Abstract
Pathological gamblers (PGs) may have high levels of impulsivity, and a correlation between substance use disorders (SUD) and impulsivity is well established. However, only a handful of studies have attempted to assess impulsivity and other impulse-spectrum traits (e.g., sensation seeking) using a variety of behavioral and self-report measures in PGs and few examined the independent impact of SUDs. We compared 30 PGs without SUD histories, 31 PGs with SUD histories and 40 control participants on self-reported impulsivity, delayed discounting, attention/memory, response inhibition, risk taking, sensation seeking and distress tolerance measures. PGs, regardless of SUD history, discounted delayed rewards at greater rates than controls. PGs also reported acting on the spur of the moment, experienced trouble planning and thinking carefully, and noted greater attention difficulties than controls. PGs with SUD took greater risks on a risk-taking task than did PGs without SUD histories, but the two groups did not differ on any other measures of impulsivity. We conclude that PGs are more impulsive than non-problem gamblers in fairly specific ways, but PGs with and without SUD histories differ on few measures. More research should focus on specific ways in which PGs exhibit impulsivity to better address impulsive behaviors in treatment.
Keywords: Pathological gambling, Substance use disorder, Impulsivity, Sensation seeking, Delay discounting
1. Introduction
Over 60% of the population of the United States gambles at least once per year (Gerstein et al., 1999), and most do so without any significant difficulties. Some, however, experience serious problems related to their gambling, and a fraction develop pathological gambling (PG). This disorder is characterized by persistent gambling behavior that significantly disrupts social, family and vocational pursuits (American Psychiatric Association, 1994), and is frequently associated with increased financial, legal and psychological problems (Ladd and Petry, 2002; Potenza et al., 2001; Shaffer et al., 2002).
Impulsivity is considered to be a prominent feature of PG, and is often thought of as a tendency to act without thinking. There are several different ways of operationalizing impulsivity (Evenden, 1999). Most studies of gambling have used paper and pencil measures, including Barratt’s impulsivity scales (Barratt, 1985; Patton et al., 1995) and Eysenck’s scale (Eysenck and Eysenck, 1978). Similarly, paper and pencil measures have also been used to examine related constructs, such as risk taking and sensation seeking (e.g., Zuckerman’s Sensation Seeking scale; Zuckerman, 1994). Together, these measures assess a variety of different aspects of impulsivity and other impulse spectrum traits, including acting without thinking, making quick decisions, lack of future orientation, failure to evaluate risks and sensation seeking. In the interests of brevity, we use the term “impulsivity” when discussing these broader traits/constructs throughout the paper.
Several studies have found correlations between PG and these self-report measures (e.g., Blaszczynski and Steel, 1998; Steel and Blaszczynski, 1998; Petry, 2001a; Vitaro et al., 1998). Some preliminary research has also revealed a relationship between self-reported impulsivity and treatment failure (Leblond et al., 2003). These studies suggest that greater impulsivity is associated with more severe gambling problems and greater psychosocial consequences of gambling.
While the relationship between PG and self-reported impulsivity is rarely in dispute, the validity of self-report measures is limited, because they are subjective and may be influenced by distorted perceptions of one’s own traits. Further, many of the studies cited above did not control for substance use disorders, despite recent prelavence data that suggests up to 73% of pathological gamblers have a lifetime comorbid alcohol use disorder and up to 38% have a lifetime substance use disorder (Petry et al., 2005). Thus, any increased impulsivity may be related to substance abuse rather than PG per se. Finally, impulsivity is not a unitary concept. Multiple components of impulsivity have been identified (Barratt, 1985; Patton et al., 1995), yet relatively little research has evaluated these various aspects of impulsivity in gamblers. The present study examined the relationship between PG and results of several behavioral and self-report measures that operationalize impulsivity in different ways.
Behaviorally-based laboratory measures may assist in better understanding the different dimensions of impulsivity in gamblers because they elicit impulsive behavior, and may prove complementary to self-report measures which require individuals to accurately characterized their own traits. Delayed discounting is defined as a tendency to discount larger, delayed rewards in favor of smaller, more immediate ones. Generally, in these choice tasks, as the delay until delivery of a reward increases, the subjective value of that reward decreases (Green et al., 1994). At issue is the rate at which a reward is discounted. More rapid discounting of delayed rewards is a measure of greater impulsivity (Green et al., 1994). Past studies have found that PGs discount delayed rewards more rapidly than non-PGs, even after accounting for substance abuse histories (Alessi and Petry, 2003; Petry, 2001b; Petry and Casarella, 1999).
The single key impulsivity paradigm (SKIP) is another behavioral-choice measure in which participants make a response to obtain money (Dougherty, 2003; Dougherty et al., 2003; Mathias et al., 2002; Swann et al., 2002). Impulsivity is reflected by the pattern and frequency of responding. Probation/paroled women with childhood aggression histories demonstrated higher impulsivity on this measure than those without aggression (Mathias et al., 2002). Forced choice paradigms similar to delayed discounting and the SKIP appear to be associated with non-planning aspects of impulsivity rather than attention, cognition or motor impulsivity (Swann et al., 2002). We included both delay discounting and SKIP in the current study.
Attention and memory measures have also been used to assess impulsivity, but they have not frequently been examined in PGs. Models that use attention and working memory define impulsivity as a deficit in one’s ability to withhold responding until accurate processing of environmental stimuli has occurred (Dougherty and Marsh, 2003; Swann et al., 2002). As many as 20% of PGs have a history of attention deficit disorder (ADD; Specker et al., 1995), and rates of ADD in PGs with co-occurring cocaine dependence are as high as 50% (Steinberg et al., 1992), suggesting that PG may be associated with significant attention difficulties. Despite these high rates of co-morbidity between PG and ADD, and relatively high impulsivity identified in both disorders, few studies have examined attention and working memory deficits in PGs. The attention and memory task included in the current study was a modification of the continuous performance task that assessed immediate and delayed attention and memory (Dougherty, 2003).
Response inhibition tasks have also been used to explore the question of impulsivity in a number of populations, and several studies have found significant relationships between response inhibition tasks and scores on paper and pencil measures of impulsivity (Finn et al., 1999; Finn et al., 2002; Horn et al., 2003; Marsh et al., 2002). Response Inhibition tasks, such as the GoStop task used in the current study, measure a participant’s ability to restrain responding when required by the rules of the task. Individuals who score higher on impulsivity measures have greater difficulty inhibiting a response to a target stimulus when they are presented with a “stop” signal, particularly as the latency between the target stimulus and the “stop” signal increases (Dougherty, 2003; Marsh et al., 2002). GoStop paradigms of impulsivity are predicated on a model of opposing neural functions in which behavioral elicitation and inhibition cues occur simultaneously (Logan et al., 1984). Behavioral expression or restraint depends on which of these neural processes is completed first (Dougherty, 2003). Marsh et al. (2002) found poorer performance on a GoStop task to be associated with higher Eysenck’s impulsiveness scale scores. Finn and colleagues (2002) found that response inhibition was similarly associated with impulsivity measures in participants with early onset alcoholism and conduct disorder. Using a stroop task, Potenza and colleagues (2003) found no differences in response inhibition between PGs and controls. However, that study focused primarily on fMRI results, and included a fairly small sample. Goudriaan and colleagues (2006), in contrast, found PGs and alcohol dependent participants had deficits in response inhibition relative to healthy controls.
Laboratory measures of risk-taking, sensation seeking and distress tolerance are also relevant to the study of impulsivity, but these tasks have rarely been investigated in gamblers. Sensation seeking is a trait characterized by a need for novel, varied and complex experiences that are often associated with significant risk taking (Langewisch and Frisch, 1998; Zuckerman, 1979). High impulsivity is associated with greater risk taking activities (e.g., suicide attempt, sexual risk taking) among PGs (Martins et al., 2004). Lejuez and colleagues (2002,2003) developed the balloon analogue risk task (BART), a computer-based measure of risk taking that is correlated with self-report measures of impulsivity and sensation seeking. Greater risk taking on the BART appears to be associated with a greater affinity for gambling and more gambling related problems (Lejuez et al., 2002).
Finally, distress tolerance has not specifically been used as a measure of impulsivity. However, it intuitively makes sense to study distress tolerance as a potential correlate of impulsivity in PGs. Recent research has examined the basis of the relationship between the experience of intense negative emotional experience and impulsive decision-making (see Cyders and Smith, 2008for a review). Further, some PGs appear to use gambling as a way to escape from negative emotional experiences (Blaszczynski and Nower, 2002; Ledgerwood and Petry, 2006a). PGs with lower distress tolerance may be impulsive because they find it more difficult to pursue longer-term resolution to interpersonal problems, and instead select a short-term or even rash solution of escape through gambling.
Our objective in the current study was to address questions about the role of impulsivity in PG. Specifically, we compared pathological gamblers (PGs) with and without a history of substance use disorders (SUD) to control participants on self-report and laboratory-based measures of impulsivity. Impulsivity measures assessed non-planning impulsivity, delay discounting, attention and memory, response inhibition, motor impulsivity, risk-taking and distress tolerance. We hypothesized that PGs would score higher on some, but possibly not all, dimensions of impulsivity compared to controls, and that participants with a past history of SUD would score higher than PGs without SUD histories. Active substance dependent individuals were excluded for the study so that recent drug use (or withdrawal) did not confound results.
2. Methods
2.1 Participants and Recruitment
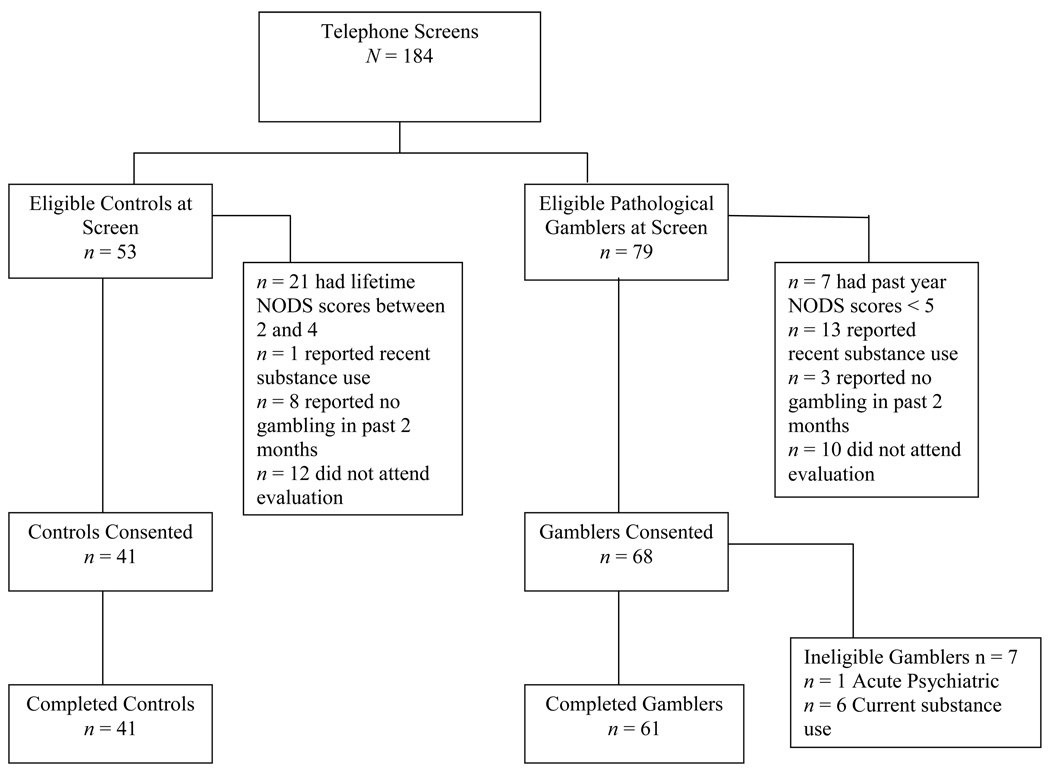
Sixty-one pathological gamblers (PGs) and 41 control participants were recruited for this study. Participants were recruited through newspaper and radio ads and a gambling treatment program. The ads asked for participants who “gambled frequently,” or “gambled recreationally” to participate in a one-day study to explore factors associated with gambling. The flow of participants through the study is presented in Figure 1.
Figure 1.
Flow of participants through the study.
PGs met current diagnostic criteria for pathological gambling (PG) as assessed by the National Opinion Research Center DSM Screen for Gambling Problems (NODS; Gerstein et al., 1999); were 18 years or older; had gambled in the past 2 months; were English speaking; and were able to provide informed consent. Participants were excluded if they had acute and severe psychiatric disorders (i.e., acute suicidality, uncontrolled psychosis). They were also excluded if they had current (i.e., within the past 12 months) substance dependence other than nicotine or caffeine, or if they provided a positive toxicology screen for any substance (including cocaine, opioids, marijuana and alcohol). Current substance abstinence was confirmed using urine toxicology tests for cocaine, opioids and marijuana using Ezscreen test sticks (Editek, Burlington, NC) and breath screening test for alcohol using Alcosensor IV Alcometer (Intoximeters, St. Louis, MO). PGs with substance use disorder (SUD) histories met DSM-IV criteria for lifetime abuse or dependence (but not past 12 months) on cocaine, opiates, marijuana or alcohol, while PGs without a SUD history did not meet criteria for abuse or dependence. Just under half (n = 27, 44.3%) of the PGs were recruited from treatment and slightly over half (n = 34, 55.7%) were non-treatment-seekers recruited from the community.
Control participants met the same inclusion and exclusion criteria as PGs without SUD histories, except that control participants were excluded if their lifetime NODS score was greater than 1. Table 1 shows demographic characteristics and shows the 3 groups did not differ significantly with respect to demographic characteristics.
Table 1.
Demographic and gambling variables for pathological gamblers with and without substance use disorder histories, and non-problem gambling control participants.
| Variable | PGs with SUD (n = 31) |
PGs without SUD (n = 30) |
Controls (n = 41) |
Test (χ2 or F) | p value |
|---|---|---|---|---|---|
| Age M (SD) | 44.5(13.3) | 48.4(14.0) | 45.7(17.9) | F (2,99) = .50 | .61 |
| Gender Women % (n) | 35.5(11) | 53.3(16) | 58.5(24) | χ2 (2, N = 102) = 3.94 | .14 |
| Race % (n) | χ2 (2, N = 102) = 0.02 | .99 | |||
| Caucasian | 87.1 (27) | 86.7 (26) | 87.8 (36) | ||
| Other | 12.9 (4) | 13.3 (4) | 12.2 (5) | ||
| Marital % (n) | χ2 (2, N = 102) = 4.40 | .11 | |||
| Married/Cohabitating | 22.6 (7) | 40.0 (12) | 46.3 (19) | ||
| Unmarried | 77.4 (24) | 60.0 (18) | 53.7 (22) | ||
| Employed full time % (n) | 51.6 (16) | 50.0 (15) | 58.5 (24) | χ2 (2, N = 102) = 0.60 | .74 |
| Income | $22,000(52,000) | $37,500(33,250) | $22,000(37,600) | χ2 (2, N = 102) = 2.61 | .27 |
| Education % (n) | χ2 (2, N = 102) = 0.06 | .97 | |||
| < 12th grade | 3.2 (1) | 3.3 (1) | 2.4 (1) | ||
| 12th grade or higher | 96.8 (30) | 96.7 (29) | 97.6 (40) | ||
| Shipley Estimated IQ M (SD) | 113.2(9.9) | 109.0(9.6) | 114.3(10.8) | F (2,99) = 2.88 | .06 |
| Gambling Items | |||||
| Past Year NODS Score M (SD) | 7.4(1.9) a | 7.1(1.6) a | 0.2(0.4) b | F (2,99) = 318.59 | .001 |
| Lifetime NODS Score M (SD) | 8.1(1.7) a | 7.7(1.7) a | 0.3(0.5)b | F (2,99) = 409.71 | .001 |
| Days gambled/Past 30 | 10(20) | 7(10) | 1(3) | χ2 (2, N = 102) = 34.30 | .001 |
| $ Gambled/Past 30 | $760(700) | $550(1,763) | $15(89) | χ2 (2, N = 102) = 45.05 | .001 |
| Age first gambled | 15(8) | 22(23) | 18(8) | χ2 (2, N = 102) = 13.41 | .001 |
| Current gambling debt | $200(19,000) | $800(14,250) | $0(0) | χ2 (2, N = 102) = 39.35 | .001 |
| Lifetime gambling debt | $30,000(97,000) | $28,000(48,000) | $0(0) | χ2 (2, N = 102) = 68.76 | .001 |
Note. Values are Median and Interquartile Range unless otherwise specified; M(SD) = Mean and Standard Deviation
Note. Superscript a is significantly more than b, p < 0.05
2.2 Procedure
This study was approved by the Institutional Review Board of the University of Connecticut Health Center, and participants provided written informed consent before participating. Participants were told that if they were ineligible for the study following a brief interview, they would be paid $20 for their time. Eligible participants were told that they would receive a minimum of $50 for completing the study plus bonuses (up to $15 extra, total $65). To encourage best possible performance on the tasks, the $15 bonus depended on how accurately they scored on computer-based measures of impulsivity. Specifically, participants who scored better than the average of previously established norms on three out of six of the computer-based tasks (described below) received a bonus.
The research assistant collected and tested urine and breath samples for the presence of drugs or alcohol. An intake interview followed that included screening and self-report measures. The participant completed the computer tasks in a sound resistant room that included a desk, chair and computer with a 14” monitor. All tasks were administered in counterbalanced order across subjects. The total duration of the assessment was approximately three hours. After completion of the tasks, the research assistant answered any questions the participant had and provided payment.
2.3 Measures
A demographic form was used to collect basic demographic information (e.g., age, gender, race, etc.). Sections of the Structured Clinical Interview for the DSM-IV (First et al., 1997) were used to assess substance abuse and dependence, and to assess exclusion criteria. To control for possible relationships between attention, working memory and IQ, participants also completed the Shipley Institute of Living Scale, a valid measure of overall intelligence (Zachary et al., 1985).
The NORC DSM Screen for Gambling Problems (NODS) was used to diagnose PG. The NODS is based on DSM-IV criteria for PG, and is a valid assessment of lifetime and current PG (Gerstein et al., 1999; Hodgins, 2004).
2.3.1. Impulsivity Self-Report
The Barratt’s Impulsiveness Scale is a 30-item self-report measure with adequate reliability and validity (Barrett, 1985; Patton et al., 1995). It has three subscales, motor, attentional and non-planning. The Zuckerman’s Sensation Seeking Scale (SSS-V), a 40-item self-report measure, was used to assess sensation seeking. The validity and reliability of the SSS-V are acceptable and have been demonstrated in previous studies (Roberti et al., 2003; Zuckerman, 1979; Zuckerman, 1994).
2.3.2. Laboratory Impulsivity Tasks
The Delayed Discounting of Monetary Rewards task administered was identical to the one developed byPetry and Casarella (1999). Delay and reward amounts were presented on a computer screen. The delayed reward was always $1000. The amount of delay varied with each presentation (delay times were 1 week, 2 weeks, 1 month, 6 months, 1 year, 5 years and 25 years). Participants chose between a hypothetical dollar amount delivered immediately, versus $1000 delivered after a specified delay (immediate dollar amounts were $1, $3.50, $8.75, $17.50, $45, $60, $80, $95, $150, $200, $250, $300, $350, $400, $450, $500, $550, $600, $650, $700, $750, $800, $850, $920, $960, $980 and $1000). Delays and monetary amounts were presented in a counter-balanced order across participants. Greater impulsivity is indicated when participants discount greater delayed rewards in favor of smaller immediate rewards at higher rates. The outcome used in the current study is Area Under the Curve (AUC), for which smaller values are associated with greater impulsivity.
Single Key Impulsivity Paradigm (SKIP) is a measure of impulsivity related to an inability to delay reward and tendency to select smaller immediate rewards over larger delayed rewards (Swann et al., 2002). It is a 20-minute computer based task in which responses (click of a computer mouse) are made to obtain a reward. This task measures the rate and pattern of responses. Participants may respond as frequently as they wish, and the amount of reward the participant earns is proportional to the amount of delay between responses. However, the total amount of money earned is not contingent on the number of responses made by the participant. Rather, points are added to the participant’s score at a rate of 1 point every 2 seconds. Participants are given visual feedback about the amount of reinforcement received with each response, so they can infer that more rapid responding leads to smaller rewards, and adjust their responding to minimize responses. Longest time duration (in seconds) between two responses assesses impulsivity on this task (Dougherty, 2003; Mathias et al., 2002).
Immediate and Delayed Memory Tasks (IMT/DMT). The IMT is a computer-based measure of attention and impulsivity (Dougherty et al., 2000; Dougherty et al., 2003). A series of five digit numbers are presented in black print on a white background on a computer screen. Each five-digit stimulus is presented for 500ms, with a blank screen presented for 500ms between stimuli. Participants respond when the number on the screen matches the one that preceded it. A hit occurs when the participant correctly detects and responds to the target stimulus before the next stimulus is presented on the screen. A miss is a failure to respond to a target stimulus when it is presented. Commission errors occur when the participant responds to catch stimuli (numbers that differ from the preceding number by only one of the five digits).
The procedures for the DMT task are nearly identical to the IMT task. However, the DMT assesses the participant’s ability to retain information in working memory following a brief delay after the presentation of interference stimuli. Each stimulus is separated by three 500ms presentations of the five-number sequence ‘12345’. In both the IMT and DMT tasks, target, catch and filler stimuli are presented. Hits, misses, commission errors, other errors and response latencies are recorded. Lower numbers of hits, shorter response latencies, and higher numbers of misses, and commission errors are indicative of greater impulsivity (Dougherty et al., 2000). We used number of commission errors on the IMT and DMT as measures of attention-impulsivity.
GoStop Impulsivity Paradigm (GoStop) was used to measure response inhibition (Dougherty, 2003). In this task, participants are presented with a consecutive series of 5-digit numbers on a computer screen. A novel stimulus (new, previously unseen set of 5-numbers) is presented followed by a target stimulus (a set of numbers identical to the novel stimulus; in black font). Participants are instructed to refrain from responding to the novel stimuli. Participants are also instructed to click the mouse when presented with the target (go) stimulus, but refrain from clicking the mouse if the target (go) stimulus font turns from black to red (i.e., a ‘stop’ signal). Target stimuli that become stop signals change from ‘go’ (black) to ‘stop’ (red) at latency intervals of 50 sec, 150 msec, 250 msec and 350 msec after onset of presentation. Half of the responses are novel stimuli, and half are targets with half of the targets turning from a ‘go’ to a ‘stop’ signal. We used percentage of inhibited responses (proportion of correctly inhibited responses to the number of stop signals presented) for each latency interval (i.e., 50 through 350 msec) as measures of response inhibition (Marsh et al., 2002).
Balloon Analogue Risk Task (BART) was used as a measure of risk taking (Lejuez et al., 2003). A computer screen displays a simulated balloon with a pump, a button labeled “collect $$$”, a “total earned” display and a “last balloon” box that displays the amount of money earned with the previous balloon. The participant is instructed to press a key several times to pump up the balloon. With each pump, the simulated balloon inflates slightly, and $0.05 is added to the score. If the balloon is pumped too much, it explodes, and the money accumulated is reset to zero. A new balloon replaces the popped one, and responding begins again. Before the balloon pops, the participant may press the “collect $$$” button at any time to have the money accumulated added to his or her total score. Each participant completed 30 trials (balloons), and the adjusted average number of responses (pumps) was used as the dependent variable. Higher scores indicate greater risk-taking.
The Paced Auditory Serial Addition Task (PASAT; Brown et al., 2002; Gronwall, 1977) is a mental arithmetic task presented in three levels of increasing duration and difficulty and was used as a distress tolerance task. The procedure used in this study was based on that used by Brown and colleagues (2002). Sequential numbers are presented on a screen, and one sums the most recent number with the one that directly preceded it. The participant then adds another number to the previously presented one (e.g., 5 + 1 [=6], + 3 [=4], + 5 [=8] and so on). A point is added to the score when s/he responds correctly. Each of the three levels last 3, 5 and 10 minutes, respectively. The arithmetic task is made more difficult with each level by decreasing the latencies between response presentations, with level 1 through 3 using latencies of 3s, 1.5s and 1s, respectively. This increasing difficulty creates greater subjective distress. In level 3, respondents are provided with an “escape” button to click if s/he wished to end the task prematurely. Additionally, participants are asked to rate their affective state immediately before and after administration of the arithmetic tasks. Specifically, participants provide ratings from 1 (not at all) to 5 (extremely) on several affective terms (e.g., anxious, irritable, nervous, hostile). The dependent variable in the current study was whether or not the participant pressed the escape button (yes vs. no). We also examined changes in affect to ensure the PASAT in fact did negatively influence affect.
2.4 Data Analysis
Initial data analysis involved assessing differences between PGs and controls on demographic variables (e.g., gender, age) and those thought to be possibly related to impulsivity (e.g., IQ), using parametric or non-parametric statistics as appropriate.
Multivariate analysis of variance (MANOVA) was used to examine the effect of the independent variable, gambling status (PG with substance use disorder history, PG without substance use disorder history, and control) on scores on three scales of the BIS, number of commission errors on the immediate and delayed attention tasks (IMT/DMT), area under the curve (AUC) calculated from delayed discounting values, SKIP longest duration between responses, adjusted pumps on the BART and total score on the sensation seeking scale. Variables were log or square root transformed as appropriate prior to analysis. Intercorrelations among these dependent variables are also presented. GoStop task data were evaluated and presented separately using repeated measures analysis of variance (ANOVA), as the values for this scale are most suited to a within subject analysis, and because of high intercorrelation between different delay times associated with this measure. The PASAT distress tolerance scale outcome is dichotomous (quit task vs. completed task) and so was analyzed using the Pearson chi-square test.
Analyses were repeated to examine substance dependence (rather than dependence and/or abuse), slight (non-significant) differences between groups on gender, and to explore potential differences between PGs recruited from treatment versus the community. Differences in statistical significance or direction from the major analyses are reported.
3. Results
3.1. Demographics, Gambling Variables and Substance Use Disorders
Table 1 presents data comparing the three groups, PGs with and without SUD, and controls, on demographic and gambling variables. The groups did not differ on age, gender, race, marital status, employment, education or income. Thus, none of these variables were included as covariates in the impulsivity analyses. A marginal, but non-significant, difference was found across groups for estimated IQ. As expected, regardless of SUD history, PGs reported greater lifetime and past year gambling symptoms, spending more money and time gambling and greater gambling debt compared with control participants. PGs with a history of SUD gambled significantly more days in the previous 30 days (U = 320.0, p < .05), and began gambling at an earlier age (U = 239.0, p < .001) than PGs without SUD histories. However, these two groups did not differ on past year or lifetime NODS scores, current or lifetime gambling debt, or amount of money spent gambling in the previous 30 days (all p’s > .05).
About half of the PGs with SUD history (n = 16, 51.6%) had a history of alcohol use disorder (abuse and or dependence) only. An additional two participants had histories of only marijuana use disorder (6.5%), and one had cocaine abuse (3.2%). The remaining participants with SUD (n = 12, 38.7%) all had a history of abuse of or dependence on more than one substance (e.g., alcohol and cocaine, or alcohol and marijuana). Table 2 presents DSM-IV substance use diagnoses for these participants.
Table 2.
DSM-IV diagnoses of pathological gamblers reporting substance use disorder history.
| Substance Use Disorder | Frequency % (N) |
|---|---|
| Alcohol Abuse Only | 25.8(8) |
| Alcohol Dependence Only | 25.8(8) |
| Marijuana Abuse Only | 3.2(1) |
| Marijuana Dependence Only | 3.2(1) |
| Cocaine Abuse Only | 3.2(1) |
| Cocaine Dependence Only | 0(0) |
| Opioid Abuse Only | 0(0) |
| Opioid Dependence Only | 0(0) |
| Alcohol and Marijuana Abuse | 3.2(1) |
| Marijuana Dependence with Alcohol Abuse | 6.5(2) |
| Marijuana Dependence with Alcohol and Cocaine Abuse | 3.2(1) |
| Cocaine Dependence with Alcohol and Marijuana Abuse | 3.2(1) |
| Alcohol and Marijuana Dependence | 6.5(2) |
| Alcohol and Cocaine Dependence | 3.2(1) |
| Alcohol, Cocaine and Marijuana Dependence | 6.5(2) |
| Cocaine, Opioid and Marijuana Dependence | 3.2(1) |
| Alcohol, Cocaine, Opioid and Marijuana Dependence | 3.2(1) |
Our primary analyses compared PGs having any lifetime substance use disorder (abuse or dependence) to those with no history of SUD. Because abuse is a fairly low threshold condition, a lack of difference between these groups might be due to the mild nature of substance abuse (compared to dependence). Thus, we also conducted secondary analyses comparing PGs with (n = 20) and without (n = 41) substance dependence to control participants. Because few stastically significant differences were found between those with and without substance dependence that were not identified in the comparison of PGs with and without a SUD, we focus on comparisons of those with and without SUD histories and briefly describe any differences that occur between the two sets of analyses.
3.2. Impulsivity
Correlations among the impulsivity measures are presented in Table 3. Significant positive correlations were found among the three scales of the BIS (Motor, Non-planning and Attention), and between the IMT and DMT commission error measures. A greater rate of discounting was associated with higher scores on the BIS Non-planning and Attention scales. A significant negative correlation was found between the BIS Motor scale and the IMT variable, suggesting that greater motor impulsivity was associated with fewer commission errors. Better response inhibition on the 150 msec delay of the Go-Stop was associated with fewer commission errors on the DMT and IMT.
Table 3.
Spearman’s rho correlations between impulsivity variables.
| Variable | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. BIS Motor | .37*** | .53*** | −.23* | −.04 | −.14 | .01 | .01 | .13 | −.12 | −.04 | .00 | .03 | −.16 |
| 2. BIS Non-planning | .61*** | −.10 | −09 | −.28** | .11 | .07 | .12 | .01 | −.10 | −.10 | .02 | −.11 | |
| 3. BIS Attention | −.09 | −.11 | −.36*** | −.02 | −.01 | .03 | .05 | −.11 | −.12 | −.09 | −.12 | ||
| 4. IMT | .63*** | .11 | .04 | .02 | .03 | .14 | −.18 | −.28** | −.13 | −.03 | |||
| 5. DMT | .11 | −.04 | −03 | .05 | .05 | −.15 | −.22* | −.09 | .03 | ||||
| 6. Discounting Area Under Curve | .03 | −.18 | −.07 | −.12 | .13 | .04 | .04 | .10 | |||||
| 7. SKIP | .08 | .18 | −.15 | −.04 | −.13 | −.16 | −.19 | ||||||
| 8. BART Pump Adj. Average | .15 | −.14 | −.08 | .03 | −.05 | −.07 | |||||||
| 9. Zuckerman Scale | −.11 | .16 | .05 | −.07 | −.19 | ||||||||
| 10. PASAT Distress Tolerance † | −.15 | .04 | .11 | .21* | |||||||||
| 11. Go-Stop 50 msec | .53 | .41*** | .18 | ||||||||||
| 12. Go-Stop 150 msec | .73*** | .53** | |||||||||||
| 13. Go-Stop 250 msec | .63*** | ||||||||||||
| 14. Go-Stop 350 msec |
p < .05
p < .01
p < .001
Point biserial correlation
Variables: 1) Barratt Impulsiveness Motor Scale; 2) Barratt Impulsiveness Non-Planning Scale; 3) Barratt Impulsiveness Attention Scale; 4) Immediate Memory Task Commission Errors; 5) Delayed Memory Scale Commission Errors; 6) Delay Discounting Area Under the Curve; 7) Single Key Impulsivity Paradigm; 8) Balloon Analogue Risk Task, Adjusted Average Pump; 9) Zuckerman Sensation Seeking Scale; 10) Paced Auditory Serial Arithmetic Task, Distress Tolerance Measure; 11) Go-Stop percent inhibited 50 msec delay; 12) Go-Stop percent inhibited 150 msec delay; 13) Go-Stop percent inhibited 250 msec delay; 14) Go-Stop percent inhibited 350 msec delay.
The three groups differed in several forms of impulsivity, and the overall MANOVA predicting impulsivity scores was statistically significant (Wilks’ Lambda F(18,182) = 4.08, p ≤; .001). Results are presented in Table 4. Significant differences across groups were found for the BIS non-planning and attention subscales, delay discounting AUC, and BART adjusted pumps.
Table 4.
MANOVA results for pathological gambling and control participants.
| Variable | Pathological Gamblers with SUD (n = 31) |
Pathological Gamblers without SUD (n = 30) |
Controls (n = 41) |
F(2,99) |
|---|---|---|---|---|
| BIS Motor | 17.7(4.0) | 17.7(3.9) | 15.9(2.9) | 2.8 |
| BIS Non-Planning | 22.3(3.9) a | 22.1(4.6) a | 19.3(2.7) b | 7.4*** |
| BIS Attention | 24.8(4.4) a | 24.6(45.1) a | 18.8(4.7) b | 18.9*** |
| Immediate Memory Task (% Commission Errors) | 25.8(15.8) | 25.9(13.8) | 28.4(10.6) | .5 |
| Delayed Memory Task (% Commission Errors) | 21.3(18.5) | 26.8(16.9) | 29.7(20.4) | 1.8 |
| Delayed Discounting AUC | .27(.19) a | .30(.19) a | .51(.19) b | 17.9*** |
| SKIP Longest Time Duration Score | 191.7(239.9) | 154.0(164.7) | 269.2(328.2) | .7 |
| BART Adjusted Pumps | 29.3(10.4) a | 21.3(13.2)b | 25.3(11.0) | 3.6* |
| Sensation Seeking Scale | 16.3(6.2) | 13.9(7.0) | 14.1(6.8) | 1.5 |
p ≤ .05
p ≤ .01
p ≤ .001
Values presented are raw means and standard deviations. Transformed variables were used in the analyses when appropriate. AUC = Area Under the Curve.
Post hoc analyses – superscript a ≠ b, p ≤ .05
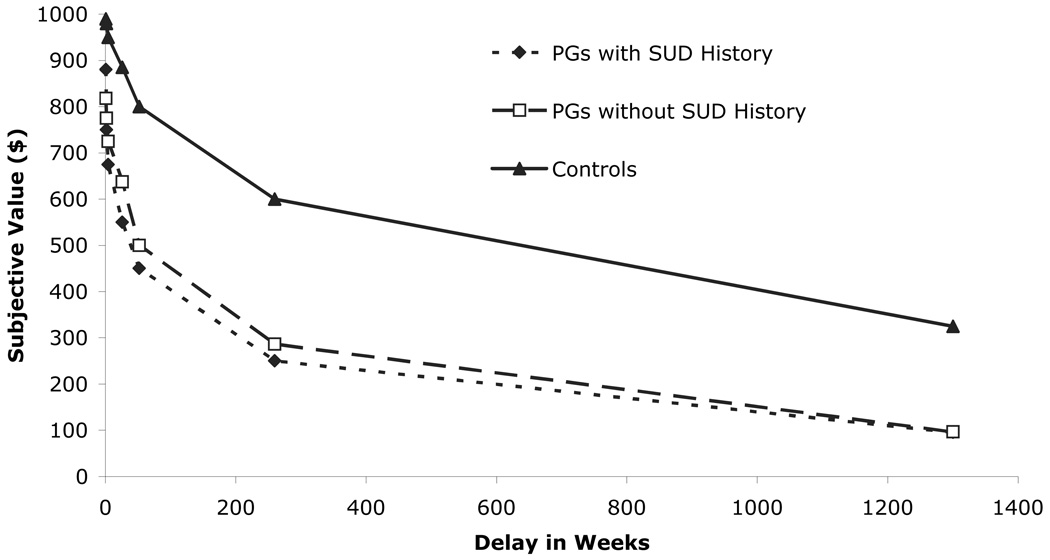
Post-hoc analyses indicated that both groups of PGs demonstrated significantly greater impulsivity on the non-planning and attention scales of the BIS than control participants, regardless of whether they had a history of SUD. Additionally, PGs, regardless of SUD history, experienced significantly lower area under the curve (AUC) values on the delayed discounting task than non-problem gamblers, indicating greater discounting of delayed rewards. To more fully appreciate the differences between PGs and controls, median crossover values for specific dollar amounts at specific delay points are presented in Figure 2.
Figure 2.
Delay discounting subjective dollar amounts and delay periods for pathological gamblers (PGs) with and without a history of substance use disorder (SUD), and control participants.
Significant differences were noted between PGs with and without SUD on the BART such that, gamblers with SUD histories pumped the balloons more times on average, suggesting that these participants took more risks during the task (p ≤ .05). However, neither group of PGs differed from controls on the BART or other measures including BIS motor, IMT or DMT commission errors, SKIP and Zuckerman Sensation Seeking Scale (all p > .05).
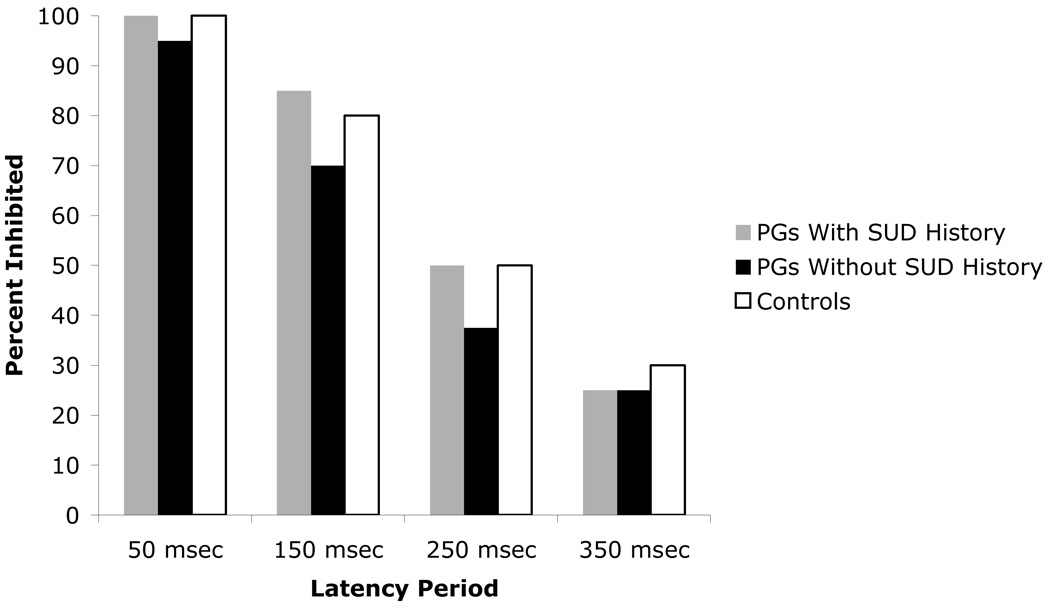
In the within subjects analysis, the three groups did not differ in their ability to inhibit their responses when presented with a stop stimulus on the GoStop task. Figure 3 shows that as the delay between the start signal and the stop signal increased (from 50 msec to 350 msec), participants experienced greater difficulty inhibiting their responses, F(3,297) = 367.04, p ≤ .001. However, this difficulty was the same across all three groups (F(2,99) = .65, p = .52), and no interactions between group and stop signal delay time (F(6,294) = .49, p = .77) were found. As there was evidence of a ceiling effect for the 50msec delay (i.e., nearly all 50msec trials resulted in appropriate response inhibition), we also conducted the analysis excluding the 50msec delay. The findings were unchanged.
Figure 3.
Median percent response inhibition at four latency periods for pathological gamblers (PGs) with and without a history of substance use disorder (SUD), and control participants.
On the PASAT, a significant increase in negative affect was noted from immediately before the task to immediately after for all three groups (all p < .05), indicating that the task had the intended purpose of causing affective distress. In total, 36.7% of the PGs with and 36.7% of the PGs without SUD quit the task, compared with 24.4% of the controls. These proportions did not differ significantly (χ2 (2, N = 101) = 1.70, p = .43) suggesting that the groups experienced similar distress tolerance levels overall. Further, there were no significant differences in the amount of time passed before participants quit the task or the task concluded (χ2 (2, N = 102) = 1.19, p = .55).
Because women were slightly over-represented in the control group, we repeated the MANOVA and the repeated measures ANOVA including gender as an independent variable. The results were unchanged.
We also re-analyzed the data using the presence or absence of substance dependence history (rather than SUD history) among the PGs. The results were largely unchanged with three exceptions. First, a significant omnibus main effect was found for the BIS Motor Impulsivity scale (F(2,99) = 3.08, p = .05) but post hoc analyses revealed no specific differences between any two specific groups. Second, the main effect for the BART was no longer statistically significant, (F(2,99) = 1.02, p = .37). Third, when we re-analyzed the PASAT data, comparing controls to PGs with and without dependence histories, 57.9% of PGs with a history of substance dependence quit the task, compared with 26.8% of PGs without a dependence history and 24.4% of controls (χ2 (2, N = 101) = 7.49, p < .05).
Finally, we also considered that PGs recruited from the community might differ from those recruited from treatment, but analyses comparing these two groups revealed no between-group differences on any measure of impulsivity (p > .05).
4. Discussion
Our study demonstrates that PGs may be more impulsive than non-problem gambling control participants in fairly specific ways. PGs discounted future, larger rewards in favor of immediate, smaller ones at a rate significantly higher than comparable control participants, a finding that is similar to past studies (Alessi and Petry, 2003; Petry, 2001a; Petry, 2001b; Petry and Casarella, 1999). Further, PGs reported acting on the spur of the moment, experiencing difficulty planning and thinking carefully and having greater difficulty maintaining attention as measured by the BIS than non-problem gamblers.
We found, however, that PGs did not have significantly more difficulty inhibiting responses when faced with a stop signal than control participants, a finding that is contrary to some prior published studies that found poorer response inhibition in PGs (Fuentes et al., 2006; Goudriaan et al., 2006). Further, PGs did not perform differently from controls on two measures of sensation seeking and risk taking, a finding that is also contrary to some (Vitaro et al., 1998) but not all (Petry, 2001b; Steel and Blaszczynski, 1998) previous studies. Some factors may account for the discrepancies between our study and some past investigations. Our participants were relatively well educated and had higher than average intelligence, two predictors of cognitive functioning that may account for similarities between the two groups. Thus, our findings may be a particularly accurate representation of gamblers and controls from the community. Whereas most prior research has included only treatment seeking gamblers, who may have more severe dysfunction and impulsivity, our study included gamblers from treatment and from the community and found no significant differences between these individuals. Additionally, with regard to sensation seeking, it is also notable that other studies have also failed to find PGs score higher than controls (e.g., Blanco et al., 1996; Powell et al., 1999), suggesting that sensation seeking may be distinguishable from impulsivity and is not necessarily characteristic of PGs.
We predicted, a priori, that PGs with SUD histories would be greater risk takers than those without, and PGs with a SUD history exhibited more risk taking behavior on the BART. Contrary to our initial hypotheses, however, PGs with and without SUD histories did not differ on any of the other measure of impulsivity assessed.
These two groups did not differ on the delayed discounting task or on self-reported impulsivity (BIS scales), response inhibition, attention/memory, sensation seeking or distress tolerance tasks in this study. The same was true when we re-analyzed the data to compare PGs with and without substance dependence history. The only exception was that PGs with dependence histories were more likely to “escape” during the distress tolerance task, suggesting that those with past substance dependence may have lower distress tolerance. Again, because our samples were well matched on demographics, our results may provide a particularly accurate representation of the differences between PGs with and without SUD histories. However, it is also important to consider that because our participants were recruited from among treatment seekers (56%) and from those who responded to newspaper ads (44%), they may differ from the general population of PGs in certain ways.
Our findings have implications for the treatment for PG. Higher impulsivity in PGs may place treatment-seeking gamblers at high risk for relapse (Ledgerwood and Petry, 2006b), and treatment failure (Leblond et al., 2003). Specifically, treatment benefits may be delayed in time, and individuals who tend toward being impulsive may disregard such future benefits for the more immediate potential benefits of gambling. This concept is consistent with our finding that PGs discount delayed rewards at a significantly greater rate than control participants. However, PGs with a SUD history appear equally impulsive to those without a SUD history, even though, in this sample, all the dual diagnosis participants were successful in reducing their problems with drug and/or alcohol.
Impulsivity may also place PGs at greater risk of co-occurring psychosocial problems that make initiation of recovery more difficult. Individuals who are more impulsive are also more likely to have greater difficulty in financial, employment, family/relationship, and legal domains. Co-occurring psychopathology may also be associated with greater impulsivity, further exacerbating such problems (Crean et al., 2000). Thus, treatment approaches for PGs should explicitly address impulsivity and psychosocial problems in addition to the gambling behavior.
Our study has some limitations. Because we selected a sample with a history of SUD, rather than active, current SUD, it is difficult to determine whether these participants are similar to the general population of PGs that may also include individuals with current drug or alcohol dependence. We purposefully excluded currently substance dependent participants because we would not be able to determine whether impulsivity is a trait of the participants, or is directly related to withdrawal from substance use or the psychoactive effects of the substance. However, some recent studies have reported a significant relationship between impulsivity and unsuccessful treatment outcomes (Moeller et al., 2001; Patkar et al., 2004), necessitating additional study on this issue. Further, due to sample size limitations, we were unable to determine whether specific drugs of abuse were more likely to be associated with greater impulsivity or risk taking. Finally, because of the relatively small groups (n’s from 30 to 40), non-significant findings should be considered carefully, as a larger sample size may have had greater statistical power to identify group differences.
Several strengths of the current study are also of note. First, this is one of only a few studies to systematically examine specific facets of impulsivity and their associations with PG. Many previous investigations have utilized a unitary or general measure of impulsivity without considering that impulsivity may be a multidimensional construct. Our study suggests that impulsivity may be multifaceted, and PGs may manifest impulsivity in fairly specific, rather than gross, ways. Second, this study is one of only a handful of investigations that explores impulsivity in PGs using primarily a behavioral task-based approach, rather than simply using subjective self-report questionnaires. Participants reading a question may not have the specific insight into their own behavior required to make judgments in all circumstances. Also, self-report questionnaires may not assess all aspects of impulsivity. Thus, utilizing a task-based approach in addition to questionnaires helps to assess impulsivity more accurately. Third, as mentioned above, we were able to obtain a control sample with very similar demographic features to our PG participants, and the two PG groups were also very similar.
In summary, the current study examined impulsivity as a multifaceted trait, and it relied mostly on behavioral assessments that did not depend on participant interpretations of their own behavior. With a greater understanding of the ways in which PGs differ from individuals who gamble without problems, we may develop treatment strategies that focus on the specific deficits or characteristics that contribute to maladaptive gambling in this population.
Acknowledgements
We thank Dr. Donald Dougherty who provided the IMT/DMT, SKIP and GO/STOP measures, Carl Lejuez who provided the BART and PASAT measures, and Marvin Zuckerman who provided the Sensation Seeking Scale. We also thank those who participated in our study.
Role of Funding Sources
This study was supported by funding from the National Center for Responsible Gaming as provided by the Institute for Research on Pathological Gambling and Related Disorders in the Division on Addictions at the Cambridge Health Alliance, a teaching affiliate of Harvard Medical School. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Center, Cambridge Health Alliance or the Institute. Additional funding was provided by R01 MH60417-Cont.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
None
References
- Alessi SM, Petry NM. Pathological gambling severity is associated with impulsivity in a delay discounting procedure. Behav Process. 2003;64:345–354. doi: 10.1016/s0376-6357(03)00150-5. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: Author; 1994. [Google Scholar]
- Barratt ES. Impulsiveness subtraits: Arousal and information processint. In: Spence JT, Izard CE, editors. Motivation, emotion and personality. North-Holland: Elsevier; 1985. pp. 137–146. [Google Scholar]
- Blanco C, Orensanz-Munoz L, Blanco-Jerez C, Saiz-Ruiz J. Pathological gambling and platelet MAO activity: A psychobiological study. Am J Psychiat. 1996;153:119–121. doi: 10.1176/ajp.153.1.119. [DOI] [PubMed] [Google Scholar]
- Blaszczynski A, Nower L. A pathways model of problem and pathological gambling. Addiction. 2002;97:487–499. doi: 10.1046/j.1360-0443.2002.00015.x. [DOI] [PubMed] [Google Scholar]
- Blaszczynski A, Steel Z. Personality disorders among Pathological gamblers. J Gambl Stud. 1998;14:51–71. doi: 10.1023/a:1023098525869. [DOI] [PubMed] [Google Scholar]
- Brown RA, Lejuez CW, Kahler CW, Strong DR. Distress tolerance and duration of past smoking cessation attempts. J Abnorm Psychol. 2002;111:180–185. [PubMed] [Google Scholar]
- Crean JP, de Wit H, Richards JB. Reward discounting as a measure of impulsive behavior in a psychiatric outpatient population. Exp Clin Psychopharm. 2000;8:155–162. doi: 10.1037//1064-1297.8.2.155. [DOI] [PubMed] [Google Scholar]
- Cyders MA, Smith GT. Emotion-based dispositions to rash action: Positive and negative urgency. Psychol Bull. 2008;134:807–828. doi: 10.1037/a0013341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty DM. Neurobehavioral Research Laboratory and Clinic. Houston TX: University of Texas Health Science Center at Houston; 2003. Laboratory behavioral measures of impulsivity: General information manual. [Google Scholar]
- Dougherty DM, Bjork JM, Harper RA, Marsh DM, Moeller FG, Mathias CW, Swann AC. Behavioral impulsivity paradigms: A comparison in hospitalized adolescents with disruptive behavior disorders. J Child Psychol Psyc. 2003;44:1145–1157. doi: 10.1111/1469-7610.00197. [DOI] [PubMed] [Google Scholar]
- Dougherty DM, Bjork JM, Marsh DM, Moeller G. A comparison between adults with conduct disorder and normal controls on a continuous performance test: Differences in impulsiveresponse characteristics. Psychol Rec. 2000;50:203–219. [Google Scholar]
- Dougherty DM, Marsh DM. Neurobehavioral Research Laboratory and Clinic. Houston, TX: University of Texas Health Center at Houston; 2003. Immediate and delayed memory tasks (IMT/DMT 2.0): A research tool for studying attention, memory, and impulsive behavior. [Google Scholar]
- Evanden JL. Varieties of impulsivity. Psychopharmacology. 1999;14:348–361. doi: 10.1007/pl00005481. [DOI] [PubMed] [Google Scholar]
- Eysenck SBG, Eysenck HJ. Impulsiveness and venturesomeness: Their position in a dimensional system of personality description. Psychol Rep. 1978;43:1247–1255. doi: 10.2466/pr0.1978.43.3f.1247. [DOI] [PubMed] [Google Scholar]
- Finn PR, Justus A, Mazas C, Steinmetz JE. Working memory, executive processes and the effects of alcohol on Go/No-Go learning: testing a model of behavioral regulation and impulsivity. Psychopharmacology. 1999;146:465–472. doi: 10.1007/pl00005492. [DOI] [PubMed] [Google Scholar]
- Finn PR, Mazas CA, Justus AN, Steinmetz J. Early-onset alcoholism with conduct disorder: Go/NoGo learning deficits, working memory capacity, and personality. Alcohol Clin Exp Res. 2002;26:186–206. [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV Axis I Disorders (SCID-I) Washington: American Psychiatric Publishing; 1997. [Google Scholar]
- Fuentes D, Tavares H, Artes R, Gorenstein C. Self-reported and neuropsychological measures of impulsivity in pathological gambling. J Int Neuropsychol Society. 2006;12:907–912. doi: 10.1017/S1355617706061091. [DOI] [PubMed] [Google Scholar]
- Gerstein D, Murphy S, Toce M, Hoffmann J, Palmer A, Johnson R, Larison C, Chuchro L, Buie T, Engelman L, Hill MA. Gambling impact and behavior study. Chicago, IL: University of Chicago; 1999. [Google Scholar]
- Goudriaan AE, Oosterlaan J, De Beurs E, Van den Brink W. Neurocognitive functions in pathological gambling: a comparison with alcohol dependence, Tourette syndrome and normal controls. Addiction. 2006;101:534–547. doi: 10.1111/j.1360-0443.2006.01380.x. [DOI] [PubMed] [Google Scholar]
- Green L, Fristoe N, Myerson J. Temporal discounting and preference reversals in choice between delayed outcomes. Psychon B Rev. 1994;1:383–389. doi: 10.3758/BF03213979. [DOI] [PubMed] [Google Scholar]
- Gronwall DMA. Paced auditory serial-addition task: A measure of recovery from concussion. Percept Motor Skills. 1977;44:367–373. doi: 10.2466/pms.1977.44.2.367. [DOI] [PubMed] [Google Scholar]
- Hodgins DC. Using the NORC DSM screen for gambling problems as an outcome measure for pathological gambling: Psychometric evaluation. Addict Behav. 2004;29:1685–1690. doi: 10.1016/j.addbeh.2004.03.017. [DOI] [PubMed] [Google Scholar]
- Horn NR, Dolan M, Elliott R, Deakin JFW, Woodruff PWR. Response inhibition and impulsivity: An fMRI study. Neuropsychologia. 2003;41:1959–1966. doi: 10.1016/s0028-3932(03)00077-0. [DOI] [PubMed] [Google Scholar]
- Ladd GT, Petry NM. Gender differences among pathological gamblers seeking treatment. Exp Clin Psychopharm. 2002;10:302–309. doi: 10.1037//1064-1297.10.3.302. [DOI] [PubMed] [Google Scholar]
- Langewisch MWJ, Frisch GR. Gambling behavior and pathology in relation to impulsivity, sensation seeking, and risky behavior in male college students. J Gambl Stud. 1998;14:245–262. [Google Scholar]
- Leblond J, Ladouceur R, Blaszczynski A. Which Pathological gamblers will complete treatment. Brit J Clin Psychol. 2003;42:205–209. doi: 10.1348/014466503321903607. [DOI] [PubMed] [Google Scholar]
- Ledgerwood DM, Petry NM. Psychological experience of gambling and subtypes of pathological gamblers. Psychiat Res. 2006a;144:17–27. doi: 10.1016/j.psychres.2005.08.017. [DOI] [PubMed] [Google Scholar]
- Ledgerwood DM, Petry NM. What do we know about relapse in pathological gambling? Clin Psychol Rev. 2006b;26:216–228. doi: 10.1016/j.cpr.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Lejuez CW, Read JP, Kahler CW, Richards JB, Ramsey SE, Stuart GL, Strong DR, Brown DA. Evaluation of a behavioral measure of risk taking: The balloon analogue risk task (BART) J Exp Psychol Appl. 2002;8:75–84. doi: 10.1037//1076-898x.8.2.75. [DOI] [PubMed] [Google Scholar]
- Logan GD, Cowan WB, Davis KA. On the ability to inhibit responses in simple and choice reactions time tasks: A model and a method. J Exp Psychol Hum Percept Perform. 1984;10:276–291. doi: 10.1037//0096-1523.10.2.276. [DOI] [PubMed] [Google Scholar]
- Marsh DM, Dougherty DM, Mathias CW, Moeller FG, Hicks LR. Comparisons of women with high and low trait impulsivity using behavioral models of response-disinhibition and reward-choice. Pers Indiv Differ. 2002;33:1291–1310. [Google Scholar]
- Martins SS, Tavares H, da Silva Lobo DS, Galetti AM, Gentil V. Pathological gambling, gender, and risk-taking behaviors. Addict Behav. 2004;29:1231–1235. doi: 10.1016/j.addbeh.2004.03.023. [DOI] [PubMed] [Google Scholar]
- Mathias CW, Dougherty DM, Marsh DM, Moeller FG, Hicks LR, Dasher K, Bar-Eli L. Laboratory measures of impulsivity: A comparison of women with or without childhood aggression. Psychol Rec. 2002;52:289–303. [Google Scholar]
- Moeller FG, Dougherty DM, Barratt ES, Schmitz JM, Swann AC, Grabowski J. The impact of impulsivity on cocaine use and retention in treatment. J Substance Abuse Treatment. 2001;21:193–198. doi: 10.1016/s0740-5472(01)00202-1. [DOI] [PubMed] [Google Scholar]
- Patkar AA, Murray HW, Mannelli P, Gottheil E, Weinstein SP, Vergare MJ. Pre-treatment measures of impulsivity, aggression and sensation seeking are associated with treatment outcome for African-American cocaine-dependent patients. J Addict Dis. 2004;23:109–122. doi: 10.1300/J069v23n02_08. [DOI] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES. Factor structure of the barratt impuliveness scale. J Clin Psychol. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Petry NM. Pathological gamblers, with and without substance use disorder, discount delayed rewards at high rates. J Abnorm Psychol. 2001a;110:482–487. doi: 10.1037//0021-843x.110.3.482. [DOI] [PubMed] [Google Scholar]
- Petry NM. Substance abuse, pathological gambling, and impulsiveness. Drug Alcohol Depend. 2001b;63:29–38. doi: 10.1016/s0376-8716(00)00188-5. [DOI] [PubMed] [Google Scholar]
- Petry NM, Casarella T. Excessive discounting of delayed rewards in substance abusers with gambling problems. Drug Alcohol Depend. 1999;56:25–32. doi: 10.1016/s0376-8716(99)00010-1. [DOI] [PubMed] [Google Scholar]
- Petry NM, Stinson FS, Grant BF. Comorbidity of DSM-IV pathological gambling and other psychiatric disorders: Results from the National Epidemiological Survey on Alcohol and Related Conditions. J Clin Psychiat. 2005;66:564–574. doi: 10.4088/jcp.v66n0504. [DOI] [PubMed] [Google Scholar]
- Potenza MN, Leung H-C, Blumberg HP, Peterson BS, Fulbright RK, Lacadie CM, Skudlarski P, Gore JC. An fMRI stroop task study of ventromedial prefrontal cortical function in pathological gamblers. Am J Psychiat. 2003;160:1990–1994. doi: 10.1176/appi.ajp.160.11.1990. [DOI] [PubMed] [Google Scholar]
- Potenza MN, Steinberg MA, McLaughlin SD, Wu R, Rounsaville BJ, O’Malley SS. Gender-related differences in the characteristics of problem gamblers using a gambling helpline. Am J Psychiat. 2001;158:1500–1505. doi: 10.1176/appi.ajp.158.9.1500. [DOI] [PubMed] [Google Scholar]
- Powell J, Hardoon K, Derevensky JL, Gupta R. Gambling and risk-taking behavior among university students. Substance Use Misuse. 1999;34:1167–1184. doi: 10.3109/10826089909039402. [DOI] [PubMed] [Google Scholar]
- Roberti JW, Storch EA, Bravata E. Further psychometric support for the Sensation Seeking Scale-Form V. J Pers Assess. 2003;81:291–292. doi: 10.1207/S15327752JPA8103_12. [DOI] [PubMed] [Google Scholar]
- Shaffer HJ, LaBrie RA, LaPlante DA, Kidman RC. The Iowa Department of Public Health Gambling Treatment Services: Four years of evidence. Boston MA: Harvard Medical School; 2002. [Google Scholar]
- Specker SM, Carlson GA, Christenson GA, Marcotte M. Impulse control disorders and attention deficit disorder in pathological gamblers. Ann Clin Psychiat. 1995;7:175–179. doi: 10.3109/10401239509149623. [DOI] [PubMed] [Google Scholar]
- Steel Z, Blaszczynski A. Impulsivity, personality disorders and pathological gambling severity. Addiction. 1998;93:895–905. doi: 10.1046/j.1360-0443.1998.93689511.x. [DOI] [PubMed] [Google Scholar]
- Steinberg MA, Kosten TA, Rounsaville BJ. Cocaine abuse and pathological gambling. Am J Addiction. 1992;1:121–132. [Google Scholar]
- Swann AC, Bjork JM, Moeller FJ, Dougherty DM. Two models of impulsivity: Relationship to personality traits and psychopathology. Biol Psychiat. 2002;51:988–994. doi: 10.1016/s0006-3223(01)01357-9. [DOI] [PubMed] [Google Scholar]
- Vitaro F, Ferland F, Jacques C, Ladouceur R. Gambling, substance use, and impulsivity during adolescence. Psychol Addict Behav. 1998;12:185–194. [Google Scholar]
- Zachary RA, Crumpton E, Spiegel DE. Estimating WAIS-R IQ from the Shipley Institute of Living Scale. J Clin Psychol. 1985;41:532–540. doi: 10.1002/1097-4679(198511)41:6<820::aid-jclp2270410616>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Zuckerman M. Sensation seeking: Beyond the optimal level of arousal. Hillsdale NJ: Erlbaum; 1979. [Google Scholar]
- Zuckerman M. Behavioral expressions and biosocial bases of personality. New York: Cambridge University Press; 1994. [Google Scholar]