Abstract
Animal studies in mice, rats, rabbits, pigs and hens demonstrated that anterior keratocytes undergo programmed cell death or apoptosis after corneal epithelial injury. Many other wound healing changes subsequently follow the keratocyte apoptosis reponse. This study evaluated early keratocyte apoptosis after corneal epithelial scrape injury in human eyes scheduled for enucleation for malignancy. Two eyes had corneal epithelial scrape one hour prior to the enucleation and another eye served as a control and had no corneal scrape prior to enucleation. One additional eye was enucleated, washed with balanced salt solution, and then had the corneal epithelium scraped one hour prior to processing for analysis. Apoptosis was identified by terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) assay and confirmed by transmission electronic microscopy (TEM). Anterior keratocyte apoptosis was detected in the three corneas that had epithelial scrape injury, but not in the control unwounded cornea. This study confirmed that keratocyte apoptosis is also an early response to corneal epithelial injury in humans and showed that tears are not essential for keratocyte apoptosis to occur in response to epithelial injury.
Keywords: apoptosis, keratocyte, corneal wound healing, epithelial scrape, human
Studies in primates and other species observed that keratocytes in the anterior stroma apparently disappear immediately following epithelial scrape injury (Dohlman et al., 1968; Campos et al. 1994; Nakayasu, 1988). Wilson and coworkers (1996) demonstrated that this phenomenon is mediated by apoptosis or programmed cell death. Apoptosis is a controlled, gentle form of cell death that leads to the formation of apoptotic bodies that contain the cellular contents of the dying cell, including lysosomal enzymes or other intracellular components that would damage surrounding cells and tissue. These apoptotic bodies diffuse into the tissue and are absorbed by other living cells. Thus, apoptosis causes minimal damage to the surrounding cells and tissue and has a critical role in tissue development, homeostasis, response to infection, and wound healing (Arends and Wyllie, 1991; Wilson and Kim, 1998; Wyllie et al., 1980; Wilson, Chaurasia and Medeiros, 2007). The anterior keratocyte apoptosis response that occurs after epithelial injury in the cornea has been hypothesized to function as a mechanism to limit the extension of viruses such as herpes simplex that infect the corneal epithelium but have the capacity to infect keratocytes, endothelial cells, and other cell types that would allow the viral infection to extend into the eye and brain (Wilson, et al., 1997). Thus, it has been demonstrated that defective keratocyte apoptosis in Stat 1 null mice is associated with more aggressive herpes simplex keratitis (Mohan et al., 2000b).
Several studies have suggested the Fas/Fas ligand system and cytokines that regulate Fas/Fas ligand are involved in regulating early keratocyte apoptosis (Mohan et al., 1997; Mohan et al., 1998; Mohan et al., 2000a; Wilson et al., 1996). Triggers that injure the epithelium initiate these cytokine-death apparatus activation pathways.
Early keratocyte apoptosis has been reported in animal models after many different types of epithelial injury, including mechanical scrape, incisions, photorefractive keratectomy (PRK), laser in situ keratomileusis (LASIK) and infection such as herpes simplex keratitis (Gao et al, 1997; Helena et al., 1998; Martínez-García et al., 2006; Mohan et al., 2003; Netto at al., 2005; Wilson et al., 1997; Wilson, et al., 2003). Virtually any epithelial injury can induce keratocyte apoptosis. For example, even mild epithelial damage from pressing a plastic ring against the cornea will trigger superficial stromal keratocyte apoptosis (Helena et al., 1998; Wilson, 1998).
The location and level of early keratocyte apoptosis in the cornea is related to the type of epithelial injury (Helena et al., 1998; Wilson, 1997). For example, photorefractive keratectomy (PRK) and laser insitu keratomeliusis (LASIK) incite different levels of early apoptosis and at different locations (Mohan, et al., 2003). Interestingly, the intensities of the subsequent wound healing events — for example, keratocyte proliferation and myofibroblast transformation — are proportional to the early apoptotic response (Mohan et al., 2003). Moreover, there is also biological variability in this response between the eyes of different animals after the same type of stimuli (Mohan et al., 2003; Netto at al., 2005). This observation is of clinical significance because these differences relate the differences in outcomes between different eyes after the same surgical procedures (Dupps and Wilson, 2006).
Animal studies in mice, rats, rabbits, pigs and hens demonstrated that anterior keratocytes undergo programmed cell death or after epithelial scrape injury (Wilson, et al., 1996; Mohan, et al., 2003; Martinez-Garcia, et al., 2006). There are no studies determining whether keratocyte apoptosis occurs in human eyes that have corneal epithelial injury. In this study, we evaluated early keratocyte apoptosis after epithelial scrape in human corneas in a unique model involving patients with normal corneas and anterior segment scheduled for enucleation or exenteration of the eye because of choroidal melanoma or orbital malignancy. These experiments were approved by the Human Subjects Division of the Institutional Review Board of the University of Washington, Seattle, WA (HSD 99-2341-A 02) and by the Ethics Commission for Research Projects in Humans from the University of São Paulo (CAPPesq – 704/02) and informed consent was obtained from each patient.
Four subjects consented to participate in this study. Two eyes had corneal epithelial scraping with a #64 Beaver Blade over the entire central cornea, sparing only 0.5 mm at the limbus, one hour before enucleation (female, age 51 and male, age 56). One eye (male, age 41) had epithelial scrape performed after enucleation and profuse washing of the globe with balanced salt solution (BSS) to remove tears. After epithelial scraping, this eye was maintained for one hour in a humidified chamber at 37° C before excision of the corneal-scleral button. One eye (female, age 46) had no epithelial scrape and served as a control. The corneal-scleral buttons were removed using 0.12 forceps and sharp Westcott scissors immediately after enucleation, with the exception of the eye that was maintained in a humidified chamber. Immediately after the excision of the corneal-scleral buttons, each was partially fixed in 4% paraformaldehyde (PFA) for 4 hours before being bisected. Half of each cornea was then cryofixed in Optimal Cutting Temperature (OCT) compound (Tissue-Tek, Torrance, CA) within a 24 mm×24 mm×5 mm mould (Fisher, Pittsburgh, PA, USA for TUNEL assay and immunohistochemistry (IHC) and half was fixed for transmission electron microscopy (TEM).
The frozen tissue blocks were stored at −85 °C until sectioning was performed. Central corneal sections (7 μm thick) were cut with a cryostat (HM 505M, Micron GmbH, Walldorf, Germany). Sections were placed on 25 mm×75 mm×1 mm microscope slides (Superfrost Plus, Fisher) and tissue sections were fixed in acetone at −20 °C for 2 minutes, dried at room temperature for 5 minutes, and then placed in balanced salt solution. A fluorescence-based TUNEL assay was performed according to the manufacturer’s instructions (ApopTag, Cat No; S7165; Intergen, Purchase, NY, USA). Positive (rabbit cornea 4 hours after epithelial scrape) and negative (rabbit cornea, unwounded) control slides were included in each assay. Photographs were obtained with a Nikon E600 fluorescent microscope (Melville, NY, USA).
Tissue for TEM was fixed in 2% paraformaldehyde and 2% glutaraldehyde in a vehicle of 1.3 sodium phosphate buffer containing 0.05% MgCl2–6H2O, pH 7.3, at 4°C for 24 hours and then washed twice with the buffered fixative vehicle for 15 minutes at room temperature. Specimens were then stored at 4°C in the fixative vehicle until they were processed. A 1.5 mm strip was removed from the center of each half corneal-scleral button fixed for TEM. The strips were again bisected and the fragments were placed in the primary fixative vehicle prior to secondary fixation. Secondary fixation was performed in 1% OsO4 in 1.0 phosphate buffer, pH 7.3, for 45 minutes at room temperature, followed by three washings in the phosphate-buffered fixative vehicle and dehydration in a graded ethanol series. The transition from 100% ethanol to epoxy was mediated by two changes of propylene oxide. An epoxy medium of Spurr’s formulation (Spurr, 1969) was used for infiltration and embedding. The fragments were mounted in flat molds and hardened at −70°C for 24 hr before sectioning. Both 1 μm thick light microscopic sections and ultra-thin TEM sections were cut. The light microscopy sections were stained with 50% modified Richardson’s stain (1% methylene blue and 1% azure II in 1% sodium borate solution diluted 1:1 with 1 dibasic sodium phosphate solution at pH 8.5) and the TEM sections were mounted on polyvinyl butyral-coated grids (Pioloform, Sigma, St Louis, MO, USA) (Robards and Wilson, 1993) and stained with saturated aqueous uranyl acetate and Reynolds lead citrate (Reynolds, 1963). TEM was performed with a model PW6020, CM10 transmission electron microscope, Philips Electronics N.V. (Eindhoven, The Netherlands).
Superficial keratocyte apoptosis was detected by the TUNEL assay under the wounded area in all scraped corneas, including the case scraped after enucleation and washing with BSS to remove tears, although there was variability in the depth that the apoptosis extended into the stroma from the anterior surface in different corneas. The TUNEL assay was negative in the control cornea, as it was in the stromal beneath intact epithelium in scraped corneas (not shown). Keratocyte apoptosis (chromatin condensation, formation of apoptotic bodies, intracellular blebbing and cellular shrinkage) was detected by transmission electron microscopy in the anterior stroma of in all scraped corneas, including the one scraped after enucleation and washing with BSS to remove tears (Figure 1B).
Figure 1.
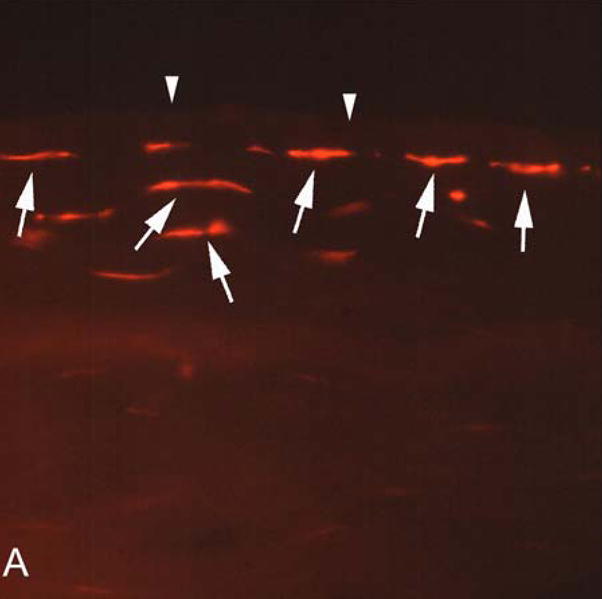
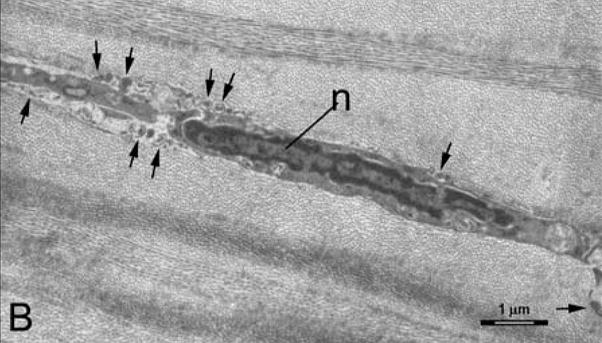
A. Keratocyte apoptosis detected in human corneas one hour after epithelial scrape injury prior to enucleation for a choroidal melanoma. TUNEL-positive keratocytes are detected (arrows) in the anterior stroma beneath Bowman’s layer (arrowheads). Magnification 400X. B. Transmission electron microscopy in a human cornea one hour after enucleation, washing with balanced salt solution, and epithelial scrape with a scalpel. Note condensation of the chromatin in the nucleus (n), cell shrinkage and formation of apoptotic bodies (arrows). Magnification 7,500X.
This study confirms keratocyte apoptosis occurs in the anterior sub-epithelial stroma immediately after epithelial scrape in human corneas, as has been reported in other animals, including mouse, rabbit and pigs. The time point after scraping used in this study was one hour, which is similar to the time course in other species. This study also confirms that tears are not essential for keratocyte apoptosis to occur after epithelial scrape injury, which is in agreement with a previous study in mice and rabbits (Mohan RR, Mohan, RR, Ambrósio Jr R, Wilson SE, Activation of keratocyte apoptosis in response to epithelial scrape injury does not require tears. Association for Research in Vision and Ophthalmology (ARVO) Annual Meeting, May, 2002. Program No. 1679). This supports the hypothesis that the stromal apoptosis response is mediated locally by modulators derived from the injured corneal epithelium and keratocytes (Mohan et al., 1997; Mohan et al., 2000a).
Acknowledgments
Supported in part by US Public Health Service grants EY010056 and EY015638 from the National Eye Institute and Research to Prevent Blindness, New York, NY. We are deeply grateful to Robert Kalina, MD, Rahul R. Mohan, PhD, Dan E. Possin, Jing Huang, MD, Marcelo V. Netto, M.D and Rajiv R. Mohan, PhD for their assistance with this study. This work was presented as part of the Thesis for the PhD Doctorate in Science by Renato Ambrósio Jr. at the University of São Paulo.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arends MJ, Wyllie AH. Apoptosis: mechanisms and roles in pathology. Int Rev Exp Pathol. 1991;32:223–54. doi: 10.1016/b978-0-12-364932-4.50010-1. [DOI] [PubMed] [Google Scholar]
- Campos M, Szerenyi K, Lee M, McDonnell JM, McDonnell PJ. Keratocyte loss after corneal deepithelialization in primates and rabbits. Arch Ophthalmol. 1994;112:254–260. doi: 10.1001/archopht.1994.01090140130034. [DOI] [PubMed] [Google Scholar]
- Dohlman CH, Gasset AR, Rose J. The effect of the absence of corneal epithelium or endothelium on stromal keratocytes. Invest Ophthalmol Vis Sci. 1968;7:520–534. [PubMed] [Google Scholar]
- Dupps WJ, Jr, Wilson SE. Biomechanics and wound healing in the cornea. Exp Eye Res. 2006;83:709–20. doi: 10.1016/j.exer.2006.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, Gelber-Schwalb TA, Addeo JV, Stern ME. Apoptosis in the rabbit cornea after photorefractive keratectomy. Cornea. 1997;16:200–208. [PubMed] [Google Scholar]
- Helena MC, Baerveldt F, Kim WJ, Wilson SE. Keratocyte apoptosis after corneal surgery. Invest Ophthalmol Vis Sci. 1998;39:276–83. [PubMed] [Google Scholar]
- Martínez-García MC, Merayo-Llovés J, Blanco-Mezquita T, Mar-Sardaña S. Wound healing following refractive surgery in hens. Exp Eye Res. 2006;83:728–35. doi: 10.1016/j.exer.2006.02.017. [DOI] [PubMed] [Google Scholar]
- Mohan RR, Hutcheon AE, Choi R, Hong J, Lee J, Mohan RR, Ambrósio R, Jr, Zieske JD, Wilson SE. Apoptosis, necrosis, proliferation, and myofibroblast generation in the stroma following LASIK and PRK. Exp Eye Res. 2003;76:71–87. doi: 10.1016/s0014-4835(02)00251-8. [DOI] [PubMed] [Google Scholar]
- Mohan RR, Kim WJ, Mohan RR, Chen L, Wilson SE. Bone morphogenic proteins 2 and 4 and their receptors in the adult human cornea. Invest Ophthalmol Vis Sci. 1998;39:2626–2636. [PubMed] [Google Scholar]
- Mohan RR, Liang Q, Kim WJ, Helena MC, Baerveldt F, Wilson SE. Apoptosis in the cornea: further characterization of Fas-Fas ligand system. Exp Eye Res. 1997;65:575–589. doi: 10.1006/exer.1997.0371. [DOI] [PubMed] [Google Scholar]
- Mohan RR, Mohan RR, Kim WJ, Stark GR, Wilson SE. Defective keratocyte apoptosis in response to epithelial injury in stat 1 null mice. Exp Eye Res. 2000b;70:485–91. doi: 10.1006/exer.1999.0807. [DOI] [PubMed] [Google Scholar]
- Mohan RR, Mohan RR, Kim WJ, Wilson SE. Modulation of TNF alpha-induced apoptosis in corneal fibroblasts by transcription factor NF-kb. Invest Ophthalmol Vis Sci. 2000a;41:1327–1336. [PubMed] [Google Scholar]
- Nakayasu K. Stromal changes following removal of epithelium in rat cornea. Jpn J Ophthalmol. 32:113–125. [PubMed] [Google Scholar]
- Netto MV, Mohan RR, Ambrósio R, Jr, Hutcheon AE, Zieske JD, Wilson SE. Wound healing in the cornea: a review of refractive surgery complications and new prospects for therapy. Cornea. 2005;24:509–22. doi: 10.1097/01.ico.0000151544.23360.17. [DOI] [PubMed] [Google Scholar]
- Reynolds ES. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963;17:208–14. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robards AW, Wilson AJ. Procedures in Electron Microscopy. Vol. 5. Wiley; New York, NY: 1993. p. 4.9. [Google Scholar]
- Spurr AR. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969;26:31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Wilson SE. Molecular cell biology for the refractive corneal surgeon: programmed cell death and wound healing. J, Refract Surg. 1997;13:171–5. doi: 10.3928/1081-597X-19970301-15. [DOI] [PubMed] [Google Scholar]
- Wilson SE. Keratocyte apoptosis in refractive surgery: Everett Kinsey Lecture. CLAO Journal. 1998;24:181–185. [PubMed] [Google Scholar]
- Wilson SE, Chaurasia SS, Medeiros FW. Apoptosis in the initiation, modulation and termination of the corneal wound healing response. Exp Eye Res. 2007;85:305–11. doi: 10.1016/j.exer.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SE, He YG, Weng J, Li Q, Vital M, Chwang EL. Epithelial injury induces keratocyte apoptosis: Hypothesized role for the interleukin-1 system in the modulation of corneal tissue organization. Exp Eye Res. 1996;62:325–338. doi: 10.1006/exer.1996.0038. [DOI] [PubMed] [Google Scholar]
- Wilson SE, Kim WJ. Keratocyte apoptosis: implications on corneal wound healing, tissue organization, and disease. Invest Ophthalmol Vis Sci. 1998;39:220–226. [PubMed] [Google Scholar]
- Wilson SE, Mohan RR, Hutcheon AE, Mohan RR, Ambrósio R, Zieske JD, Hong J, Lee J. Effect of ectopic epithelial tissue within the stroma on keratocyte apoptosis, mitosis, and myofibroblast transformation. Exp Eye Res. 2003;76:193–201. doi: 10.1016/s0014-4835(02)00277-4. [DOI] [PubMed] [Google Scholar]
- Wilson SE, Pedroza L, Beuerman R, Hill JM. Herpes simplex virus type-1 infection of corneal epithelial cells induces apoptosis of the underlying keratocytes. Exp Eye Res. 1997;64:775–779. doi: 10.1006/exer.1996.0266. [DOI] [PubMed] [Google Scholar]
- Wyllie AH, Kerr JF, Currie AR. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]


