Abstract
The ability to image the newborn brain during development has provided new information regarding the effects of injury on brain development at different vulnerable time periods. Studies in animal models of brain injury correlate beautifully with what is now observed in the human newborn. We now know that injury at term results in a predilection for gray matter injury while injury in the premature brain results in a white matter predominant pattern although recent evidence suggests a blurring of this distinction. These injuries affect how the brain matures subsequently and again, imaging has led to new insights that allow us to match function and structure. This review will focus on these patterns of injury that are so critically determined by age at insult. In addition, this review will highlight how the brain responds to these insults with changes in connectivity that have profound functional consequences.
Over the last decade, advances in magnetic resonance imaging technology provide an unprecedented view of the brain in critically ill newborns, revealing important insights into the age-dependent responses of the immature brain to injury. These observations build on the fundamental concept of selective vulnerability in the developing brain. Until recently, experimental observations suggest that patterns of histopathological injury result from the vulnerability of specific cell populations during certain times in development. In the preterm brain, early lineage oligodendroglia, such as the oligodendroglial precursors are vulnerable to an ischemic insult that does not affect more mature oligodendrocytes. Subplate neurons, a transient mature population of neurons present in the immature brain are uniquely vulnerable to excitotoxic and ischemic insults, whereas other neurons like cortical projection neurons remain unaffected. In the term newborn brain, projection neurons in the striatum are sensitive to hypoxic ischemic injury while interneurons like the neuronal nitric oxide synthase containing interneurons remain invulnerable to the insult. These selective cell vulnerabilities are reflected on pathological examination after a hypoxic ischemic insult such that the preterm brain will have gliosis of the white matter while the term brain will have these destructive changes in deep gray nuclei like basal ganglia and thalamus (Figure 1 and Figure 2). However, more recent experimental observations, and those made with advanced imaging in the newborn, has led to a blurring of the “gray-white” (term- preterm) dichotomy: white matter injury is increasingly recognized in the term baby and injury to gray matter structures, such as the thalamus and cerebellum are being appreciated in the preterm brain. Furthermore, the mechanisms of vulnerability in the developing brain must be considered in the setting of normal brain development. This review will examine how connectivity of selectively vulnerable cell populations and circuits in the developing brain shapes an expression of injury that can be visualized in vivo with brain imaging and ultimately determines the affected child’s neurodevelopmental outcome.
Figure 1.
A. Brain development from early in premature life to term-equivalent age. Axial T2 weighted and color coded fractional anisotropy maps in a premature newborn delivered at 28 weeks gestation and scanned at 30 weeks postmenstrual age and again at 39 weeks. On the T2 weighted images note the dramatic increase in gyration of the cerebral cortex and early myelination in the posterior limb of the internal capsule. The color coded fractional anisotropy maps represent the “directionality” of water diffusion, with brighter areas indicating more directionality (higher FA). Water diffusion in the right-left plane is coloured red, superior-inferior in blue, and anterior –posterior in green. Note the loss of FA in the cerebral cortex from preterm to term-equivalent age, consistent with the normal loss of cortical radial organization. White matter tracts such as the corticospinal tracts (blue region in the posterior limb of the internal capsule) and optic radiations (green tract from the thalamus to calcarine cortex) are more clearly delineated with higher FA at term-equivalent age.
B. Predominant Patterns of Brain Injury in Preterm and Term Newborns. In the preterm newborn delivered at 28 weeks gestation and scanned at 30 weeks postmenstrual age, the axial image from the spoiled gradient echo volumetric scan demonstrates several foci of abnormal T1 hyperintensity in the periventricular white matter consistent with focal non-cystic white matter injury (blue arrows; note lesions are evident bilaterally). In the term newborn with encephalopathy following acute profound asphyxia, the axial image from the T1-weighted sequence on the tenth day of life demonstrates abnormal T1 hyperintensity in the basal nuclei and perirolandic cortex in the characteristic basal nuclei predominant pattern of injury (yellow arrows; note abnormalities are evident bilaterally).
Figure 2. Patterns of Brain Injury.
Deep Nuclei and Watershed Patterns of Brain Injury (A–C): MRI scan demonstrating the basal nuclei predominant pattern of injury (from Figure 1). Neuropathology from the first day of life in a term newborn following acute profound asphyxia. On gross pathology (A) injury is evident as brown discoloration in the thalami, basal ganglia, hippocampi, and cerebral cortex in a vascular watershed distribution (arrow). On hematoxylin and eosin stained microscopic sections, diffuse neuronal injury in the thalamus (B) is evident with eosinophilic (dead) neurons (arrowhead), and in the cerebellar cortex (C) with eosinophilic Purkinje cells (arrowhead).
White Matter Injury (D–F): MRI scan demonstrating white matter injury (from Figure 1). Neuropathology from the second month of life demonstrating the cystic and diffuse components of periventricular leukomalacia. On gross pathology (D) cystic degeneration is present in the periventricular white matter injury (arrow). On hematoxylin and eosin stained microscopy sections, the cyst (E) (arrowhead) contains macrophages and is surrounded by marked astrogliosis and calcifications. In a section of white matter remote from the cyst (F), there is a marked paucity of myelin and astrogliosis.
Images courtesy of Dr. Glenda Hendson (University of British Columbia).
SPECTRUM OF FUNCTIONAL CONSEQUENCES OF NEONATAL BRAIN INJURY
The functional consequences of early injury to the developing brain may involve multiple developmental domains: motor, cognition and behaviour, vision and hearing. Functional motor deficits are often described as cerebral palsy, a non-progressive disorder of motor function or posture originating in early life. Disabilities in other developmental domains often co-occur with cerebral palsy and include sensorineural hearing loss, cortical blindness and, cognitive and learning difficulties.1 Yet, these deficits are now recognized in some childhood survivors of perinatal hypoxic-ischemic brain injury even in the absence of cerebral palsy.2 The incidence of disability and the pattern of neurodevelopmental deficits differ in preterm and term survivors of early hypoxic ischemic brain injury. For example, severe white matter injury in the premature newborn results in the clinical picture of spastic diplegia (a form of cerebral palsy affecting function in the legs more than the arms) and visual dysfunction, often accompanied by deficits in cognition and learning, whereas acute profound asphyxia in the term newborn often results in the clinical picture of spastic quadriparesis (a severe form of cerebral palsy affecting the arms, legs and lower cranial nerve functions) and mental retardation. In addition to the major motor and cognitive disabilities that can be diagnosed in early childhood, a complete assessment of neurocognitive outcomes needs to consider domains that are more readily assessed as children develop to school age: learning (including writing, reading, and math), executive functions, behaviour and social competence. Impairments in these domains are often only detected with the increased demands of school and peer groups.3 “Quality of life”, an individual’s subjective perception of physical and psychological health, is an important aspect of outcome that is only beginning to be probed following neonatal brain injury. Thus, the consequence of neonatal brain injury impacts the child’s function in multiple environments- family, school, employment, and society.
MAGNETIC RESONANCE IMAGING OF BRAIN INJURY & DEVELOPMENT IN NEWBORNS
Magnetic resonance imaging (MRI) is now the “gold-standard” for safe and reliable diagnosis of injury in the newborn brain and at the same time has led to insights regarding normal brain maturation.4 The available data suggest that focal brain abnormalities (or “injuries”) in the newborn can be detected clearly with conventional MRI. In the newborn, acquired brain abnormalities, such as stroke and WMI, are often indicated by discrete (focal) areas of MR signal abnormality, the extent of which corresponds closely to histopathological changes on post-mortem examination.5–7 Recent observations with advanced MRI techniques indicate that these focal injuries, and clinical conditions that affect energy-substrate delivery to the brain, can even impede maturation of the brain in areas that appear normal on conventional MRI.8, 9 Therefore areas of damage and disturbed development might be more extensive than appear when using conventional MRI.
Advanced MRI techniques, such as high-resolution MRI, MR spectroscopic imaging (MRSI) and diffusion tensor imaging (DTI), now provide quantifiable assessments of neonatal brain development in vivo. High-resolution MRI can now be used to quantify volumes of cerebral structures, allowing measures of brain structural growth. MRSI measures regional brain biochemistry, including N-acetylaspartate (NAA) and lactate. Changes in these metabolites reflect changes associated with normal brain development (e.g. increasing NAA/choline) and are regionally specific.10–12 NAA, an acetylated amino acid found primarily in high concentrations in neurons, increases with advancing cerebral maturity.10 Lactate is observed in the brains of premature newborns in the absence of overt brain injury, and becomes undetectable by term-age.10 Levels of NAA and lactate can be quantified or are expressed relative to the choline peak. In addition to their application to studying brain development, these metabolites also provide an important measure of brain injury. For example, lactate is elevated with disturbances in cerebral energy substrate delivery and oxidative metabolism.13 Following neonatal hypoxic-ischemic brain injury the elevations in lactate and reductions in NAA are predictive of adverse neurodevelopment outcomes such as cognitive deficits like mental retardation, and neuromotor disability like spastic quadriparesis.14
DTI characterizes the three-dimensional spatial distribution of water diffusion in each voxel of the MR image.15 Water diffusion is summarized in each voxel by two parameters: average diffusivity (Dav) and fractional anisotropy (FA). These measures provide a sensitive reflector of regional brain microstructural development. With increasing maturity, Dav decreases,9, 15 apparently due to a decrease in water content and to developing neuronal and glial cell membranes restricting proton diffusion.15, 16 FA is a measure of the directionality of proton diffusion, and with advancing brain development changes differently in gray and white matter. In the gray matter of the cerebral cortex, FA is high early in the third trimester, reflecting the radial organization of the cerebral cortex.17, 18 As this organization is lost, FA decreases and becomes undetectable by term.17, 18 In the white matter, FA increases with advancing brain development, particularly with the maturation of the oligodendrocyte lineage and early events of myelination, providing a sensitive measure of white matter microstructural development (Figure 1).9, 19, 20
PATTERNS OF BRAIN INJURY IN THE PREMATURE NEWBORN
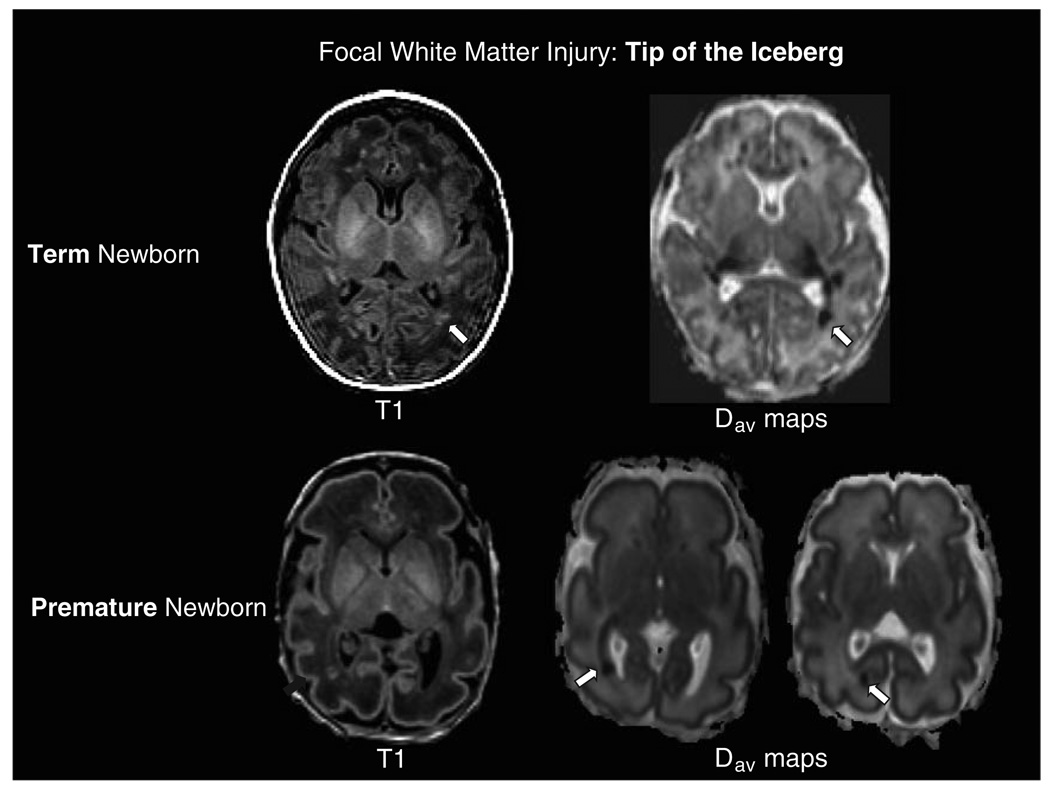
Injury to the premature brain can be either ischemic or hemorrhagic. Because the very premature brain circulation is pressure passive,21 it is more likely to experience rupture of vessels in the germinal matrix with extreme changes in blood flow velocity resulting in intraventricular hemorrhage. Injury to the vulnerable white matter can be caused by a variety of factors such as prolonged periods of hypoxia or ischemia (e.g. hypotension) 22 or exposure to infections and inflammation.23, 24 This injury will be based primarily in the white matter and will be focal or diffuse with areas of gliosis or necrosis depending on the severity of the metabolic disturbance. With the increasing application of MRI to the clinical assessment of brain injury in the premature newborn, the “focal non-cystic white matter injury” is recognized as one of the most common patterns of brain injury in this population.25, 26 On conventional MRI, focal non-cystic WMI appears as areas of hyperintensity on T1-weighted MR images (Figure 2 and Figure 3). This type of WMI is distinct from cystic periventricular leukomalacia (PVL), a more severe abnormality that refers specifically to cystic regions of necrosis in the periventricular white matter that are well detected by brain ultrasound.27 Over the past decade, there has been a shift of white matter injury away from PVL and toward the more focal WMI for unclear reasons but possibly depending on how long the newborn has been ventilated or whether the baby was exposed to an inflammatory insult.23, 24, 28, 29 While early PVL has been associated with spastic diplegia and cognitive disability in early childhood, focal non-cystic WMI is now observed in half of premature newborns on MRI.26, 30 It is becoming increasingly clear that these focal abnormalities seen on MRI and DTI are the “tip of the iceberg” and are associated with significant visual, motor and cognitive dysfunction as profound as those associated previously with PVL.26, 31–33
Figure 3.
Focal White Matter Injury- Tip of the Iceberg. Term Newborn: Axial images from the T1-weighted sequence and the average diffusivity maps (Dav) in a term newborn with encephalopathy and white matter injury. In addition to the discreet focus of white matter injury in the optic radiations on the T1-weighted image (arrow), note the more extensive abnormality of restricted diffusion (hypointensity on the average diffusivity map, arrow) extending from the focal white matter lesion along the optic radiations to the thalamus. Similar findings are evident with a smaller lesion contralaterally. Premature Newborn: Axial images from a premature newborn at 31 weeks postmenstrual age demonstrate a focus of white matter injury in the optic radiations on the T1-weighted spoiled gradient echo volumetric sequence. The axial average diffusivity images demonstrate the focus of white matter injury as an area of restricted diffusion (hypointensity, arrow) accompanied by extension of the restricted diffusion along the optic radiations from the thalamus to the calcarine cortex (arrow). These images highlight the effects of focal white matter lesions on “connected” white matter pathways such as the optic radiations-from the thalamus to the calcarine cortex.
All premature newborns are at risk for WMI and that it is not necessary to have a specific ischemic episode preceding the occurrence of WMI. In fact, current definitions of hypotension in the premature newborn do not adequately predict the occurrence of PVL.34 Several lines of evidence now indicate that the risks of white matter injury, as well as adverse neurodevelopmental outcome, in the premature newborn are significantly altered by systemic illness and by critical care therapies. For example, in preterm newborns who develop chronic lung disease due to lack of maturity of lung development at birth necessitating prolonged treatment with mechanical ventilation and supplemental oxygen, cognitive outcome at 8 years of age is impaired even after controlling for birthweight and neurological complications at birth.35 Postnatal infection in preterm newborns is also associated with impaired neurodevelopmental outcomes 36 and recurrent postnatal infection is now recognized as an important risk for progressive WMI.23 Recent observations suggest that motor impairments seen more commonly in preterm infants with serious systemic infections are mediated by white matter abnormalities.24 However, other observations suggest that both postnatal infection and WMI are independently associated with adverse neurodevelopmental outcomes.26
Premature newborns in critical care often require drug therapy, and postnatal exposure to therapeutic corticosteroids for the treatment of chronic lung disease from lung immaturity may impair brain growth, though this effect may be limited to early treatment with dexamethasone.37, 38 A dramatic decline in the incidence of cystic PVL can be achieved, at least in part, by a decrease in days of mechanical ventilation,29 possibly by avoiding hypocarbic alkalosis.39 In an observational study, less WMI was seen in premature newborns exposed to prolonged indomethacin therapy for closure of a patent ductus arteriosus (nonclosure of the fetal connection between two of the major arteries near the heart).40 These data highlight the important potential of newborn brain imaging to identify critical care therapies that can be applied with “brain protective” effects. It is unclear how drugs given to avoid pain, to prevent or treat infection, to accelerate maturity of the lung, to treat seizures might affect brain development. While these treatments have little long term effects in a mature individual, in the developing baby, these drugs can alter brain development by accelerating programmed cell death and synaptic plasticity resulting in profound impairments of cognition and sensory motor integration.41
SELECTIVE VULNERABILITY IN THE PREMATURE NEWBORN BRAIN
Over the last decade it has become recognized that WMI is due to the selective vulnerability of specific developmentally regulated cell populations: late oligodendrocyte progenitor cells and subplate neurons.27 Two mechanisms proposed for the selective vulnerability of these developmentally regulated cell types are oxidative stress and excitotoxicity.42–44 Importantly, late oligodendrocyte progenitor cells express NMDA and AMPA receptors and are protected by blocking these receptors.43, 45 Since these cells are immature, they lack the receptor subunits that regulate calcium flux resulting in increased vulnerability to glutamate exposure.46, 47 Additionally, subplate neurons are the first neurons of the cerebral cortex to express these receptors making them more vulnerable to ischemic challenges that trigger excitotoxicity.48, 49
Back et al. have demonstrated the selective vulnerability of the late oligodendrocyte progenitor cell to hypoxic ischemic injury.50 The late oligodendrocyte progenitor is the cell type predominating in the white matter through the high-risk period for white matter injury in the premature newborn.51 In a sheep model, the distribution of these susceptible oligodendrocyte progenitor cells underlies the spatial anatomy of WMI.52 In this model, the distribution of these vulnerable cells were more important than limitations in cerebral blood flow in determining the distribution of WMI. Neurons in the subplate zone (subplate neurons) are another developmentally regulated cell population that plays a critical role in visual thalamocortical development.42 McQuillen et al. have determined that subplate neurons are selectively vulnerable to neonatal hypoxia-ischemia in a rodent model of brain injury that is particularly relevant to injury in the human premature newborn.42, 53 In humans, like the oligodendrocyte progenitors, subplate neuron numbers peak at the onset of vulnerability for WMI.54 Ultimately, a long term neuropathological sequelae of WMI in the premature newborn is a failure of normal myelination in the periventricular regions.27 Recent observations suggest that this myelination failure is the result of both delayed degeneration of the oligodendrocyte progenitor as well as an arrest in the maturation of the oligodendrocyte precursor pool. 55 Additionally, the persistence of oligodendrocyte precursors, a “susceptible” cell population, maintains a whiter matter vulnerability to recurrent insults, such as that due to hypoxia-ischemia.55 In addition to the myelination failure of chronic white matter injury in the premature newborn, axonal damage is now recognized pathologically,56 and with newborn diffusion tensor imaging studies.57 As will be discussed below, WMI may itself diffusely impair the subsequent development of brain white matter and gray matter structures such as corpus callosum and the thalamus.
PATTERNS OF BRAIN INJURY IN THE TERM NEWBORN
MRI allows insight into the timing and heterogeneity of brain injury associated with neonatal encephalopathy in the term newborn. Previous retrospective studies suggested that neonatal encephalopathy is primarily related to antenatal risk factors Such as maternal hypothyroidism, preeclampsia, maternal infection, etc.58, 59 However, in some term newborns with encephalopathy a sentinel hypoxic-ischemic event can be identified. Even when a sentinel event is not identified by clinical history, two large prospective cohort studies of term newborns with encephalopathy evaluated with MRI demonstrate that brain injury actually occurs at or near birth.60, 61 Since the injury is recent, it may be amenable to post-natal interventions, such as hypothermia, in the first days of life. It also indicates the need to discover the causal pathway from antenatal risks and perinatal injury, so that new prevention and recovery strategies can be implemented.
A remarkable regional vulnerability is observed in the brain of term newborns following hypoxia-ischemia, resulting in two major patterns of injury detectable by MRI: (1) a watershed predominant pattern involving the white matter, particularly in the vascular boundary zones, extending to cortical gray matter when severe, and (2) a basal nuclei predominant pattern involving the deep gray nuclei and perirolandic cortex, extending to the total cortex when severe (Figure 1 and Figure 2).27, 53, 62, 63 These patterns result in primarily cognitive disabilities in the former 64 and severe motor disabilities in the latter.61, 65 In fact, the pattern of brain injury on MRI is even more predictive of neurodevelopmental outcome than the severity of the lesions.60, 61
SELECTIVE VULNERABILITY IN THE TERM NEWBORN
The term newborn will experience an hypoxic ischemic insult when there is placental disruption, prolonged nuchal cord compression, or severe trauma to the head and neck. The term newborn is also at high risk for both ischemic and hemorrhagic stroke due possibly in part to the prothrombotic state of the mother and the maternal-fetal unit. These insults result in vulnerability of specific neuronal populations in the term brain, and like white matter vulnerability in the preterm brain, hypoxia ischemia and inflammation result in excitotoxicity and oxidative stress. In the term brain, there is an over-expression of certain glutamate receptors in selective regions like the basal ganglia. The NMDA glutamate receptor subtype is the predominant mediator of this type of injury, exerting its effects via coupling to neuronal nitric oxide synthase containing neurons (nNOS) in the post synaptic density complex. NMDA receptor subunit composition changes with development with the NR2B subunit predominating early, followed by increasing expression of NR2A. NMDA receptors with NR2B have slower deactivation and higher conductance, thus conferring a greater vulnerability to excitotoxicity in early postnatal development. Following hypoxia-ischemia there are differential effects on NMDA receptor subunit composition and these effects differ by age. This interaction ultimately results in generation of both nitrogen and oxygen free radicals that in turn injure nearby cells. The newborn brain is rich in free iron, and lacking in antioxidant defences (such as glutathione peroxidase and superoxide dismutase) thereby providing a fertile environment for oxidative damage.53
NEWBORN BRAIN INJURY IS NOT ALL “GRAY & WHITE”
PREMATURE NEWBORN BRAIN INJURY IS NOT ALL “WHITE”
In addition to focal brain injuries, premature newborns may have impairment in brain development. These acquired brain abnormalities may be even more common than the focal brain “injuries” identified by areas of signal abnormality on MRI, and are not limited to the white matter. It is unclear whether these white matter abnormalities cause the impairment in brain maturation or whether the brain, for some unknown genetic or environmental reason is more susceptible to both injury and maldevelopment. When severe, these diffuse abnormalities are apparent on qualitative assessment of MRI images by a paucity of white matter, expansion of the ventricles (ventriculomegaly), impaired gyral development, or enlarged subarachnoid spaces.66 Yet, even when examined using quantitative MRI measures of brain volumes at term-equivalent age most premature newborns do not have these dramatic brain abnormalities. 67 Furthermore, up to 20% with adverse cognitive and motor outcomes in childhood do not have significant qualitative abnormalities on neonatal MRI.26 In these cases, it is possible that the brain is imaged at a time when signal abnormalities have been replaced by tissue loss that is not as easily detected on qualitative MRI.
Abnormal brain structural development is detected in premature newborns as early as term-equivalent age as measured by high-resolution MRI to quantify brain volumes.68 The pattern of regional volume abnormalities at term-equivalent age is related to the degree of prematurity and some aspects of the newborn’s systemic conditions (e.g. chronic lung disease).67, 68 Yet many of the processes resulting in abnormal brain development are largely unknown. When assessed by high-resolution MRI to quantify brain volumes at 8 years of age, premature birth is associated with smaller brain volumes relative to term-born children; these structural differences in brain development are associated with abnormal cognitive development.38, 69 Although some studies show a dramatic recovery of brain volumes as the child grows, there still appears to be gender specific abnormalities that persist through adulthood.70
Additionally, the presence of WMI at term-equivalent age is strongly associated with abnormal gray matter volumes, including smaller volumes of the cerebral cortex.68, 71 Preterm newborn scanned at term-equivalent age also have smaller basal nuclei; reductions in thalamic and lentiform volumes were most striking in newborns with “focal” brain lesions, such as WMI.72 Abnormalities of the cerebellum are also being increasingly recognized in premature newborns. Premature newborns with supratentorial pathology such as hemorrhagic parenchymal infarction and PVL have reduced cerebellar volume,73 with unilateral cerebral injury associated with smaller contralateral cerebellar hemisphere volume, including gray and white matter components.74 These data highlight the important connectivity of gray and white matter structures and of supratentorial structures with the cerebellum and suggest that there are systems preferential degeneration that occurs over time after a neonatal insult.
More sophisticated quantitative brain imaging methods are now available to detect acquired abnormalities of brain development in the newborn period. For example, using serial DTI, focal WMI prior to term age is followed by diffuse abnormalities of white matter development as the premature newborns develop to term-equivalent age (Figure 3).9 The volume and pattern of thalamo-cortical connections may also be disrupted in premature newborns with WMI on MRI, resulting in visual dysfunction.75 Importantly, microstructural abnormalities in particular white matter regions correspond with subsequent motor and cognitive impairments.32 Furthermore, regional tissue losses, such as smaller hippocampal volumes, are linked to abnormalities in cognitive development, such as working memory deficits.76, 77 Using sophisticated DTI techniques to measure microstructural development of the cerebellum, significant developmental changes in cerebellar grey and white matter were observed in patients with supratentorial intraventricular hemhorrhage.78These observations highlight the importance of connections at multiple anatomical levels (cortex-thalamus/basal ganglia-cerebellum/brainstem) when considering the potential impact of acquired lesions on brain development. The recent ability to image the subplate zone 79 and cortical microstructure,17, 18 opens up new avenues to determine the impact of focal injuries on subsequent gray matter and white matter development.
TERM NEWBORN BRAIN INJURY IS NOT ALL “GRAY”
While WMI is the characteristic pattern of brain injury in premature newborns, it is increasingly recognized in populations of term newborns (Figure 3). In a recent study of 48 term newborns with encephalopathy studied with MRI at 72 hours of life eleven (23%) had focal non-cystic WMI.80 The WMI demonstrated restricted diffusion on apparent diffusion coefficient maps in 10 of 11 newborns, suggesting that these lesions were acquired near birth. As newborns with WMI had milder encephalopathy relative to other newborns in the cohort, these lesions may have been under-detected in the past. An increasing severity of WMI was associated with lower gestational age at birth suggesting that brain maturation is an important determinant of this injury pattern.80 Delayed white matter degeneration, extending past the first week of life, is also seen in sequential studies of term newborns with encephalopathy,81 particularly those with basal nuclei injuries.82 This “progressive” white matter damage might follow basal nuclei injury in a manner analogous to corticospinal tract degeneration evident on MRI following cerebral infarction in the term newborn (i.e. Wallerian degeneration).83, 84 In the term newborn with arterial infarction, white matter tract abnormalities consistent with Wallerian degeneration, remote from gray matter damage, are predictive of subsequent motor outcomes, particularly hemiplegia.83, 84
Full-term infants with congenital heart disease (CHD) also have a strikingly high incidence of WMI on MRI and at autopsy.85–90 The WMI observed in these term newborns has strikingly similar imaging characteristics to that reported in preterms.26, 85 As described above, the pattern of WMI is attributed to developmentally regulated cell populations vulnerable to ischemia and oxidative stress.27, 42, 52 Though predominant injury to neurons would be the expected response to these insults in term newborns with CHD,61 WMI nonetheless occurs frequently. Similar to premature newborns, those with CHD are certainly at risk of impaired delivery of energy substrates due to ischemia, inflammation, and oxidative stress, particularly with cardiopulmonary bypass. Recent data acquired with MRI, DTI and MR spectroscopic imaging suggests that in utero brain development is delayed in newborns with two types of CHD: D-transposition of the great arteries (TGA) and single ventricle physiology (SVP), including Hypoplastic Left Heart Syndrome where the left ventricle is malformed or absent.8 The pattern of lower NAA/choline, higher average diffusivity and lower white matter fractional anisotropy seen in these newborns is congruous with findings in premature newborns at an earlier age.9, 11, 12 Neuropathology data in newborns with CHD, and a recent in vivo MRI study, also indicate that they are more likely to be microcephalic and have an immature cortical mantle.91–93 Newborns with TGA and SVP have impaired in utero brain growth, possibly related to impaired fetal cerebral oxygen delivery.94–96 Parallel to findings in the premature newborn, where WMI is associated with more widespread impairments in gray and white matter development, brain injury in newborns with CHD prior to surgery also impairs the subsequent development of the corticospinal tracts.97 Together these data highlight the important connection between gray and white matter injuries in term newborns, and of focal injuries with widespread abnormalities in subsequent brain development.
FROM VULNERABILITY TO CONNECTIVITY
Developing neurons are highly dependent on trophic support for survival such that target deprivation will result in degeneration. This is clearly seen in newborns with perinatal stroke where infarction of the cortex leads to degeneration of the corticospinal tracts.84 In the newborn brain, this Wallerian neurodegeneration occurs over a long period of time (at least months).98 Data from rodent studies indicate that early injury in the hippocampus will result in fimbria fornix and dorsolateral septal nuclei degeneration in a stepwise fashion over time as evidenced by DTI maps and histological studies (Figure 4).99 These data support the hypothesis of delayed neurodegeneration in a systems preferential manner that occurs after an initial insult. This delayed neurodegeneration results in impairments in the human newborn that evolve over time into complex disabilities reflected by the systems affected. For example, injury to the cortex after an ischemic insult will result in thalamic damage that occurs over time due to loss of trophic support. This type of injury results in profound impairments in sensory motor integration.99
Figure 4.
(A) Neural Network Degeneration: Fiber tracking, overlayed on direction encoded fractional anisotropy maps, reveals significant connection with axons entering the septum in a control C57BL6 mouse images on postnatal day 42 (i and iii). Following neonatal hypoxia ischemia at postnatal day seven in a C57BL6 mouse (ii and iv), almost no ipsilateral fimbria connections remain through the ipsilateral column of fornix to the septum (yellow tracks) while robust contralateral axonal connections remain (red tracks).
(B)Imaging Connectivity in Neonatal Stroke: Following stroke in the neonatal period (i), a minimal decrease in fractional anisotropy (FA) in the ipsilateral posterior limb of the internal capsule (PLIC) and cerebral peduncle (CP) is seen at birth, with complete loss of FA at 3 months (white arrows) (FA: fractional anisotropy map; DEC: Direction encode color map). Note the loss of high signal on the FA map in the position of the PLIC, also evident as a loss of blue signal (superior-inferior tracks) on the DEC map. When the 3 month scan is mapped against templated uninjured controls (ii), the deficit in the ipsilateral PLIC and CP is evident (red signal at arrow). Severe pruning of ipsilateral fibers (yellow) tracking through the PLIC and cerebral peduncle at 3 months of age (iii) portends diagnosis of severe hemiplegia at 2 years of age. Fiber tracking in another patient shows mild differences at 3 months; this patient developed a milder gross motor deficit (iv).
Images courtesy of Dr. Frances Northington.
CONCLUSIONS: IMPLICATIONS FOR REPAIR
Advances in newborn brain imaging have afforded tremendous insights into the age-dependent response of the brain to neurological insult. With this unprecedented view of the brain in critically ill newborns, there is increasing recognition that clinical care practices in the intensive care nursery have beneficial or detrimental consequences on brain development and injury. A better understanding of the clinical factors that impact brain development and injury will allow us to directly improve the neurodevelopmental outcome of newborns at highest risk of neurodevelopmental impairments, such as those born prematurely, full term newborns with encephalopathy, and those with congenital heart birth defects. In particular, a clearer view of the connectivity of selectively vulnerable cell populations and circuits in the developing brain will enable the consideration of therapeutic approaches that extend past the first hours of an acute insult. This view of affected brain systems will also provide essential prognostic information regarding long-term functional outcomes to allow caregivers to best care for these newborns as they develop through childhood. Moreover, the ability to identify and quantify brain injury, particularly those changes in “connected” structures that extend beyond the focal abnormalities visible on MRI, will lay the foundation for testing new strategies for preventing or treating brain injury in these populations. 100
Acknowledgements
NS 35902, NS40117 to DMF and Canadian Institutes for Health Research (CIHR; CHI 151135), March of Dimes Foundation (#5-FY05-1231) to SPM. SPM is supported by a CIHR Clinician Scientist Phase 2 award and a Michael Smith Foundation for Health Research Scholar award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Marlow N, Rose AS, Rands CE, et al. Neuropsychological and educational problems at school age associated with neonatal encephalopathy. Arch Dis Child Fetal Neonatal Ed. 2005;90:F380–F387. doi: 10.1136/adc.2004.067520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gonzalez FF, Miller SP. Does perinatal asphyxia impair cognitive function without cerebral palsy? Arch Dis Child Fetal Neonatal Ed. 2006;91:F454–F459. doi: 10.1136/adc.2005.092445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Handel M, Swaab H, de Vries LS, et al. Long-term cognitive and behavioral consequences of neonatal encephalopathy following perinatal asphyxia: a review. Eur J Pediatr. 2007;166:645–654. doi: 10.1007/s00431-007-0437-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ment LR, Bada HS, Barnes P, et al. Practice parameter: neuroimaging of the neonate: report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology. 2002;58:1726–1738. doi: 10.1212/wnl.58.12.1726. [DOI] [PubMed] [Google Scholar]
- 5.Schouman-Claeys E, Henry-Feugeas MC, Roset F, et al. Periventricular leukomalacia: correlation between MR imaging and autopsy findings during the first 2 months of life. Radiology. 1993;189:59–64. doi: 10.1148/radiology.189.1.8372220. [DOI] [PubMed] [Google Scholar]
- 6.Hope PL, Gould SJ, Howard S, et al. Precision of ultrasound diagnosis of pathologically verified lesions in the brains of very preterm infants. Dev Med Child Neurol. 1988;30:457–471. doi: 10.1111/j.1469-8749.1988.tb04773.x. [DOI] [PubMed] [Google Scholar]
- 7.Felderhoff-Mueser U, Rutherford MA, Squier WV, et al. Relationship between MR imaging and histopathologic findings of the brain in extremely sick preterm infants. Ajnr. American Journal of Neuroradiology. 1999;20:1349–1357. [PMC free article] [PubMed] [Google Scholar]
- 8.Miller SP, McQuillen PS, Hamrick S, et al. Abnormal brain development in newborns with congenital heart disease. N Engl J Med. 2007;357:1928–1938. doi: 10.1056/NEJMoa067393. [DOI] [PubMed] [Google Scholar]
- 9.Miller SP, Vigneron DB, Henry RG, et al. Serial quantitative diffusion tensor MRI of the premature brain: Development in newborns with and without injury. J Magn Reson Imaging. 2002;16:621–632. doi: 10.1002/jmri.10205. [DOI] [PubMed] [Google Scholar]
- 10.Kreis R, Hofmann L, Kuhlmann B, et al. Brain metabolite composition during early human brain development as measured by quantitative in vivo 1H magnetic resonance spectroscopy. Magn Reson Med. 2002;48:949–958. doi: 10.1002/mrm.10304. [DOI] [PubMed] [Google Scholar]
- 11.Vigneron DB. Magnetic resonance spectroscopic imaging of human brain development. Neuroimaging Clin N Am. 2006;16:75–85. doi: 10.1016/j.nic.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 12.Vigneron DB, Barkovich AJ, Noworolski SM, et al. Three-dimensional proton mr spectroscopic imaging of premature and term neonates. AJNR Am J Neuroradiol. 2001;22:1424–1433. [PMC free article] [PubMed] [Google Scholar]
- 13.Kasischke KA, Vishwasrao HD, Fisher PJ, et al. Neural activity triggers neuronal oxidative metabolism followed by astrocytic glycolysis. Science. 2004;305:99–103. doi: 10.1126/science.1096485. [DOI] [PubMed] [Google Scholar]
- 14.Miller SP, Newton N, Ferriero DM, et al. Predictors of 30-month outcome after perinatal depression: role of proton MRS and socioeconomic factors. Pediatr Res. 2002;52:71–77. doi: 10.1203/00006450-200207000-00014. [DOI] [PubMed] [Google Scholar]
- 15.Mukherjee P, Miller JH, Shimony JS, et al. Diffusion-tensor MR imaging of gray and white matter development during normal human brain maturation. AJNR Am J Neuroradiol. 2002;23:1445–1456. [PMC free article] [PubMed] [Google Scholar]
- 16.Beaulieu C. The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed. 2002;15:435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- 17.Deipolyi AR, Mukherjee P, Gill K, et al. Comparing microstructural and macrostructural development of the cerebral cortex in premature newborns: Diffusion tensor imaging versus cortical gyration. Neuroimage. 2005;27:579–586. doi: 10.1016/j.neuroimage.2005.04.027. [DOI] [PubMed] [Google Scholar]
- 18.McKinstry RC, Mathur A, Miller JH, et al. Radial organization of developing preterm human cerebral cortex revealed by non-invasive water diffusion anisotropy MRI. Cereb Cortex. 2002;12:1237–1243. doi: 10.1093/cercor/12.12.1237. [DOI] [PubMed] [Google Scholar]
- 19.Drobyshevsky A, Song SK, Gamkrelidze G, et al. Developmental changes in diffusion anisotropy coincide with immature oligodendrocyte progression and maturation of compound action potential. J Neurosci. 2005;25:5988–5997. doi: 10.1523/JNEUROSCI.4983-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prayer D, Barkovich AJ, Kirschner DA, et al. Visualization of nonstructural changes in early white matter development on diffusion-weighted MR images: evidence supporting premyelination anisotropy. AJNR Am J Neuroradiol. 2001;22:1572–1576. [PMC free article] [PubMed] [Google Scholar]
- 21.Limperopoulos C, Gauvreau KK, O'Leary H, et al. Cerebral hemodynamic changes during intensive care of preterm infants. Pediatrics. 2008;122:e1006–e1013. doi: 10.1542/peds.2008-0768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chau V, Poskitt KJ, McFadden DE, et al. Effect of Chorioamnionitis on Brain Development and Injury in Premature Newborns. Ann. Neurol. 2009 doi: 10.1002/ana.21713. In Press. [DOI] [PubMed] [Google Scholar]
- 23.Glass HC, Bonifacio SL, Chau V, et al. Recurrent postnatal infections are associated with progressive white matter injury in premature infants. Pediatrics. 2008;122:299–305. doi: 10.1542/peds.2007-2184. [DOI] [PubMed] [Google Scholar]
- 24.Shah DK, Doyle LW, Anderson PJ, et al. Adverse neurodevelopment in preterm infants with postnatal sepsis or necrotizing enterocolitis is mediated by white matter abnormalities on magnetic resonance imaging at term. J Pediatr. 2008;153:170–175. doi: 10.1016/j.jpeds.2008.02.033. [DOI] [PubMed] [Google Scholar]
- 25.Cornette LG, Tanner SF, Ramenghi LA, et al. Magnetic resonance imaging of the infant brain: anatomical characteristics and clinical significance of punctate lesions. Arch Dis Child Fetal Neonatal Ed. 2002;86:F171–F177. doi: 10.1136/fn.86.3.F171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller SP, Ferriero DM, Leonard C, et al. Early Brain Injury in Premature Newborns Detected with MRI: Relationship with Early Neurodevelopmental Outcome. J Pediatr. 2005;147:609–616. doi: 10.1016/j.jpeds.2005.06.033. [DOI] [PubMed] [Google Scholar]
- 27.Volpe JJ. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol. 2009;8:110–124. doi: 10.1016/S1474-4422(08)70294-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aziz K, Vickar DB, Sauve RS, et al. Province-based study of neurologic disability of children weighing 500 through 1249 grams at birth in relation to neonatal cerebral ultrasound findings. Pediatrics. 1995;95:837–844. [PubMed] [Google Scholar]
- 29.Hamrick SE, Miller SP, Leonard C, et al. Trends in severe brain injury and neurodevelopmental outcome in premature newborn infants: the role of cystic periventricular leukomalacia. J Pediatr. 2004;145:593–599. doi: 10.1016/j.jpeds.2004.05.042. [DOI] [PubMed] [Google Scholar]
- 30.Miller SP, Cozzio CC, Goldstein RB, et al. Comparing the diagnosis of white matter injury in premature newborns with serial MR imaging and transfontanel ultrasonography findings. AJNR Am J Neuroradiol. 2003;24:1661–1669. [PMC free article] [PubMed] [Google Scholar]
- 31.Woodward LJ, Anderson PJ, Austin NC, et al. Neonatal MRI to predict neurodevelopmental outcomes in preterm infants. N Engl J Med. 2006;355:685–694. doi: 10.1056/NEJMoa053792. [DOI] [PubMed] [Google Scholar]
- 32.Counsell SJ, Edwards AD, Chew AT, et al. Specific relations between neurodevelopmental abilities and white matter microstructure in children born preterm. Brain. 2008;131:3201–3208. doi: 10.1093/brain/awn268. [DOI] [PubMed] [Google Scholar]
- 33.Krishnan ML, Dyet LE, Boardman JP, et al. Relationship between white matter apparent diffusion coefficients in preterm infants at term-equivalent age and developmental outcome at 2 years. Pediatrics. 2007;120:e604–e609. doi: 10.1542/peds.2006-3054. [DOI] [PubMed] [Google Scholar]
- 34.Limperopoulos C, Bassan H, Kalish LA, et al. Current definitions of hypotension do not predict abnormal cranial ultrasound findings in preterm infants. Pediatrics. 2007;120:966–977. doi: 10.1542/peds.2007-0075. [DOI] [PubMed] [Google Scholar]
- 35.Short EJ, Klein NK, Lewis BA, et al. Cognitive and academic consequences of bronchopulmonary dysplasia and very low birth weight: 8-year-old outcomes. Pediatrics. 2003;112:e359. doi: 10.1542/peds.112.5.e359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stoll BJ, Hansen NI, Adams-Chapman I, et al. Neurodevelopmental and growth impairment among extremely low-birth-weight infants with neonatal infection. JAMA. 2004;292:2357–2365. doi: 10.1001/jama.292.19.2357. [DOI] [PubMed] [Google Scholar]
- 37.Murphy BP, Inder TE, Huppi PS, et al. Impaired cerebral cortical gray matter growth after treatment with dexamethasone for neonatal chronic lung disease. Pediatrics. 2001;107:217–221. doi: 10.1542/peds.107.2.217. [DOI] [PubMed] [Google Scholar]
- 38.Lodygensky GA, Rademaker K, Zimine S, et al. Structural and functional brain development after hydrocortisone treatment for neonatal chronic lung disease. Pediatrics. 2005;116:1–7. doi: 10.1542/peds.2004-1275. [DOI] [PubMed] [Google Scholar]
- 39.Fujimoto S, Togari H, Yamaguchi N, et al. Hypocarbia and cystic periventricular leukomalacia in premature infants. Arch Dis Child. 1994;71:F107–F110. doi: 10.1136/fn.71.2.f107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miller SP, Mayer EE, Clyman RI, et al. Prolonged indomethacin exposure is associated with decreased white matter injury detected with magnetic resonance imaging in premature newborns at 24 to 28 weeks' gestation at birth. Pediatrics. 2006;117:1626–1631. doi: 10.1542/peds.2005-1767. [DOI] [PubMed] [Google Scholar]
- 41.Gressens P, Rogido M, Paindaveine B, et al. The impact of neonatal intensive care practices on the developing brain. Journal of Pediatrics. 2002;140:646–653. doi: 10.1067/mpd.2002.123214. [DOI] [PubMed] [Google Scholar]
- 42.McQuillen PS, Sheldon RA, Shatz CJ, et al. Selective vulnerability of subplate neurons after early neonatal hypoxia-ischemia. J Neurosci. 2003;23:3308–3315. doi: 10.1523/JNEUROSCI.23-08-03308.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Follett PL, Rosenberg PA, Volpe JJ, et al. NBQX attenuates excitotoxic injury in developing white matter. J Neurosci. 2000;20:9235–9241. doi: 10.1523/JNEUROSCI.20-24-09235.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Back SA, Gan X, Li Y, et al. Maturation-dependent vulnerability of oligodendrocytes to oxidative stress-induced death caused by glutathione depletion. J Neurosci. 1998;18:6241–6253. doi: 10.1523/JNEUROSCI.18-16-06241.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Manning SM, Talos DM, Zhou C, et al. NMDA receptor blockade with memantine attenuates white matter injury in a rat model of periventricular leukomalacia. J Neurosci. 2008;28:6670–6678. doi: 10.1523/JNEUROSCI.1702-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Follett PL, Deng W, Dai W, et al. Glutamate receptor-mediated oligodendrocyte toxicity in periventricular leukomalacia: a protective role for topiramate. J Neurosci. 2004;24:4412–4420. doi: 10.1523/JNEUROSCI.0477-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Deng W, Rosenberg PA, Volpe JJ, et al. Calcium-permeable AMPA/kainate receptors mediate toxicity and preconditioning by oxygen-glucose deprivation in oligodendrocyte precursors. Proc Natl Acad Sci U S A. 2003;100:6801–6806. doi: 10.1073/pnas.1136624100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Catalano SM, Chang CK, Shatz CJ. Activity-dependent regulation of NMDAR1 immunoreactivity in the developing visual cortex. J Neurosci. 1997;17:8376–8390. doi: 10.1523/JNEUROSCI.17-21-08376.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Talos DM, Follett PL, Folkerth RD, et al. Developmental regulation of alpha-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid receptor subunit expression in forebrain and relationship to regional susceptibility to hypoxic/ischemic injury. II. Human cerebral white matter and cortex. J Comp Neurol. 2006;497:61–77. doi: 10.1002/cne.20978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Back SA, Han BH, Luo NL, et al. Selective vulnerability of late oligodendrocyte progenitors to hypoxia- ischemia. J Neurosci. 2002;22:455–463. doi: 10.1523/JNEUROSCI.22-02-00455.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Back SA, Luo NL, Borenstein NS, et al. Late oligodendrocyte progenitors coincide with the developmental window of vulnerability for human perinatal white matter injury. J Neurosci. 2001;21:1302–1312. doi: 10.1523/JNEUROSCI.21-04-01302.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Riddle A, Luo NL, Manese M, et al. Spatial heterogeneity in oligodendrocyte lineage maturation and not cerebral blood flow predicts fetal ovine periventricular white matter injury. J Neurosci. 2006;26:3045–3055. doi: 10.1523/JNEUROSCI.5200-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McQuillen PS, Ferriero DM. Selective vulnerability in the developing central nervous system. Pediatr Neurol. 2004;30:227–235. doi: 10.1016/j.pediatrneurol.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 54.Kostovic I, Judas M, Rados M, et al. Laminar organization of the human fetal cerebrum revealed by histochemical markers and magnetic resonance imaging. Cereb Cortex. 2002;12:536–544. doi: 10.1093/cercor/12.5.536. [DOI] [PubMed] [Google Scholar]
- 55.Segovia KN, McClure M, Moravec M, et al. Arrested oligodendrocyte lineage maturation in chronic perinatal white matter injury. Ann Neurol. 2008;63:520–530. doi: 10.1002/ana.21359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Haynes RL, Billiards SS, Borenstein NS, et al. Diffuse axonal injury in periventricular leukomalacia as determined by apoptotic marker fractin. Pediatr Res. 2008;63:656–661. doi: 10.1203/PDR.0b013e31816c825c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Counsell SJ, Shen Y, Boardman JP, et al. Axial and radial diffusivity in preterm infants who have diffuse white matter changes on magnetic resonance imaging at term-equivalent age. Pediatrics. 2006;117:376–386. doi: 10.1542/peds.2005-0820. [DOI] [PubMed] [Google Scholar]
- 58.Badawi N, Kurinczuk JJ, Keogh JM, et al. Intrapartum risk factors for newborn encephalopathy: the Western Australian case-control study. BMJ. 1998;317:1554–1558. doi: 10.1136/bmj.317.7172.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Badawi N, Kurinczuk JJ, Keogh JM, et al. Antepartum risk factors for newborn encephalopathy: the Western Australian case-control study. BMJ. 1998;317:1549–1553. doi: 10.1136/bmj.317.7172.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cowan F, Rutherford M, Groenendaal F, et al. Origin and timing of brain lesions in term infants with neonatal encephalopathy. Lancet. 2003;361:736–742. doi: 10.1016/S0140-6736(03)12658-X. [DOI] [PubMed] [Google Scholar]
- 61.Miller SP, Ramaswamy V, Michelson D, et al. Patterns of brain injury in term neonatal ncephalopathy. J Pediatr. 2005;146:453–460. doi: 10.1016/j.jpeds.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 62.Barkovich AJ, Hajnal BL, Vigneron D, et al. Prediction of neuromotor outcome in perinatal asphyxia: evaluation of MR scoring systems. AJNR. American Journal of Neuroradiology. 1998;19:143–149. [PMC free article] [PubMed] [Google Scholar]
- 63.Sie LT, van der Knaap MS, Oosting J, et al. MR patterns of hypoxic-ischemic brain damage after prenatal, perinatal or postnatal asphyxia. Neuropediatrics. 2000;31:128–136. doi: 10.1055/s-2000-7496. [DOI] [PubMed] [Google Scholar]
- 64.Steinman KJ, Gorno-Tempini ML, Glidden DV, et al. Neonatal watershed brain injury on magnetic resonance imaging correlates with verbal IQ at 4 years. Pediatrics. 2009;123:1025–1030. doi: 10.1542/peds.2008-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Roland EH, Poskitt K, Rodriguez E, et al. Perinatal hypoxic-ischemic thalamic injury: clinical features and neuroimaging. Ann Neurol. 1998;44:161–166. doi: 10.1002/ana.410440205. [DOI] [PubMed] [Google Scholar]
- 66.Inder TE, Wells SJ, Mogridge NB, et al. Defining the nature of the cerebral abnormalities in the premature infant: a qualitative magnetic resonance imaging study. J Pediatr. 2003;143:171–179. doi: 10.1067/S0022-3476(03)00357-3. [DOI] [PubMed] [Google Scholar]
- 67.Boardman JP, Counsell SJ, Rueckert D, et al. Early growth in brain volume is preserved in the majority of preterm infants. Ann Neurol. 2007;62:185–192. doi: 10.1002/ana.21171. [DOI] [PubMed] [Google Scholar]
- 68.Inder TE, Warfield SK, Wang H, et al. Abnormal cerebral structure is present at term in premature infants. Pediatrics. 2005;115:286–294. doi: 10.1542/peds.2004-0326. [DOI] [PubMed] [Google Scholar]
- 69.Peterson BS, Vohr B, Staib LH, et al. Regional brain volume abnormalities and long-term cognitive outcome in preterm infants. JAMA. 2000;284:1939–1947. doi: 10.1001/jama.284.15.1939. [DOI] [PubMed] [Google Scholar]
- 70.Kesler SR, Reiss AL, Vohr B, et al. Brain volume reductions within multiple cognitive systems in male preterm children at age twelve. J Pediatr. 2008;152:513–520. doi: 10.1016/j.jpeds.2007.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Inder TE, Huppi PS, Warfield S, et al. Periventricular white matter injury in the premature infant is followed by reduced cerebral cortical gray matter volume at term. Ann. Neurol. 1999;46:755–760. doi: 10.1002/1531-8249(199911)46:5<755::aid-ana11>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 72.Srinivasan L, Dutta R, Counsell SJ, et al. Quantification of deep gray matter in preterm infants at term-equivalent age using manual volumetry of 3-tesla magnetic resonance images. Pediatrics. 2007;119:759–765. doi: 10.1542/peds.2006-2508. [DOI] [PubMed] [Google Scholar]
- 73.Srinivasan L, Allsop J, Counsell SJ, et al. Smaller cerebellar volumes in very preterm infants at term-equivalent age are associated with the presence of supratentorial lesions. AJNR Am J Neuroradiol. 2006;27:573–579. [PMC free article] [PubMed] [Google Scholar]
- 74.Limperopoulos C, Soul JS, Haidar H, et al. Impaired trophic interactions between the cerebellum and the cerebrum among preterm infants. Pediatrics. 2005;116:844–850. doi: 10.1542/peds.2004-2282. [DOI] [PubMed] [Google Scholar]
- 75.Counsell SJ, Dyet LE, Larkman DJ, et al. Thalamo-cortical connectivity in children born preterm mapped using probabilistic magnetic resonance tractography. Neuroimage. 2007;34:896–904. doi: 10.1016/j.neuroimage.2006.09.036. [DOI] [PubMed] [Google Scholar]
- 76.Beauchamp MH, Thompson DK, Howard K, et al. Preterm infant hippocampal volumes correlate with later working memory deficits. Brain. 2008;131:2986–2994. doi: 10.1093/brain/awn227. [DOI] [PubMed] [Google Scholar]
- 77.Thompson DK, Wood SJ, Doyle LW, et al. Neonate hippocampal volumes: prematurity, perinatal predictors, and 2-year outcome. Ann Neurol. 2008;63:642–651. doi: 10.1002/ana.21367. [DOI] [PubMed] [Google Scholar]
- 78.Tam EW, Ferriero DM, Xu D, et al. Cerebellar development in the preterm neonate: effect of supratentorial brain injury. Pediatr Res. 2009 doi: 10.1203/PDR.0b013e3181a1fb3d. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Maas LC, Mukherjee P, Carballido-Gamio J, et al. Early laminar organization of the human cerebrum demonstrated with diffusion tensor imaging in extremely premature infants. Neuroimage. 2004;22:1134–1140. doi: 10.1016/j.neuroimage.2004.02.035. [DOI] [PubMed] [Google Scholar]
- 80.Li AM, Chau V, Poskitt KJ, et al. White Matter Injury in Term Newborns with Neonatal Encephalopathy. Pediatr Res. 2009;65(1):85–89. doi: 10.1203/PDR.0b013e31818912d2. [DOI] [PubMed] [Google Scholar]
- 81.Neil JJ, Inder TE. Detection of wallerian degeneration in a newborn by diffusion magnetic resonance imaging (MRI) J Child Neurol. 2006;21:115–118. doi: 10.1177/08830738060210021501. [DOI] [PubMed] [Google Scholar]
- 82.Barkovich AJ, Miller SP, Bartha A, et al. MR imaging, MR spectroscopy, and diffusion tensor imaging of sequential studies in neonates with encephalopathy. AJNR Am J Neuroradiol. 2006;27:533–547. [PMC free article] [PubMed] [Google Scholar]
- 83.De Vries LS, Van der Grond J, Van Haastert IC, et al. Prediction of outcome in new-born infants with arterial ischaemic stroke using diffusion-weighted magnetic resonance imaging. Neuropediatrics. 2005;36:12–20. doi: 10.1055/s-2005-837544. [DOI] [PubMed] [Google Scholar]
- 84.Kirton A, Shroff M, Visvanathan T, et al. Quantified corticospinal tract diffusion restriction predicts neonatal stroke outcome. Stroke. 2007;38:974–980. doi: 10.1161/01.STR.0000258101.67119.72. [DOI] [PubMed] [Google Scholar]
- 85.McQuillen PS, Barkovich AJ, Hamrick SE, et al. Temporal and Anatomic Risk Profile of Brain Injury With Neonatal Repair of Congenital Heart Defects. Stroke. 2007;38:736–741. doi: 10.1161/01.STR.0000247941.41234.90. [DOI] [PubMed] [Google Scholar]
- 86.Mahle WT, Tavani F, Zimmerman RA, et al. An MRI study of neurological injury before and after congenital heart surgery. Circulation. 2002;106:I109–I114. [PubMed] [Google Scholar]
- 87.Galli KK, Zimmerman RA, Jarvik GP, et al. Periventricular leukomalacia is common after neonatal cardiac surgery. J Thorac Cardiovasc Surg. 2004;127:692–704. doi: 10.1016/j.jtcvs.2003.09.053. [DOI] [PubMed] [Google Scholar]
- 88.Gilles FH, Leviton A, Jammes J. Age-dependent changes in white matter in congenital heart disease. J Neuropathol Exp Neurol. 1973;32:179. [Google Scholar]
- 89.Kinney HC, Panigrahy A, Newburger JW, et al. Hypoxic-ischemic brain injury in infants with congenital heart disease dying after cardiac surgery. Acta Neuropathol (Berl) 2005;110:563–578. doi: 10.1007/s00401-005-1077-6. [DOI] [PubMed] [Google Scholar]
- 90.Miller G, Mamourian AC, Tesman JR, et al. Long-term MRI changes in brain after pediatric open heart surgery. J Child Neurol. 1994;9:390–397. doi: 10.1177/088307389400900411. [DOI] [PubMed] [Google Scholar]
- 91.Rosenthal GL. Patterns of prenatal growth among infants with cardiovascular malformations: possible fetal hemodynamic effects. Am J Epidemiol. 1996;143:505–513. doi: 10.1093/oxfordjournals.aje.a008771. [DOI] [PubMed] [Google Scholar]
- 92.Glauser TA, Rorke LB, Weinberg PM, et al. Congenital brain anomalies associated with the hypoplastic left heart syndrome. Pediatrics. 1990;85:984–990. [PubMed] [Google Scholar]
- 93.Licht DJ, Shera DM, Clancy RR, et al. Brain maturation is delayed in infants with complex congenital heart defects. J Thorac Cardiovasc Surg. 2009;137:529–536. doi: 10.1016/j.jtcvs.2008.10.025. discussion 536-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Donofrio MT, Bremer YA, Schieken RM, et al. Autoregulation of cerebral blood flow in fetuses with congenital heart disease: the brain sparing effect. Pediatr Cardiol. 2003;24:436–443. doi: 10.1007/s00246-002-0404-0. [DOI] [PubMed] [Google Scholar]
- 95.Jouannic JM, Benachi A, Bonnet D, et al. Middle cerebral artery Doppler in fetuses with transposition of the great arteries. Ultrasound Obstet Gynecol. 2002;20:122–124. doi: 10.1046/j.1469-0705.2002.00756.x. [DOI] [PubMed] [Google Scholar]
- 96.Rudolph A. Congenital Diseases of the Heart: Clinical-Physiological Considerations. 2nd ed. Armonk, New York: Futura Publishing Company; 2001. [Google Scholar]
- 97.Partridge SC, Vigneron DB, Charlton NN, et al. Pyramidal tract maturation after brain injury in newborns with heart disease. Ann Neurol. 2006;59:640–651. doi: 10.1002/ana.20772. [DOI] [PubMed] [Google Scholar]
- 98.Northington FJ, Ferriero DM, Flock DL, et al. Delayed neurodegeneration in neonatal rat thalamus after hypoxia-ischemia is apoptosis. J Neurosci. 2001;21:1931–1938. doi: 10.1523/JNEUROSCI.21-06-01931.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Stone BS, Zhang J, Mack DW, et al. Delayed neural network degeneration after neonatal hypoxia-ischemia. Ann Neurol. 2008;64:535–546. doi: 10.1002/ana.21517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sherlock RL, McQuillen PS, Miller SP. Preventing brain injury in newborns with congenital heart disease: brain imaging and innovative trial designs. Stroke. 2009;40:327–332. doi: 10.1161/STROKEAHA.108.522664. [DOI] [PubMed] [Google Scholar]