Abstract
Background and purpose:
Tumour necrosis factor-related apoptosis-inducing ligand (TRAIL) triggers apoptotic death in a variety of cancer cells without marked toxicity to most normal cells. We previously reported that wogonin, a potent anticancer agent from a Chinese herb, up-regulates p53 in prostate cancer cells. In this study, the effects of combinations of TRAIL and wogonin on a human prostate cancer cell line LNCaP, resistant to TRAIL, was evaluated for evidence of synergy in triggering apoptosis.
Experimental approach:
Western blot assay and the ‘comet’ assay were used to study the underlying mechanisms of cell death and search for any mechanisms of enhancement of TRAIL-induced apoptosis in the presence of wogonin.
Key results:
During combined treatment with wogonin and TRAIL, cytotoxicity, poly(ADP-ribose) polymerase cleavage and caspase activation were associated with up-regulation of p53 through DNA damage and reactive oxygen species (ROS) generation. N-acetylcysteine (NAC), an antioxidant, inhibited ROS generation and synergistic interaction between TRAIL and wogonin. Experimental results in human colon cancer HCT116 cells demonstrated that p53-dependent Puma up-regulation played an important role; deficiency in either p53 or Puma prevented wogonin-enhanced TRAIL-induced apoptosis.
Conclusions and implications:
The present studies suggest that wogonin enhances TRAIL-induced cytotoxicity through up-regulation of p53 and Puma, mediated by ROS.
Keywords: wogonin, TRAIL, apoptosis, ROS, DNA damage, p53, Puma
Introduction
Prostate cancer has become the most frequently diagnosed non-cutaneous neoplasm and second leading cause of cancer-related deaths among men in the United States. It is estimated that in the year 2007, 218 890 new cases will be diagnosed and 27 050 men will die from prostate cancer in the United States alone (Albano et al., 2007). Thus, developing novel treatment options for prostate cancer has become an important medical need. The use of phytonutrients as anticancer agents has gained considerable importance in recent years. Several studies from our laboratory and by others have suggested that HawngQium, especially its constituent polyphenols, possesses chemopreventive and therapeutic potential against prostate cancer (Lee et al., 2008). Much of the anticancer and/or cancer chemopreventive effects of HawngQium are attributed to its major polyphenol, wogonin (Lee et al., 2008).
Wogonin (C16H12O5; Figure 1), one of the main active compounds of Scutellaria baicalensis, has been found to be effective in inhibiting tumour growth, such as human ovarian cancer cell A2780, human promyeloleukemic cell HL-60, human hepatocellular carcinoma cell SK-HEP-1, and human prostate cancer cell LNCaP (Chen et al., 2002; Lee et al., 2002; 2008; Li et al., 2003). Wogonin has also been found to have anti-inflammatory activities in vitro as well as in vivo (Chi et al., 2001). We previously observed that p53-dependent up-regulation of Puma, a member of the Bcl-2 family of proteins, and subsequent oligomerization of Bax play important roles in the sensitivity of cancer cells to apoptosis induced by caspase activation through wogonin (Lee et al., 2008).
Figure 1.

Chemical structure of wogonin.
Members of the tumour necrosis factor (TNF) receptor superfamily, including TNF receptor, Fas and TNF-related apoptosis-inducing ligand (TRAIL) receptor, share similar conserved structures (Locksley et al., 2001). Although TNF-α and FasL can trigger apoptosis in some solid tumours, their clinical usage has been limited by the risk of lethal systemic inflammation and hepatotoxicity respectively (Fiers, 1991; Ogasawara et al., 1993). In contrast, recombinant soluble human TRAIL has shown a profound apoptotic effect, without toxicity, in human hepatocytes in vitro and in vivo (Hao et al., 2004). These results indicate that this form of TRAIL may prove to be a safe and effective biological agent for cancer therapy in humans. However, an obstacle to effective therapy is that prostate cancer, similar to many other cancers, develops resistance to TRAIL (Pei et al., 2004; Shankar and Srivastava, 2004). Thus, researchers are currently seeking to identify TRAIL sensitizers capable of overcoming TRAIL resistance in cancer cells. Novel agents are needed to overcome the resistance to improve TRAIL efficacy. Recently, various agents, including DNA-damaging agents such as ionizing irradiation and many anticancer drugs (Wang and El-deiry, 2003), histone deacetylase inhibitors (Nakata et al., 2004), IFN-α (Shigeno et al., 2003), and proteasome inhibitors (He et al., 2004), have been reported to sensitize tumours to TRAIL-induced apoptosis.
In this study, we examine the potential sensitizing effects of wogonin to TRAIL-mediated apoptosis in human prostate cancer cells. Here, for the first time we present evidence that combination of non-apoptosis-inducing doses of wogonin and TRAIL leads to the apoptosis of LNCaP cells. Wogonin is an effective sensitizer to TRAIL-mediated apoptosis in LNCaP cells. The sensitizing effect of wogonin on TRAIL is associated with generation of ROS, DNA damage and up-regulation of p53 and Puma protein levels. Our data suggest that both extrinsic and intrinsic pathways are involved in apoptosis induced by combined treatment with wogonin and TRAIL.
Methods
Cell culture
Human prostate adenocarcinoma LNCaP and PC-3, and human prostate carcinoma DU-145 cells were purchased from American Tissue Type Culture Collection (ATCC, Manassas, VA, USA). Cells were cultured in RPMI 1640 medium (Invitrogen, Carlsbad, CA, USA) with 10% fetal bovine serum (HyClone, Logan, UT, USA), 1 mmol·L−1 L-glutamine, and 26 mmol·L−1 sodium bicarbonate for monolayer cell culture. Rat prostate endothelial cells (YPEN-1) were obtained from ATCC. The cells were cultured in Dulbecco's modified Eagle's medium supplemented with 5% heat-inactivated fetal bovine serum (Gibco-BRL, Gaithersburg, MD, USA), glutamine at 233.6 mg·mL−1, penicillin–streptomycin at 72 mg·mL−1, and amphotericin B at 0.25 mg·mL−1, and were adjusted to pH 7.4–7.6 with NaHCO3 in an atmosphere of 5% CO2. p53-containing (p53+/+) and p53-deficient (p53−/−) HCT116 human colon carcinoma cell lines, Puma-containing (Puma+/+) and Puma-deficient (Puma−/−) HCT116 cell lines, and Bax-containing (Bax+/−) and Bax-deficient (Bax−/−) HCT116 cell lines were kindly provided by Dr Bert Vogelstein (Johns Hopkins University, Baltimore, MD, USA). These cell lines were cultured in McCoy's 5A medium (Gibco-BRL) containing 10% fetal bovine serum and antibiotics. The dishes containing cells were kept in a 37°C humidified incubator with 5% CO2.
Drug treatment
Wogonin was purchased from LKT Laboratories. (St. Louis, MO). The caspase inhibitors zDEVD-fmk (caspase-3 inhibitor), zLEHD-fmk (caspase-9 inhibitor) and zIETD-fmk (caspase-8 inhibitor) were purchased from R&D systems (Minneapolis, MN, USA). These caspase inhibitors were prepared and dissolved in dimethylsulphoxide (DMSO) and applied to the cells at 25 µmol·L−1. Treatment of cells with drugs was accomplished by aspirating the medium and replacing it with medium containing these drugs.
Determination of cell viability
One or two days prior to the experiment, cells were plated into 60-mm dishes at a density of 1 × 105 cells per plate in 5 mL tissue culture medium in triplicate. For Trypan blue exclusion assay, trypsinized cells were pelleted and resuspended in 0.2 mL of medium, 0.5 mL of 0.4% Trypan blue solution and 0.3 mL of phosphate-buffered saline solution (PBS). The samples were mixed thoroughly, incubated at room temperature for 15 min, and examined under a light microscope. At least 300 cells were counted for each survival determination.
Production of recombinant TRAIL
A human TRAIL cDNA fragment (amino acids 114–281) obtained by RT-PCR was cloned into a pET-23d (Novagen, Madison, WI, USA) plasmid, and His-tagged TRAIL protein was purified using the Qiagen express protein purification system (Qiagen, Valencia, CA, USA).
Measurement of ROS generation
Reactive oxygen species generation in control and wogonin-treated cells was measured by flow cytometry following staining with a chloro methyl derivative of di-chloro di-hydro fluorescein diacetate (CMH2DCFDA). Briefly, the desired cell line was seeded in six-well plates (1 × 105 cells per well), allowed to attach overnight and exposed to DMSO (control) or desired concentrations of wogonin for specified time periods. The cells were stained with 5 µmol·L−1 CMH2DCFDA for 30 min at 37°C, and the fluorescence was detected by a fluorescence microscope. Alternatively, the fluorescence intensity of dichlorofluorescein in cells was determined using the flow cytometer (Becton Dickinson and Co.).
DNA damage assay
Wogonin-induced DNA damage was assessed by the alkaline single-cell gel electrophoresis (‘comet’ assay) method (Olive and Banath, 1990). LNCaP cells were stimulated, trypsinized and embedded into 0.5% low-melting agarose on glass microscope slides. After treatment with alkaline lysis buffer, slides were subjected to electrophoresis, stained with propidium iodide (PI), and analysed by epifluorescence microscopy. For quantification of DNA damage, fluorescence intensities (from PI staining) of the head and tail portions were obtained from each comet image, and the percentage intensity of the tail portion was multiplied by the length of the tail (in µm) (DNA migration) to yield a tail moment.
RNA interference by siRNA of p53 and Puma
To knock-down p53 or Puma gene expression, p53 siRNA, Puma siRNA or control siRNA was purchased from Santa Cruz Biotechnology. LNCaP cells were seeded in six-well plate at a density of 2 × 105 cells per well in 2 mL complete RPMI 1640 growth medium. Twenty-four hours later, when cells reached 70–90% confluence, growth medium was replaced with 0.8 mL per well Opti-MEM transfection medium (Invitrogen) and 0.2 mL siRNA transfection mix. Transfections were carried out according to Invitrogen's oligofectamine protocol. After transfection, cells were incubated for 48 h at 37°C in a 5% CO2 humidified atmosphere and the interference of p53 or Puma expression was confirmed by immunoblotting using anti-p53 or anti-Puma antibody respectively.
Immunoblot analysis
Proteins were separated by SDS-PAGE and electrophoretically transferred to nitrocellulose membrane. The nitrocellulose membrane was blocked with 5% non-fat dry milk in PBS-Tween-20 (0.1%, v/v) at 4°C overnight. The membrane was incubated with primary antibody (diluted according to the manufacturer's instructions) for 2 h. Horseradish peroxidase conjugated anti-rabbit or anti-mouse IgG was used as the secondary antibody. Immunoreactive protein was visualized by the chemiluminescence protocol (ECL, Amersham, Arlington Heights, IL).
Statistical analysis
Statistical analysis was carried out using Graphpad InStat 3 software (GraphPad Software, Inc., San Diego, CA, USA). Results were considered statistically significant at P < 0.05.
Materials
Anti-caspase-3 antibody and anti-p53 antibody were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Anti-caspase-8 antibody, anti-phospho-H2A.X antibody, anti-H2A.X antibody, and anti-Puma antibody were purchased from Cell Signaling (Beverly, MA, USA). Anti-caspase-9 antibody was purchased from Upstate Biotechnology (Lake Placid, NY, USA). Anti-PARP antibody was purchased from Biomol Research Laboratory (Plymouth Meeting, PA, USA). Anti-actin antibody was purchased from MP Biomedicals (Solon, OH, USA). For the secondary antibodies, anti-mouse-IgG-HRP and anti-rabbit-IgG-HRP were purchased from Santa Cruz Biotechnology.
Results
Differential TRAIL sensitivity in human prostate cancer cells
Flavonoids are diphenylpropanes commonly found in plants. More than 4000 flavonoids have been found and many are components of the human diet. However, many biological activities of flavonoids are still undefined. Wogonin is one of the major flavonoids produced by S. baicalensis (Figure 1) and earlier studies demonstrated that wogonin sensitizes TRAIL-resistant leukaemia cells (Fas et al., 2006). In this study, we examined whether wogonin sensitizes TRAIL-resistant prostate cancer cells and possible mechanisms of such sensitization. To determine TRAIL sensitivity in prostate cancer cells, DU-145 and LNCaP cells were treated with various concentrations of TRAIL for 6 h. Figure 2A shows significant concentration-dependent reduction of the viability of DU-145 cells, but not of LNCaP cells. Figure 2B shows TRAIL-induced poly(ADP-ribose) polymerase-1 (PARP-1) cleavage, the hallmark of apoptosis, in DU-145 cells but not in LNCaP cells. These results are consistent with previous studies showing that LNCaP was resistant, while DU-145 was sensitive, to TRAIL-induced apoptosis (Kim and Lee, 2007).
Figure 2.

TRAIL-induced cytotoxicity and PARP-1 cleavage in DU-145 and LNCaP cells. Cells were treated with various concentrations (10–200 ng·mL−1) of TRAIL for 6 h. (A) Survival was analysed by the Trypan blue dye exclusion assay as described in Methods. Error bars represent the mean ± SE from three separate experiments. Asterisk * or ** represents a statistically significant difference between control and TRAIL-treated cells at P < 0.05 or P < 0.01 respectively. (B) Lysates containing equal amounts of protein (20 µg) were separated by SDS-PAGE and immunoblotted with anti-PARP-1 antibody. Actin is shown as an internal standard.
Combined treatment with wogonin and TRAIL induces apoptosis in LNCaP cells, but not in YPEN-1 cells
Prior to investigating the effect of combined treatment with wogonin and TRAIL on cell viability, we examined whether wogonin alone induced cytotoxicity in LNCaP cells. Cells were treated with various concentrations (10–100 µmol·L−1) of wogonin for 24 h. As shown in Figure 3A, wogonin induced cytotoxicity in a dose-dependent manner with an IC50 of 57 µmol·L−1. We also observed minimal cytotoxicity with 10–25 µmol·L−1 wogonin. Next, we examined the effect of wogonin (5–25 µmol·L−1) in combination with 50 ng·mL−1 TRAIL on cell viability. Wogonin strongly synergized with TRAIL to induce cytotoxicity in a dose-dependent manner (Figure 3B). The typical morphological changes of apoptotic cell death were observed under phase-contrast microscopy (data not shown). Similar results were observed by Annexin V and PI staining assay. Data from fluorescence activated cell sorting (FACS) analysis show that apoptotic death occurred during combined treatment with TRAIL and wogonin (data not shown). Figure 3C shows that no cleavage of PARP-1 occurred during treatment with wogonin or TRAIL alone. However, PARP-1 cleavage was clearly observed after combined treatment with wogonin and TRAIL. Unlike the LNCaP cells, no PARP-1 cleavage was observed by combined treatment with wogonin and TRAIL in a non-cancer, rat prostate epithelial cell line, YPEN-1 (Figure 3D).
Figure 3.

Combined treatment with wogonin and TRAIL induces apoptotic death in LNCaP cells, but not in YPEN-1 cells. (A) LNCaP cells were treated with DMSO (sham control) or various concentrations (10–100 µmol·L−1) of wogonin for 24 h. (B) LNCaP cells were treated with various concentrations (5–25 µmol·L−1) of wogonin in combination with 50 ng·mL−1 TRAIL for 24 h. Cell viability was determined by Trypan blue dye exclusion assay. Error bars represent the mean ± SE from three separate experiments. * or **Significant difference between control and wogonin-treated cells at P < 0.05 or P < 0.01 respectively. (C and D) LNCaP (C) and YPEN-1 (D) cells were treated with wogonin alone, TRAIL alone, or wogonin in combination with TRAIL for 24 h. Lysates containing equal amounts of protein (20 µg) were separated by SDS-PAGE and immunoblotted with anti-PARP-1 antibody. Actin was used to confirm the equal amount of proteins loaded in each lane.
Combined treatment with wogonin and TRAIL leads to caspase activation
To confirm the activation of apoptotic signals by combined treatment with wogonin and TRAIL, we performed Western blot analysis of PARP-1 and caspases. The cleavage of procaspases is an indication of their activation. We therefore examined the cleavage of caspase-8, caspase-3, caspase-9 and PARP-1 in LNCaP cells by Western blot analysis. Treatment of LNCaP cells with wogonin or TRAIL alone resulted in no or minimal cleavage of caspase-8, caspase-3, caspase-9 and PARP-1 (Figure 4A). In contrast, the combination of wogonin and TRAIL led to the cleavage of caspase-8, caspase-3, caspase-9 and PARP-1. PARP-1 cleavage was increased as a function of exposure time with TRAIL and wogonin (Figure 4A). The cleavage of caspases was increased by increasing wogonin concentrations (Figure 4B). We further examined the role of caspases in cell survival during treatment with TRAIL and wogonin. Figure 4C clearly demonstrates that inhibitors of caspases protected cells from apoptotic death during treatment with TRAIL and wogonin. These results suggest that TRAIL plus wogonin-induced apoptosis is associated with the activation of caspase-8, -9 and -3.
Figure 4.
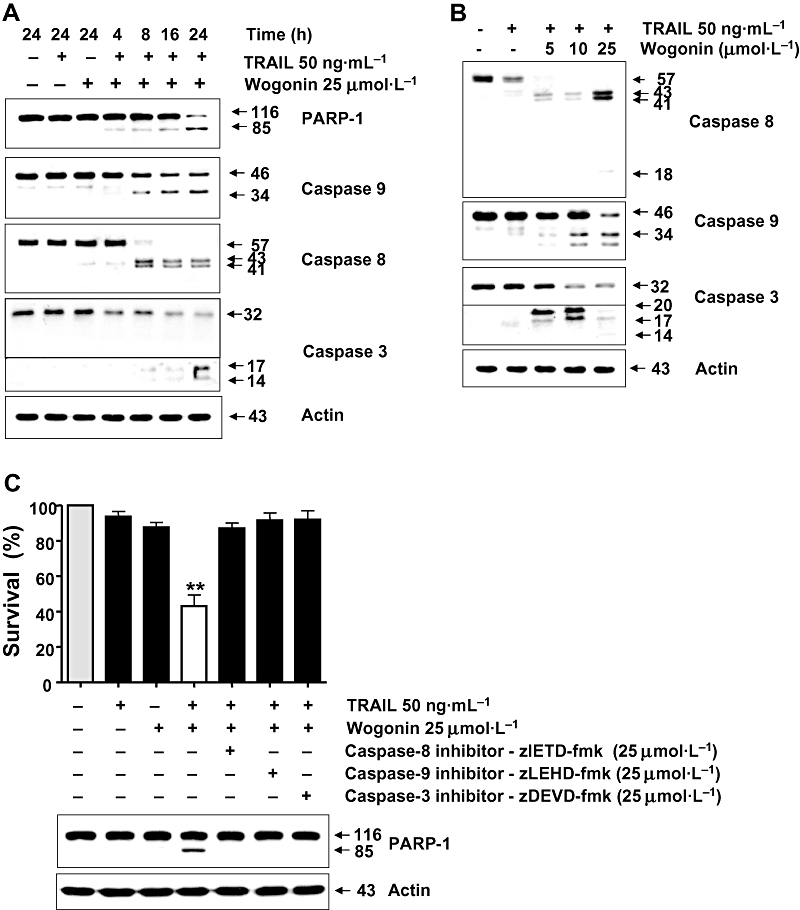
Combined treatment with wogonin and TRAIL activates caspases in LNCaP cells. (A) Cells were treated with 25 µmol·L−1 wogonin and/or 50 ng·mL−1 TRAIL for various times (4–24 h). (B) Cells were treated with 50 ng·mL−1 TRAIL alone or TRAIL in combination with wogonin (5–25 µmol·L−1) for 24 h. Lysates containing equal amounts of protein (20 µg) were separated by SDS-PAGE and immunoblotted with anti-PARP-1, anti-caspase-8, anti-caspase-9, or anti-caspase-3 antibody. (C) Cells were treated with 25 µmol·L−1 wogonin and/or 50 ng·mL−1 TRAIL for 24 h with or without pretreatment with caspase inhibitor for 30 min. Cell survival was determined as described in Figure 2A. Inhibitors of caspases significantly (P < 0.01) protect cells from apoptotic death during treatment with TRAIL and wogonin. Lysates containing equal amounts of protein (20 µg) were separated by SDS-PAGE and immunoblotted with anti-PARP-1 antibody (lower panel). Actin was used to confirm the equal amount of proteins loaded in each lane.
Generation of ROS by wogonin
Next, we attempted to clarify the mechanisms underlying apoptosis induced by wogonin in combination with TRAIL. Previous studies have shown that several chemopreventive agents induce apoptosis in cancer cells through generation of ROS (Singh et al., 2005; Shankar and Srivastava, 2007) and we therefore examined whether wogonin induced ROS production. LNCaP cells were treated with H2O2 or wogonin and the intracellular level of ROS was determined by using the CMH2DCFDA fluorescence probe. Figure 5A shows that significant fluorescence signals were detected in cells which were exposed to H2O2 or wogonin. Wogonin-induced ROS production occurred in a dose-dependent manner (Figure 5B). Interestingly, the time course studies showed that there was a biphasic increase of the intracellular level of ROS during wogonin treatment (Figure 5C). ROS production increased as a function of time and reached a maximum level at 20 min after wogonin treatment. ROS production then rapidly decreased within 1 h and increased again within 4 h during continuous treatment with wogonin. Similar results were observed in a variety of cell lines (PC-3, HCT116, HCT116 p53−/−, HCT116 Puma−/− and HCT116 Bax−/−) (Figure 5D). TRAIL alone did not significantly increase ROS production (Figure 6A). Pretreatment of LNCaP cells with N-acetylcysteine (NAC), an antioxidant, inhibited wogonin-induced ROS production (Figure 6A), and prevented cell death induced by combined treatment with wogonin and TRAIL (Figure 6B), as well as PARP-1 cleavage and elevation of p53 and Puma levels (Figure 6C).
Figure 5.

Wogonin induces dose- and time-dependent ROS production. (A) LNCaP cells were exposed to 50 µmol·L−1 H2O2 or wogonin (25 or 50 µmol·L−1) for 1 h and then treated with CMH2DCFDA (25 µmol·L−1) for 30 min. Morphological features were analysed with a phase-contrast microscope and fluorescent signals were detected with fluorescence microscopy. Control represents untreated cells. (B) LNCaP cells were treated with various concentrations (5–50 µmol·L−1) of wogonin for various times (1–4 h) and then incubated with CMH2DCFDA (25 µmol·L−1) for 30 min. *, ** or *** Significant (P < 0.05) increase in fluorescence intensity in concentration and time-dependent manner. (C and D) LNCaP, PC-3, HCT116, HCT p53−/−, HCT Puma−/− and HCTBax−/− cells were treated with wogonin (25 or 50 µmol·L−1) for various times and then incubated with CMH2DCFDA (25 µmol·L−1) for 30 min. ROS production was measured as fluorescence units at excitation wavelength of 485 nm and emission at 530 nm in a fluorescence plate reader. * or ** Significant (P < 0.05) increase in fluorescence intensity in concentration or cell line-dependent manner.
Figure 6.

Effect of N-acetylcysteine (NAC) on wogonin in combination with TRAIL-induced ROS production, viability, and PARP-1 cleavage. Cells were pretreated with 4 mmol·L−1 NAC for 2 h followed by treatment with 25 µmol·L−1 wogonin or/and 50 ng·mL−1 TRAIL for 24 h. (A) Intracellular level of ROS was measured by FACS analysis. Error bars represent the mean ± SE from three separate experiments. *Significant effect of NAC on cells treated with TRAIL + wogonin (P < 0.05). (B) Survival was analysed by the Trypan blue dye exclusion assay as described in Methods. Error bars represent the mean ± SE from three separate experiments. *Significant difference between wogonin and TRAIL + wogonin-treated cells (P < 0.05). **Significant effect of NAC on cells treated with TRAIL + wogonin (P < 0.05). (C) Lysates containing equal amounts of protein (20 µg) were separated by SDS-PAGE and immunoblotted with anti-PARP-1, anti-p53, or anti-Puma antibody. Actin was used to confirm the equal amount of proteins loaded in each lane.
Wogonin-induced DNA damage and phosphorylation of H2A.X
We hypothesized that wogonin-induced ROS may lead to DNA strand breaks which trigger the apoptosis signal transduction pathway. To test this hypothesis, LNCaP cells were treated with 50 µmol·L−1 wogonin for 20 min and analysed with the ‘comet’ assay. After treatment with wogonin, DNA was isolated and analysed with agarose gel electrophoresis. Gel slabs were stained with PI. Image analysis was carried out under a fluorescence microscope attached to a black and white CCD video camera. The parameter used to evaluate DNA damage was tail moment. Figures 7A and B show that wogonin treatment caused an increase in tail moments (P < 0.01), indicating an increase in DNA damage in the wogonin-treated cells. Next, we examined phosphorylation of the histone H2A.X, a hallmark of DNA damage, during wogonin treatment. H2A.X is a variant form of histone H2A that is directly phosphorylated at Ser139 by an activated ATM (ataxia telangiectasia mutated) kinase, marking an early event in response to DNA damage, and is also known to play a critical role in the retention of DNA repair factors at DNA-damaged sites (Burma et al., 2001). Figures 7C and D show that a dose- and time-dependent accumulation of wogonin induced phosphorylation of Ser139-H2A.X in LNCaP cells. Taken together, these findings suggest that wogonin causes DNA damage leading to serine 139 phosphorylation of H2A.X, which is also known as an early marker of apoptosis induction.
Figure 7.

Wogonin-induced DNA damage and phosphorylation of H2A.X in LNCaP cells. (A) Cells were treated with 50 µmol·L−1 wogonin for 20 min. After treatment, cells were embedded into an agarose gel and the proteins and lipids were removed by exposing the gel to an alkaline NaOH solution. DNA fragments were electrically separated from the nucleus (comet head) and visualized by staining with propidium iodide (PI). DNA damage is seen in the form of a comet tail (indicated by arrow) under a fluorescence microscope. (B) For DNA damage quantification, PI intensities of the head and tail portions were obtained from each comet image, and the percentage intensity of the tail portion was multiplied by the size of the tail (DNA migration) to yield a tail moment. n, avg and sem represent the number of individual measurements, average, and SEM respectively. (C and D) Cells were treated with various concentrations (5–50 µmol·L−1) of wogonin for 24 h or various times (1–24 h) with 50 µmol·L−1 wogonin. Equal amounts of protein (20 µg) from cell lysates were separated by SDS-PAGE and immunoblotted with anti-phospho-H2A.X or anti-H2A.X antibody. Actin was shown as an internal standard.
Wogonin up-regulates the intracellular level of p53 and Puma and enhances TRAIL-induced apoptotic death
As H2A.X is a substrate for ATM/ATR (ataxia telangiectasia-mutated/ATM-Rad3 related), our data suggest that wogonin induces activation of ATM/ATR in LNCaP cells. This possibility was examined with p53, another substrate for ATM/ATR. Figure 8A shows that wogonin induced phosphorylation of p53 at Ser15, Ser46 and Ser392 residues. Figure 8A also shows the accumulation of p53 during treatment with wogonin. These results suggest that phosphorylation of p53 affects the stabilization of p53. It has been well known that p53 can mediate the apoptotic response to chemotherapeutic agents. Several mechanisms of apoptosis induction by p53 have been identified involving transcriptional and/or non-transcriptional regulation of its downstream effectors (Yee and Vousden, 2005). For example, p53 is known to induce apoptosis by transcriptional up-regulation of pro-apoptotic proteins such as Noxa, Puma, Bax, Apaf-1, and by transcriptional repression of Bcl-2 and inhibitors of apoptosis (Yee and Vousden, 2005). We previously reported that p53-dependent transcriptional induction of Puma plays an important role in wogonin-induced apoptotic death (Lee et al., 2008). In this study, we examined whether combined treatment with wogonin and TRAIL-induced apoptosis is mediated through up-regulation of p53 and Puma. Figure 8B shows that, consistent with previous observations, the intracellular level of p53 and Puma protein was increased during treatment with wogonin. The accumulation of these proteins was increased by combined treatment with wogonin and TRAIL (Figures 8C and D). We further examined whether p53 and Puma play an important role in wogonin in combination with TRAIL-induced apoptosis by using small interfering RNA (siRNA) for p53 and Puma. Figure 8E shows that expression of p53 or Puma was effectively reduced by transfection with either siRNA for p53 or siRNA for Puma respectively. Knock-down of p53 or Puma significantly inhibited apoptosis induced by wogonin in combination with TRAIL, as shown by PARP-1 cleavage. To confirm the involvement of p53 in the synergy between TRAIL and wogonin, three sets of experiments were performed. In the first set, we used PC-3 (p53 null) and DU-145 (p53 mutant) cells which were treated with wogonin and TRAIL. As shown in Figures 9A–D, wogonin did not increase TRAIL-induced apoptotic death. In the second set, two HCT116 human colon adenocarcinoma cell lines, one containing a wild-type p53 (p53+/+) and the other containing a p53-deleted derivative (p53−/−), were co-treated with wogonin and TRAIL. Cell viability assay and Western blot analysis revealed that wogonin synergized with TRAIL to increase both cytotoxicity and the intracellular level of p53 and PARP-1 cleavage in HCT116 p53+/+, but not in HCT116 p53−/− cells (Figures 10A and B). In the third set of experiments, to further evaluate the apoptotic cell death associated with Puma expression, we employed cells expressing Puma (HCT116 Puma+/+) and not expressing Puma (HCT116 Puma−/−). These cells were co-treated with wogonin and TRAIL; and the cell viability, PARP-1 cleavage and the intracellular levels of p53 and Puma were examined. Figures 10C and D show that wogonin promoted TRAIL-induced cell death and PARP-1 cleavage in HCT116 Puma+/+ cells, but not in HCT116 Puma−/− cells. Taken together, these results suggest that up-regulation of p53 and Puma is required for sensitization to TRAIL-induced apoptosis by wogonin.
Figure 8.
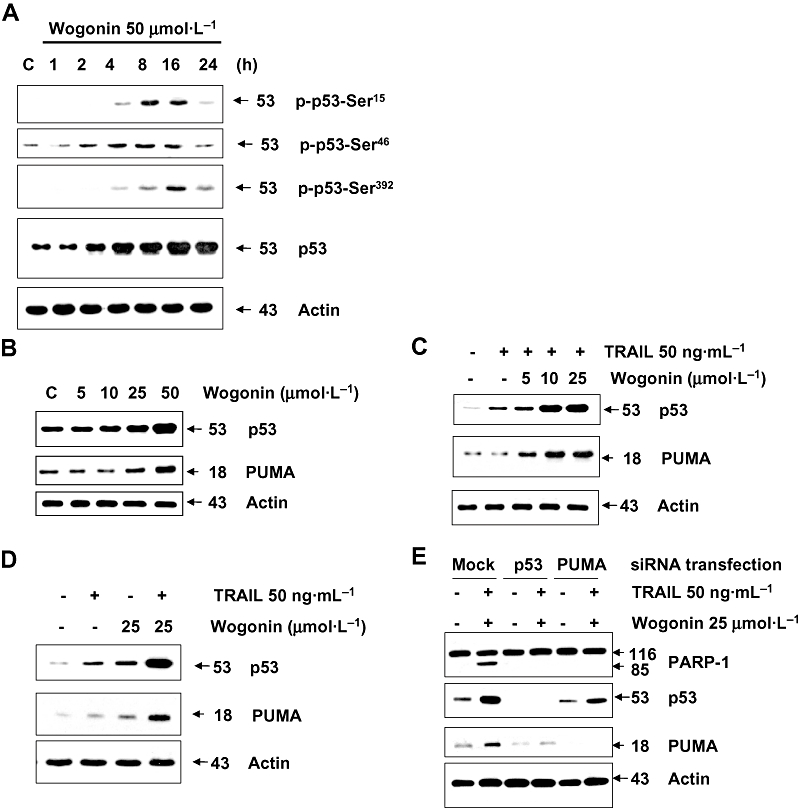
Wogonin induces phosphorylation of p53 and increases intracellular level of p53 and Puma in the presence or absence of TRAIL in LNCaP cells. (A) Cells were treated with 50 µmol·L−1 wogonin for various times (1–24 h). Equal amounts of protein (20 µg) from cell lysates were separated by SDS-PAGE and immunoblotted with anti-phospho-Ser15-p53, anti-phospho-Ser46-p53, anti-phospho-Ser392-p53 or anti-p53 antibody. Actin was shown as an internal standard. (B) Cells were treated with various concentrations (5–50 µmol·L−1) of wogonin for 24 h, and then cells were harvested. (C and D) Cells were treated with 5, 10 or 25 µmol·L−1 wogonin in the presence or absence of TRAIL (50 ng·mL−1) for 24 h. (E) Cells were transfected with siRNA for p53 or Puma and then treated with TRAIL (50 ng·mL−1) in combination with wogonin (25 µmol·L−1) for 24 h. Lysates containing equal amounts of protein (20 µg) were separated by SDS-PAGE and immunoblotted with anti-p53 or anti-Puma antibody. Actin was used to confirm the equal amount of proteins loaded in each lane.
Figure 9.

Role of p53 in wogonin-enhanced TRAIL cytotoxicity in PC-3 and DU-145 cells. Cells were treated with wogonin (25 or 50 µmol·L−1) and/or TRAIL (50 ng·mL−1) for 6 h. (A and C) PC-3 (A) or DU-145 (C) cell viability was determined using the Trypan blue dye exclusion assay. Error bars represent the mean ± SE from three separate experiments. (B and D) Equal amounts of protein (20 µg) from PC-3 (B) or DU-145 (D) cell lysates were separated by SDS-PAGE and immunoblotted with anti-PARP-1, anti-p53 or anti-Puma antibody. Actin was shown as an internal standard.
Figure 10.

Involvement of p53 and Puma in combination of wogonin and TRAIL-induced apoptosis. (A) and (B) HCT116 p53+/+ and HCT116 p53−/− cells were treated with wogonin (25 µmol·L−1) and/or TRAIL (2.5 ng·mL−1) for 6 h. (A) Cell viability was determined using the Trypan blue dye exclusion assay. Error bars represent the mean ± SE from three separate experiments. *Significant difference between TRAIL and TRAIL + wogonin-treated cells at P < 0.05. (B) Equal amounts of protein (20 µg) from cell lysates were separated by SDS-PAGE and immunoblotted with anti-PARP-1 or anti-p53 antibody. Actin was shown as an internal standard. (C) and (D) HCT116 Puma+/+ and HCT116 Puma−/− cells were treated with wogonin (25 µmol·L−1) of wogonin and/or TRAIL (2.5 ng·mL−1) for 6 h. (C) Cell viability was determined using the Trypan blue dye exclusion assay. Error bars represent the mean ± SE from three separate experiments. *Significant difference between TRAIL and TRAIL + wogonin-treated cells at P < 0.05. (D) Equal amounts of protein (20 µg) from cell lysates were separated by SDS-PAGE and immunoblotted with anti-PARP-1, anti-p53 or anti-Puma antibody. Actin is shown as an internal standard.
Role of Bax in apoptosis induced by wogonin in combination with TRAIL
To further examine whether apoptosis induced by combined treatment with wogonin and TRAIL depends on the presence of Bax, we employed HCT116 Bax+/− as well as HCT116 Bax−/− cells. As shown in Figures 11A and B, compared with HCT116 Bax+/− cells, HCT116 Bax−/− cells were resistant to PARP-1 cleavage (apoptosis) and caspase 8 activation. As shown in Figures 11C and D, TRAIL-induced cell death and PARP-1 cleavage were promoted by treatment with wogonin in HCT116 Bax+/− cells, but not in HCT116 Bax−/− cells, even though the combination significantly enhanced the intracellular levels of p53 and Puma in both cell lines.
Figure 11.

Role of Bax in TRAIL in combination with wogonin-induced apoptosis. (A) and (B) HCT116 Bax+/− and HCT116 Bax−/− cells were treated with TRAIL (10–200 ng·mL−1) for 6 h. Equal amounts of protein (20 µg) from cell lysates were separated by SDS-PAGE and immunoblotted with anti-PARP-1 or anti-caspase-8 antibody. Actin was shown as an internal standard. (C) and (D) HCT116 Bax+/− and HCT116 Bax−/− cells were treated with wogonin (25 µmol·L−1) of wogonin and/or TRAIL (2.5 ng·mL−1) for 6 h. (C) Cell viability was determined using the Trypan blue dye exclusion assay. Error bars represent the mean ± SE from three separate experiments. *Significant difference between TRAIL and TRAIL + wogonin-treated cells at P < 0.05. (D) Equal amounts of protein (20 µg) from cell lysates were separated by SDS-PAGE and immunoblotted with anti-PARP-1, anti-p53, anti-Puma or anti-Bax antibody. Actin was shown as an internal standard.
A model for the mechanism of wogonin-promoted TRAIL cytotoxicity
A scheme including our experimental data and other already published data is shown in Figure 12. Our studies revealed that Bax was a key mediator between wogonin-induced up-regulation of p53 and PUMA and TRAIL-induced caspase activation. Wogonin induced ROS production and activated the intrinsic pathway (mitochondria mediated activation of caspase-9), while TRAIL bound to receptors and activated the extrinsic pathway (death-receptor mediated activation of caspase-8). When cells are treated with wogonin in combination with TRAIL, these two death signals are merged and the activation of caspases is amplified.
Figure 12.

Diagram of mechanisms underlying the synergy between wogonin and TRAIL in inducing apoptosis.
Discussion
The regulation of apoptotic cell signalling pathways by chemotherapeutic drugs, radiation, hyperthermia and chemopreventive agents have been shown to sensitize cancer cells to TRAIL treatment (Kim et al., 2005; 2008; Fas et al., 2006; Kim and Lee, 2007; Yoo et al., 2008). In this study, we investigated the effect of wogonin, a naturally occurring chemopreventive agent, on TRAIL-induced apoptosis in human cancer cells. We show for the first time that sub-toxic doses of wogonin sensitize cells to TRAIL-induced apoptosis by up-regulating p53 and Puma through ROS-associated DNA damage.
We observed that ROS generation occurs during treatment with wogonin. ROS, including the superoxide anion, hydrogen peroxide and hydroxyl radical, are known to mediate apoptosis induced by some cancer chemopreventive and therapeutic agents (Davis et al., 2001). Intracellular ROS may interact with cellular membrane lipids, proteins, and DNA and cause oxidative injury (Valko et al., 2006). Recently, Yu et al. (2007) also reported that treatment with wogonin induces apoptosis in hepatoma cells and that the initial signal for wogonin-induced apoptosis is derived from ROS. These observations are consistent with our results which demonstrate that wogonin induces a dose- and time-dependent increase in ROS in LNCaP cells. However, recent studies show that an increase in intracellular ROS production is observed by treatment with nor-wogonin, but not with wogonin in human leukaemia HL-60 cells (Chow et al., 2008). This discrepancy may be due to cell line differences. Nevertheless, dose-dependent DNA strand breaks were observed in LNCaP cells exposed to wogonin. Wogonin-induced ROS generation was evident as early as 10 min during treatment with wogonin, and a significant increase in the protein levels of p53 was observed after 1 h. Pretreatment with NAC, an antioxidant, blocked the wogonin-induced ROS generation and increase in p53 expression (Figure 6). Finally, pretreatment of LNCaP cells with NAC blocked the induction of cell death by treatment with wogonin and TRAIL. These results suggest that ROS act as upstream signalling molecules for the initiation of wogonin-induced p53 expression and are critical for the sensitization of the cells to TRAIL-induced apoptosis.
Wogonin has been reported to suppress proliferation or various cancer cells by causing cell cycle arrest, apoptosis, or both (Baumann et al., 2008; Lee et al., 2008). For example, wogonin-induced cell cycle arrest is associated with suppression of cyclin D1 and dephosphorylation of Akt (Chung et al., 2008). Furthermore, up-regulation of Bax and down-regulation of Bcl-2 occur during wogonin-induced apoptosis in human hepatoma cells (Wang et al., 2006). Our recent studies have shown that wogonin up-regulates the expression of p53 and Puma in prostate cancer cells (Figure 7; Lee et al., 2008). Although we observed that wogonin induced phosphorylation of p53 in LNCaP cells (Figure 8A), we need further study to find out whether phosphorylation of p53 plays an important role in the stabilization of p53. We previously demonstrated that treatment of LNCaP cells with wogonin resulted in translocation of Bax and p53 to the mitochondria, release of cytochrome c, and activation of caspase-3 leading to apoptosis (Lee et al., 2008).
p53 is the most frequent target of genetic alterations in human cancers, with mutations occurring in almost 50% of all human tumours (Cariello et al., 1994). It has been suggested that p53 may play an important role in DNA repair, cell cycle arrest and apoptosis under conditions of environmental stress (Levine, 1997; Oren, 1999). Following DNA damage, p53 is phosphorylated and acetylated at a number of sites. Its phosphorylation represents an early cellular response to a variety of genotoxic stresses and promotes both the accumulation and functional activation of p53 (Shieh et al., 1997). Besides phosphorylation and acetylation, the binding of p53 to DNA is modulated by a redox regulation mechanism (Gaiddon et al., 1999). The increased cellular p53 protein levels resulting from exposure to various genotoxic agents are due mainly to an increase in the stability of p53 protein rather than an increase in the levels of p53 mRNA. However, it has been suggested that an increase in p53 protein stability is not solely responsible for the recruitment of p53 in response to genotoxic stress; it is more likely that the p53 genotoxic stress response is a complex cellular process regulated at transcriptional mRNA stability levels. p53 protein regulates apoptosis through both transcriptional-dependent and independent mechanisms. Through transcription-dependent pathways, p53 functions as a transactivator to up-regulate downstream pro-apoptotic genes (e.g. Bax, Noxa and Puma) and functions as a repressor to down-regulate anti-apoptotic genes (e.g. Bcl-2), promoting apoptosis. Bax induces apoptosis by enhancing the release of mitochondrial proteins (e.g. cytochrome c and Smac/DIABLO) to cytosol. Through transcription-independent pathways, p53 has a direct apoptogenic role when it translocates to the mitochondria in response to cellular stress, resulting in apoptosis via interaction with antiapoptotic Bcl-2 and Bcl-XL proteins that alter the mitochondrial membrane potential and induce cytochrome c and Smac/DIABLO release into the cytosol with resultant caspase activation (Yang et al., 2006). In our previous studies, we observed that p53-dependent transcriptional induction of PUMA and oligomerization of Bax resulted in mitochondrial outer membrane permeabilization and activation of caspase during wogonin treatment.
Free radicals can cause extensive chemical modifications and alterations in DNA and nucleoproteins, including modified bases and sugars and even strand breaks (Jaruga et al., 1994). DNA damage can cause cell death by induction of apoptosis via various signalling pathways (Roos and Kain, 2006). In cell models, DNA damage activates ATM and ATR proteins, which signal downstream to checkpoint kinases, such as CHK1 and CHK2, and tumour suppressor gene p53. As discussed previously, p53 is a major player in the apoptotic response of cells, because it induces transcriptional activation of pro-apoptotic factors such as Fas, Puma and Bax and inhibition of pro-survival factors such as Bcl-2 and Bcl-xl (Roos and Kain, 2006). A fundamental question which remains unanswered is how wogonin treatment increases the intracellular level of ROS. At the present time, we can only speculate about the mechanism of wogonin-induced ROS production. It is possible that ROS production is resulted from a consequence of the peroxide activation of wogonin in which peroxidase-catalysed one-electron oxidation of the wogonin phenolic ring and/or interaction of this phenolic moiety with reactive radicals yields its phenoxyl radical. Wogonin can also act as an antioxidant (Tai et al., 2005). It may act as an effective donor of electrons for scavenging reactive peroxyl radicals as indicated below.
Reactivity of wogonin phenoxyl radicals (Wogonin-O) towards different biomolecules (Reaction 2) may be dependent upon redox states in cells.
Wogonin phenoxyl radicals may enter the redox cycle to potentially increase ROS and subsequently cause oxidative DNA damage. The other possibility is that wogonin may act as an uncoupler of oxidative phosphorylation, induce ROS generation and disrupt mitochondrial membrane potentials. Another possibility is that wogonin oxidizes intracellular thiol-containing reducing agents like glutathione and thioredoxin, and thereby allows the accumulation of ROS.
Although we are far from understanding how wogonin promotes TRAIL cytotoxicity, we postulate that wogonin acts as an effective donor of electrons for scavenging reactive peroxyl radicals and that wogonin phenoxyl radicals enter the redox cycle to potentially increase ROS and subsequently cause oxidative DNA damage. DNA damage activates the ATM/ATR-p53-PUMA-Bax oligomerization-cytochrome c release-caspase-9 activation pathway. The activation of the intrinsic pathway by wogonin may promote the TRAIL-induced extrinsic pathway (Figure 12). This interaction is probably a key to unveiling the mechanism of the synergy exerted by the combination of wogonin and TRAIL to induce apoptosis. We believe that this model will provide a framework for future studies.
Acknowledgments
This work was supported by the following grants: NCI grant funds (CA95191, CA96989 and CA121395), DOD prostate program funds (PC020530 and PC040833), Susan G. Komen Breast Cancer Foundation fund (BCTR60306).
Glossary
Abbreviations:
- NAC
N-acetylcysteine
- PAGE
polyacrylamide gel electrophoresis
- PARP
poly(ADP-ribose) polymerase
- PBS
phosphate-buffered saline
- ROS
reactive oxygen species
- SDS
sodium dodecyl sulphate
- TNF
tumour necrosis factor
- TRAIL
TNF-related apoptosis-inducing ligand
Footnotes
Conflict of interest
The authors state no conflict of interest.
References
- Albano JD, Ward E, Jemal A, Anderson R, Cokkinides VE, Murray T, et al. Cancer mortality in the United States by education level and race. J Natl Cancer Inst. 2007;99:1384–1394. doi: 10.1093/jnci/djm127. [DOI] [PubMed] [Google Scholar]
- Baumann S, Fas SC, Giaisi M, Müller WW, Merling A, Gülow K, et al. Wogonin preferentially kills malignant lymphocytes and suppresses T-cell tumor growth by inducing PLCgamma1- and Ca2+-dependent apoptosis. Blood. 2008;111:2354–2363. doi: 10.1182/blood-2007-06-096198. [DOI] [PubMed] [Google Scholar]
- Burma S, Chen BP, Murphy M, Kurimasa A, Chen DJ. Phosphorylates histone H2A.X in response to DNA double-strand breaks. J Biol Chem. 2001;276:42462–42467. doi: 10.1074/jbc.C100466200. [DOI] [PubMed] [Google Scholar]
- Cariello NF, Beroud C, Soussi T. Database and software for the analysis of mutations at the human p53 gene. Nucleic Acids Res. 1994;22:3450–3459. [PMC free article] [PubMed] [Google Scholar]
- Chen YC, Shen SC, Lee WR, Lin HY, Ko CH, Shih CM, et al. Wogonin and fisetin induction of apoptosis through activation of caspase 3 cascade and alternative expression of p21Cip1 protein in hepatocellular carcinoma cells SK-HEP-1. Arch Toxicol. 2002;76:351–359. doi: 10.1007/s00204-002-0346-6. [DOI] [PubMed] [Google Scholar]
- Chi YS, Cheon BS, Kim HP. Effect of wogonin, a plant flavone from Scutellaria radix, on the suppression of cyclooxygenase-2 and the induction of inducible nitric oxide synthase in lipopolysaccharide-treated RAW 264.7 cells. Biochem Pharmacol. 2001;61:1195–1203. doi: 10.1016/s0006-2952(01)00597-4. [DOI] [PubMed] [Google Scholar]
- Chow JM, Huang GC, Shen SC, Wu CY, Lin CW, Chen YC. Differential apoptotic effect of wogonin and nor-wogonin via stimulation of ROS production in human leukemia cells. J Cell Biochem. 2008;103:1394–1404. doi: 10.1002/jcb.21528. [DOI] [PubMed] [Google Scholar]
- Chung H, Jung YM, Shin DH, Lee JY, Oh MY, Kim HJ, et al. Anticancer effects of wogonin in both estrogen receptor-positive and -negative human breast cancer cell lines in vitro and in nude mice xenografts. Int J Cancer. 2008;122:816–822. doi: 10.1002/ijc.23182. [DOI] [PubMed] [Google Scholar]
- Davis W, Jr, Ronai Z, Tew KD. Cellular thiols and reactive oxygen species in drug-induced apoptosis. J Pharmacol Exp Ther. 2001;296:1–6. [PubMed] [Google Scholar]
- Fas SC, Baumann S, Zhu JY, Giaisi M, Treiber MK, Mahlknecht U, et al. Wogonin sensitizes resistant malignant cells to TNFalpha- and TRAIL-induced apoptosis. Blood. 2006;108:3700–3706. doi: 10.1182/blood-2006-03-011973. [DOI] [PubMed] [Google Scholar]
- Fiers W. Tumor necrosis factor. Characterization at the molecular, cellular and in vivo level. FEBS Lett. 1991;285:199–212. doi: 10.1016/0014-5793(91)80803-b. [DOI] [PubMed] [Google Scholar]
- Gaiddon C, Moorthy NC, Prives C. Ref-1 regulation the transactivation and pro apoptotic functions of p53 in vivo. EMBO J. 1999;18:5609–5621. doi: 10.1093/emboj/18.20.5609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao C, Song JH, Hsi B, Lewis J, Song DK, Petruk KC, et al. TRAIL inhibits tumor growth but is nontoxic to human hepatocytes in chimeric mice. Cancer Res. 2004;64:8502–8506. doi: 10.1158/0008-5472.CAN-04-2599. [DOI] [PubMed] [Google Scholar]
- He Q, Huang Y, Sheikh MS. Proteasome inhibitor MG132 upregulates death receptor 5 and cooperates with Apo2L/TRAIL to induce apoptosis in Bax-proficient and -deficient cells. Oncogene. 2004;23:2554–2558. doi: 10.1038/sj.onc.1207351. [DOI] [PubMed] [Google Scholar]
- Jaruga P, Zastawny TH, Skokowski J, Dizdaroglu M, Olinski R. Oxidative DNA base damage and antioxidant enzyme activities in human lung cancer. FEBS Lett. 1994;341:59–64. doi: 10.1016/0014-5793(94)80240-8. [DOI] [PubMed] [Google Scholar]
- Kim YH, Lee YJ. TRAIL apoptosis is enhanced by quercetin through Akt dephosphorylation. J Cell Biochem. 2007;100:998–1009. doi: 10.1002/jcb.21098. [DOI] [PubMed] [Google Scholar]
- Kim KM, Song JJ, An JY, Kwon YT, Lee YJ. Pretreatment of acetylsalicylic acid promotes tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis by down-regulating BCL-2 gene expression. J Biol Chem. 2005;280:41047–41056. doi: 10.1074/jbc.M503713200. [DOI] [PubMed] [Google Scholar]
- Kim SC, Park SS, Lee YJ. Effect of UV irradiation on colorectal cancer cells with acquired TRAIL resistance. J Cell Biochem. 2008;104:1172–1180. doi: 10.1002/jcb.21682. [DOI] [PubMed] [Google Scholar]
- Lee DH, Kim C, Zhang L, Lee YJ. Role of p53, PUMA, and Bax in wogonin-induced apoptosis in human cancer cells. Biochem Pharmacol. 2008;75:2020–2033. doi: 10.1016/j.bcp.2008.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WR, Shen SC, Lin HY, Hou WC, Yang LL, Chen YC. Wogonin and fisetin induce apoptosis in human promyeloleukemic cells, accompanied by a decrease of reactive oxygen species, and activation of caspase 3 and Ca(2+)-dependent endonuclease. Biochem Pharmacol. 2002;63:225–236. doi: 10.1016/s0006-2952(01)00876-0. [DOI] [PubMed] [Google Scholar]
- Levine AJ. p53, the cellular gatekeeper for growth and division. Cell. 1997;88:323–331. doi: 10.1016/s0092-8674(00)81871-1. [DOI] [PubMed] [Google Scholar]
- Li DR, Hou HX, Zhang W, Li L. Effects of wogonin on inducing apoptosis of human ovarian cancer A2780 cells and telomerase activity. Ai Zheng. 2003;22:801–805. [PubMed] [Google Scholar]
- Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell. 2001;104:487–501. doi: 10.1016/s0092-8674(01)00237-9. [DOI] [PubMed] [Google Scholar]
- Nakata S, Yoshida T, Horinaka M, Shiraishi T, Wakada M, Sakai T. Histone deacetylase inhibitors upregulate death receptor 5/TRAIL-R2 and sensitize apoptosis induced by TRAIL/APO2-L in human malignant tumor cells. Oncogene. 2004;23:6261–6271. doi: 10.1038/sj.onc.1207830. [DOI] [PubMed] [Google Scholar]
- Ogasawara J, Watanabe-Fukunaga R, Adachi M, Matsuzawa A, Kasugai T, Kitamura Y, et al. Lethal effect of the anti-Fas antibody in mice. Nature. 1993;364:806–809. doi: 10.1038/364806a0. [DOI] [PubMed] [Google Scholar]
- Olive PL, Banath JP. Durand RE Heterogeneity in radiation-induced DNA damage and repair in tumor and normal cells measured using the ‘comet’ assay. Radiat Res. 1990;122:86–94. [PubMed] [Google Scholar]
- Oren M. Regulation of the p53 tumor suppressor protein. J Biol Chem. 1999;274:36031–36034. doi: 10.1074/jbc.274.51.36031. [DOI] [PubMed] [Google Scholar]
- Pei Z, Chu L, Zou W, Zhang Z, Qiu S, Qi R, et al. An oncolytic adenoviral vector of Smac increases antitumor activity of TRAIL against HCC in human cells and in mice. Hepatology. 2004;39:1371–1381. doi: 10.1002/hep.20203. [DOI] [PubMed] [Google Scholar]
- Roos WP, Kain B. DNA damage-induced cell death by apoptosis. Trends Mol Med. 2006;12:440–450. doi: 10.1016/j.molmed.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Shankar S, Srivastava R. Enhancement of therapeutic potential of TRAIL by cancer chemotherapy and irradiation: mechanisms and clinical implications. Drug Resist Updat. 2004;7:139–156. doi: 10.1016/j.drup.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Shankar S, Srivastava RK. Involvement of Bcl-2 family members, phosphatidylinositol 3′-kinase/AKT and mitochondrial p53 in curcumin (diferulolylmethane)-induced apoptosis in Prostate Cancer. Inter J Oncol. 2007;30:905–918. [PubMed] [Google Scholar]
- Shieh SY, Ikeda M, Taya Y, Prives C. DNA damage-induced phosphorylation of p53 alleviates inhibition by MDM2. Cell. 1997;91:325–334. doi: 10.1016/s0092-8674(00)80416-x. [DOI] [PubMed] [Google Scholar]
- Shigeno M, Nakao K, Ichikawa T, Suzuki K, Kawakami A, Abiru S, et al. Interferon-α sensitizes human hepatoma cells to TRAIL-induced apoptosis through DR5 upregulation and NF-κB inactivation. Oncogene. 2003;22:1653–1662. doi: 10.1038/sj.onc.1206139. [DOI] [PubMed] [Google Scholar]
- Singh SV, Srivastava SK, Choi S, Lew KL, Antosiewicz J, Xiao D, et al. Sulforaphane-induced cell death in human prostate cancer cells is initiated by reactive oxygen species. J Biol Chem. 2005;280:19911–19924. doi: 10.1074/jbc.M412443200. [DOI] [PubMed] [Google Scholar]
- Tai MC, Tsang SY, Chang LY, Xue H. Therapeutic potential of wogonin: a naturally occurring flavonoid. CNS Drug Rev. 2005;11:141–150. doi: 10.1111/j.1527-3458.2005.tb00266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact. 2006;160:1–40. doi: 10.1016/j.cbi.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Wang S, El-Deiry WS. Requirement of p53 targets in chemosensitization of colonic carcinoma to death ligand therapy. Proc Natl Acad Sci. 2003;100:15095–15100. doi: 10.1073/pnas.2435285100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Guo Q, You Q, Zhang K, Yang Y, Yu J, et al. Involvement of bax/bcl-2 in wogonin-induced apoptosis of human hepatoma cell line SMMC-7721. Anticancer Drugs. 2006;17:797–805. doi: 10.1097/01.cad.0000217431.64118.3f. [DOI] [PubMed] [Google Scholar]
- Yang X, Fraser M, Moll UM, Basak A, Tsang BK. Akt-mediated cisplatin resistance in ovarian cancer: modulation of p53 action on caspase-dependent mitochondrial death pathway. Cancer Res. 2006;66:3126–3136. doi: 10.1158/0008-5472.CAN-05-0425. [DOI] [PubMed] [Google Scholar]
- Yee KS, Vousden KH. Complicating the complexity of p53. Carcinogenesis. 2005;26:1317–1322. doi: 10.1093/carcin/bgi122. [DOI] [PubMed] [Google Scholar]
- Yoo J, Park SS, Lee YJ. Pretreatment of docetaxel enhances TRAIL-mediated apoptosis in prostate cancer cells. J Cell Biochem. 2008;104:1636–1646. doi: 10.1002/jcb.21729. [DOI] [PubMed] [Google Scholar]
- Yu JQ, Liu HB, Tian DZ, Liu YW, Lei JC, Zou GL. Changes in mitochondrial membrane potential and reactive oxygen species during wogonin-induced cell death in human hepatoma cells. Hepatol Res. 2007;37:68–76. doi: 10.1111/j.1872-034X.2007.00003.x. [DOI] [PubMed] [Google Scholar]


