Abstract
Background and purpose:
Human pancreatic carcinoma is a highly malignant cancer. Previous studies have shown that the decoy receptor 3 (DcR3) for Fas ligand (FasL) plays significant roles in tumour progression and immune suppression. In the present study, we evaluated the anti-cancer activity of a natural compound, denbinobin (5-hydroxy-3,7-dimethoxy-1,4-phenanthraquinone), through decreasing DcR3 levels in human pancreatic adenocarcinoma cell lines.
Experimental approach:
We used immunoprecipitation and ELISA assays to examine DcR3 levels, and used FACS to determine the percentage of cells with a sub-G1 DNA content.
Key results:
AsPC-1 and BxPC-3 human pancreatic cancer cells express high levels of DcR3. Denbinobin concentration-dependently decreased DcR3 levels in BxPC-3 cells. MTT and flow cytometry assays indicated that BxPC-3 was FasL-resistant because high concentrations (100 ng·mL−1) of soluble FasL did not inhibit cell growth. However, combinations of denbinobin (3 µmol·L−1) with lower concentrations of soluble FasL (10, 30 and 50 ng·mL−1) or membrane-bound FasL, were synergistic on cell growth inhibition and apoptosis. Exogenous excess DcR3 reversed this synergistic effect. We observed no significant increase in the levels of surface Fas, cleaved forms of caspase-8, -3, -9, Bax, Bid, Bcl-xL, cytochrome c or mitochondrial membrane potentials following denbinobin treatment. However, denbinobin treatment increased the levels of apoptosis-inducing factor.
Conclusions and implications:
Denbinobin and FasL trigger a synergistic cytotoxic effect in human pancreatic adenocarcinoma cells. Denbinobin mediated a decrease in levels of DcR3, which played a major role in this synergistic effect, and also increased caspase-independent apoptosis, via apoptosis-inducing factor.
Keywords: Fas ligand, decoy receptor 3, denbinobin, synergistic effect, apoptosis
Introduction
Recent statistics for the United States indicate that human pancreatic carcinoma has the highest fatality rate of all cancers and ranks fourth among all cancer-related deaths (Jemal et al., 2008). Surgical resection is the only cure, but more than 80% of pancreatic cancer patients have inoperable cancer (Ko et al., 2007). This disease is usually diagnosed at a late, incurable stage and the 5 year survival rate is less than 5% (Ko et al., 2007; O'Reilly and Abou-Alfa, 2007). In addition, pancreatic cancer is relatively resistant to many forms of therapy. Recent studies of the use of cytotoxic therapy, such as gemcitabine/fluoropyrimidine or platinum-based therapy, have achieved median survival times of fewer than 10 months (Heinemann et al., 2007; O'Reilly and Abou-Alfa, 2007). The 1 year survival rate of patients following gemcitabine/radiation treatment was also very low (<35%) (Mishra et al., 2005; Russo et al., 2007). Development of an effective therapeutic agent for pancreatic cancer is therefore a high priority.
The binding of Fas to Fas ligand (FasL) is an important trigger for apoptosis. During carcinogenesis, there is a balance between anti-tumour immunity and tumour-originated pro-inflammatory activity, a process that weakens anti-tumour immunity (Kim et al., 2006). When host-mediated anti-tumour activity is weaker, tumour cells undergo immune escape and grow rapidly (Lin and Karin, 2007). Thus, resistance to apoptosis is believed to be one of the hallmarks of cancer (Hanahan and Weinberg, 2000). Recent studies show that pancreatic and other cancer cells, in spite of their expression of Fas, develop mechanisms that make them resistant to FasL/Fas-mediated growth inhibition (Walker et al., 1997; von Bernstorff et al., 1999; Elsasser-Beile et al., 2003). Several mechanisms may be responsible for the decreased sensitivity to Fas-mediated apoptosis, such as down-regulation of Fas (which is associated with a p53 mutation) or up-regulation of the decoy receptor for FasL [decoy receptor 3 (DcR3)] (Kim et al., 2006). In addition, surviving tumour cells increase the expression of FasL and this leads to a counterattack against Fas-sensitive immune cells and, ultimately, to tumour progression and metastasis (Kim et al., 2006; 2007;).
The DcR3 is one of the tumour necrosis factor receptor superfamily. The binding of DcR3 to FasL, LIGHT and TL1A neutralizes the pro-apoptotic effects of Fas-FasL, LIGHT-LTβR and TL1A-DR3 (Pitti et al., 1998; Yu et al., 1999; Roth et al., 2001; Migone et al., 2002; Tsuji et al., 2003; Yang et al., 2004). Recent evidence has demonstrated that DcR3 is overexpressed in cells from malignant tumours, such as those of the oesophagus, stomach, glioma, lung, colon, rectum and pancreas (Pitti et al., 1998; Bai et al., 2000; Ohshima et al., 2000; Roth et al., 2001; Takahama et al., 2002; Tsuji et al., 2003). In addition, high serum levels of DcR3 have been detected in many cancer patients (Wu et al., 2003). A recent study demonstrated a correlation between the level of DcR3 and FasL-induced apoptosis (Li et al., 2007). DcR3 is also an important modulator of angiogenesis (Yang et al., 2004).
Therefore, it is possible that overexpression of DcR3 may promote growth and survival of tumour cells. In support of this, tumour cells that were engineered to release large amounts of DcR3 were protected from FasL-induced apoptosis and chemotaxis, resulting in decreased immune cell infiltration in glioma xenografts (Roth et al., 2001). In addition, an elevated level of DcR3 has been associated with poor prognoses in cancer patients (Takahama et al., 2002).
Denbinobin (5-hydroxy-3,7-dimethoxy-1,4-phenanthraquinone), a biologically active, natural product isolated from Ephemerantha lonchophylla, has anti-inflammatory, antioxidant and anti-tumourigenic activities (Lee et al., 1995; Chen et al., 1999; Lin et al., 2001; Huang et al., 2005). In this study, we examined the effects of denbinobin on the expression of DcR3 in human pancreatic cancer cells and assessed the cytotoxic effect of denbinobin when it was combined with FasL.
Methods
Cell culture
Human pancreatic adenocarcinoma cells BxPC-3 and AsPC-1, human pancreatic epithelioid carcinoma PANC-1, human colon adenocarcinoma cells SW480 and HT-29, human T cell leukaemia Jurkat, Clone E6-1 were obtained from the American Type Culture Collection (Manassas, VA, USA), and cultured in the media recommended by the vendor (RPMI-1640 medium for BxPC-3, AsPC-1 and Jurkat cells; Dulbecco's modified Eagle's medium for PANC-1 cells; Minimum essential medium Eagle for HT-29 cells; Leibovitz's L-15 medium for SW480 cells) supplemented with 10% (v/v) foetal bovine serum (InvitrogenTM Life Technologies, Carlsbad, CA, USA), 100 U·mL−1 of penicillin and 100 µg·mL−1 of streptomycin (Biological Industries, Kibbutz Beit Haemek, Israel) at 37°C in a humidified atmosphere of 5% CO2 in air. SW480 cells were maintained at 37°C without CO2.
Cell viability assay
Cell viability was measured by the colourimetric 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay. Cells (1 × 104) were cultured in 100 µL medium in 96-well plates and incubated with vehicle or test compound for 48 h. After various treatments, 1 mg·mL−1 MTT was added to culture plates and incubated at 37°C for an additional 2 h. Then cells were pelleted and lysed in 100 µL dimethyl sulphoxide. The absorbance at 550 nm was measured on a microplate reader. To assess cell death mediated by membrane-bound FasL (mFasL), 1 × 104 BxPC3 cells were seeded in 96-well plates containing 100 µL medium. After 24 h, the medium was replaced by the one containing denbinobin 3 µmol·L−1 and/or 5 × 103 Jurkat cells, fixed with 2% paraformaldehyde in phosphate-buffered saline (PBS) at 4°C for 1 h. After 48 h incubation, MTT assays were performed as described above. Each experiment was performed in duplicate and repeated five times.
Flow cytometry
Cells were harvested, washed twice with FACS washing buffer (1% foetal bovine serum and 0.1% NaN3 in PBS), incubated with antibodies at 4°C for 30 min, and washed three times with FACS washing buffer. Fluorescence of cells was then analysed using a FACScan flow cytometer (Becton Dickinson, Mountain View, CA, USA). To detect cell cycle progression, the cells were incubated with or without the indicated agent for 24 h, washed twice with ice-cold PBS, collected by centrifugation and fixed in 70% (v/v) ethanol for at least 2 h at −20°C. The cells were then incubated with 0.2 mL of DNA extraction buffer (0.2 mol·L−1 Na2HPO4 and 0.1 mol·L−1 citric acid buffer, pH 7.8) for 30 min at room temperature, centrifuged at 3500 ×g for 1 min at 25°C, and resuspended in 1 mL of propidium iodide staining buffer (0.1% Triton X-100, 100 µg·mL−1 of RNase A, 80 µg·mL−1 of propidium iodide in PBS), incubated at 37°C for 30 min in the dark, then sorted by flow cytometry (FACScan; Becton Dickinson, Bedford, MA) and analysed using CellQuest software (Becton Dickinson). The cell cycle distribution is shown as the percentage of cells containing G0/G1, S, G2 and M DNA as judged by propidium iodide staining. The apoptotic population was determined as the percentage of cells with a sub-G1 (<G1) DNA content.
Determination of the change of mitochondrial membrane potential
Changes of mitochondrial membrane potential were assessed using a fluorometric probe, rhodamine 6G, as previously described (Chen et al., 2008). Briefly, cells were treated with vehicle or denbinobin for 12 or 24 h. Thirty minutes before the termination of incubation, a rhodamine 6G solution (10 µmol·L−1) was added to the cell suspension and incubated for the last 30 min at 37°C. The cells were harvested, and the accumulation of rhodamine 6G was determined using FACScan flow cytometry.
Western blot analysis
Cells were incubated for 10 min at 4°C in lysis buffer (20 mmol·L−1 HEPES, pH 7.4, 2 mmol·L−1 EGTA, 50 mmol·L−1β-glycerophosphate, 0.1% Triton X-100, 10% glycerol, 1 mmol·L−1 DTT, 1 µg·mL−1 of leupeptin, 5 µg·mL−1 of aprotinin, 1 mmol·L−1 phenylmethylsulphonyl fluoride and 1 mmol·L−1 sodium orthovanadate), then were scraped off, incubated on ice for a further 10 min, and centrifuged at 100 g for 30 min at 4°C. The whole cell extract (120 µg of proteins) was mixed with an equal volume of SDS sample buffer (62.5 mmol·L−1 Tris-HCl, pH 6.8, 2% SDS, 1% glycerol, 300 mmol·L−1 2-mercaptoethanol and 0.00125% bromophenol blue) and the mixture heated at 95°C for 5 min, separated by electrophoresis on 10% SDS gels, and the proteins transferred onto polyvinylidene fluoride membranes. Immunoblotting was performed using the relevant rabbit or mouse antibody and the corresponding horseradish peroxidase-conjugated second antibody, following by detection using ECL reagents (Amersham Biosciences) and exposure to photographic film.
Immunoprecipitation assay
Cell culture supernatants were collected and concentrated 30-fold (v/v) on an Amicon Ultra centrifugal filter device (Millipore, Billerica, MA, USA), then 5 mg of concentrated supernatant was immunoprecipitated overnight at 4°C with 1 µg of mouse monoclonal anti-DcR3 antibody and A/G-agarose beads. The precipitated beads were washed three times with 1 mL of ice-cold cell lysis buffer, and the immune complex resolved by 10% SDS-PAGE gel electrophoresis, followed by immunoblotting assay using anti-DcR3 antibody.
Small interference RNA suppression assay
Cells (1 × 106) were plated in 6 cm dishes in 2 mL medium without serum 1 day before transfection. The cells were transfected with 160 nmol·L−1 DcR3 small interference RNA (siRNA) duplexes or with DcR3 non-silence control using Lipofectamine 2000. The siRNA-transfected cells were incubated for 48 h after transfection before analysis. The 21-mer siRNAs were synthesized by Invitrogen. The DcR3 siRNA sequences were: sense sequence, 5′-GCC AGG CUC UUC CUC CCA UdTdT-3′; antisense sequence, 5′-AUG GGA GGA AGA GCC UGG CdTdT-3′. The non-silence control siRNA sequences were: sense sequence, 5′-GCC CGC UUU CCC UCA GCA UdTdT-3′; antisense sequence, 5′-AUG CUG AGG GAA AGC GGG C-3′.
ELISA assay
Cell culture supernatants were collected at various time points. Levels of DcR3 were quantified using commercial ELISA kits (R&D Systems, Minneapolis, MN, USA), according to the vendor's instructions.
Data analysis
The data are expressed as the mean ± SEM and were analysed statistically using one-way anova. When anova showed significant differences between groups, Tukey's post hoc test was used to determine the specific pairs of groups showing statistically significant differences. A P-value of less than 0.05 was considered statistically significant.
Materials
Denbinobin was extracted and purified by one of our colleagues (C.C.C.) and the purity was more than 98% by HPLC and NMR analyses. Rabbit polyclonal antibodies specific for caspase-9, Bid and mouse monoclonal anti-human caspase-8 were purchased form Cell Signaling Technology (Danvers, MA, USA). Rabbit polyclonal anti-endonuclease G and other chemicals were obtained from Sigma-Aldrich (St. Louis, MO, USA). Mouse monoclonal antibodies specific for DcR3, FasL, Fas, Bax, apoptosis-inducing factor (AIF), Bcl-xL, cytochrome c and GAPDH were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Mouse monoclonal anti-caspase-3 was obtained from Imgenex (San Diego, CA, USA). Recombinant human soluble FasL (sFasL) was purchased from PeproTech Asia (Rehovot, Israel). ELISA kit for human DcR3 and human DcR3/Fc chimera were purchased from R&D Systems (Minneapolis, MN, USA).
Results
DcR3 expression in human pancreatic carcinoma cells
We used immunoprecipitation and ELISA assays to investigate the presence of DcR3 in different human pancreatic cancer cells. Human colon cancer cell line HT-29 was the negative control and line SW480 was the positive control for DcR3 expression. As shown in Figure 1A, we detected a high level of DcR3 protein in two human pancreatic adenocarcinoma cell lines (AsPC-1 and BxPC-3), but not in another human pancreatic carcinoma cell line (PANC-1) (Figure 1 A and B). A previous study also showed that BxPC-3 cells express high levels of DcR3 (Yang et al., 2005). Thus, we selected the BxPC-3 cells for the subsequent experiments.
Figure 1.
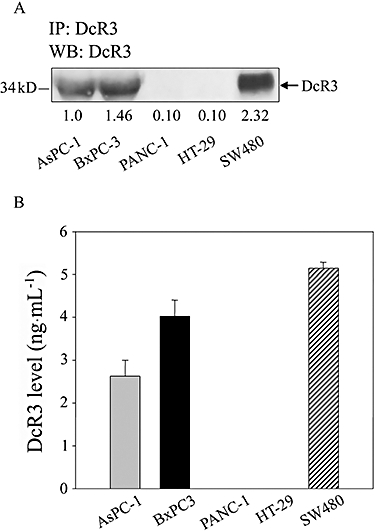
Immunoprecipitation and ELISA assessment of decoy receptor 3 (DcR3) protein expression in three pancreatic cancer cell lines. (A) Equal amounts of concentrated media from cells were immunoprecipitated (IP) with 1 µg of the DcR3 antibody, followed by immunblot (WB) analysis. (B) Cells (1 × 105) were cultured in 24-well plates. After 24 h, we collected supernatants and measured the DcR3 levels by ELISA. HT-29 or SW480 cells were used as negative and positive controls for DcR3 expression respectively. Data represent the mean ± SEM from five independent experiments.
Denbinobin decreased DcR3 expression and synergistically enhanced sFasL-induced apoptosis in human pancreatic cancer cells
Denbinobin has been reported to induce an anti-cancer effect in colon cancer, lung cancer and leukaemia cells (Huang et al., 2005; Yang et al., 2005; Chen et al., 2008; Kuo et al., 2008). Our results show that denbinobin (0.3–3 µmol·L−1; 24 h) decreased DcR3 expression in a concentration-dependent manner (Figure 2A). These treatments had no significant effect on cell viability, assessed using the MTT assay (Figure 2B).
Figure 2.
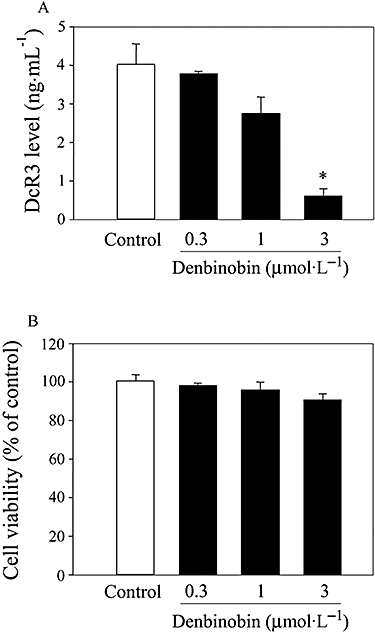
Denbinobin decreased decoy receptor 3 (DcR3) expression in BxPC-3 cells. Cells (1 × 105) were cultured in 24-well plates for 24 h and then treated with denbinobin as indicated concentrations for another 24 h. Culture media were collected and the levels of DcR3 were measured by ELISA. Data represent the mean ± SEM from four independent experiments. *P < 0.05 as compared with the control group.
A previous study showed that 100 ng·mL−1 sFasL only caused a mild (less 20%) growth inhibition in BxPC-3 cells (Tsuji et al., 2003). Thus, we studied whether the denbinobin-mediated decrease in DcR3 would affect sFasL resistance in human pancreatic cancer cells. We first measured sFasL-mediated cell growth inhibition in different human pancreatic cancer cells. As shown in Figure 3A, treatment with 100 ng·mL−1 sFasL for 48 h did not affect cell growth in BxPC-3 and AsPC-1 cells, but significantly inhibited growth of PANC-1 cells (cells that do not express DcR3). Also, incubation with a range of concentrations of sFasL (10–100 ng·mL−1) for 48 h did not significantly affect growth of BxPC-3 cells (Figure 3B, left-hand panel). In addition, treatment of these cells with 3 µmol·L−1 denbinobin for 48 h only mildly suppressed cell viability to 85% of control. By contrast, combination of this low concentration of denbinobin with sFasL (50 ng·mL−1) dramatically reduced viability to less than 20% of control (Figure 3B, centre panel). This Figure also shows that synergistic effects on cell viability were also observed with lower concentrations of sFasL (30, 10 ng·mL−1), combined with denbinobin (3 µmol·L−1). Moreover, these synergistic effects of denbinobin and FasL on cell growth were reversed by adding exogenous DcR3 (20 ng·mL−1; Figure 3B, centre panel).
Figure 3.
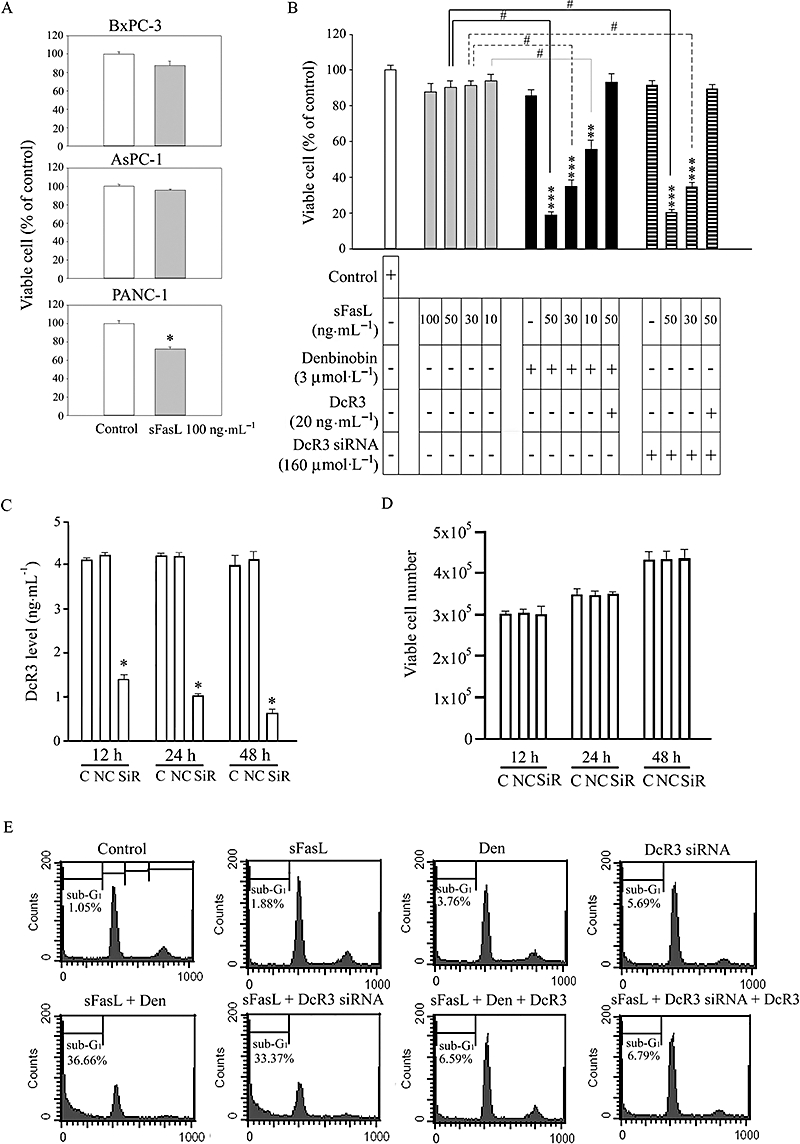
Denbinobin synergistically enhanced soluble recombinant human Fas ligand (sFasL)-induced apoptosis in human pancreatic cancer cells. (A) Cells (1 × 104) were treated with sFasL 100 ng·mL−1 for 48 h and viable cell numbers were examined by the MTT assay. (B) BxPC-3 cells (1 × 104) were cultured for 24 h. Cell viability after treatment with different concentrations of sFasL, 3 µmol·L−1 denbinobin and transfection of 160 nmol·L−1 DcR3 siRNA in the absence or presence of decoy receptor 3 (DcR3)/Fc (20 ng·mL−1) for 24 h were detected by MTT assay. Data represent the mean ± SEM from four independent experiments. **P < 0.01 and ***P < 0.001 as compared with the control group respectively. #P < 0.01 as compared with different groups as indicated. (C) BxPC-3 cells (1 × 105) were transiently transfected with 160 nmol·L−1 DcR3 siRNA (SiR) or non-silence control siRNA (NC), then supernatants were collected at the indicated time and DcR3 levels measured by ELISA. The data are the mean ± SEM for five separate experiments. *P < 0.05 compared with the control group respectively. (D) Cells (2 × 105) were transfected as indicated for different time periods and viable cell numbers measured using the MTT assay. The data are the mean ± SEM for five separate experiments. (E) BxPC-3 cells were treated with different reagents as indicated for 24 h. Then, the cells were fixed and stained with propidium iodide to analyse DNA content by the FACScan flow cytometer. The cell cycle phase (sub-G1, G0/G1, S, G2/M) is indicated. Sub-G1 phase is indicative of apoptosis. Three independent experiments were performed.
To validate further the DcR3 dependence of this synergistic effect, we transfected BxPC-3 cells with DcR3 siRNA (160 nmol·L−1) to test whether inhibition of DcR3 expression by siRNA, combined with sFasL treatment could cause a similar synergistic inhibition of cell growth. Figure 3C shows that transfection of BxPC-3 cells with DcR3 siRNA did significantly reduce DcR3 levels, this effect being first seen at 12 h and maintained for at least 48 h. Figure 3D shows that transfection with siRNA for DcR3 by itself had no effect on cell viability when compared with the control group over a 48 h incubation. However, when BxPC-3 cells were transfected with DcR3 siRNA for 24 h and then were treated with lower concentrations of sFasL (30, 50 ng·mL−1) for 24 h, there was a clearly synergistic inhibition of cell viability (Figure 3B, right hand panel). Note also that this decreased viability was reversed by addition of exogenous DcR3 (20 ng·mL−1).
FACS analysis of cell cycle distribution shows that denbinobin (3 µmol·L−1) and sFasL (50 ng·mL−1) separately had no effect on the number of BxPC3 cells in the sub-G1 phase (Figure 3E). However, when given together, these compounds acted synergistically to increase the number of cells in sub-G1 phase (more than 30%) and this effect was reversed by exogenous DcR3 (20 ng·mL−1) treatment. Similar synergistic effects on cell numbers in sub-G1 phase were observed for cells transfected with DcR3 siRNA when combined with sFasL treatment; and again 20 ng·mL−1 exogenous DcR3 reversed this synergistic effect (Figure 3E). Taken together, these results indicate that the synergistic effect of denbinobin combined with sFasL to inhibit cell growth and induce apoptosis in BxPC-3 cells was dependent on decreased DcR3 levels.
Denbinobin exerted DcR3-dependent synergism with mFasL to induce apoptosis
mFasL, which mediates apoptosis by oligomerizing the Fas receptor of target cells, is the primary mediator of apoptosis in the immune system (Tsuji et al., 2003). A previous study reported that phytohemagglutinin A (PHA, 10 µg·mL−1) stimulated expression of mFasL in null Jurkat cells (Xerri et al., 1997). Thus, we initially used this established system to evaluate the effect of denbinobin with the mFasL. Figure 4A shows that significant FasL staining was seen following PHA treatment of Jurkat cells. Activated Jurkat cells co-cultured with BxPC3 significantly inhibited viability to just under 80% control (Figure 4B). Both groups with combined treatment, that is, denbinobin or DcR3 siRNA transfection plus activated Jurkat cells, synergistically and markedly reduced viability of BxPC3 cells to about 10% of control values (Figure 4B). Cell cycle distribution analysis indicated that the combination treatments induced the accumulation of a significant number of cells in the sub-G1 phase compared with those in the single treatment groups (Figure 4C), whether denbinobin or DcR3 siRNA transfection was combined with mFasL on Jurkat cells. As observed before, exogenous DcR3 (20 ng·mL−1) reversed the effect of the combination treatments (Figure 4B and C). These results suggest that denbinobin can act synergistically with the mFasL to inhibit cell growth and induce apoptosis, via lowering levels of DcR3.
Figure 4.
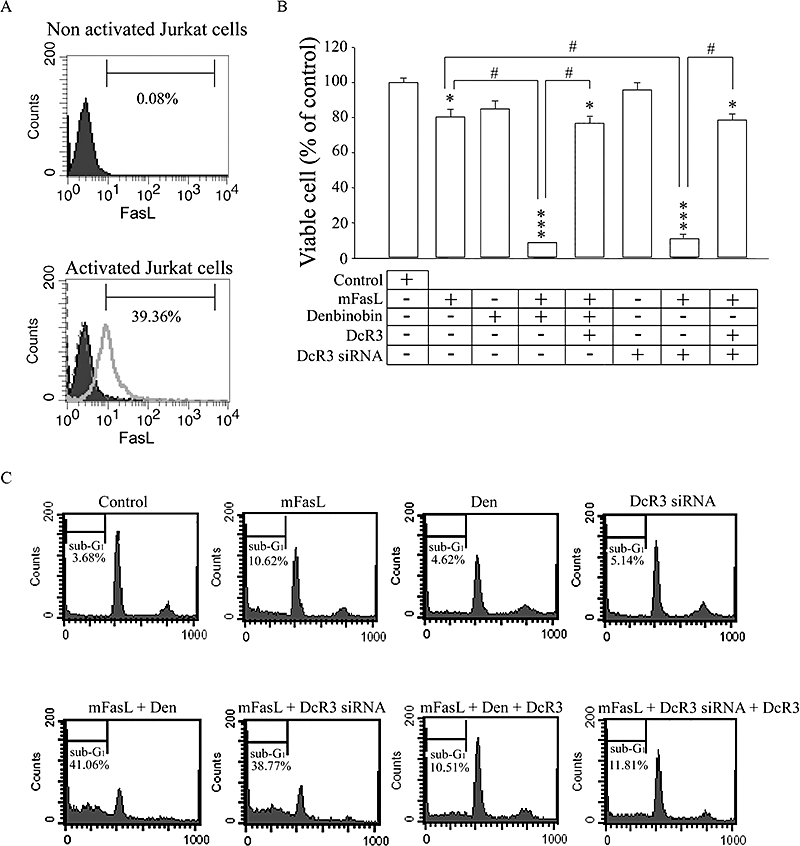
Denbinobin synergized with membrane-bound Fas ligand (mFasL) on activated Jurkat T cells, in a decoy receptor 3 (DcR3)-dependent manner. (A) Jurkat T cells were cultured in the absence (solid black area) or presence (grey line) of 10 µg·mL−1 of phytohemagglutinin A for 2 h. After incubation, cells were treated with specific FasL antibody to detect the surface expression of FasL by FACScan flow cytometry. Jurkat T cells marked with anti-mouse FITC antibody (dashed grey line) served as the negative control. (B) BxPC-3 cells were seeded onto 24-well plates at a density of 1 × 104 per well. In another group, BxPC-3 cells were transfected with DcR3 siRNA (160 nmol·L−1). After 24 h, medium was replaced with medium containing 3 µmol·L−1 denbinobin and/or 20 ng·mL−1 DcR3/Fc or 5 × 103 paraformaldehyde-fixed activated Jurkat T cells. MTT assays were performed after 24 h co-culturing. Data represent the mean ± SEM from four experiments. *P < 0.05 and ***P < 0.001 as compared with the control group respectively. #P < 0.01 as compared with different groups as indicated. (C) BxPC-3 cells were treated with vehicle (control), 3 µmol·L−1 denbinobin (Den) or transfected with 160 nmol·L−1 DcR3 siRNA as indicated; these cells were then co-cultured with activated Jurkat cells (as a source of mFasL) for 24 h. Analysis of DNA content was performed by a FACScan flow cytometer. Sub-G1 phase was indicative of apoptosis. Three independent experiments were performed.
Denbinobin alone induced apoptotic effects by increasing AIF release, but not through increasing Fas expression or activation of extrinsic/intrinsic pathway
Because denbinobin alone suppressed viable cell growth to 85% of the control (Figure 3B), we looked for the mechanisms of this denbinobin-mediated cytotoxic effect in BxPC-3 cells. We first investigated whether treatment with denbinobin alone could increase Fas expression on human pancreatic cancer cells and thus contribute to the synergistic apoptotic effect with FasL. Using FACScan analysis, no significant increase of Fas was detected on any of the three human pancreatic cancer cell lines after treatment with 3 µmol·L−1 denbinobin for 12 h or 24 h (Figure 5).
Figure 5.
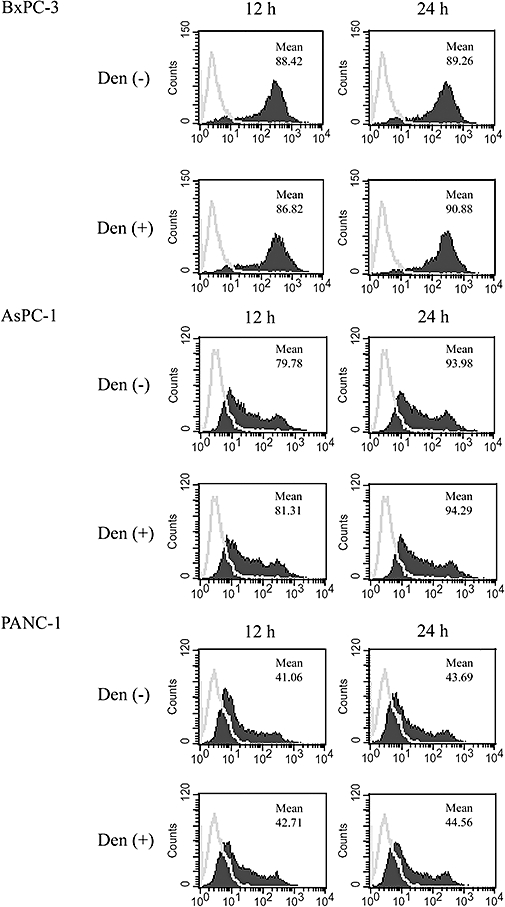
Denbinobin did not change Fas expression on human pancreatic tumour cells. Cells (1 × 106) incubated in the absence (grey line) or presence (solid black area) of 3 µmol·L−1 of denbinobin for 12 or 24 h. After incubation, cells were treated with specific Fas antibody to detect the surface expression of Fas by FACScan cytometry. The mean values of fluorescence intensity are indicated. The results shown are representative of three experiments with similar results.
The process of FasL/Fas-mediated apoptosis has been well characterized (von Reyher et al., 1998). FasL binding to Fas triggers the major extrinsic signal pathway by the formation of the death-inducing signalling complex to activate caspase-8, leading to the rapid activation of caspase-3 and cell death. Another signal pathway, the minor intrinsic pathway, activates the mitochondrial apoptotic activity (Krammer et al., 1998; von Reyher et al., 1998). We therefore looked for effects of denbinobin on caspases in BxPC-3 cells. As shown in Figure 6, incubation of BxPC-3 cells with 50 ng·mL−1 sFasL induced significant cleavages of caspase-8 and caspase-3 but not of caspase-9, whereas incubation with denbinobin alone showed no obvious cleavages of caspases-8, -3 or -9, suggesting that denbinobin by itself did not trigger the extrinsic pathway in BxPC-3 cells. However, the combination of denbinobin and sFasL increased cleavage of caspase-8 and caspase-3 (2.09 ± 0.32-fold vs. 1.65 ± 0.27-fold and 3.86 ± 0.43-fold vs. 2.93 ± 0.35-fold of control respectively). These results suggest that FasL was more effective via the extrinsic pathway when DcR3 levels were reduced by denbinobin.
Figure 6.
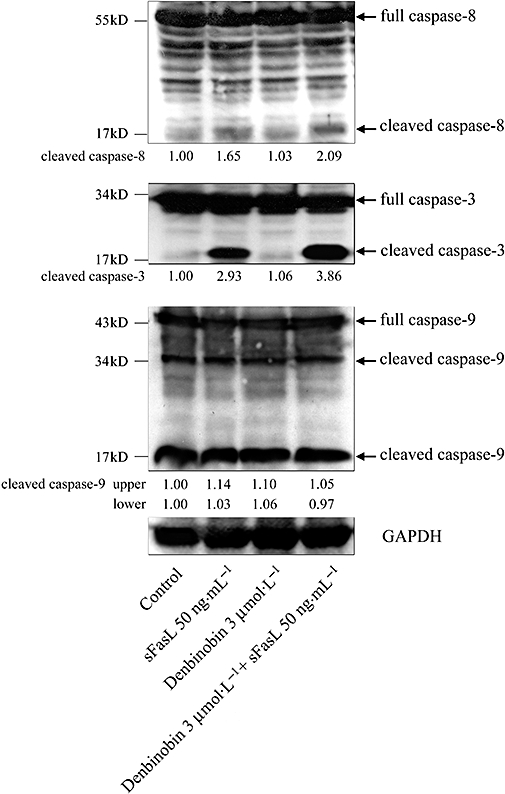
Denbinobin increased the Fas ligand (FasL)-mediated cleavage of caspase-8 and caspase-3 in BxPC-3 cells. Cells were incubated with soluble FasL (sFasL) (50 ng·mL−1), denbinobin (3 µmol·L−1) or both as indicated for 24 h. Cells were harvested and prepared for quantification of the protein levels of caspases-8, -3, -9 and GAPDH (internal control) by Western blot analysis. The numbers below each record are the mean quantitative data obtained by a densitometer. The results shown are representative of three independent experiments.
In another set of experiments, the mitochondrial membrane potential in BxPC-3 cells was unaffected by incubation with denbinobin alone (3 µmol·L−1) for up to 24 h (Figure 7A). A number of proteins are critically involved in apoptosis and the effects of sFasL, denbinobin and their combination on some of these proteins were studied, relative to control cells (Figure 7B). The results showed that sFasL, denbinobin or the combination did not change levels of Bax, endonuclease G, the cleaved form of Bid, Bcl-xL or cytochrome c. Only the levels of AIF were significantly increased by denbinobin alone, but not by sFasL alone. These results suggest that treatment with denbinobin alone induced its apoptotic effect via increasing AIF release, but not through activation of the extrinsic or the intrinsic pathway.
Figure 7.
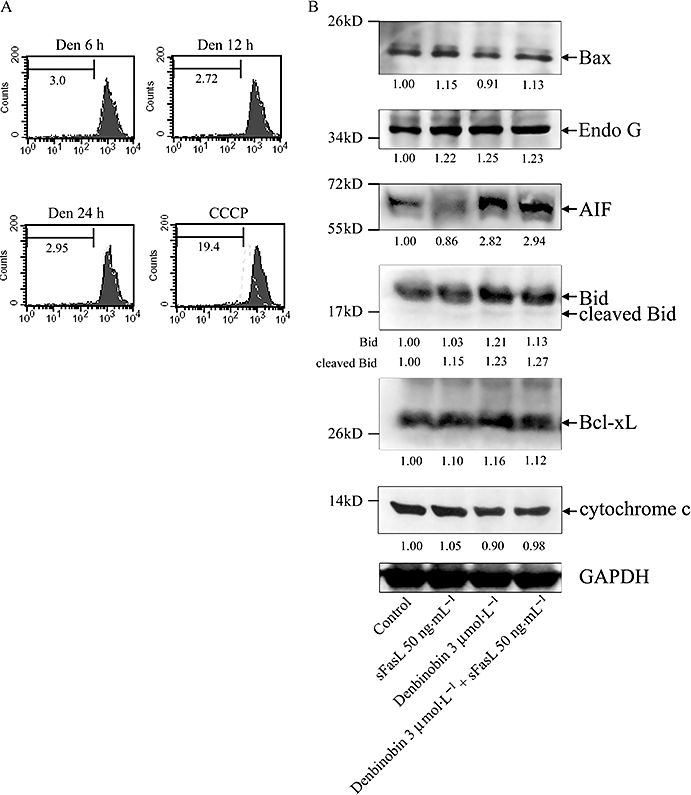
Denbinobin-induced apoptosis involved apoptosis-inducing factor (AIF). (A) BxPC-3 cells were treated with vehicle (solid black area) or 3 µmol·L−1 denbinobin (dashed grey line) for indicated times. After treatment, the mitochondrial membrane potential was assessed, as described in the Methods. Data acquisition and analysis were performed on a FACScan flow cytometry. 10 µmol·L−1 carbonyl cyanide m-chlorophenylhydrazone (CCCP) was used as positive control. (B) BxPC-3 cells were incubated with soluble Fas ligand (sFasL) (50 ng·mL−1), denbinobin (3 µmol·L−1) or both as indicated for 24 h. Then, cells were harvested and prepared for quantification of the protein levels of Bax, endonuclease G (Endo G), AIF, Bid, Bcl-xL, cytochrome c and GAPDH (internal control) by Western blot analysis. Quantitative data obtained by a densitometer are given below the records. The results shown are representative of three independent experiments.
In summary, our results demonstrated that denbinobin and FasL triggered a synergistic cytotoxic effect in human pancreatic adenocarcinoma BxPC-3 cells. The denbinobin-mediated decrease in the level of DcR3 played a primary role in this synergistic effect. However, denbinobin could also trigger a cytotoxic effect via a mechanism involving increased release of AIF.
Discussion
Tumour escape and recurrence are major impediments for successful cancer therapy. Several studies have indicated that down-regulation of Fas expression or loss of Fas function correlates with tumour progression in humans (Walker et al., 1997; von Reyher et al., 1998; von Bernstorff et al., 1999). However, other studies showed that the malignant cells in pancreatic adenocarcinoma (von Bernstorff et al., 1999), colon cancer (von Reyher et al., 1998), hepatocellular carcinoma (Krammer et al., 1998), acute lymphoblastic leukaemia (Inaba et al., 2005), stomach cancer (Lee et al., 2003), prostate cancer (Hyer et al., 2002) and non-small cell lung cancer (Lee et al., 1999) did not undergo apoptosis despite Fas expression. This suggests that, in cancer cells, the normal regulatory pathways leading to Fas-mediated apoptosis are disrupted. There are two major mechanisms by which tumour cells can develop decreased sensitivity to Fas-mediated apoptosis: (i) cells may down-regulate or disrupt the function of Fas expression on the cell surface (von Bernstorff et al., 1999; Ivanov et al., 2003); and (ii) cells may disrupt Fas-mediated apoptotic signalling by secreting DcR3 (Pitti et al., 1998; Li et al., 2007) or by increasing the expression of cellular-FLICE inhibitory protein (Elnemr et al., 2001; Lee et al., 2003). DcR3 plays a major role in allowing cancer cells to evade attack by the immune system (Pitti et al., 1998; Elnemr et al., 2001; Li et al., 2007). Additional studies have demonstrated that there is a significant correlation between the overexpression of DcR3 and resistance to FasL-mediated apoptosis in cancer cells (Pitti et al., 1998; Elnemr et al., 2001; Shen et al., 2005). Thus, DcR3 is a potential target for treatment of cancer (Wu et al., 2003; Shen et al., 2005).
In this study we demonstrated that denbinobin, a biologically active natural product isolated from E. lonchophylla, decreased the levels of DcR3 in human pancreatic adenocarcinoma cells. We also showed that combined treatment with denbinobin and FasL had a synergistic effect on inhibition of growth of pancreatic cancer cells and on apoptosis. Denbinobin alone also caused a minor suppression of cell growth in pancreatic cancer cells. This led us to ask whether the denbinobin/FasL-mediated synergistic effect was mainly attributable to the denbinobin-mediated reduction of DcR3 levels or to a direct cytotoxic effect. To investigate this question, we treated pancreatic cancer cells with DcR3 siRNA and exogenous DcR3. The results showed that transfection of DcR3 siRNA into BxPC-3 cells mimicked denbinobin-mediated synergy with FasL and that exogenous DcR3 reversed this effect (Figure 3E). This indicates that the denbinobin/FasL synergism results from a reduction in the level of DcR3. It is accepted that mFasL is the primary mediator of apoptosis in the immune system. Thus, to mimic the mediation of cytotoxicity by infiltrating T cells, we co-cultured pancreatic cancer cells with PHA-activated Jurkat cells that express mFasL. Synergy was again observed after both combination treatments (denbinobin or DcR3 siRNA plus PHA-activated Jurkat cells) on inhibition of cell growth and apoptosis in this co-culture system (Figure 4B and C) and excess DcR3 treatment reversed these effects. We also found that combined denbinobin/FasL treatment increased the cleavage of caspase-8 and caspase-3, compared with cleavage following FasL alone. Taken together, our results suggest that the denbinobin-mediated decrease in the levels of DcR3 significantly enhanced FasL-mediated apoptosis in human pancreatic cancer cells.
However, these results do not rule out the contribution of denbinobin-mediated direct cytotoxic effects in denbinobin/FasL treatment. Western blot analysis showed no significant effect of denbinobin on cleavage of caspase-8, -3, and -9. This implied that a denbinobin-mediated cytotoxic effect did not occur through the extrinsic pathway. In the intrinsic pathway, it has been suggested that the Bcl-2 family proteins are involved in the regulation of apoptosis (Shih et al., 2003). Previous reports indicated that there are two opposing factions of the Bcl-2 family: pro-survival proteins (such as Bcl-2, Bcl-xL) and pro-apoptotic proteins (such as Bax, Bak) (Adams and Cory, 1998). Bcl-2 directly or indirectly prevents the release of cytochrome c from mitochondria (Adams and Cory, 1998). On the other hand, Bax directly triggers cytochrome c release from mitochondria via channel-forming activity, and thereby activates caspase-9, which in turn activates caspase-3 and -7 (Adams and Cory, 1998; Yang et al., 2005). In our study, denbinobin had no effect on the levels of caspase-9, Bcl-xL, Bid, Bax, cytochrome c and mitochondrial membrane potential, indicating that the cytotoxic effects mediated by denbinobin alone did not occur through activation of the intrinsic pathway.
Our results thus suggest that denbinobin may mediate a caspase-independent apoptotic mechanism. It was recently suggested that translocation of apoptogenic factors (such as endonuclease G, AIF) from the mitochondria into the cytosol and then the nucleus, is an indication of caspase-independent cell death (Cande et al., 2002; Shih et al., 2003). In normal cells, compartmentalization of endonuclease G in the mitochondrial intermembrane space prevents genomic DNA degradation. In response to apoptotic signals, mitochondria release endonuclease G and this cleaves chromatin DNA into nucleosomal fragments, independently of caspases (Li et al., 2001). Bid, a member of the pro-apoptotic Bcl-2 family, plays a pivotal role in the release of endonuclease G (Li et al., 2001). In the present study, we have not observed significant changes in endonuclease G and cleaved Bid in response to denbinobin treatment, suggesting that denbinobin did not regulate endonuclease G-related mechanisms. AIF was first identified as a mitochondrial flavoprotein that, upon apoptotic stimulation, translocates to the nucleus and induces large-scale DNA fragmentation and chromatin condensation (Susin et al., 1999). Our results showed that denbinobin treatment mediated a significant increase in the levels of AIF. Similar results have been described in the human colorectal cancer cell line, HCT-116 (Chen et al., 2008). This indicates that denbinobin has a caspase-independent cytotoxic effect in human pancreatic cancer cells.
In summary, our study demonstrated that treatment with combinations of denbinobin and FasL treatment triggered a synergistic cytotoxic effect in human pancreatic adenocarcinoma cells. Denbinobin mediated a decrease in levels of DcR3, which plays a major role in this synergistic effect, and also increased caspase-independent apoptosis. These results indicate that denbinobin has potential applications in the treatment of human pancreatic cancer.
Acknowledgments
Grant support: National Science Council of Taiwan (NSC96-2320-B002-034 and NSC97-2320-B-002-019-MY3).
Glossary
Abbreviations:
- AIF
apoptosis-inducing factor
- DcR3
decoy receptor 3
- DR3
death receptor 3
- FasL
Fas ligand
- LTβR
lymphotoxin β receptor
- mFasL
membrane-bound FasL
- sFasL
soluble FasL
Footnotes
Statement of conflicts of interest
The authors state no conflict of interest.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Figure S1 Effects of cytotoxic agents on DcR3 expression in BxPC-3 cells. Cells (1 × 105) were cultured in 24-well plates for 24 h and treated with paclitaxel or doxorubicin at the indicated concentration for another 24 h, then the culture medium was collected and DcR3 levels measured by ELISA. The data are the mean ± S.E.M. for three separate experiments. * p < 0.05 compared to the control group.
Figure S2 Cytotoxic effects of denbinobin combined with paclitaxel or doxorubicin treatment in (A) BxPC-3 and (B) PANC-1 human pancreatic cancer cell lines. Cells (1 × 105) were cultured in 24-well plates for 24 h and treated with different drugs at indicated concentration for another 24 h. Cell viability compared to the control group was estimated using the MTT assay. The data are the mean ± S.E.M. for three separate experiments. * p < 0.05 compared to the control group.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Adams JM, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science. 1998;281:1322–1326. doi: 10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]
- Bai C, Connolly B, Metzker ML, Hilliard CA, Liu X, Sandig V, et al. Overexpression of M68/DcR3 in human gastrointestinal tract tumors independent of gene amplification and its location in a four-gene cluster. Proc Natl Acad Sci U S A. 2000;97:1230–1235. doi: 10.1073/pnas.97.3.1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Bernstorff W, Spanjaard RA, Chan AK, Lockhart DC, Sadanaga N, Wood I, et al. Pancreatic cancer cells can evade immune surveillance via nonfunctional Fas (APO-1/CD95) receptors and aberrant expression of functional Fas ligand. Surgery. 1999;125:73–84. doi: 10.1067/msy.2099.93570. [DOI] [PubMed] [Google Scholar]
- Cande C, Cohen I, Daugas E, Ravagnan L, Larochette N, Zamzami N, et al. Apoptosis-inducing factor (AIF): a novel caspase-independent death effector released from mitochondria. Biochimie. 2002;84:215–222. doi: 10.1016/s0300-9084(02)01374-3. [DOI] [PubMed] [Google Scholar]
- Chen HY, Shiao MS, Huang YL, Shen CC, Lin YL, Kuo YH, et al. Antioxidant principles from Ephemerantha lonchophylla. J Nat Prod. 1999;62:1225–1227. doi: 10.1021/np990025f. [DOI] [PubMed] [Google Scholar]
- Chen TH, Pan SL, Guh JH, Chen CC, Huang YT, Pai HC, et al. Denbinobin induces apoptosis by apoptosis-inducing factor releasing and DNA damage in human colorectal cancer HCT-116 cells. Naunyn Schmiedebergs Arch Pharmacol. 2008;378:447–457. doi: 10.1007/s00210-008-0324-5. [DOI] [PubMed] [Google Scholar]
- Elnemr A, Ohta T, Yachie A, Kayahara M, Kitagawa H, Fujimura T, et al. Human pancreatic cancer cells disable function of Fas receptors at several levels in Fas signal transduction pathway. Int J Oncol. 2001;18:311–316. doi: 10.3892/ijo.18.2.311. [DOI] [PubMed] [Google Scholar]
- Elsasser-Beile U, Gierschner D, Welchner T, Wetterauer U. Different expression of Fas and Fas ligand in tumor infiltrating and peripheral lymphocytes of patients with renal cell carcinomas. Anticancer Res. 2003;23:433–437. [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Heinemann V, Labianca R, Hinke A, Louvet C. Increased survival using platinum analog combined with gemcitabine as compared to single-agent gemcitabine in advanced pancreatic cancer: pooled analysis of two randomized trials, the GERCOR/GISCAD intergroup study and a German multicenter study. Ann Oncol. 2007;18:1652–1659. doi: 10.1093/annonc/mdm283. [DOI] [PubMed] [Google Scholar]
- Huang YC, Guh JH, Teng CM. Denbinobin-mediated anticancer effect in human K562 leukemia cells: role in tubulin polymerization and Bcr-Abl activity. J Biomed Sci. 2005;12:113–121. doi: 10.1007/s11373-004-8171-y. [DOI] [PubMed] [Google Scholar]
- Hyer ML, Sudarshan S, Kim Y, Reed JC, Dong JY, Schwartz DA, et al. Downregulation of c-FLIP sensitizes DU145 prostate cancer cells to Fas-mediated apoptosis. Cancer Biol Ther. 2002;1:401–406. doi: 10.4161/cbt.1.4.15. [DOI] [PubMed] [Google Scholar]
- Inaba H, Shimada K, Zhou YW, Ido M, Buck S, Yonehara S, et al. Acquisition of Fas resistance by Fas receptor mutation in a childhood B-precursor acute lymphoblastic leukemia cell line, MML-1. Int J Oncol. 2005;27:573–579. [PubMed] [Google Scholar]
- Ivanov VN, Lopez Bergami P, Maulit G, Sato TA, Sassoon D, Ronai Z. FAP-1 association with Fas (Apo-1) inhibits Fas expression on the cell surface. Mol Cell Biol. 2003;23:3623–3635. doi: 10.1128/MCB.23.10.3623-3635.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- Kim R, Emi M, Tanabe K, Arihiro K. Tumor-driven evolution of immunosuppressive networks during malignant progression. Cancer Res. 2006;66:5527–5536. doi: 10.1158/0008-5472.CAN-05-4128. [DOI] [PubMed] [Google Scholar]
- Kim R, Emi M, Tanabe K. Cancer immunoediting from immune surveillance to immune escape. Immunology. 2007;121:1–14. doi: 10.1111/j.1365-2567.2007.02587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko AH, Quivey JM, Venook AP, Bergsland EK, Dito E, Schillinger B, et al. A phase II study of fixed-dose rate gemcitabine plus low-dose cisplatin followed by consolidative chemoradiation for locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2007;68:809–816. doi: 10.1016/j.ijrobp.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Krammer PH, Galle PR, Moller P, Debatin KM. CD95 (APO-1/Fas)-mediated apoptosis in normal and malignant liver, colon, and hematopoietic cells. Adv Cancer Res. 1998;75:251–273. doi: 10.1016/s0065-230x(08)60744-7. [DOI] [PubMed] [Google Scholar]
- Kuo CT, Hsu MJ, Chen BC, Chen CC, Teng CM, Pan SL, et al. Denbinobin induces apoptosis in human lung adenocarcinoma cells via Akt inactivation, Bad activation, and mitochondrial dysfunction. Toxicol Lett. 2008;177:48–58. doi: 10.1016/j.toxlet.2007.12.009. [DOI] [PubMed] [Google Scholar]
- Lee SH, Shin MS, Park WS, Kim SY, Kim HS, Han JY, et al. Alterations of Fas (Apo-1/CD95) gene in non-small cell lung cancer. Oncogene. 1999;18:3754–3760. doi: 10.1038/sj.onc.1202769. [DOI] [PubMed] [Google Scholar]
- Lee SH, Kim HS, Kim SY, Lee YS, Park WS, Kim SH, et al. Increased expression of FLIP, an inhibitor of Fas-mediated apoptosis, in stomach cancer. APMIS. 2003;111:309–314. doi: 10.1034/j.1600-0463.2003.1110203.x. [DOI] [PubMed] [Google Scholar]
- Lee YH, Park JD, Baek NI, Kim SI, Ahn BZ. In vitro and in vivo antitumoral phenanthrenes from the aerial parts of Dendrobium nobile. Planta Med. 1995;61:178–180. doi: 10.1055/s-2006-958043. [DOI] [PubMed] [Google Scholar]
- Li L, Luo X, Wang X. Endonuclease G is an apoptotic DNase when released from mitochondria. Nature. 2001;412:95–99. doi: 10.1038/35083620. [DOI] [PubMed] [Google Scholar]
- Li W, Zhang C, Chen C, Zhuang G. Correlation between expression of DcR3 on tumor cells and sensitivity to FasL. Cell Mol Immunol. 2007;4:455–460. [PubMed] [Google Scholar]
- Lin TH, Chang SJ, Chen CC, Wang JP, Tsao LT. Two phenanthraquinones from Dendrobium moniliforme. J Nat Prod. 2001;64:1084–1086. doi: 10.1021/np010016i. [DOI] [PubMed] [Google Scholar]
- Lin WW, Karin M. A cytokine-mediated link between innate immunity, inflammation, and cancer. J Clin Invest. 2007;117:1175–1183. doi: 10.1172/JCI31537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migone TS, Zhang J, Luo X, Zhuang L, Chen C, Hu B, et al. TL1A is a TNF-like ligand for DR3 and TR6/DcR3 and functions as a T cell costimulator. Immunity. 2002;16:479–492. doi: 10.1016/s1074-7613(02)00283-2. [DOI] [PubMed] [Google Scholar]
- Mishra G, Butler J, Ho C, Melin S, Case LD, Ennever PR, et al. Phase II trial of induction gemcitabine/CPT-11 followed by a twice-weekly infusion of gemcitabine and concurrent external beam radiation for the treatment of locally advanced pancreatic cancer. Am J Clin Oncol. 2005;28:345–350. doi: 10.1097/01.coc.0000159559.42311.c5. [DOI] [PubMed] [Google Scholar]
- O'Reilly EM, Abou-Alfa GK. Cytotoxic therapy for advanced pancreatic adenocarcinoma. Semin Oncol. 2007;34:347–353. doi: 10.1053/j.seminoncol.2007.05.009. [DOI] [PubMed] [Google Scholar]
- Ohshima K, Haraoka S, Sugihara M, Suzumiya J, Kawasaki C, Kanda M, et al. Amplification and expression of a decoy receptor for Fas ligand (DcR3) in virus (EBV or HTLV-1) associated lymphomas. Cancer Lett. 2000;160:89–97. doi: 10.1016/s0304-3835(00)00567-x. [DOI] [PubMed] [Google Scholar]
- Pitti RM, Marsters SA, Lawrence DA, Roy M, Kischkel FC, Dowd P, et al. Genomic amplification of a decoy receptor for Fas ligand in lung and colon cancer. Nature. 1998;396:699–703. doi: 10.1038/25387. [DOI] [PubMed] [Google Scholar]
- von Reyher U, Strater J, Kittstein W, Gschwendt M, Krammer PH, Möller P. Colon carcinoma cells use different mechanisms to escape CD95-mediated apoptosis. Cancer Res. 1998;58:526–534. [PubMed] [Google Scholar]
- Roth W, Isenmann S, Nakamura M, Platten M, Wick W, Kleihues P, et al. Soluble decoy receptor 3 is expressed by malignant gliomas and suppresses CD95 ligand-induced apoptosis and chemotaxis. Cancer Res. 2001;61:2759–2765. [PubMed] [Google Scholar]
- Russo S, Butler J, Ove R, Blackstock AW. Locally advanced pancreatic cancer: a review. Semin Oncol. 2007;34:327–334. doi: 10.1053/j.seminoncol.2007.05.007. [DOI] [PubMed] [Google Scholar]
- Shen HW, Gao SL, Wu YL, Peng SY. Overexpression of decoy receptor 3 in hepatocellular carcinoma and its association with resistance to Fas ligand-mediated apoptosis. World J Gastroenterol. 2005;11:5926–5930. doi: 10.3748/wjg.v11.i38.5926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih CM, Wu JS, Ko WC, Wang LF, Wei YH, Liang HF, et al. Mitochondria-mediated caspase-independent apoptosis induced by cadmium in normal human lung cells. J Cell Biochem. 2003;89:335–347. doi: 10.1002/jcb.10488. [DOI] [PubMed] [Google Scholar]
- Susin SA, Lorenzo HK, Zamzami N, Marzo I, Snow BE, Brothers GM, et al. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature. 1999;397:441–446. doi: 10.1038/17135. [DOI] [PubMed] [Google Scholar]
- Takahama Y, Yamada Y, Emoto K, Fujimoto H, Takayama T, Ueno M, et al. The prognostic significance of overexpression of the decoy receptor for Fas ligand (DcR3) in patients with gastric carcinomas. Gastric Cancer. 2002;5:61–68. doi: 10.1007/s101200200011. [DOI] [PubMed] [Google Scholar]
- Tsuji S, Hosotani R, Yonehara S, Masui T, Tulachan SS, Nakajima S, et al. Endogenous decoy receptor 3 blocks the growth inhibition signals mediated by Fas ligand in human pancreatic adenocarcinoma. Int J Cancer. 2003;106:17–25. doi: 10.1002/ijc.11170. [DOI] [PubMed] [Google Scholar]
- Walker PR, Saas P, Dietrich PY. Role of Fas ligand (CD95L) in immune escape: the tumor cell strikes back. J Immunol. 1997;158:4521–4524. [PubMed] [Google Scholar]
- Wu Y, Han B, Sheng H, Lin M, Moore PA, Zhang J, et al. Clinical significance of detecting elevated serum DcR3/TR6/M68 in malignant tumor patients. Int J Cancer. 2003;105:724–732. doi: 10.1002/ijc.11138. [DOI] [PubMed] [Google Scholar]
- Xerri L, Devilard E, Hassoun J, Haddad P, Birg F. Malignant and reactive cells from human lymphomas frequently express Fas ligand but display a different sensitivity to Fas-mediated apoptosis. Leukemia. 1997;11:1868–1877. doi: 10.1038/sj.leu.2400815. [DOI] [PubMed] [Google Scholar]
- Yang CR, Hsieh SL, Teng CM, Ho FM, Su WL, Lin WW. Soluble decoy receptor 3 induces angiogenesis by neutralization of TL1A, a cytokine belonging to TNF superfamily and exhibiting angiostatic action. Cancer Res. 2004;64:1122–1129. doi: 10.1158/0008-5472.can-03-0609. [DOI] [PubMed] [Google Scholar]
- Yang KC, Uen YH, Suk FM, Liang YC, Wang YJ, Ho YS, et al. Molecular mechanisms of denbinobin-induced anti-tumorigenesis effect in colon cancer cells. World J Gastroenterol. 2005;11:3040–3045. doi: 10.3748/wjg.v11.i20.3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu KY, Kwon B, Ni J, Zhai Y, Ebner R, Kwon BS. A newly identified member of tumor necrosis factor receptor superfamily (TR6) suppresses LIGHT-mediated apoptosis. J Biol Chem. 1999;274:13733–13736. doi: 10.1074/jbc.274.20.13733. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


