Abstract
The endogenous protein, tumour necrosis factor receptor apoptosis-inducing ligand (TRAIL), induces apoptosis in a wide variety of transformed and cancer cells but has little or no effect on normal cells. Therefore, TRAIL is considered to be a tumour-selective, apoptosis-inducing cytokine and a promising new candidate for cancer prevention and treatment. Some cancer cells are however resistant to TRAIL-induced apoptosis, but treatment in combination with conventional chemotherapeutic drugs or radiation generally restores TRAIL sensitivity in those cells. A novel class of molecules exhibiting synergy with TRAIL but devoid of major side effects are emerging as alternative approaches to treat resistant cancer cells, including natural antioxidants such as sulphoraphane or the flavonoids curcumin, quercetin, resveratrol, baicalein and wogonin. In this issue of the BJP, Lee et al. demonstrate that treatment of TRAIL-resistant cancer cells with wogonin restores TRAIL-induced cell death in a reactive oxygen species-dependent manner through up-regulation of p53 and Puma.
Keywords: TRAIL, antioxidants, apoptosis, wogonin, ROS, cell death, tumour, flavonoid, p53, Puma
The endogenous protein, tumour necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL), is a member of the TNF superfamily and has been shown to induce apoptosis in cancer cells but not normal cells; for this reason, TRAIL has become a recognized target for cancer therapy (Ashkenazi et al., 2008). TRAIL binds to TRAIL-R1 or TRAIL-R2, two death domain-containing receptors, also called DR4 and DR5, to trigger apoptosis. Unfortunately, a considerable range of cancer cells, especially some highly malignant tumours, are resistant to induction of apoptosis by TRAIL. Resistance to TRAIL can occur at different points in the signalling pathways of TRAIL-induced apoptosis, including mutations in the death receptors, defects in the molecules involved in formation of the death-inducing signalling complex (DISC), dysregulation of DISC activation by TRAIL receptor antagonists or by over-expression of cellular FLICE-like inhibitory protein (cFLIP) (Merino et al., 2007). Therefore, developing strategies to overcome such resistance are important for the successful use of TRAIL for cancer therapy.
A large number of studies have revealed that combination of TRAIL with chemotherapy or radiotherapy significantly enhances cytotoxicity to tumours or reverses the resistance to monotherapy (Merino et al., 2007). However, the use of conventional chemotherapeutic drugs or radiotherapy in association with TRAIL could be limited due to their severe toxic side effects and their potential to induce cell death processes in both malignant and non-malignant cells (Meurette et al., 2006). In recent years, efforts have been focused on the development of biologically based strategies to enhance the anti-tumour activity of TRAIL without the toxic side effects of chemotherapy or radiotherapy (Ashkenazi et al., 2008). Among these, the flavonoids and glucosinolates, which are ‘natural antioxidants’ known to scavenge free radicals, have emerged as promising compounds to overcome resistance of cancer cells to TRAIL, while having little or no effect on normal cells (Ishibashi and Ohtsuki, 2008). For example, curcumin, which is the active component of tumeric, has been shown to enhance TRAIL-induced apoptosis in a variety of in vitro models including breast, ovarian, prostate and pancreatic cancer cells (Reuter et al., 2008). Resveratrol which is found in numerous plant species, including mulberries, peanuts and grapes can also enhance TRAIL-induced apoptosis in prostate, colon and melanoma cancer cells (Ishibashi and Ohtsuki, 2008). Resveratrol-induced sensitization to TRAIL was shown to occur through multiple mechanisms including cFLIP down-regulation in melanoma cells (Ivanov et al., 2008) or redistribution of agonistic TRAIL receptors within lipid rafts in colon carcinoma cells (Delmas et al., 2004). Other antioxidants found to enhance the apoptotic properties of TRAIL include sulphoraphane, indole-3-carbinol, apigenin and quercetin (Ishibashi and Ohtsuki, 2008).
Induction of procaspase-3, procaspase-8 and procaspase-9 cleavage followed by Bid truncation and cytochrome c release from the mitochondria is probably the most frequent mechanism by which these antioxidants are found to enhance TRAIL-induced apoptosis. Strikingly, in a large number of reports, TRAIL-induced sensitization by flavonoids or sulphoraphane was also associated with production of reactive oxygen species (ROS). Likewise, sulphoraphane (from broccoli) was found to enhance TRAIL-induced apoptosis in a prostate cancer cell line (PC-3) through the generation of intracellular ROS, leading to collapse of mitochondrial membrane potential, activation of caspase-3 and caspase-9, and up-regulation of DR4 and DR5 (Ishibashi and Ohtsuki, 2008). Another study showed that resveratrol in combination with TRAIL resulted in generation of ROS, translocation of Bax to mitochondria and subsequent drop in mitochondrial membrane potential, release of mitochondrial proteins into the cytosol, activation of effector caspase-3 and caspase-9, and induction of apoptosis. More recently, ROS-induced up-regulation of DR5 was also reported to account for TRAIL-induced sensitization by baicalein (Taniguchi et al., 2008). The common theme from these studies, with a range of antioxidants in combination with TRAIL, is that generation of ROS seems to trigger signal transduction culminating in cell cycle arrest and/or apoptosis.
In this issue of the BJP, Lee et al. (2009) provide strong experimental evidences that wogonin, which is one of the main active compounds of Scutellaria baicalensis, enhances TRAIL-induced cytotoxicity through up-regulating two crucial proteins, p53 and Puma, mediated by ROS and induced by DNA damage. This up-regulation leads to enhanced Bax activation and cell death. Blocking ROS generation, or a deficiency in p53, Puma or Bax failed to restore sensitivity to TRAIL in cells stimulated with wogonin. ROS generation was, however, not inhibited in cells lacking p53, Bax or Puma, nor in cells in which p53 was mutated, indicating that ROS production drives p53 regulation. Furthermore, authors show that phosphorylation of H2Ax at serine 139 occurs before p53 up-regulation. This finding is compatible with an earlier report showing that several flavonoids such as quercetin, genistein, but not biochanin A nor daidzein, activate the DNA-damage response pathway (Ye et al., 2004). This result is particularly interesting as TRAIL itself was recently shown to activate the Chk2 DNA-damage response pathway leading to Bax activation in a caspase-dependent feedback loop but p53-independent manner (Solier et al., 2009). Altogether, the findings reported by Lee et al. (2009) highlight a novel molecular crosstalk between TRAIL and compounds exhibiting sensitizing activities such as natural antioxidants, radiotherapy or conventional chemotherapeutic drugs (Figure 1).
Figure 1.
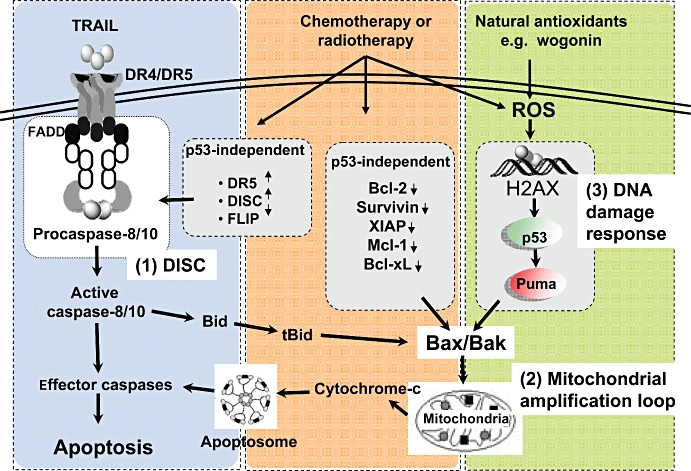
Overview of how wogonin enhances tumour necrosis factor receptor apoptosis-inducing ligand (TRAIL)-induced apoptosis in TRAIL resistant tumour cells. The inset diagram indicates the processes involved in the enhancement by wogonin of the TRAIL-induced apoptotic mechanism which include: (i) death-inducing signalling complex (DISC) formation; (ii) mitochondrial loop activation; and (iii) DNA damage. FADD, Fas–associated protein with death domain; FLIP, FLICE-like inhibitory protein; ROS, reactive oxygen species; XIAP, X-linked inhibitor of apoptosis protein.
More work is however clearly needed to assess the reality of using these natural antioxidants in combination therapy with TRAIL to treat patients suffering from cancer. TRAIL-based agonists are being assessed in clinical trials (Ashkenazi et al., 2008). Most recombinant human TRAIL or agonist antibodies targeting DR4 (mapatumumab) and DR5 (lexatumumab, mapatumumab, AMG-655, LBY135, CS-1008) have now been assessed in phase I trials and shown to be generally well tolerated with minimum toxicity. Phase II trials are now in progress in association with conventional chemotherapeutic compounds and the results are eagerly awaited.
Importantly, antioxidants such as the one reported in this review may be an alternative to chemotherapy or radiotherapy for the use of TRAIL agonists. However, it remains to be determined whether these findings will be clinically applicable as a wide range of tumor cells, corresponding to approximately 50% of the tumors found in patients, have mutations in p53. Besides, pharmacokinetic studies suggest that oral administration of many of these natural antioxidants results in low bioavailability. However, pharmacologically active concentrations may be achievable in tissues that are directly exposed to such compounds, such as the colon and skin. With this in mind, additional efforts are required to address the therapeutic potential of combination therapy using TRAIL and natural antioxidants in animal models of cancer. The findings reported here may nevertheless pave the way to novel alternative therapeutic approaches using TRAIL agonists.
Glossary
Abbreviations:
- cFLIP
cellular FLICE-like inhibitory protein
- DISC
death-inducing signalling complex
- ROS
reactive oxygen species
- TRAIL
TNF receptor apoptosis inducing ligand
Footnotes
References
- Ashkenazi A, Holland P, Eckhardt SG. Ligand-based targeting of apoptosis in cancer: the potential of recombinant human apoptosis ligand 2/Tumor necrosis factor-related apoptosis-inducing ligand (rhApo2L/TRAIL) J Clin Oncol. 2008;26:3621–3630. doi: 10.1200/JCO.2007.15.7198. [DOI] [PubMed] [Google Scholar]
- Delmas D, Rébé C, Micheau O, Athias A, Gambert P, Grazide S, et al. Redistribution of CD95, DR4 and DR5 in rafts accounts for the synergistic toxicity of resveratrol and death receptor ligands in colon carcinoma cells. Oncogene. 2004;23:8979–8986. doi: 10.1038/sj.onc.1208086. [DOI] [PubMed] [Google Scholar]
- Ishibashi M, Ohtsuki T. Studies on search for bioactive natural products targeting TRAIL signaling leading to tumor cell apoptosis. Med Res Rev. 2008;28:688–714. doi: 10.1002/med.20123. [DOI] [PubMed] [Google Scholar]
- Ivanov VN, Partridge MA, Johnson GE, Huang SX, Zhou H, Hei TK. Resveratrol sensitizes melanomas to TRAIL through modulation of antiapoptotic gene expression. Exp Cell Res. 2008;314:1163–1176. doi: 10.1016/j.yexcr.2007.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D-H, Rhee JG, Lee YJ. Reactive oxygen species up-regulate p53 and Puma; a possible mechanism for apoptosis during combined treatment with TRAIL and wogonin. Br J Pharmacol. 2009;157:1189–1202. doi: 10.1111/j.1476-5381.2009.00245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merino D, Lalaoui N, Morizot A, Solary E, Micheau O. TRAIL in cancer therapy: present and future challenges. Expert Opin Ther Targets. 2007;11:1299–1314. doi: 10.1517/14728222.11.10.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meurette O, Fontaine A, Rebillard A, Le Moigne G, Lamy T, Lagadic-Gossmann D, et al. Cytotoxicity of TRAIL/anticancer drug combinations in human normal cells. Ann N Y Acad Sci. 2006;1090:209–216. doi: 10.1196/annals.1378.023. [DOI] [PubMed] [Google Scholar]
- Reuter S, Eifes S, Dicato M, Aggarwal BB, Diederich M. Modulation of anti-apoptotic and survival pathways by curcumin as a strategy to induce apoptosis in cancer cells. Biochem Pharmacol. 2008;76:1340–1351. doi: 10.1016/j.bcp.2008.07.031. [DOI] [PubMed] [Google Scholar]
- Solier S, Sordet O, Kohn KW, Pommier Y. Death receptor-induced activation of the Chk2- and histone H2AX-associated DNA damage response pathways. Mol Cell Biol. 2009;29:68–82. doi: 10.1128/MCB.00581-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi H, Yoshida T, Horinaka M, Yasuda T, Goda AE, Konishi M, et al. Baicalein overcomes tumor necrosis factor-related apoptosis-inducing ligand resistance via two different cell-specific pathways in cancer cells but not in normal cells. Cancer Res. 2008;68:8918–8927. doi: 10.1158/0008-5472.CAN-08-1120. [DOI] [PubMed] [Google Scholar]
- Ye R, Goodarzi AA, Kurz EU, Saito S, Higashimoto Y, Lavin MF, et al. The isoflavonoids genistein and quercetin activate different stress signaling pathways as shown by analysis of site-specific phosphorylation of ATM, p53 and histone H2AX. DNA Repair (Amst) 2004;3:235–244. doi: 10.1016/j.dnarep.2003.10.014. [DOI] [PubMed] [Google Scholar]


