Abstract
Background and purpose:
Disodium 2,4-disulphophenyl-N-tert-butylnitrone (NXY-059) was neuroprotective in experimental stroke models but ineffective in a large clinical trial. This first-ever individual animal meta-analysis was used to assess the preclinical studies.
Experimental approach:
Studies were obtained from AstraZeneca and PubMed searches. Data for each animal were obtained from the lead author of each study and/or AstraZeneca. Published summary data were used if individual data were not available. Infarct volume and motor impairment were standardized to reflect different species and scales. Standardized mean difference (SMD), coefficients from multilevel models and 95% confidence intervals (95% CI) are presented.
Key results:
Fifteen studies (26 conditions, 12 laboratories) involving rats (544), mice (9) and marmosets (32) were identified (NXY-059: 332, control: 253) with individual data for 442 animals. Four studies were unpublished. Studies variably used randomization (40%), blinding of surgeon (53%) and outcome assessor (67%). NXY-059 reduced total (SMD −1.17, 95% CI −1.50 to −0.84), cortical (SMD −2.17, 95% CI −2.99 to −1.34) and subcortical (−1.43, 95% CI −2.20 to −0.86) lesion volume; efficacy was seen in transient, permanent and thrombotic ischaemia, up to 180 min post occlusion. NXY-059 reduced motor impairment (SMD −1.66, 95% CI −2.18 to −1.14) and neglect. Evidence for performance, attrition and publication bias was present.
Conclusions and implications:
NXY-059 was neuroprotective in experimental stroke although bias may have resulted in efficacy being overestimated. Efficacy in young, healthy, male animals is a poor predictor of clinical outcome. We suggest the use of preclinical meta-analysis before initiation of future clinical trials.
Keywords: stroke, meta-analysis, neuroprotection, animal models, cerebral ischaemia, NXY-059
Introduction
Disodium 2,4-disulphophenyl-N-tert-butylnitrone (NXY-059) is a nitrone-based compound that traps free radicals (Maples et al., 2001; Williams et al., 2007). Several preclinical studies have reported that NXY-059 reduces infarct volume and motor impairment in experimental models of stroke using rodents, rabbits and primates. These findings have been reported from several laboratories. The beneficial effects have been reported for permanent and transient models of ischaemia, and lesion volume was reduced in both cortex and sub-cortex. Treatment appeared to be effective when given up to 4 h post onset of occlusion. NXY-059 was considered by some (Green, 2008) to fulfil the Stroke Therapy Academic Industry Roundtable (STAIR) criteria for demonstrating neuroprotection (STAIR, 1999).
In view of the positive preclinical data, clinical development was initiated with phase I (Edenius et al., 2002; Strid et al., 2002) and phase II (Lees et al., 2001; 2003;) studies, these culminating in two phase III randomized controlled trials (Lees et al., 2006; Shuaib et al., 2007). In the first trial (SAINT 1), NXY-059 reduced combined death and dependency in comparison with placebo (Lees et al., 2006). Although the trial was positive using its pre-specified statistical approach (Cochran–Mantel–Haenszel test, P= 0.038), post hoc analyses using more conventional binary or ordinal (OAST Collaboration, 2007) approaches were neutral (Koziol and Feng, 2006). In a second and larger trial (SAINT 2), NXY-059 had no significant treatment effect (Shuaib et al., 2007) and the results from the combined trials were similarly negative (Diener et al., 2008).
The conflicting results obtained in the two SAINT trials are most easily explained if SAINT I is considered to have had a false positive finding, a hypothesis supported by its weak statistical significance. More difficult to explain is the difference between the apparent positive preclinical studies and neutral clinical development. One possible explanation is that the preclinical studies may have varied in their findings with undue weight being given to positive rather than neutral ones. To assess this further, we performed a meta-analysis of the preclinical studies using individual data from each animal. Although meta-analyses of preclinical studies based on published summary data are becoming more common (Willmot et al., 2005a; Gibson et al., 2007), analyses based on individual subjects are considered the gold standard (Stewart and Parmar, 1993), but have never been performed previously in animal studies.
Methods
Study identification
Completed studies that investigated the effect of NXY-059 in experimental models of stroke were identified primarily from AstraZeneca (AZ), the developer of NXY-059, and electronic searches of PubMed (last on 29 March 2008) using the term ‘NXY-059’. Studies were included if they had been reported by the end of 2007. Reference lists of earlier reviews and identified trial publications were also checked for additional trials. Publications could be in any language.
Study selection
Completed controlled studies in animals, whether randomized or not, were included. Animals had to have been treated with NXY-059 versus control/placebo.
Data extraction
The following information on the study design was extracted from study reports (provided by AZ), publications and the lead author: species, experimental model (transient, permanent, global thromboembolic), design, whether the study was randomized or pseudo-randomized, whether surgeons were blinded to treatment and whether outcomes were assessed blinded to treatment. Individual data were obtained for each animal, these including: sex, weight, lesion volume, motor impairment (and scale), temperature during treatment and vital status. Information on treatment was also obtained: time to treatment from occlusion, loading and maintenance doses of NXY-059, duration of treatment and plasma concentration.
Where individual animal data (IAD) were not present, published summary (aggregate or group) data were used. In some cases, it was necessary to estimate summary data from published graphs (e.g. lesion volume in Yoshimoto et al., 2002a,b;). Analyses involving dose assessment used the total dose [loading dose + (maintenance dose × treatment length]. Deaths were included whether spontaneous or resulting from early culling because of poor health. Studies were considered randomized if animals were numbered before study commencement and a randomization code was used to choose which animal would be the next experimental subject; if animals were ‘picked at random’ from a cage, studies were considered as pseudo-randomized as this approach is open to bias.
The methodological quality of each study was assessed using an 9-point score based on the STAIR (1999) rating as described previously (Macleod et al., 2005; Willmot et al., 2005a,b; Gibson et al., 2006). One point was given for written evidence of each of the following criteria: presence of randomization (0.5 given for pseudo-randomization), monitoring of physiological parameters, assessment of dose–response relationship, assessment of optimal time window, masked outcome measurement, assessment of outcome at days 1–3, assessment of outcome at days 1–30, combined measurement of lesion volume and functional outcome and additionally to the STAIR rating – masked surgery.
Data analysis
Data analysis comprised two stages. First, IAD and summary data were analysed together and, second, IAD were analysed separately. The analysis of summary data and IAD used random effects models to produce standardized mean differences (SMD, for continuous or ordinal data, http://www.cochrane-net.org/openlearning/html/modA1-4.htm) and 95% confidence intervals. Random effects models were used as biological heterogeneity was expected due to the varied nature of studies involving different species, models of ischaemia, time to treatment and doses of NXY-059. Statistical heterogeneity was calculated using the I2 statistic. The presence of publication bias was assessed using a funnel plot and Egger's test (Egger et al., 1997).
For the IAD analysis, data were entered into an Excel spreadsheet with each row containing data for an individual animal. Multilevel models were built to compare NXY-059 with control taking into account the differences between trials. Infarct volume was standardized (score-mean/standard deviation) to account for differences between the brain and infarct sizes in different species. Similarly, motor impairment was standardized as different scales were used, although all were based on that from Bederson et al. (1986). Coefficients for dose are given per 100 mg·kg−1. For analyses of dose/concentration and concentration/response relationships, NXY-059 concentrations were identified reflecting steady-state levels, typically at 24 h. All analyses were carried out in Stata version 8.
Results
Study identification
Fifteen completed studies fulfilled the inclusion criteria (Figure 1); these included 26 separate experiments involving 585 animals and came from 12 different laboratories in five countries (Canada, Japan, Sweden, UK, USA). Four of the studies were unpublished (Maples, 1996; Green, 2002; Sydserff, 2002; Zhao et al., 2002). Investigators from five studies (89 animals), all non-commercial, did not share IAD and the publications did not give data in a format suitable for inclusion (Lapchak et al., 2002a,b; 2004;); these studies, which involved rabbits, were therefore excluded (Figure 1).
Figure 1.
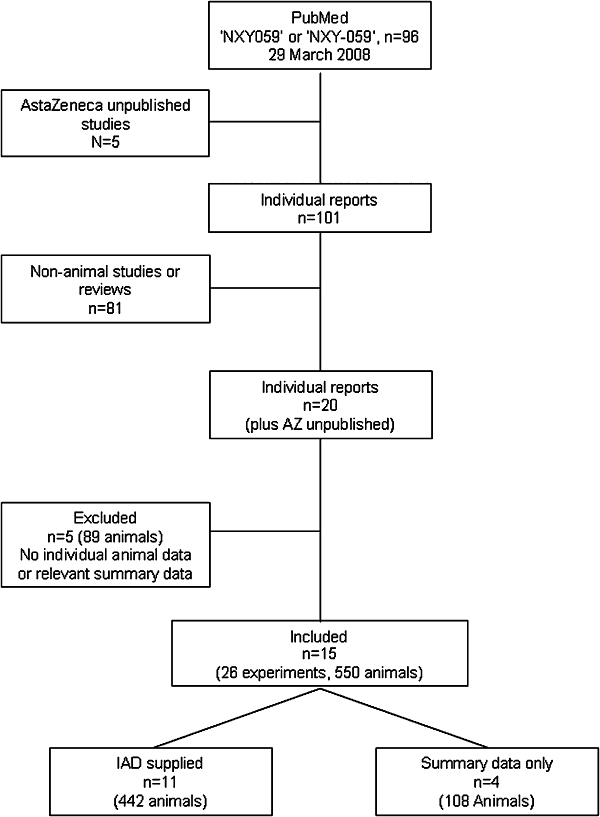
Flow chart of study identification and inclusions and exclusions.
Study design
The studies involved 585 animals (NXY-059 332, control 253) from three species: mice (9 animals), rats (544, from several strains – Long Evans, spontaneously hypertensive, Sprague-Dawley, Wistar) and marmosets (32 animals) (Table 1); individual data were available for 442 (76%) animals. The studies varied in size involving between 9 and 46 (median 12) animals. Seven out of 15 (47%) studies were randomized, three studies were pseudo-randomized and five did not involve randomization. Surgeons were blinded to treatment in 6/15 (40%) studies; outcome assessors were recorded as being blinded to treatment in 8/15 (53%) studies. A variety of anaesthetic agents were used: alphaxolone/alphadolone acetate, chloral hydrate, halothane in N2O/O2, isoflurane and pentobarbital; of these, only pentobarbitone may be considered as neuroprotective in its own right. Studies involved different models of ischaemia (temporary, permanent, thrombotic), times from onset of occlusion to treatment (5–480 min; median 90 min), length of treatment (21.75–72 h) and loading (0.3–200 mg·kg−1) and maintenance (0.3–200 mg·kg−1 h) doses of NXY-059 (Table 1).
Table 1.
Studies included in the analysis
| Laboratory | Species | Sex | Anaesthetic | Random | Surgery blinded | Outcome blinded | Temperature control | Model | Time after occlusion onset (min) | NXY-059 load (mg·kg−1) | NXY-059 maintenance (mg·kg−1h) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Maples (1996) | Sunnyvale, CA, USA | Rat (Sprague-Dawley) | Male | Pentobarbital 40 mg·kg−1 | Yes | NR | NR | Yes | MCAo-p (cautery) | 30 | 100 i.v. | 4.12 i.v. for 23 h |
| Kuroda et al. (1999) (3 studies) | Lund Sweden | Rat (Wistar) | Male | Halothane (1–3%) in N2O/O2 (70/30) | No | NR | NR | Yes | MCAo-t (2 h, time–response) | 180, 300, 480 | 0.3, 3, 30 | 0.3, 3, 30 for 24 h |
| Marshall et al. (2001) | Cambridge, UK | Marmoset (Common) | Both | Alphaxolone (13.5 mg·kg−1 i.m.)/alphadolone acetate (4.5 mg·kg−1 i.m.) | Pseudo | Yes | Yes | Yes | MCAo-p (cautery) | 5 | 28 i.v. | 18 s.c. for 48 h |
| Zhao et al. (2001) | Calgary, Canada | Rat (SHR) | Male | Halothane/N2O/O2 (2/70/28%) | Yes | Yes | Yes | Yes | MCAo-p (dose– response) | 5 | 30–60 i.v. | 30–60 i.v. for 24 h |
| Aronowski (2002) (see Green, 2002) (4 studies) | Houston, TX, USA | Rat (Long Evans) and Rat (SHR) | Male | Chloral hydrate and Isoflurane | NR | NR | NR | Yes | MCA/CCAo-t (3 h) and MCA/CCAo-p | 15 | 60 i.v. | 50 i.v. for 24 h and 60 i.v. for 24 h |
| Sydserff et al. (2002) (3 studies) | Worcester, MA, USA | Rat (Wistar) | Male | Halothane (2–6%)/O2 (1.0 L·min−1)/N2O (1.5 L·min−1) | Pseudo | Yes | Yes | Yes | MCAo-p (time– response) and MCAo-t (2 h, dose–response) | 5–240 135 | 53.8 s.c., 32.5–75.4 s.c. | 50 s.c. for 24 h, 30–70 s.c. for 23 h 55 min and 3–30 i.v. for 21 h 45 min |
| Zhao et al. (2002) | Calgary, Canada | Rat (SHR) | Male | Halothane/N2O/O2 (2/70/28%) | Yes | Yes | Yes | MCAo-p | 5 | 120 i.v. | 120 i.v. for 24 h | |
| Sydserff (2002) | Worcester, MA, USA | Rat (Sprague-Dawley) | Male | Chloral hydrate (400 mg·kg−1) | Yes | NR | Yes | MCAo-t (30 min) | 30 | 30 i.v. | 30 i.v. for 72 h | |
| Yoshimoto et al. (2002a) | Honolulu, USA | Rat (Wistar) | Male | Halothane (1.5–4%)/N2O (70/30) | NR | NR | NR | MCAo-t (2 h, filament, time–response) | 125, 180 | 30 i.v. | 30 i.v. for 24 h | |
| Yoshimoto et al. (2002b) | Honolulu, USA | Rat (Wistar) | Male | Halothane/N2O/O2 (1.5–4/70/30) | Yes | NR | NR | MCAo-t (2 h, filament) | 180 | 30 i.v. | 30 i.v. for 24 h | |
| Han et al. (2003) | Honolulu, USA | Rat (Wistar) | Male | Halothane/N2O/O2 (70/30) | NR | NR | NR | MCAo-t (2 h) | 180 | 30 i.v. | 30 i.v. | |
| Marshall et al. (2003) | Cambridge, UK | Marmoset (Common) | Both | Alphaxolone (13.5 mg·kg−1 i.m.)/alphadolone acetate (4.5 mg·kg−1 i.m.) | Pseudo | Yes | Yes | MCAo-p (cautery) | 240 | 77 i.v. + 154 s.c. | 32 for 48 h | |
| Wang and Shuaib (2004) | Edmonton, Canada | Rat (Wistar) | Male | Halothane (1.5–3%)/O2/N2O | Yes | Yes | Yes | MCAo-e | 90 | 65, 200 i.v. | 65, 200 i.v. for 4 h | |
| Balogh et al. (2005) | Budapest, Hungary | Mouse (NMRI) | Male | Chloral hydrate (550 mg·kg−1 i.p.) | Yes | No | Yes | MCAo-p | 30 | 10 i.p. | – | |
| Takizawa et al. (2007) | Japan | Rat (Sprague-Dawley) | Male | Halothane | No | No | No | Yes | MCAo (photo thrombotic) | 0 and 30 | 3.10 mg·kg−1 | – |
i.m., intramuscular; i.p., intraperitoneal; i.v., intravenous; MCAo, middle cerebral artery occlusion; MCAo-e, embolic occlusion MCAo; MCAo-p, permanent MCAo; MCAo-t, transient MCAo; NMRI, Naval Medical Research Institute; NR, not recorded; s.c., subcutaneous; SHR, spontaneously hypertensive.
Following onset of occlusion, five studies confirmed that cerebral blood flow was significantly reduced, either using visual inspection of the middle cerebral artery to confirm occlusion post-mortem (Marshall et al., 2001; 2003;) or using laser doppler methods (Green, 2002; Zhao et al., 2001; 2002;).
Lesion volume
Fifteen studies (26 experiments) had data on total infarct volume (Table 2). Visual and statistical assessment of publication bias using a funnel plot (Figure 2) and Egger's test (Egger et al., 1997) showed significant bias (P < 0.0001) with the majority of included studies showing beneficial effects for NXY-059.
Table 2.
Experimental results for included studies
| Animals, total (NXY-059 : control) | Individual animal data | Weight (g), NXY-059 Control | Temperature max, NXY-059 Control | Exclusions from analysis, NXY-059 Control | Death, NXY-059 Control | Lesion volume timing (h) | Lesion total, NXY-059 Control | Motor score, NXY-059 Control | Quality/8 | Comment for NXY-059 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Maples (1996) | 46 (24:24) | ✓ | 332 (22) 341 (22) | – | 0 1 | 1 1 | 23 | 29 (62) 57 (64) | – | 3 | Reduced infarct volume |
| Kuroda et al. (1999) | 38 (28:10) | ✓ | 310 (11)322 (17) | 38.0 (0.2) 37.9 (0.3) | 0 0 | 0 0 | 7 days | 120 (113)296 (82) | 0.8 (0.9)2.4 (0.7) | 6 | Dose-dependent reduction in infarct volume |
| Kuroda et al. (1999) | 15 (8:7) | ✓ | 326 (14) 327 (8) | 37.9 (0.3) 38.0 (0.2) | 0 0 | 0 0 | 7 days | 74 (107)202 (24) | 0.5 (0.8)2.4 (0.8) | 6 | Reduced infarct volume |
| Kuroda et al. (1999) | 21 (16:5) | ✓ | 328 (11) 327 (7) | 38.0 (0.1) 38.1 (0.1) | 0 0 | 0 0 | 48 | 155 (119)289 (64) | 2.2 (0.7) 2.4 (0.7) | 6 | Reduced infarct volume at 3 h |
| Marshall et al. (2001) | 12 (6:6) | ✓ | – | – | 0 1 | 0 0 | 20 weeks | 340 (155)158 (107) | – | 6.5 | Reduced motor impairment and neglect |
| Zhao et al. (2001) | 31 (21:10) | ✓ | 275 (26) 269 (13) | – | 0 0 | 0 0 | 24 | 40 (14)57 (16) | – | 6 | No effect on infarct volume at 30 mg·kg−1; significant reduction of infarct volume at 60 mg·kg−1 |
| Aronowski 2002 (see Green, 2002) | 20 (10:10) | ✓ | – | – | 0 0 | 0 0 | 72 | 123 (49) 109 (53) | – | 2 | No effect on infarct volume in MCA/CCAo-t. |
| Aronowski (2002) (see Green, 2002) | 10 (6:4) | ✓ | – | – | 0 0 | 0 0 | 72 | 87 (44) 51 (33) | 2 | No effect on infarct volume in MCA/CCAo-p. | |
| Aronowski (2002) (see Green, 2002) | 17 (10:7) | ✓ | 263 (12) 263 (12) | 36.7 (0.2) 36.9 (0.1) | 0 0 | 2 0 | 72 | 143 (26) 131 (39) | – | 2 | No effect on infarct volume, BP or temperature. |
| Aronowski (2002) (see Green, 2002) | 17 (9:8) | ✓ | 250 (15)273 (20) | 36.5 (0) 36.5 (0) | 0 0 | 1 0 | 72 | 88 (39) 105 (41) | – | 2 | No effect on infarct volume, BP or temperature. Also, no effect in positive control (caffeine/ethanol) |
| Sydserff et al. (2002) | 47 (39:8) | ✓ | – | – | 0 0 | 0 0 | 24 | 124 (47)226 (34) | – | 6.5 | Dose-dependent reduction in infarct volume linearly to plasma concentration |
| Sydserff et al. (2002) | 37 (28:9) | ✓ | 310 (6) 307 (7) | 37.2 (0.5) 36.9 (0.9) | 1 1 | 3 0 | 24 | 92 (66)200 (39) | 4.2 (1.8)5.8 (2.1) | 6.5 | |
| Sydserff et al. (2002) | 56 (28:28) | ✓ | 304 (13) 309 (10) | – | 0 0 | 0 0 | 48 | 77 (49)156 (58) | 3.5 (1.5)5.8 (1.2) | 6.5 | Dose-dependent decrease in infarct volume and motor impairment |
| Zhao et al. (2002) | 20 (10:10) | ✓ | 232 (16) 229 (23) | – | 0 0 | 0 0 | 24 | 99 (19) 94 (12) | – | 5 | |
| Sydserff (2002) | 10 (5:5) | ✓ | – | – | 0 0 | 0 0 | 72 | 82 (38)147 (27) | – | 4 | Reduced infarct volume |
| Yoshimoto et al. (2002b) | 10 (6:4) | ✗ | [260–310] | – | – | – | 2 h 5 min | 12 (10)33 (5) | – | 3 | Temperature higher in NXY-059 group higher at 4 h. Reduced infarct volume, secondary decline in mitochondrial respiratory function and mitochondrial release of cytochrome c, but no effect on calcium-induced mitochondrial swelling |
| Yoshimoto et al. (2002b) | 10 (6:4) | ✗ | [260–310] | – | – | – | 3 | 10 (4)33 (5) | – | 3 | |
| Yoshimoto et al. (2002a) | 12 (6:6) | ✗ | [260–310] | – | – | – | 4 | 13 (4.9) 14 (4.9) | – | 4 | Reduced infarct volume and neuronal mitochondrial cytochrome c release, and normal fall in p-Akt |
| Yoshimoto et al. (2002a) | 13 (7:6) | ✗ | [260–310] | – | – | – | 24 | 16 (5.3)26 (14.7) | – | 4 | |
| Yoshimoto et al. (2002a) | 13 (6:7) | ✗ | [260–310] | – | – | – | 48 | 15 (2.4)30 (10.6) | – | 4 | |
| Han et al. (2003) | 20 (10:10) | ✗ | [300–350] | – | – | – | 24 | 13 (8)37 (5) | – | 1 | Reduced cytochrome c release |
| Marshall et al. (2003) | 25 (13:12) | ✓ | 389 (28) 407 (44) | – | 0 0 | 2 2 | 11 weeks | 234 (100) 329 (147) | – | 6.5 | Reduced infarct volume, functional outcome and neglect |
| Wang and Shuaib (2004) | 24 (16:8) | ✓ | 386 (32) 375 (47.5) | – | 0 0 | 4 3 | 48 | 31% (9)43% (15) | 2.5 (0.5) 2.8 (0.8) | 4 | High-dose NXY-059 showed a higher number of deaths than low dose. Low dose produced a greater protective effect |
| Balogh et al. (2005) | 9 (4:5) | ✓ | 29 (1) 31 (1) | – | 0 0 | 0 0 | 48 | 295 (13) 306 (11) | – | 3 | No significant effect |
| Takizawa et al. (2007) | 20 (10:5) | ✗ | [306 (12)] | – | 0 0 | 0 0 | 24 | 223 (19) 244 (10) | – | 3 | Low dose reduced motor impairment but not infarct volume |
| Takizawa et al. (2007) | 20 (10:5) | ✗ | [306 (12)] | – | 0 0 | 0 0 | 24 | 192 (10)244 (10) | – | 3 | High dose reduced infarct volume and motor impairment |
Mean (SD) or median (IQR); statistically significant differences in lesion volume and motor score between the treatment groups are shown in bold (t-test). Figures for lesion size are generally infarct volume in mm3. However, in some cases values are those given for the particular measure made (e.g. MAP2 kinase staining in case of Yoshimoto et al., 2002a).
NXY-059, disodium 2,4-disulphophenyl-N-tert-butylnitrone.
Figure 2.

Funnel plot of NXY-059 on total infarct volume incorporating both individual animal data and summary data as assessment of publication bias, Egger et al. (1997) test: P < 0.0001. NXY-059, disodium 2,4-disulphophenyl-N-tert-butylnitrone.
In the combined IAD and summary analysis (Table 3), NXY-059 reduced total, cortical and subcortical lesion volumes by one to two standard deviations between the means. Although NXY-059 reduced lesion volume in rats and marmosets, no effect was seen in mice; however, the point estimate (SMD −0.91) for mice suggests that this is due to a type II error as only nine animals were studied (Balogh et al., 2005). Comparable results were found when using IAD alone; this analysis shows the coefficient by total dose of NXY-059 with adjustment for time to treatment (Table 4).
Table 3.
Effect of NXY-059 on infarct volume by brain regional, stroke model, animal species, time from occlusion to treatment onset, length of treatment, loading dose and maintenance dose
| Group | Studies | Experiments | Animals | SMD | 95% CI | P-value | Heterogeneity P-value |
|---|---|---|---|---|---|---|---|
| Total volume | 15 | 26 | 585 | −1.17 | −1.50 to −0.84 | <0.0001 | <0.0001 |
| Cortical | 5 | 6 | 145 | −2.17 | −2.99 to −1.34 | <0.0001 | <0.0001 |
| Subcortical | 5 | 6 | 145 | −1.43 | −2.00 to −0.86 | <0.0001 | 0.05 |
| Species | |||||||
| Mice | 1 | 1 | 9 | −0.91 | −2.31 to 0.49 | 0.20 | – |
| Rats | 12 | 23 | 544 | −1.05 | −1.53 to −0.57 | <0.0001 | <0.0001 |
| Marmosets | 2 | 2 | 32 | −0.95 | −1.70 to −0.21 | 0.01 | 0.45 |
| Model | |||||||
| Permanent | 8 | 13 | 302 | −1.05 | −1.53 to −0.57 | <0.0001 | <0.0001 |
| Transient | 6 | 12 | 259 | −1.33 | 1.84 to −0.82 | <0.0001 | <0.0001 |
| Thrombotic | 1 | 1 | 24 | −1.08 | −2.00 to −0.16 | 0.02 | 0.47 |
| Start time (min) | |||||||
| −30 | 9 | 18 | 282 | −0.87 | −1.36 to −0.38 | 0.001 | <0.0001 |
| >30 to −60 | 1 | 1 | 10 | −2.36 | −4.29 to −0.43 | 0.02 | – |
| >60 to −120 | 1 | 2 | 34 | −1.21 | −2.03 to −0.40 | 0.003 | 0.63 |
| >120 to −180 | 4 | 9 | 208 | −1.47 | −2.04 to −0.89 | <0.0001 | 0.001 |
| 240 | 2 | 2 | 30 | −2.31 | −5.79 to 1.18 | 0.19 | 0.02 |
| 300 | 1 | 1 | 10 | −1.53 | −3.27 to 0.19 | 0.08 | – |
| 480 | 1 | 1 | 11 | −0.91 | −2.30 to 0.48 | 0.20 | – |
| Treatment length (h) | |||||||
| 0† | 2 | 3 | 39 | −2.19 | −4.21 to −0.16 | 0.03 | 0.006 |
| >0 to −24 | 10 | 19 | 489 | −1.08 | −1.44 to −0.71 | <0.0001 | <0.0001 |
| 48 | 3 | 3 | 47 | −1.13 | −1.76 to −0.50 | <0.0001 | 0.50 |
| 72 | 1 | 1 | 10 | −1.94 | −3.51 to −0.38 | 0.02 | – |
| Loading dose (mg·kg−1) | |||||||
| No load | 1 | 3 | 77 | −0.60 | −1.07 to −0.12 | 0.01 | 0.66 |
| ≤30 | 9 | 18 | 231 | −1.69 | −2.18 to −1.20 | <0.0001 | 0.004 |
| >30 to ≤60 | 3 | 12 | 154 | −0.88 | −1.58 to −0.18 | 0.01 | <0.0001 |
| >60 to ≤120 | 5 | 5 | 111 | −0.74 | −1.44 to −0.04 | 0.04 | 0.03 |
| 200 | 1 | 1 | 12 | −0.77 | −2.02 to 0.48 | 0.23 | – |
| Maintenance dose (mg·kg−1) | |||||||
| No maintenance | 2 | 3 | 39 | −2.19 | −4.21 to −0.16 | 0.03 | 0.01 |
| ≤30 | 9 | 20 | 326 | −1.23 | −1.63 to −0.82 | <0.0001 | 0.001 |
| >30 to ≤60 | 4 | 12 | 164 | −0.94 | −1.60 to −0.28 | 0.01 | <0.0001 |
| >60 to ≤120 | 3 | 3 | 44 | −1.07 | −2.69 to 0.56 | 0.20 | 0.01 |
| 200 | 1 | 1 | 12 | −0.77 | −2.02 to 0.48 | 0.23 | – |
Data are standardized mean difference (SMD), 95% confidence intervals (95% CI) and significance for effect and heterogeneity. Results in bold are statistically significant.
Two studies administered one or two loading doses only, without maintenance.
NXY-059, disodium 2,4-disulphophenyl-N-tert-butylnitrone.
Table 4.
Effect of NXY-059 on infarct volume by brain region subdivided by animal species and sex, and model of ischaemia
| Group | Experiments | Animals | Coefficient | 95% CI | P-value |
|---|---|---|---|---|---|
| Species | |||||
| Total | 18 | 442 | −0.03 | −0.04 to −0.02 | <0.0001 |
| Mice | 1 | 9 | −8.67 | −19.69 to 2.36 | 0.12 |
| Rats | 15 | 401 | −0.03 | −0.04 to −0.02 | <0.0001 |
| Marmosets | 2 | 32 | −0.06 | −0.10 to −0.01 | 0.01 |
| Males | 2 | 17 | −0.02 | −0.06 to 0.01 | 0.13 |
| Females | 2 | 15 | −0.05 | −0.09 to −0.005 | 0.03 |
| Cortical† | 4 | 111 | −0.08 | −0.11 to −0.06 | <0.0001 |
| Rats | 2 | 79 | −0.13 | −0.16 to −0.10 | <0.0001 |
| Marmosets | 2 | 32 | −0.04 | −0.09 to 0.003 | 0.07 |
| Males | 2 | 17 | −0.03 | −0.10 to 0.03 | 0.32 |
| Females | 2 | 15 | −0.04 | −0.08 to 0.01 | 0.13 |
| Subcortical† | 4 | 111 | −0.10 | −0.13 to −0.08 | <0.0001 |
| Rats | 2 | 79 | −0.13 | −0.16 to −0.10 | <0.0001 |
| Marmosets | 2 | 32 | −0.07 | −0.11 to −0.02 | 0.002 |
| Males | 2 | 17 | −0.06 | −0.13 to 0.01 | 0.11 |
| Females | 2 | 15 | −0.07 | −0.11 to −0.03 | 0.001 |
| Model | |||||
| Total | |||||
| Transient | 6 | 160 | −0.05 | −0.08 to −0.03 | <0.0001 |
| Permanent | 11 | 258 | −0.10 | −0.13 to −0.08 | <0.0001 |
| Thrombotic | 1 | 24 | −0.06 | −0.15 to 0.03 | 0.18 |
No data were available for cortical/subcortical volumes in mice. Analyses based on individual animal data only. Data are coefficients per 100 mg·kg−1 of total administered NXY-059 dose with standardization for model and adjustment for time to treatment; 95% confidence intervals (95% CI) and significance. Results in bold are statistically significant.
Permanent models only.
NXY-059, disodium 2,4-disulphophenyl-N-tert-butylnitrone.
In combined IAD and summary analyses, total lesion volume was reduced to a similar extent in models of transient, permanent and thrombotic ischaemia (although the latter finding is based on only one study) (Table 3, Figure 3). In the adjusted IAD analysis, NXY-059 reduced total lesion volume in transient and permanent models (but not in the thrombotic study, probably reflecting the different methods of analysis) (Table 4). Both cortical and subcortical lesion volumes were less with NXY-059 in permanent models of ischaemia.
Figure 3.

Forest plot of NXY-059 on total infarct volume incorporating both individual animal data and summary data. NXY-059, disodium 2,4-disulphophenyl-N-tert-butylnitrone.
Reductions in total lesion volume were seen with treatment commenced between 5 and 180 min post onset of ischaemia; declining trends to efficacy were seen beyond this out to 480 min, but the limited number of animals prevents further interpretation (Table 3). Treatment with NXY-049 for 48 or 72 h did not appear to increase efficacy over and above that seen with 24 h (similar confidence intervals around the effects). Similarly, there was no evidence for dose–response effects in respect of either loading or maintenance dose. Eighteen out of 255 (7%) animals receiving NXY-059 had no lesion in comparison with 0/163 (0%) receiving control (P= 0.001). These findings came from two studies (four experiments); in both cases, surgeons and outcome assessors were unblinded to treatments (Maples, 1996; Kuroda et al., 1999).
When IAD data for lesion volume were assessed by markers of study quality, important observations were present. Total lesion volume was only reduced in studies with pseudo-randomization, but not those with true or no randomization (Table 5). The blinding of surgeons to treatment did not appear to alter treatment effects on total lesion volume. When considering the seven studies involving randomization (pseudo or full), surgeon and outcome-blinding, and monitoring of physiological variables, lesion volume was significantly reduced (−0.04, 95% confidence interval −0.05 to −0.02). Lesion volume was reduced in studies where a non-neuroprotective anaesthetic agent was used. However, efficacy was only seen in normotensive rather than hypertensive animals, and no studies in old animals were identified (Table 5, Figure 4).
Table 5.
Effect of NXY-059 on infarct volume by markers of study quality
| Group | Experiments | Animals | Coefficient | 95% CI | P-value | Interaction test P-value |
|---|---|---|---|---|---|---|
| Randomization | ||||||
| Yes | 6 | 140 | −0.02 | −0.03 to 0.003 | 0.11 | – |
| Pseudo | 5 | 167 | −0.10 | −0.13 to −0.08 | <0.0001 | <0.0001* |
| No | 7 | 135 | −0.02 | −0.05 to 0.01 | 0.16 | 0.99* |
| Blinded surgery | 0.10 | |||||
| Yes | 8 | 242 | −0.04 | −0.05 to −0.02 | <0.0001 | |
| No | 10 | 200 | −0.02 | −0.05 to −0.002 | 0.03 | |
| Outcome blinded | 0.12 | |||||
| Yes | 10 | 261 | −0.04 | −0.05 to −0.02 | <0.0001 | |
| No | 8 | 181 | −0.02 | −0.04 to 0.01 | 0.16 | |
| Anaesthetic | 0.19 | |||||
| Neuroprotective | 1 | 46 | −0.23 | −0.51 to 0.06 | 0.12 | |
| Not neuroprotective | 17 | 396 | −0.04 | −0.05 to −0.02 | <0.0001 | |
| Blood pressure | <0.0001 | |||||
| Hypertensive | 5 | 92 | −0.002 | −0.02 to 0.02 | 0.88 | |
| Normotensive | 13 | 350 | −0.07 | −0.09 to −0.05 | <0.0001 | |
| Quality score | ||||||
| 2 | 4 | 61 | 0.02 | −0.02 to 0.05 | 0.34 | <0.0001† |
| 3 | 2 | 55 | −0.20 | −0.47 to 0.06 | 0.14 | 0.34† |
| 4 | 2 | 34 | −0.06 | −0.11 to −0.02 | 0.003 | 0.13† |
| 5 | 1 | 20 | 0.01 | −0.02 to 0.04 | 0.50 | <0.0001† |
| 6 | 4 | 105 | −0.07 | −0.11 to −0.34 | <0.0001 | 0.62† |
| 6.5 | 5 | 167 | −0.10 | −0.13 to −0.08 | <0.0001 | – |
Analyses based on individual animal data only. Data are coefficients per 100 mg·kg−1 of total administered NXY-059 dose with standardization for model and adjustment for time to treatment; 95% confidence intervals (95% CI) and significance. Results in bold are statistically significant.
In comparison with randomized studies.
in comparison with the highest quality studies.
NXY-059, disodium 2,4-disulphophenyl-N-tert-butylnitrone.
Figure 4.
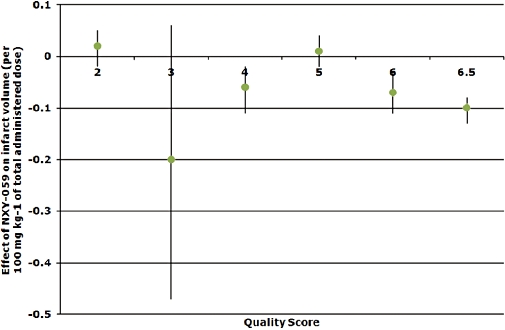
Relationship between study quality and infarct volume, coefficients per 100 mg·kg−1 of total administered NXY-059 dose. NXY-059, disodium 2,4-disulphophenyl-N-tert-butylnitrone.
Clinical phase III trials commenced in May 2003; total lesion volume was significantly reduced by NXY-059 in the 15 studies completed by this time (−0.03, 95% confidence interval −0.04 to −0.02).
Motor impairment and neglect
Data on motor impairment were available for six studies in rats (Table 6), this being recorded at 24 h post onset of occlusion in all but one study (48 h; Kuroda et al., 1999). In adjusted analyses based on IAD, impairment was reduced in rats (Table 6), this effect being present in transient and permanent but not thrombotic models.
Table 6.
Effect of NXY-059 on motor impairment in rats by model of ischaemia
| Group | Experiments | Animals | Coefficient | 95% CI | P-value |
|---|---|---|---|---|---|
| Rats | 6 | 180 | −0.06 | −0.10 to −0.02 | 0.001 |
| Transient | 4 | 130 | −0.10 | −0.16 to −0.04 | 0.001 |
| Permanent | 1 | 31 | −0.05 | −0.10 to −0.002 | 0.04 |
| Thrombotic | 1 | 19 | −0.02 | −0.14 to 0.09 | 0.67 |
Analyses based on individual animal data only. Data are coefficients per 100 mg·kg−1 of total administered NXY-059 dose with standardization for model and adjustment for time to treatment; 95% confidence intervals (95% CI) and significance. Results in bold are statistically significant.
NXY-059, disodium 2,4-disulphophenyl-N-tert-butylnitrone.
When considering two primate studies in marmosets (Marshall et al., 2001; 2003;), motor impairment was similar at baseline and reduced at 3 weeks post treatment in NXY-059-treated animals (Table 7). Similarly, neglect at 3 weeks was less in NXY-059-treated primates, an effect that was significant in females with a trend to reduced neglect being seen in males. Results for data at 10 weeks were not available for individual animals, so no analysis was possible, although both marmoset studies individually were positive at 10 weeks (Marshall et al., 2001; 2003;).
Table 7.
Effect of NXY-059 on motor impairment and neglect in marmosets (permanent ischaemia)
| Group | n | Coefficient | 95% CI | P-value |
|---|---|---|---|---|
| Motor | ||||
| Baseline | 32 | 0.03 | −0.43 to 0.49 | 0.90 |
| 3 weeks | 32 | 3.20 | 0.12 to 6.29 | 0.04 |
| Males | 17 | 2.54 | −0.58 to 5.67 | 0.11 |
| Females | 15 | 3.17 | −2.05 to 8.40 | 0.24 |
| Neglect | ||||
| Baseline | 32 | 0.004 | −0.003 to 0.01 | 0.24 |
| 3 weeks | 32 | 5.99 | 2.05 to 9.94 | 0.003 |
| Males | 17 | 5.11 | −0.72 to 10.94 | 0.09 |
| Females | 15 | 6.67 | 1.47 to 11.86 | 0.01 |
Analyses based on individual animal data only. Data are coefficients by presence or absence of NXY-059; 95% confidence intervals (95% CI) and significance. Results in bold are statistically significant.
NXY-059, disodium 2,4-disulphophenyl-N-tert-butylnitrone.
Death
No difference in death rates were present between NXY-059 (13/281, 5%) and control (6/176, 4%) animals (P= 0.4); however, death was not recorded in four studies.
NXY-059 plasma concentration
The relationship between NXY-059 dose and plasma concentration was assessed in five studies on rats (Zhao et al., 2001; Sydserff et al., 2002; Wang and Shuaib, 2004) and two on marmosets (Marshall et al., 2001; 2003;) (Table 8); some studies did not involve stroke models (Maples, 1997). A linear concentration–dose relationship was seen (Figure 5) with [NXY-059]total in plasma (mg·L−1) = 1.57 × maintenance dose (mg·kg−1 h) (P < 0.0001); a comparable relationship based on a sub-set of these studies was used by AZ and Centaur Pharmaceuticals in preparing for human studies, [NXY-059]total in plasma (µg·mL−1) = 1.7 × maintenance dose (Maples, 1997). The unbound fraction of NXY-059 in blood varies between species; figures of 30% for rats (Zhao et al., 2001; Sydserff et al., 2002; Wang and Shuaib, 2004) and 70% for marmosets (Marshall et al., 2001; 2003;) were used in calculations to assess the relationship between unbound NXY-059 and total stroke lesion size. As a result, lower maintenance doses of NXY-059 could be used in marmosets than rats to achieve the same unbound plasma concentration. Using measured (Marshall et al., 2001) or estimated unbound concentrations, Figure 6 shows the negative correlation between the effect of NXY-059 on total lesion volume and unbound plasma concentration [SMD =−0.13 − 0.02 × unbound plasma concentration (mg·L−1); P < 0.0001].
Table 8.
Plasma concentration of NXY-059
| Species | Model | n | Average weight (g) | NXY-059 dose, maintenance mg·kg−1h | Administration (h) | Total (NXY-059) in plasma, mean (SD) mg·L−1 | SMD | |
|---|---|---|---|---|---|---|---|---|
| Marshall et al. (2001) | Marmoset (common) | Permanent | 2 | ∼12 months | 16 | 24 | 41.6 (4.3) | −1.39 |
| Marshall et al. (2003) | Marmoset (common) | Permanent | 2 | 397 | 32 | 24 | 117.5 (7.0) | −0.76 |
| Zhao et al. (2001) | Rat (SHR) | Permanent | 10 | 269 | 0 | 24 | 0 | – |
| Permanent | 9 | 275 | 30 | 24 | 30.6 (19.9) | −0.91 | ||
| Permanent | 10 | 275 | 60 | 24 | 149.1 (78.9) | −1.29 | ||
| Sydserff et al. (2002) | Rat (Wistar) | Permanent | 2 | 313 | 50 | 24 | 122.4 (−) | – |
| Transient | 8 | 304 | 10 | 21.75 | 10.9 (3.2) | −0.64 | ||
| Transient | 10 | 309 | 0 | 21.75 | 0 | – | ||
| Permanent | 8 | 307 | 30 | 24 | 74.7 (37.8) | 0.24 | ||
| Permanent | 8 | 311 | 50 | 24 | 147.6 (56.1) | −1.09 | ||
| Permanent | 8 | 311 | 70 | 24 | 171.6 (73.3) | −2.35 | ||
| Zhao et al. (2002) | Rat (SHR) | Permanent | 10 | 229 | 0 | 24 | 0 | – |
| Permanent | 10 | 232 | 120 | 24 | 64.5 (30.8) | 0.29 | ||
| Wang and Shuaib (2004)‡ | Rat (Wistar) | Thrombotic | 4 | 383 | 65 | 4 | 100.7 (18.3) | −1.44 |
| Thrombotic | 4 | 383 | 200 | 4 | 399.6 (78.6) | −0.77 | ||
| Maples (1997)† | Rat (Sprague-Dawley) | † | 4 | – | 10 | 672 | 23.5 (5.4) | † |
| † | 4 | – | 55 | 672 | 116.7 (23.0) | † | ||
| † | 4 | – | 300 | 672 | 502.0 (75.0) | † |
Unbound fractions are calculated as a proportion of total concentration: marmosets 70%, rats 30%.
Maples: safety study in non-stroked animals.
Wang studies are excluded from further plasma analyses as the measurement was carried out much earlier than the others.
NXY-059, disodium 2,4-disulphophenyl-N-tert-butylnitrone; SHR, spontaneously hypertensive rats; SMD, standardized mean differences.
Figure 5.
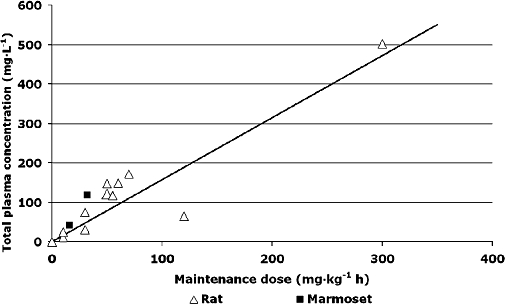
Relationship between total plasma concentration and maintenance dose of NXY-059. Equation of best fit [NXY-059]total in plasma (mg·L−1) = 1.57 × maintenance dose (mg·kg−1 h). NXY-059, disodium 2,4-disulphophenyl-N-tert-butylnitrone.
Figure 6.
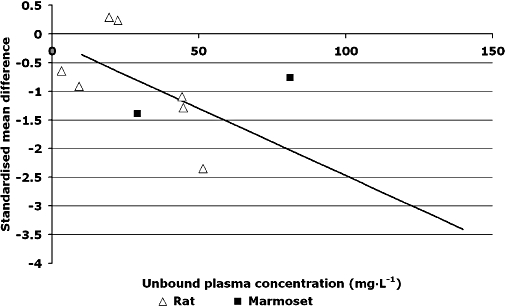
Relationship between unbound plasma concentration (mg·L−1) and standardized mean difference in infarct volume. Equation of best fit standardized mean difference=−0.13 − 0.02 × unbound plasma concentration (mg·L−1).
Discussion
This meta-analysis is the first to use IAD in any area of medicine. The results confirm, on the basis of the available data, that NXY-059 is neuroprotective in preclinical models of stroke. Specifically, treatment with NXY-059 was associated with reduced total, cortical and subcortical lesion volumes. The beneficial effects on lesion volume were seen in three species (significant in rats and marmosets, a trend in the few studied mice), and in three models of stroke – transient, permanent and thrombotic. Treatment was effective when started between 5 and 180 min after the onset of ischaemia; interestingly, the length of treatment did not appear to matter. Surprisingly, there was no evidence for a dose–response relationship in respect of both loading and maintenance dose, although such a relationship was observed in an individual study in rats designed to examine this relationship in both transient and permanent ischaemia (Sydserff et al., 2002). Furthermore, a dose relationship was observed using the plasma concentration (Figure 5) and plasma concentration has previously been shown to relate linearly to the administered dose in an individual study in rats (Sydserff et al., 2002).
In addition to effects on lesion volume, NXY-059 treatment was associated with reductions in motor impairment (in two species) and neglect (a higher cortical function) in marmosets. These data come from multiple laboratories, both commercial and academic, and were published in quality peer-reviewed journals. Superficially, these results appear to fulfil the modified STAIR criteria for demonstration of preclinical activity (see Green, 2008). And yet, the positive results in 585 animals did not translate into positive clinical data in 5028 patients with acute ischaemic stroke (Diener et al., 2008).
The data show some concerns when outcomes are judged by animal sex and blood pressure status. The interaction between sex and response was only available to study in marmosets; while females showed significant reductions in total and subcortical lesion volumes and neglect (but only a trend in cortical lesion volume and motor impairment), NXY-059 did not significantly reduce any of these parameters in males (although trends were present for many of these) (Tables 4 and 7). Nevertheless, it is likely that these findings reflect limited statistical power rather than real differences in response by sex as male rats did respond to NXY-059; point estimates for effect sizes were similar for male and female marmosets, and no differences in efficacy were seen between men and women in the SAINT-I and -II trials (Lees et al., 2006; Shuaib et al., 2007). Of more concern is the observation that only normotensive rats responded to NXY-059 (Table 5); no effect was apparent in hypertensive rats with a point estimate approximating to 0. Although this finding could also reflect a type II error, the finding was present in 92 rats across five experiments. Whether spontaneously hypertensive rats are a good model of experimental stroke is probably a moot point as the heterogeneity present in human stroke demands that interventions should be effective in a wide range of species, subspecies and co-morbidities.
It is important to assess the validity of this meta-analysis, based in the main on data from individual animals. Individual subject meta-analyses are considered the gold standard (Stewart and Parmar, 1993), partly because they allow analyses to be performed at the level of individuals rather than studies. The data on NXY-059 allowed subgroups to be studied (e.g. effects by species, sex, stroke model, treatment time, treatment length and dose) as well as interactions between variables. As individual data were not available for all animals we also used summary approaches to meta-analysis. The findings for the subset of studies with IAD were compatible with the overall summary results with any differences between these two likely to reflect the small number of studies and animals in certain analyses.
The integrity of meta-analyses depends on identifying all relevant studies, whether published or not, and then obtaining data from each study. Publication bias can lead effect size to be overestimated (if neutral or negative studies are not included) or underestimated if positive data relating to unpatented or newly patented interventions are not published. In the case of NXY-059, it is unlikely that positive data were missed as AZ shared all data known to them, including that from four unpublished studies (of which two were neutral). Importantly, AZ encouraged publication when supplying NXY-059 to independent laboratories [A.R. Green, pers. comm.], so the failure to publish may lie with either investigators, or journal referees or editors. However, data from other neutral or negative studies may have been absent as publication bias was detected using standard statistical techniques (Egger et al., 1997). Indeed, IAD from several studies were not made available by the authors (Lapchak et al., 2002a,b; 2004;) and the published data were not presented in a form suitable for inclusion in this meta-analysis. Whether these data could have contributed to the meta-analysis is unclear as it was based on a very different methodological approach, namely determining the dose of blood clot required to cause a particular lesion in the presence or absence of NXY-059. Nevertheless, it is possible that other unpublished studies exist, perhaps where NXY-059 was used as a ‘positive control’, as occurred in two included studies (Balogh et al., 2005; Takizawa et al., 2007) where one study was neutral and the other positive.
The validity of a meta-analysis is also dependent on the quality of included studies. Key factors in study quality include randomization, and blinding of the surgeon/experimenter and outcome assessor. Absence of these measures leads to selection, performance and observer bias respectively. In the case of NXY-059, there was no direct evidence that the absence of randomization and blinding led to biased results (Table 5), why studies involving pseudo-randomization were the only type to be positive is unclear, but critically there was no evidence for a gradient in efficiency from randomized to unrandomized. Nevertheless, the absence of any lesions in some animals given NXY-059 could reflect such experimental bias so that investigators were not ‘blinded’ to treatment assignment, either at the stage of surgery [so that occlusion of the middle cerebral artery (MCAo) might have been incomplete], or when outcomes were assessed. Exclusion of performance bias can be estimated experimentally by confirming that MCAo has occurred, either by direct visualization (Marshall et al., 2001; 2003;) or through assessment of blood flow using laser Doppler techniques, as done in three studies (Zhao et al., 2001; 2002; Green, 2002).
Overall study quality can be summarized as a composite score encompassing randomization and blinding factors as well as others; previous meta-analyses have found that efficacy may only be present in low-quality studies (Crossley et al., 2008). Importantly, the beneficial effects of NXY-059 on lesion volume were present across the range of study quality (Table 5). However, other forms of potential bias were present, including the exclusion from analysis of some enrolled animals (‘attrition bias’), as occurred in one study (Sydserff et al., 2002). Additionally, enrolled animals that died spontaneously or were culled for welfare reasons were not always included in analyses (Maples, 1996; Marshall et al., 2001; 2003; Green, 2002; Sydserff et al., 2002; Wang and Shuaib, 2004), thereby preventing analysis by intention-to-treat. This issue raises the question of whether all outcomes should include death, a practice that is standard in clinical trials, for example, with the modified Rankin Scale; in this respect, death is usually assigned a ‘worst’ value. A further deficit in all studies was the absence of a sample size calculation.
In summary, this meta-analysis of preclinical studies and based on data from individual animals finds that NXY-059 does have neuroprotectant properties; in particular, lesion volume, motor impairment and neglect were all reduced, although differential efficacy by sex and blood pressure status introduces some uncertainty into the robustness of these findings. The discrepancy between this conclusion and the overall neutral findings of large clinical trials (Diener et al., 2008) needs explaining. Several possibilities exist, including: (i) the results of preclinical studies are simply not relevant to humans; (ii) effective concentrations of NXY-059 in rats and marmosets were not predictive of the required concentrations needed in man; and (iii) NXY-059 has restricted access to brain tissue (Kuroda et al., 1999; Green et al., 2006). Nevertheless, the presence of several potential sources of bias in the preclinical work, especially performance, attrition and publication bias, is worrying, as is the absence of sample size calculations for most studies. Such deficits may have led to overestimation of the preclinical efficacy of NXY-059.
It has to be remembered that several of the NXY-059 preclinical studies are from the last decade with two antedating the STAIR criteria of 1999. As such, it might be harsh to judge these studies by today's standards. Nevertheless, a number of recommendations arise for the future conduct of preclinical stroke studies. First, they must embody the general principles by which clinical trials avoid bias, these resting on proper concealment of allocation (Schulz and Grimes, 2002), randomization, ‘blinding’ of the surgeon and outcome assessor to treatment assignment. Drugs should always be compared with a matching placebo, and the investigators should remain ignorant of treatment assignment until all experiments have been completed, outcomes assessed and the database locked. Studies should be designed on the basis of sample size calculations, so that they are of a sufficient size to exclude false negative findings at the 80–90% level. Occlusion of cerebral arteries should be confirmed visually or using approaches such as laser doppler. Journal editors and reviewers need to ensure that information on the above factors is always given in publications. The type and design of studies also needs to be reviewed. For example, the majority of NXY-059 experiments (18 of 34; 282 of 585 animals) tested short delays (5–30 min) between onset of ischaemia and treatment, a situation that does not mirror human stroke. Future developments should focus on studies with longer delays to treatment. Finally, sufficient numbers of female and older animals, and those with co-morbidites (such as hypertension) should be studied.
Following the initial submission of this paper, a meta-analysis of the quality of the preclinical NXY-059 data was published (Macleod et al., 2008). While several of the conclusions were similar to those published here, there are several major differences in the two studies. First, as pointed out earlier, this is the first study performed using IAD; the Macleod study used only measures of outcome lesion results from published studies. The Macleod et al. (2008) study was primarily concerned with study quality and its relationship to the outcome measures. No attempt was made to analyse neuroprotection data on the basis of time of drug administration, type of ischaemia model or dose administered. Furthermore, only the current investigation analysed both published and crucially the unpublished studies, both positive and neutral. While Macleod et al. (2008) assessed the ‘quality score’ of the study, their decision to not contact the authors for information, but rather relying on the publication, sometimes led to errors in the score given (although we would concur that all experimental details should always be included in publications, however obvious the investigators may deem them to be). For example, the Marshall et al. (2001; 2003;) studies are suggested to be unblinded whereas these were fully blinded for both allocation and outcome measures. Surprisingly, there are also other errors in the quality scores given by Macleod et al. (2008). The Marshall et al. (2001; 2003;) studies are marked down for not stating that there was temperature control of the animals, when this was clearly stated to have taken place in those publications, and points were awarded for blinded induction of ischaemia in the Yoshimoto et al. (2002a) study when there is no published evidence that this procedure was followed. These errors weaken the accuracy of the graphs presented by Macleod et al. (2008) that relate study quality to size of neuroprotection. Interestingly, we failed to find a close association between some aspects of quality, such as blinding and outcome measures.
In conclusion, we suggest that at the completion of the preclinical studies, a formal systematic review with quantitative meta-analysis (ideally using IAD) of all known studies should be performed to assess the overall effect of treatment and potential modifying factors. This meta-analysis should inform the decision to proceed to clinical studies. Researchers should be willing to share data with such meta-analyses, especially as most source studies will have been funded from either the developing pharmaceutical company or from public or charity sources (of note, the NXY-059 studies where data were not shared were funded by several organizations, including the US National Institutes of Health/National Institutes of Neurological Disorders and Stroke, US Christopher Reeve Paralysis Foundation, and US Veterans Affairs). In summary, the comprehensive reporting of results, sharing of data and a continuous improvement of experimental standards are essential to reduce the risk of having further neutral clinical trials.
Acknowledgments
We thank collaborators who shared IAD: Gyorgy Balogh and Krisztina Vukics (Hungary), Chenxu Wang and Ashfaq Shuaib (Canada), Zonghang Zhao and Alistair Buchan (Canada) and AZ for allowing full access to internal company reports.
Glossary
Abbreviations:
- AZ
AstraZeneca
- CI
confidence intervals
- IAD
individual animal data
- MCAo
middle cerebral artery occlusion
- NXY-059
disodium 2,4-disulphophenyl-N-tert-butylnitrone
- SHR
spontaneously hypertensive rats
- SMD
standardized mean differences
- STAIR
Stroke Therapy Academic Industry Roundtable
Footnotes
Conflicts of interest
A.R.G. was formerly employed by AZ and was the preclinical leader in the NXY-059 development team. P.M.W.B. chaired the Data Monitoring Committee of one of the phase II trials of NXY-059 (Lees et al., 2003) and served on the Data Monitoring Committees of the SAINT I, II and CHANT trials.(Lees et al., 2006; Lyden et al., 2007; Shuaib et al., 2007). L.J.G. is funded, in part, by the Medical Research Council (G0501797, G0501997). P.M.W.B. is Stroke Association Professor of Stroke Medicine.
References
- Balogh GT, Vukics K, Konczol A, Kis-Varga A, Gere A, Fischer J. Nitrone derivatives of trolox as neuroprotective agents. Bioorg Med Chem Lett. 2005;15:3012–3015. doi: 10.1016/j.bmcl.2005.04.043. [DOI] [PubMed] [Google Scholar]
- Bederson JB, Pitts LH, Tsuji M, Nishimura MC, Davis RL, Bartkowski H. Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination. Stroke. 1986;17:472–476. doi: 10.1161/01.str.17.3.472. [DOI] [PubMed] [Google Scholar]
- Crossley NA, Sena E, Goehler J, Horn J, van der Worp B, Bath PMB, et al. Empirical evidence of bias in the design of experimental stroke studies: a meta-epidemiologic approach. Stroke. 2008;39:929–934. doi: 10.1161/STROKEAHA.107.498725. [DOI] [PubMed] [Google Scholar]
- Diener HC, Lees KR, Lyden P, Grotta J, Davalos A, Davis SM, et al. NXY-059 for the treatment of acute stroke: pooled analysis of the SAINT I and II Trials. Stroke. 2008;39:1751–1758. doi: 10.1161/STROKEAHA.107.503334. [DOI] [PubMed] [Google Scholar]
- Edenius C, Strid S, Borga O, Breitholtz-Emanuelsson A, Lanbeck Vallén K, Fransson B. Pharmacokinetics of NXY-059, a nitrone-based free radical trapping agent, in health young and eldery subjects. J Stroke Cerebrovasc Dis. 2002;11:34–43. doi: 10.1053/jscd.2002.123973. [DOI] [PubMed] [Google Scholar]
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Br Med J. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson CL, Gray LJ, Murphy SP, Bath PMB. Estrogen and experimental ischemic stroke: a systematic review. J Cereb Blood Flow Metab. 2006;26:1103–1113. doi: 10.1038/sj.jcbfm.9600270. [DOI] [PubMed] [Google Scholar]
- Gibson CL, Gray LJ, Bath PMW, Murphy S. Progesterone for the treatment of experimental brain injury; a systematic review. Brain. 2007;131:318–328. doi: 10.1093/brain/awm183. [DOI] [PubMed] [Google Scholar]
- Green AR. NXY-059: Effect in a transient and permanent focal ischemia model in rats. AstraZeneca Internal Report D1550-SP-0001.
- Green AR. Pharmacological approaches to acute ischaemic stroke: reperfusion certainly, neuroprotection possibly. Br J Pharmacol. 2008;153:S325–S338. doi: 10.1038/sj.bjp.0707594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green AR, Lanbeck-Vallén K, Ashwood T, Lundquist S, Lindström Böö E, Jonasson H, et al. Brain penetration of the novel free radical trapping neuroprotectant NXY-059 in rats subjected to permanent focal ischemia. Brain Res. 2006;1072:224–226. doi: 10.1016/j.brainres.2005.12.035. [DOI] [PubMed] [Google Scholar]
- Han M, He QP, Yong G, Siesjo BK, Li PA. NXY-059, a nitrone with free radical trapping properties inhibits release of cytochrome C after focal cerebral ischemia. Cell Mol Biol. 2003;49:1249–1252. [PubMed] [Google Scholar]
- Koziol JA, Feng AC. On the analysis and interpretation of outcome measures in stroke clinical trials. Lessons from the SAINT I study of NXY-059 for acute ischemic stroke. Stroke. 2006;37:2644–2647. doi: 10.1161/01.STR.0000241106.81293.2b. [DOI] [PubMed] [Google Scholar]
- Kuroda S, Tsuchidate R, Smith M-L, Maples KR, Siejo BK. Neuroprotective effects of a novel nitrone, NXY-059, after transient focal cerebral ischemia in the rat. J Cereb Blood Flow Metab. 1999;19:778–787. doi: 10.1097/00004647-199907000-00008. [DOI] [PubMed] [Google Scholar]
- Lapchak PA, Araugo DM, Song D, Wei J, Zivin JA. Neuroprotective effects of the spin trap agent disodium-[(tert-butylimino)methyl] benzene-1,3-disulfonate N-oxide (generic NXY-059) in rabbit small clot embolic stroke model. Combination studies with the thrombolytic tissue plasminogen activitor. Stroke. 2002a;33:1411–1415. doi: 10.1161/01.str.0000015346.00054.8b. [DOI] [PubMed] [Google Scholar]
- Lapchak PA, Araugo DM, Song D, Wei J, Purdy R, Zivin JA. Effects of the spin trap agent disodium-[(tert-butylimino)methyl]benzene-1,3-disulfonate N-oxide (generic NXY-059) on intracerebral hemorrhage in a rabbit large clot embolic stroke model. Combination studies with tissue plasminogen activator. Stroke. 2002b;33:1665–1670. doi: 10.1161/01.str.0000017145.22806.aa. [DOI] [PubMed] [Google Scholar]
- Lapchak PA, Song D, Wei J, Zivin JA. Coadministration of NXY-059 and tenecteplase six hours following embolic strokes in rabbits improves clinical rating scores. Exp Neurol. 2004;188:279–285. doi: 10.1016/j.expneurol.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Lees KR, Sharma AK, Barer D, Ford GA, Kostulas V, Cheng YF, et al. Tolerability and Pharmacokenetics of the nitrone NXY-059 in patients with acute stroke. Stroke. 2001;32:675–680. doi: 10.1161/01.str.32.3.675. [DOI] [PubMed] [Google Scholar]
- Lees KR, Barer D, Ford GA, Hacke W, Kostulas V, Sharma AK, et al. Tolerability of NXY-059 at higher target concentrations in patients with acute stroke. Stroke. 2003;34:482–487. doi: 10.1161/01.str.0000053032.14223.81. [DOI] [PubMed] [Google Scholar]
- Lees KR, Zivin JA, Ashwood T, Davalos A, Davis SM, Diener H-C, et al. NXY-059 for acute ischemic stroke. N Engl J Med. 2006;354:588–599. doi: 10.1056/NEJMoa052980. [DOI] [PubMed] [Google Scholar]
- Lyden PD, Shuaib A, Lees KR, Davalos A, Davis SM, Diener H-C, et al. Safety and tolerability of NXY-059 for acute intracerebral haemorrhage. The CHANT Trial. Stroke. 2007;38:2262–2269. doi: 10.1161/STROKEAHA.106.472746. [DOI] [PubMed] [Google Scholar]
- Macleod MR, O'Collins T, Horky LL, Howells DW, Donnan GA. Systematic review and meta-analysis of the efficacy of FK506 in experimental stroke. J Cereb Blood Flow Metab. 2005;25:713–721. doi: 10.1038/sj.jcbfm.9600064. [DOI] [PubMed] [Google Scholar]
- Macleod MR, van der Worp B, Sena ES, Howells DW, Dirnagl U, Donnan GA. Evidence for the efficacy of NXY-059 in experimental focal cerebral ischemia is confounded by study quality. Stroke. 2008;39:2824–2829. doi: 10.1161/STROKEAHA.108.515957. [DOI] [PubMed] [Google Scholar]
- Maples KR. NXY-059 reduces cortical infarct volume when given 30 min after a permanent middle cerebral artery occlusion. Astra Internal Report 805-61 AN 030-31.
- Maples KR. Estimation of NXY-059 plasma levels following continuous intravenous infusion at 0.30, 3.0, or 30 mg/kg/h. Centaur Internal Report BOR1-R1.
- Maples KR, Ma F, Zhang YK. Comparison of the radical trapping ability of PBN, S-PBN and NXY-059. Free Radic Res. 2001;43:417–426. doi: 10.1080/10715760100300351. [DOI] [PubMed] [Google Scholar]
- Marshall JWB, Duffin KJ, Green AR, Ridley RM. NXY-059, a free radical-trapping agent, substantially lessens the functional disability resulting from cerebral ischemia in a primate species. Stroke. 2001;32:190–198. doi: 10.1161/01.str.32.1.190. [DOI] [PubMed] [Google Scholar]
- Marshall JWB, Cummings RM, Bowes LJ, Ridley RM, Green AR. Functional and Histological Evidence for the protective effect of NXY-059 in a primate model of stroke when given 4 hours after occlusion. Stroke. 2003;34:2228–2233. doi: 10.1161/01.STR.0000087790.79851.A8. [DOI] [PubMed] [Google Scholar]
- Schulz KF, Grimes DA. Allocation concealment in randomized trials: defending against deciphering. Lancet. 2002;359:614–618. doi: 10.1016/S0140-6736(02)07750-4. [DOI] [PubMed] [Google Scholar]
- Shuaib A, Lees KR, Lyden P, Grotta J, Davalos A, Davis SMD, et al. NXY-059 for the treatment of acute ischemic stroke. N Engl J Med. 2007;357:562–571. doi: 10.1056/NEJMoa070240. [DOI] [PubMed] [Google Scholar]
- Stewart LA, Parmar MK. Meta-analysis of the literature or of individual patient data: is there a difference? Lancet. 1993;341:418–422. doi: 10.1016/0140-6736(93)93004-k. [DOI] [PubMed] [Google Scholar]
- Strid S, Borga O, Edenius C, Jostell K-G, Odergren T, Weil A. Pharmacokinetics in renally impaired subjects of NXY-059, a nitrone-based, free-radical trapping agent developed for the treatment of acute stroke. J Clin Pharmacol. 2002;58:409–415. doi: 10.1007/s00228-002-0478-x. [DOI] [PubMed] [Google Scholar]
- Stroke Therapy Academic Industry Roundtable (STAIR) Recommendations for standards regarding preclinical neuroprotective and restorative drug development. Stroke. 1999;30:2752–2758. doi: 10.1161/01.str.30.12.2752. [DOI] [PubMed] [Google Scholar]
- Sydserff S. NXY-059: effects on infarct volume in a transient middle cerebral artery occlusion model. AstraZeneca Report D1550-SP-0002-01.
- Sydserff SG, Borelli AR, Green AR, Cross AJ. Effect of NXY-059 on infarct volume after transient or permanent middle cerebral artery occlusion in the rat; studies on dose, plasma concentration and therapeutic time window. Br J Pharmacol. 2002;135:103–112. doi: 10.1038/sj.bjp.0704449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takizawa S, Izuhara Y, Kitao Y, Hori O, Ogawa S, Morita Y, et al. A novel inhibitor of advanced glycation and endoplasmic reticulum stress reduces infarct volume in rat focal cerebral ischemia. Brain Res. 2007;1183:124–137. doi: 10.1016/j.brainres.2007.07.006. [DOI] [PubMed] [Google Scholar]
- The Optimising Analysis of Stroke Trials (OAST) Collaboration. Can we improve the statistical analysis of stroke trials? Statistical re-analysis of functional outcomes in stroke trials. Stroke. 2007;38:1911–1915. doi: 10.1161/STROKEAHA.106.474080. [DOI] [PubMed] [Google Scholar]
- Wang CX, Shuaib A. NXY-059: a neuroprotective agent in acute stroke. Int J Clin Pract. 2004;58:964–969. doi: 10.1111/j.1368-5031.2004.00245.x. [DOI] [PubMed] [Google Scholar]
- Williams HE, Claybourn M, Green AR. Investigating the radical trapping ability of NXY-059, S-PBN and PBN. Free Radic Res. 2007;49:1047–1052. doi: 10.1080/10715760701557161. [DOI] [PubMed] [Google Scholar]
- Willmot M, Gray L, Gibson C, Murphy S, Bath PMW. Systematic review of nitric oxide donors and L-arginine in experimental stroke; effects on infarct size and cerebral blood flow. Nitric Oxide. 2005a;12:141–149. doi: 10.1016/j.niox.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Willmot M, Gibson C, Gray L, Murphy S, Bath PMW. Nitric oxide synthase inhibitors in experimental stroke and their effects on infarct size and cerebral blood flow; a systematic review. Free Rad Biol Med. 2005b;39:412–425. doi: 10.1016/j.freeradbiomed.2005.03.028. [DOI] [PubMed] [Google Scholar]
- Yoshimoto T, Kanakaraj P, Ma JY, Cheng M, Kerr I, Malaiyandi L, et al. NXY-059 maintains Akt activation and inhibits release of cytochrome C after focal cerebral ischemia. Brain Res. 2002a;947:191–198. doi: 10.1016/s0006-8993(02)02922-0. [DOI] [PubMed] [Google Scholar]
- Yoshimoto T, Kristian T, Hu B, Ouyang Y-B, Siesjo BK. Effect of NXY-059 on secondary mitochondrial dsyfunction after transient focal ischemia; comparison with cyclosporin A. Brain Res. 2002b;932:99–109. doi: 10.1016/s0006-8993(02)02286-2. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Cheng M, Maples KR, Ma JY, Buchan AM. NXY-059 a novel free radical trapping compound, reduces cortical infarction after permanent focal cerebral ischemia in the rat. Brain Res. 2001;909:46–50. doi: 10.1016/s0006-8993(01)02618-x. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Cheng M, Maples KR, Ying Ma J, Buchan AM. Continuous IV infusion of NXY-059 at 120 mg/kg/h fails to achieve expected exposure levels and thus does not reduce cortical infarction after permanent focal cerebral ischemia in the rat. Internal Report to AstraZeneca.


