Abstract
In the recent years, a wide range of metabonomic analytical techniques are widely used in the modern research of traditional Chinese medicine (TCM). At the same time, the international community has attached increasing importance to TCM toxicity problems. Thus, many studies have been implemented to investigate the toxicity mechanisms of TCM. Among these studies, many metabonomic-based methods have been implemented to facilitate TCM toxicity investigation. At present, the most prevailing methods for TCM toxicity research are mainly single analysis techniques using only one analytical means. These techniques include nuclear magnetic resonance (NMR), gas chromatography-mass spectrometry (GC-MS), and liquid chromatography-mass spectrometry (LC-MS), etc.; with these techniques, some favourable outcomes have been gained in the toxic reaction studies of TCM, such as the action target organs assay, the establishment of action pattern, the elucidation of action mechanism and the exploration of action material foundation. However, every analytical technique has its advantages and drawbacks, no existing analytical technique can be versatile. Multi-analysed techniques can partially overcome the shortcomings of single-analysed techniques. Combination of GC-MS and LC-MS metabolic profiling approaches has unravelled the pathological outcomes of aristolochic acid-induced nephrotoxicity, which can not be achieved by single-analysed techniques. It is believed that with the further development of metabonomic analytical techniques, especially multi-analysed techniques, metabonomics will greatly promote TCM toxicity research and be beneficial to the modernization of TCM in terms of extending the application of modern means in the TCM safety assessment, assisting the formulation of TCM safety norms and establishing the international standards indicators.
Keywords: traditional Chinese medicine, toxicity, metabonomics, nuclear magnetic resonance, high-performance liquid chromatography, gas chromatography, high-performance capillary electrophoresis, mass spectrometry
Introduction
Metabonomics is an emerging subject of the post-genome era, which, together with genomics, transcriptomics and proteomics, jointly constitutes the ‘Systems Biology’ (Nicholson and Wilson, 2003). Metabonomics is the branch of science concerned with the quantitative understandings of the metabolite component of integrated living systems and its dynamic responses to the changes of both endogenous factors (such as physiology and development) and exogenous factors (such as environmental factors and xenobiotics) (Tang and Wang, 2006). Recently, as a novel systemic approach to study metabolic profile and accelerate the course of drug development, metabonomics has achieved great growth, which is attracting more and more concerns from the academic community (Figure 1). The main subjects of metabonomics are a variety of predominantly low molecular weight metabolites (MW < 1000) that serve as the substrates and products of metabolic pathways, and its main biological samples are urine, plasma and serum. Integrity of metabonomic processes includes sample collection and pretreatment, data collection and analysis, and metabolic variation interpretation. Generally, the main technologies metabonomics relied are the nuclear magnetic resonance (NMR)-based method, chromatography-based method and mass spectrometry (MS)-based method (Tang and Wang, 2006). Depending on a series analysis of different sample spectra and combination with chemical pattern recognition methods, metabonomics can be used to identify organisms in pathophysiological state, gene function, drug toxicity and efficacy, and associated biomarkers (Nicholoson et al., 2002).
Figure 1.
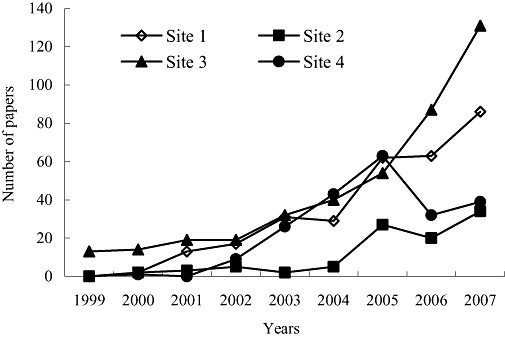
Number of metabonomics-related papers published between January 2000 and December 2007. Generally, metabonomics is gaining more and more attention as showed by the number of related papers published by several main international well-known academic databases. Databases are searched using ISI Web of sciencedirect (site 1), interscience (site 2), acs (site 3) and ebscohost (site 4). Search term used was: ‘metabolomic or metabolomics or metabonomic or metabonomics or metabolite profiling’.
Advanced combination of analytical techniques with chemometric tools (such as pattern recognition and expert systems analysis, etc.) is the basic method for metabonomic study, and accurate, sensitive and high-throughput chemical analytical methods for metabolic analysis underlie the metabonomic study (Mao and Yuan, 2007). Whether an analytical technique is suitable for certain metabolic study has direct impact on the experimental results, and finally determines the effectiveness on interpretation of various metabolic changes in biological systems stimulated or disturbed by xenobiotic. Therefore, the development of analytical techniques plays a critical role on the issue as to whether metabonomics could be further extended to broader and deeper application. At present, there are a variety of analytical techniques used in traditional Chinese medicine (TCM) metabolic analysis, including NMR (Bailey et al., 2004; Zhang et al., 2006), gas chromatography (GC) (Chen et al., 2005a), high-performance liquid chromatography (HPLC) (Hu et al., 2006a), high-performance capillary electrophoresis (HPCE) (Xu et al., 2005) and MS (Ni et al., 2007).
TCM has a long history and for a long time has been accepted by the academic community and patients as superior and a unique valuable property in China. However, in recent years, incident reports about TCM toxicity appear occasionally; TCM is facing severe challenges (Vanherweghem et al., 1993; Besheer et al., 2008; Laird et al., 2008). Therefore, it is indispensable to strengthen TCM toxicity research. However, the complexity of TCM constituents and action mechanisms make it difficult to fully study by traditional TCM safety assessment methods, which has become the constraints against TCM toxicity study.
Fortunately, as a systemic approach, metabonomics adopts a ‘top-down’ strategy to reflect the function of organisms from terminal symptoms of metabolic network and understand metabolic changes of a complete system caused by interventions in holistic context (Nicholson, 2006). This properly consists with the holistic thinking of TCM, suggesting the possibility of mutual infiltration between metabonomic study on drug and differential treatment of TCM. Therefore, in the study of TCM toxicity, metabonomics play an increasingly important role.
This paper reviews several metabonomic analytical techniques widely used in TCM modern research in recent years, and preliminarily summarizes their applications to toxicity studies on TCM. Lack of space forbids further exploration of the topic here; thus, the authors solely present analytical techniques selectively. For more details, readers could refer to Li et al. (2006a) together with Dunn and Ellis (2005) for a comprehensive understanding of metabonomic analytical techniques; for individual technique, interested readers could also refer to Lu et al. (2008) for liquid chromatography (LC), Griffin (2003) for NMR, Pasikanti et al. (2008) for GC, García-Pérez et al. (2008) for capillary electrophoresis (CE), Ackermann et al. (2006) for MS and Rousseau et al. (2008) for chemometric techniques.
Metabonomic techniques widely used in TCM modern research
The comprehensive investigation of the metabolites is being complicated by its enormous complexity and dynamics, which drive people to improve and develop all kinds of analytical techniques to approach or even achieve ‘versatile’ analysis. Currently, a number of different analytical strategies have been employed, with the ultimate goal of measuring a large fraction or all of the metabolites present. These strategies can be broadly classified as chromatography-based, MS-based and NMR-based according to the major detection methods used (Tang and Wang, 2006). Among these strategies, there are many analytical techniques widely used in TCM modern research, such as LC for quality control of TCM, high-throughput TCM fingerprint, qualitative and quantitative determination of active components in TCM, NMR for biological fingerprint and elaboration of complicated TCM science theories, GC for establishment of experimental animal model, CE for pharmacokinetic study of TCM, and MS (often combined with other methods, such as GC, LC) for assisting other analytical methods in TCM modern research.
LC
HPLC
Because HPLC is low-cost, easy to use, high sensitive, not limited by sample volatility and stability, as well as of favourable separating power, it has been widely used in biological sample analyses and TCM modern research, which, however, are nearly target component analyses rather than fingerprint analyses of the entire samples combined with chemometrics. Attempting to achieve high-throughput fingerprint analysis, we must develop generic methods based on HPLC to improve analytical efficiency (Yang et al., 2002; 2004; 2005; Pham-Tuan et al., 2003). Recently, there are a series of improved HPLCs that appeared one after another as technology developments, for fingerprint analysis of TCM and other spheres of TCM modern research, such as capillary HPLC (CHPLC) for qualitative and quantitative determination of active components in TCM (Zhao et al., 2008a), hydrophilic interaction chromatography for comprehensive quality control of TCM (Jin et al., 2009), ultra high-performance liquid chromatography (UPLC) for identification of active compounds in TCM (Liu et al., 2007a), monolithic columns for biological fingerprinting analysis of TCM (Wang et al. 2007b).
Multidimensional liquid separation systems
Complex samples [such as Chinese herbal medicines (CHMs), urine, plasma, etc.] can not be retained in the C18 column that resulted from very complex polar materials (e.g. low polar materials similar to natural products, amphoteric substances, acidic or alkaline substances, etc.) they contain. Therefore, it is impossible to retain and separate all these different metabolites with merely a single chromatographic column. Multidimensional liquid separation systems using two or more liquid chromatographic separation modes stand a good chance to be a promising powerful approach to enrich, separate and quantify a large variety of exogenous and endogenous compounds in complex biological samples and TCM with powerful separation ability, high resolution and sensitivity, high peak capacity, and excellent detectability in comparison to one-dimension HPLC. Up to now, although there are not so much applications in TCM modern research, several effective multidimensional liquid separation systems have been developed and applied to separation and identification of compounds in TCM, such as two-dimensional LC (LC × LC) in Psoralea corylifolia (Chen et al., 2005b) and Rheum palmatum L. (Hu et al., 2006a), comprehensive two-dimensional capillary LC and CE for resolution of neutral components in Cheng-Qi-Tang (Zhang et al., 2001), and multidimensional counter-current chromatography system in Ginkgo biloba L. and Hippophae rhamnoides L (Yang et al., 1998), etc.
NMR
Ever since Nicholson introduced NMR-based metabonomics in 1999 (Nicholson et al., 1999), NMR has already been one of the main analytical techniques for metabolic research. In comparison with MS and HPLC, NMR-based method has evident advantages (Tang and Wang, 2006; Lenz and Wilson, 2007): (i) non-invasive and non-destructive to samples, making it possible to facilitate in vivo and in situ studies; (ii) quantitative and simultaneous detection method, thus not biased for any molecules; (iii) high throughput and can measure up to 400 samples per 24 h with flow-injection technology; (iv) rich dynamic molecular information including metabolite structure, concentration, molecular dynamics, interactions, pH and compartmentation when diffusion-editing techniques are employed; (v) require little or no sample preparation; and (vi) good resolution and reproducibility. On account of the above descriptions, NMR-based method is very suitable for analysis of complex compositions in metabolites, especially for the TCM modern research. One representative illustration is given by Bailey et al. (2004). They used 1H NMR spectroscopy and chemometrics, such as partial least squares discriminant analysis (PLS-DA), to analyse the extracts of Artemisia annua with the purpose of predicting the anti-plasmodial activity of this TCM. Such approach allowed to classify TCM samples from different sources according to anti-plasmodial activity without prior knowledge of this activity. Recently, considerable works on drug development through NMR-based method have met with tremendous success. Table 1 roughly summarizes the application of NMR-based metabonomics to drug development in Western medicine and TCM.
Table 1.
The application of NMR-based metabonomics to drug development
| Aspects | Objectives | References |
|---|---|---|
| Identification and authentication of drug action pattern | Distinguish the metabolic conditions of animal models between different species and strains, identify human in disease state and construct suitable animal model for research of human disease, drug efficacy and toxicity. | Aardema and MacGregor, 2002 |
| Drug action mechanism study | Investigate the role of toxicants in toxic lesion and how they produce these toxic effects. | Slim et al., 2002; Williams and Lock, 2004 |
| Preclinical drug toxicity evaluation | Establish new drug safety assessment norms, reduce toxic effect, provide scientific basis for new drugs toxic effect level on human health and anticipate this effect level of new drugs for sale. | Lindon et al., 2003; 2005; Bollard et al., 2005 TCM modern research |
| TCM modern research | Integrate (‘assemble’) a range of information systems to explain complicated TCM science theories. | Zhang et al., 2006; Li et al., 2007b |
NMR, nuclear magnetic resonance; TCM, traditional Chinese medicine.
GC
The advantage of GC clearly lies in its high sensitivity of detection for almost all the volatile chemical compounds or non-volatile compounds readily derivatized. The important uses of GC have been for investigating volatile compounds in TCM and biological samples, such as urine, blood plasma, blood serum, etc. For instance, Chen et al. (2005a) used a high dose of hydrocortisone to establish an animal model that suffered from ‘kidney deficiency syndromes’, an early stage of obesity and diabetes in TCM science. In order to establish urine metabolic patterns of the treatment rats, they used a metabonomic approach consisting of GC-MS and multivariate statistical technique. The results indicated that clear and consistent biochemical changes following hydrocortisone intervention under controlled conditions could be identified using chemometric analysis. This study shows that this metabonomic approach could be used as a potentially powerful tool for early diagnosis to investigate the biochemical changes of certain physiopathologic conditions such as metabolic syndrome.
In GC analysis of volatile compounds in TCM, sample pretreatment is the crucial step. A variety of novel sample pretreatment techniques with significant advantages over conventional methods continue to emerge (Dong et al., 2007b; Tam et al., 2007; Yu et al., 2007c). At present, the coupling of capillary GC to MS proved to be a potentially useful method based on its high sensitivity, peak resolution and reproducibility (Pasikanti et al., 2008). More importantly, structural information and selectivity available from MS made the combination of MS with GC coupled with chemometric analytical method the most effective technique for analysis of TCM (Liang et al., 2005). These advantages are not provided by other chromatography-based methods (such as LC) (Weckwerth and Fiehn, 2002; Boernsen et al., 2005) and NMR-based methods (Pasikanti et al., 2008) due to the poor overall reproducibility and low sensitivity respective. Recently, two-dimensional GC (GC × GC) gradually shows some advantages in comparison to other GC-based analytical techniques because of its superior resolution, and very sensitive detection (Adahchour et al., 2006a,b,c,d;). Although it is still not very popular right now, to some extent, separation and analysis in GC × GC system is more powerful than in one: (i) it has more peak capacity and can accommodate more complex mixtures; (ii) component identification is potentially more reliable; and (iii) separations are likely to be more structured in two dimensions (Phillips and Beens, 1999). Consequently, even though it is not yet so an important analytical technique, GC × GC will probably gain due attention in practice. Table 2 lists some applications of GC analysis to volatile components of TCM.
Table 2.
Some applications of GC analysis to volatile components of TCM
| Analytical strategies | Applications | Analytes | References |
|---|---|---|---|
| GC-MS | Metabonomic study for the assessment of TCM. | Biochemical modification | Chen et al., 2005a |
| GC-MS | Optimize and compare different methods for extraction of volatile compounds in TCM. | α-copaene, cyperene, β-selinene, β-cyperone, α-cyperone | Tam et al., 2007 |
| HS-SPME and GC-MS | Pharmacokinetics study of paeonol in the TCM | Paeonol | Dong et al., 2007a |
| GC-MS and CMC | Screen and analyse TCM components in vitro. | Ligustilide, butylidenephthalide | Liang et al., 2005 |
| GC-MS, HPLC and LC-MS | Investigate the relationship between chemical components of Si-wu decoction (SDE) and proliferation-promoting effects on rat marrow-derived mesenchymal stem cells (MSCs). | Ligustilide, palmitic acid methyl ester, stearic acid ethyl, etc. | Zeng, et al., 2008 |
| GC-MS and LC-MS | Unravel the pathological outcomes of aristolochic acid-induced nephrotoxicity. | Pathological changes in | Ni et al., 2007 |
| GC × GC-TOF-MS | Analysis of Artemisia annua L. volatile oil | Terpene compounds, etc. | Ma et al., 2007 |
| GC-MS-AMWFA and HP-5MS-PTRIs | Identification of volatile components in different essential oils from Clematis species. | n-hexadecanoic acid, (Z,Z)-9-12-octadecadienoic acid | Zeng, et al., 2007 |
| GC-MS-NMF or GC-MS analysis combining with OP technique | Chromatographic fingerprint analysis. | Fingerprints of houttuynia cordata Thumb. | Gao et al., 2005a; Zeng, et al., 2006 |
| GC × GC-TOF-MS and GC × GC-FID | Determination of TCM volatile oils from different geographical origins | Monoterpenes, oxygenated sesquiterpenes | Qiu et al., 2007 |
| MAE-GC-MS | Study on the chromatographic fingerprint of Fructus xanthii. | Fingerprints of Fructus xanthii | Ruan and Li, 2007 |
| PHWE-SPME-GC-MS | TCM quality assessment: Quantitative determination of active volatile compounds in TCM | Eucalyptol, camphor, borneol | Dong et al., 2007b |
| MISPE combined with GC-MS | Preparation of molecularly imprinted adsorptive resin for trapping of ligustrazine from the traditional Chinese herb Ligusticum chuanxiong Hort. | Ligustrazine | Guo et al., 2008 |
CMC, cell membrane chromatography; GC-MS, gas chromatography-mass spectrometry; GC-MS-AMWFA, gas chromatography-mass spectrometry combined with alternative moving window factor analysis; HPLC, high-performance liquid chromatography; HP-5MS-PTRIs, temperature-programmed retention indices (PTRIs) on HP-5MS column; HS-SPME, headspace solid-phase microextraction; LC-MS, liquid chromatography-mass spectrometry; MAE-GC-MS, microwave assisted extraction coupled with gas chromatography-mass spectrometry; MISPE, molecularly imprinted solid-phase extraction; OP, orthogonal projection; PHWE-SPME-GC-MS, pressurized hot water extraction followed by solid-phase microextraction and gas chromatography-mass spectrometry; TOF-MS, time-of-flight mass spectrometry; TCM, traditional Chinese medicine.
CE
CE, also known as HPCE or capillary electroseparation method, was introduced in the early 1980s (Jorgenson and Lukacs, 1983). Thereafter, CE has developed rapidly and became a powerful tool for separation and purification. HPCE technique is the combination of classic electrophoretic technique and modern micro-column separation technique; it integrates some advantages between high-voltage electrophoresis and HPLC (e.g. high speed and short analysis time, low solvent and sample consumption, excellent separation efficiency and easy to automate) (Ronda et al., 2008); therefore, it was widely used in rapid analyses, from bioactive compounds analysis (Huang et al., 2004), DNA identification (Chang et al., 2008) to determination of single cell and analysis of virus (Zhu et al., 2008).
HPCE applied to TCM modern research
HPCE enjoys some advantages in comparison to HPLC, such as shorter analysis time, less sample and solvent consumption as described above; furthermore, it is more appropriate for separating ‘dirty’ samples simultaneously and lower operating cost. However, the overall sensitivity of HPCE is about two orders of magnitude lower than that of HPLC, particularly when dealing with spectroscopic detection means, such as UV–vis spectroscopy and fluorescence (Liu et al., 2007b; Feng et al., 2008), which limits its extensive application to TCM modern research. Accordingly, it receives rare application in metabonomics currently. Despite this disappointment, it does possess brilliant prospects in TCM modern research, for instance, component analyses of CHMs (see Table 3) and Chinese materia medica preparation (Hu et al., 2006b; Li et al., 2006b; Yu et al., 2006), identification of CHM and Chinese medicine processed products (Cao et al., 2005; Zougagh et al., 2005), establishment of TCM fingerprinting (Yu et al., 2007a), chiral drug analysis of TCM (Kvasnička et al., 2005), and pharmacokinetic study of TCM (Tegtmeier et al., 2000).
Table 3.
The applications of HPCE in active components analysis of TCMs
| TCMs | Active components | Objectives | Methods | References |
|---|---|---|---|---|
| Herba Leonuri | Flavonoids | Separation and quantitative determination of kaempferol, rutin, hyperoside, quercitrin, quercetin. | CE | Xu et al., 2005 |
| Garcinia atroviridis Griff | Acid and lactone | Separation and analysis of hydroxycitric acid and hydroxycitric acid lactone. | CZE | Muensritharam et al., 2008 |
| Macleaya cordata (Willd.) R. Br. and Chelidonium majus L. | Alkaloids | Separation and quantitative determination of chelerythrine and sanguinarine. | NACE-LIF | Liu et al., 2006b |
| Lonicera japonica Thumb. | Phenolic acids and flavones | Separation and analysis of hyperoside, chlorogenic acid, luteolin and caffeic acid. | CE-ED | Peng et al., 2005 |
| Cortex fraxini | Polyphenol | Simultaneous determination of active components in herbal medicines by MEKC with Tween 20. | MEKC | Wang et al. 2007a |
| Sceletium tablets | Mesembrine | Quantitative determination of psychoactive alkaloids. | CZE | Patnala and Kanfer, 2008 |
| Passiflora incarnata | Glycoside | Quantification of the Flavonoid Glycosides. | CE | Marchart et al., 2003 |
| Hedyotis diffusa | Organic acid | Simultaneous determination of key bioactive components in TCM. | CE | Cheung et al., 2006 |
| Moutan Cortex | Glycosides and sugars | Determination of glycosides and sugars. | CE-ED | Chen et al., 2006a |
| Red clover (Trifolium pratense). | Flavones | Quantitative determination of daidzein, genistein and biochanin A. | CE-ED | Peng and Ye, 2006 |
| Japanese dock root and fleeceflower root | Anthraquinones and saccharides | Simultaneous determination of emodin, chrysophanol and their 8-β-D-Glucosides | CZE | Koyama et al., 2003 |
| Three citrus plants | Coumarins | Separation and determination of xanthoxyletin and xanthoxylin. | MEKC | Wang et al., 2003 |
| Several families of herbal drugs (Herba Chelidonii, Caulis Mahoniae, et al.) and their relevant medicinal preparations | Alkaloids | Quantitative determination of protoberberine alkaloids including berberine, palmatine, and jatrorrhizine. | NACE | Gao et al., 2005b |
| Rabdosia japonica (Burm.f.) Hara var. Glaucocalyx (Maxin.) Hara | Triterpenes | Separation and determination of bioactive triterpenes in Chinese herbs. | NACE | Qi et al., 2006b |
| Cordyceps sinensis (Berk.) | Nucleosides | Simultaneous determination of cytosine, uracil, uridine, hypoxanthine, 2′-deoxyuridine, inosine, guanosine, thymidine, adenine, adenosine, and cordycepin. | CEC | Yang et al., 2007 |
| Liquorice roots | Glycyrrhizins | Separation and analysis of glycyrrhizin, 18β-glycyrrhetic acid and 18α-glycyrrhetic acid. | CZE | Sabbioni et al., 2005 |
| Aristolochia | Organic acid | Determination of aristolochic acid. | CE-LIF | Hsieh et al., 2006 |
| Glycyrrhiza uralensis Fisch root | Glycyrrhizic acid | Separation of active components in TCM by MECC. | MECC | Li et al., 1999 |
| Herba Epimedii, Ying-Yang-Huo | Flavonoids | Quantitative determination of flavonoids. | CZE | Liu et al., 2006a |
CE, capillary electrophoresis; CEC, cations exchange capacity; CE-ED, capillary electrophoresis coupled with electrochemical detection; CE-LIF, capillary electrophoresis in conjunction with laser-induced fluorescence detection; CZE, capillary zone electrophoresis; HPCE, high-performance capillary electrophoresis; MECC, micellar electrokinetic capillary chromatography; MEKC, micellar electrokinetic capillary; NACE, nonaqueous capillary electrophoresis; TCM, traditional Chinese medicine.
MS
Generally, MS is frequently coupled with other separative techniques; therefore, it is a detecting technique rather than a separative one in most cases. However, MS also can be served as a technique combining separative and detecting attributes because it is also used on its own sometimes. Actually, no existing technique including MS is capable of holistic analysis and detection, the pros and cons of the currently used MS methods are indicated as follows: (i) cons attributes (Tang and Wang, 2006): (1) fairly extensive sample preparations; (2) invasive and destructive (to samples); (3) pre-knowledge required about samples; (4) high recurrent expenditure; and (3) fairly expensive equipment, etc.; (ii) pros attributes: (1) although it requires laborious sample pre-treatment (hydrolysation, derivatization), MS-based methods is the gold standard for the identification and quantification of volatile and thermally stable components when hyphenated with GC (Ceglarek et al., 2009); (2) the above problems can be overcome by hyphenation with LC (Chace and Kalas, 2005; Dunn et al., 2005); (3) available for ionization of polar to non-polar components [such as electrospray ionization (ESI), atmospheric pressure chemical ionization and atmospheric pressure photo ionization] (Marquet, 2002; Baumann et al., 2005); and (4) sensitive and selective detection, multi-analyte analysis, and the ability to provide structural information (Ackermann et al., 2006). As discussed above, MS is continually used with other analytical techniques in a hyphenated way; therefore, there are tremendous numbers of applications in TCM modern research. Some representative examples are illustrated by: (i) HPLC-ESI time-of-flight mass spectrometry (HPLC-ESI-TOF-MS) in pharmacokinetics research for understanding TCM efficacy (Zhang et al., 2008); (ii) LC-MS and GC-MS in TCM toxicity research (Chen et al., 2006b; Ni et al., 2007); (iii) HPLC-diode array evaporative light scattering detector (HPLC-DAD-ELSD) and HPLC-GS-MS2 in TCM fingerprint analysis for quality control (Li et al., 2007c); (iv) ESI-TOF-MS with HPLC-DAD-ELSD in screening of TCM interactive components (Qi et al., 2006a); (v) UPLC combined with quadrupole time-of-flight tandem mass spectrometry (UPLC Q-TOF MS) in evaluation of the efficacy of TCM preparation (Zhao et al., 2008b); and (vi) HPLC-DAD-ESI-MS in investigation of antibacterial modes of action (Yu et al., 2007b).
Applications in TCM toxicity research
In 1993, Belgium researchers (Vanherweghem et al., 1993) reported that a number of young women had suffered from chronic interstitial nephritis, a disease mainly regarded as ‘rapidly progressive interstitial renal fibrosis’ in morphology, after following the slimming treatments containing Chinese herbal ingredients such as Stephanie tetrandra, Magnolia officinalis and Aristolochia manshuriensis. They called it ‘Chinese herbs nephropathy (CHN)’. Later, some scholars (Gillerot et al., 2001) indicated the main cause of CHN that patients had taken the CHM containing aristolochic acid (AA) ingredients which could induce the so-called CHN, and they considered it was more appropriate to characterize the disease as ‘aristolochic acid nephropathy’ rather than CHN. Consequently, such TCM has been forbidden for medication. Some domestic scholars (Yang, 2007) complain: ‘The international judgments merely based on the use of a single TCM toxic constituent are not scientific.’, ‘TCM export encounters double standards abroad in an awkward predicament.’ But since the international community has put forward this issue, there are undoubtedly somewhat rationalities existing. Subsequently, adverse reaction reports of TCM are mounting up both at home and abroad in recent years (Besheer et al., 2008; Laird et al., 2008), making TCM security issues in international attention.
Admittedly, the toxicity of TCM is mostly caused by excessive or wrong medication. However, the complexity of TCM constituents and action mechanisms make it difficult to comprehensively study. The general procedure of traditional TCM safety assessment is to medicate a certain number of experimental animals or a certain amount of analyte, and thereafter observe the characteristics of toxic effects induced by the dosed TCM. Limited by experimental methods and conditions, this tactics remains confined to the toxic study of a single TCM chemical constituent, which has become the constraint against further TCM toxicity study. If TCM wants to achieve de facto modernization and be accepted by the world, application of novel analytical techniques for toxicity study is becoming the most imperative and essential thing we must perform.
As a systemic approach, metabonomics is fairly in accordance with the holistic thought of TCM science theories. On the one hand, it lays aside complicated interaction between biochemical molecules and discards unfavourable methods that merely use a single or a few indicators on pathological and physiological changes. On the other hand, it adopts ‘top-down’ strategy to reflect the function of organisms from terminal symptoms of metabolic network in holistic context, which in turn facilitates the interpretation of metabolic changes of a complete system caused by interventions (Nicholson, 2006). Therefore, this strategy provides a possibility of mutual infiltration between metabonomic study on drug and differential treatment of TCM. At present, metabonomics has been playing an increasingly important role in TCM toxicology research.
General flows of metabonomic investigation in TCM toxicity research
Compared to other spheres to which metabonomics is applied in TCM modern research, there are merely a few applications in TCM toxicity research through metabonomic methods up to now. Although the methods they used are much different from each other in detail, they all follow four general flows (Figure 2):
Figure 2.

Flow chart of the metabonomic investigation in TCM toxicity research. GC, gas chromatography; HPLC, high-performance liquid chromatography; MS, mass spectrometry; NMR, nuclear magnetic resonance; TCm, traditional Chinese medicine; UPLC, ultra high-performance liquid chromatography.
Pretreatment
TCM handling
TCM is unique in that its pharmacological and toxicological effects can be greatly fluctuant with different collecting ways, processing technologies and prescriptions, such as collecting in different habitats and periods, prescriptions with different compatibility programs or active fractions may yield wide different or even opposite effects (Li et al., 2008b).
Experimental animal treatment (Zhang et al., 2006)
In complicated theory system research of TCM science, establishment of experimental animal model is an intensive and decisive step for further researches because the so-called symptom in TCM science (i.e. kidney deficiency syndromes, syndrome of Qi deficiency, blood stasis syndrome, etc.) is a complicated multi-factor-interactional, dialectic result induced in holistic level (Li et al., 2007b). Accordingly, use of single or just a few compounds or methods is incapable of exactly ‘reproducing’ authentic toxic-pathological status in animal model. No matter how frustrating the present establishment of animal model is, metabonomics in TCM toxicity research can provide valuable feedback information for reassessment of animal model.
Sample collection and preparation
This step is equal to routine metabonomic analysis. Conventional metabonomics is carried out on biofluids which can provide an integrated view of the whole systems biology. And the most frequently used biofluids include urine, blood plasma and serum, etc. However, there is a wide range of biofluids that can be, and have been, investigated, including seminal fluids, lung aspirates and dialysis fluids, blister and cyst fluids, digestive fluids, synovial fluid, amniotic fluid and cerebrospinal fluid (Lenz and Wilson, 2007).
Data analysis and detection
There are three main methods for data acquisition and detection, such as NMR-based methods, MS-based methods and chromatography-based methods (Tang and Wang, 2006). As mentioned above, all these methods have their intrinsic advantages and disadvantages; thus, hyphenation among them becomes indispensable, which in turn results in the compatible problem among these methods. Up to the present, this problem still fails to be overcome commendably; further studies still need to be implemented to improve these methods.
Data mining
The use of multiple analytical techniques in combination may offer a better strategy for identifying a broadened spectrum of important metabolites associated with TCM perturbation. However, it is technically challenging to integrate data from different analytical methods which measure different types of biochemical molecules generated from diverse aspects of a system (Ni et al., 2007). How will valuable information of interest be read and how will big pictures emerge from the sea of biological data obtained by various analytical tools? By virtue of a variety of chemometric tools, scientists partially solved the problems. Among the chemometric tools used, principal components analysis (PCA) enjoys the most extensively used one (Rousseau et al., 2008). Nevertheless, different laboratories use different standardization methods to integrate their data acquired from single or multiple analytical methods respectively. This makes exchanging data between laboratories difficult and disappointing (Kanani et al., 2008). Moreover, there are 12,870 kinds of TCM resources (Li et al., 2008b); obviously, it is more or less insufficient to interpret data merely by chemometric tools for such huge resources. Therefore, establishment of TCM-related database with uniform data interpretation standard became an immediate mission. Such database should embody as many TCM resources as possible, and provide all kinds of information including basic theories (e.g. action mechanisms, biomarkers, matter foundation of drug effect, target organs), scientific prescription guide (e.g. comparison of differential toxicity induced by variable compatibility programs and processing technologies), quality control standards, etc. And most of all, these information should be given in a united standard, for instance, TCM named with united nomenclature, symptoms interpretation with united TCM science theoretical basis, etc. At present, lack of standardization is the most disillusionary obstructor for real data mining.
Toxic-pathologic interpretation
Interpretation of related information can be easily accessed through combination with toxicogenomics and toxicoprotenomics, and further exploring link between these ‘toxicomics’ greatly facilitates TCM modern research (Aardema and MacGregor, 2002; Ekins et al., 2005). Finally, this interpretation, in turn, can be used to reassess the pretreatment step to formulate the best program which can yield the best outcome and facilitate our studies on TCM toxicity or therapy with TCM for patients. All these steps constitute the so-called TCM toxicity metabonomic study circle.
Single-analysed techniques
At present, the most prevailing methods for TCM toxicity research are mainly single-analysed techniques using only one analytical means. The techniques used are composed of NMR, GC-MS, LC-MS, etc. They play an increasingly important role in the studies of TCM toxic reaction, including action target organs assay, establishment of action pattern, and elucidation of action mechanism and exploration of action material foundation. There are several TCMs which have hitherto been investigated by single-analysed techniques.
Manchurian dutchmanspipe stem
Metabonomic method is a promising new technology in TCM toxicity research. Up to the present, Aristolochia manshuriensis-induced nephrotoxicity is the most intensive topic in TCM toxicity research. Chromatography-based analytical technique can be applied to metabonomic profiling for toxicology study of AA; such metabonomic profiling can act as a preclinical protocol which allows early treatment before symptoms were observed. In these studies, the urine of rats is the most thriving sample (Chan and Cai, 2008; Chan et al., 2008). Chan and Cai (2008) used LC-MS metabonomic technique coupled with PCA to find variations in urine profiles between the control and the dosed rats. PCA data showed clear differences in metabolic profiles between the control and the dosed rats analysed by LC-MS. Two potential biomarkers, kynurenic acid and hippuric acid, were identified by high-resolution mass measurement and MS-MS analyses on a Qq-TOF. They believed that changes in dosed urine profile could have occurred before symptoms were observed. Therefore, such methodology may act as a preclinical protocol for early treatment.
In addition, model toxins can be used to investigate the subacute toxicity of AA by comparing urinary profiles interfered by known model toxicants and AA at various time intervals. In Zhang's study, 35 male Wistar rats were divided into seven groups after acclimation for 7 days (Zhang et al., 2006): Model groups [injected intraperitoneally with a single dose of NaCrO4, HgCl2, 2-bromoethanamine hydrobromide (BEA), or administered with an oral dose of α-naphthylisothiocyanate (ANIT), hydrazine dihydrochloride (HYD)], AA group (dosed intraperitoneally with 10 mg·kg−1 body weight each day during the 5 day time interval and housed in a normal way for another 5 days) and control group (intraperitoneally with 0.9% saline). Also, visual comparison of the NMR spectra showed that AA caused a renal proximal tubular and papillary lesion and a slight hepatic impair. Pattern recognition analysis indicated that the renal proximal tubule lesion was the main damage induced by AA, and the renal toxicity induced by AA to the rat was a time-dependent and dosage-accumulative process by monitoring the toxicological processes from onset, development and part-recovery.
Cinnabar and realgar
High reliability of NMR-based metabonomic approach integrated with other techniques can facilitate understanding of the biochemical effects induced by TCM. Wei et al. (2008, 2009) used an integrated metabonomic approach, including 1H NMR spectra, liver and kidney histopathology examination and serum clinical chemistry analyses, to investigate the toxicological mechanisms of cinnabar and realgar in rats respectively. They found that both TCMs could perturb energy metabolism, impair amino acid metabolism and affect the gut microflora environment in a time-dependent way. They also found that glutathione could be a potential biomarker of realgar-induced oxidative injury.
Aconitum carmichaelii Debx
Li et al. (2008a) used NMR-based metabonomic methodology to study the effects of Hei-Shun-Pian, the processed lateral root of Aconitum carmichaelii Debx. (Ranunculaceae), on the metabonomic profile of rats, so as to discuss the mechanism of toxicology and to find out the potential biomarkers of the toxic effects. The experiment discovered that the urinary levels of taurine in the high-dosed and middle-dosed groups decreased dramatically in the early stage of the dosing period. While at the later stage of the dosing period, the taurine increased and gradually reached the original level at the end of experiment. The recovery of taurine level might be explained as the effect of decreased intoxication, due to the increased tolerance or metabolic capability of aconitum alkaloids in Hei-Shun-Pian, along with the time of administration. However, no metabolic differences were observed between low-dosed and control groups until the later stage of the dosing period when a slight increase in urinary taurine level was observed, suggesting an indication of accumulated toxicity of Hei-Shun-Pian. In the present study, the decrease of urine taurine along with the alternation of citrate, glucose and amino acids suggests that the decoction of Hei-Shun-Pian has the cardiac toxicity; thus, the experimental results clearly showed that the high and middle doses of the decoctions of Hei-Shun-Pian could cause significant changes in the metabolism of rats and had toxicities on the heart and liver.
Breviscapine
In toxicokinetics research, finding the metabolites of candidate drug and identification of the drug metabolites are critical steps for further TCM toxicity investigation. Also, the compounds that exist only in post-dose samples are responsible for differentiating the control and dosed samples, which could help to detect the drug metabolites. Ran et al. (2006) applied LC coupled with ion trap MS combined with PCA to studies of TCM scutellarin in rat urine. They found that this methodology could be used to detect potential drug metabolites. This research has detected two potential metabolites of scutellarin and found nine ions responsible for the gender variation and one ion for the dosage variation.
Tripterygium wilfordii Hook
Metabonomics is time-saving and convenient for studying TCM toxicity. Furthermore, biological indicators in the metabolites often correspond to the lesion of specific sites. Therefore, application of metabonomics to TCM toxicity can obtain better understanding of site of action and action process, and ultimately achieve early prediction of TCM toxicity in drug development. With the purpose of exploring the renal toxicity of triptolid, Li et al. (2007a) analysed the influences of oral dosing of triptolide on endogenetic metabolites in rat urine using NMR coupled with pattern recognition technique and PCA. The results showed concentrations of acetate, betaine and acetone in urine increased after dosed with triptolide, which suggested the damages on the renal cortex S1, papilla and cortex S3 respectively. These data clearly demonstrate the renal toxicity of triptolide and its site of action and action process. PCA revealed that the metabonomic profile of triptolide-treated group was obviously different from that of control one. The metabolites variation showed that the metabonomic profile of urine closely related with the renal toxicity of triptolide. This study has successfully indicated that different sites and the extent of the lesion and organ damage could be associated with the corresponding changes in metabonomic profile.
Multi-analysed techniques
There is no existing analytical technique that can be a versatile analysis up to present. Every analytical technique has its advantages and drawbacks. Nevertheless, multi-analysed technique which applies different metabonomic analytical methods and integrates different dataset from these multi-analytical platforms, may capture different sets of endogenous metabolites with unique biological significance, therefore, it is technically feasible for comprehensive understanding of complex biochemical variations of living systems induced by TCM. Generally, multi-analysed technique is more close to the so-called versatile analysis in comparison with single-analysed technique (Ni et al., 2007).
GC-MS is widely used in metabolic fingerprint analysis of TCM owing to its high detecting sensitivity, besides the availability of existing citable and standard spectral libraries for reference, for almost all the volatile chemical compounds or non-volatile compounds readily derivatized in TCM (Want et al., 2007). Accordingly, it is convenient to obtain qualitative results for metabolic components and abundant analytical information under TCM intervention by GC-MS analysis. However, GC-MS has its own restrictions since the analytes have to be volatile and most biomolecules require chemical derivatization (Boernsen et al., 2005). Thus, it fails to supply metabolic information of most non-volatile compounds which are hard to derivatize, for instance, organic diphosphates, cofactors or metabolites larger than trisaccharides (Weckwerth and Fiehn, 2002). In addition, derivatization requires extensive sample preparation which is time-consuming and laborious and, more importantly, induces loss of sample (Pasikanti et al., 2008).
Differing from GC-MS, LC analysis obviates the thermolysis of chemical compositions and the restriction of samples volatility and stability; thus, it should be of powerful separation and high sensitivity. But there is limited analytical information one can obtain by LC analysis; moreover, LC analysis is time-consuming and interfered by sample matrix (Liu et al., 2007b).
Therefore, to achieve a more perfect effect, it is indispensable to associate GC-MS with LC in metabonomic analysis to integrate the advantages of the two techniques for TCM toxicity research. This method is potentially applicable to toxicological study, providing comprehensive understanding of living systems in response to xenobiotic (such as TCM, etc.) intervention.
Ni et al. (2007) combined GC-MS and LC-MS metabolic profiling approaches to unravel the pathological outcomes of AA-induced nephrotoxicity. They analysed the urine samples of Wistar rats by GC-MS and LC-MS in combination with PCA and orthogonal projection to latent structure-discriminant analysis (OPLSDA). They found that: (i) significant variations of homocysteine and methionine were detected by LC-MS analysis, whereas changes in cystine and cysteine concentration were observed by GC-MS analysis. Then they combined the results obtained from the two analytical methods together, which consequently turned out to be a disturbed metabolic pathway (Figure 3A); (ii) the negative OPLSDA correlation coefficients of the fatty acids including caprylic acid, valeric acid and arachidonic acid showed that phospholipase A2 (PLA2) was greatly inhibited by AA, which in turn induced a reduced level of arachidonic acid (Figure 3B); therefore, they assumed that depletion of arachidonic acid might be one of the fundamental causes of acute renal failure induced by AA; (iii) compared with the healthy control animals, the crucial substances of tricarboxylic acids (TCA) cycle, for example, citrate, iso-citrate and aconite, were significantly decreased (Figure 3C), suggesting that AA might inhibit the activity of these compounds, ultimately repressing energy metabolism. This study revealed that AA led to direct cytotoxic effect and enzyme inhibition, and to the significant alteration of gut-motivated metabolites and energy metabolism, and ultimately induced a disruption of the kidney-related metabolic regulatory network and renal function.
Figure 3.
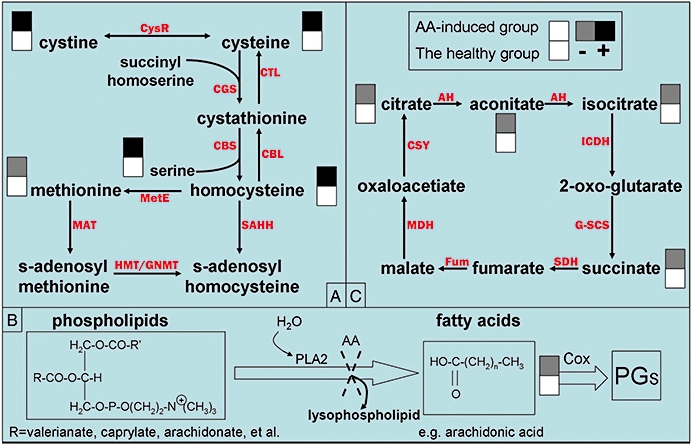
The perturbed metabolic pathways in response to aristolochic acid (AA) exposure (modified from Ni et al., 2007): (A) AA-induced metabolic disorder of amino acids; (B) AA perturbed tricarboxylic acids (TCA) cycle; (C) PLA2-related mechanisms of AA-induced nephrotoxicity. Dark square denotes an elevated concentration of metabolites present in the urine from rats exposed to AA, whereas gray square means a reduced level of metabolites in this group as compared to the healthy. The red abbreviations adjacent to the arrows are consistent with the corresponding enzymes, in detail, AH: aconitate hydratase; CBL: cystathionine beta-lyase; CBS: cystathionine beta-synthase; CGS: cystathionine gamma-synthase; CSY: citrate (Si)-synthase; CTL: cystathionine gamma-lyase; CysR: cystine reductase; Fum: fumarate hydratase; GNMT: glycine N-methyltransferase; G-SCS: succinate–CoA ligase (GDP-forming); HMT: homocysteine S-methyltransferase; ICDH: isocitrate dehydrogenase (NADP(+)); MAT: methionine adenosyltransferase; MDH: malate dehydrogenase; MetE: 5-methyltetrahydropteroyltriglutamate–homocysteine S-methyltransferase; SAHH: adenosylhomocysteinase; SDH: succinate dehydrogenase (ubiquinone).
Prospects
In recent years, TCM toxicity problems have attracted a wide range of concerns, which arouses many toxicity studies on TCM. Nevertheless, the present studies on mechanism of toxic action remain merely confined to a single chemical constituent in TCM. It must be impossible to acquire comprehensive understanding about TCM toxicity retained by means of restrictive experimental conditions and methods. In addition, there is no standard and objective basis for TCM toxicity evaluation and no uniform standard for safety assessment up to now. All these factors seriously hinder the development of TCM toxicology research currently. Therefore, with implementation of the modernization of TCM, we urgently desiderate strengthening TCM toxicology studies, formulating TCM safety assessment norms, extending further application of modern scientific and technological means to commonly used TCM safety assessment and establishing international standards and safety assessment criteria and indicators. Clearly, metabonomics based on a variety of high-throughput analytical techniques provides a novel technological means for the security study on TCM. There are different kinds of analytical techniques widely used in TCM modern research, but a few of which are applied to the study of TCM toxicity. The predicament is largely attributable to two reasons: first, there are still more or less shortcomings and inadequacies in these analytical techniques; second, lack of profound awareness of the great benefits of metabonomics in that metabonomics is an emerging discipline, the applications of which in various fields are still in its start-up stage. Thus, with the development of various existing analytical techniques along with the emergence of novel technologies, as well as with deeper understanding, metabonomics based on various analytical techniques will play an increasingly important role in TCM toxicology research.
Acknowledgments
This project was supported by National Key Technology R&D Program 2006BAD27B04.
Glossary
Abbreviations:
- AA
aristolochic acid
- AAN
aristolochic acid nephropathy
- ANIT
α-naphthylisothiocyanate
- APCI
atmospheric pressure chemical ionization
- APPI
atmospheric pressure photo ionization
- BEA
2-bromoethanamine hydrobromide
- CE
capillary electrophoresis
- CESM
capillary electroseparation method
- CHMs
Chinese herbal medicines
- CHN
Chinese herbs nephropathy
- CHPLC
capillary high-performance liquid chromatography
- ESI
electrospray ionization
- GC
gas chromatography
- GC × GC
two-dimensional gas chromatography
- GC-MS
gas chromatography-mass spectrometry
- HILIC
hydrophilic interaction chromatography
- HPCE
high-performance capillary electrophoresis
- HPLC
high-performance liquid chromatography
- HPLC-DAD-ELSD
high-performance liquid chromatography-diode array evaporative light scattering detector
- HPLC-ESI-TOF-MS
high-performance liquid chromatography-electrospray ionization time-of-flight mass spectrometry
- HYD
hydrazine dihydrochloride
- LC
liquid chromatography
- LC × LC
two-dimensional liquid chromatography
- LC-MS
liquid chromatography-mass spectrometry
- MDCCC
multidimensional counter-current chromatography
- MS
mass spectrometry
- NMR
nuclear magnetic resonance
- OPLSDA
orthogonal projection to latent structure-discriminant analysis
- PCA
principal components analysis
- PLA2
phospholipase A2
- PLS-DA
partial least squares discriminant analysis
- TCA
tricarboxylic acids
- TCM
traditional Chinese medicine
- UPLC Q-TOF MS
ultra high-performance liquid chromatography combined with quadrupole time-of-flight tandem mass spectrometry
- UPLC
ultra high-performance liquid chromatography
Conflict of interest
The authors state no conflict of interest.
References
- Aardema MJ, MacGregor JT. Toxicology and genetic toxicology in the new era of ‘toxicogenomics’: impact of ‘-omics’ technologies. Mutat Res. 2002;499:13–25. doi: 10.1016/s0027-5107(01)00292-5. [DOI] [PubMed] [Google Scholar]
- Ackermann BL, Hale JE, Duffin KL. The role of mass spectrometry in biomarker discovery and measurement. Curr Drug Metab. 2006;7:525–539. doi: 10.2174/138920006777697918. [DOI] [PubMed] [Google Scholar]
- Adahchour M, Beens J, Vreuls RJJ, Brinkman UATh. Recent developments in comprehensive two-dimensional gas chromatography (GC × GC): I. Introduction and instrumental set-up. TrAC-Trend Anal Chem. 2006a;25:438–454. [Google Scholar]
- Adahchour M, Beens J, Vreuls RJJ, Brinkman UATh. Recent developments in comprehensive two-dimensional gas chromatography (GC × GC): II. Modulation and detection. TrAC-Trend Anal Chem. 2006b;25:540–553. [Google Scholar]
- Adahchour M, Beens J, Vreuls RJJ, Brinkman UATh. Recent developments in comprehensive two-dimensional gas chromatography (GC × GC): III. Applications for petrochemicals and organohalogens. TrAC-Trend Anal Chem. 2006c;25:726–741. [Google Scholar]
- Adahchour M, Beens J, Vreuls RJJ, Brinkman UATh. ). Recent developments in comprehensive two-dimensional gas chromatography (GC × GC): IV. Further applications, conclusions and perspectives. TrAC-Trend Anal Chem. 2006d;25:821–840. [Google Scholar]
- Bailey NJC, Wang Y, Sampson J, Davis W, Whitcombe I, Hylands PJ, et al. Prediction of anti-plasmodial activity of Artemisia annua extracts: application of 1H NMR spectroscopy and chemometrics. J Pharm Biomed Anal. 2004;35:117–126. doi: 10.1016/j.jpba.2003.12.024. [DOI] [PubMed] [Google Scholar]
- Baumann S, Ceglarek U, Fiedler GM, Lembcke J, Leichtle A, Thiery J. Standardized approach to proteome profiling of human serum based on magnetic bead separation and matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Clin Chem. 2005;51:973–980. doi: 10.1373/clinchem.2004.047308. [DOI] [PubMed] [Google Scholar]
- Besheer J, Overstreet DH, Grondin JJM, Hodge CW, Lee DYW. The herbal medicine NPI-025 reduces ethanol self-administration and reinstatement. Alcohol Clin Exp Res. 2008;32(Suppl. 1):86A–86A. [Google Scholar]
- Boernsen KO, Gatzek S, Imbert G. Controlled protein precipitation in combination with chip-based nanospray infusion mass spectrometry. An approach for metabolomics profiling of plasma. Anal Chem. 2005;77:7255–7264. doi: 10.1021/ac0508604. [DOI] [PubMed] [Google Scholar]
- Bollard ME, Keun HC, Beckonert O, Ebbels TMD, Antti H, Nicholls AW, et al. Comparative metabonomics of differential hydrazine toxicity in the rat and mouse. Toxicol Appl Pharmacol. 2005;204:135–151. doi: 10.1016/j.taap.2004.06.031. [DOI] [PubMed] [Google Scholar]
- Cao YH, Wang Y, Ye JN. Differentiation of Swertia Mussotii Franch from Artemisiae Capillaris Herba by capillary electrophoresis with electrochemical detection. J Pharm Biomed Anal. 2005;39:60–65. doi: 10.1016/j.jpba.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Ceglarek U, Leichtle A, Brügel M, Kortz L, Brauer R, Bresler K, et al. Challenges, developments in tandem mass spectrometry based clinical metabolomics. Mol Cell Endocrinol. 2009;301:266–271. doi: 10.1016/j.mce.2008.10.013. [DOI] [PubMed] [Google Scholar]
- Chace DH, Kalas TA. A biochemical perspective on the use of tandem mass spectrometry for newborn screening and clinical testing. Clin Biochem. 2005;38:296–309. doi: 10.1016/j.clinbiochem.2005.01.017. [DOI] [PubMed] [Google Scholar]
- Chan W, Cai ZW. Aristolochic acid induced changes in the metabolic profile of rat urine. J Pharm Biomed Anal. 2008;46:757–762. doi: 10.1016/j.jpba.2007.11.042. [DOI] [PubMed] [Google Scholar]
- Chan W, Lee KC, Liu N, Wong RNS, Liu HW, Cai ZW. Liquid chromatography/mass spectrometry for metabonomics investigation of the biochemical effects induced by aristolochic acid in rats: the use of information-dependent acquisition for biomarker identification. Rapid Commun Mass Spectrom. 2008;22:873–880. doi: 10.1002/rcm.3438. [DOI] [PubMed] [Google Scholar]
- Chang PL, Hsieh WS, Chiang CL, Yen-Liberman B, Procop GW, Chang HT, et al. Identification of individual DNA molecule of Mycobacterium tuberculosis by nested PCR-RFLP and capillary electrophoresis. Talanta. 2008;77:182–188. doi: 10.1016/j.talanta.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Chen MJ, Zhao LP, Jia W. Metabonomic study on the biochemical profiles of a hydrocortisone-induced animal model. J Proteome Res. 2005a;4:2391–2396. doi: 10.1021/pr050158o. [DOI] [PubMed] [Google Scholar]
- Chen XG, Kong L, Su XY, Pan CS, Ye ML, Zou HF. Integration of ion-exchange chromatography fractionation with reversed-phase liquid chromatography-atmospheric pressure chemical ionization mass spectrometer and matrix-assisted laser desorption/ionization time-of-flight mass spectrometry for isolation and identification of compounds in Psoralea corylifolia. J Chromatogr A. 2005b;1089:87–100. doi: 10.1016/j.chroma.2005.06.067. [DOI] [PubMed] [Google Scholar]
- Chen G, Zhang LY, Zhu YZ. Determination of glycosides and sugars in Moutan Cortex by capillary electrophoresis with electrochemical detection. J Pharm Biomed Anal. 2006a;41:129–134. doi: 10.1016/j.jpba.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Chen MJ, Su MM, Zhao LP, Jiang J, Liu P, Cheng JY, et al. Metabonomic study of aristolochic acid-induced nephrotoxicity in rats. J Proteome Res. 2006b;5:995–1002. doi: 10.1021/pr050404w. [DOI] [PubMed] [Google Scholar]
- Cheung HY, Cheung SH, Lawa ML, Lai WP. Simultaneous determination of key bioactive components in Hedyotis diffusa by capillary electrophoresis. J Chromatogr B. 2006;834:195–198. doi: 10.1016/j.jchromb.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Dong L, Deng CH, Wang JY, Shen XZ. Fast determination of paeonol in plasma by headspace solid-phase microextraction followed by gas chromatography – mass spectrometry. Anal Chim Acta. 2007a;585:76–80. doi: 10.1016/j.aca.2006.12.024. [DOI] [PubMed] [Google Scholar]
- Dong L, Wang JY, Deng CH, Shen XZ. Gas chromatography-mass spectrometry following pressurized hot water extraction and solid-phase microextraction for quantification of eucalyptol, camphor, and borneol in Chrysanthemum flowers. J Sep Sci. 2007b;30:86–89. doi: 10.1002/jssc.200600207. [DOI] [PubMed] [Google Scholar]
- Dunn WB, Ellis DI. Metabolomics: current analytical platforms and methodologies. TrAC-Trend Anal Chem. 2005;24:285–294. [Google Scholar]
- Dunn WB, Bailey NJC, Johnson HE. Measuring the metabolome: current analytical technologies. Analyst. 2005;130:606–625. doi: 10.1039/b418288j. [DOI] [PubMed] [Google Scholar]
- Ekins S, Nikolsky Y, Nikolskaya T. Techniques: application of systems biology to absorption, distribution, metabolism, excretion and toxicity. Trends Pharmacol Sci. 2005;26:202–209. doi: 10.1016/j.tips.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Feng HY, Li XJ, Hou SR, Zheng N, Hu ZB, Yuan ZB. On-line concentration of trace genistein by acid barrage stacking in capillary electrophoresis with UV detection. Chinese Chem Lett. 2008;19:973–976. [Google Scholar]
- Gao HT, Li TH, Chen K, Li WG, Bi X. Overlapping spectra resolution using non-negative matrix factorization. Talanta. 2005a;66:65–73. doi: 10.1016/j.talanta.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Gao WH, Lin SY, Jia L, Guo XK, Chen XG, Hu ZD. Analysis of protoberberine alkaloids in several herbal drugs and related medicinal preparations by non-aqueous capillary electrophoresis. J Sep Sci. 2005b;28:92–97. doi: 10.1002/jssc.200401863. [DOI] [PubMed] [Google Scholar]
- García-Pérez I, Vallejo M, García A, Legido-Quigley C, Barbas C. Metabolic fingerprinting with capillary electrophoresis. J Chromatogr A. 2008;1204:130–139. doi: 10.1016/j.chroma.2008.07.025. [DOI] [PubMed] [Google Scholar]
- Gillerot G, Jadoul M, Arlt VM, van Ypersele de Strihou C, Schmeiser HM, But PPH, et al. Aristolochic acid nephropathy in a Chinese patient: time to abandon the term ‘Chinese herbs nephropathy’? Am J Kidney Dis. 2001;38:1–5. doi: 10.1053/ajkd.2001.28624. [DOI] [PubMed] [Google Scholar]
- Griffin JL. Metabonomics: NMR spectroscopy and pattern recognition analysis of body fuids and tissues for characterisation of xenobiotic toxicity and disease diagnosis. Curr Opin Chem Biol. 2003;7:648–654. doi: 10.1016/j.cbpa.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Guo ZF, Guo TT, Guo MF. Preparation of molecularly imprinted adsorptive resin for trapping of ligustrazine from the traditional Chinese herb Ligusticum chuanxiong Hort. Anal Chim Acta. 2008;612:136–143. doi: 10.1016/j.aca.2008.02.039. [DOI] [PubMed] [Google Scholar]
- Hsieh SC, Huang MF, Lin BS, Chang HT. Determination of aristolochic acid in Chinese herbal medicine by capillary electrophoresis with laser-induced fluorescence detection. J Chromatogr A. 2006;1105:127–134. doi: 10.1016/j.chroma.2005.07.056. [DOI] [PubMed] [Google Scholar]
- Hu LH, Li X, Feng S, Kong L, Su XY, Chen XG, et al. Comprehensive two-dimensional HPLC to study the interaction of multiple components in Rheum palmatum L. with HSA by coupling a silica-bonded HSA column to a silica monolithic ODS column. J Sep Sci. 2006a;29:881–888. doi: 10.1002/jssc.200500442. [DOI] [PubMed] [Google Scholar]
- Hu Z, He LC, Zhang J, Luo GA. Determination of three bile acids in artificial Calculus Bovis and its medicinal preparations by micellar electrokinetic capillary electrophoresis. J Chromatogr B. 2006b;837:11–17. doi: 10.1016/j.jchromb.2006.03.050. [DOI] [PubMed] [Google Scholar]
- Huang XD, Kong L, Li X, Chen XG, Guo M, Zou HF. Strategy for analysis and screening of bioactive compounds in traditional Chinese medicines. J Chromatogr B. 2004;812:71–84. doi: 10.1016/j.jchromb.2004.06.046. [DOI] [PubMed] [Google Scholar]
- Jin Y, Liang T, Fu Q, Xiao YS, Feng JT, Ke YX, et al. Fingerprint analysis of Ligusticum chuanxiong using hydrophilic interaction chromatography and reversed-phase liquid chromatography. J Chromatogr A. 2009;1216:2136–2141. doi: 10.1016/j.chroma.2008.04.010. [DOI] [PubMed] [Google Scholar]
- Jorgenson JW, Lukacs KD. Capillary zone electrophoresis. Science. 1983;222:266–272. doi: 10.1126/science.6623076. [DOI] [PubMed] [Google Scholar]
- Kanani H, Chrysanthopoulos PK, Klapa MI. Standardizing GC–MS metabolomics. J Chromatogr B. 2008;871:191–201. doi: 10.1016/j.jchromb.2008.04.049. [DOI] [PubMed] [Google Scholar]
- Koyama J, Morita I, Kawanishi K, Tagahara K, Kobayashi N. Capillary electrophoresis for simultaneous determination of emodin, chrysophanol, and their 8-β-D-glucosides. Chem Pharm Bull (Tokyo) 2003;51:418–420. doi: 10.1248/cpb.51.418. [DOI] [PubMed] [Google Scholar]
- Kvasnička F, Bíba B, Cvak L. Capillary zone electrophoresis separation of enantiomers of lisuride. J Chromatogr A. 2005;1066:255–258. doi: 10.1016/j.chroma.2005.01.020. [DOI] [PubMed] [Google Scholar]
- Laird AR, Ramchandani N, Degoma EM, Avula B, Khan IA, Gesundheit NA. Hepatitis associated with the use of an herbal supplement (Polygonum Multiflorum) mimicking iron-overload syndrome. J Clin Gastroenterol. 2008;42:861–862. doi: 10.1097/MCG.0b013e3181492515. [DOI] [PubMed] [Google Scholar]
- Lenz EM, Wilson ID. Analytical strategies in metabonomics. J Proteome Res. 2007;6:443–458. doi: 10.1021/pr0605217. [DOI] [PubMed] [Google Scholar]
- Li GB, Zhang HY, Fan YQ, Zhao L, Hu ZD. Migration behavior and separation of active components in Glycyrrhiza uralensis Fisch and its commercial extract by micellar electrokinetic capillary chromatography. J Chromatogr A. 1999;863:105–114. doi: 10.1016/s0021-9673(99)00945-0. [DOI] [PubMed] [Google Scholar]
- Li FM, Xiong ZL, Lu XM, Qin F, Li XQ. Strategy and chromatographic technology of quality control for traditional Chinese medicines. Chin J Chromatogr. 2006a;24:537–544. [PubMed] [Google Scholar]
- Li YQ, He XJ, Qi SD, Gao WH, Chen XG, Hu ZD. Separation and determination of strychnine and brucine in Strychnos nux-vomica L. and its preparation by nonaqueous capillary electrophoresis. J Pharm Biomed Anal. 2006b;41:400–407. doi: 10.1016/j.jpba.2005.11.036. [DOI] [PubMed] [Google Scholar]
- Li JX, Hua J, He CC. Metabonomic study on the toxicity of traditional Chinese medicine (I): renal toxicity of triptolide. Asia Pac Tradit Med. 2007a;3:41–45. [Google Scholar]
- Li L, Wang JN, Ren JX, Xiang JF, Tang YL, Liu JX, et al. Metabonomics analysis of the urine of rats with Qi deficiency and blood stasis syndrome based on NMR techniques. Chinese Sci Bull. 2007b;52:3068–3073. [Google Scholar]
- Li YP, Hu Z, He LC. An approach to develop binary chromatographic fingerprints of the total alkaloids from Caulophyllum robustum by high performance liquid chromatography/diode array detector and gas chromatography/mass spectrometry. J Pharm Biomed Anal. 2007c;43:1667–1672. doi: 10.1016/j.jpba.2006.12.028. [DOI] [PubMed] [Google Scholar]
- Li L, Sun B, Zhang Q, Fang JJ, Ma KP, Li Y, et al. Metabonomic study on the toxicity of Hei-Shun-pian, the processed lateral root of Aconitum carmichaelii Debx. (Ranunculaceae) J Ethnopharmacol. 2008a;116:561–568. doi: 10.1016/j.jep.2008.01.014. [DOI] [PubMed] [Google Scholar]
- Li WF, Jiang JG, Chen J. Chinese medicine and its modernization demands. Arch Med Res. 2008b;39:246–251. doi: 10.1016/j.arcmed.2007.09.011. [DOI] [PubMed] [Google Scholar]
- Liang MJ, He LC, Yang GD. Screening, analysis and in vitro vasodilatation of effective components from Ligusticum Chuanxiong. Life Sci. 2005;78:128–133. doi: 10.1016/j.lfs.2005.04.038. [DOI] [PubMed] [Google Scholar]
- Lindon JC, Nicholson JK, Holmes E, Antti H, Bollard ME, Keun H, et al. Contemporary issues in toxicology the role of metabonomics in toxicology and its evaluation by the COMET project. Toxicol Appl Pharmacol. 2003;187:137–146. doi: 10.1016/s0041-008x(02)00079-0. [DOI] [PubMed] [Google Scholar]
- Lindon JC, Keun HC, Ebbels TMD, Pearce JMT, Holmes E, Nicholson JK. The Consortium for Metabonomic Toxicology (COMET): aims, activities and achievements. Pharmacogenomics. 2005;6:691–699. doi: 10.2217/14622416.6.7.691. [DOI] [PubMed] [Google Scholar]
- Liu JJ, Li SP, Wang YT. Optimization for quantitative determination of four flavonoids in Epimedium by capillary zone electrophoresis coupled with diode array detection using central composite design. J Chromatogr A. 2006a;1103:344–349. doi: 10.1016/j.chroma.2005.11.036. [DOI] [PubMed] [Google Scholar]
- Liu Q, Liu YJ, Guo ML, Luo XB, Yao SZ. A simple and sensitive method of nonaqueous capillary electrophoresis with laser-induced native fluorescence detection for the analysis of chelerythrine and sanguinarine in Chinese herbal medicines. Talanta. 2006b;70:202–207. doi: 10.1016/j.talanta.2006.02.049. [DOI] [PubMed] [Google Scholar]
- Liu M, Li YG, Chou GX, Cheng XM, Zhang M, Wang ZT. Extraction and ultra-performance liquid chromatography of hydrophilic and lipophilic bioactive components in a Chinese herb Radix Salviae Miltiorrhizae. J Chromatogr A. 2007a;1157:51–55. doi: 10.1016/j.chroma.2007.05.018. [DOI] [PubMed] [Google Scholar]
- Liu XM, Zhang JS, Chen XG. Separation and determination of three water-soluble compounds in Salvia miltiorrhiza Bunge and two related traditional medicinal preparations by flow injection-capillary electrophoresis. J Chromatogr B. 2007b;852:325–332. doi: 10.1016/j.jchromb.2007.01.034. [DOI] [PubMed] [Google Scholar]
- Lu X, Zhao XJ, Bai CM, Zhao CX, Lu G, Xu GW. LC–MS-based metabonomics analysis. J Chromatogr B. 2008;866:64–76. doi: 10.1016/j.jchromb.2007.10.022. [DOI] [PubMed] [Google Scholar]
- Ma CF, Wang HH, Lu X, Li HF, Liu BY, Xu GW. Analysis of Artemisia annua L. volatile oil by comprehensive two-dimensional gas chromatography time-of-flight mass spectrometry. J Chromatogr A. 2007;1150:50–53. doi: 10.1016/j.chroma.2006.08.080. [DOI] [PubMed] [Google Scholar]
- Mao Y, Yuan BJ. Current status and prospect of metabonomics. Chin J New Drugs. 2007;16:1005–1010. [Google Scholar]
- Marchart E, Krenn L, Kopp B. Quantification of the flavonoid glycosides in Passiflora incarnata by capillary electrophoresis. Planta Med. 2003;69:452–456. doi: 10.1055/s-2003-39699. [DOI] [PubMed] [Google Scholar]
- Marquet P. Progress of liquid chromatography-mass spectrometry in clinical and forensic toxicology. Ther Drug Monit. 2002;24:255–276. doi: 10.1097/00007691-200204000-00008. [DOI] [PubMed] [Google Scholar]
- Muensritharam L, Tolieng V, Chaichantipyuth C, Petsom A, Nhujak T. Capillary zone electrophoresis for separation and analysis of hydroxycitric acid and hydroxycitric acid lactone: application to herbal products of Garcinia atroviridis Griff. J Pharm Biomed Anal. 2008;46:577–582. doi: 10.1016/j.jpba.2007.11.008. [DOI] [PubMed] [Google Scholar]
- Ni Y, Su MM, Qiu YP, Chen MJ, Liu YM, Zhao AH, et al. Metabolic profiling using combined GC–MS and LC–MS provides a systems understanding of aristolochic acid-induced nephrotoxicity in rat. FEBS Lett. 2007;581:707–711. doi: 10.1016/j.febslet.2007.01.036. [DOI] [PubMed] [Google Scholar]
- Nicholoson JK, Connelly J, Lindon JC, Holmes E. Metabonomics: a platform for studying drug toxicity and gene function. Nat Rev. 2002;1:153–161. doi: 10.1038/nrd728. [DOI] [PubMed] [Google Scholar]
- Nicholson JK. Global systems biology, personalized medicine and molecular epidemiology. Mol Syst Biol. 2006;2:52–57. doi: 10.1038/msb4100095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson JK, Wilson ID. Understanding ‘global’ systems biology: metabonomics and the continuum of metabolism. Nat Rev. 2003;2:668–676. doi: 10.1038/nrd1157. [DOI] [PubMed] [Google Scholar]
- Nicholson JK, Lindon JC, Holmes E. Metabonomics': understanding the metabolic responses of living systems to pathophysiological stimuli via multivariate statistical analysis of biological NMR spectroscopic data. Xenobiotica. 1999;29:1181–1189. doi: 10.1080/004982599238047. [DOI] [PubMed] [Google Scholar]
- Pasikanti KK, Ho PC, Chan ECY. Gas chromatography/mass spectrometry in metabolic profiling of biological fluids. J Chromatogr B. 2008;871:202–211. doi: 10.1016/j.jchromb.2008.04.033. [DOI] [PubMed] [Google Scholar]
- Patnala S, Kanfer I. A capillary zone electrophoresis method for the assay and quality control of mesembrine in Sceletium tablets. J Pharm Biomed Anal. 2008;48:440–446. doi: 10.1016/j.jpba.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Peng YY, Ye JN. Determination of isoflavones in red clover by capillary electrophoresis with electrochemical detection. Fitoterapia. 2006;77:171–178. doi: 10.1016/j.fitote.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Peng YY, Liu FH, Ye JN. Determination of phenolic acids and flavones in Lonicera japonica thumb, by capillary electrophoresis with electrochemical detection. Electroanal. 2005;17:356–362. [Google Scholar]
- Pham-Tuan H, Kaskavelis L, Daykin CA, Janssen HG. Method development in high-performance liquid chromatography for high-throughput profiling and metabonomic studies of biofluid samples. J Chromatogr B. 2003;789:283–301. doi: 10.1016/s1570-0232(03)00077-1. [DOI] [PubMed] [Google Scholar]
- Phillips JB, Beens J. Comprehensive two-dimensional gas chromatography: a hyphenated method with strong coupling between the two dimensions. J Chromatogr A. 1999;856:331–347. doi: 10.1016/s0021-9673(99)00815-8. [DOI] [PubMed] [Google Scholar]
- Qi LW, Li P, Li SL, Sheng LH, Li RY, Song Y, et al. Screening and identification of permeable components in a combined prescription of Danggui Buxue decoction using a liposome equilibrium dialysis system followed by HPLC and LC-MS. J Sep Sci. 2006a;29:2211–2220. doi: 10.1002/jssc.200600107. [DOI] [PubMed] [Google Scholar]
- Qi SD, Ding L, Tian K, Chen XG, Hu ZD. Novel and simple nonaqueous capillary electrophoresis separation and determination bioactive triterpenes in Chinese herbs. J Pharm Biomed Anal. 2006b;40:35–41. doi: 10.1016/j.jpba.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Qiu YQ, Lu X, Pang T, Zhu SK, Kong HW, Xu GW. Study of traditional Chinese medicine volatile oils from different geographical origins by comprehensive two-dimensional gas chromatography-time-of-flight mass spectrometry (GC × GC-TOFMS) in combination with multivariate analysis. J Pharm Biomed Anal. 2007;43:1721–1727. doi: 10.1016/j.jpba.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Ran XR, Si SZ, Liang QL, Luo GA. Application of the reversed-phase liquid chromatography coupled to ion trap mass spectrometry with principal components analysis for metabonomics studies of scutellarin in rat urine. Chinese Chem Let. 2006;17:1225–1228. [Google Scholar]
- Ronda F, Rodríguez-Nogales JM, Sancho D, Gómez BOYM. Multivariate optimisation of a capillary electrophoretic method for the separation of glutenins. Application to quantitative analysis of the endosperm storage proteins in wheat. Food Chem. 2008;108:287–296. [Google Scholar]
- Rousseau R, Govaerts B, Verleysen M, Boulanger B. Comparison of some chemometric tools for metabonomics biomarker identification. Chemometr Intell Lab. 2008;91:54–66. [Google Scholar]
- Ruan GH, Li GK. The study on the chromatographic fingerprint of Fructus xanthii by microwave assisted extraction coupled with GC–MS. J Chromatogr B. 2007;850:241–248. doi: 10.1016/j.jchromb.2006.11.036. [DOI] [PubMed] [Google Scholar]
- Sabbioni C, Mandrioli R, Ferranti A, Bugamelli F, Saracino MA, Forti GC, et al. Separation and analysis of glycyrrhizin, 18β-glycyrrhetic acid and 18a-glycyrrhetic acid in liquorice roots by means of capillary zone electrophoresis. J Chromatogr A. 2005;1081:65–71. doi: 10.1016/j.chroma.2005.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slim RM, Robertson DG, Albassam M, Reily MD, Robosky L, Dethloff LA. Effect of dexamethasone on the metabonomics profile associated with phosphodiesterase inhibitor-induced vascular lesions in rats. Toxicol Appl Pharmacol. 2002;183:108–116. [PubMed] [Google Scholar]
- Tam CU, Yang FQ, Zhang QW, Guan J, Li SP. Optimization and comparison of three methods for extraction of volatile compounds from Cyperus rotundus evaluated by gas chromatography–mass spectrometry. J Pharm Biomed Anal. 2007;44:444–449. doi: 10.1016/j.jpba.2006.10.026. [DOI] [PubMed] [Google Scholar]
- Tang HR, Wang YL. Metabonomics: a revolution in progress. Prog Biochem Biophys. 2006;33:401–417. [Google Scholar]
- Tegtmeier M, Muhlau A, Ducker D, Runkel M, Legrum W. A new method of capillary electrophoresis for metabolites of coumarin. Pharmazie. 2000;55:94–96. [PubMed] [Google Scholar]
- Vanherweghem JL, Tielemans C, Abramowicz D, Depierreux M, Vanhaelen-Fastre R, Vanhaelen M, et al. Rapidly progressive interstitial renal fibrosis in young woman: association with slimming regimen including Chinese herbs. Lancet. 1993;341:387–391. doi: 10.1016/0140-6736(93)92984-2. [DOI] [PubMed] [Google Scholar]
- Wang SF, Ju Y, Chen XG, Hu ZD. Separation and determination of coumarins in the root bark of three citrus plants by micellar electrokinetic capillary chromatography. Planta Med. 2003;69:483–486. doi: 10.1055/s-2003-39697. [DOI] [PubMed] [Google Scholar]
- Wang XK, He YZ, Qian LL. Determination of polyphenol components in herbal medicines by micellar electrokinetic capillary chromatography with Tween 20. Talanta. 2007a;74:1–6. doi: 10.1016/j.talanta.2007.05.023. [DOI] [PubMed] [Google Scholar]
- Wang Y, Kong L, Hu LH, Lei XY, Yang L, Chou GX, et al. Biological fingerprinting analysis of the traditional Chinese prescription Longdan Xiegan Decoction by on/off-line comprehensive two-dimensional biochromatography. J Chromatogr B. 2007b;860:185–194. doi: 10.1016/j.jchromb.2007.10.027. [DOI] [PubMed] [Google Scholar]
- Want EJ, Nordströlm A, Morita H, Siuzdak G. From exogenous to endogenous: the inevitable imprint of mass spectrometry in metabolomics. J Proteome Res. 2007;6:459–468. doi: 10.1021/pr060505+. [DOI] [PubMed] [Google Scholar]
- Weckwerth W, Fiehn O. Can we discover novel pathways using metabolomic analysis? Curr Opin Biotechnol. 2002;13:156–160. doi: 10.1016/s0958-1669(02)00299-9. [DOI] [PubMed] [Google Scholar]
- Wei L, Liao PQ, Wu HF, Li XJ, Pei FK, Li WS, et al. Toxicological effects of cinnabar in rats by NMR-based metabolic profiling of urine and serum. Toxicol Appl Pharmacol. 2008;227:417–429. doi: 10.1016/j.taap.2007.11.015. [DOI] [PubMed] [Google Scholar]
- Wei L, Liao PQ, Wu HF, Li XJ, Pei FK, Li WS, et al. Metabolic profiling studies on the toxicological effects of realgar in rats by 1H NMR spectroscopy. Toxicol Appl Pharmacol. 2009;234:314–325. doi: 10.1016/j.taap.2008.11.010. [DOI] [PubMed] [Google Scholar]
- Williams RE, Lock EA. D-serine-induced nephrotoxicity: possible interaction with tyrosine metabolism. Toxicology. 2004;201:231–238. doi: 10.1016/j.tox.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Xu XQ, Ye HZ, Wang W, Chen GN. An improved method for the quantitation of flavonoids in Herba Leonuri by capillary electrophoresis. J Agric Food Chem. 2005;53:5853–5857. doi: 10.1021/jf0504216. [DOI] [PubMed] [Google Scholar]
- Yang FQ, Li SP, Li P, Wang YT. Optimization of CEC for simultaneous determination of eleven nucleosides and nucleobases in Cordyceps using central composite design. Electrophoresis. 2007;28:1681–1688. doi: 10.1002/elps.200600416. [DOI] [PubMed] [Google Scholar]
- Yang FQ, Quan J, Zhang TY, Ito Y. Multidimensional counter-current chromatographic system and its application. J Chromatogr A. 1998;803:298–301. doi: 10.1016/s0021-9673(97)01273-9. [DOI] [PubMed] [Google Scholar]
- Yang J, Xu GW, Kong HW, Zheng YF, Pang T, Yang Q. Artificial neural network classification based on high-performance liquid chromatography of urinary and serum nucleosides for the clinical diagnosis of cancer. J Chromatogr B. 2002;780:27–33. doi: 10.1016/s1570-0232(02)00408-7. [DOI] [PubMed] [Google Scholar]
- Yang J, Xu GW, Zheng YF, Kong HW, Pang T, Lv S, et al. Diagnosis of liver cancer using HPLC-based metabonomics avoiding false-positive result from hepatitis and hepatocirrhosis diseases. J Chromatogr B. 2004;813:59–65. doi: 10.1016/j.jchromb.2004.09.032. [DOI] [PubMed] [Google Scholar]
- Yang J, Xu GW, Zheng YF, Kong HW, Wang C, Zhao XJ, et al. Strategy for metabonomics research based on high-performance liquid chromatography and liquid chromatography coupled with tandem mass spectrometry. J Chromatogr A. 2005;1084:214–221. doi: 10.1016/j.chroma.2004.10.100. [DOI] [PubMed] [Google Scholar]
- Yang XW. Discovery strategy for toxic-invited constituents and toxic-effective substances on Chinese material medica based on processes of metabolism and disposition in intra-body. China Journal of Traditional Chinese Medicine and Pharmacy. 2007;22:67–72. [PubMed] [Google Scholar]
- Yu K, Wang YW, Cheng YY. Determination of paeonol and paeoniflorin in Chinese medicine Cortex Moutan and ‘Shuangdan’ granule by micellar electrokinetic capillary chromatography. J Pharm Biomed Anal. 2006;40:1257–1262. doi: 10.1016/j.jpba.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Yu K, Gong YF, Lin ZY, Cheng YY. Quantitative analysis and chromatographic fingerprinting for the quality evaluation of Scutellaria baicalensis Georgi using capillary electrophoresis. J Pharm Biomed Anal. 2007a;43:540–548. doi: 10.1016/j.jpba.2006.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Yi ZB, Liang YZ. Validate antibacterial mode and find main bioactive components of traditional Chinese medicine Aquilegia oxysepala. Bioorgan Med Chem. 2007b;17:1855–1859. doi: 10.1016/j.bmcl.2007.01.032. [DOI] [PubMed] [Google Scholar]
- Yu YJ, Huang TM, Yang B, Liu X, Duan GL. Development of gas chromatography – mass spectrometry with microwave distillation and simultaneous solid-phase microextraction for rapid determination of volatile constituents in ginger. J Pharm Biomed Anal. 2007c;43:24–31. doi: 10.1016/j.jpba.2006.06.037. [DOI] [PubMed] [Google Scholar]
- Zeng HP, Wang TT, Chen W, Wang CY, Chen DF, Shen JG. Characterization of chemical components in extracts from Si-wu decoction with proliferation-promoting effects on rat mesenchymal stem cells. Bioorg Med Chem. 2008;16:5109–5114. doi: 10.1016/j.bmc.2008.03.024. [DOI] [PubMed] [Google Scholar]
- Zeng YX, Zhao CX, Liang YZ, Yang H, Fang HZ, Yi LZ, et al. Comparative analysis of volatile components from Clematis species growing in China. Anal Chim Acta. 2007;595:328–339. doi: 10.1016/j.aca.2006.12.022. [DOI] [PubMed] [Google Scholar]
- Zeng ZD, Liang YZ, Zhang T, Chau FT, Wang YL. Orthogonal projection (OP) technique applied to pattern recognition of fingerprints of the herbal medicine houttuynia cordata Thunb and its final injection products. Anal Bional Chem. 2006;385:392–400. doi: 10.1007/s00216-006-0405-6. [DOI] [PubMed] [Google Scholar]
- Zhang X, Qi LW, Yi L, Li P, Wen XD, Yu QT. Screening and identification of potential bioactive components in a combined prescription of Danggui Buxue decoction using cell extraction coupled with high performance liquid chromatography. Biomed Chromatogr. 2008;22:157–163. doi: 10.1002/bmc.910. [DOI] [PubMed] [Google Scholar]
- Zhang XM, Hu HL, Xu SY, Yang XH, Zhang J. Comprehensive two-dimensional capillary LC and CE for resolution of neutral components in traditional Chinese medicines. J Sep Sci. 2001;24:385–391. [Google Scholar]
- Zhang XY, Wu HF, Liao PQ, Li XJ, Ni JZ, Pei FK. NMR-based metabonomic study on the subacute toxicity of aristolochic acid in rats. Food Chem Toxicol. 2006;44:1006–1014. doi: 10.1016/j.fct.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Zhao J, Yu QT, Li P, Zhou P, Zhang YJ, Wang W. Determination of nine active components in Radix Hedysari and Radix Astragali using capillary HPLC with diode array detection and MS detection. J Sep Sci. 2008a;31:255–261. doi: 10.1002/jssc.200700379. [DOI] [PubMed] [Google Scholar]
- Zhao XJ, Zhang Y, Meng XL, Yin PY, Deng C, Chen J, et al. Effect of a traditional Chinese medicine preparation Xindi soft capsule on rat model of acute blood stasis: a urinary metabonomics study based on liquid chromatography-mass spectrometry. J Chromatogr B. 2008b;873:151–158. doi: 10.1016/j.jchromb.2008.08.010. [DOI] [PubMed] [Google Scholar]
- Zhu L, Tse CH, Wong VWS, Chim AML, Leung KS, Chan HLY. A complete genomic analysis of hepatitis B virus genotypes and mutations in HBeAg-negative chronic hepatitis B in China. J Viral Hepatitis. 2008;15:449–458. doi: 10.1111/j.1365-2893.2008.00967.x. [DOI] [PubMed] [Google Scholar]
- Zougagh M, Simonet BM, Ríos A, Valcárcel M. Use of non-aqueous capillary electrophoresis for the quality control of commercial saffron samples. J Chromatogr A. 2005;1085:293–298. doi: 10.1016/j.chroma.2005.06.041. [DOI] [PubMed] [Google Scholar]


