Abstract
Background and purpose:
P2X receptors are widely expressed in cells of the immune system with varying functions. This study sought to characterize P2X receptor expression in the LAD2 human mast cell line and human lung mast cells (HLMCs).
Experimental approach:
Reverse transcriptase polymerase chain reaction (RT-PCR) and patch clamp studies were used to characterize P2X expression in mast cells using a range of pharmacological tools.
Key results:
RT-PCR revealed P2X1, P2X4 and P2X7 transcripts in both cell types; mRNA for P2X6 was also detected in LAD2 cells. Under whole-cell patch clamp conditions, rapid application of ATP (1–1000 µM) to cells clamped at −60 mV consistently evoked inward currents in both types of cells. Brief application of ATP (1 s) evoked a rapidly desensitizing P2X1-like current in both cell types. This current was also elicited by αβmethylene ATP (10 µM, 94% cells, n= 31) and was antagonized in LAD2 cells by NF 449 (1 µM) and pyridoxal phosphate-6-azo(benzene-2,4-disulphonic acid) (1–10 µM). A P2X7-like non-desensitizing current in response to high concentrations of ATP (1–5 mM) was also seen in both cell types (96% LAD2, n= 24; 54% HLMCs, n= 24) which was antagonized by AZ11645373 (1 µM). P2X7-like responses were also evoked in LAD2 cells by 2′(3′)-0-(4-benzoylbenzoyl)ATP (300 µM). A P2X4-like current was evoked by 100 µM ATP (80% LAD2, n= 10; 21% HLMCs, n= 29), the amplitude and duration of which was potentiated by ivermectin (3 µM).
Conclusion and implications:
Our data confirmed the presence of functional P2X1, P2X4 and P2X7 receptors in LAD2 cells and HLMCs.
Keywords: mast cells, human, lung, LAD2, P2X1 receptors, P2X4 receptors, P2X7 receptors, ATP, ivermectin, AZ11645373
Introduction
Mast cells are pluripotent immune cells which participate in a wide variety of physiological and pathological processes including asthma and allergy (Bradding et al., 2006; Rivera and Gilfillan et al., 2006; Brown et al. 2008). They can be activated through both FcεRI-dependent and FcεRI-independent pathways (Gilfillan and Tkaczyk, 2006). FcεRI-dependent activation triggers depletion of Ca2+ from internal stores, leading to an influx of extracellular Ca2+ which is crucial for the release of both pre-formed and newly generated mediators. There is increasing realization however, that mast cells also express an array of other classes of receptors which can either directly activate mast cells or modify IgE-dependent signalling; receptors which contribute to Ca2+ signalling in particular, would be expected to modulate degranulation, release of eicosanoids and the transcription and release of chemokines and cytokines. The entry of extracellular Ca2+ through non-selective cation channels such as P2X receptors is therefore likely to affect mast cell activation and moreover is probable as nucleotides, which are released through cell injury, platelet activation and cell death are likely to be present around the mast cells in inflamed tissues (Bodin and Burnstock, 2001).
The P2 receptor family which is composed of seven ionotropic P2X receptors (P2X1-7) (North, 2002) and eight metabotropic P2Y receptors (P2Y1,2,4,6,11–14) (von Kugelgen, 2006; nomenclature follows Alexander et al., 2008; Collingridge et al., 2009) is well represented in cells of the haematopoietic lineage (Di et al., 2001; Lemoli et al., 2004; Wang et al., 2004; Tolhurst et al. 2005). P2X receptors specifically have been described in many immune cells including platelets, lymphocytes and macrophages (Di et al., 2001) where they have been implicated in the regulation of a variety of functions including platelet aggregation, apoptosis, migration and cytokine release (Burnstock, 2002; Hechler et al., 2003). In particular it has been shown in macrophages that P2X7 activation induces the release of IL-1β (Ferrari et al., 1997).
Nucleotides, acting at P2 receptors, have previously been shown to influence mast cell function, including histamine secretion, chemotaxis, cytokine generation and apoptosis (Cockcroft and Gomperts, 1979; 1980; Tatham and Lindau et al., 1990; McCloskey et al., 1999; Schulman et al., 1999; Feng et al., 2004; Bulanova et al., 2005. We still know little about the molecular identity of the P2 receptor subtypes involved in these cellular events; however, robust expression of P2X7 in rodent mast cells has been well documented and can account for some of the ATP-mediated functions recorded. It is still to be determined if these results extrapolate to human mast cells.
We hypothesized that human mast cells possess functional P2X receptors which might therefore contribute to the extracellular Ca2+ influx responsible for the initiation of mast cell activation and secretion. This concept is supported by one of our earlier studies using GeneChip microarrays, showing the presence of P2X1 and P2X4 in human cord blood-derived, skin and lung mast cells (Bradding et al., 2003).
The principal aim of this study was to investigate the expression of P2X receptors in human lung mast cells (HLMCs) and the leukaemia-derived human mast cell line, LAD2 (Kirshenbaum et al., 2003). RT-PCR experiments on mRNA isolated from multiple donors showed that genes for P2X1, P2X4 and P2X7 are constitutively expressed in differentiated mast cells. Whole-cell patch-clamp recordings from isolated cells showed further that functional channels with the expected pharmacological and biophysical properties of P2X1, P2X4 and P2X7 receptors were present at the plasma membrane and that when bound to extracellular ATP, the receptors induced robust cationic inward currents.
Methods
Preparation and maintenance of HLMCs and a human mast cell line
All patients donating lung tissue gave written informed consent, and the study had approval from the Leicestershire Research Ethics Committee. HLMC were dispersed enzymatically from macroscopically normal lung obtained within 1 h of resection for lung cancer and purified using immunoaffinity magnetic selection (Dynabeads) as described previously (Sanmugalingam et al., 2000). The final mast cell purity, assessed using metachromatic staining, was >99% with cell viability >98% (monitored by exclusion of Trypan blue). HLMC were cultured in DMEM/Glutamax/HEPES containing 1% antibiotic/antimycotic solution, 1% non-essential amino acids, 10% fetal calf serum, 100 ng·mL−1 human stem cell factor, 50 ng·mL−1 IL-6 and 10 ng·mL−1, IL-10. Half the media was replaced every 7 days. Cells were maintained at 37 °C in an incubator with a humidified atmosphere of 5% CO2
The LAD2 mast cell line, derived from a patient with mast cell leukemia, was a gift from Dr. D. Metcalfe [National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD for details see Kirshenbaum et al. (2003)] and cultured in StemPro-34 SFM serum-free complete medium (Invitrogen Life Technologies), with 100 ng·mL−1 human stem cell factor as previously described (Wykes et al., 2007). Half the medium was replaced every 7 days.
RT-PCR
Total RNA from LAD2 cells and HLMC from three donors were isolated using QIAshredder and RNeasy kit (Qiagen, Crawley, UK). For LAD2 cells, 1 µg of total RNA was treated with DNAse I (Invitrogen Ltd., Paisley,UK) and subsequently reverse transcribed using SuperScript™III Reverse Transcriptase (Invitrogen Ltd.) and oligo(dT)21 in a final reaction volume of 20 µL. For negative control, SuperScript™III Reverse Transcriptase was replaced with H2O. PCR reactions (30 cycles) using BIOTAQ™ DNA Polymerase (1.25 unit·reaction−1) were then performed as instructed by the manufacturer (Bioline, London, UK) with 1 µL cDNA or 1 µL negative control. The sequence of the primers used for PCR amplifications on LAD2 cDNA were as follows: P2X1 [annealing temperature (AT) 58°C] forward 5′-CGTCATCGGGTGGGTGTTTCTCTA-3′, reverse 5′-AGGGCGCGGGATGTCGTCA-3′; P2X2 (AT 59°C) forward 5′-GGGCCCCGAGAGCTCCATCATC-3′, reverse 5′-GCAGGCAGGTCCAGGTCACAGTCC-3′; P2X3 (58°C)forward 5′-ACTGGCCGCTGCGTGAACTACA-3′, reverse 5′-CACGTCGAAGCGGATGCCAAAAG-3′; P2X4 (58°C) forward 5′-CGGCACCCACAGCAACGGAGTCT-3′, reverse 5′-TGTATCGAGGCGGCGGAAGGAGTA-3′; P2X5 (AT 55°C) forward 5′-GGCCCCAAGAACCACTACTGC-3′, reverse 5′-CCTCGGCCTCCTGGGAACTGTCT-3′; P2X6 (58°C) forward 5′-AGCCCCTACTGTCCCGTGTTCC-3′, reverse 5′-GCCTTGGCCTCCTCATACTTTGTC-3′; P2X7 (55°C) forward 5′-CCGGCCACAACTACACCACGAG-3′, reverse 5′-GGCCAGACCGAAGTAGGAGAGG-3′; human β-actin (57°C) forward 5′-TGGTGGGCATGGGTCAGAAG-3′, reverse 5′-GTCCCGGCCAGCCAGGTCCAG-3′.
For HMLC, 500 ng of total RNA was converted to cDNA (BioRad iScript kit, BioRad, Hemel Hempstead, UK). PCR reactions (35 cycles) using Thermo-Start PCR master mix were then performed as instructed by the manufacturer (ABgene Ltd., Epsom, UK). The sequence of the primers used for PCR amplifications on HLMC cDNA were as followed: P2X1 (55°C) forward 5′-TTTCATCGTGACCCCGAAGCAG-3′, reverse 5′-TCAAAGCGAATCCCAAACACC-3′; P2X4 (55°C) forward 5′-ACAGCAACGGAGTCTCAACAGG-3′, reverse 5′-CCTTCCCAAACACAATGATGTCG-3′and P2X7 (55°C) forward 5′-TGCGATGGACTTCACAGATTTG-3′, reverse 5′-TGCCCTTCACTCTTCGGAAAC-3′. For analysis, all PCR products were run on 1.5% agarose gel containing ethidium bromide.
Electrophysiological recordings
Standard whole-cell patch clamp recordings were performed at room temperature using either an EPC10 amplifier and Pulse acquisition software (HEKA, Lambrecht, Germany) or Axopatch 200B amplifier and pClamp9 software (Molecular Devices, Sunnyvale, CA, USA). Cells were plated on poly-L-lysine-treated coverslips and continuously superfused with an external solution consisting of (in mM) 147 NaCl, 10 HEPES, 16 glucose, 2 KCl, 2 CaCl2 and 1 MgCl2 (pH 7.3, NaOH). Where stated, this was replaced with a low divalent external solution consisting of (in mM) 147 NaCl, 10 HEPES, 13 glucose, 0.2 CaCl2 and 2 KCl (pH 7.3, NaOH). Recording electrodes were pulled from borosilicate glass (resistance: 3–6 MΩ) and backfilled with a standard internal solution consisting of (in mM) 135 Cs-glutamate, 8 NaCl, 10 EGTA, 10 HEPES, 3.6 CaCl2 and 2 MgATP (pH 7.3, CsOH). Electrode tips were forward filled with the same solution but omitting the MgATP.
To study the reproducibility of αβmethylene ATP (αβmeATP)-evoked currents, where stated some recordings were performed using the perforated patch configuration established by adding amphotericin B (120 mg·mL−1) to an internal solution consisting of (in mM): K-gluconate 140, NaCl 5, HEPES 10, EGTA 9, (pH adjusted to 7.3 with KOH). The external solution consisted of (in mM): NaCl 150, KCl 2.5, HEPES 10, CaCl2 2.5 and MgCl2 1 (pH adjusted to 7.3 with NaOH).
Agonists and antagonists were delivered using either an RSC fast-flow system (BioLogic Science Instruments, Grenoble, France) or a U-tube perfusion system (Evans and Kennedy, 1994). Antagonists were superfused (pyridoxal phosphate-6-azo(benzene-2,4-disulphonic acid (PPADS), 3 min; NF449, 5 min, KN-62 10–20 min) and/or applied via the fast-flow system (AZ11645373, 1 min) before and during application of agonist. Membrane potential was clamped at −60 mV in all experiments except where the voltage-dependence of agonist-induced currents was examined by ramping the membrane potential from −60 mV to +50 mV over 450 ms. Leak currents were recorded by applying the same ramp protocol in the absence of agonist and were then subtracted from the current–voltage traces. All current–voltage traces corrected for a −9 mV liquid junction potential.
Materials
DMEM/Glutamax/HEPES, antibiotic/antimycotic solution, MEM non-essential amino acids and fetal calf serum obtained from Invitrogen Life Technologies. Stem cell factor, IL-6 and IL-10 were obtained from R&D, Abingdon, UK. Nucleotides, suramin, PPADS, poly-L-lysine, ivermectin, EGTA, D-glutamic acid and HEPES were purchased from Sigma-Aldrich. Physiological salts, sodium hydroxide and glucose were purchased from BDH Anachem. CsOH was purchased from ICN Biomedicals. AZ11645373 was a generous gift from AstraZeneca R&D Charnwood, UK.
Results
HLMC and LAD2 express P2X1, P2X4 and P2X7
RT-PCR experiments were carried out to determine the mRNA expression for P2X receptors in LAD2 and HLMC. Strong expression was seen for P2X1, P2X4 and P2X7 receptors in both the LAD2 human mast cell line and HLMCs obtained from three different donors (Figure 1). This is in accordance with findings of an earlier study using GeneChip microarrays which indicated the presence of P2X1 and P2X4 in human cord blood-derived, skin and lung mast cells (Bradding et al., 2003). Low levels of P2X6 receptors were also found in the LAD2 cells.
Figure 1.
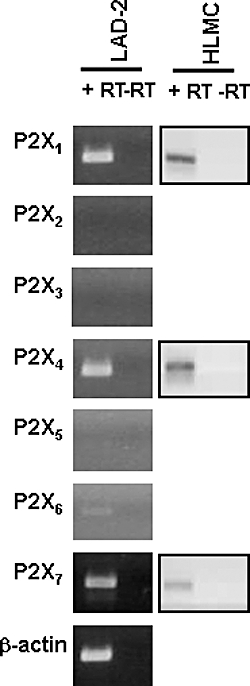
P2X receptor expression in LAD2 cells and HLMCs. RT-PCR on total RNA isolated from LAD2 cells and HMLCs. Data for HLMC are representative of similar results obtained from three donors. Both types of human mast cells revealed the presence of P2X1, P2X4 and P2X7 receptor transcripts. P2X6 receptor transcripts were also identified in LAD2 cells. (+RT) and (–RT) indicate the presence or the absence of reverse transcription reaction. The housekeeping gene β-actin was used as an internal control of integrity of the samples. HLMCs, human lung mast cells.
Presence of a P2X1-like current in LAD2 cells
To investigate the biophysical and pharmacological properties of native P2X receptors expressed in human mast cells, we conducted whole-cell and perforated-patch clamp recording experiments in LAD2 cells and cultured HLMCs. Brief applications (1–2 s) of ATP (0.1–100 µM) to isolated LAD2 cells voltage-clamped at −60 mV elicited a rapidly activating and rapidly desensitizing inward current (Figure 2A). The amplitude of the current evoked by the first application of ATP showed high variability from cell to cell probably reflecting different levels of pre-existing desensitization. Incubation of cells with suramin (20 µM, 1.5–3 h) prior to commencing the recordings, reduced this cell-to-cell variability and under these conditions the mean current evoked by the first application of 1 µM ATP was −44.3 ± 4.2 pA·pF−1 (n= 5). Pre-incubation with apyrase (0.32 U·mL−1 type VII) was similarly effective in decreasing this pre-existing desensitization, consistent with the view that it is caused by even nanomolar levels of ATP (Rettinger and Schmalzing, 2003) released into the growth media by dying or spontaneously active cells.
Figure 2.
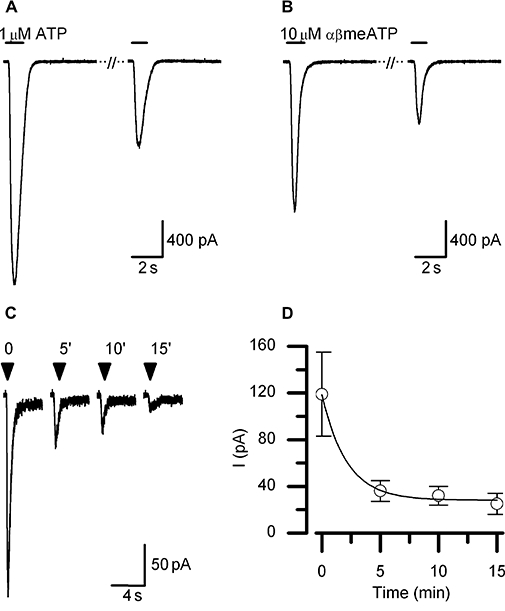
P2X1-like current in LAD2 cells. A rapidly activating and fast desensitizing current is elicited by both ATP (A) and αβmeATP (B) in the whole-cell configuration. The time of agonist application is indicated by bars drawn above the current traces. Consecutive current traces shown represent the first and second application of agonist given to the same cell with a 5 min interval. The second response to agonist was always significantly attenuated compared with the first, consistent with ‘rundown’ of the receptor. (C) Receptor rundown is also seen in response to repeated applications of αβmeATP under perforated patch recording conditions. Currents shown are from a single cells, agonist was applied for 2 s at times indicated by arrow heads. (D) Mean data from n= 7 experiments like that shown in (C), showing the time course of receptor rundown. The line drawn through the data points represents the best fit exponential decline. All currents were recorded at a holding potential of −60 mV unless otherwise stated. αβmeATP, αβmethylene ATP.
In 94% of suramin pretreated cells, a current with similar kinetics and amplitude was also elicited by the ATP analogue, αβmeATP (10 µM) (Figure 2B; mean amplitude −35.5 ± 5.6 pA·pF−1, n= 29). The decline of the current evoked by a 5 s application of 10 µM αβmeATP was best fitted by a single exponential with a time constant of 288 ± 32 ms (n= 7). Responses to repeated applications of agonist, given at 5 min intervals, ran down (Figure 2A,B). This apparent irreversible loss of receptor and lack of full recovery from desensitization was also observed under perforated-patch recording conditions (Figure 2C) and followed a mono-exponential time course with a time constant of 127 s (Figure 2D). This rapid rundown of the responses precluded detailed analysis of rank order of potency of agonists for this receptor or a detailed analysis of the current voltage-relationship. We were however, able to confirm that the currents evoked by 1 µM ATP could be reversibly antagonized by PPADS (1–10 µM) (Figure 3A,B) and were also antagonized by the P2X1-selective antagonist NF 449 (Rettinger et al., 2005) (Figure 3C,D). Taken together with the RT-PCR data, these results indicate that LAD2 cells express functional P2X1 receptors.
Figure 3.
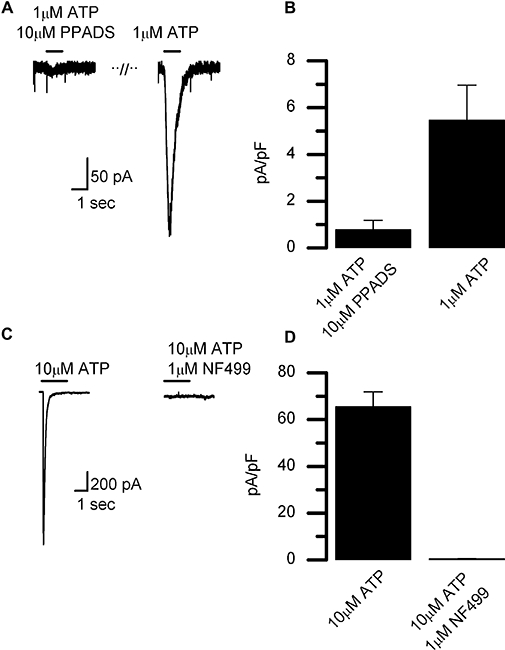
Antagonism of P2X1-like current in LAD2 cells by PPADS and NF 449. (A) Representative current traces recorded in response to a 1 s application of ATP with PPADS and then again after a 10 min washout of the antagonist. Cells were pre-equilibrated with the antagonist with PPADS (10 µM) by superfusion for 3 min at the start of the experiment. (B) Summary of PPADS (10 µM) antagonism of the ATP (1 µM) induced current, expressed as mean peak currents ± SEM (n= 4). (C) Current induced by ATP (10 µM) in the presence or absence of NF 449 (1 µM, superfused 5 min before and during agonist application). (D) Summary of NF 449 antagonism of the ATP-induced current, data expressed as mean current ± SEM. Nucleotides applied for duration indicated by bars above traces. Currents were recorded at −60 mV. PPADS, pyridoxal phosphate-6-azo(benzene-2,4-disulphonic acid.
Presence of a P2X4-like current in LAD2 cells
Application of a moderately high concentration of ATP (100 µM) elicited a biphasic current in 58% of cells tested (n= 19). The first response evoked consisted of an initial fast component with properties similar to the P2X1-like current followed by a more sustained phase. Responses to subsequent applications of 100 µM ATP (1 min intervals) lacked the initial fast component and consisted of a sustained current which ran down over time in the whole-cell configuration (Figure 4A). If the first application of 100 µM ATP was preceded by a 5 s conditioning application of 10 µM αβmeATP to desensitize any existing P2X1 receptors, then the resulting current evoked by ATP lacked the initial peak and consisted of only a slowly desensitizing current (Figure 4B). This type of current was also observed in the presence the P2X7-selective antagonist AZ11645373 (Stokes et al., 2006) (mean amplitude 106 ± 19 pA (n= 11); Figure S2). From the molecular expression patterns of P2X receptors in these cells along with the kinetics of the response, it therefore seemed likely a P2X4-like receptor was responsible for this slowly desensitizing current. Homomeric P2X4 receptors are insensitive to αβmeATP at the concentrations used in this study (10 µM), but reportedly gain sensitivity to this agonist in heteromeric assemblies with P2X1 (Nicke et al., 2005). The rapid desensitization kinetics of the currents recorded with αβmeATP in our studies are strikingly different to the slow desensitization properties reported for heteromeric P2X1/4 receptors (Nicke et al., 2005) consistent with the view that LAD2 cells express homomeric P2X1 receptors. Further evidence against the existence of heteromeric assemblies of P2X1/4 in LAD2 cells comes from the relative insensitivity of the current evoked by 100 µM ATP in these cells to the antagonist suramin (10–100 µM, n= 3 data not shown); both P2X1 homomeric channels and P2X1/4 channels are fully blocked by these concentrations of suramin (Nicke et al., 2005).
Figure 4.
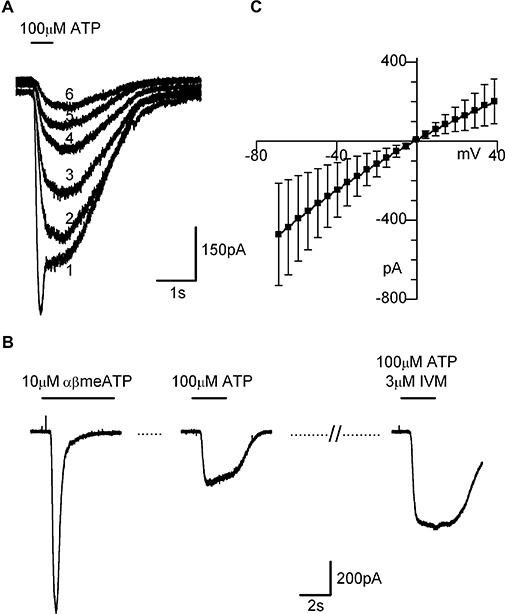
P2X4-like current in LAD2 cells. (A) Overlayed currents recorded in a single cell in response to repeated (numbered 1–6) brief applications 100 µM ATP (indicated by bar drawn above). Note the first current elicited by agonist application (labelled 1) has a biphasic time course of desensisitization; the initial fast component is missing from subsequent traces. (B) Example of ivermectin (IVM) potentiation of the slowly desensitizing current evoked by 100 µM ATP. For this experiment, any contributions from P2X1-like responses were removed by desensitizing the receptor with a conditioning application of αβmeATP (10 µM, left panel), 100 µM ATP was then applied (middle panel), followed by 3 min ivermectinapplication (3 µM) and subsequent ATP+ivermectin application (right panel). (C) Current–voltage relationship of ivermectin-potentiated current, mean ± SEM (n= 3). αβmeATP, αβmethylene ATP.
Uniquely among the P2X receptors, homomeric P2X4 receptors have been shown to be potentiated by ivermectin (Khakh et al., 1999) with both an increase in peak current (Toulme et al., 2006) and prolongation of the current (Priel and Silberberg, 2004). Treatment with ivermectin (3 µM, 3 min) significantly potentiated (1.8 ± 0.2-fold, n= 7) the slowly desensitizing current seen in response to 100 µM ATP and markedly slowed the deactivation kinetics following washout of the agonist (Figure 4B). The current–voltage relationship of the ivermectin-potentiated current showed moderate inward rectification and reversed near 0 mV as expected for a non-selective cation current (Figure 4C). Taken together, the kinetics and pharmacology of the responses evoked by 100 µM ATP indicate that LAD2 cells express functional P2X4 receptors as well as homomeric P2X1 receptors.
Presence of a P2X7-like current in LAD2 cells
P2X7 receptors are distinguished from other types of P2X receptors by their low affinity for ATP, requiring concentrations in excess of 1 mM to be activated, their high affinity for 2′(3′)-0-(4-benzoylbenzoyl)ATP (BzATP), their sensitivity to external divalent cations and their tendency to run-up rather than run-down upon repeated agonist application (North, 2002). Consistent with the known properties of P2X7 receptors, repeated application of 1–5 mM ATP in a low divalent external solution to LAD2 cells evoked a slowly activating current whose amplitude increased with each successive application (96% of cells, n= 24) (Figure 5A). A current showing similar characteristics could also be elicited by BzATP (300 µM, 100% cells, n= 8) (Figure 5B). The current–voltage relationship was characteristic of a P2X7-like channel (Rassendren et al., 1997), showing no rectification and a reversal potential of 0 mV (Figure 5C). Moreover, the current was antagonized by AZ11645373, which markedly reduced the mean facilitated current in response to 1 mM ATP (Figure 5D,E). Pre-incubation of the cells for 15–25 min with KN-62 (1 µM), a non-competitive antagonist at human P2X7 receptors (Gargett and Wiley, 1997; Humphreys et al., 1998), also abolished currents evoked by 5 mM ATP (n= 5). Finally, LAD2 cells were also shown to exhibit another characteristic property of P2X7 receptors (North, 2002), namely the uptake of ethidium bromide in response to receptor activation (ATP 5 mM, Figure S1). Thus in agreement with the molecular data, electrophysiological and dye uptake studies of the purinoceptors present in LAD2 cells indicate the cells express functional P2X1, P2X4 and P2X7 receptors.
Figure 5.
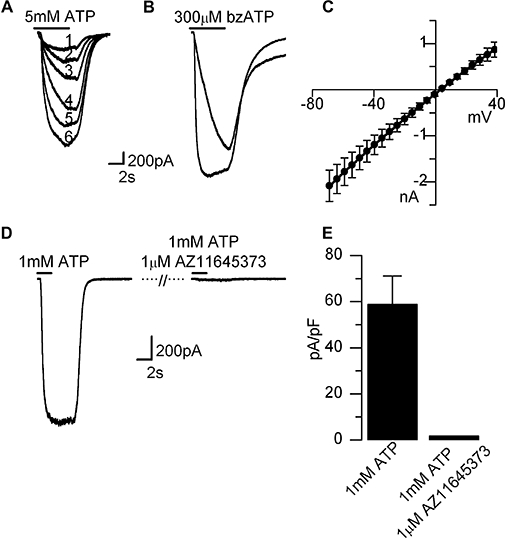
P2X7-like current in LAD2 cells. (A) Superimposed traces (labelled 1–6) from a single cell showing facilitation of P2X7-like responses in response to repeated applications of ATP (5 mM), and BzATP (300 µM) (B). (C) Current–voltage relationship of the ATP evoked current expressed as mean ± SEM (n= 5). (D) Near complete antagonism of ATP (1 mM) induced current with AZ11645373 (1 µM applied for 1 min prior to ATP+antagonist application). (D) Summary of inhibition of ATP (1 mM) induced current by AZ11645373 (1 µM), expressed as mean current ± SEM (n= 4). All recordings made in low divalent external solution to maximize P2X7 activation. Nucleotides applied for durations indicated in bars above traces. Currents were recorded at −60 mV unless otherwise stated. BzATP, 2′(3′)-0-(4-benzoylbenzoyl)ATP.
P2X currents in HLMCs
Functional expression of P2X receptors in HLMCs was then investigated using similar electrophysiology experiments. Consistent with our results in LAD2 cells, a rapidly activating and fast desensitizing P2X1-like current was also elicited by either ATP or αβmeATP in HLMCs (Figure 6A). αβmeATP (10 µM) produced a current in 94% of cells (n= 31) pre-incubated with suramin (20 µM, 1.5–3 h) with a mean current amplitude of −13.4 ± 2.7 pA·pF−1 (pooled results from two donors, n= 29) which desensitized with a mono-exponential time course (τ= 241 ± 17 ms, n= 13). Raising the ATP concentration to 100 µM evoked a slowly desensitizing P2X4-like current in only 21 % of these cells. The effect of ivermectin on these responses was more varied than that observed in LAD2 cells; 80% (n= 5) showed an alteration in the current following ivermectin treatment. In three out of four cells tested, ivermectin increased the peak amplitude of the current evoked by 100 µM ATP (overall an average of 3.0 ± 1.7-fold increase). However, more consistently, ivermectin slowed the current deactivation kinetics resulting in a 13.5 ± 6.6-fold increase in the current remaining 3 s after termination of the ATP (Figure 6B).
Figure 6.
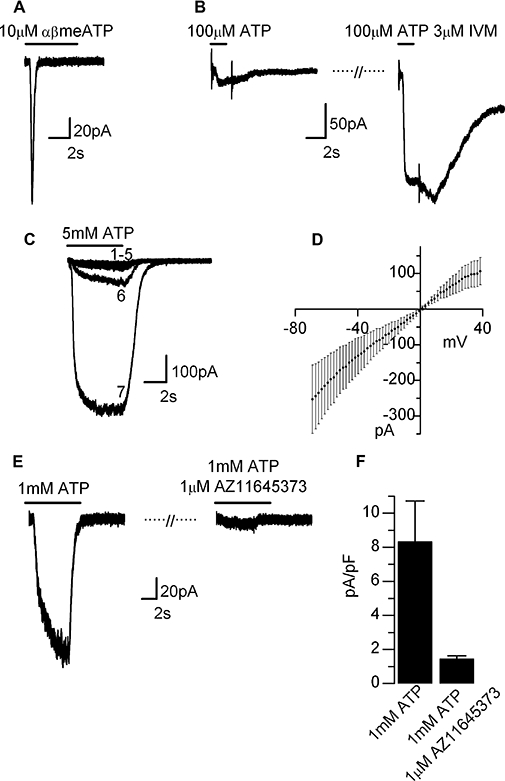
P2X currents in HLMCs. (A) Rapidly desensitizing P2X1-like current seen in response to αβmeATP. (B) Example of a P2X4-like current elicited by ATP (100 µM) showing a characteristic increase in amplitude and duration following ivermectin (IVM) treatment [3 µM superfused for 3 min before application of ATP (100 µM)+ivermectin (3 µM)]. (C) Facilitating P2X7-like currents elicited by repeated application of 5 mM ATP (1 min intervals, labelled 1–6). Maximum facilitation of the response (labelled 7) was achieved following a 1 min continuous application of 5 mM ATP. (D) Current–voltage relationship of ATP (5 mM) induced current, means ± SEM (n= 5). (E) Antagonism of ATP (1 mM) induced currents by AZ 11645373 (1 min application of antagonist prior to ATP+antagonist application). (F) Summary of antagonism of ATP (1 mM) induced current by AZ11645373, expressed as mean currents ± SEM (n= 6). Recordings made in low divalent external solution to maximize currents through P2X7 receptors. Nucleotides applied for durations indicated by bars drawn over the traces. Currents were recorded at −60 mV unless otherwise stated. HLMCs, human lung mast cells.
Application of high concentrations of ATP (5 mM) to HLMCs evoked a P2X7-like current with characteristic slow kinetics and which facilitated on repeated agonist-applications (Figure 6C). A current with these properties was seen in 54 % of cells tested (n= 24, pooled results three donors). The variability in the expression of P2X7-like currents was observed from cell-to-cell rather than donor-to-donor, and was therefore unlikely to be a consequence of loss-of-function polymorphisms in the P2X7 gene (Fernando et al., 2006). In responding cells, the average current before facilitation was 16.5 ± 2.0 pA·pF−1 compared with 183 ± 56 pA·pF−1 (n= 11) post facilitation. The current–voltage relationship of the facilitated current was linear with a reversal potential at 0 mV (Figure 6D). As observed with the P2X7-like current in LAD2 cells, the facilitating current observed in HLMCs was abolished by 1 µM AZ11645373 (Figure 6E,F).
Discussion
This study provides the first functional evidence for the expression of P2X receptors in HLMC and the LAD2 cell line. The molecular and electrophysiological data show that both these types of human mast cells express three distinct subtypes of P2X receptors with properties which closely resemble those described for homomeric P2X1, P2X4 and P2X7 receptors (North, 2002).
Homomeric P2X1 receptors are characterized by their fast activation and desensitization kinetics, high affinity for ATP and αβmeATP (EC50∼1 µM) and high sensitivity to blockade by the non-selective P2 antagonists PPADS as well as the P2X1-selective antagonist NF 449 (Evans et al., 1995; Rettinger et al., 2005). These properties correspond closely with those observed for responses evoked by low concentrations (1–10 µM) of ATP and αβmeATP in our human mast cells. In common with previous reports on native and expressed P2X1 receptors (Evans et al., 1995; Lewis and Evans, 2000; Sim et al., 2007), the P2X1-like currents evoked in both the LAD2 cells and HLMC showed rapid ‘run-down’ upon repeated application of agonist with consequent loss of responsiveness to low concentrations of ATP. Unlike P2X1-receptors in smooth muscle cells (Lewis and Evans, 2000), rundown in the mast cells was not prevented by using the perforated patch recording technique, indicating that it was not caused by loss of cytoplasmic components in the whole-cell recording configuration. It is therefore more likely to represent a desensitization process with a very slow recovery time, possibly associated with receptor internalization (Dutton et al., 2000; Ennion and Evans, 2001). Activation of this slow desensitization process by endogenous ATP, released into the culture media by spontaneously active (Osipchuk and Cahalan, 1992) or dying cells, is likely to account for the cell-to-cell variability in responsiveness we observed in both types of mast cells and would explain why pretreatment with apyrase or a reversible antagonist reduces this variability. This pre-existing desensitization of P2X1 receptors could also account for failure of previous studies on mast cells to detect the presence of P2X1-like receptors (Tatham and Lindau, 1990; Schulman et al., 1999).
P2X1 receptors are also reported to form heteromeric assemblies with P2X5 or P2X4 receptors (North, 2002; Nicke et al., 2005; Rettinger et al., 2005; Lalo et al., 2008). Neither LAD2 nor HLMCs expressed mRNA for P2X5, we can therefore discount the presence of P2X1/5 receptors; both, however, expressed P2X4 mRNA, raising the possibility for the expression of P2X1/4 receptors. The rapid desensitization of responses to αβmeATP observed in our studies are, however, very different to the sustained responses reported for P2X1/4 receptors (Nicke et al., 2005), supporting the notion that in human mast cells, as in macrophages (Sim et al., 2007), co-expression of P2X1 and P2X4 does not necessarily lead to the formation of heteromeric receptors but instead to the expression of two separate populations of homomeric receptors.
Increasing the concentration of applied ATP to moderate levels (100 µM) revealed the presence of a second P2X-like current in LAD2 and HLMCs which had distinct pharmacology and slow desensitization kinetics compared with the P2X1-receptors. This second receptor subtype was αβmeATP-insensitive and was potentiated and prolonged by ivermectin, two characteristic features of homomeric P2X4 receptors (Khakh et al., 1999; North, 2002). Moreover, like heterologously expressed P2X4 receptors (Fountain and North, 2006), the P2X4-like currents in human mast cells declined in amplitude with repeated agonist application under whole-cell recording conditions and was not antagonized by AZ11645373. Given that we also found mRNA for P2X4, we concluded that P2X4 receptors account for this second component of ATP-induced current in human mast cells.
Increasing the concentration of ATP to mM levels revealed a third component P2X-mediated current in the human mast cells with biophysical and pharmacological properties which closely resemble cloned human P2X7 receptors and native P2X7-like receptors in other immune cells (Rassendren et al., 1997; North, 2002). Thus BzATP was an effective agonist at the P2X7-like receptor in LAD2 cells and HLMCs, but as observed previously for cloned human P2X7 receptors, the potency was significantly less than at rodent P2X7 (Rassendren et al., 1997) and the human P2X7-selective antagonist AZ11645373 (Stokes et al., 2006) effectively abolished the currents evoked by 1 mM ATP in both the LAD2 cells and HLMC. Consistent with the known pharmacology of human P2X7 receptors (Gargett and Wiley, 1997; Humphreys et al., 1998), KN-62 was also found to antagonize the current evoked by 5 mM ATP in LAD2 cells. Other characteristic features of cloned human homomeric P2X7 receptors including sensitivity to extracellular divalent cations, changes in their time course and increasing amplitude with repeated agonist application and linear current–voltage relationship were also observed in the human mast cells. Thus our electrophysiological data along with the RT-PCR data strongly indicate that HLMC and LAD2 cells express functional P2X7-like receptors. In contrast to our findings, Schulman et al. (1999) failed to detect the presence of P2X7 receptors in HLMCs. The main difference between our study and theirs was that they used freshly isolated HLMCs, while we used cells treated with stem cell factor and IL-6 to prevent apoptosis and sustain them in culture. Thus four possible explanations for the differences between the results of the two studies are (i) the expression of P2X7 receptors was up-regulated by the culture conditions; (ii) P2X7-receptor expressing cells are selectively killed during cell isolation procedures by exposure to high levels of ATP present from damaged and dying cells; (iii) P2X receptor expression varies among subtypes of mast cells present in human lung (Oskeritzian et al., 2005); or (iv) HLMCs used in the Schulman study were inadvertently isolated from a group of donors homozygous for one of the loss-of-function polymorphisms in the P2X7 gene (Fernando et al., 2006).
Mast cells are notoriously heterogeneous, as their phenotype is strongly influenced by the environment of the tissue in which they reside, by cell-to-cell contact and differences in the concentration of stem cell factor and other cytokines and chemokines to which they are exposed (Metz and Maurer, 2007). Interestingly another study which used human mast cells derived from cord blood mononuclear cells, cultured under similar conditions to ours, also found mRNA for P2X1, P2X4 and varying levels of P2X7 (Feng et al., 2004). As expression levels of P2X7 and P2X4 receptors are known to be dynamically regulated in other immune cells in response to pro- and anti-inflammatory stimuli (Humphreys and Dubyak, 1998; La et al., 2003; Raouf et al., 2007; Wilson et al., 2007), it seems highly plausible that the expression of these receptors in human mast cells may also be regulated within the lung. Dynamic regulation of P2X7 and P2X4 in maturing or activated mast cells could also explain why we found differences in the percentages of HLMCs expressing functional P2X4 (21%) versus P2X7 receptors (54%) compared with P2X1 (94%).
Co-expression of P2X4 and P2X7 is found in many other types of immune cells as well as non-immune cells (Dubyak, 2007). Within the lung, receptors with properties resembling P2X4 and P2X7 have been described in macrophages and ciliated epithelia (Rassendren et al., 1997; Bowler et al., 2003; Ma et al., 2006). Recently, evidence for the existence of heteromeric P2X4/7 receptors with a mixed pharmacology has emerged (Guo et al., 2007). Notably these receptors have a higher sensitivity to BzATP than ATP and are allosterically modulated by ivermectin. Whether these heteromeric receptors are able to regulate the full complement of downstream signalling pathways described for P2X7 receptors, including the ability to form ethidium and YO-PRO-1 permeable pores has not been investigated. Unfortunately, without the expression of dominant negative subunits like those used by Guo et al. (2007), distinguishing between a mixed population of heteromeric assemblies and homomeric assemblies of P2X4/7, P2X4 and P2X7 in HLMCs and LAD2 cells is not possible.
The presence of P2X7 receptors in rodent mast cells is well established. Early studies in rat peritoneal mast cells identified an ATP4−-gated ion channel which had a large pore conductance and linear current–voltage relationship, that caused calcium influx, degranulation, histamine release, permeabilization of the membrane and dye uptake (Bennett et al., 1981; Tatham et al., 1988). RT-PCR, Western blotting and calcium imaging studies further confirmed the presence of P2X7 receptors in murine bone marrow-derived mast cells and moreover showed them to up-regulate transcription and trigger release of pro-inflammatory cytokines and to induce mast cell apoptosis (Bulanova et al., 2005). Interestingly, the RT-PCR data of Bulanova et al. (2005) also indicated the presence of P2X1 and P2X4 receptors consistent with the our data in human mast cells. However, functional evidence for the expression of the receptors is lacking and was unlikely to have been detected as most experiments were performed with high and relatively long applications of ATP which would desensitize P2X1 and homomeric P2X4 receptors.
Mast cells are strongly implicated in the pathogenesis of many allergic diseases, including asthma via their release of mediators into surrounding tissues (Bradding et al., 2006). In areas of inflammation, such as the site of an allergic reaction, there are many sources of ATP such as ruptured blood vessels, platelets and even activated mast cells themselves (Beigi et al., 1999; Adriaensen and Timmermans, 2004). By expressing a mixture of P2X1, P2X4 and P2X7 receptors with their high-to-low affinity for ATP, HLMCs and a growing number of immune cells found to express the same repertoire of receptors, would be able to tailor their response to ATP in a concentration-dependent manner. Thus submicromolar levels of ATP would selectively activate P2X1 receptors, which although rapidly desensitizing, can none-the-less produce significant calcium influx (Evans et al., 1996; Egan and Khakh, 2004) and which may synergize with signalling by other classes of receptors (Vial et al., 2002; Fung et al., 2007), including P2Y receptors and IgE receptors (Schulman et al., 1999; Kuehn and Gilfillan, 2007), to significantly modulate mast cell function. Increasing ATP concentrations to micromolar levels would recruit signalling via P2X4, which like P2X1 receptors, have high calcium permeability (Egan and Khakh, 2004) but are much less rapidly desensitized and therefore can produce long lasting changes in intracellular calcium independently of other receptor regulated pathways present in mast cells. Increasing ATP concentrations yet further to millimolar levels would lead to activation of P2X7 receptor signalling cascades (Chen and Brosnan, 2006), cytokine generation and finally apoptosis and attenuation of the inflammatory response in that particular region of the lung (Bulanova et al., 2005). Thus via their effects either independently or in consort with other signalling pathways, P2X receptors are likely to play an important role in mast cell signalling and could represent novel targets for mast cell stabilizing drugs used in treatment of chronic inflammatory diseases such as asthma.
Glossary
Abbreviations:
- αβmeATP
αβmethylene ATP
- BzATP
2′(3′)-0-(4-benzoylbenzoyl)ATP
- HLMCs
human lung mast cells
- PPADS
pyridoxal phosphate-6-azo(benzene-2,4-disulphonic acid
- RT-PCR
reverse transcriptase polymerase chain reaction
Conflicts of interest
None.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Figure S1 Ethidium bromide uptake in LAD-2 cells in response to 5 mM ATP. Time course of ethidium bromide uptake and intracellular fluorescence increase. Traces represent the mean ± SEM (n = 66 cells). Cells were plated on poly-L lysine coated coverslips and perfused with a bath solution containing (in mM) 147 NaCl, 10 HEPES, 16 Glucose, 2 KCl, 2 CaCl2 and 1 MgCl2 (pH 7.3, NaOH) supplemented with 25 μM ethidium bromide. 5 mM ATP applied at t = 100 s. Experiments carried out on a Nikon confocal imaging system.
Figure S2 P2X1 and P2X4 like currents in LAD-2 cells in the presence of 1 μM AZ11645373. Cells superfused with 1 μM AZ11645373 for at least 1 min prior to agonist applications. Recordings made in the whole cell configuration at a holding potential of −60 mV.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Adriaensen D, Timmermans JP. Purinergic signalling in the lung: important in asthma and COPD? Curr Opin Pharmacol. 2004;4:207–214. doi: 10.1016/j.coph.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 3rd edn. Br J Pharmacol. 2008;153(Suppl. 2):S1–S209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beigi R, Kobatake E, Aizawa M, Dubyak GR. Detection of local ATP release from activated platelets using cell surface-attached firefly luciferase. Am J Physiol. 1999;276:C267–C278. doi: 10.1152/ajpcell.1999.276.1.C267. [DOI] [PubMed] [Google Scholar]
- Bennett JP, Cockcroft S, Gomperts BD. Rat mast cells permeabilized with ATP secrete histamine in response to calcium ions buffered in the micromolar range. J Physiol. 1981;317:335–345. doi: 10.1113/jphysiol.1981.sp013828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodin P, Burnstock G. Purinergic signalling: ATP release. Neurochem Res. 2001;26:959–969. doi: 10.1023/a:1012388618693. [DOI] [PubMed] [Google Scholar]
- Bowler JW, Bailey RJ, North RA, Surprenant A. P2X4, P2Y1 and P2Y2 receptors on rat alveolar macrophages. Br J Pharmacol. 2003;140:567–575. doi: 10.1038/sj.bjp.0705459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradding P, Okayama Y, Kambe N, Saito H. Ion channel gene expression in human lung, skin, and cord blood-derived mast cells. J Leukocyte Biology. 2003;73:614–620. doi: 10.1189/jlb.1202602. [DOI] [PubMed] [Google Scholar]
- Bradding P, Walls AF, Holgate ST. The role of the mast cell in the pathophysiology of asthma. J Allergy Clin Immunol. 2006;117:1277–1284. doi: 10.1016/j.jaci.2006.02.039. [DOI] [PubMed] [Google Scholar]
- Brown JM, Wilson TM, Metcalfe DD. The mast cell and allergic diseases: role in pathogenesis and implications for therapy. Clin Exp Allergy. 2008;38:4–18. doi: 10.1111/j.1365-2222.2007.02886.x. [DOI] [PubMed] [Google Scholar]
- Bulanova E, Budagian V, Orinska Z, Hein M, Petersen F, Thon L, et al. Extracellular ATP induces cytokine expression and apoptosis through P2X7 receptor in murine mast cells. J Immunol. 2005;174:3880–3890. doi: 10.4049/jimmunol.174.7.3880. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Purinergic signaling and vascular cell proliferation and death. Arterioscler Thromb Vasc Biol. 2002;22:364–373. doi: 10.1161/hq0302.105360. [DOI] [PubMed] [Google Scholar]
- Chen L, Brosnan CF. Regulation of immune response by P2X7 receptor. Crit Rev Immunol. 2006;26:499–513. doi: 10.1615/critrevimmunol.v26.i6.30. [DOI] [PubMed] [Google Scholar]
- Cockcroft S, Gomperts BD. ATP induces nucleotide permeability in rat mast cells. Nature. 1979;279:541–542. doi: 10.1038/279541a0. [DOI] [PubMed] [Google Scholar]
- Cockcroft S, Gomperts BD. The ATP4-receptor of rat mast cells. Biochem J. 1980;188:789–798. doi: 10.1042/bj1880789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collingridge GL, Olsen RW, Peters J, Spedding M. A nomenclature for ligand-gated ion channels. Neuropharmacology. 2009;56:2–5. doi: 10.1016/j.neuropharm.2008.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di VF, Chiozzi P, Ferrari D, Falzoni S, Sanz JM, Morelli A, et al. Nucleotide receptors: an emerging family of regulatory molecules in blood cells. Blood. 2001;97:587–600. doi: 10.1182/blood.v97.3.587. [DOI] [PubMed] [Google Scholar]
- Dubyak GR. Go it alone no more – P2X7 joins the society of heteromeric ATP-gated receptor channels. Mol Pharmacol. 2007;72:1402–1405. doi: 10.1124/mol.107.042077. [DOI] [PubMed] [Google Scholar]
- Dutton JL, Poronnik P, Li GH, Holding CA, Worthington RA, Vandenberg RJ, et al. P2X1 receptor membrane redistribution and down-regulation visualized by using receptor-coupled green fluorescent protein chimeras. Neuropharmacology. 2000;39:2054–2066. doi: 10.1016/s0028-3908(00)00058-7. [DOI] [PubMed] [Google Scholar]
- Egan TM, Khakh BS. Contribution of calcium ions to P2X channel responses. J Neurosci. 2004;24:3413–3420. doi: 10.1523/JNEUROSCI.5429-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennion SJ, Evans RJ. Agonist-stimulated internalisation of the ligand-gated ion channel P2X1 in rat vas deferens. FEBS Lett. 2001;489:154–158. doi: 10.1016/s0014-5793(01)02102-0. [DOI] [PubMed] [Google Scholar]
- Evans RJ, Kennedy C. Characterization of P2-purinoceptors in the smooth muscle of the rat tail artery: a comparison between contractile and electrophysiological responses. Br J Pharmacol. 1994;113:853–860. doi: 10.1111/j.1476-5381.1994.tb17071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans RJ, Lewis C, Buell G, Valera S, North RA, Surprenant A. Pharmacological characterization of heterologously expressed ATP-gated cation channels (P2x purinoceptors) Mol Pharmacol. 1995;48:178–183. [PubMed] [Google Scholar]
- Evans RJ, Lewis C, Virginio C, Lundstrom K, Buell G, Surprenant A, et al. Ionic permeability of, and divalent cation effects on, two ATP-gated cation channels (P2X receptors) expressed in mammalian cells. J Physiol (Lond) 1996;497:413–422. doi: 10.1113/jphysiol.1996.sp021777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng C, Mery AG, Beller EM, Favot C, Boyce JA. Adenine nucleotides inhibit cytokine generation by human mast cells through a Gs-coupled receptor. J Immunol. 2004;173:7539–7547. doi: 10.4049/jimmunol.173.12.7539. [DOI] [PubMed] [Google Scholar]
- Fernando SL, Saunders BM, Sluyter R, Skarratt KK, Goldberg H, Marks GB, et al. A polymorphism in the P2X7 gene increases susceptibility to extrapulmonary tuberculosis. Am J Respir Crit Care Med. 2006;175:360–366. doi: 10.1164/rccm.200607-970OC. [DOI] [PubMed] [Google Scholar]
- Ferrari D, Chiozzi P, Falzoni S, Dal SM, Melchiorri L, Baricordi OR, et al. Extracellular ATP triggers IL-1 beta release by activating the purinergic P2Z receptor of human macrophages. J Immunol. 1997;159:1451–1458. [PubMed] [Google Scholar]
- Fountain SJ, North RA. A C-terminal lysine that controls human P2X4 receptor desensitization. J Biol Chem. 2006;281:15044–15049. doi: 10.1074/jbc.M600442200. [DOI] [PubMed] [Google Scholar]
- Fung CY, Cendana C, Farndale RW, Mahaut-Smith MP. Primary and secondary agonists can use P2X(1) receptors as a major pathway to increase intracellular Ca(2+) in the human platelet. J Thromb Haemost. 2007;5:910–917. doi: 10.1111/j.1538-7836.2007.02525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gargett CE, Wiley JS. ) The isoquinoline derivative KN-62 a potent antagonist of the P2Z-receptor of human lymphocytes. Br J Pharmacol. 1997;120:1483–1490. doi: 10.1038/sj.bjp.0701081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilfillan AM, Tkaczyk C. Integrated signalling pathways for mast-cell activation. Nat Rev Immunol. 2006;6:218–230. doi: 10.1038/nri1782. [DOI] [PubMed] [Google Scholar]
- Guo C, Masin M, Qureshi OS, Murrell-Lagnado RD. Evidence for functional P2X4/P2X7 heteromeric receptors. Mol Pharmacol. 2007;72:1447–1456. doi: 10.1124/mol.107.035980. [DOI] [PubMed] [Google Scholar]
- Hechler B, Lenain N, Marchese P, Vial C, Heim V, Freund M, et al. A role of the fast ATP-gated P2X1 cation channel in thrombosis of small arteries in vivo. J Exp Med. 2003;198:661–667. doi: 10.1084/jem.20030144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys BD, Dubyak GR. Modulation of P2X7 nucleotide receptor expression by pro- and anti-inflammatory stimuli in THP-1 monocytes. J Leukoc Biol. 1998;64:265–273. doi: 10.1002/jlb.64.2.265. [DOI] [PubMed] [Google Scholar]
- Humphreys BD, Virginio C, Surprenant A, Rice J, Dubyak GR. Isoguinolines as antagonists of the P2X7 nucleotide receptor: high selectivity for the human versus rat receptor homologues. Mol Pharmacol. 1998;54:22–32. doi: 10.1124/mol.54.1.22. [DOI] [PubMed] [Google Scholar]
- Khakh BS, Proctor WR, Dunwiddie TV, Labarca C, Lester HA. Allosteric control of gating and kinetics at P2X(4) receptor channels. J Neurosci. 1999;19:7289–7299. doi: 10.1523/JNEUROSCI.19-17-07289.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirshenbaum AS, Akin C, Wu Y, Rottem M, Goff JP, Beaven MA, et al. Characterization of novel stem cell factor responsive human mast cell lines LAD 1 and 2 established from a patient with mast cell sarcoma/leukemia; activation following aggregation of Fc[var epsilon]RI or Fc[gamma]RI. Leukemia Res. 2003;27:677–682. doi: 10.1016/s0145-2126(02)00343-0. [DOI] [PubMed] [Google Scholar]
- Kuehn HS, Gilfillan AM. G protein-coupled receptors and the modification of FcepsilonRI-mediated mast cell activation. Immunol Lett. 2007;113:59–69. doi: 10.1016/j.imlet.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Kugelgen I. Pharmacological profiles of cloned mammalian P2Y-receptor subtypes. Pharmacol Ther. 2006;110:415–432. doi: 10.1016/j.pharmthera.2005.08.014. [DOI] [PubMed] [Google Scholar]
- La SA, Ferrari D, Di VF, Idzko M, Norgauer J, Girolomoni G. Alerting and tuning the immune response by extracellular nucleotides. J Leukoc Biol. 2003;73:339–343. doi: 10.1189/jlb.0802418. [DOI] [PubMed] [Google Scholar]
- Lalo U, Pankratov Y, Wichert SP, Rossner MJ, North RA, Kirchhoff F, et al. P2X1 and P2X5 subunits form the functional P2X receptor in mouse cortical astrocytes. J Neurosci. 2008;28:5473–5480. doi: 10.1523/JNEUROSCI.1149-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemoli RM, Ferrari D, Fogli M, Rossi L, Pizzirani C, Forchap S, et al. Extracellular nucleotides are potent stimulators of human hematopoietic stem cells in vitro and in vivo. Blood. 2004;104:1662–1670. doi: 10.1182/blood-2004-03-0834. [DOI] [PubMed] [Google Scholar]
- Lewis CJ, Evans RJ. Lack of run-down of smooth muscle P2X receptor currents recorded with the amphotericin permeabilized patch technique, physiological and pharmacological characterization of the properties of mesenteric artery P2X receptor ion channels. Br J Pharmacol. 2000;131:1659–1666. doi: 10.1038/sj.bjp.0703744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma W, Korngreen A, Weil S, Cohen EB, Priel A, Kuzin L, et al. Pore properties and pharmacological features of the P2X receptor channel in airway ciliated cells. J Physiol. 2006;571:503–517. doi: 10.1113/jphysiol.2005.103408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCloskey MA, Fan Y, Luther S. Chemotaxis of rat mast cells toward adenine nucleotides. J Immunol. 1999;163:970–977. [PubMed] [Google Scholar]
- Metz M, Maurer M. Mast cells – key effector cells in immune responses. Trends Immunol. 2007;28:234–241. doi: 10.1016/j.it.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Nicke A, Kerschensteiner D, Soto F. Biochemical and functional evidence for heteromeric assembly of P2X1 and P2X4 subunits. J Neurochem. 2005;92:925–933. doi: 10.1111/j.1471-4159.2004.02939.x. [DOI] [PubMed] [Google Scholar]
- North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- Osipchuk Y, Cahalan M. Cell-to-cell spread of calcium signals mediated by ATP receptors in mast cells. Nature. 1992;359:241–244. doi: 10.1038/359241a0. [DOI] [PubMed] [Google Scholar]
- Oskeritzian CA, Zhao W, Min HK, Xia HZ, Pozez A, Kiev J, et al. Surface CD88 functionally distinguishes the MCTC from the MCT type of human lung mast cell. J Allergy Clin Immunol. 2005;115:1162–1168. doi: 10.1016/j.jaci.2005.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priel A, Silberberg SD. Mechanism of ivermectin facilitation of human P2X4 receptor channels. J Gen Physiol. 2004;123:281–293. doi: 10.1085/jgp.200308986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raouf R, Chabot-Dore AJ, Ase AR, Blais D, Seguela P. Differential regulation of microglial P2X4 and P2X7 ATP receptors following LPS-induced activation. Neuropharmacology. 2007;53:496–504. doi: 10.1016/j.neuropharm.2007.06.010. [DOI] [PubMed] [Google Scholar]
- Rassendren F, Buell GN, Virginio C, Collo G, North RA, Surprenant A. The permeabilizing ATP receptor, P2X7. Cloning and expression of a human cDNA. J Biol Chem. 1997;272:5482–5486. doi: 10.1074/jbc.272.9.5482. [DOI] [PubMed] [Google Scholar]
- Rettinger J, Schmalzing G. Activation and desensitization of the recombinant P2X1 receptor at nanomolar ATP concentrations. J Gen Physiol. 2003;121:451–461. doi: 10.1085/jgp.200208730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rettinger J, Braun K, Hochmann H, Kassack MU, Ullmann H, Nickel P, et al. Profiling at recombinant homomeric and heteromeric rat P2X receptors identifies the suramin analogue NF449 as a highly potent P2X1 receptor antagonist. Neuropharmacology. 2005;48:461–468. doi: 10.1016/j.neuropharm.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Rivera J, Gilfillan AM. Molecular regulation of mast cell activation. J Allergy Clin Immunol. 2006;117:1214–1225. doi: 10.1016/j.jaci.2006.04.015. [DOI] [PubMed] [Google Scholar]
- Sanmugalingam D, Wardlaw AJ, Bradding P. Adhesion of human lung mast cells to bronchial epithelium: evidence for a novel carbohydrate-mediated mechanism. J Leuk Biol. 2000;68:38–46. [PubMed] [Google Scholar]
- Schulman ES, Glaum MC, Post T, Wang Y, Raible DG, Mohanty J, et al. ATP modulates anti-IgE-induced release of histamine from human lung mast cells. Am J Respir Cell Mol Biol. 1999;20:530–537. doi: 10.1165/ajrcmb.20.3.3387. [DOI] [PubMed] [Google Scholar]
- Sim JA, Park CK, Oh SB, Evans RJ, North RA. P2X1 and P2X4 receptor currents in mouse macrophages. Br J Pharmacol. 2007;152:1283–1290. doi: 10.1038/sj.bjp.0707504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes L, Jiang LH, Alcaraz L, Bent J, Bowers K, Fagura M, et al. Characterization of a selective and potent antagonist of human P2X(7) receptors, AZ11645373. Br J Pharmacol. 2006;149:880–887. doi: 10.1038/sj.bjp.0706933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatham PE, Cusack NJ, Gomperts BD. Characterisation of the ATP4-receptor that mediates permeabilisation of rat mast cells. Eur J Pharmacol. 1988;147:13–21. doi: 10.1016/0014-2999(88)90628-0. [DOI] [PubMed] [Google Scholar]
- Tatham PER, Lindau M. ATP-induced pore formation in the plasma membrane of rat peritoneal mast cells. J Gen Physiol. 1990;95:459–476. doi: 10.1085/jgp.95.3.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolhurst G, Vial C, Leon C, Gachet C, Evans RJ, Mahaut-Smith MP. Interplay between P2Y(1), P2Y(12), and P2X(1) receptors in the activation of megakaryocyte cation influx currents by ADP: evidence that the primary megakaryocyte represents a fully functional model of platelet P2 receptor signaling. Blood. 2005;106:1644–1651. doi: 10.1182/blood-2005-02-0725. [DOI] [PubMed] [Google Scholar]
- Toulme E, Soto F, Garret M, Boue-Grabot E. Functional properties of internalization-deficient P2X4 receptors reveal a novel mechanism of ligand-gated channel facilitation by ivermectin. Mol Pharmacol. 2006;69:576–587. doi: 10.1124/mol.105.018812. [DOI] [PubMed] [Google Scholar]
- Vial C, Rolf MG, Mahaut-Smith MP, Evans RJ. A study of P2X1 receptor function in murine megakaryocytes and human platelets reveals synergy with P2Y receptors. Br J Pharmacol. 2002;135:363–372. doi: 10.1038/sj.bjp.0704486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Jacobsen SE, Bengtsson A, Erlinge D. P2 receptor mRNA expression profiles in human lymphocytes, monocytes and CD34+ stem and progenitor cells. BMC Immunol. 2004;5:16. doi: 10.1186/1471-2172-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson HL, Varcoe RW, Stokes L, Holland KL, Francis SE, Dower SK, et al. P2X receptor characterization and IL-1/IL-1Ra release from human endothelial cells. Br J Pharmacol. 2007;151:115–127. doi: 10.1038/sj.bjp.0707213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wykes RC, Lee M, Duffy SM, Yang W, Seward EP, Bradding P. Functional transient receptor potential melastatin 7 channels are critical for human mast cell survival. J Immunol. 2007;179:4045–4052. doi: 10.4049/jimmunol.179.6.4045. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


