Abstract
Estrogens and nitric oxide (NO) exert wide-ranging effects on brain function. Recent evidence suggested that one important mechanism for the regulation of NO production may reside in the differential coupling of the calcium-activated neuronal NO synthase (nNOS) to glutamate N-Methyl-D-Aspartate (NMDA) receptor channels harboring NR2B subunits by the scaffolding protein postsynaptic density-95 (PSD95), and that estrogens promote the formation of this ternary complex. Here, we demonstrate that 30-min estradiol-treatment triggers the production of NO by physically and functionally coupling NMDA receptors to nNOS in primary neurons of the rat preoptic region in vitro. The ability of estradiol to activate neuronal NO signaling in preoptic neurons and to promote changes in protein-protein interactions is blocked by ICI 182,780, an estrogen receptor antagonist. In addition, blockade of NMDA receptor NR2B subunit activity with ifenprodil or disruption of PSD95 synthesis in preoptic neurons by treatment with an antisense oligodeoxynucleotide inhibited the estradiol-promoted stimulation of NO release in cultured preoptic neurons. Thus, estrogen receptor-mediated stimulation of the nNOS/PSD95/NMDA receptor complex assembly is likely to be a critical component of the signaling process by which estradiol facilitates coupling of glutamatergic fluxes for NO production in neurons.
Keywords: Animals; Cells, Cultured; Estradiol; physiology; Female; Male; Neurons; cytology; enzymology; metabolism; Nitric Oxide; biosynthesis; chemistry; Nitric Oxide Synthase Type I; chemistry; metabolism; Protein Binding; physiology; Rats; Rats, Sprague-Dawley; Receptors, Estrogen; chemistry; physiology; Receptors, N-Methyl-D-Aspartate; chemistry; metabolism
Keywords: nNOS, NMDA receptor, estradiol, estrogen receptor, PSD-95, hypothalamus
Introduction
Estradiol is the most biologically prevalent and active compound of a class of steroids called estrogens, and it exerts potent and wide-ranging effects on the brain (Maggi et al. 2004). Besides their well-known effects on the hypothalamic-pituitary-gonadal axis, estrogens have multiple and complex influences on brain structure and physiology (Maggi et al. 2004; Woolley 2007). Natural fluctuations of estrogen levels across the estrous cycle have been shown to cause cyclic changes in dendritic spine density and synaptogenesis in the rat hippocampus (Woolley and McEwen 1992). Likewise, estradiol treatment in ovariectomized female rats controls dynamic changes in spine density in the hippocampus (Gould et al. 1990) and hypothalamus (Calizo and Flanagan-Cato 2000). In addition to these structural effects on neuronal connectivity, recent studies showed estrogens to play a distinct role in controlling nitric oxide (NO) production (Lamar et al. 1999; d’Anglemont de Tassigny et al. 2007).
NO is a major messenger molecule involved in various cellular functions in the brain such as apoptosis, differentiation, development, synaptic plasticity and neurosecretion (Prast and Philippu 2001; Boehning and Snyder 2003; McCann et al. 2003). Neuronal NO synthase (nNOS) is the predominant source of NO in neurons (Boehning and Snyder 2003). Neuronal NOS is primarily activated by its interaction with the Ca2+-calmodulin complex when intracellular Ca2+ increases (Bredt and Snyder 1990; Abu-Soud et al. 1994). Although there are several distinct calcium pools at the synapse, only Ca2+ influx through the N-Methyl-D-Aspartate (NMDA) glutamate receptor efficiently activates nNOS (Kiedrowski et al. 1992) as calmodulin is physically associated to the NMDA receptor (Hisatsune et al. 1997). A physical approximation of nNOS to the NMDA receptor determines this specificity and is only permitted via postsynaptic density protein PSD95 (Christopherson et al. 1999), which acts as an adaptor protein via PDZ (PSD-95/Discs-large/Zona occludens) domain interaction (Christopherson et al. 1999; Sattler et al. 1999; Aarts et al. 2002). Therefore, NO production highly depends on i) NMDA receptor activation (Bredt and Snyder 1989; Garthwaite and Boulton 1995), and ii) nNOS subcellular localization within the neuron.
Interestingly, we have recently reported that estrogens promote cyclic fluctuations in the association between nNOS and the NMDA receptor subunit 2B (NR2B) in the preoptic region of the hypothalamus in female rats during the ovarian cycle (d’Anglemont de Tassigny et al. 2007). Thus, one mechanism involved in the regulation by estradiol of nNOS activity in neurons may reside in its increased coupling to PSD-95/NR2B complex to facilitate the access to the Ca2+-calmodulin located just underneath the NMDA receptor. However, the molecular mechanisms underlying these changes remain elusive, and in particular, whether classical estrogen receptors activation is required to promote nNOS/NMDA receptor complex formation and thereby coupling glutamatergic fluxes with NO production is unknown.
Using neurons-containing primary cultures from the preoptic region of neonate rats, we report here a role for estrogen receptors in regulating protein-protein interactions, the induction of nNOS/NMDA receptor complex formation and its translation into NO release in response to the gonadal steroid estradiol. This effect on NO production is dependent on the activation of NR2B NMDA receptor subunits by glutamate and on PSD-95 expression.
Materials and Methods
Primary neuron-containing cultures from the preoptic region
Primary cultures were prepared from newborn (P0) Sprague-Dawley rats (Janvier Saint-Berthevin, France). Both male and female pups were used for experiments. Even though, circulating testosterone at birth is already higher in males as compared to females (Amateau and McCarthy 2004), hormone exposure is too short to mediate masculinization of the P0 male brain and to promote irreversible changes in hormone-sensitive neurons (Finn et al. 1996). After decapitation and removal of the brain, the meninges and optic chiasm were discarded and the preoptic region was isolated under a binocular magnifying glass with Wecker’s scissors (Moria, France). The external limits for this dissection (adapted to P0 brain size from adult brain) are: lateral, the external border of the Medial Preoptic Area (MPO); dorsal, the internal border of anterior commissures (aco); and antero-posterior limits are +0.95 to −0.51 mm from bregma, according to the Swanson Atlas (Swanson 1996). Preoptic region explants were placed in ice-cold Dulbecco’s Modified Eagle Medium (DMEM, Invitrogen). Each explant was cut into 5–6 smaller pieces and incubated for 1 hr at 37°C and a 5% CO2 atmosphere in DMEM containing papain (33 U/ml, 3126, Worthington-Cooper, Lakewood, NJ), deoxyribonuclease I (DNase I, 125 U/ml, D4527, Sigma) and L-cysteine (2.5mM, Sigma) for papain activation. Papain-incubated tissues were washed twice in DMEM with the trypsin inhibitor ovomucoid (1.54 mg/ml, 109878, Roche Diagnostics), DNase I (125 U/ml, Sigma) and bovine serum albumin (0.62 mg/ml, A7906, Sigma) to end the enzymatic reaction. The fragments were crushed through a 20 μm nylon mesh (Sefar America Inc., Kansas City, MO) and the dissociated cells were centrifuged at 90xg for 10 min and resuspended in serum-free neuronal defined medium consisting of Neurobasal-A medium without phenol red (12340-015, Invitrogen) with 2% (v/v) B-27 supplement (17504-044, Invitrogen), 1% (v/v) GlutaMAX (35050-038, Invitrogen) and 2% (v/v) antibiotics (penicillin/streptomycin, 10378-016, Invitrogen). Cells were counted with a hemacytometer (Thoma Cell, Marienfield, Germany). For immunoprecipitation and immunoblotting experiments, 2×106 cells were plated in 10 cm-diameter poly-L-lysine (MW > 300,000, P5899, Sigma)-coated dishes (Falcon). Primary cell cultures used for fluorescent experiments were plated onto 12mm-diameter poly-L-lysine-coated coverslips in 24-well-plates (Falcon) with a density of 4×105 cells per well. Primary cultures were maintained in an incubator at 37°C and 5% CO2 for 9 days in vitro (DIV; time of plating, DIV0), their medium was changed after 2 days of culture and subsequently 3 times a week. 17-β-estradiol was obtained from Sigma (E-8875). ICI 182,780 (ICI, 1047) and ifenprodil (0545) were supplied by Tocris (Ellisville, MO).
Immunohistofluorescence
Coverslips with adhered cells were immersed in 4% paraformaldehyde and 5% sucrose in 0.1M PBS pH7.4, warmed to 37°C for 10min, washed three times in PBS, and permeabilized with 50% ethanol for 60min at 4°C. The cells were then washed three times in PBS and incubated in 5% bovine albumin (A7906, Sigma) in 0.3% Triton X-100 PBS (PBST) for 60min at RT with agitation. After blocking, both sheep polyclonal anti-nNOS (1:3000; Dr. PC Emson (Medical Research Council, Laboratory for Molecular Research, Cambridge, UK) (Herbison et al. 1996) and rabbit polyclonal anti-NR2B (1:500; 71–8600, Zymed Laboratories, San Francisco, CA) (Christopherson et al. 1999) were applied in 5% bovine albumin PBST, and the cultures were incubated overnight at 4°C. Coverslips were extensively washed in PBST and incubated in PBST containing secondary anti-sheep Alexa Fluor 568 conjugate (1:500) and biotinylated anti-rabbit (1:500) for 60min at room temperature with agitation. After three washes, cultures were incubated with a streptavidin Alexa Fluor 488 conjugate (1:500) in PBST for 60min at RT. After washing, coverslips were mounted on slides in Permafluor medium (434990, Immunon, Pittsburgh, PA). Images were acquired with a TCS SP confocal system (Leica, Nussloch, Germany).
Protein extraction and coimmunoprecipitation
After treatment, cells were briefly washed with ice-cold PBS and snap frozen on dry ice. They were lysed in 500μl freshly prepared lysis buffer (pH 7.4, 25mM Tris, 50mM β-glycerophosphate, 1.5mM EGTA, 0.5mM EDTA, 1mM sodium pyrophosphate, 1mM sodium orthovanadate, 10μg/ml leupeptin and pepstatin, 10μg/ml aprotinin, 100μg/ml PMSF, and 1% Triton X-100). Protein concentrations of cell lysate were determined using the Bradford method (BioRad, Hercules, CA). Equal amounts of protein (500 μg) in a total volume of 750μl lysis buffer were incubated with gentle rocking at 4°C overnight with 1 μg of rabbit polyclonal IgG anti-nNOS (sc-8309; Santa Cruz, CA) or 2 μg of mouse monoclonal anti-PSD95 (MA1-045 and MA1-046; Affinity BioReagents, Golden, CO) (Kornau et al. 1995). Thereafter, 60 μl of protein A-sepharose beads in lysis buffer (1:1 blend) was added to each sample and incubated for an additional 3h with gentle rocking at 4°C. The sepharose beads were pelleted by brief centrifugation, the supernatant was collected and 375μl of 3X sample buffer (187mM Tris-Base, 9% SDS, 15% glycerol, 15% β-mercaptoethanol and bromophenol blue, pH 6.8 in 1X final) was added to it for analysis of non-immunoprecipitated proteins. The beads were washed three times with ice-cold lysis buffer and boiled for 5 min in 50μl of 2X sample buffer. Beads alone incubated with proteins extracts were used as negative controls. When necessary, the samples were stored at −80°C until use.
Western blotting analysis
Samples were reboiled for 5 min after thawing and electrophoresed for 75min at 150 V in 7% Tris-acetate, or for 50 min at 200 V in 4–12% MES precast SDS-polyacrylamide gels according to the protocol supplied with the NuPAGE system (Invitrogen, Carlsbald, CA). After size-fractionation, the proteins were transferred onto polyvinylidene difluoride (PVDF) 0.2 μm pore-size membranes (LC2002, Invitrogen) in the blot module of the NuPAGE sytem (Invitrogen) for 75 min at room temperature (RT). Blots were blocked for 1 hr in TBS with 0.05% Tween 20 (TBST) and 5% non-fat milk at RT reacted overnight at 4°C with primary antibody against nNOS (1:500; sc-8309), PSD95 (1:500; MA1-046), NR2B (1:500; 71–8600 Zymed Laboratories, San Francisco, CA) or NR2A (1:500; AB1555P Chemicon), and washed four times with TBST before being exposed to horseradish peroxidase-conjugated secondary antibodies diluted in 5% non-fat milk TBST for 1 hr at RT. The immunoreactions were detected with enhanced chemiluminescence (NEL101, PerkinElmer, Boston, MA). When stripping and reprobing were required, membranes were incubated in a stripping solution (62.5 mM Tris-HCl, 2% SDS, pH 6.7, 100 mM β-mercaptoethanol) for 30 min with gentle rocking at 65°C. HRP-conjugated secondary antibody was used to verify that all former immunoreactivity was successfully stripped off. Specific bands densitometry was semi-quantified using Scion Image software.
Measurement of NO production in primary cultures from the preoptic region using the NO-sensitive fluorescent indicator DAF-FM
Relative changes in cytosolic nitric oxide concentration in preoptic neurons were monitored using the fluorescent nitric oxide probe DAF-FM (4-amino-5-methylamine-2′, 7′-difluorofluorescine, Molecular probes). DAF-FM is converted via an NO-specific mechanism to an intensely fluorescent triazole derivative (Itoh et al. 2000). Cells were loaded with 10 μM DAF-FM for 1 h in serum-free neuronal defined medium at 37°C and 5% CO2 atmosphere, rinsed in pre-warmed PBS and incubated an additional 30 min in 1 ml neuronal defined medium with or without test substances.
For morphological analysis, the cultured cells were fixed for 15 min at room temperature by adding 1ml of 8% paraformaldehyde in 0.1M PBS pH7.4. Thereafter, the cells were rinsed in PBS and incubated for 1–2 min with 0.02% Hoechst 33258 bis-benzimide (H3569, Molecular Probes) in PBS to stain cell nuclei. DAF-FM-treated cultures were imaged using a AxioCam MRm camera (Zeiss, Germany) attached to a Zeiss Imager.Z1 fluorescent microscope. Nine images from random uniformly distributed fields of cells were acquired per coverslip, with a Plan-Apochromat 20X 0.8 objective. For each image, the total number of cells as well as the number of DAF-FM fluorescent cells was counted. The results obtained from the analysis of the nine fields per coverslip were added, and the percentage of cells bearing DAF-FM fluorescence calculated. For each experimental condition, at least six coverslips were analyzed, and the results were averaged.
Primary cell culture treatment with antisense oligodeoxynucleotides (ODNs)
Fifteen-nucleotide oligomer phosphorothioated antisense ODNs (5′-GAATGGGTCACCTCC-3′) corresponding to nucleotides 435–449 of rat PSD-95/SAP90 (Gen-Bank accession number M96853) were synthesised by MWG Biotech (Roissy, France). This ODN sequence was previously shown to specifically inhibit PSD-95 expression in neurons of the rat preoptic region in vitro (d’Anglemont de Tassigny et al. 2007). ODNs (5 μM) were added to the culture medium during feeding at 2, 4, 6 and 8 DIV. Cultures were used for DAF-FM experiments at 9 DIV.
Statistics
The differences between several groups were analysed by one-way ANOVA followed by the Student-Newman-Keuls multiple comparison test for unequal replication. Before statistical analysis, percentages were subjected to arc-sine transformation to convert them from binomial to normal distribution. The level of significance was set at p < 0.05.
Results
NMDA receptor NR2B subunit is expressed by NO-producing neurons of the preoptic region in vitro
To determine whether hypothalamic preoptic neurons in vitro express nNOS, we subjected primary cultures prepared from newborn rat preoptic regions to immunocytochemical procedures. Immunohistofluorescence–confocal microscopy studies showed that a subpopulation of isolated neurons in culture expressed nNOS at 9 DIV (Fig. 1A). Further analysis showed that virtually all preoptic nNOS-expressing neurons in vitro also expressed the NMDA receptor NR2B subunit (Fig. 1A) which is known to interact with nNOS (Christopherson et al. 1999; Sattler et al. 1999; Aarts et al. 2002). In contrast, only few NR2B-immunoreactive primary preoptic neurons expressed nNOS (Fig. 1A).
Figure 1.
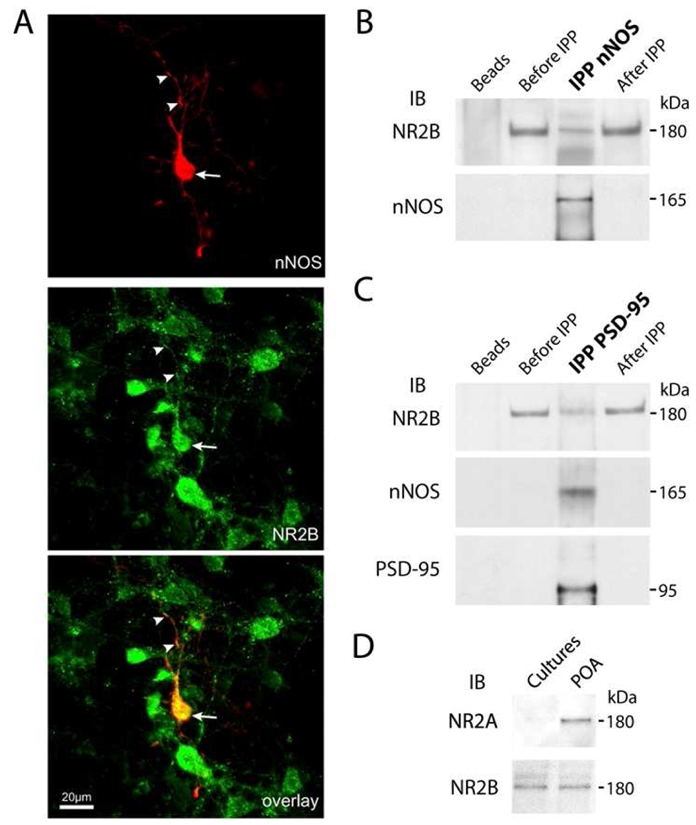
Neuronal NOS and NMDA receptor subunit NR2B are expressed and interact physically in a subset of hypothalamic neurons of the preoptic region in vitro. A, Confocal microscopy image illustrating the co-localization of NR2B (green) and nNOS (red) immunofluorescent stainings in cultured neurons derived from the preoptic region from new born rats. Note the expression of the NR2B immunoreactive material both at soma (arrow) and dendrites (arrowheads) in nNOS neurons in vitro. Scale bar: 20 μm. B–C, Neuronal NOS, NR2B and PSD-95 coimmunoprecipitate from primary cultures of preoptic neurons. Solubilized rat preoptic region primary culture homogenates were immunoprecipitated (IPP) with an antibody to nNOS (B) or PSD-95 (C). Starting material before IPP, immunoprecipitated proteins and depleted fraction after IPP were analyzed by immunoblotting (IB) for NR2B. Then immunoblots were stripped and reprobed with antibodies to nNOS and/or PSD-95. Beads alone incubated with proteins extracts were used as a negative control. D, Absence of detectable NR2A protein expression in primary cultures of preoptic neurons as assessed by straight Western blot analyses. Protein extracts from the adult preoptic region (POA) served as a positive control, and the effective amount of protein loaded is represented by NR2B blot. In B–D, all experiments were performed at least in tree independent cultures.
nNOS is physically associated with NMDA receptor NR2B subunit and PSD-95 in preoptic neurons
To investigate whether nNOS is actually able to interact with NR2B in cultured preoptic neurons as it does in cortical neurons (Sattler et al. 1999; Aarts et al. 2002), solubilized hypothalamic primary culture extracts were subjected to coimmunoprecipitation and immunoblot assays. While NR2B receptors were detected readily using straight Western blotting (Fig. 1 B–C), nNOS and PSD-95 could only be detected after immunoprecipitation (Fig. 1 B–C). Immunoprecipitation with nNOS antibodies resulted in the coprecipitation of NR2B (Fig. 1B). Because nNOS contains a N-terminal PSD/Discs-large/Zona-Occludens-1 homologous (PDZ)-binding domain capable to bind to a similar PDZ domain from the postsynaptic density protein PSD-95, which in turns binds to the cytosolic tail of the NMDA receptor (Christopherson et al. 1999), we next determined whether nNOS and NR2B physically interact with PSD-95. Both NR2B and nNOS coimmunoprecipitated with PSD-95 (Fig. 1C). These results thus suggest that physical association of nNOS with NMDA receptors involves the scaffolding protein PSD95 and the assembly of a ternary complex, as reported previously in other systems in vitro (Christopherson et al. 1999; Sattler et al. 1999; Aarts et al. 2002) and in the hypothalamus in vivo (d’Anglemont de Tassigny et al. 2007). Interestingly, additional Western blot experiments showed that cultured preoptic neurons at 9 DIV do not express detectable levels of NR2A (Fig. 1D), which is known to complex also PSD95 (Sans et al. 2000; Liu et al. 2004; Cui et al. 2007). Altogether these results suggest that association of nNOS with NMDA receptors is likely to involve the specific participation of NR2B in primary cultures of neurons from the preoptic region.
Estradiol promotes nNOS/NR2B complex formation in preoptic neurons
To explore the possible role of estrogens in triggering the physical linkage of nNOS to NR2B in hypothalamic neurons, additional experiments were performed on cultured preoptic neurons. Treatment of hypothalamic cultures with physiological levels of estradiol (1 nM) for 30 min or 48h and immunoprecipitation of the protein extracts with rabbit polyclonal antibodies to nNOS, followed by Western blot analysis to NR2B, showed an increased physical approximation of nNOS and NR2B after 30 min estradiol-treatment when compared to control cultures (Fig. 2; n = 4 independent experiments, one Way ANOVA, p = 0.025 control vs. 30-min estradiol-treatment). A similar trend was monitored after 48-h estradiol-treatment but did not reach statistical significance (Fig. 2; n = 4 independent experiments, one Way ANOVA, p = 0.057 control vs. 48-h estradiol-treatment).
Figure 2.
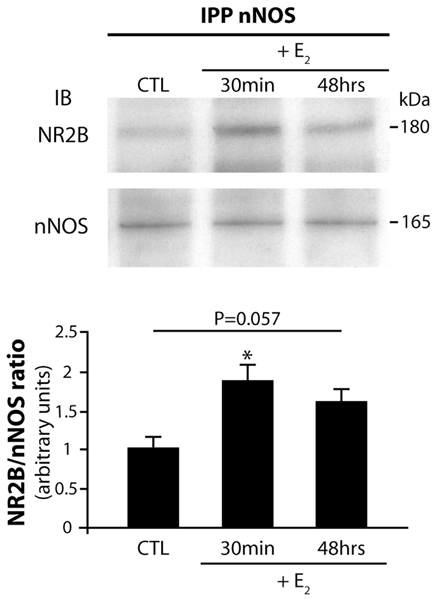
Estradiol promotes nNOS/NR2B complex formation in neurons of the preoptic region in vitro. Treatment of preoptic region neuronal cultures with 17-b-estradiol (E2) results in an increased physical association of nNOS with NR2B. Neurons were cultured in neuronal defined medium and were exposed to E2 1 nM for 30 min or 48 h or to ethanol for control cultures (CTL). Upper panel, 500 μg of proteins per condition were immunoprecipitated (IPP) with a specific nNOS antibody, electrophoresed and immunobloted (IB) for NR2B. Then the immunoblot was stripped and reprobed with antibodies to nNOS to ensure that equal amounts of nNOS protein had been immunoprecipitated and loaded on the gel. Lower panel, bar graph showing the quantitative analysis of the differential NR2B/nNOS association among E2 treatment (n = 4 independent experiments; * p = 0.025 vs CTL). Error bars indicate SEM. Statistical differences were established using a one way ANOVA by the Student-Newman-Keuls multiple comparison test.
Estradiol promotes neuronal NO formation via estrogen receptor activation
We next investigated whether estradiol-mediated increase in physical approximation of nNOS with NMDA receptor NR2B subunit is correlated with changes in NO formation in cultured preoptic neurons. To provide microscopic visualization of NOS catalytic activity, we used the NO-sensitive fluorescent indicator DAF-FM (Kojima et al. 1999; Itoh et al. 2000; Canabal et al. 2007). Application of 1 nM estradiol for 30 min induced a two-fold increase in the relative number of cells exhibiting DAF-FM fluorescence (Fig. 3A–B). Estradiol-induced increase in NO production was completely blocked by the treatment of neuronal cultures with an estrogen receptor antagonist, ICI 182,780 (1 μM), for 30 min before exposing them to estradiol for an additional 30 min (Fig. 3B). Control experiments confirmed that the increase in DAF-FM fluorescence was attributable to NO production, because the NOS inhibitor L-NAME (1 mM) blocked the estradiol-induced DAF-FM fluorescent increase (Fig. 3B). Interestingly, coimmunoprecipitation experiments showed that ICI 182,780 treatment also abrogates estradiol-induced nNOS/NR2B association in hypothalamic preoptic neurons in culture (Fig. 3C), whereas ICI 182,780 alone had no effect on nNOS association with NR2B (Fig. 3C).
Figure 3.
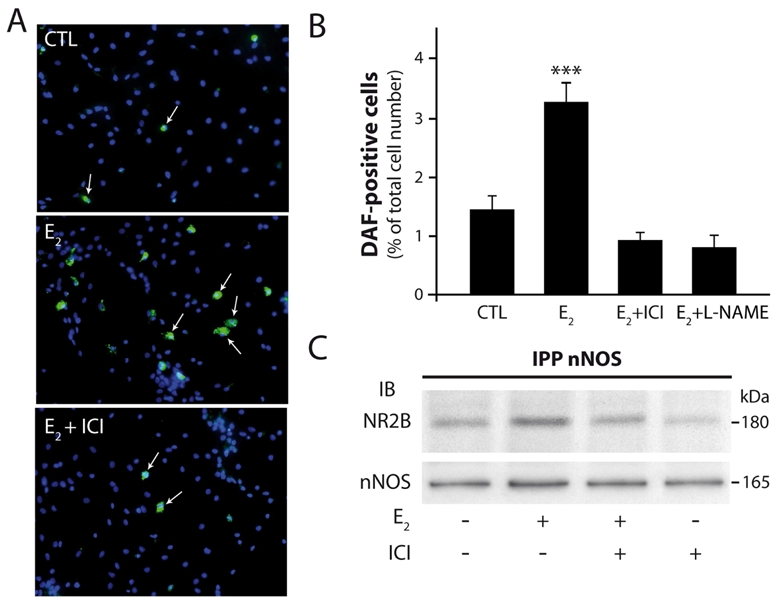
Estradiol stimulates NOS catalytic activity in hypothalamic preoptic neurons in vitro. Both estradiol-stimulated NO formation and nNOS/NR2B complex formation requires estrogen receptor activation in cultured neurons of the preoptic region. Neurons were treated with 17-b-estradiol (E2) 1 nM for 30 min and exposed to ICI 182,780 1 μM, a pure estrogen receptor antagonist, or to L-NAME 1 mM, a NOS inhibitor, for 30 min before this treatment. A, An example of fluorescent images of purified neurons loaded with the NO-sensitive dye DAF-FM (arrows) in control- (CTL) 17-b-estradiol- and 17-b-estradiol plus ICI 182,780-treated conditions (E2 + ICI). Scale bar, 40 μm. B, Summary graph showing that the pure estrogen receptor inhibitor ICI 182,780 and the NOS inhibitor L-NAME significantly reduced the amplitude of 17-b-estradiol-induced DAF fluorescent increase in neurons. ***, p < 0.001. Statistical differences were established using a one way ANOVA by the Student-Newman- Keuls multiple comparison test; n = 6–8 independent observations. C, ICI 182,780 Inhibits 17-b-estradiol-stimulated NR2B/nNOS complex formation in neurons of the rat preoptic region in culture. After treatment proteins were extracted and processed as indicated above in Figure 2. IPP, immunoprecipitation; IB, immunoblot.
Estradiol-promoted NO synthesis requires both NR2B-containing NMDA receptors activity and PSD-95 expression
At this stage of our results, we showed that ligand-activated estrogen receptors stimulate both the catalytic activity of nNOS and its physical association with NMDA receptors, however, whether these two events are causally related remains unknown. Because nNOS activity is primarily regulated by Ca2+ influx through the NMDA receptor (Garthwaite et al. 1988; Bredt and Snyder 1990), we performed experiments to determine whether estradiol-mediated increase in nNOS catalytic activity requires NMDA receptor function. Cultured preoptic neurons were exposed to estradiol and ifenprodil, a specific antagonist of NR2B-containing NMDA receptors (Williams 1993). This antagonist was previously shown to greatly reduce NMDA receptor-mediated response in hypothalamic neurons (Panatier et al. 2006). Figure 4 shows that 30-min pretreatment of cultured neurons with ifenprodil at 3 and 10 μM both suppress the stimulatory effect of estradiol on NOS activity.
Figure 4.
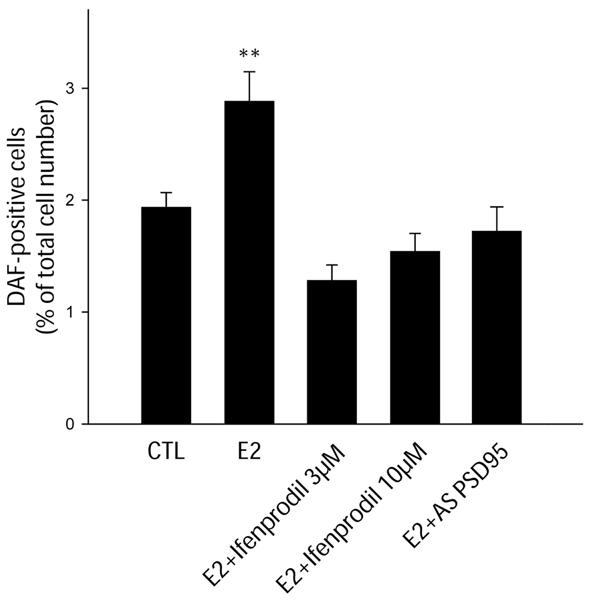
Estradiol requires both NR2B-containing NMDA receptors activity and PSD-95 expression to mediate its stimulatory effect on NO production in hypothalamic preoptic neurons in vitro. Neurons were treated with 17-b-estradiol (E2) 1 nM for 30 min and exposed to ifenprodil (3 and 10 μM), a NR2B antagonist, or PSD-95 antisense ODNs 5 μM for 30 min or 7 d, respectively, before this treatment. Statistical differences were established using a one way ANOVA by the Student-Newman-Keuls multiple comparison test. **, p < 0.01, n = 4–6 independent observations.
Having shown that estrogens may promote neuronal NO synthesis by physically and functionally coupling nNOS to NMDA receptors, we next investigated whether this system requires the scaffolding protein PSD-95. To hamper PSD-95 synthesis, we used antisense ODNs previously shown to specifically inhibit PSD-95 expression in primary neurons of the rat preoptic region in culture conditions identical to those used in the present study (d’Anglemont de Tassigny et al. 2007). Application of the ODN in the culture media (5 μM for 7 d) significantly impaired the capability of estradiol to increase nNOS catalytic activity in vitro (Figure 4). Altogether, these results suggest that coupling of nNOS to NMDA receptors via PSD-95 through estrogen receptors-dependent mechanisms is likely to be an integral component of the process by which nNOS is activated by estradiol in neurons of the hypothalamic preoptic region in vitro.
Discussion
The present results indicate that ligand-mediated stimulation of estrogen receptors in hypothalamic preoptic region neurons leads to activation of NO signaling in nNOS-expressing cells. The cellular underpinnings of this activation include physical approximation of nNOS with NMDA receptor NR2B subunit and activation of nNOS by a mechanism that appears to require the activity of NMDA receptors, which constitutes the main stimulatory calcium influx pathway for this enzyme (Garthwaite et al. 1988; Bredt and Snyder 1990). Our findings identify a distinct role for estrogen receptors in the modulation of inter-neuronal communication processes involving glutamatergic NMDA receptor activation of NO signaling. This pathway may be used by endogenous estrogens to alternate coupling and uncoupling of glutamatergic fluxes for NO production during the ovarian-cycle in the female rat, thereby regulating NOergic neurotransmission and properties of synaptic transmission (Savchenko et al. 1997; Wang et al. 2005).
It was recently shown that high endogenous estrogen levels in female rats coincide with increase in both the physical association of nNOS to NR2B and the magnitude of NO release in the preoptic region (d’Anglemont de Tassigny et al. 2007). By now showing that the estradiol-induced NO release is dependent of the NR2B-containing NMDA receptors activity within neurons, our current results expand our previous in vivo observations and suggest that the two events are causally linked (Figure 5). In addition to demonstrating the interdependent involvement of nNOS/NMDA receptor coupling in estradiol-induced activation of neuronal NO signaling by glutamate, our NOS catalytic activity assays and coimmunoprecipitation analyses showed that classical estrogen receptor signaling may directly contribute to this process. The estrogen receptor antagonist ICI 182,780 that competitively inhibits binding of estradiol to the estrogen receptor and impairs receptor dimerisation (Osborne et al. 2004) indeed prevented both estradiol-stimulated NR2B/nNOS complex formation and NO production in cultured preoptic neurons (Figure 5).
Figure 5.
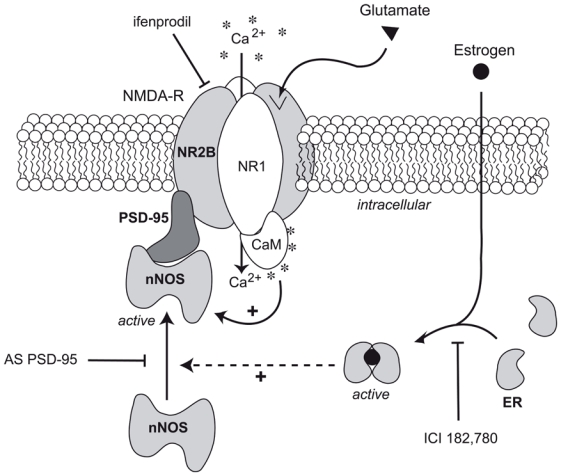
Schematic representation of the possible estradiol-mediated changes in proteinprotein interactions involved in the control of nNOS activity in neurons of the preoptic region. Neuronal NOS activity is primarily regulated by increases in the local intracellular [Ca2+], which activates nNOS through calmoduling (CaM) binding (Bredt and Snyder 1990). Importantly, Ca2+ influx through the NMDA receptor (NMDA-R) but not other Ca2+ influx pathways efficiently promotes NO synthesis (Garthwaite et al. 1988; Bredt and Snyder 1990) One estrogen receptor (ER)-dependent mechanism used for the regulation of nNOS activity may reside in the alternative coupling and uncoupling of the enzyme to NR2B-containing NMDA receptors channels by the scaffolding protein PSD-95, in the presence or the absence of estrogens, respectively. PSD-95 acts as an adaptor protein, thereby physically and functionally coupling NMDA receptors to nNOS and thus enables to couple glutamatergic fluxes with NO production. NO is formed enzymatically from L-arginine (L-Arg) in equimolar amounts with L-citrulline (L-Cit) by nNOS. Functional glutamate NMDA receptors are composed of NR1 and NR2 subunits (Hollmann and Heinemann 1994). The estrogen receptor antagonist ICI 182,780 competitively inhibits binding of estradiol to the estrogen receptor and impairs receptor dimerisation (Osborne et al. 2004); Antisense oligodeoxynucleotides to PSD-95 (AS PSD-95) selectively decreases PSD-95 expression in primary cultures of preoptic neurons (d’Anglemont de Tassigny et al. 2007); infenprodil is a specific antagonist of NR2B-containing NMDA receptors (Williams 1993).
It was demonstrated previously that physical coupling of nNOS to NMDA receptors involves the scaffolding protein PSD-95 and the assembly of the ternary complex (Christopherson et al. 1999) that efficiently couples Ca2+ influx, via NMDA receptors, to NO synthesis and activity (Sattler et al. 1999; Aarts et al. 2002; Ishii et al. 2006). Since impairment of PSD-95 synthesis via treatment with an antisense ODN in the culture media blunts the estradiol-promoted activation of NOS activity without affecting basal NOS activity, our data point to PSD-95 as a key component of the signaling pathway by which estradiol mediates its stimulatory effects on NO production in preoptic neurons. Furthermore, we demonstrate that blocking the NR2B-containing NMDA receptors with ifenprodil also prevents the estradiol-induced NO production. This suggests that the estradiol - NMDA receptor- NO pathway is essential in our in vitro system. The NMDA receptor subunit 2A (NR2A) that is expressed in the preoptic region (Gore et al. 2000) has also been shown to interact with PSD95 (Sans et al. 2000; Liu et al. 2004; Cui et al. 2007). Intriguingly, our data show that while NR2A is expressed in the hypothalamus of adult rats, this NMDA receptor subunit is not expressed at detectable levels in primary cultures of preoptic neurons obtained from newborns. Differences in neuronal maturation could explain this apparent discrepancy, as shown previously for other neuronal populations both in vitro (Brewer et al. 2007) and in vivo (Liu et al. 2004). These results together with the blockade of estrogen-promoted NO release by micromolar concentrations of ifenprodil strongly suggest that NR2B-containing NMDA channels are involved in the coupling of glumatergic fluxes for NO production in preoptic neurons.
We know very little about the mechanism used by estrogen receptors to promote the recruitment of nNOS to the NMDA receptor. A non-genomic action of estradiol could be involved in this mechanism, as changes in nNOS/PSD-95/NR2B are observable within 30 minutes. The question of whether the non-genomic effects of estradiol are mediated by membrane-bound estrogen receptors may be of low relevance since the plasma membrane is not a barrier for estradiol to enter into cells (Warner and Gustafsson 2006). However, the nature and location of the receptor might have a profound effect on its ability to induce the nNOS/PSD-95/NR2B ternary complex formation. The use of ICI 182,780 prevents the estradiol-induced nNOS/NR2B association and subsequent NO release. In addition to bind to classical estrogen receptors, ICI 182,780 was shown to act on the recently discovered transmembrane estrogen receptor GPR30 (Prossnitz et al. 2008). While ICI 182,780 blocks the classical ERα and ERβ, it activates GPR30 (Filardo et al. 2000; Filardo et al. 2002; Thomas et al. 2005). Our results thus almost certainly discard the participation of GPR30 membrane estrogen receptor. There is some evidence that estradiol acts in neurons to stimulate rapid PSD-95 new protein synthesis by promoting protein translation of dendrite-localized mRNA transcripts via the Akt/protein kinase B pathway (Akama and McEwen 2003). Estradiol also may mediate coalescence of cytoskeleton-tethered nNOS (Haraguchi et al. 2000) to PSD-95 through spine formation (Amateau and McCarthy 2002; Abraham et al. 2004), which would require remodeling of the actin cytoskeleton (Hering and Sheng 2001). Nonetheless, additional studies are required to determine whether such phenomena occur within the hypothalamus to modulate the nNOS subcellular localization.
A key physiological output for the estradiol effect on nNOS/NMDA receptor association and NO production in neurons of the preoptic region is the onset of the preovulatory surge. The central control of reproduction operates through the timely activation of gonadotropin-releasing hormone (GnRH) neurons, the final pathway for neural control of ovulation (Ojeda et al. 2002; Herbison and Neill 2006). GnRH neurons’ activity is finely tuned by several factors in response to sex steroids feedback. In females, a prolonged high concentration of estrogens induce the preovulatory GnRH/LH surge by modulating neuronal pathways upstream to GnRH neurons that are located within the preoptic region of the hypothalamus in rodents (Herbison and Neill 2006). Estradiol interacts with glutamate-containing pathways of the preoptic region to mediate their stimulatory effect on GnRH secretion (Brann and Mahesh 1991; Jarry et al. 1992). These effects involve activation of NMDA receptors (Ojeda et al. 1990; Brann and Mahesh 1991) and require the production of NO (Bonavera et al. 1993; Brenman et al. 1996; d’Anglemont de Tassigny et al. 2007). Previous studies performed in rodents have described the relationship existing between GnRH neurons and NO (Bhat et al. 1995; Herbison et al. 1996; Clasadonte et al. 2008) and have demonstrated that endogenous NO production was capable of influencing GnRH neuronal activity in the preoptic region (Clasadonte et al. 2008). Moreover, most nNOS neurons of the preoptic region express both estrogen receptor α(Scordalakes et al. 2002; Sato et al. 2005) and NR2B-containing NMDA receptors (d’Anglemont de Tassigny et al. 2007). It thus becomes increasingly clear that these neurons may be critical for the estrogen positive feedback to GnRH neurons.
In conclusion, we demonstrate in vitro that estradiol stimulates neuronal NO production by inducing physical association of nNOS to NMDA receptors in an estrogen receptor-dependent manner. Our results together with previous studies identify a distinct role for estrogens and estrogen receptors in modulating the coupling of glutamatergic fluxes for NO production in neurons of the CNS.
Acknowledgments
This research was supported by the Institut National de la Santé et de la Recherche Médicale (Inserm, France) grant U837, the Fondation pour le Recherche Médicale (Equipe FRM), l’Agence Nationale de la Recherche (ANR), the Université Lille 2, the imaging Core of IFR114. XdAdT and CC were Ph.D. students supported by a fellowship from Inserm and the Région Nord Pas de Calais. We thank Dr. PC Emson (Medical Research Council, Laboratory for Molecular Research, Cambridge, UK) for his generous supply of antibodies against nNOS. We thank Dr. Amanda Sferruzzi-Perri for comments on the manuscript.
References
- Aarts M, Liu Y, Liu L, Besshoh S, Arundine M, Gurd JW, Wang YT, Salter MW, Tymianski M. Treatment of ischemic brain damage by perturbing NMDA receptor- PSD-95 protein interactions. Science. 2002;298:846–850. doi: 10.1126/science.1072873. [DOI] [PubMed] [Google Scholar]
- Abraham IM, Todman MG, Korach KS, Herbison AE. Critical in vivo roles for classical estrogen receptors in rapid estrogen actions on intracellular signaling in mouse brain. Endocrinology. 2004;145:3055–3061. doi: 10.1210/en.2003-1676. [DOI] [PubMed] [Google Scholar]
- Abu-Soud HM, Yoho LL, Stuehr DJ. Calmodulin controls neuronal nitric-oxide synthase by a dual mechanism. Activation of intra- and interdomain electron transfer. J Biol Chem. 1994;269:32047–32050. [PubMed] [Google Scholar]
- Akama KT, McEwen BS. Estrogen stimulates postsynaptic density-95 rapid protein synthesis via the Akt/protein kinase B pathway. J Neurosci. 2003;23:2333–2339. doi: 10.1523/JNEUROSCI.23-06-02333.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amateau SK, McCarthy MM. A novel mechanism of dendritic spine plasticity involving estradiol induction of prostaglandin-E2. J Neurosci. 2002;22:8586–8596. doi: 10.1523/JNEUROSCI.22-19-08586.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amateau SK, McCarthy MM. Induction of PGE2 by estradiol mediates developmental masculinization of sex behavior. Nat Neurosci. 2004;7:643–650. doi: 10.1038/nn1254. [DOI] [PubMed] [Google Scholar]
- Bhat GK, Mahesh VB, Lamar CA, Ping L, Aguan K, Brann DW. Histochemical localization of nitric oxide neurons in the hypothalamus: association with gonadotropin-releasing hormone neurons and co-localization with N-methyl-D-aspartate receptors. Neuroendocrinology. 1995;62:187–197. doi: 10.1159/000127004. [DOI] [PubMed] [Google Scholar]
- Boehning D, Snyder SH. Novel neural modulators. Annu Rev Neurosci. 2003;26:105–131. doi: 10.1146/annurev.neuro.26.041002.131047. [DOI] [PubMed] [Google Scholar]
- Bonavera JJ, Sahu A, Kalra PS, Kalra SP. Evidence that nitric oxide may mediate the ovarian steroid-induced luteinizing hormone surge: involvement of excitatory amino acids. Endocrinology. 1993;133:2481–2487. doi: 10.1210/endo.133.6.8243268. [DOI] [PubMed] [Google Scholar]
- Brann DW, Mahesh VB. Endogenous excitatory amino acid involvement in the preovulatory and steroid-induced surge of gonadotropins in the female rat. Endocrinology. 1991;128:1541–1547. doi: 10.1210/endo-128-3-1541. [DOI] [PubMed] [Google Scholar]
- Bredt DS, Snyder SH. Nitric oxide mediates glutamate-linked enhancement of cGMP levels in the cerebellum. Proc Natl Acad Sci USA. 1989;86:9030–9033. doi: 10.1073/pnas.86.22.9030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredt DS, Snyder SH. Isolation of nitric oxide synthetase, a calmodulin-requiring enzyme. Proc Natl Acad Sci USA. 1990;87:682–685. doi: 10.1073/pnas.87.2.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenman JE, Chao DS, Gee SH, McGee AW, Craven SE, Santillano DR, Wu Z, Huang F, Xia H, Peters MF, Froehner SC, Bredt DS. Interaction of nitric oxide synthase with the postsynaptic density protein PSD-95 and alpha1-syntrophin mediated by PDZ domains. Cell. 1996;84:757–767. doi: 10.1016/s0092-8674(00)81053-3. [DOI] [PubMed] [Google Scholar]
- Brewer LD, Thibault O, Staton J, Thibault V, Rogers JT, Garcia-Ramos G, Kraner S, Landfield PW, Porter NM. Increased vulnerability of hippocampal neurons with age in culture: temporal association with increases in NMDA receptor current, NR2A subunit expression and recruitment of L-type calcium channels. Brain Res. 2007;1151:20–31. doi: 10.1016/j.brainres.2007.03.020. [DOI] [PubMed] [Google Scholar]
- Calizo LH, Flanagan-Cato LM. Estrogen selectively regulates spine density within the dendritic arbor of rat ventromedial hypothalamic neurons. J Neurosci. 2000;20:1589–1596. doi: 10.1523/JNEUROSCI.20-04-01589.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canabal DD, Song Z, Potian JG, Beuve A, McArdle JJ, Routh VH. Glucose, insulin, and leptin signaling pathways modulate nitric oxide synthesis in glucose-inhibited neurons in the ventromedial hypothalamus. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1418–R1428. doi: 10.1152/ajpregu.00216.2006. [DOI] [PubMed] [Google Scholar]
- Christopherson KS, Hillier BJ, Lim WA, Bredt DS. PSD-95 assembles a ternary complex with the N-methyl-D-aspartic acid receptor and a bivalent neuronal NO synthase PDZ domain. J Biol Chem. 1999;274:27467–27473. doi: 10.1074/jbc.274.39.27467. [DOI] [PubMed] [Google Scholar]
- Clasadonte J, Poulain P, Beauvillain JC, Prevot V. Activation of neuronal nitric oxide release inhibits spontaneous firing in adult gonadotropin-releasing hormone neurons: a possible local synchronizing signal. Endocrinology. 2008;149:587–596. doi: 10.1210/en.2007-1260. [DOI] [PubMed] [Google Scholar]
- Cui H, Hayashi A, Sun HS, Belmares MP, Cobey C, Phan T, Schweizer J, Salter MW, Wang YT, Tasker RA, Garman D, Rabinowitz J, Lu PS, Tymianski M. PDZ protein interactions underlying NMDA receptor-mediated excitotoxicity and neuroprotection by PSD-95 inhibitors. J Neurosci. 2007;27:9901–9915. doi: 10.1523/JNEUROSCI.1464-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d’Anglemont de Tassigny X, Campagne C, Dehouck B, Leroy D, Holstein GR, Beauvillain JC, Buee-Scherrer V, Prevot V. Coupling of neuronal nitric oxide synthase to NMDA receptors via postsynaptic density-95 depends on estrogen and contributes to the central control of adult female reproduction. J Neurosci. 2007;27:6103–6114. doi: 10.1523/JNEUROSCI.5595-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filardo EJ, Quinn JA, Bland KI, Frackelton AR., Jr Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Mol Endocrinol. 2000;14:1649–1660. doi: 10.1210/mend.14.10.0532. [DOI] [PubMed] [Google Scholar]
- Filardo EJ, Quinn JA, Frackelton AR, Jr, Bland KI. Estrogen action via the G protein-coupled receptor, GPR30: stimulation of adenylyl cyclase and cAMP-mediated attenuation of the epidermal growth factor receptor-to-MAPK signaling axis. Mol Endocrinol. 2002;16:70–84. doi: 10.1210/mend.16.1.0758. [DOI] [PubMed] [Google Scholar]
- Finn PD, McFall TB, Clifton DK, Steiner RA. Sexual differentiation of galanin gene expression in gonadotropin-releasing hormone neurons. Endocrinology. 1996;137:4767–4772. doi: 10.1210/endo.137.11.8895345. [DOI] [PubMed] [Google Scholar]
- Garthwaite J, Boulton CL. Nitric oxide signaling in the central nervous system. Annu Rev Physiol. 1995;57:683–706. doi: 10.1146/annurev.ph.57.030195.003343. [DOI] [PubMed] [Google Scholar]
- Garthwaite J, Charles SL, Chess-Williams R. Endothelium-derived relaxing factor release on activation of NMDA receptors suggests role as intercellular messenger in the brain. Nature. 1988;336:385–388. doi: 10.1038/336385a0. [DOI] [PubMed] [Google Scholar]
- Gore AC, Yeung G, Morrison JH, Oung T. Neuroendocrine aging in the female rat: the changing relationship of hypothalamic gonadotropin-releasing hormone neurons and N-methyl-D-aspartate receptors. Endocrinology. 2000;141:4757–4767. doi: 10.1210/endo.141.12.7841. [DOI] [PubMed] [Google Scholar]
- Gould E, Woolley CS, Frankfurt M, McEwen BS. Gonadal steroids regulate dendritic spine density in hippocampal pyramidal cells in adulthood. J Neurosci. 1990;10:1286–1291. doi: 10.1523/JNEUROSCI.10-04-01286.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haraguchi K, Satoh K, Yanai H, Hamada F, Kawabuchi M, Akiyama T. The hDLG-associated protein DAP interacts with dynein light chain and neuronal nitric oxide synthase. Genes Cells. 2000;5:905–911. doi: 10.1046/j.1365-2443.2000.00374.x. [DOI] [PubMed] [Google Scholar]
- Herbison AE, Neill JD. Knobil and Neill’s Physiology of Reproduction. 3. Elsevier; 2006. Physiology of the Gonadotropin-Releasing Hormone Neuronal Network; pp. 1415–1482. [Google Scholar]
- Herbison AE, Simonian SX, Norris PJ, Emson PC. Relationship of neuronal nitric oxide synthase immunoreactivity to GnRH neurons in the ovariectomized and intact female rat. J Neuroendocrinol. 1996;8:73–82. doi: 10.1111/j.1365-2826.1996.tb00688.x. [DOI] [PubMed] [Google Scholar]
- Hering H, Sheng M. Dendritic spines: structure, dynamics and regulation. Nat Rev Neurosci. 2001;2:880–888. doi: 10.1038/35104061. [DOI] [PubMed] [Google Scholar]
- Hisatsune C, Umemori H, Inoue T, Michikawa T, Kohda K, Mikoshiba K, Yamamoto T. Phosphorylation-dependent regulation of N-methyl-D-aspartate receptors by calmodulin. J Biol Chem. 1997;272:20805–20810. doi: 10.1074/jbc.272.33.20805. [DOI] [PubMed] [Google Scholar]
- Hollmann M, Heinemann S. Cloned glutamate receptors. Annu Rev Neurosci. 1994;17:31–108. doi: 10.1146/annurev.ne.17.030194.000335. [DOI] [PubMed] [Google Scholar]
- Ishii H, Shibuya K, Ohta Y, Mukai H, Uchino S, Takata N, Rose JA, Kawato S. Enhancement of nitric oxide production by association of nitric oxide synthase with N-methyl-D-aspartate receptors via postsynaptic density 95 in genetically engineered Chinese hamster ovary cells: real-time fluorescence imaging using nitric oxide sensitive dye. J Neurochem. 2006;96:1531–1539. doi: 10.1111/j.1471-4159.2006.03656.x. [DOI] [PubMed] [Google Scholar]
- Itoh Y, Ma FH, Hoshi H, Oka M, Noda K, Ukai Y, Kojima H, Nagano T, Toda N. Determination and bioimaging method for nitric oxide in biological specimens by diaminofluorescein fluorometry. Anal Biochem. 2000;287:203–209. doi: 10.1006/abio.2000.4859. [DOI] [PubMed] [Google Scholar]
- Jarry H, Hirsch B, Leonhardt S, Wuttke W. Amino acid neurotransmitter release in the preoptic area of rats during the positive feedback actions of estradiol on LH release. Neuroendocrinology. 1992;56:133–140. doi: 10.1159/000126220. [DOI] [PubMed] [Google Scholar]
- Kiedrowski L, Costa E, Wroblewski JT. Glutamate receptor agonists stimulate nitric oxide synthase in primary cultures of cerebellar granule cells. J Neurochem. 1992;58:335–341. doi: 10.1111/j.1471-4159.1992.tb09315.x. [DOI] [PubMed] [Google Scholar]
- Kojima H, Urano Y, Kikuchi K, Higuchi T, Hirata Y, Nagano T. Fluorescent Indicators for Imaging Nitric Oxide Production. Angew Chem Int Ed Engl. 1999;38:3209–3212. doi: 10.1002/(sici)1521-3773(19991102)38:21<3209::aid-anie3209>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Kornau HC, Schenker LT, Kennedy MB, Seeburg PH. Domain interaction between NMDA receptor subunits and the postsynaptic density protein PSD-95. Science. 1995;269:1737–1740. doi: 10.1126/science.7569905. [DOI] [PubMed] [Google Scholar]
- Lamar CA, Bhat GK, Mahesh VB, Brann DW. Evidence that neuronal nitric oxide synthase but not heme oxygenase increases in the hypothalamus on proestrus afternoon. Neuroendocrinology. 1999;70:360–367. doi: 10.1159/000054497. [DOI] [PubMed] [Google Scholar]
- Liu XB, Murray KD, Jones EG. Switching of NMDA receptor 2A and 2B subunits at thalamic and cortical synapses during early postnatal development. J Neurosci. 2004;24:8885–8895. doi: 10.1523/JNEUROSCI.2476-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi A, Ciana P, Belcredito S, Vegeto E. Estrogens in the nervous system: mechanisms and nonreproductive functions. Annu Rev Physiol. 2004;66:291–313. doi: 10.1146/annurev.physiol.66.032802.154945. [DOI] [PubMed] [Google Scholar]
- McCann SM, Haens G, Mastronardi C, Walczewska A, Karanth S, Rettori V, Yu WH. The role of nitric oxide (NO) in control of LHRH release that mediates gonadotropin release and sexual behavior. Curr Pharm Des. 2003;9:381–390. doi: 10.2174/1381612033391766. [DOI] [PubMed] [Google Scholar]
- Ojeda SR, Urbanski HF, Costa ME, Hill DF, Moholt-Siebert M. Involvement of transforming growth factor alpha in the release of luteinizing hormone-releasing hormone from the developing female hypothalamus. Proc Natl Acad Sci USA. 1990;87:9698–9702. doi: 10.1073/pnas.87.24.9698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojeda SR, Terasawa E, Pfaff D, Arnold A, Etgen A, Fahrbach S, Moss R, Rubin R. Neuroendocrine regulation of puberty. Elsevier; New York: 2002. pp. 589–659. [Google Scholar]
- Osborne CK, Wakeling A, Nicholson RI. Fulvestrant: an oestrogen receptor antagonist with a novel mechanism of action. Br J Cancer. 2004;90(Suppl 1):S2–S6. doi: 10.1038/sj.bjc.6601629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panatier A, Theodosis DT, Mothet JP, Touquet B, Pollegioni L, Poulain DA, Oliet SH. Glia-derived D-serine controls NMDA receptor activity and synaptic memory. Cell. 2006;125:775–784. doi: 10.1016/j.cell.2006.02.051. [DOI] [PubMed] [Google Scholar]
- Prast H, Philippu A. Nitric oxide as modulator of neuronal function. Prog Neurobiol. 2001;64:51–68. doi: 10.1016/s0301-0082(00)00044-7. [DOI] [PubMed] [Google Scholar]
- Prossnitz ER, Oprea TI, Sklar LA, Arterburn JB. The ins and outs of GPR30: a transmembrane estrogen receptor. J Steroid Biochem Mol Biol. 2008;109:350–353. doi: 10.1016/j.jsbmb.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sans N, Petralia RS, Wang YX, Blahos J, 2nd, Hell JW, Wenthold RJ. A developmental change in NMDA receptor-associated proteins at hippocampal synapses. J Neurosci. 2000;20:1260–1271. doi: 10.1523/JNEUROSCI.20-03-01260.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato S, Braham CS, Putnam SK, Hull EM. Neuronal nitric oxide synthase and gonadal steroid interaction in the MPOA of male rats: co-localization and testosterone-induced restoration of copulation and nNOS-immunoreactivity. Brain Res. 2005;1043:205–213. doi: 10.1016/j.brainres.2005.02.074. [DOI] [PubMed] [Google Scholar]
- Sattler R, Xiong Z, Lu WY, Hafner M, MacDonald JF, Tymianski M. Specific coupling of NMDA receptor activation to nitric oxide neurotoxicity by PSD-95 protein. Science. 1999;284:1845–1848. doi: 10.1126/science.284.5421.1845. [DOI] [PubMed] [Google Scholar]
- Savchenko A, Barnes S, Kramer RH. Cyclic-nucleotide-gated channels mediate synaptic feedback by nitric oxide. Nature. 1997;390:694–698. doi: 10.1038/37803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scordalakes EM, Shetty SJ, Rissman EF. Roles of estrogen receptor alpha and androgen receptor in the regulation of neuronal nitric oxide synthase. J Comp Neurol. 2002;453:336–344. doi: 10.1002/cne.10413. [DOI] [PubMed] [Google Scholar]
- Swanson LW. Structure of the rat brain. Elsevier Science Publishers; Amsterdam: 1996. [Google Scholar]
- Thomas P, Pang Y, Filardo EJ, Dong J. Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells. Endocrinology. 2005;146:624–632. doi: 10.1210/en.2004-1064. [DOI] [PubMed] [Google Scholar]
- Wang HG, Lu FM, Jin I, Udo H, Kandel ER, de Vente J, Walter U, Lohmann SM, Hawkins RD, Antonova I. Presynaptic and postsynaptic roles of NO, cGK, and RhoA in long-lasting potentiation and aggregation of synaptic proteins. Neuron. 2005;45:389–403. doi: 10.1016/j.neuron.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Warner M, Gustafsson JA. Nongenomic effects of estrogen: why all the uncertainty? Steroids. 2006;71:91–95. doi: 10.1016/j.steroids.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Williams K. Ifenprodil discriminates subtypes of the N-methyl-D-aspartate receptor: selectivity and mechanisms at recombinant heteromeric receptors. Mol Pharmacol. 1993;44:851–859. [PubMed] [Google Scholar]
- Woolley CS. Acute effects of estrogen on neuronal physiology. Annu Rev Pharmacol Toxicol. 2007;47:657–680. doi: 10.1146/annurev.pharmtox.47.120505.105219. [DOI] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS. Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat. J Neurosci. 1992;12:2549–2554. doi: 10.1523/JNEUROSCI.12-07-02549.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]


