophthalmology and Visual Science. 2000;41:2967–2971. [PubMed] [Google Scholar]
- 24.Ikeda K, Tanihara H, Honda Y, Tatsuno T, Nogushi H, Nakayama C. BDNF attenuates retinal cell death caused by chemically induced hypoxia in rats. Investigative Ophthalmology and Visual Science. 1999;40:2130–2140. [PubMed] [Google Scholar]
- 25.Weibel D, Kreutzberg GW, Schwab ME. Brain-derived neurotrophic factor (BDNF) prevents lesion-induced axonal die-back in young rat optic nerve. Brain Research. 1995;679:249–254. doi: 10.1016/0006-8993(95)00238-l. [DOI] [PubMed] [Google Scholar]
- 26.Pernet V, Di-Polo A. Synergistic action of brain-derived neurotrophic factor and lens injury promotes retinal ganglion cell survival, but leads to optic nerve dystrophy in vivo. Brain. 2006;129:1014–1026. doi: 10.1093/brain/awl015. [DOI] [PubMed] [Google Scholar]
- 27.Peinado-Ramon P, Salvador M, Villegas-Perez MP, Vidal-Sanz M. Effects of axotomy and intraocular administration of NT-4, NT-3, and Brain-derived Neurotrophic factor on the survival of adult rat retinal ganglion cells. Investigative Ophthalmology and Visual Science. 1996;37:489–500. [PubMed] [Google Scholar]
- 28.Watanabe M, Tokita Y, Kato M, Fukuda Y. Intravitreal injections of neurotrophic factors and forskolin enhance survival and axonal regeneration of axotomized beta ganglion cells in cat retina. Neuroscience. 2003;116:733–742. doi: 10.1016/s0306-4522(02)00562-6. [DOI] [PubMed] [Google Scholar]
- 29.Martin KR, Quigley HA, Zack DJ, Levkovitch-Verbin H. Gene therapy with brain-derived neurotrophic factor as a protection: retinal ganglion cells in a rat model of glaucoma. Investigative Ophthalmology and Visual Science. 2003;44:4357–4365. doi: 10.1167/iovs.02-1332. [DOI] [PubMed] [Google Scholar]
- 30.Di-Polo A, Aigner LJ, Dunn RJ, Bray GM, Aguayo AJ. Prolonged delivery of brain-derived neurotrophic factor by adenovirus-infected Muller cells temporarily rescues injured retinal ganglion cells. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:3978–3983. doi: 10.1073/pnas.95.7.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Colter DC, Class R, DiGirolamo CM, Prockop DJ. Rapid expansion of recycling stem cells in cultures of plastic-adherent cells from human bone marrow. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:3213–3218. doi: 10.1073/pnas.070034097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Herzog EL, Krause DS. Plasticity of marrow-derived stem cells. Blood. 2003;102:3483–3493. doi: 10.1182/blood-2003-05-1664. [DOI] [PubMed] [Google Scholar]
- 33.Kicic A, Shen WY, Wilson AS, Constable IJ, Robertson T, Rakoczy PE. Differentiation of marrow stromal cells into photoreceptors in the rat eye. Journal of Neuroscience. 2003;23:7742–7749. doi: 10.1523/JNEUROSCI.23-21-07742.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Azizi SA, Stokes D, Augelli BJ, DiGirolamo C, Prockop DJ. Engraftment and migration of human bone marrow stromal cells implanted in the brains of albino rats -similarities to astrocyte grafts. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:3908–3913. doi: 10.1073/pnas.95.7.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kopen GC, Prockop DJ, Phinney DG. Marrow stromal cells migrate throughout forebrain and cerebellum, and they differentite into astrocytes after injection into neonatal mouse brains. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:10711–10716. doi: 10.1073/pnas.96.19.10711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brazelton TR, Rossi FM, Keshet GL, Blau HM. From marrow to brain: expression of neuronal phenotypes in adult mice. Science. 2000;290:1775–1782. doi: 10.1126/science.290.5497.1775. [DOI] [PubMed] [Google Scholar]
- 37.Jiang Y, Jahagidar BN, Reinhardt RL, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 38.Sanchez-Ramos J, Song S, Cardoza-Pelaez F, et al. Adult bone marrow stromal cells differentiate into neural cells in vitro. Experimental Neurology. 2000;164:247–256. doi: 10.1006/exnr.2000.7389. [DOI] [PubMed] [Google Scholar]
- 39.Woodbury D, Schwarz EJ, Prockop DJ, Black IB. Adult rat and human bone marrow stromal cells differentiate into neurons. Journal of Neuroscience Research. 2000;61:364–370. doi: 10.1002/1097-4547(20000815)61:4<364::AID-JNR2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 40.Jiang Y, Henderson D, Blackstad M, Chen A, Miller RF, Verfaille CM. Neuroectodermal differentiation from mouse multipotent adult progenitor cells. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:11854–11860. doi: 10.1073/pnas.1834196100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wislet-Gendebien S, Hans G, Leprince P, Rigo JM, Moonen G, Rogister B. Plasticity of cultured mesenchymal stem cells: swith from nestin-positive to excitable neuron-like phenotype. Stem Cells. 2005;23 doi: 10.1634/stemcells.2004-0149. [DOI] [PubMed] [Google Scholar]
- 42.Otani A, Kinder K, Ewalt K, Otero FJ, Schimmel P, Friedlander M. Bone marrow-derived stem cells target retinal astrocytes and can promote or inhibit retinal angiogenesis. Nature Medicine. 2002;8:1004–1010. doi: 10.1038/nm744. [DOI] [PubMed] [Google Scholar]
- 43.Otani A, Dorrell MI, Kinder K, et al. Rescue of retinal degeneration by intravitreally injected adult bone marrow-deprived lineage-negative hematopoietic stem cells. Journal of Clinical Investigation. 2004;114:765–774. doi: 10.1172/JCI21686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Anderson MG, Libby RT, Gould DB, Smith RS, John SW. High-dose radiation with bone marrow transfer prevets neurodegeneration in an inherited glaucoma. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:4566–4571. doi: 10.1073/pnas.0407357102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lindsay RM. Nerve Growth Factors (NGF, BDNF) Enhance Axonal Regeneration buy are not Required for Survival of Adult Sensory Neurons. Journal of Neuroscience. 1988;8:2394–2405. doi: 10.1523/JNEUROSCI.08-07-02394.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tuttle R, Matthew WD. Neurotrophins affect the pattern of DRG neurite growth in a bioassay that presents a choice of CNS and PNS substrates. Development. 1995;121:1301–1309. doi: 10.1242/dev.121.5.1301. [DOI] [PubMed] [Google Scholar]
- 47.Klocker N, Kermer P, Weishaupt JH, Labes M, Ankerhold R, Bahr M. Brain-derived neurotrophic factor-mediated neuroprotection of adult rat retinal ganglion cells in vivo does not exclusively depend on phosphatidy-inositol-3′-kinase/protein kinase B signaling. Journal of Neuroscience. 2000;20:6962–6967. doi: 10.1523/JNEUROSCI.20-18-06962.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Khalyfa A, Chlon T, Qiang H, Agarwal N, Cooper NG. Microarray reveals complement components are regulated in the serum-deprived rat retinal ganglion cell line. Molecular Vision. 2007;13:293–308. [PMC free article] [PubMed] [Google Scholar]
- 49.Ju WK, Liu Q, Kim KY, et al. Elevated hydrostatic pressure triggers mitochondrial fission and decreases cellular ATP in differentiated RGC-5 cells. Investigative Ophthalmology and Visual Science. 2007;48:2145–2151. doi: 10.1167/iovs.06-0573. [DOI] [PubMed] [Google Scholar]
- 50.Keuhn MH, Kim CY, Ostojic J, et al. Retinal synthesis and deposition of complement components induced by ocular hypertension. Experimental Eye Research. 2006;83:620–628. doi: 10.1016/j.exer.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 51.Abu-Amero KK, Morales J, Bosley TM. Mitochondrial abnormalities in patients with primary open-angle glaucoma. Investigative Ophthalmology and Visual Science. 2006;47:2533–2541. doi: 10.1167/iovs.05-1639. [DOI] [PubMed] [Google Scholar]
- 52.Okoye G, Zimmer J, Sung J, et al. Increased expression of brain-derived neurotrophic factor preserves retinal function and slows cell death from rhodopsin mutation or oxidative damage. J Neurosci. 2003;23:4164–4172. doi: 10.1523/JNEUROSCI.23-10-04164.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhao X, Rebeck GW, Hoe HS, Andrews PM. Tarenflurbil protection from cytotoxicity is associated with an upregulation of neurotrophins. J Alzheimers Dis. 2008;15:397–407. doi: 10.3233/jad-2008-15306. [DOI] [PubMed] [Google Scholar]
- 54.Gabaizadeh R, Staecker H, Liu W, Van De Water TR. BDNF protection of auditory neurons from cisplatin involves changes in intracellular levels of both reactive oxygen species and glutathione. Brain Res Mol Brain Res. 1997;50:71–78. doi: 10.1016/s0169-328x(97)00173-3. [DOI] [PubMed] [Google Scholar]
- 55.Yamasaki M, Mishima HK, Yamashita H, et al. Neuroprotective effects of erythropoietin on glutamate and nitric oxide toxicity in primary cultured retinal ganglion cells. Brain Research. 2005;1050:15–26. doi: 10.1016/j.brainres.2005.05.037. [DOI] [PubMed] [Google Scholar]
- 56.Castillo B, Jr, del Cerro M, Breakefield XO, et al. Retinal ganglion cell survival is promoted by genetically modified astrocytes designed to secrete brain-derived neurotrophic factor (BDNF) Brain Res. 1994;647:30–36. doi: 10.1016/0006-8993(94)91395-1. [DOI] [PubMed] [Google Scholar]
- 57.Di Polo A, Aigner LJ, Dunn RJ, Bray GM, Aguayo AJ. Prolonged delivery of brain-derived neurotrophic factor by adenovirus-infected Muller cells temporarily rescues injured retinal ganglion cells. Proc Natl Acad Sci U S A. 1998;95:3978–3983. doi: 10.1073/pnas.95.7.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bemelmans AP, Husson I, Jaquet M, Mallet J, Kosofsky BE, Gressens P. Lentiviral-mediated gene transfer of brain-derived neurotrophic factor is neuroprotective in a mouse model of neonatal excitotoxic challenge. J Neurosci Res. 2006;83:50–60. doi: 10.1002/jnr.20704. [DOI] [PubMed] [Google Scholar]
- 59.Vecino E, Garcia-Grespo D, Garcia M, Martinez-Millan L, Sharma SC, Carrascal E. Rat retinal ganglion cells co-express brain derived neurotrophic factor (BDNF) and its receptor TrkB. Vision Research. 2002;42:151–157. doi: 10.1016/s0042-6989(01)00251-6. [DOI] [PubMed] [Google Scholar]
- 60.Herzog KH, von-Bartheld CS. Contributions of the optic tectum and the retina as sources of brain-derived neurotrophic factor for retinal ganglion cells in the chick embryo. Journal of Neuroscience. 1998;18:2891–2906. doi: 10.1523/JNEUROSCI.18-08-02891.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ginty DD, Segal RA. Retrograde neurotrophin signaling: Trk-ing along the axon. Current Opinion in Neurobiology. 2002;12:268–274. doi: 10.1016/s0959-4388(02)00326-4. [DOI] [PubMed] [Google Scholar]
- 62.Quigley HA, McKinnon S, Zack DJ, et al. Retrograde axonal transport of BDNF in retinal ganglion cells is blocked by acute IOP elevation in rats. Investigative Ophthalmology and Visual Science. 2000;41:3460–3466. [PubMed] [Google Scholar]
- 63.Pease ME, McKinnon SJ, Quigley HA, Kerrigan-Baumrind LA, Zack DJ. Obstructed axonal transport of BDNF and its receptor TrkB in experimental glaucoma. Investigative Ophthalmology and Visual Science. 2000;41:764–774. [PubMed] [Google Scholar]
- 64.Sgodda M, Aurich H, Kleist S, et al. Hepatocyte differentiation of mesenchymal stem cells from rat peritoneal adipose tissue in vitro and in vivo. Exp Cell Res. 2007;313:2875–2886. doi: 10.1016/j.yexcr.2007.05.020. [DOI] [PubMed] [Google Scholar]


