Abstract
The purinergic system is composed of mononucleosides, mononucleoside polyphosphates and dinucleoside polyphosphates as agonists, as well as the respective purinergic receptors. Interest in the role of the purinergic system in cardiovascular physiology and pathophysiology is on the rise. This review focuses on the overall impact of dinucleoside polyphosphates in the purinergic system. Platelets, adrenal glands, endothelial cells, cardiomyocytes and tubular cells release dinucleoside polyphosphates. Plasma concentrations of dinucleoside polyphosphates are sufficient to cause direct vasoregulatory effects and to induce proliferative effects on vascular smooth muscle cells and mesangial cells. In addition, increased plasma concentrations of a dinucleoside polyphosphate were recently demonstrated in juvenile hypertensive patients. In conclusion, the current literature accentuates the strong physiological and pathophysiological impact of dinucleoside polyphosphates on the cardiovascular system.
Keywords: dinucleoside polyphosphates, purinergic system, cardiovascular system, extracellular mediators, intracellular mediators, vascular tone, proliferation
Introduction
Interest in the functional roles of nucleotides and the underlying purinergic system in cardiovascular physiology and pathophysiology continues to grow (Gabriels et al., 2002). The purinergic system consists of mononucleosides, mononucleoside polyphosphates and dinucleoside polyphosphates as agonists, as well as the respective purinergic receptors, which possess very different functions (Ralevic and Burnstock, 1998). The purinergic signalling system that controls vascular regulation displays a high degree of complexity.
Purinergic receptor system (purinoceptors)
The complexity of the purinergic signalling system is partially due to the large number of agonists that constitute this system. This is further complicated by the diversity of the purinergic receptors (purinoceptors), including the subtypes of the P1, P2X and P2Y receptors, as well as formation of heteropolymeric P2X ion channels (Nori et al., 1998; Robertson et al., 2001), and P2X splicing variants (Cheewatrakoolpong et al., 2005; Koshimizu et al., 2006). Additionally, there are numerous soluble and membrane-bound ecto-nucleotidases (Yegutkin et al., 2000; 2007; Linden, 2001; Leipziger, 2003) that transform one active purinoceptor agonist into an active agonist for another purinoceptor. In the last several years, our understanding of the role(s) the purinergic system plays in normal and pathological cardiovascular physiology has been vastly increased by the identification of new purinergic agonists and cloning of several purine receptor subtypes.
The potent actions of purine mononucleoside polyphosphates on cardiovascular system vessels were first described in 1929 (Drury and Szent-Györgyi, 1929). Since then, the impact of mononucleoside polyphosphates in various vasoregulatory processes, like immunomodulatory and prothrombotic responses in the cardiovascular system, have been described in detail elsewhere (Burnstock, 2002; Moore and MacKenzie, 2007).
Dinucleoside polyphosphates as mediators of the purinergic system
In recent years, interest in dinucleoside polyphosphates as strong purinergic agonists has been growing. Dinucleoside polyphosphates mediate vascular tone regulation (Schlüter et al., 1994; Jankowski et al., 2005), as well as vascular smooth muscle and mesangial cell proliferation (McLennan, 1992; Heidenreich et al., 1995).
Occurrence and physiological effects of dinucleoside polyphosphates
Molecular structure of dinucleoside polyphosphates
Dinucleoside polyphosphates (XpnX) consist of two nucleotides (ribosylated nucleic acids), which are interconnected by a polyphosphate chain of two to seven phosphates through phosphoester bonds at the 5′-position of two ribose moieties (where X = adenosine and/or guanosine, or uridine; n= number of phosphate groups). Figure 1 shows the molecular structure of P1,P4 uridine adenosine tetraphosphate (Up4A) as an example of a dinucleoside polyphosphates. In comparison with mononucleoside polyphosphates, dinucleoside polyphosphates have relatively long half-lives. Furthermore, metabolites of dinucleoside polyphosphates may serve as potential sources of extracellular ATP and other purines.
Figure 1.
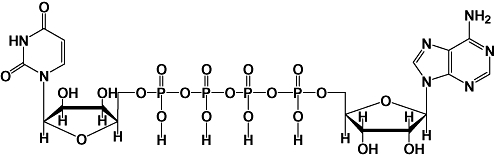
Molecular structure of uridine (5′)-adenosine (5′) tetraphosphate (Up4A).
Occurrence of dinucleoside polyphosphates
P1,P4 diadenosine tetraphosphate (Ap4A) was the first diadenosine polyphosphate to be identified in mammalian tissue (Rapaport and Zamecnik, 1976), was subsequently also identified in human platelets (Flodgaard and Klenow, 1982; Lüthje and Ogilvie, 1983). Following these early discoveries, numerous different nucleotides have been isolated from human tissues. For example, diadenosine polyphosphates (ApnA, with n= 2–7) were isolated from body fluids and cells (Pintor et al., 1992a; Schlüter et al., 1994; 1998; Hoyle et al., 1996; Jankowski et al., 1999; 2001a; 2003a;). Dinucleoside polyphosphates are released into the circulation from several cell types, including activated platelets (Flodgaard and Klenow, 1982; Lüthje and Ogilvie, 1983; Jankowski et al., 1999), chromaffin cells of the adrenal glands (Rodriguez del Castillo et al., 1988; Castillo et al., 1992; Pintor et al., 1991; 1992a;), tubular cells (Jankowski et al., 2007a; 2008;) or from synaptic vesicles (Zimmermann et al., 1993). ApnA are important neurotransmitter molecules in the nervous system (Delicado et al., 2006), and the importance of purines for neurotransmission in general was recently reviewed in an excellent manner by G Burnstock (Burnstock, 2007). In addition, ApnA stimulate different responses in the cardiovascular system, including control of vascular tone and prevention of platelet aggregation (Flores et al., 1999). Release of dinucleoside polyphosphates may result in local concentrations in the range of 10−5 mol·L−1 or even higher (Ogilvie, 1992). Diadenosine polyphosphates have a direct effect on the vascular tone (Busse et al., 1988; Schlüter et al., 1994; Ralevic et al., 1995; Hoyle et al., 1996; Inscho et al., 1998; van der Giet et al., 1998; Jankowski et al., 1999; Luo et al., 1999b; Gabriels et al., 2000; Lewis and Evans, 2000). In the vasculature of isolated perfused rat kidney, Ap5A and Ap6A were effective at a concentration of 10−9 mol·L−1, and contractions in aortic rings were elicited at 10−8 mol·L−1. Intra-aortic injection in the rat caused a prolonged increase in blood pressure (Schlüter et al., 1994). The vasoconstrictive action of Ap7A on the vasculature of the isolated perfused rat kidney Ap7A is lower than that of Ap6A. The threshold of the vasoconstrictive action of Ap7A is in the range of 10−5 mol·L−1 (Jankowski et al., 1999). Vasoconstriction induced by the diadenosine polyphosphates is mediated by an increase in intracellular free calcium ions, [Ca2+]i (Tepel et al., 1996; 1997;).
Recently, interest in uridine (5′)-adenosine (5′) tetraphosphate (Up4A) (Figure 1) has increased (Jankowski et al., 2005). Up4A was isolated from the supernatant of stimulated human endothelium and was identified by mass-spectrometry. Stimulation with adenosine 5′-triphosphate (ATP), uridine 5′-triphosphate (UTP), acetylcholine, endothelin, A23187 and mechanical stress releases Up4A from endothelium, suggesting that Up4A contributes to vascular regulation. Up4A plasma concentrations found in healthy subjects are high enough to cause vasoconstriction. Up4A is the first endogenous dinucleoside polyphosphate isolated from living organisms, which contains both purine and pyrimidine moieties. Several lines of evidence strongly suggest that Up4A has a functional role in the cardiovascular system, including its vasoactive effects (Figure 2), plasma concentrations and its release upon endothelial stimulation.
Figure 2.
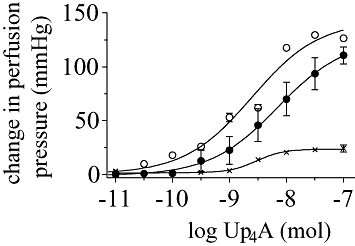
Change in perfusion pressure in the isolated perfused rat kidney induced by Up4A alone (•), with α,β-methylene ATP (×) and with L-NAME (○) [Figure adapted from Jankowski et al. (2005)].
Vasodilatory effects of dinucleoside polyphosphates
A small number of dinucleoside polyphosphates have vasodilatory effects, for example, Ap2A on the tone of isolated mesenteric arterial bed of rats (Ralevic and Burnstock, 1996). Ap3A and Ap4A both induce vasodilation upon perfusion of arteries containing endothelium, whereas Ap4A causes vasoconstriction in arteries from which the endothelium has been removed (Busse et al., 1988). Arterial infusion of Ap4A produced a dose-dependent decrease of systemic blood pressure and coronary vascular resistance (Nakae et al., 1996). These vasodilatory effects of dinucleoside polyphosphates are mediated via endothelial A2 receptors (Ralevic and Burnstock, 1996) and endothelial metabotropic P2Y receptors (Ralevic et al., 1995; Hilderman and Christensen, 1998; Rump et al., 1998; Gabriels et al., 2000; Malmsjo et al., 2000b). Stimulation of endothelial P2Y1, P2Y2 and P2Y4 receptors causes endothelium-derived hyperpolarizing factor- and NO-mediated dilatation (Malmsjo et al., 2000a,b; Mombouli and Vanhoutte, 1993; Vanhoutte, 1991). Ohata et al. reported that in aortic strips in situ[Ca2+]i waves within endothelial cells are induced by ATP via the P2Y1 purinoceptor, but not by the P2Y2 purinoceptor (Ohata et al., 1997).
In addition to endothelium-dependent dilation effects, Ap4A and Ap5A were also demonstrated to directly affect vascular smooth muscle cell (VSMC) dilation. Dilation induced by both dinucleoside polyphosphates was mediated by cAMP, which affects a decrease of [Ca2+]I, possibly through activation of K+ channels (Sumiyoshi et al., 1997). Direct, endothelium-independent vasodilation induced by purinoceptor agonists was also observed in intrapulmonary arteries isolated from newborn piglets and found to be mediated by a P2Y receptor on the VSMCs (McMillan et al., 1999).
The different functional effects of Ap4A (constriction in some smooth muscle and relaxation in others) may be explained by the action of a specific Ap4A hydrolase. This Ap4A hydrolase hydrolyses Ap4A in an asymmetric fashion to yield AMP, which is dephosphorylated (Walker and Hilderman, 1993) to yield adenosine, and ATP, which is converted to AMP and inorganic pyrophosphate (Lüthje and Ogilvie, 1985; 1988; Ogilvie et al., 1989; Hankin et al., 1995; Thorne et al., 1995). This enzyme is presumed to be involved in the regulation of the intracellular adenosine, AMP, ADP and ATP levels (Guranowski and Sillero, 1992). Thus, it is possible that the application of Ap4A may produce adenosine, AMP, ADP and ATP, leading to stimulation of multiple receptors such as nucleotide receptors and adenosine receptors. Conversely, there was no significant difference in the potency of Ap4A and Ap5A to induce relaxation of the guinea pig left atrium (Hoyle et al., 1996). Sumiyoshi et al. (1997) also observed almost equal potency for Ap4A- and Ap5A-induced coronary vasorelaxation. However, it is still possible that Ap4A might stimulate receptors in its intact form (i.e. without degradation) because it has been reported that ApnA are substance with long half-lives (Busse et al., 1988; Pohl et al., 1991; Hoyle et al., 1996). In agreement with this speculation, the specific and saturable membrane receptors for Ap4A have been reported to be present in brain, cardiac, liver, kidney, spleen and adipose tissue (Hilderman et al., 1991; Walker and Hilderman, 1993).
Proliferative effects of dinucleoside polyphosphates on VSMCs
Dinucleoside polyphosphates do not only directly influence the vascular physiology, but also increase the proliferation rate of VSMCs. Growth-stimulating effects of nucleoside polyphosphates have been demonstrated in numerous types of vascular beds (Erlinge, 1998; Jankowski et al., 2001b; Verspohl et al., 2004; Jankowski et al., 2007a,b;). The ATP-induced proliferation of VSMCs is coupled to a Gq-protein and triggers phosphoinositide hydrolysis with subsequent activation of protein kinase C, serine/threonine-kinase Raf-1 and mitogen-activated protein kinase (MAPK) (Yu et al., 1996; Wilden et al., 1998). Tu et al. observed that P2Y2 receptor stimulation involves the activation of Ras/Raf/MEK/MAPK pathway, which is modulated by [Ca2+]i, protein kinase C and tyrosine kinase (Tu et al., 2000). ATP-stimulated proliferation of coronary artery smooth muscle cells requires independent activation of both the extracellular signal-regulated kinase (ERK/MAPK) cascade and phosphatidylinositol 3-kinase (PI3K) signalling pathways (Wilden et al., 1998). P2Y2 receptor stimulation results in increased c-fos mRNA expression in cultured aortic smooth muscle cells and stimulates proliferation of vascular tissue (Malam-Souley et al., 1996). Ap4A is equipotent to ATP for induction of these effects (Erlinge et al., 1995).
Vascular smooth muscle cell proliferation and c-fos proto-oncogene expression are induced by Ap3A, Ap4A, Ap5A (Jankowski et al., 2001b), ApnG and GpnG (n= 3–6) (Schlüter et al., 1998) as well as Ap2A, Ap2G and Gp2G (Jankowski et al., 2001a). In vascular tissues, the proliferative effect of the diguanosine polyphosphates GpnG (with n= 3–6) is significantly stronger than that of ATP (Schlüter et al., 1998). Micromolar concentrations of Ap3A, Ap4A, Ap5A and Ap6A also stimulate growth in rat glomerular mesangial cells (Heidenreich et al., 1995; Schulze-Lohoff et al., 1995). Moreover, these diadenosine polyphosphates potentiate the growth response to platelet-derived growth factor, but not to insulin-like growth factor-1 (Heidenreich et al., 1995).
Effects of dinucleoside polyphosphates on platelet aggregation
The dinucleoside polyphosphates are potent antagonists of ADP-induced platelet aggregation (Jankowski et al., 1999). These inhibitory effects of dinucleoside polyphosphates on platelet aggregation are mediated by the P2Y1, the P2Y12 and the P2X1 receptors and appears to be via competitive inhibition of ADP and ATP, with Ap4A having a Ki of approximately 0.7 mmol·L−1 (Kunapuli and Daniel, 1998). Because plasma Ap4A concentrations are significantly lower than the Ki, a systemic impact is unlikely. However, the interaction may be caused by local effects as the concentrations close to platelets are significantly higher than plasma concentrations. A comparison of the homologous series of ApnA compounds with phosphate chain lengths from two to six revealed Ap5A as the most potent inhibitor of ADP-induced platelet aggregation, followed by Ap6A and Ap4A, which were more potent than Ap3A or Ap2A. Inhibition of platelet aggregation by dinucleoside polyphosphates is proposed to be due to direct competition between the dinucleoside polyphosphates and ADP at a specific receptor site on the platelet membrane (Harrison et al., 1975; Kunapuli, 1998). Dinucleoside polyphosphates inhibit release of ADP from blood platelets with a potency that decreases with decreasing chain length. Thus, dinucleoside polyphosphates in platelets may fulfil an anti-aggregatory role. In human neutrophils, Ap3A, Ap4A, Ap5A and Ap6A produce an increase in intracellular free calcium via a G-protein-coupled receptor (Pintor et al., 1997).
Release of dinucleoside polyphosphates
Up to millimolar range concentrations of dinucleoside polyphosphates are stored in secretory granules from platelets, in adrenal chromaffin cells and in central nervous synaptosomes (Flodgaard and Klenow, 1982; Lüthje and Ogilvie, 1983; Rodriguez del Castillo et al., 1988; Pintor et al., 1991). The intracellular concentration of diadenosine (5′, 5′) tetraphosphate (Ap4A) during normal growth (Garrison and Barne, 1992) correlates directly with the proliferative state of the cell or tissue (Rapaport and Zamecnik, 1976; Remy, 1992). Ap4A levels are known to respond to cellular stresses, such as oxidation and heat shock. Ap4A has been described as an alarmone that signals the onset of cellular and metabolic stress, although its precise role remains unclear (Brevet et al., 1985; Garrison et al., 1986; Remy, 1992).
Metabolism of dinucleoside polyphosphates
Subsequent inactivation of the released nucleotides is thought to be mainly regulated by vascular endothelial (Marcus et al., 2003) and lymphoid (Heptinstall et al., 2005) membrane-bound nucleoside triphosphate diphosphohydrolase (NTPDase; also known as ecto-ATPDase, CD39) and ecto-5′-nucleotidase (CD73). Ectohydrolases are present on a broad variety of cell types, including aortic endothelial cells (Mateo et al., 1997a,b;), chromaffin cells (Gasmi et al., 1998), rat mesangial cells, bovine corneal epithelial cells, the human hepatocellular liver carcinoma cell line (Hep-G2) and periodontal cells (von Drygalski and Ogilvie, 2000). The human diphosphorylated inositol phosphate phosphohydrolase was shown to be a candidate for regulating signalling of diadenosine polyphosphates by hydrolysis of Ap5A and Ap6A in preference to other diadenosine polyphosphates (Safrany et al., 1999). The enzymatic breakdown of dinucleoside polyphosphates leads to generation of mononucleotides and nucleotides that, in turn, are biologically active in vascular tissues. In contrast to these traditional paradigms that focus on nucleotide-inactivating mechanisms, it has now become clear that nucleotide-phosphorylating enzymes adenylate kinase and NDP kinase are also co-expressed on the cell surface and finely control the purinergic signalling cascade via two counter-balancing pathways, ATP-inactivating and ATP-regenerating respectively (Yegutkin et al., 2000; Yegutkin and Burnstock, 2000). The identification of a complex mixture of nucleotide pyrophosphatase/phosphodiesterase (NPP), NTPDase, adenylate kinase and other soluble purinergic enzymes freely circulating in the bloodstream adds another level of complexity to the understanding of the regulatory mechanisms of purine homeostasis within the vasculature (Birk et al., 2002; Yegutkin et al., 2003; 2007; 2008;). The agonistic effects of nucleotides are obviously mediated by complex mechanisms, including: (i) dinucleoside polyphosphate receptor pathway; (ii) inhibition of ecto-adenylate kinase activity; and (iii) generation of biologically active ATP and adenosine.
Table 1 gives a characteristic overview of endogenous dinucleoside polyphosphates that have been identified in human tissues and cells.
Table 1.
Isolations, identifications and characterizations of dinucleoside polyphosphates in human tissues and their receptor-mediated vascular effects
| Compound | Isolation from human tissue | Known vascular effects and receptor type involved (in brackets) | References |
|---|---|---|---|
| Ap2A | Myocardium Platelets Placenta Adrenal glands | Vasodilation (A2) and vasoconstriction (A1) in coronary arteries and renal vessels Proliferation of vascular smooth muscle cells (P2Y) | Hoyle et al. (1996); van der Giet et al. (1997a); Luo et al. (1999a); Jankowski et al. (2001a,b,c; 2003b;) |
| Ap2G | Platelets | Proliferation of vascular smooth muscle cells (P2Y) | Jankowski et al. (2001a) |
| Gp2G | Platelets | Proliferation of vascular smooth muscle cells (P2Y) | Jankowski et al. (2001a) |
| Ap3A | Myocardium Platelets Placenta Adrenal glands Plasma Proximal tubule Epithelial cells | Vasodilation (P2Y and A2) and vasoconstriction (A1 and P2X) in coronary arteries, renal vessels and mesenteric vessels Proliferation of vascular smooth muscle cells (P2Y) | Lüthje and Ogilvie (1983); Ogilvie and Jakob (1983); Schlüter et al. (1994); van der Giet et al. (1997a); Luo et al. (1999a); Jankowski et al. (2001a,b,c; 2003a,b; 2007a;) |
| Ap3G | Platelets | Vasoconstriction in renal vessels (P2X) Proliferation of vascular smooth muscle cells (P2Y) | Schlüter et al. (1998) |
| Gp3G | Platelets | Proliferation of vascular smooth muscle cells (P2Y) | Schlüter et al. (1998) |
| Ap4A | Adrenal gland Plasma Platelets Brain Proximal tubule epithelial cells | Vasoconstriction in renal vessels (P2X, A1) and mesenteric vessels (P2X) Proliferation of vascular smooth muscle cells (P2Y) | Ogilvie et al. (1989); Pintor et al. (1992a); van der Giet et al. (1997a; 1998;); Jankowski et al. (2001a,b; 2003a,b;) |
| Ap4G | Platelets | Vasoconstriction in renal vessels (P2X) Proliferation of vascular smooth muscle cells (P2Y) | Schlüter et al. (1998) |
| Gp4G | Platelets | Vasoconstriction of renal vessels (P2X) Proliferation of vascular smooth muscle cells (P2Y) | Schlüter et al. (1998) |
| Ap5A | Adrenal glands Plasma Platelets Placenta Brain Proximal tubule epithelial cells | Proliferation in vascular smooth muscle cells (P2Y) Vasoconstriction in renal vessels (P2X) and coronary arteries (P2X) Vasodilation in coronary arteries (P2Y) | Pintor et al. (1992a); Schlüter et al. (1994); van der Giet et al. (1997a; 1999; 2001; 2002;); Jovanovic et al. (1998); Jankowski et al. (2001a,b,c; 2003a,b; 2007a;); |
| Ap5G | Platelets | Vasoconstriction in renal vessels (P2X) Proliferation of vascular smooth muscle cells (P2Y) Vasoconstriction in renal vessels (P2X) and coronary arteries (P2X) Vasodilation in coronary arteries (P2Y) | Schlüter et al. (1998); van der Giet et al. (2001; 2002;) |
| Gp5G | Platelets | Proliferation of vascular smooth muscle cells (P2Y) | Schlüter et al. (1998); van der Giet et al. (2001; 2002;) |
| Ap6A | Adrenals Plasma Platelets Red blood cells Placenta Proximal tubule epithelial cells | Proliferation of vascular smooth muscle cells (P2Y) Vasoconstriction in renal vessels (P2X) and coronary arteries (P2X) Vasodilation in coronary arteries (P2Y) | Pintor et al. (1992b); Schlüter et al. (1994); van der Giet et al. (1997a; 1998; 2001; 2002;); Jankowski et al. (2001a,b.c; 2003a.b; 2007a) |
| Ap6G | Platelets | Vasoconstriction in renal vessels (P2X) and coronary vessels (P2X) Vasodilation in coronary arteries (P2Y) Proliferation of vascular smooth muscle cells (P2Y) | Schlüter et al. (1998); van der Giet et al. (2001; 2002;) |
| Gp6G | Platelets | Proliferation of vascular smooth muscle cells (P2Y) | Schlüter et al. (1998); van der Giet et al. (2001; 2002;) |
| Ap7A | Platelets | Vasoconstriction in renal vessels (P2X) | Jankowski et al. (1999) |
| Up4A | Endothelial cells Tubule cells | Vasoconstriction and proliferation | Jankowski et al. (2005; 2007b; 2008;); Gui et al. (2008) |
Purinoceptor system
The physiological and pathophysiological effects of mononucleosides, mononucleoside polyphosphates and dinucleoside polyphosphates are mediated via nucleotide-selective receptors. Essentially two major purine receptors subfamilies have been described based on pharmacological, functional and cloning data (Ralevic, 2000; Burnstock, 2002). P1 receptors are preferably activated by adenosine, while P2 receptors are activated by ATP, ADP, UTP and UDP and also by dinucleoside polyphosphates. Using molecular, biochemical and pharmacological criteria P1 receptors have been further subdivided into four subgroups: A1, A2A, A2B and A3 according to molecular, biochemical and pharmacological criteria. The group of P2 receptors is divided into P2X and P2Y (Figure 3) receptors according to their molecular structure and the signal transduction pathways they regulate. Selective agonists or antagonists discriminating adequately between families of P2X and P2Y receptors, or between subtypes of receptors within each of these groups have been discovered. Some selective antagonists include the P2X7 antagonist [3H]-2-cyano-1-[(1S)-1-phenylethyl]-3-quinolin-5-ylguanidine (Donnelly-Roberts et al., 2008), the P2Y1-selective antagonist N6-methyl 2′-deoxyadenosine 3′,5′-bisphosphate (Boyer et al., 1998) and the potent and relatively selective P2X1 antagonist diinosine pentaphosphate (Ip5I) (King et al., 1999). Purinoceptors are characterized by high plasticity, and they are dynamically regulated during development. The purinoceptor system plays a general role as a sympathetic regulator of vasomotor tone (Kennedy, 1996; Ralevic, 2000). The purinoceptor system that controls vascular homeostasis displays a high degree of complexity.
Figure 3.
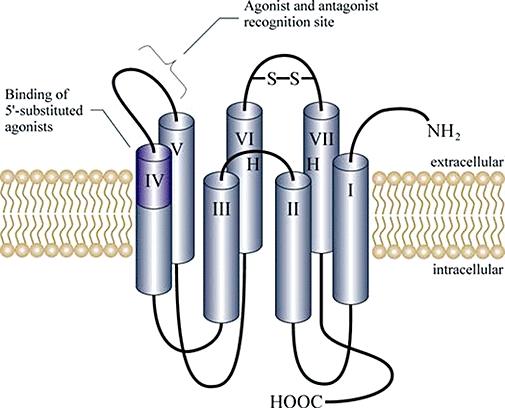
Structure of P2Y membrane receptors. P2Y are G-protein-coupled receptors (Fields and Burnstock, 2006), which act via a downstream signalling cascade including G-proteins and inositol triphosphate among other factors (Barnard and Simon, 2001).
P2X receptors mediating dinucleoside polyphosphate effects
P2X receptors are ligand-gated ion channels, which are opened by purinergic messengers (Bo et al., 2000), thus mediating rapid changes in the membrane permeability of monovalent and divalent cations (Bean, 1992; Dubyak and el-Moatassim, 1993; Burnstock, 2006; Erb et al., 2006). Stimulation of ionotropic P2X receptors induces an influx of Na+-ions and Ca2+-ions into the cytosol. The increase of the concentration of these ions triggers depolarization of the membrane potential, which opens potential operated Ca2+-channels (Usune et al., 1996). The resulting Ca2+-influx increases [Ca2+]i, which affects the constriction of the VSMCs (Tepel et al., 1997). There are seven P2X receptor cDNAs currently known, P2X1–P2X7 (Khakh et al., 2001). When expressed individually in heterologous systems, P2X1 and P2X3 subunits form channels activated by ATP or α,β-methylene ATP, whereas P2X2, P2X4 and P2X5 form channels activated by ATP but not α,β-methylene ATP (North and Surprenant, 2000). P2X1 and P2X3 receptors are characterized by strong and rapid desensitization, whereas desensitization of P2X2, P2X4 and P2X6 receptors is relatively modest (Ralevic and Burnstock, 1998). Therefore, P2X1-induced vasoconstrictions are transient, whereas P2X2- or P2X4-mediated vasoconstrictions are permanent as long as the agonist is present.
The ion-gating pore is formed by the aggregation of three P2X monomers (Nicke et al., 1998). Co-expression of P2X subtypes in heterologous expression systems can result in the formation of heteropolymeric P2X trimers (Nori et al., 1998). Therefore, it can be assumed that heteropolymeric channel formation is also possible in vivo. Heteropolymeric P2X receptors clearly differ from their homomeric relatives (Torres et al., 1998; Bianchi et al., 1999) and may therefore constitute an important mechanism for generating functional diversity of ATP- and dinucleoside polyphosphate-mediated responses. The P2X1 forms large, approximately elliptical clusters on the smooth muscle cells of mesenteric, renal and pulmonary arteries as well as in the aorta and in veins, which are restricted to the adventitial surface of the media. At the adventitial surface, the large clusters are immediately apposed to sympathetic varicosities. In the pulmonary artery, large receptor clusters were found throughout the media of the vessel. Smaller, spherical P2X1 clusters occur throughout the media of arteries of all sizes. The small P2X1 clusters are not associated with varicosities (Hansen et al., 1999). This observation may point to a paracrine role of purinergic agonists. P2X1, P2X2 and P2X4 are co-expressed in smooth muscle cells of coronary vessels as well as in peripheral vessels, including aorta, pulmonary artery, renal artery, femoral artery, internal and external iliac arteries. The co-expression of P2X receptor subtypes substantiates the possibility of a heteropolymeric assembly of the P2X ion channels. In contrast, no mRNA transcripts of P2X1, P2X2 or P2X4 were found in the superior mesenteric artery (Nori et al., 1998). A further aspect contributing to the complexity of the purinergic system is the existence of P2X splice variants. In the human bladder cells, Hardy et al. detected a P2X1 receptor splice variant, which lacks part of the second transmembrane domain. It was suggested that isoforms might be potential sites for modifying or regulating putative purinergic activation (Hardy et al., 2000). This view is supported by the observation of Chen et al., who published that ATP-induced currents in cells expressing P2X2/1 and P2X2/2 variants were large and desensitized rapidly, whereas the current in those cells expressing the P2X2/3 variant was much smaller and desensitized more slowly (Chen et al., 2000). Alternatively, spliced P2X4 RNAs in human smooth muscle cells were identified (Dhulipala et al., 1998).
The P2X4 receptor is the most prominent P2X receptor in human vascular endothelial cells from umbilical veins, aorta, pulmonary artery and skin microvessels. Vascular endothelial cells are continuously exposed to variations in blood flow, which plays an important role in vessel growth or regression and in the local development of atherosclerosis. The shear stress that occurs during changes in blood flow leads to substantial release of ATP and dinucleoside polyphosphates from endothelial cells (Burnstock, 1999; Jankowski et al., 2005), and these purines might mediate alterations in the balance between proliferation and apoptosis. This endothelium-dependent response to ATP is absent in atherosclerotic patients. Consequently, P2X4 mRNA expression was much higher in these cells than was the expression of other subtypes, including P2X1, P2X3, P2X5 and P2X7 (Yamamoto et al., 2000). P2X2 receptors are located on neurons and on endothelial cells in rat blood vessels (Hansen et al., 1999; Zemkova et al., 2004).
The calcium-permeable P2X1 receptor is considered the principal mediator of vasoconstriction (Kennedy, 1996), with P2X1 protein clusters on the adventitial surface of blood vessels immediately adjacent to sympathetic nerve varicosities (Hansen et al., 1999). However, P2X1 transcripts co-localize with P2X2, P2X4 and P2X5 mRNA in muscle cells of a number of blood vessels, which alludes the added presence of heteromeric P2X receptors (Lewis and Evans, 2000; 2001; Pulvirenti et al., 2000; Turner et al., 2003). For example, heteromeric P2X1/5 receptors have been implicated in vasoconstriction of submucosal arterioles in the guinea pig (Surprenant et al., 2000).
P2Y receptors mediating dinucleoside polyphosphate effects
P2Y receptors are 7-membrane-spanning proteins (King et al., 2000; Abbracchio et al., 2006; Burnstock, 2006; Erb et al., 2006). Some common mechanisms of signal transduction shared by most 7-membrane-spanning receptors include activation of phospholipase C and/or regulation of adenyl cyclase activity (Burnstock, 2006; Erb et al., 2006). P2Y receptors do not act directly by inducing a cation influx, but via a downstream signalling cascade including G-proteins and inositol triphosphate among other factors (Barnard and Simon, 2001).
P2X and G-protein-coupled P2Y receptors expressed in VSMCs were reported to mediate vasoconstriction (Inscho et al., 1998; Fukumitsu et al., 1999; Hillaire-Buys et al., 1999; McMillan et al., 1999; Shen et al., 2004). The vasoconstriction inducing P2Y receptor is probably coupled to PLC-β1 via Gαq/11 and to PLC-β3 via Gβγ3 (Murthy and Makhlouf, 1998a,b;). In human coronary arteries, extracellular nucleotides elicit constriction primarily by activation of P2X and P2Y2 receptors, whereas a role for P2Y1 and P2Y6 receptors was excluded (Malmsjo et al., 2000b). UTP- and ATP-induced vasoconstriction in intrapulmonary arteries is consistent with activation of the P2Y4 receptor subtype (McMillan et al., 1999), which is sensitive to Ap4A. Dinucleoside polyphosphate vasoconstriction is also mediated by the adenosine A1 receptor (Vahlensieck et al., 1996; van der Giet et al., 1997b; 1998; 1999; Gabriels et al., 2000). Because the vasoconstrictive effects of Ap2A and Ap3A, but not the vasoconstrictive effect of Ap5A, are mediated by A1 receptors (van der Giet et al., 1997b), vasoconstriction is likely due to a direct effect of intact dinucleoside polyphosphates on the A1 receptor rather than effects caused by dinucleoside polyphosphate metabolites.
Purinoceptor heteromerization
These principally different mechanisms led to the terminology metabotropic and ionotropic used for P2Y receptors and P2X receptors respectively. The purine receptor subtypes have been characterized according to their molecular structures and according to their pharmacological characteristics, both of which show considerable differences. Different purinoceptor subtypes may be expressed in the same cell type, and heteromeric receptors are formed among different purinoceptor subtypes (Torres et al., 1998; Le et al., 1999; Barnard and Simon, 2001). These purinoceptor heteromeres may show pharmacological properties quite different from those of purinoceptor homomeres (Torres et al., 1998). The ability to form heteromeres has been demonstrated both in P2X receptors (Le et al., 1999) and in P2Y receptors (Barnard and Simon, 2001) and contributes to the diversity of pharmacological properties manifested by the purinoceptor family.
Distribution of purinoceptor
Characterization of P2X and P2Y expression in vascular endothelium has been an especially active area of investigation in recent years. Different purine nucleotides activate endothelial cells in distinct ways, suggesting that at least two different endothelial purinoceptors exist. Pirotton et al. (1996) initially demonstrated and others have since confirmed (Bultmann et al., 1997) that both P2Y1 and P2Y2 receptors are co-expressed in endothelial cells from bovine aorta. P2Y receptors have also been identified in endothelial cells from rat cerebral vessels (Vigne et al., 2000; Mistry et al., 2003) and from mesenteric arteries (Malmsjo et al., 2000a,b;). These findings have been confirmed also in rat renal glomeruli (Harada et al., 2000). Currently, it remains unclear whether other purinoceptor subtypes are also expressed in endothelial cells. Undoubtedly, the endothelial P2Y1 receptor subtype mediates vasodilation (Bultmann et al., 1997; Malmsjo et al., 2000b; Vigne et al., 2000). In addition, the P2Y2 and P2Y4 receptor subtypes may also play a role in vasodilation (Malmsjo et al., 2000b).
Specific dinucleoside polyphosphate receptors
Dinucleoside polyphosphates have the capacity to potentiate signalling effects via P2 receptors (primarily, via P2X1, P2X3 and P2Y1 subtypes), although the existence of specific dinucleoside polyphosphate receptors has also been proposed (Flores et al., 1999; Delicado et al., 2006). However, the specificity of dinucleoside polyphosphate receptors and their implication into multiple extracellular signals are still poorly understood, primarily due to the complexity of the purinergic signalling cascade. The effects of the dinucleoside polyphosphates are blocked by desensitization of P2X receptors with α,β-methylene ATP subtype (Bo et al., 1998; Kunapuli and Daniel, 1998; Wang et al., 2002) or blockade by diinosine pentaphosphates (Ip5I) (Hoyle et al., 1997).
Uridine adenosine tetraphosphate (Up4A)
Physiological and pathophysiological effects of Up4A
Mean total plasma Up4A concentrations are significantly increased in juvenile hypertensives compared with juvenile normotensive subjects (Jankowski et al., 2007b). Accordingly, Up4A shows a significant association with juvenile hypertension. The plasma Up4A concentrations correlate with left ventricular mass and intima media wall thickness in hypertensive subjects. The increased intima media thickness may be related to proliferative effects of Up4A, as Up4A has been demonstrated to increase human VSMC proliferation. Up4A is obviously an important risk factor of juvenile hypertension. Furthermore, Up4A was identified in renal tissue (Jankowski et al., 2008). Stimulation of tubule cells with oleoyl-2-acetyl-sn-glycerol (OAG) increases the release rate of Up4A from tubule cells about 10-fold. Up4A acts as a strong vasoconstrictive mediator on afferent arterioles, but has no significant effect on the tone of efferent arterioles, suggesting a functional role of Up4A as an autocrine hormone for glomerular perfusion. Because of the predominant effect of the Up4A on afferent arterioles, Up4A may decrease glomerular perfusion, intra-glomerular pressure and, hence, glomerular filtration rate. The release of Up4A from renal tubular cells may be an additional mechanism whereby tubular cells could affect renal perfusion. Up4A release may further contribute to renal vascular autoregulation mechanisms. Up4A obviously plays a role in renal haemodynamics and blood pressure regulation.
Moreover, Up4A stimulated contraction of isolated rat pulmonary arteries in a concentration-dependent manner (Gui et al., 2008). Up4A is potent as UTP and UDP in arteries without endothelium, while much more effective than UTP and UDP in preparations with endothelium. The vasoconstrictor effect of Up4A is inhibited by suramin, but not by P1,P5 diinosine pentaphosphate (Ip5I) or desensitization of P2X receptors with α,β-methylene-ATP. Up4A-induced contraction is inhibited by pretreatment with thapsigargin, nitrendipine or EGTA, but unaffected by the specific Rho-kinase inhibitor (S)-(+)-2-methyl-1-[(4-methyl-5-iso-quinolinyl) sulfonyl]-homopiperazine (H-1152) (Ikenoya et al., 2002). Furthermore, unlike ATP and UTP, Up4A does not induce relaxation of preparations with endothelium precontracted with phenylephrine. Up4A is obviously a potent vasoconstrictor, but not a vasodilator, of the rat pulmonary artery. It is likely that Up4A acts through a suramin-sensitive P2Y receptor. The contractile effect of Up4A involves the entry of extracellular Ca2+ and release of Ca2+ from intracellular stores, but not Ca2+ sensitization via the RhoA/Rho-kinase pathway. Up4A, therefore, may be important for regulation of pulmonary vascular tone.
Plasma concentrations of dinucleoside polyphosphates
Physiologically relevant concentrations of dinucleoside polyphosphates are present in human plasma. The mean total plasma diadenosine polyphosphate concentrations (µmol·L−1; mean ± SEM) in cubital veins of normotensive subjects are 0.89 ± 0.59 for Ap3A, 0.72 ± 0.72 for Ap4A, 0.33 ± 0.24 for Ap5A and 0.18 ± 0.18 for Ap6A (Jankowski et al., 2003a). In adrenal venous plasma, significantly higher diadenosine polyphosphate concentrations are detectable than in plasma from the infrarenal and suprarenal vena cava. Mean total plasma Up4A concentrations are in the range of 55.5 ± 15.2 nmol·L−1 (Jankowski et al., 2005). Adrenal medulla (Jankowski et al., 2003a) and endothelial cells (Jankowski et al., 2005) are obviously a source of plasma dinucleoside polyphosphates in humans.
Therapeutic aspects of the purinergic system
There have been promising developments concerning purinergic anti-thrombotic drugs (Cattaneo, 2006; Gachet, 2006; Gachet et al., 2006). Platelets are known to express P2Y1, P2Y12 and P2X1 receptors (Hollopeter et al., 2001). Clinical trials like CURE (Yusuf et al., 2001) and CREDO (Beinart et al., 2005) have provided clear evidence that the purinergic anti-thrombotic drugs clopidogrel and ticlopidine reduce the risks of recurrent strokes and heart attacks, especially when combined with aspirin (Kam and Nethery, 2003; Kunapuli et al., 2003).
Moreover, dinucleoside polyphosphates have been shown to possess beneficial properties in the treatment of various diseases, such as chronic obstructive pulmonary disease. Dinucleoside polyphosphates facilitate the clearance of mucous secretions from the lungs of mammals, including humans, being treated for diseases, such as cystic fibrosis (Picher and Boucher, 2000) and chronic bronchitis (Picher and Boucher, 2000). Furthermore, properties of diadenosine polyphosphates may serve to help in the treatment of some ocular pathologies like dry eye (Yerxa et al., 2002; Guzman-Aranguez et al., 2007) and retinal detachment (Guzman-Aranguez et al., 2007). Recent findings showing increased dinucleoside polyphosphate concentration in hypertensive patients (Jankowski et al., 2007b) may provide novel therapeutic approaches for hypertension in the future.
Patented therapeutic effects of dinucleoside polyphosphates
Some potential therapeutic effects of dinucleoside polyphosphates are protected by international patents. For example, Stutts et al. claim the rights to the therapeutic effects of dinucleoside polyphosphates in the context of asthma, bronchiectasis, post-operative mucous retention, pneumonia and primary ciliary dyskinesia (Stutts et al., 1995). Moreover, prevention and treatment of pneumonia in immobilized patients using dinucleoside polyphosphates are protected by Jacobus and Leighton (1996). Further patents claim treatment of sinusitis (Jacobus et al., 1998a,b; Jacobus et al., 1996), otitis media (Drutz et al., 1996) and nasolacrimal duct obstruction (Yerxa and Brown, 2003) with dinucleoside polyphosphates.
Acknowledgments
This review was supported by a grant from the German Research Foundation (DFG, Ja-972 /11-1; JJ), Federal Ministry of Education and Research (BMBF; 01GR0807); JJ), by a Rahel-Hirsch-scholarship from the Charité (VJ) and by the Sonnenfeld Foundation (JJ and VJ).
Glossary
Abbreviations:
- ERK
extracellular signal-regulated kinase
- H-1152
(S)-(+)-2-methyl-1-[(4-methyl-5-iso-quinolinyl) sulfonyl]-homopiperazine: Rho-kinase inhibitor
- Hep-G2
human hepatocellular liver carcinoma cell line
- MAPK
mitogen-activated protein kinase
- OAG
oleoyl-2-acetyl-sn-glycerol
- PI3K
phosphatidylinositol 3-kinase
- Raf-1
serine/threonine-specific kinase (EC 2.7.11.1)
- MAP
kinase kinase kinase (MAP3K)
- VSMC
vascular smooth muscle cell
- XpnX
dinucleoside polyphosphates [with X = adenosine, guanosine, inosine or uridine; n= number of phosphate groups (p)]
Conflicts of interest
None.
References
- Abbracchio MP, Burnstock G, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, et al. International Union of Pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol Rev. 2006;58:281–341. doi: 10.1124/pr.58.3.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnard EA, Simon J. An elusive receptor is finally caught: P2Y(12′), an important drug target in platelets. Trends Pharmacol Sci. 2001;22:388–391. doi: 10.1016/s0165-6147(00)01759-4. [DOI] [PubMed] [Google Scholar]
- Bean BP. Pharmacology and electrophysiology of ATP-activated ion channels. Trends Pharmacol Sci. 1992;13:87–90. doi: 10.1016/0165-6147(92)90032-2. [DOI] [PubMed] [Google Scholar]
- Beinart SC, Kolm P, Veledar E, Zhang Z, Mahoney EM, Bouin O, et al. Long-term cost effectiveness of early and sustained dual oral antiplatelet therapy with clopidogrel given for up to one year after percutaneous coronary intervention results: from the Clopidogrel for the Reduction of Events During Observation (CREDO) trial. J Am Coll Cardiol. 2005;46:761–769. doi: 10.1016/j.jacc.2005.03.073. [DOI] [PubMed] [Google Scholar]
- Bianchi BR, Lynch KJ, Touma E, Niforatos W, Burgard EC, Alexander KM, et al. Pharmacological characterization of recombinant human and rat P2X receptor subtypes. Eur J Pharmacol. 1999;376:127–138. doi: 10.1016/s0014-2999(99)00350-7. [DOI] [PubMed] [Google Scholar]
- Birk AV, Bubman D, Broekman MJ, Robertson HD, Drosopoulos JH, Marcus AJ, et al. Role of a novel soluble nucleotide phospho-hydrolase from sheep plasma in inhibition of platelet reactivity: hemostasis, thrombosis, and vascular biology. J Lab Clin Med. 2002;139:116–124. doi: 10.1067/mlc.2002.121334. [DOI] [PubMed] [Google Scholar]
- Bo X, Sexton A, Xiang Z, Nori SL, Burnstock G. Pharmacological and histochemical evidence for P2X receptors in human umbilical vessels. Eur J Pharmacol. 1998;353:59–65. doi: 10.1016/s0014-2999(98)00383-5. [DOI] [PubMed] [Google Scholar]
- Bo X, Schoepfer R, Burnstock G. Molecular cloning and characterization of a novel ATP P2X receptor subtype from embryonic chick skeletal muscle. J Biol Chem. 2000;275:14401–14407. doi: 10.1074/jbc.275.19.14401. [DOI] [PubMed] [Google Scholar]
- Boyer JL, Mohanram A, Camaioni E, Jacobson KA, Harden TK. Competitive and selective antagonism of P2Y1 receptors by N6-methyl 2′-deoxyadenosine 3′,5′-bisphosphate. Br J Pharmacol. 1998;124:1–3. doi: 10.1038/sj.bjp.0701837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brevet A, Plateau P, Best-Belpomme M, Blanquet S. Variation of Ap4A and other dinucleoside polyphosphates in stressed Drosophila cells. J Biol Chem. 1985;260:15566–15570. [PubMed] [Google Scholar]
- Bultmann R, Hansmann G, Tuluc F, Starke K. Vasoconstrictor and vasodilator effects of guanine nucleotides in the rat aorta. Naunyn Schmiedebergs Arch Pharmacol. 1997;356:653–661. doi: 10.1007/pl00005102. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Release of vasoactive substances from endothelial cells by shear stress and purinergic mechanosensory transduction. J Anat. 1999;194:335–342. doi: 10.1046/j.1469-7580.1999.19430335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. Purinergic signaling and vascular cell proliferation and death. Arterioscler Thromb Vasc Biol. 2002;22:364–373. doi: 10.1161/hq0302.105360. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Purinergic signalling. Br J Pharmacol. 2006;147:S172–S181. doi: 10.1038/sj.bjp.0706429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev. 2007;87:659–797. doi: 10.1152/physrev.00043.2006. [DOI] [PubMed] [Google Scholar]
- Busse R, Ogilvie A, Pohl U. Vasomotor activity of diadenosine triphosphate and diadenosine tetraphosphate in isolated arteries. Am J Physiol. 1988;254(5):H828–H832. doi: 10.1152/ajpheart.1988.254.5.H828. Pt 2. [DOI] [PubMed] [Google Scholar]
- Castillo CJ, Moro MA, Del Valle M, Sillero A, Garcia AG, Sillero MA. Diadenosine tetraphosphate is co-released with ATP and catecholamines from bovine adrenal medulla. J Neurochem. 1992;59:723–732. doi: 10.1111/j.1471-4159.1992.tb09428.x. [DOI] [PubMed] [Google Scholar]
- Cattaneo M. ADP receptors: inhibitory strategies for antiplatelet therapy. Drug News Perspect. 2006;19:253–259. doi: 10.1358/dnp.2006.19.5.985936. [DOI] [PubMed] [Google Scholar]
- Cheewatrakoolpong B, Gilchrest H, Anthes JC, Greenfeder S. Identification and characterization of splice variants of the human P2X7 ATP channel. Biochem Biophys Res Commun. 2005;332:17–27. doi: 10.1016/j.bbrc.2005.04.087. [DOI] [PubMed] [Google Scholar]
- Chen C, Parker MS, Barnes AP, Deininger P, Bobbin RP. Functional expression of three P2X(2) receptor splice variants from guinea pig cochlea. J Neurophysiol. 2000;83:1502–1509. doi: 10.1152/jn.2000.83.3.1502. [DOI] [PubMed] [Google Scholar]
- Delicado EG, Miras-Portugal MT, Carrasquero LM, Leon D, Perez-Sen R, Gualix J. Dinucleoside polyphosphates and their interaction with other nucleotide signaling pathways. Pflugers Arch. 2006;452:563–572. doi: 10.1007/s00424-006-0066-5. [DOI] [PubMed] [Google Scholar]
- Dhulipala PD, Wang YX, Kotlikoff MI. The human P2X4 receptor gene is alternatively spliced. Gene. 1998;207:259–266. doi: 10.1016/s0378-1119(97)00647-1. [DOI] [PubMed] [Google Scholar]
- Donnelly-Roberts DL, Namovic MT, Surber B, Vaidyanathan SX, Perez-Medrano A, Wang Y, et al. [(3)H]A-804598 ([(3)H]2-cyano-1-[(1S)-1-phenylethyl]-3-quinolin-5-ylguanidine) is a novel, potent, and selective antagonist radioligand for P2X7 receptors. Neuropharmacology. 2008;56:223–229. doi: 10.1016/j.neuropharm.2008.06.012. [DOI] [PubMed] [Google Scholar]
- Drury AN, Szent-Györgyi A. The physiological activity of adenine com-pounds with especial reference to their action upon the mammalian heart. J Physiol. 1929;68:213–237. doi: 10.1113/jphysiol.1929.sp002608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drutz D, Rideout J, Jacobus K. Method of treating otitis media with uridine triphosphates and related compounds. USA: US-Pat. 6,423,694, Inspire Pharmaceuticals, Inc.
- von Drygalski A, Ogilvie A. Ecto-diadenosine 5′,5‴-P1,P4-tetraphosphate (Ap4A)-hydrolase is expressed as an ectoenzyme in a variety of mammalian and human cells and adds new aspects to the turnover of Ap4A. Biofactors. 2000;11:179–187. doi: 10.1002/biof.5520110304. [DOI] [PubMed] [Google Scholar]
- Dubyak GR, el-Moatassim C. Signal transduction via P2-purinergic receptors for extracellular ATP and other nucleotides. Am J Physiol. 1993;265:C577–C606. doi: 10.1152/ajpcell.1993.265.3.C577. [DOI] [PubMed] [Google Scholar]
- Erb L, Liao Z, Seye CI, Weisman GA. P2 receptors: intracellular signaling. Pflugers Arch. 2006;452:552–562. doi: 10.1007/s00424-006-0069-2. [DOI] [PubMed] [Google Scholar]
- Erlinge D. Extracellular ATP: a growth factor for vascular smooth muscle cells. Gen Pharmacol. 1998;31:1–8. doi: 10.1016/s0306-3623(97)00420-5. [DOI] [PubMed] [Google Scholar]
- Erlinge D, You J, Wahlestedt C, Edvinsson L. Characterisation of an ATP receptor mediating mitogenesis in vascular smooth muscle cells. Eur J Pharmacol. 1995;289:135–149. doi: 10.1016/0922-4106(95)90178-7. [DOI] [PubMed] [Google Scholar]
- Fields RD, Burnstock G. Purinergic signalling in neuron-glia interactions. Nat Rev Neurosci. 2006;7:423–436. doi: 10.1038/nrn1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flodgaard H, Klenow H. Abundant amounts of diadenosine 5′,5‴-P1,P4-tetraphosphate are present and releasable, but metabolically inactive, in human platelets. Biochem J. 1982;208:737–742. doi: 10.1042/bj2080737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores NA, Stavrou BM, Sheridan DJ. The effects of diadenosine polyphosphates on the cardiovascular system. Cardiovasc Res. 1999;42:15–26. doi: 10.1016/s0008-6363(99)00004-8. [DOI] [PubMed] [Google Scholar]
- Fukumitsu A, Takano Y, Iki A, Honda K, Saito R, Katsuragi T, et al. Endogenous ATP released by electrical field stimulation causes contraction via P2x- and P2y-purinoceptors in the isolated tail artery of rats. Jpn J Pharmacol. 1999;81:375–380. doi: 10.1254/jjp.81.375. [DOI] [PubMed] [Google Scholar]
- Gabriels G, Endlich K, Rahn KH, Schlatter E, Steinhausen M. In vivo effects of diadenosine polyphosphates on rat renal microcirculation. Kidney Int. 2000;57:2476–2484. doi: 10.1046/j.1523-1755.2000.00106.x. [DOI] [PubMed] [Google Scholar]
- Gabriels G, Rahn KH, Schlatter E, Steinmetz M. Mesenteric and renal vascular effects of diadenosine polyphosphates (ApnA) Cardiovasc Res. 2002;56:22–32. doi: 10.1016/s0008-6363(02)00533-3. [DOI] [PubMed] [Google Scholar]
- Gachet C. Regulation of platelet functions by P2 receptors. Annu Rev Pharmacol Toxicol. 2006;46:277–300. doi: 10.1146/annurev.pharmtox.46.120604.141207. [DOI] [PubMed] [Google Scholar]
- Gachet C, Leon C, Hechler B. The platelet P2 receptors in arterial thrombosis. Blood Cells Mol Dis. 2006;36:223–227. doi: 10.1016/j.bcmd.2005.12.024. [DOI] [PubMed] [Google Scholar]
- Garrison PN, Barnes LD. Determination of dinucleoside polyphosphates. In: McLennan AG, editor. AP4A and Other Dinucleoside Polyphosphates. Boca Raton, FL: CRC Press; 1992. pp. 29–61. [Google Scholar]
- Garrison PN, Mathis SA, Barnes LD. In vivo levels of diadenosine tetraphosphate and adenosine tetraphospho-guanosine in Physarum polycephalum during the cell cycle and oxidative stress. Mol Cell Biol. 1986;6:1179–1186. doi: 10.1128/mcb.6.4.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasmi L, Cartwright JL, McLennan AG. The hydrolytic activity of bovine adrenal medullary plasma membranes towards diadenosine polyphosphates is due to alkaline phosphodiesterase-I. Biochim Biophys Acta. 1998;1405:121–127. doi: 10.1016/s0167-4889(98)00097-4. [DOI] [PubMed] [Google Scholar]
- van der Giet M, Khattab M, Borgel J, Schlüter H, Zidek W. Differential effects of diadenosine phosphates on purinoceptors in the rat isolated perfused kidney. Br J Pharmacol. 1997a;120:1453–1460. doi: 10.1038/sj.bjp.0701074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Giet M, Khattab M, Börgel J, Schlüter H, Zidek W. Differential effects of diadenosine phosphates on purinoceptors in the rat isolated perfused kidney. Br J Pharmacol. 1997b;120:1453–1460. doi: 10.1038/sj.bjp.0701074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Giet M, Jankowski J, Schlüter H, Zidek W, Tepel M. Mediation of the vasoactive properties of diadenosine tetraphosphate via various purinoceptors. J Hypertens. 1998;16:1939–1943. doi: 10.1097/00004872-199816121-00013. [DOI] [PubMed] [Google Scholar]
- van der Giet M, Cinkilic O, Jankowski J, Tepel M, Zidek W, Schlüter H. Evidence for two different P2X-receptors mediating vasoconstriction of Ap5A and Ap6A in the isolated perfused rat kidney. Br J Pharmacol. 1999;127:1463–1469. doi: 10.1038/sj.bjp.0702667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Giet M, Westhoff T, Cinkilic O, Jankowski J, Schlüter H, Zidek W, et al. The critical role of adenosine and guanosine in the affinity of dinucleoside polyphosphates to P2X-receptors in the isolated perfused rat kidney. Br J Pharmacol. 2001;132:467–474. doi: 10.1038/sj.bjp.0703817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Giet M, Schmidt S, Tölle M, Jankowski J, Schlüter H, Zidek W, et al. Effects of dinucleoside polyphosphates on regulation of coronary vascular tone. Eur J Pharmacol. 2002;448:207–213. doi: 10.1016/s0014-2999(02)01986-6. [DOI] [PubMed] [Google Scholar]
- Gui Y, Walsh MP, Jankowski V, Jankowski J, Zheng XL. Up4A stimulates endothelium-independent contraction of isolated rat pulmonary artery. Am J Physiol Lung Cell Mol Physiol. 2008;194:L733–L738. doi: 10.1152/ajplung.00403.2007. [DOI] [PubMed] [Google Scholar]
- Guranowski A, Sillero A. Enzymes Cleaving Dinucleoside Polyphosphates. Boca Raton, FL: CRC Press; 1992. [Google Scholar]
- Guzman-Aranguez A, Crooke A, Peral A, Hoyle CH, Pintor J. Dinucleoside polyphosphates in the eye: from physiology to therapeutics. Prog Retin Eye Res. 2007;26:674–687. doi: 10.1016/j.preteyeres.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Hankin S, Matthew N, Thorne H, McLennan AG. Diadenosine 5′,5‴-P1,P4-tetraphosphate hydrolase is present in human erythrocytes, leukocytes and platelets. Int J Biochem Cell Biol. 1995;27:201–206. doi: 10.1016/1357-2725(94)00076-n. [DOI] [PubMed] [Google Scholar]
- Hansen MA, Dutton JL, Balcar VJ, Barden JA, Bennett MR. P2X (purinergic) receptor distributions in rat blood vessels. J Auton Nerv Syst. 1999;75:147–155. doi: 10.1016/s0165-1838(98)00189-1. [DOI] [PubMed] [Google Scholar]
- Harada H, Chan CM, Loesch A, Unwin R, Burnstock G. Induction of proliferation and apoptotic cell death via P2Y and P2X receptors, respectively, in rat glomerular mesangial cells. Kidney Int. 2000;57:949–958. doi: 10.1046/j.1523-1755.2000.00911.x. [DOI] [PubMed] [Google Scholar]
- Hardy LA, Harvey IJ, Chambers P, Gillespie JI. A putative alternatively spliced variant of the P2X(1) purinoreceptor in human bladder. Exp Physiol. 2000;85:461–463. [PubMed] [Google Scholar]
- Harrison MJ, Brossmer R, Goody RS. Inhibition of platelet aggregation and the platelet release reaction by alpha, omega diadenosine polyphosphates. FEBS Lett. 1975;54:57–60. doi: 10.1016/0014-5793(75)81067-2. [DOI] [PubMed] [Google Scholar]
- Heidenreich S, Tepel M, Schlüter H, Harrach B, Zidek W. Regulation of rat mesangial cell growth by diadenosine phosphates. J Clin Invest. 1995;95:2862–2867. doi: 10.1172/JCI117992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heptinstall S, Johnson A, Glenn JR, White AE. Adenine nucleotide metabolism in human blood – important roles for leukocytes and erythrocytes. J Thromb Haemost. 2005;3:2331–2339. doi: 10.1111/j.1538-7836.2005.01489.x. [DOI] [PubMed] [Google Scholar]
- Hilderman RH, Christensen EF. P1,P4-diadenosine 5′ tetraphosphate induces nitric oxide release from bovine aortic endothelial cells. FEBS Lett. 1998;427:320–324. doi: 10.1016/s0014-5793(98)00454-2. [DOI] [PubMed] [Google Scholar]
- Hilderman RH, Martin M, Zimmerman JK, Pivorun EB. Identification of a unique membrane receptor for adenosine 5′,5‴-P1,P4- tetraphosphate. J Biol Chem. 1991;266:6915–6918. [PubMed] [Google Scholar]
- Hillaire-Buys D, Dietz S, Chapal J, Petit P, Loubatieres-Mariani MM. Involvement of P2X and P2U receptors in the constrictor effect of ATP on the pancreatic vascular bed. J Soc Biol. 1999;193:57–61. [PubMed] [Google Scholar]
- Hollopeter G, Jantzen HM, Vincent D, Li G, England L, Ramakrishnan V, et al. Identification of the platelet ADP receptor targeted by antithrombotic drugs. Nature. 2001;409:202–207. doi: 10.1038/35051599. [DOI] [PubMed] [Google Scholar]
- Hoyle CH, Ziganshin AU, Pintor J, Burnstock G. The activation of P1- and P2-purinoceptors in the guinea-pig left atrium by diadenosine polyphosphates. Br J Pharmacol. 1996;118:1294–1300. doi: 10.1111/j.1476-5381.1996.tb15536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyle CH, Pintor J, Gualix J, Miras-Portugal MT. Antagonism of P2X receptors in guinea-pig vas deferens by diinosine pentaphosphate. Eur J Pharmacol. 1997;333:R1–R2. doi: 10.1016/s0014-2999(97)01129-1. [DOI] [PubMed] [Google Scholar]
- Ikenoya M, Hidaka H, Hosoya T, Suzuki M, Yamamoto N, Sasaki Y. Inhibition of rho-kinase-induced myristoylated alanine-rich C kinase substrate (MARCKS) phosphorylation in human neuronal cells by H-1152, a novel and specific Rho-kinase inhibitor. J Neurochem. 2002;81:9–16. doi: 10.1046/j.1471-4159.2002.00801.x. [DOI] [PubMed] [Google Scholar]
- Inscho EW, Cook AK, Mui V, Miller J. Direct assessment of renal microvascular responses to P2-purinoceptor agonists. Am J Physiol. 1998;274:F718–F727. doi: 10.1152/ajprenal.1998.274.4.F718. [DOI] [PubMed] [Google Scholar]
- Jacobus K, Rideout J, Yerxa B, Pendergast W, Siddiqi S, Drutz D. Method of treating sinusitis with uridine triphosphates and related compounds. USA: US-Pat. 5,789,391, Inspire Pharmaceuticals, Inc.
- Jacobus K, Rideout J, Yerxa B, Pendergast W, Siddiqi S, Drutz D. Method for treating sinusitis with uridine triphosphates and related compounds. USA: US-Pat. 5,981,506, Inspire Pharmaceuticals, Inc.
- Jacobus K, Rideout J, Yerxa B, Pendergast W, Siddiqi S, Drutz D. Method of treating sinusitis with uridine triphosphates and related compounds. USA: US-Pat. 5,958,897, Inspire Pharmaceuticals.
- Jacobus KM, Leighton HJ. Method of preventing or treating pneumonia in immobilized patients with uridine triphosphates and related compounds. USA: US-Pat. 5,763,447, Inspire Pharmaceuticals (Durham, NC.
- Jankowski J, Tepel M, van der Giet M, Tente IM, Henning L, Junker R, et al. Identification and characterization of P1, P7-diadenosine-5′-heptaphosphate from human platelets. J Biol Chem. 1999;274:23926–23931. doi: 10.1074/jbc.274.34.23926. [DOI] [PubMed] [Google Scholar]
- Jankowski J, Hagemann J, Tepel M, van der Giet M, Stephan N, Henning L, et al. Dinucleotides as growth promoting extracellulary mediators: presence of dinucleoside diphosphates Ap2A, Ap2G and Gp2G in releasable granlues of platelets. J Biol Chem. 2001a;276:8904–8909. doi: 10.1074/jbc.M009527200. [DOI] [PubMed] [Google Scholar]
- Jankowski J, Hagemann J, Yoon MS, van der Giet M, Stephan N, Zidek W, et al. Increased vascular growth in hemodialysis patients induced by platelet-derived diadenosine polyphosphates. Kidney Int. 2001b;59:1134–1141. doi: 10.1046/j.1523-1755.2001.0590031134.x. [DOI] [PubMed] [Google Scholar]
- Jankowski J, Yoon MS, Stephan N, Zidek W, Schlüter H. Vasoactive diadenosine polyphosphates in human placenta: possible candidates in the pathophysiology of pre-eclampsia? J Hypertens. 2001c;19:567–573. doi: 10.1097/00004872-200103001-00008. [DOI] [PubMed] [Google Scholar]
- Jankowski J, Jankowski V, Laufer U, van der Giet M, Henning L, Tepel M, et al. Identification and quantification of diadenosine polyphosphate concentrations in human plasma. Arterioscler Thromb Vasc Biol. 2003a;23:1231–1238. doi: 10.1161/01.ATV.0000075913.00428.FD. [DOI] [PubMed] [Google Scholar]
- Jankowski J, Jankowski V, Seibt B, Henning L, Zidek W, Schlüter H. Identification of dinucleoside polyphosphates in adrenal glands. Biochem Biophys Res Commun. 2003b;304:365–370. doi: 10.1016/s0006-291x(03)00596-5. [DOI] [PubMed] [Google Scholar]
- Jankowski V, Tölle M, Vanholder R, Schönfelder G, van der Giet M, Henning L, et al. Identification of uridine adenosine tetraphosphate (Up4A) as an endothelium-derived vasoconstrictive factor. Nat Med. 2005;11:223–227. doi: 10.1038/nm1188. [DOI] [PubMed] [Google Scholar]
- Jankowski V, Karadogan S, Vanholder R, Nofer JR, Herget-Rosenthal S, van der Giet M, et al. Paracrine stimulation of vascular smooth muscle proliferation by diadenosine polyphosphates released from proximal tubule epithelial cells. Kidney Int. 2007a;71:994–1000. doi: 10.1038/sj.ki.5002186. [DOI] [PubMed] [Google Scholar]
- Jankowski V, Meyer AA, Schlattmann P, Gui Y, Zheng XL, Stamcou I, et al. Increased uridine adenosine tetraphosphate concentrations in plasma of juvenile hypertensives. Arterioscler Thromb Vasc Biol. 2007b;27:1776–1781. doi: 10.1161/ATVBAHA.107.143958. [DOI] [PubMed] [Google Scholar]
- Jankowski V, Patzak A, Herget-Rosenthal S, Tran TN, Lai EY, Gunthner T, et al. Uridine adenosine tetraphosphate acts as an autocrine hormone affecting glomerular filtration rate. J Mol Med. 2008;83:333–340. doi: 10.1007/s00109-008-0306-6. [DOI] [PubMed] [Google Scholar]
- Jovanovic A, Jovanovic S, Mays DC, Lipsky JJ, Terzic A. Diadenosine 5′,5″-P1,P5-pentaphosphate harbors the properties of a signaling molecule in the heart. FEBS Lett. 1998;423:314–318. doi: 10.1016/s0014-5793(98)00114-8. [DOI] [PubMed] [Google Scholar]
- Kam PC, Nethery CM. The thienopyridine derivatives (platelet adenosine diphosphate receptor antagonists), pharmacology and clinical developments. Anaesthesia. 2003;58:28–35. doi: 10.1046/j.1365-2044.2003.02960.x. [DOI] [PubMed] [Google Scholar]
- Kennedy C. ATP as a cotransmitter in perivascular sympathetic nerves. J Auton Pharmacol. 1996;16:337–340. doi: 10.1111/j.1474-8673.1996.tb00048.x. [DOI] [PubMed] [Google Scholar]
- Khakh BS, Burnstock G, Kennedy C, King BF, North RA, Seguela P, et al. International union of pharmacology. XXIV. Current status of the nomenclature and properties of P2X receptors and their subunits. Pharmacol Rev. 2001;53:107–118. [PubMed] [Google Scholar]
- King BF, Liu M, Pintor J, Gualix J, Miras-Portugal MT, Burnstock G. Diinosine pentaphosphate (IP5I) is a potent antagonist at recombinant rat P2X1 receptors. Br J Pharmacol. 1999;128:981–988. doi: 10.1038/sj.bjp.0702876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King BF, Burnstock G, Boyer JL, Boeynaems J, Weisman GA, Kennedy C, et al. Nucleotide receptors: P2Y receptors. In: Girdlestone D, editor. The IUPHAR Compendium of Receptor Characterization and Classification. IUPHAR Media; 2000. pp. 306–320. 2000. [Google Scholar]
- Koshimizu TA, Kretschmannova K, He ML, Ueno S, Tanoue A, Yanagihara N, et al. Carboxyl-terminal splicing enhances physical interactions between the cytoplasmic tails of purinergic P2X receptors. Mol Pharmacol. 2006;69:1588–1598. doi: 10.1124/mol.105.019802. [DOI] [PubMed] [Google Scholar]
- Kunapuli SP. Multiple P2 receptor subtypes on platelets: a new interpretation of their function [In Process Citation] Trends Pharmacol Sci. 1998;19:391–394. doi: 10.1016/s0165-6147(98)01248-6. [DOI] [PubMed] [Google Scholar]
- Kunapuli SP, Daniel JL. P2 receptor subtypes in the cardiovascular system. Biochem J. 1998;336:513–523. doi: 10.1042/bj3360513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunapuli SP, Ding Z, Dorsam RT, Kim S, Murugappan S, Quinton TM. ADP receptors – targets for developing antithrombotic agents. Curr Pharm Des. 2003;9:2303–2316. doi: 10.2174/1381612033453947. [DOI] [PubMed] [Google Scholar]
- Le KT, Boue-Grabot E, Archambault V, Seguela P. Functional and biochemical evidence for heteromeric ATP-gated channels composed of P2X1 and P2X5 subunits. J Biol Chem. 1999;274:15415–15419. doi: 10.1074/jbc.274.22.15415. [DOI] [PubMed] [Google Scholar]
- Leipziger J. Control of epithelial transport via luminal P2 receptors. Am J Physiol Renal Physiol. 2003;284:F419–432. doi: 10.1152/ajprenal.00075.2002. [DOI] [PubMed] [Google Scholar]
- Lewis CJ, Evans RJ. Lack of run-down of smooth muscle P2X receptor currents recorded with the amphotericin permeabilized patch technique, physiological and pharmacological characterization of the properties of mesenteric artery P2X receptor ion channels. Br J Pharmacol. 2000;131:1659–1666. doi: 10.1038/sj.bjp.0703744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis CJ, Evans RJ. P2X receptor immunoreactivity in different arteries from the femoral, pulmonary, cerebral, coronary and renal circulations. J Vasc Res. 2001;38:332–340. doi: 10.1159/000051064. [DOI] [PubMed] [Google Scholar]
- Linden J. Molecular approach to adenosine receptors: receptor-mediated mechanisms of tissue protection. Annu Rev Pharmacol Toxicol. 2001;41:775–787. doi: 10.1146/annurev.pharmtox.41.1.775. [DOI] [PubMed] [Google Scholar]
- Luo J, Jankowski J, Knobloch M, van der Giet M, Gardanis K, Russ T, et al. Identification and characterization of diadenosine 5′,5‴-P1,P2-diphosphate and diadenosine 5′,5‴-P1,P3-triphosphate in human myocardial tissue. FASEB J. 1999a;13:695–705. doi: 10.1096/fasebj.13.6.695. [DOI] [PubMed] [Google Scholar]
- Luo J, Jankowski J, Tepel M, von der Giet M, Zidek W, Schlüter H. Identification of diadenosine hexaphosphate in human erythrocytes. Hypertension. 1999b;34:872–875. doi: 10.1161/01.hyp.34.4.872. [DOI] [PubMed] [Google Scholar]
- Lüthje J, Ogilvie A. The presence of diadenosine 5′,5‴-P1,P3-triphosphate (Ap3A) in human platelets. Biochem Biophys Res Commun. 1983;115:253–260. doi: 10.1016/0006-291x(83)90997-x. [DOI] [PubMed] [Google Scholar]
- Lüthje J, Ogilvie A. Catabolism of Ap3A and Ap4A in human plasma. Purification and characterization of a glycoprotein complex with 5′-nucleotide phosphodiesterase activity. Eur J Biochem. 1985;149:119–127. doi: 10.1111/j.1432-1033.1985.tb08901.x. [DOI] [PubMed] [Google Scholar]
- Lüthje J, Ogilvie A. Catabolism of Ap4A and Ap3A in whole blood. The dinucleotides are long-lived signal molecules in the blood ending up as intracellular ATP in the erythrocytes. Eur J Biochem. 1988;173:241–245. doi: 10.1111/j.1432-1033.1988.tb13990.x. [DOI] [PubMed] [Google Scholar]
- McLennan AG. Ap4A and Other Dinucleoside Polyphosphates. Boca Raton, FL: CRC Press, Inc.; 1992. [Google Scholar]
- McMillan MR, Burnstock G, Haworth SG. Vasoconstriction of intrapulmonary arteries to P2-receptor nucleotides in normal and pulmonary hypertensive newborn piglets. Br J Pharmacol. 1999;128:549–555. doi: 10.1038/sj.bjp.0702814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malam-Souley R, Seye C, Gadeau AP, Loirand G, Pillois X, Campan M, et al. Nucleotide receptor P2u partially mediates ATP-induced cell cycle progression of aortic smooth muscle cells. J Cell Physiol. 1996;166:57–65. doi: 10.1002/(SICI)1097-4652(199601)166:1<57::AID-JCP7>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Malmsjo M, Edvinsson L, Erlinge D. P2X receptors counteract the vasodilatory effects of endothelium derived hyperpolarising factor. Eur J Pharmacol. 2000a;390:173–180. doi: 10.1016/s0014-2999(00)00010-8. [DOI] [PubMed] [Google Scholar]
- Malmsjo M, Hou M, Harden TK, Pendergast W, Pantev E, Edvinsson L, et al. Characterization of contractile P2 receptors in human coronary arteries by use of the stable pyrimidines uridine 5′-O-thiodiphosphate and uridine 5′-O-3-thiotriphosphate. J Pharmacol Exp Ther. 2000b;293:755–760. [PubMed] [Google Scholar]
- Marcus AJ, Broekman MJ, Drosopoulos JH, Islam N, Pinsky DJ, Sesti C, et al. Metabolic control of excessive extracellular nucleotide accumulation by CD39/ecto-nucleotidase-1: implications for ischemic vascular diseases. J Pharmacol Exp Ther. 2003;305:9–16. doi: 10.1124/jpet.102.043729. [DOI] [PubMed] [Google Scholar]
- Mateo J, Miras-Portugal MT, Rotllan P. Ecto-enzymatic hydrolysis of diadenosine polyphosphates by cultured adrenomedullary vascular endothelial cells. Am J Physiol. 1997a;273:C918–C927. doi: 10.1152/ajpcell.1997.273.3.C918. [DOI] [PubMed] [Google Scholar]
- Mateo J, Rotllan P, Marti E, De Aranda G, Solsona I, Miras-Portugal C, et al. Diadenosine polyphosphate hydrolase from presynaptic plasma membranes of Torpedo electric organ. Biochem J. 1997b;323:677–684. doi: 10.1042/bj3230677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mistry H, Gitlin JM, Mitchell JA, Hiley CR. Endothelium-dependent relaxation and endothelial hyperpolarization by P2Y receptor agonists in rat-isolated mesenteric artery. Br J Pharmacol. 2003;139:661–671. doi: 10.1038/sj.bjp.0705271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mombouli JV, Vanhoutte PM. Purinergic endothelium-dependent and -independent contractions in rat aorta. Hypertension. 1993;22:577–583. doi: 10.1161/01.hyp.22.4.577. [DOI] [PubMed] [Google Scholar]
- Moore SF, MacKenzie AB. Murine macrophage P2X7 receptors support rapid prothrombotic responses. Cell Signal. 2007;19:855–866. doi: 10.1016/j.cellsig.2006.10.010. [DOI] [PubMed] [Google Scholar]
- Murthy KS, Makhlouf GM. Coexpression of ligand-gated P2X and G protein-coupled P2Y receptors in smooth muscle. Preferential activation of P2Y receptors coupled to phospholipase C (PLC)-beta1 via Galphaq/11 and to PLC-beta3 via Gbetagammai3. J Biol Chem. 1998a;273:4695–4704. doi: 10.1074/jbc.273.8.4695. [DOI] [PubMed] [Google Scholar]
- Murthy KS, Makhlouf GM. Differential regulation of phospholipase A2 (PLA2)-dependent Ca2+ signaling in smooth muscle by cAMP- and cGMP-dependent protein kinases. Inhibitory phosphorylation of PLA2 by cyclic nucleotide-dependent protein kinases. J Biol Chem. 1998b;273:34519–34526. doi: 10.1074/jbc.273.51.34519. [DOI] [PubMed] [Google Scholar]
- Nakae I, Takahashi M, Takaoka A, Liu Q, Matsumoto T, Amano M, et al. Coronary effects of diadenosine tetraphosphate resemble those of adenosine in anesthetized pigs: involvement of ATP-sensitive potassium channels. J Cardiovasc Pharmacol. 1996;28:124–133. doi: 10.1097/00005344-199607000-00019. [DOI] [PubMed] [Google Scholar]
- Nicke A, Baumert HG, Rettinger J, Eichele A, Lambrecht G, Mutschler E, et al. P2X1 and P2X3 receptors form stable trimers: a novel structural motif of ligand-gated ion channels. EMBO J. 1998;17:3016–3028. doi: 10.1093/emboj/17.11.3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nori S, Fumagalli L, Bo X, Bogdanov Y, Burnstock G. Coexpression of mRNAs for P2X1, P2X2 and P2X4 receptors in rat vascular smooth muscle: an in situ hybridization and RT-PCR study. J Vasc Res. 1998;35:179–185. doi: 10.1159/000025582. [DOI] [PubMed] [Google Scholar]
- North RA, Surprenant A. Pharmacology of cloned P2X receptors. Annu Rev Pharmacol Toxicol. 2000;40:563–580. doi: 10.1146/annurev.pharmtox.40.1.563. [DOI] [PubMed] [Google Scholar]
- Ogilvie A. Extracellular functions of ApnA. In: McLennan AG, editor. Ap4A and Other Dinucleoside Polyphosphates. Boca Raton, FL: CRC Press Inc.; 1992. pp. 229–273. [Google Scholar]
- Ogilvie A, Jakob P. Diadenosine 5′,5‴-P1,P3-triphosphate in eukaryotic cells: identification and quantitation. Anal Biochem. 1983;134:382–392. doi: 10.1016/0003-2697(83)90313-5. [DOI] [PubMed] [Google Scholar]
- Ogilvie A, Luthje J, Pohl U, Busse R. Identification and partial characterization of an adenosine(5′)tetraphospho(5′)adenosine hydrolase on intact bovine aortic endothelial cells. Biochem J. 1989;259:97–103. doi: 10.1042/bj2590097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohata H, Ujike Y, Momose K. Confocal imaging analysis of ATP-induced Ca2+ response in individual endothelial cells of the artery in situ. Am J Physiol. 1997;272:C1980–C1987. doi: 10.1152/ajpcell.1997.272.6.C1980. [DOI] [PubMed] [Google Scholar]
- Picher M, Boucher RC. Biochemical evidence for an ecto alkaline phosphodiesterase I in human airways. Am J Respir Cell Mol Biol. 2000;23:255–261. doi: 10.1165/ajrcmb.23.2.4088. [DOI] [PubMed] [Google Scholar]
- Pintor J, Torres M, Miras-Portugal MT. Carbachol induced release of diadenosine polyphosphates – Ap4A and Ap5A – from perfused bovine adrenal medulla and isolated chromaffin cells. Life Sci. 1991;48:2317–2324. doi: 10.1016/0024-3205(91)90268-g. [DOI] [PubMed] [Google Scholar]
- Pintor J, Diaz-Rey MA, Torres M, Miras-Portugal MT. Presence of diadenosine polyphosphates – Ap4A and Ap5A – in rat brain synaptic terminals. Ca2+ dependent release evoked by 4-aminopyridine and veratridine. Neurosci Lett. 1992a;136:141–144. doi: 10.1016/0304-3940(92)90034-5. [DOI] [PubMed] [Google Scholar]
- Pintor J, Rotllan P, Torres M, Miras-Portugal MT. Characterization and quantification of diadenosine hexaphosphate in chromaffin cells: granular storage and secretagogue-induced release. Anal Biochem. 1992b;200:296–300. doi: 10.1016/0003-2697(92)90469-n. [DOI] [PubMed] [Google Scholar]
- Pintor J, Gualix J, Miras-Portugal MT. Dinucleotide receptor modulation by protein kinases (protein kinases A and C) and protein phosphatases in rat brain synaptic terminals. J Neurochem. 1997;68:2552–2557. doi: 10.1046/j.1471-4159.1997.68062552.x. [DOI] [PubMed] [Google Scholar]
- Pirotton S, Communi D, Motte S, Janssens R, Boeynaems JM. Endothelial P2-purinoceptors: subtypes and signal transduction. J Auton Pharmacol. 1996;16:353–356. doi: 10.1111/j.1474-8673.1996.tb00052.x. [DOI] [PubMed] [Google Scholar]
- Pohl U, Ogilvie A, Lamontagne D, Busse R. Potent effects of AP3A and AP4A on coronary resistance and autacoid release of intact rabbit hearts. Am J Physiol. 1991;260:H1692–H1697. doi: 10.1152/ajpheart.1991.260.5.H1692. [DOI] [PubMed] [Google Scholar]
- Pulvirenti TJ, Yin JL, Chaufour X, McLachlan C, Hambly BD, Bennett MR, et al. P2X (purinergic) receptor redistribution in rabbit aorta following injury to endothelial cells and cholesterol feeding. J Neurocytol. 2000;29:623–631. doi: 10.1023/a:1010828302936. [DOI] [PubMed] [Google Scholar]
- Ralevic V. P2 receptors in the central and peripheral nervous systems modulating sympathetic vasomotor tone. J Auton Nerv Syst. 2000;81:205–211. doi: 10.1016/s0165-1838(00)00139-9. [DOI] [PubMed] [Google Scholar]
- Ralevic V, Burnstock G. Discrimination by PPADS between endothelial P2Y- and P2U-purinoceptors in the rat isolated mesenteric arterial bed. Br J Pharmacol. 1996;118:428–434. doi: 10.1111/j.1476-5381.1996.tb15420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- Ralevic V, Hoyle CH, Burnstock G. Pivotal role of phosphate chain length in vasoconstrictor versus vasodilator actions of adenine dinucleotides in rat mesenteric arteries. J Physiol (Lond) 1995;483:703–713. doi: 10.1113/jphysiol.1995.sp020615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapaport E, Zamecnik PC. Presence of diadenosine 5′,5‴-P1, P4-tetraphosphate (Ap4A) in mamalian cells in levels varying widely with proliferative activity of the tissue: a possible positive ‘pleiotypic activator. Proc Natl Acad Sci USA. 1976;73:3984–3988. doi: 10.1073/pnas.73.11.3984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remy P. Intracellular Functions of ApnN: Eukaryotes. Boca Raton, FL: CRC Press; 1992. [Google Scholar]
- Robertson SJ, Ennion SJ, Evans RJ, Edwards FA. Synaptic P2X receptors. Curr Opin Neurobiol. 2001;11:378–386. doi: 10.1016/s0959-4388(00)00222-1. [DOI] [PubMed] [Google Scholar]
- Rodriguez del Castillo A, Torres M, Delicado EG, Miras-Portugal MT. Subcellular distribution studies of diadenosine polyphosphates – Ap4A and Ap5A – in bovine adrenal medulla: presence in chromaffin granules. J Neurochem. 1988;51:1696–1703. doi: 10.1111/j.1471-4159.1988.tb01147.x. [DOI] [PubMed] [Google Scholar]
- Rump LC, Oberhauser V, von Kugelgen I. Purinoceptors mediate renal vasodilation by nitric oxide dependent and independent mechanisms. Kidney Int. 1998;54:473–481. doi: 10.1046/j.1523-1755.1998.00002.x. [DOI] [PubMed] [Google Scholar]
- Safrany ST, Ingram SW, Cartwright JL, Falck JR, McLennan AG, Barnes LD, et al. The diadenosine hexaphosphate hydrolases from Schizosaccharomyces pombe and Saccharomyces cerevisiae are homologues of the human diphosphoinositol polyphosphate phosphohydrolase. Overlapping substrate specificities in a MutT-type protein. J Biol Chem. 1999;274:21735–21740. doi: 10.1074/jbc.274.31.21735. [DOI] [PubMed] [Google Scholar]
- Schlüter H, Offers E, Brüggemann G, van der Giet M, Tepel M, Nordhoff E, et al. Diadenosine phosphates and the physiological control of blood pressure. Nature. 1994;367:186–188. doi: 10.1038/367186a0. [DOI] [PubMed] [Google Scholar]
- Schlüter H, Gross I, Bachmann J, Kaufmann R, van der Giet M, Tepel M, et al. Adenosine(5′) oligophospho-(5′) guanosines and guanosine(5′) oligophospho-(5′) guanosines in human platelets. J Clin Invest. 1998;101:682–688. doi: 10.1172/JCI119882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze-Lohoff E, Zanner S, Ogilvie A, Sterzel RB. Vasoactive diadenosine polyphosphates promote growth of cultured renal mesangial cells. Hypertension. 1995;26:899–904. doi: 10.1161/01.hyp.26.6.899. [DOI] [PubMed] [Google Scholar]
- Shen J, Seye CI, Wang M, Weisman GA, Wilden PA, Sturek M. Cloning, up-regulation, and mitogenic role of porcine P2Y2 receptor in coronary artery smooth muscle cells. Mol Pharmacol. 2004;66:1265–1274. doi: 10.1124/mol.104.002642. [DOI] [PubMed] [Google Scholar]
- Stutts I, Monroe J, Boucher J, Richard C, Eduardo RAC. Dinucleotides useful for the treatment of cystic fibrosis and for hydrating mucus secretions. USA: US-Pat. 5,635,160, The University of North Carolina at Chapel Hill.
- Sumiyoshi R, Nishimura J, Kawasaki J, Kobayashi S, Takahashi S, Kanaide H. Diadenosine polyphosphates directly relax porcine coronary arterial smooth muscle. J Pharmacol Exp Ther. 1997;283:548–556. [PubMed] [Google Scholar]
- Surprenant A, Schneider DA, Wilson HL, Galligan JJ, North RA. Functional properties of heteromeric P2X(1/5) receptors expressed in HEK cells and excitatory junction potentials in guinea-pig submucosal arterioles. J Auton Nerv Syst. 2000;81:249–263. doi: 10.1016/s0165-1838(00)00123-5. [DOI] [PubMed] [Google Scholar]
- Tepel M, Lowe S, Nofer JR, Assmann G, Schlüter H, Zidek W. Diadenosine polyphosphates regulate cytosolic calcium in human fibroblast cells by interaction with P2x purinoceptors coupled to phospholipase C. Biochim Biophys Acta. 1996;1312:145–150. doi: 10.1016/0167-4889(96)00035-3. [DOI] [PubMed] [Google Scholar]
- Tepel M, Jankowski J, Schlüter H, Bachmann J, van der Giet M, Ruess C, et al. Diadenosine polyphosphates' action on calcium and vessel contraction. Am J Hypertens. 1997;10:1404–1410. doi: 10.1016/s0895-7061(97)80305-6. [DOI] [PubMed] [Google Scholar]
- Thorne NM, Hankin S, Wilkinson MC, Nunez C, Barraclough R, McLennan AG. Human diadenosine 5′,5‴-P1,P4-tetraphosphate pyrophosphohydrolase is a member of the MutT family of nucleotide pyrophosphatases. Biochem J. 1995;311:717–721. doi: 10.1042/bj3110717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres GE, Haines WR, Egan TM, Voigt MM. Co-expression of P2X1 and P2X5 receptor subunits reveals a novel ATP-gated ion channel. Mol Pharmacol. 1998;54:989–993. doi: 10.1124/mol.54.6.989. [DOI] [PubMed] [Google Scholar]
- Tu MT, Luo SF, Wang CC, Chien CS, Chiu CT, Lin CC, et al. P2Y(2) receptor-mediated proliferation of C(6) glioma cells via activation of Ras/Raf/MEK/MAPK pathway. Br J Pharmacol. 2000;129:1481–1489. doi: 10.1038/sj.bjp.0703182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner CM, Vonend O, Chan C, Burnstock G, Unwin RJ. The pattern of distribution of selected ATP-sensitive P2 receptor subtypes in normal rat kidney: an immunohistological study. Cells Tissues Organs. 2003;175:105–117. doi: 10.1159/000073754. [DOI] [PubMed] [Google Scholar]
- Usune S, Katsuragi T, Furukawa T. Effects of PPADS and suramin on contractions and cytoplasmic Ca2+ changes evoked by Ap4A, ATP and alpha, beta-methylene ATP in guinea-pig urinary bladder. Br J Pharmacol. 1996;117:698–702. doi: 10.1111/j.1476-5381.1996.tb15246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahlensieck U, Boknik P, Knapp J, Linck B, Muller FU, Neumann J, et al. Negative chronotropic and inotropic effects exerted by diadenosine hexaphosphate (Ap6A) via A1-adenosine receptors. Br J Pharmacol. 1996;119:835–844. doi: 10.1111/j.1476-5381.1996.tb15748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhoutte PM. Platelet-derived serotonin, the endothelium, and cardiovascular disease. J Cardiovasc Pharmacol. 1991;17:S6–S12. [PubMed] [Google Scholar]
- Verspohl EJ, Hagemann J, Lempka M. Vascular smooth muscle cells (VSMC) proliferation of streptozotocin-diabetic animals induced by diadenosine polyphosphates. Exp Clin Endocrinol Diabetes. 2004;112:142–147. doi: 10.1055/s-2004-817823. [DOI] [PubMed] [Google Scholar]
- Vigne P, Breittmayer JP, Frelin C. Diadenosine polyphosphates as antagonists of the endogenous P2Y1 receptor in rat brain capillary endothelial cells of the B7 and B10 clones. Br J Pharmacol. 2000;129:1506–1512. doi: 10.1038/sj.bjp.0703228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J, Hilderman RH. Identification of a serine protease which activates the mouse heart adenosine 5′,5‴,P1,P4-tetraphosphate receptor. Biochemistry. 1993;32:3119–3123. doi: 10.1021/bi00063a025. [DOI] [PubMed] [Google Scholar]
- Wang L, Karlsson L, Moses S, Hultgardh-Nilsson A, Andersson M, Borna C, et al. P2 receptor expression profiles in human vascular smooth muscle and endothelial cells. J Cardiovasc Pharmacol. 2002;40:841–853. doi: 10.1097/00005344-200212000-00005. [DOI] [PubMed] [Google Scholar]
- Wilden PA, Agazie YM, Kaufman R, Halenda SP. ATP-stimulated smooth muscle cell proliferation requires independent ERK and PI3K signaling pathways. Am J Physiol. 1998;275:H1209–H1215. doi: 10.1152/ajpheart.1998.275.4.H1209. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Korenaga R, Kamiya A, Qi Z, Sokabe M, Ando J. P2X4 receptors mediate ATP-induced calcium influx in human vascular endothelial cells. Am J Physiol Heart Circ Physiol. 2000;279:H285–H292. doi: 10.1152/ajpheart.2000.279.1.H285. [DOI] [PubMed] [Google Scholar]
- Yegutkin G, Bodin P, Burnstock G. Effect of shear stress on the release of soluble ecto-enzymes ATPase and 5′-nucleotidase along with endogenous ATP from vascular endothelial cells. Br J Pharmacol. 2000;129:921–926. doi: 10.1038/sj.bjp.0703136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yegutkin G, Jankowski J, Jalkanen S, Gunthner T, Zidek W, Jankowski V. Dinucleotide polyphosphates contribute to purinergic signalling via inhibition of adenylate kinase activity. Biosci Rep. 2008;28:189–194. doi: 10.1042/BSR20080052. [DOI] [PubMed] [Google Scholar]
- Yegutkin GG, Burnstock G. Inhibitory effects of some purinergic agents on ecto-ATPase activity and pattern of stepwise ATP hydrolysis in rat liver plasma membranes. Biochim Biophys Acta. 2000;1466:234–244. doi: 10.1016/s0005-2736(00)00165-6. [DOI] [PubMed] [Google Scholar]
- Yegutkin GG, Samburski SS, Jalkanen S. Soluble purine-converting enzymes circulate in human blood and regulate extracellular ATP level via counteracting pyrophosphatase and phosphotransfer reactions. FASEB J. 2003;17:1328–1330. doi: 10.1096/fj.02-1136fje. [DOI] [PubMed] [Google Scholar]
- Yegutkin GG, Samburski SS, Mortensen SP, Jalkanen S, Gonzalez-Alonso J. Intravascular ADP and soluble nucleotidases contribute to acute prothrombotic state during vigorous exercise in humans. J Physiol. 2007;579:553–564. doi: 10.1113/jphysiol.2006.119453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yerxa B, Brown E. Di(uridine 5′)-tetraphosphate and salts thereof. USA: US-Pat. 20040014713.
- Yerxa BR, Mundasad M, Sylvester RN, Garden JC, Cooper M, Kellerman DJ. Ocular safety of INS365 ophthalmic solution, a P2Y2 agonist, in patients with mild to moderate dry eye disease. Adv Exp Med Biol. 2002;506:1251–1257. doi: 10.1007/978-1-4615-0717-8_180. [DOI] [PubMed] [Google Scholar]
- Yu SM, Chen SF, Lau YT, Yang CM, Chen JC. Mechanism of extracellular ATP-induced proliferation of vascular smooth muscle cells. Mol Pharmacol. 1996;50:1000–1009. [PubMed] [Google Scholar]
- Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med. 2001;345:494–502. doi: 10.1056/NEJMoa010746. [DOI] [PubMed] [Google Scholar]
- Zemkova H, He ML, Koshimizu TA, Stojilkovic SS. Identification of ectodomain regions contributing to gating, deactivation, and resensitization of purinergic P2X receptors. J Neurosci. 2004;24:6968–6978. doi: 10.1523/JNEUROSCI.1471-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann H, Volknandt W, Wittich B, Hausinger A. Synaptic vesicle life cycle and synaptic turnover. J Physiol Paris. 1993;87:159–170. doi: 10.1016/0928-4257(93)90027-q. [DOI] [PubMed] [Google Scholar]


