Abstract
Objective
Numerous studies have implicated the hippocampus and prefrontal cortex in schizophrenia. However, precisely which subregions of the hippocampus and prefrontal cortex are abnormal remain unknown. Our study goal was to investigate structure of the anterior hippocampus, posterior hippocampus, dorsolateral prefrontal cortex (DLPFC), and orbitofrontal cortex (OFC) simultaneously in thirty-eight patients with schizophrenia and twenty-nine controls to determine which of these subregions are abnormal in schizophrenia. As an exploratory study goal, we investigated the relation of neurocognition to brain structure in schizophrenia patients.
Method
We generated detailed structural magnetic resonance imaging data and compared hippocampal and prefrontal subregional structural brain volumes between schizophrenia and control groups. We obtained a neurocognitive test battery in schizophrenia patients and studied the association of abnormal brain structures to neurocognition.
Results
Structural brain abnormalities were pinpointed to the left anterior hippocampus and left OFC in schizophrenia patients, which were both significantly reduced in volume. The DLPFC and posterior hippocampus, though numerically decreased in volume, were not significantly decreased. Anterior hippocampal volumes were more strongly associated with OFC volumes in schizophrenia patients compared to controls. By contrast, DLPFC volume was unrelated to anterior or posterior hippocampal volume. Both the anterior hippocampus and OFC were independently related to cognitive abnormalities common in schizophrenia, including indices of verbal, language, and executive function. The DLPFC and posterior hippocampal volume were unrelated to cognitive measures.
Conclusions
These findings highlight related abnormalities of the anterior hippocampus and OFC in schizophrenia, which may shed light on the pathophysiology of the disorder.
Keywords: hippocampus, schizophrenia, prefrontal cortex, magnetic resonance imaging, neuropsychology
1. INTRODUCTION
Although multiple brain areas have been implicated in schizophrenia, the frontal cortex and hippocampal formation are consistently found to be abnormal (Shenton, Dickey, Frumin, & McCarley, 2001). The association of medial temporal lobe and frontal lobe structural brain abnormalities with cognitive deficits in schizophrenia have led researchers to hypothesize that dysfunction in this network may be central to illness pathophysiology (Weinberger, Berman, Suddath, & Torrey, 1992). For example, volume deficit of the frontal lobes are well established in schizophrenia and have been associated not only with impairments in executive functioning and working memory, but also in functions traditionally ascribed to the temporal lobes, including immediate and long-term memory, verbal memory, and verbal function (Baare et al., 1999; Sanfilipo et al., 2002a). Likewise, medial temporal lobe volume deficits exist and have been associated with impairment of cognitive functions traditionally deemed “frontal” in schizophrenia patients, including executive functioning (Bilder et al., 1995; Weinberger et al., 1992).
Biological plausibility for this network is highlighted by anatomic tracing studies in primates and rodents that have shown precise connections linking the temporal and prefrontal brain regions, including a monosynaptic projection from the anterior medial temporal lobe to the medial frontal lobe including OFC. The projections have been specified as originating in primarily the anterior hippocampus (via the subiculum and CA1 subfield) and projecting via a monosynaptic, ipsilateral, excitatory pathway to the ventral medial OFC (Rosene & Van Hoesen, 1977). Using morphometric shape analysis of the anterior hippocampus, volumetric methods, or high-resolution functional imaging of basal metabolism(Csernansky et al., 1998; Narr et al., 2004; Schobel et al., 2009; Szeszko et al., 2002), the CA1 subfield and subiculum subregions have been implicated in schizophrenia, highlighting the potential relevance of this neuronal pathway to the pathophysiology of the disorder.
The second projection between the hippocampus and prefrontal cortex involves a bidirectional polysynaptic pathway connecting the dorsolateral prefrontal cortex (DLPFC) to the posterior hippocampus via the parahippocampal gyrus, transitional cortices, and the pre-subiculum(Goldman-Rakic, Selemon, & Schwartz, 1984); it is not a part of the anterior hippocampal/medial frontal network. There is also anatomic evidence to support specific posterior hippocampal volume reduction in schizophrenia (Velakoulis et al., 2001), while still others find no differential effect of anterior or posterior hippocampal volume (Weiss, Dewitt, Goff, Ditman, & Heckers, 2005).
Evidence for this functional segregation in the hippocampus is suggested by recent functional imaging studies which show that the anterior hippocampus is primarily activated by tasks involving the display of novel stimuli or the process of mental encoding (Lepage, Habib, & Tulving, 1998; Strange, Fletcher, Henson, Friston, & Dolan, 1999), a process posited to be functionally abnormal, resulting in ‘aberrant salience’, during acute psychosis (Kapur, 2003). In contrast, posterior hippocampal function appears to be most linked to spatial memory function(Colombo, Fernandez, Nakamura, & Gross, 1998; Small, 2002). A longitudinal neuroimaging study of ultra high risk or prodromal cases has shown progressive decreases in left medial temporal lobe volume and left OFC volume with the onset of psychosis, suggesting a link between the anterior hippocampus and orbitofrontal cortex (OFC) in first episode psychosis (Pantelis et al., 2003).
Given the lack of empirical literature which has investigated the structural association of the hippocampus (anterior and posterior) and prefrontal cortex (ventral medial orbitofrontal and dorsolateral) in schizophrenia, based upon the above considerations, our study aimed to find structural evidence of anterior hippocampal and medial prefrontal cortical brain abnormalities in schizophrenia. Our primary study hypothesis was that the volumes of the anterior hippocampus and OFC would be specifically reduced in schizophrenia, compared to the DLPFC and posterior hippocampal subregions, which would be either less affected (DLPFC) or unchanged (posterior hippocampus). Our second study hypothesis was that volumes in anterior hippocampus and OFC would be correlated in an ipsilateral fashion in both patient and control groups, reflecting their anatomic neuronal association, but that this association would be enhanced within the schizophrenia patient group, reflecting pathology in two linked brain regions. We next hypothesized that this association would show a greater degree of correlation on the left than right, owing to the left-sided predominant findings in the literature (Shenton et al., 2001). Finally, as an exploratory study goal, we sought to explore the volumetric relationships of these subregions to neuropsychological domains most affected in schizophrenia: language, memory, and executive function.
2. Methods and Materials
2.1 Participants
Thirty-eight patients with diagnoses of schizophrenia or schizoaffective disorder (DSM-IV, American Psychiatric Association, 1994) by the Diagnostic Interview for Genetic Studies (DIGS)(Nurnberger et al., 1994)and twenty-nine controls who were comparable in sex and age were enrolled from Columbia University Medical Center and the New York State Psychiatric Institute inpatient schizophrenia research unit. Dr. Jill Harkavy-Friedman supervised interviewer training. The interviewers had experience in mental health treatment at a Master’s level or above. Training consisted of initial calibration using training tapes/programs followed by reliability testing using videotaped and live interviews rated with a qualified co-rater. Raters were required to achieve adequately high reliability (i.e., Kappa > .85 for diagnosis and > .75 for individual psychotic symptom ratings). Training was followed by ongoing reliability testing, with 20% of all diagnostic and symptom ratings co-rated by a 2nd rater. Inter-rater reliability on assessments was maintained by monthly meetings and review ratings to prevent drift. All control subjects and patients were medically healthy by history, physical examination and routine laboratory evaluation, including urine toxicology examination. Subjects were excluded who met DIGS criteria for current substance abuse or lifetime substance dependence. Three patients and three controls were eliminated from analyses for motion artifacts in their scans. All subjects were deemed competent to participate in voluntary research and signed informed consent. The protocol was approved by Columbia University's IRB. See Table 1 for subject demographics.
Table 1.
Subject Demographics
| Schizophrenia N = 38 | Control N = 29 | ||
|---|---|---|---|
| Gender (M / F) | 25 / 13 | 22 / 7 | χ2 .797 p =.37 |
| Mean Age (SD) | 32.3 (10.6) | 31.3 (9.2) | T(65,.025)=−.398 p =.69 |
| Education Mean Yrs (SD) | 13.3 (2.4) | 14.7 (3.2) | T(65,.025)=1.44 p=.16 |
| Mean Duration of Illness Yrs (SD) | 10.8 (9.2) | n/a | -- |
| Mean PANSS Negative Symptom Score (SD) | 13.6 (6.4) | n/a | -- |
| Mean PANSS Positive Symptom Score (SD) | 15.2 (6.2) | n/a | -- |
| Mean PANSS General Symptom Score (SD) | 28.6 (9.6) | n/a | -- |
2.2 Imaging protocol
Participants were scanned with a Signa Advantage system (GE Healthcare Technologies, Waukesha, Wisconsin, USA) operating at 1.5 T. The series used for this study was acquired as a 3D SPGR, with contiguous axial slices of 1.5mm with 0.86*0.86mm pixels within-slice, acquisition time to echo (TE) 5msecs; repetition time (TR) 34msecs; flip angle 45%; field of view 22 × 22cm; bandwidth 16 kHz; matrix 256 × 256 × 124 slices; slice thickness 1.5 mm. Scans were acquired with axial slices aligned with the plane connecting the anterior and posterior commissures.
2.3 Neuropsychological and symptom data
Patients were comprehensively assessed across neuropsychological domains by a single rater (RAS). A cognitive test battery was administered to patients. Our measures first included the Weschler Adult Intelligence Scale-Revised (Wechsler, 1981), including tests of full-scale, performance, and verbal IQ. We then used the WAIS summary scores, including verbal comprehension, perceptual processing, working memory, and processing speed, to measure broad indices of functions that have been localized to both frontal and limbic regions. Verbal long-term declarative memory was evaluated with the Weschler Memory Scale-Revised (Wechsler, 1987). Additional tests of executive function included the Wisconsin Card Sorting Test (Heaton, 1981); and Trail-making Test B (Reitan, 1958). Psychiatric symptoms were evaluated using the Positive and Negative Syndrome Scale (Kay, Fiszbein, & Opler, 1987). Controls were screened using a standard checklist using the non-patient version of the Structured Clinical Interview for DSM-IV. All patients were on stable doses of antipsychotic medication at the time of neuropsychological testing; for purposes of this study, medication exposure was coded into a categorical variable ‘yes/no’ and therefore detailed dosing and medication information for individual patients was not available.
2.4 Image analysis: Segmentation of whole brain and ROIs
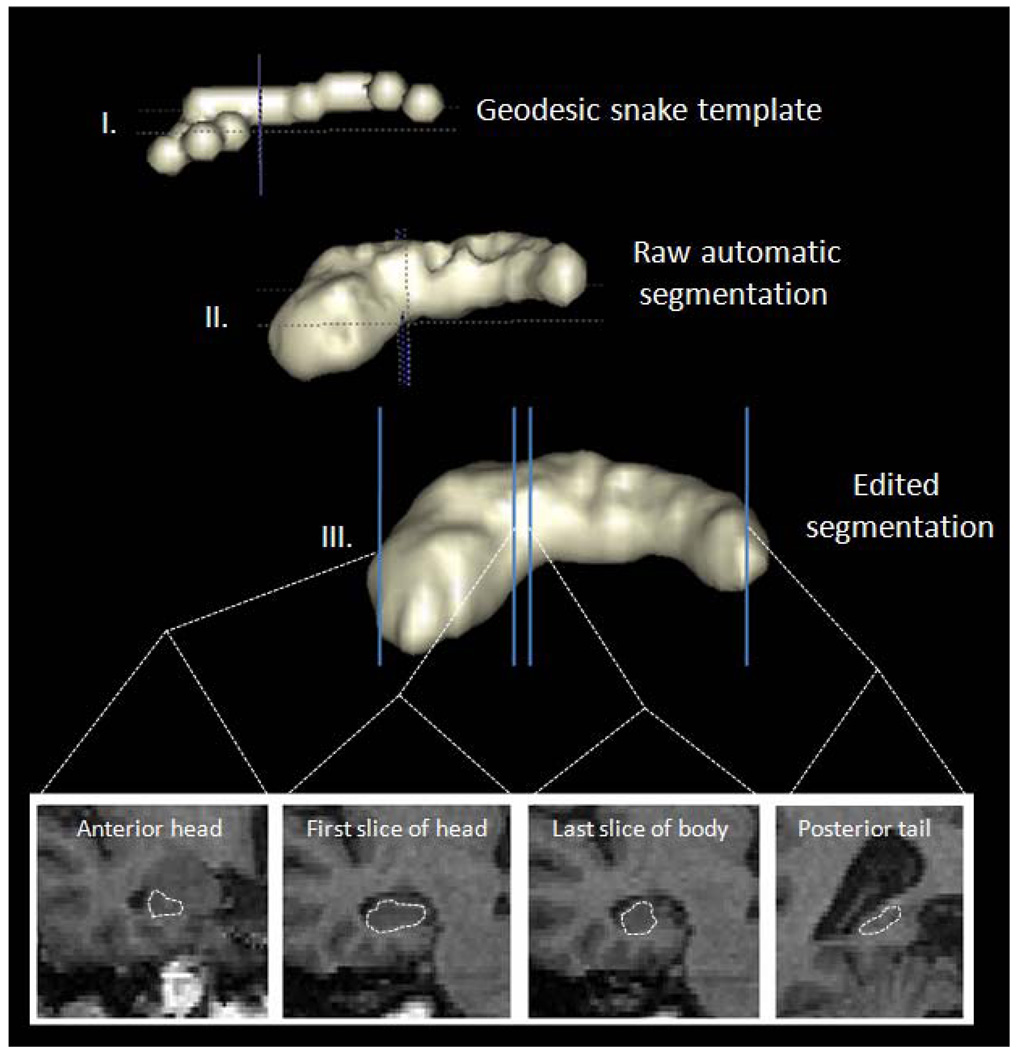
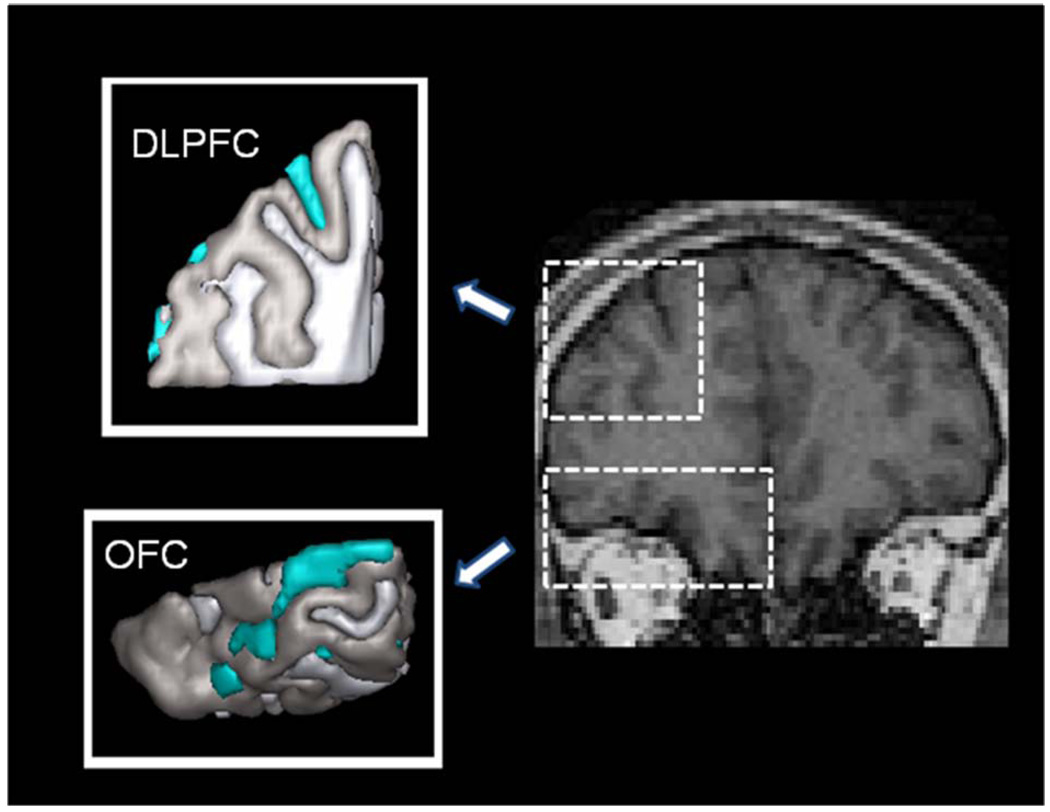
All images were coded into series numbers for blinded analyses. We used the 1) Brain Extraction tool (BET) of FSL to isolate the whole brain from extracranial tissues after applying an inhomogeneity correction (Smith, 2002); 2) FAST for automatic tissue segmentation (Zhang, Brady, & Smith, 2001); and 3) ITK-SNAP for semi-automated tri-planar region of interest volumetric analysis of whole brain, OFC gray matter (OFC), DLPFC gray matter (DLPFC) and hippocampus (Yushkevich et al., 2006) [Figure 1 and Figure 2]. The hippocampus, OFC and DLPFC were lateralized into right and left volumes, and the hippocampus was further subdivided into anterior and posterior subregions.
Figure 1.
Semiautomatic segmentation of anterior and posterior hippocampus with ITK-SNAP
Figure 2.
Dorsolateral prefrontal and orbitofrontal cortical regions of interest and anatomic definitions of the structures.
After using BET, whole brains were post-processed to eliminate any remaining extracranial voxels using ITK-SNAP. Three raters evaluated whole brain volumes (M.A.K., S.A.S., and K.A.V.), which included ventricle and sulcal CSF. Prior to data analysis, a reliability series was completed evaluating a total of fifteen scans: three from each of the five subjects. Interrater and intrarater reliability for whole brain volumes were both 0.99.
The hippocampus was segmented in a semi-automated fashion according to a triplanar method as previously described in Preussner, Weiss, and Chakos et al (Chakos et al., 2005; Pruessner et al., 2000; Weiss et al., 2005) [Figure 1]. ITK-SNAP parameters were set to region competition mode with default values. The hippocampal region of interest was constructed to maximize gray matter pixels in the body of the structure while preserving a clear inferior boundary with the white matter of the parahippocampal gyrus and clear lateral boundary with the temporal horn of the lateral ventricle, so as to not oversegment the structure. After localization of the hippocampus in three planes in the first step, the snake was initialized with a bubble setting of 2.0 to 2.2 along the anterior and poster axis of the structure. The snake was then manually evolved using the default settings of ballon force 1.0 and curvature force of .20. Hippocampal segmentations included the hippocampus proper, the subiculum, the fimbria and subsplenial gyrus and were subject to manual post-processing for anatomic fidelity according to the following criteria: We used the sagittal plane to separate the hippocampus from the amygdala, with the alveus and/or the uncal recess of the temporal horn to separate the structures. More medially in the sagittal plane, the axial view was used at the level of the tuber cinerium, mamillary bodies and optic tracts. This corresponds to the level of the hippocampal–amygdala transition area in the axial plane. This area was used as the boundary between the hippocampus and amygdala in the axial plane, which corresponds to the superior–medial boundary of the structure as viewed in the coronal plane (Convit et al., 1999). The hippocampal body was defined in the coronal plane from the inferior–lateral border to medial, with the contour following (and thus excluding) the parahippocampal gyrus. The anterior boundary of the hippocampus was the anterior convexity of the hippocampal alveus, which was previously delineated in the sagittal plane. The superior hippocampal border was the alveus. The inferior border continued to be the parahippocampal region, and the lateral border was the temporal horn of the lateral ventricle or temporal stem. Anterior hippocampus was separated from posterior hippocampus at the first appearance of the uncus in the coronal plane, consistent with the methodology as described in detail in Weiss [Figure 1] (Weiss et al., 2005) Our ITK-SNAP settings were kept constant for each brain (Yushkevich et al., 2006). The primary advantage of using the geodesic snake is to eliminate variability in choosing hippocampal boundaries along the body of the structure, thereby improving the reliability of our measurements. All hippocampal measurements were completed by a single rater (M.A.K.), with an intrarater reliability of 0.94. We defined the PFC as the full cortical volume anterior to the first appearance of the corpus callosum in the coronal plane (Lawrie et al., 2001). Our analysis was completed by a single rater (M.A.K.) with an intrarater reliability of 0.99. We manually traced the OFC in the coronal plane on the gray scale images , and once again completed measurements using a single rater (M.A.K) with an intrarater reliability of 0.98. The posterior boundary was defined as the last slice in which either of the medial posterior orbitofrontal gyri was visible. The superior rostral sulcus was used as the medial boundary anteriorly; the olfactory sulcus was used as the medial boundary posteriorly. Superior borders were defined by a line between the medial and lateral boundaries that bisected the white matter.
The DLPFC measurement was aimed at isolating Brodmann’s areas 9 and 46 (Al-Hakim, 2006). As the gyral and sulcal patterns of these areas are highly variable as viewed in the coronal plane, we used the frontal and temporal pole as landmarks to define the anterior and posterior boundaries. More specifically, we defined the DLPFC as occupying 40% of the area between the poles as previously described (Al-Hakim, 2006). Two raters evaluated the DLPFC volumes (M.A.K. and K.A.V.), with an interrater reliability of 0.98 and intrarater reliability of 0.99.
2.5 Statistical analysis
Statistical analysis was conducted using SPSS Version 15 (SPSS, Chicago). All statistical tests were two-sided with alpha set to 0.05.
We constructed a repeated-measures multivariate analysis of co-variance (MANCOVA), using status (schizophrenia versus control) and gender as between-group factors and hemisphere (left versus right) and subregions (anterior hippocampus, posterior hippocampus, OFC, and DLPFC) as within subject factors, all corrected for whole brain volume. Significant main effects and interactions were explored using post-hoc t-tests. We next conducted separate bivariate correlational analyses (Pearson’s product correlation coefficients) of these same structures in the patient and control groups—one overall, and one for each hemisphere—to evaluate the volumetric relationship between these structures between study groups. Last, we conducted exploratory bivariate correlational analyses (Pearson’s r) of left-sided brain structures found to be abnormal in the above analyses with the WAIS-R, WMS-R, WCST, and Trails B neuropsychological tests (table 2).
Table 2.
Exploratory Correlational Analysis of neuropsychological function with left hippocampus and prefrontal cortical structure
| Left Anterior Hippocampus | Left Posterior Hippocam pus | Left OFC Gray Matter | Left DLPFC Gray Matter | |
|---|---|---|---|---|
| Neuropsychological Test | r | r | r | r |
| WAIS Full-Scale IQ | .39* | −.08 | .48** | .12 |
| WAIS Verbal IQ | .42* | −.14 | .42* | .10 |
| WAIS Performance IQ | .31 | .00 | .50** | .12 |
| WAIS Verbal Comprehension Index | .41* | −.11 | .35 | .10 |
| WAIS Perceptual Organization Index | .35 | −.04 | .61** | .25 |
| WAIS Working Memory Index | .33 | −.02 | .44* | −.07 |
| WAIS Processing Speed Index | .28 | .04 | .25 | −.06 |
| WCST Errors | −.26 | −.07 | −.36* | −.10 |
| WCST Categories | .34 | .15 | .41* | .08 |
| Trails B Seconds | −.42* | −.28 | −.44* | −.04 |
| Trails B Errors | −.32 | .04 | −.51** | −.18 |
Uncorrected P value <.05
Uncorrected P value <.01
Uncorrected P value<.001
3. RESULTS
3.1 Mapping disease-related structural abnormalities within the hippocampal-prefrontal circuit
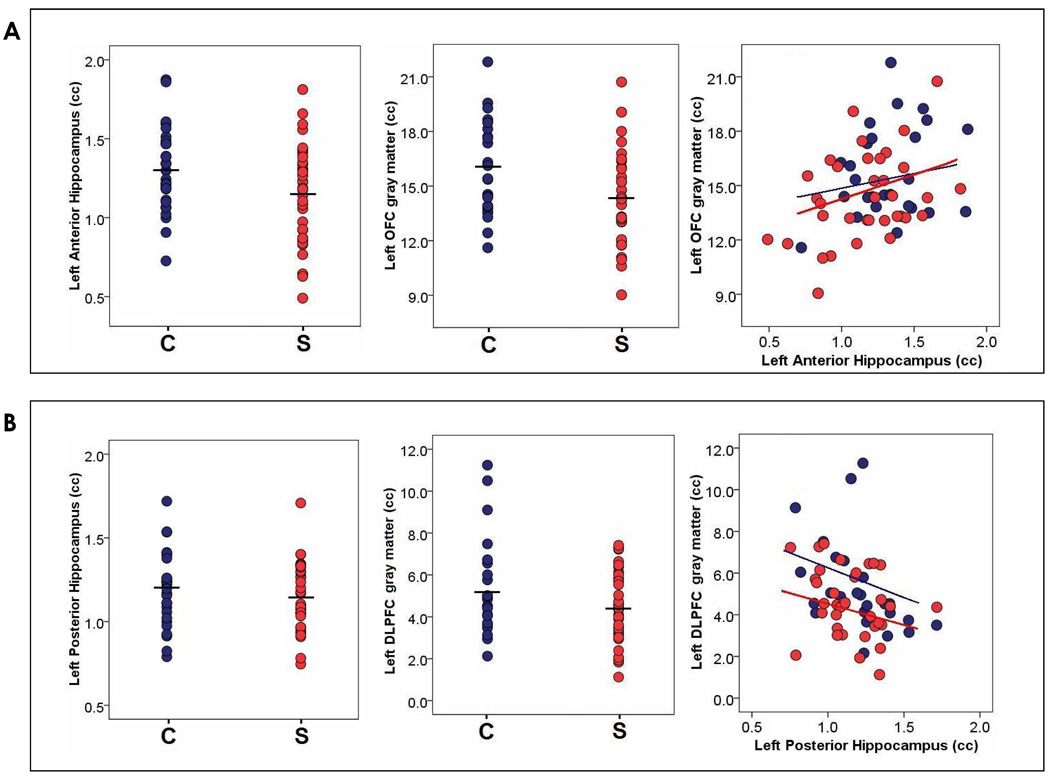
The MANCOVA of the four brain regions corrected for whole brain volume identified an interaction between hemisphere and status (F=6.0, df=1, 57 p=.02), with patients having smaller left-sided brain structures than controls. There was no interaction between brain subregion and status (F=2.1, df=3, 55 p=.11); however, there was a hemisphere by subregion by status interaction (F=3.7 df=3, 55 p=.02). Post-hoc t tests revealed this interaction to be driven by volume decreases specifically in the left anterior hippocampus (t=2.1, df=59, p= 0.04) and left OFC gray matter (t=2.1, df=59, p= 0.04) in the patient group. The left posterior hippocampus, DLPFC, and right-sided brain structures were numerically decreased in patients compared to controls but these differences did not reach statistical significance.
3.2 Mapping disease-related structural abnormalities between the hippocampus and PFC
We measured the association of volumes of the total anterior hippocampus with total OFC in both the patient and control groups, as well as the relationship of the left and right anterior and posterior hippocampus with the left and right OFC and DLPFC. As hypothesized, the volume of the anterior hippocampus was moderately associated in schizophrenia patients with that of the OFC (r=.38, p=.02) but not with the DLPFC (r=−.03, p=.86). This anatomic relationship was found specifically in patients in the left (r=.38, p=.02) and trended towards significance on the right side (r=.33, p=.06). Anterior hippocampal volume, though weakly associated in controls as predicted with OFC volume(r=.25, p=.21), did not reach the level of association found in patients (Figure 3). The analysis of the posterior hippocampus showed that its volume was not associated with dorsolateral prefrontal cortical volume in controls (r=−.30, p=.12) or patients (r=−.29, p=.09).
Figure 3.
Finally, as an exploratory study goal, we examined the associations between regional brain volumes and performance on neuropsychological tests within the schizophrenia patient group. Results revealed that both left anterior hippocampal and left OFC structure were related in a linear fashion to several neuropsychological indices, including three of the four WAIS scale summary scores, including verbal comprehension, perceptual processing, and working memory (table 2). Left dorsolateral prefrontal cortical volumes and posterior hippocampal volumes were not related to any of the neurocognitive domains. Neurocognitive function on the WMS-R was not significantly associated with brain volumes. Right sided structures were unrelated to the cognitive measures.
4. DISCUSSION
This study used detailed magnetic resonance imaging to examine the structure of separate subregions of the hippocampus and of the prefrontal cortex in patients with schizophrenia and in healthy controls. It also examined the inter-correlations of these volumes and their relationship to cognitive functioning in schizophrenia. The consistent and robust findings of this study centered on localized volume reductions in the left anterior hippocampus and the left OFC in the patient group.
These volumes were strongly correlated in the patients and they were linearly related with working memory and executive function in the patient group. These left anterior hippocampus and left OFC volume reductions explained an overall brain volume reduction for the patient group. There were numeric volume reductions in the DLPFC, the left posterior hippocampus, and in right hemispheric structures; however, none of these were to the same degree as on the left and did not reach statistical significance and, as mentioned, were not associated in the patient or the control groups.
These results are consistent with previous structural imaging studies showing decreased medial temporal and OFC volumes in chronic schizophrenia patients(Shenton et al., 2001) (Venkatasubramanian, Jayakumar, Gangadhar, & Keshavan, 2008). This study furthermore supports the notion of anterior-specific defects in hippocampal volume in schizophrenia patients, which is more controversial(Thoma et al., 2009; Weiss et al., 2005). This study had the benefit of a larger sample than several other published studies and, despite the inherent limitations of visualization of this complex structure, we nonetheless used triplanar criteria to determine hippocampal boundaries and achieved a high reliability in measurement.
We did not find any significant volume reduction in the DLPFC, but this result in no way precludes more subtle structural or functional abnormalities in this regions. Advancements in methodology will certainly reveal other abnormalities in subgroups of schizophrenia cases. Novel techniques such as surface analysis have recently shown cortical thinning of DLPFC during the transition to psychosis(Sun et al., 2009). Cognitive challenge studies report abnormal activation in the DLPFC during executive function and memory tasks in schizophrenia patients(Ragland et al., 1998). The DLPFC is positioned to receive information from the ventral fronto-temporal network(Moghaddam & Homayoun, 2007), so our results are not inconsistent with these findings; OFC damage may very well contribute to a DLPFC dysfunction that is not reflected in gross structural abnormality within DLPFC.
Our exploratory analysis of the relationship of these structural abnormalities to neuropsychological performance helps generate hypotheses of the left anterior hippocampus and the left OFC in cognitive dysfunction in schizophrenia. Namely, volumetric deficit of both of these regions are associated with deficits in executive function and verbal performance. Our findings were in the expected direction and are consistent with emerging evidence that a ventral-medial frontal-temporal network is relevant to cognitive dysfunction in schizophrenia(Matsui et al., 2008). The absence of an association of brain structures in our sample with the WMS-R, despite hippocampal structural abnormalities, is not surprising, since studies that have attempted to link memory function and hippocampal volume deficits in schizophrenia patients have been inconsistent in establishing this relationship(Bilder et al., 1995; Goldberg, Torrey, Berman, & Weinberger, 1994; Sanfilipo et al., 2002b).
On the other hand, structural defects as well as neurochemical abnormalities including increased glutamate in the anterior hippocampus have been implicated in executive function deficits in studies of first-episode patients and in the affected twin in studies of monozygotic twins discordant for schizophrenia(Bilder et al., 1995; Rusch et al., 2008; Szeszko et al., 2003), as well as with episodic memory in chronic schizophrenia(Thoma et al., 2009). Recent findings also highlight the relation of the hippocampus to defects in verbal memory in the ultra high-risk state(Hurlemann et al., 2008). These findings are also consistent with animal models of schizophrenia based on neonatal structural lesions to the hippocampus that occur early in development which manifest electrophysiologic abnormalities in the prefrontal cortex as well as cognitive dysfunction in later adolescence(Lipska & Weinberger, 2002).
These results of frontal and medial temporal lobe abnormalities are consistent with our earlier metabolic study in medication free schizophrenia patients in the resting state, which showed left prefrontal hypoperfusion and hippocampal hyperactivation; we subsequently showed this pattern was more robust in sporadic schizophrenia cases(Malaspina et al., 2004; Malaspina et al., 1999). A number of disease models have postulated that abnormal increases in hippocampal activity might exist in the disease(Benes, 1999; Krieckhaus, Donahoe, & Morgan, 1992) and that hippocampal hyperactivity might influence the function of other cortical regions(Moore, West, & Grace, 1999). Several individual imaging studies have provided evidence documenting both hyperactivity in resting neural states (for example, see (Heckers et al., 1998) and volume reduction of the hippocampus in schizophrenia, though the precise relation of the two variables in a single study has not been investigated to date. Our findings identify an association between the anterior hippocampus and the OFC present in established illness which links the hippocampus to other cortical regions that may account for deficits in the disorder. Though speculative, if we interpret our findings in the context of the body of literature delineating anatomical connections between prefrontal and limbic subregions, given the dense monosynaptic ipsilateral connection of the anterior hippocampus to the OFC, we hypothesize that hyperfunction of the anterior hippocampus is associated with OFC and ventral cortical hyperfunction found in schizophrenia(Ragland et al., 2004; Schobel et al., 2009). The OFC cortex itself is a part of the ventral striatal network that stimulates a range of downstream areas, including subcortical dopaminergic centers, such as the striatum(Ongur, An, & Price, 1998) and subcortical regions implicated in emotional and cognitive processing(Barbas, 2000). Thus, our findings can be taken as structural evidence supporting the hypothesis of a hyperfunctioning network active in schizophrenia which could plausibly be associated with the elevated dopaminergic state characteristic of schizophrenia(Abi-Dargham et al., 1998).
In summary, our findings advance the hypothesis of a dysfunctional fronto-temporal network in schizophrenia based upon dysfunction of medial/ventral limbic-cortical brain structures. Of course, in complex disorders such as schizophrenia, causal claims that rely on cross-sectional structural imaging studies must be made with extreme caution. Our study has several important limitations. Our exploratory analysis of neuropsychological function with brain structure was completed in the patient group only, limiting our ability to directly compare the degree to which neuropsychological function was impaired in the patient group relative to that of the healthy comparison group. Further, the patient group was imaged and tested while on stable doses of antipsychotic medication for which detailed medication information was not available, though this is unlikely to have caused a substantial difference in our primary study outcomes(Keefe et al., 2007; Velakoulis et al., 2006). Longitudinal imaging studies of first-episode patients which combine modalities (e.g., structural and functional) and which are based upon anatomical patterns of connectivity between the hippocampus and frontal cortex will further our knowledge of the anatomic localization of hippocampal and prefrontal cortical pathology in schizophrenia.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abi-Dargham A, Gil R, Krystal J, Baldwin RM, Seibyl JP, Bowers M, van Dyck CH, Charney DS, Innis RB, Laruelle M. Increased striatal dopamine transmission in schizophrenia: confirmation in a second cohort. American Journal of Psychiatry. 1998;155:761–767. doi: 10.1176/ajp.155.6.761. [DOI] [PubMed] [Google Scholar]
- Al-Hakim R, Nain D, Melonakos J, Tannenbaum A, Fallon J. A Dorsolateral Prefrontal Cortex Semi-Automatic Segmenter. Proc SPIE Medical Imaging. 2006 [Google Scholar]
- Baare WF, HulshoffPol HE, Hijman R, Mali WP, Viergever MA, Kahn RS. Volumetric analysis of frontal lobe regions in schizophrenia: relation to cognitive function and symptomatology. Biol Psychiatry. 1999;45:1597–1605. doi: 10.1016/s0006-3223(98)00266-2. [DOI] [PubMed] [Google Scholar]
- Barbas H. Connections underlying the synthesis of cognition, memory, and emotion in primate prefrontal cortices. Brain Res Bull. 2000;52:319–330. doi: 10.1016/s0361-9230(99)00245-2. [DOI] [PubMed] [Google Scholar]
- Benes FM. Evidence for altered trisynaptic circuitry in schizophrenic hippocampus. Biological Psychiatry. 1999;46:589–599. doi: 10.1016/s0006-3223(99)00136-5. [DOI] [PubMed] [Google Scholar]
- Bilder RM, Bogerts B, Ashtari M, Wu H, Alvir JM, Jody D, Reiter G, Bell L, Lieberman JA. Anterior hippocampal volume reductions predict frontal lobe dysfunction in first episode schizophrenia. Schizophr Res. 1995;17:47–58. doi: 10.1016/0920-9964(95)00028-k. [DOI] [PubMed] [Google Scholar]
- Chakos MH, Schobel SA, Gu H, Gerig G, Bradford D, Charles C, Lieberman JA. Duration of illness and treatment effects on hippocampal volume in male patients with schizophrenia. Br J Psychiatry. 2005;186:26–31. doi: 10.1192/bjp.186.1.26. [DOI] [PubMed] [Google Scholar]
- Colombo M, Fernandez T, Nakamura K, Gross CG. Functional differentiation along the anterior-posterior axis of the hippocampus in monkeys. J Neurophysiol. 1998;80:1002–1005. doi: 10.1152/jn.1998.80.2.1002. [DOI] [PubMed] [Google Scholar]
- Convit A, McHugh P, Wolf OT, de Leon MJ, Bobinski M, De Santi S, Roche A, Tsui W. MRI volume of the amygdala: a reliable method allowing separation from the hippocampal formation. Psychiatry Res. 1999;90:113–123. doi: 10.1016/s0925-4927(99)00007-4. [DOI] [PubMed] [Google Scholar]
- Csernansky JG, Joshi S, Wang L, Haller JW, Gado M, Miller JP, Grenander U, Miller MI. Hippocampal morphometry in schizophrenia by high dimensional brain mapping. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:11406–11411. doi: 10.1073/pnas.95.19.11406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg TE, Torrey EF, Berman KF, Weinberger DR. Relations between neuropsychological performance and brain morphological and physiological measures in monozygotic twins discordant for schizophrenia. Psychiatry Res. 1994;55:51–61. doi: 10.1016/0925-4927(94)90011-6. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Selemon LD, Schwartz ML. Dual pathways connecting the dorsolateral prefrontal cortex with the hippocampal formation and parahippocampal cortex in the rhesus monkey. Neuroscience. 1984;12:719–743. doi: 10.1016/0306-4522(84)90166-0. [DOI] [PubMed] [Google Scholar]
- Heaton RK. Wisconsin Card Sorting Test (WCST) Odessa FL: Psychological Assessment Resources; 1981. [Google Scholar]
- Heckers S, Rauch SL, Goff D, Savage CR, Schacter DL, Fischman AJ, Alpert NM. Impaired recruitment of the hippocampus during conscious recollection in schizophrenia.[see comment] Nature Neuroscience. 1998;1:318–323. doi: 10.1038/1137. [DOI] [PubMed] [Google Scholar]
- Hurlemann R, Jessen F, Wagner M, Frommann I, Ruhrmann S, Brockhaus A, Picker H, Scheef L, Block W, Schild HH, Moller-Hartmann W, Krug B, Falkai P, Klosterkotter J, Maier W. Interrelated neuropsychological and anatomical evidence of hippocampal pathology in the at-risk mental state. Psychol Med. 2008;38:843–851. doi: 10.1017/S0033291708003279. [DOI] [PubMed] [Google Scholar]
- Kapur S. Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia. Am J Psychiatry. 2003;160:13–23. doi: 10.1176/appi.ajp.160.1.13. [DOI] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Keefe RS, Bilder RM, Davis SM, Harvey PD, Palmer BW, Gold JM, Meltzer HY, Green MF, Capuano G, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, Davis CE, Hsiao JK, Lieberman JA. Neurocognitive effects of antipsychotic medications in patients with chronic schizophrenia in the CATIE Trial. Arch Gen Psychiatry. 2007;64:633–647. doi: 10.1001/archpsyc.64.6.633. [DOI] [PubMed] [Google Scholar]
- Krieckhaus EE, Donahoe JW, Morgan MA. Paranoid schizophrenia may be caused by dopamine hyperactivity of CA1 hippocampus. Biological Psychiatry. 1992;31:560–570. doi: 10.1016/0006-3223(92)90242-r. [DOI] [PubMed] [Google Scholar]
- Lawrie SM, Whalley HC, Abukmeil SS, Kestelman JN, Donnelly L, Miller P, Best JJ, Owens DG, Johnstone EC. Brain structure, genetic liability, and psychotic symptoms in subjects at high risk of developing schizophrenia. Biological Psychiatry. 2001;49:811–823. doi: 10.1016/s0006-3223(00)01117-3. [DOI] [PubMed] [Google Scholar]
- Lepage M, Habib R, Tulving E. Hippocampal PET activations of memory encoding and retrieval: the HIPER model. Hippocampus. 1998;8:313–322. doi: 10.1002/(SICI)1098-1063(1998)8:4<313::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Lipska BK, Weinberger DR. A neurodevelopmental model of schizophrenia: neonatal disconnection of the hippocampus. Neurotox Res. 2002;4:469–475. doi: 10.1080/1029842021000022089. [DOI] [PubMed] [Google Scholar]
- Malaspina D, Harkavy-Friedman J, Corcoran C, Mujica-Parodi L, Printz D, Gorman JM, Van Heertum R. Resting neural activity distinguishes subgroups of schizophrenia patients. Biological Psychiatry. 2004;56:931–937. doi: 10.1016/j.biopsych.2004.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaspina D, Storer S, Furman V, Esser P, Printz D, Berman A, Lignelli A, Gorman J, Van Heertum R. SPECT study of visual fixation in schizophrenia and comparison subjects. Biol Psychiatry. 1999;46:89–93. doi: 10.1016/s0006-3223(98)00306-0. [DOI] [PubMed] [Google Scholar]
- Matsui M, Suzuki M, Zhou SY, Takahashi T, Kawasaki Y, Yuuki H, Kato K, Kurachi M. The relationship between prefrontal brain volume and characteristics of memory strategy in schizophrenia spectrum disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1854–1862. doi: 10.1016/j.pnpbp.2008.08.018. [DOI] [PubMed] [Google Scholar]
- Moghaddam B, Homayoun H. Divergent Plasticity of Prefrontal Cortex Networks. Neuropsychopharmacology. 2007 doi: 10.1038/sj.npp.1301554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore H, West AR, Grace AA. The regulation of forebrain dopamine transmission: relevance to the pathophysiology and psychopathology of schizophrenia. Biol Psychiatry. 1999;46:40–55. doi: 10.1016/s0006-3223(99)00078-5. [DOI] [PubMed] [Google Scholar]
- Narr KL, Thompson PM, Szeszko P, Robinson D, Jang S, Woods RP, Kim S, Hayashi KM, Asunction D, Toga AW, Bilder RM. Regional specificity of hippocampal volume reductions in first-episode schizophrenia. Neuroimage. 2004;21:1563–1575. doi: 10.1016/j.neuroimage.2003.11.011. [DOI] [PubMed] [Google Scholar]
- Nurnberger JI, Jr, Blehar MC, Kaufmann CA, York-Cooler C, Simpson SG, Harkavy-Friedman J, Severe JB, Malaspina D, Reich T. Diagnostic interview for genetic studies.Rationale, unique features, and training. NIMH Genetics Initiative. Arch Gen Psychiatry. 1994;51:849–859. doi: 10.1001/archpsyc.1994.03950110009002. discussion 863–4. [DOI] [PubMed] [Google Scholar]
- Ongur D, An X, Price JL. Prefrontal cortical projections to the hypothalamus in macaque monkeys.[see comment] Journal of Comparative Neurology. 1998;401:480–505. [PubMed] [Google Scholar]
- Pantelis C, Velakoulis D, McGorry PD, Wood SJ, Suckling J, Phillips LJ, Yung AR, Bullmore ET, Brewer W, Soulsby B, Desmond P, McGuire PK. Neuroanatomical abnormalities before and after onset of psychosis: a cross-sectional and longitudinal MRI comparison. Lancet. 2003;361:281–288. doi: 10.1016/S0140-6736(03)12323-9. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Li LM, Serles W, Pruessner M, Collins DL, Kabani N, Lupien S, Evans AC. Volumetry of hippocampus and amygdala with high-resolution MRI and three-dimensional analysis software: minimizing the discrepancies between laboratories. Cereb Cortex. 2000;10:433–442. doi: 10.1093/cercor/10.4.433. [DOI] [PubMed] [Google Scholar]
- Ragland JD, Gur RC, Glahn DC, Censits DM, Smith RJ, Lazarev MG, Alavi A, Gur RE. Frontotemporal cerebral blood flow change during executive and declarative memory tasks in schizophrenia: a positron emission tomography study. Neuropsychology. 1998;12:399–413. doi: 10.1037//0894-4105.12.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragland JD, Gur RC, Valdez J, Turetsky BI, Elliott M, Kohler C, Siegel S, Kanes S, Gur RE. Event-related fMRI of frontotemporal activity during word encoding and recognition in schizophrenia. Am J Psychiatry. 2004;161:1004–1015. doi: 10.1176/appi.ajp.161.6.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitan RM. Validity of the Trail making Test as an indicator of organic brain damage. Perceptual and Motor Skills. 1958;8:271–276. [Google Scholar]
- Rosene DL, Van Hoesen GW. Hippocampal efferents reach widespread areas of cerebral cortex and amygdala in the rhesus monkey. Science. 1977;198:315–317. doi: 10.1126/science.410102. [DOI] [PubMed] [Google Scholar]
- Rusch N, Tebartz van Elst L, Valerius G, Buchert M, Thiel T, Ebert D, Hennig J, Olbrich HM. Neurochemical and structural correlates of executive dysfunction in schizophrenia. Schizophr Res. 2008;99:155–163. doi: 10.1016/j.schres.2007.05.024. [DOI] [PubMed] [Google Scholar]
- Sanfilipo M, Lafargue T, Rusinek H, Arena L, Loneragan C, Lautin A, Rotrosen J, Wolkin A. Cognitive performance in schizophrenia: relationship to regional brain volumes and psychiatric symptoms. Psychiatry Res. 2002a;116:1–23. doi: 10.1016/s0925-4927(02)00046-x. [DOI] [PubMed] [Google Scholar]
- Sanfilipo M, Lafargue T, Rusinek H, Arena L, Loneragan C, Lautin A, Rotrosen J, Wolkin A. Cognitive performance in schizophrenia: relationship to regional brain volumes and psychiatric symptoms. Psychiatry Research. 2002b;116:1–23. doi: 10.1016/s0925-4927(02)00046-x. [DOI] [PubMed] [Google Scholar]
- Schobel S, Lewandowski N, Corcoran C, Moore H, Brown T, Malaspina D, Small S. Differential Targeting of the CA1 subfield of The Hippocampal Formation by Schizophrenia and Related Psychotic Disorders. Archives of General Psychiatry. 2009 doi: 10.1001/archgenpsychiatry.2009.115. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenton ME, Dickey CC, Frumin M, McCarley RW. A review of MRI findings in schizophrenia. Schizophrenia Research. 2001;49:1–52. doi: 10.1016/s0920-9964(01)00163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small SA. The longitudinal axis of the hippocampal formation: its anatomy, circuitry, and role in cognitive function. Rev Neurosci. 2002;13:183–194. doi: 10.1515/revneuro.2002.13.2.183. [DOI] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Human Brain Mapping. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange BA, Fletcher PC, Henson RN, Friston KJ, Dolan RJ. Segregating the functions of human hippocampus. Proc Natl Acad Sci U S A. 1999;96:4034–4039. doi: 10.1073/pnas.96.7.4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D, Phillips L, Velakoulis D, Yung A, McGorry PD, Wood SJ, van Erp TG, Thompson PM, Toga AW, Cannon TD, Pantelis C. Progressive brain structural changes mapped as psychosis develops in 'at risk' individuals. Schizophr Res. 2009;108:85–92. doi: 10.1016/j.schres.2008.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szeszko PR, Goldberg E, Gunduz-Bruce H, Ashtari M, Robinson D, Malhotra AK, Lencz T, Bates J, Crandall DT, Kane JM, Bilder RM. Smaller anterior hippocampal formation volume in antipsychotic-naive patients with first-episode schizophrenia. Am J Psychiatry. 2003;160:2190–2197. doi: 10.1176/appi.ajp.160.12.2190. [DOI] [PubMed] [Google Scholar]
- Szeszko PR, Strous RD, Goldman RS, Ashtari M, Knuth KH, Lieberman JA, Bilder RM. Neuropsychological correlates of hippocampal volumes in patients experiencing a first episode of schizophrenia. American Journal of Psychiatry. 2002;159:217–226. doi: 10.1176/appi.ajp.159.2.217. [DOI] [PubMed] [Google Scholar]
- Thoma RJ, Monnig M, Hanlon FM, Miller GA, Petropoulos H, Mayer AR, Yeo R, Euler M, Lysne P, Moses SN, Canive JM. Hippocampus volume and episodic memory in schizophrenia. J Int Neuropsychol Soc. 2009;15:182–195. doi: 10.1017/S1355617709090225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velakoulis D, Stuart GW, Wood SJ, Smith DJ, Brewer WJ, Desmond P, Singh B, Copolov D, Pantelis C. Selective bilateral hippocampal volume loss in chronic schizophrenia. Biological Psychiatry. 2001;50:531–539. doi: 10.1016/s0006-3223(01)01121-0. [DOI] [PubMed] [Google Scholar]
- Velakoulis D, Wood SJ, Wong MT, McGorry PD, Yung A, Phillips L, Smith D, Brewer W, Proffitt T, Desmond P, Pantelis C. Hippocampal and amygdala volumes according to psychosis stage and diagnosis: a magnetic resonance imaging study of chronic schizophrenia, first-episode psychosis, and ultra-high-risk individuals. Archives of General Psychiatry. 2006;63:139–149. doi: 10.1001/archpsyc.63.2.139. [DOI] [PubMed] [Google Scholar]
- Venkatasubramanian G, Jayakumar PN, Gangadhar BN, Keshavan MS. Automated MRI parcellation study of regional volume and thickness of prefrontal cortex (PFC) in antipsychotic-naive schizophrenia. Acta Psychiatr Scand. 2008;117:420–431. doi: 10.1111/j.1600-0447.2008.01198.x. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale-Revised. New York: The Psychological Corporation; 1981. [Google Scholar]
- Wechsler D. San Antonio: The Psychological Corporation; 1987. WMS-R.Wechsler memory Scale-Revised Manual. [Google Scholar]
- Weinberger DR, Berman KF, Suddath R, Torrey EF. Evidence of dysfunction of a prefrontal-limbic network in schizophrenia: a magnetic resonance imaging and regional cerebral blood flow study of discordant monozygotic twins. Am J Psychiatry. 1992;149:890–897. doi: 10.1176/ajp.149.7.890. [DOI] [PubMed] [Google Scholar]
- Weiss AP, Dewitt I, Goff D, Ditman T, Heckers S. Anterior and posterior hippocampal volumes in schizophrenia. Schizophr Res. 2005;73:103–112. doi: 10.1016/j.schres.2004.05.018. [DOI] [PubMed] [Google Scholar]
- Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, Gerig G. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage. 2006;31:1116–1128. doi: 10.1016/j.neuroimage.2006.01.015. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Transactions on Medical Imaging. 2001;20:45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]