Abstract
Background and purpose:
Bovine glycomacropeptide (BGMP) is a natural milk peptide that is produced naturally in the gastrointestinal tract during digestion. Glycomacropepide has intestinal anti-inflammatory activity, but the mechanism of action is unknown. Here we have characterized the effects of BGMP on monocytes.
Experimental approach:
We have used human THP-1 cells as an in vitro monocyte model. The effect of BGMP on the secretion of tumour necrosis factor (TNF), interleukin (IL)-1β and IL-8 was assessed, as well as the involvement of the NF-κB and MAP kinase signalling pathways. The stimulatory effect of BGMP was also tested in human peripheral blood monocytes.
Key results:
BGMP up-regulated the secretion of TNF, IL-1β and IL-8 in a concentration-dependent fashion. The biological activity was exerted by the intact peptide, because cytokine secretion was not affected by protease inhibitors. The secretion of IL-8 and specially TNF and IL-1β was blocked by PD98059, SP600125, SB203580 and Bay11-7082, suggesting the involvement of the MAP kinases p38, c-Jun N-terminal kinase and ERK and particularly the NF-κB pathway, although IL-8 secretion was independent of p38. BGMP was shown to elicit the phosphorylation of IκB-α and the nuclear translocation of the NF-κB subunits p50 and p65. The effect of BGMP on cytokine secretion was validated in human primary blood monocytes.
Conclusions and implications:
BGMP stimulates human monocytes, operating via MAP kinase and NF-κB pathways. BGMP may exert an indirect intestinal anti-inflammatory effect by potentiating host defences against invading microorganisms.
Keywords: bovine glycomacropeptide, THP-1 cells, NF-κB
Introduction
The concept of milk as a biologically active fluid was established a long time ago (Newby et al., 1982), and in the last decades the therapeutic value of milk proteins and peptides has been widely described (Brody, 2000; Cross and Gill, 2000; Clare et al., 2003; Florisa et al., 2003; Zimecki and Kruzel, 2007). Bovine glycomacropeptide (BGMP) or casein macropeptide is one of the biologically active components of milk (Brody, 2000; Zimecki and Kruzel, 2007). BGMP is a 64-amino-acid peptide, corresponding to amino acids 106–169 of κ-casein, which contains N-acetylneuraminic (sialic) acid and is physiologically obtained in the stomach of neonates (and adults) by the chymosin digestion of native protein (Brody, 2000; Zimecki and Kruzel, 2007). The industrial source of BGMP is milk whey, produced during the cheese-making process, in which BGMP is the result of the action of the rennet chymosin (Brody, 2000).
BGMP is currently added to infant formulas and, due to its low content of aromatic amino acids including phenylalanine, it has been proposed to be useful in the elaboration of products for individuals with phenylketonuria (Lim et al., 2007). In addition, BGMP is included in toothpaste because of its anti-cariogenic properties; it has antibacterial activity, preventing dental caries and tooth demineralization, while promoting enamel mineralization (Aimutis, 2004).
A wide array of additional BGMP activities have been described, including its ability to bind cholera and Escherichia coli enterotoxins, the inhibition of bacterial and viral adhesions, the promotion of bifidobacterial growth and the modulation of immune system responses (Brody, 2000; Nakajima et al., 2005; Bruck et al., 2006). In this regard, we have established the anti-inflammatory effect of BGMP in a rat model of colitis (Daddaoua et al., 2005) and recently also in experimental ileitis (Requena et al., 2008). Interestingly, it has been recently reported that BGMP does not induce T cell -mediated immune responses in vivo, unlike the native protein κ-casein, even when administered as a polymer (Mikkelsen et al., 2006). There are some studies available on the effect of BGMP on cells of the immune system in vitro, most of which have focused on lymphocytes. Thus, BGMP has been claimed to inhibit lipopolysaccharide (LPS)-induced splenocyte proliferation (Otani and Monnai, 1993; Mikkelsen et al., 2005), to suppress interleukin (IL)-2 receptor expression in mouse CD4+ T cells (Otani et al., 1996) and to block serum IgG antibody production by mouse lymphocytes (Monnai et al., 1998).
There are only two studies focused on the effect of BGMP on macrophages showing that BGMP augments the secretion of IL-1receptor antagonist (IL-1ra), without affecting IL-1β, in a mouse monocytic cell line (Monnai and Otani, 1997), and that it enhances the proliferation and phagocytic activity of human macrophage-like cells (Li and Mine, 2004). Of note, the intestinal anti-inflammatory effect of BGMP is associated with a marked decrease in intestinal tumour necrosis factor (TNF) and IL-1β, cytokines mainly derived from monocytes/macrophages, suggesting that this cell type may be affected directly or indirectly (i.e. via hydrolytic peptide fragments) by BGMP (Daddaoua et al., 2005; Requena et al., 2008). Thus, we designed the present study to characterize the immunoregulatory effect of BGMP in the innate immune response mediated by monocyte/macrophages and to determine the intracellular signalling pathways that could be regulated by this peptide. Specifically, we focused on the pro-inflammatory cytokines expressed by monocytes/macrophages, which play an important role in intestinal inflammation, expecting a down-regulation by BGMP. We used the human monocyte THP-1 cell line as a model and validated the main results in human primary blood monocytes. This study demonstrates that BGMP did not inhibit and actually enhanced the expression of TNF, IL-1β and IL-8 in monocytes and that its mechanism of action is related to the stimulation of the MAP kinase (MAPK) and the IκB/NF-κB signal transduction pathways.
Methods
Cell culture
The human monocytic cell line THP1 (ECACC 88081201) was cultured in RPMI-1640 medium supplemented with 10% (v·v−1) heat-inactivated foetal bovine serum (FBS), 2 mmol·L−1 glutamine, 100 U·L−1 penicillin, 0.1 mg·mL−1 streptomycin, 2.5 µg·mL−1 amphotericin B and 0.05 mmol·L−1 mercaptoethanol. Cells were incubated under humidified 5% CO2 atmosphere at 37°C. The cells were cultured at a concentration of 106 cells·mL−1.
Monocytes were isolated from human blood obtained from volunteers. All the participants provided an informed consent and the protocol was approved by the Research Ethics Committee of the University of Granada. In brief, heparinized peripheral blood was diluted 1:2 with sterile saline and separated using Ficoll (GE Healthcare, Barcelona, Spain). The mononuclear cell preparation was washed once in sterile Hank's balanced salt solution and placed on a culture dish for 1 h. Adherent cells were retrieved and plated at a density of 106 cells·mL−1 for assay. Human primary monocytes were cultured in DMEM medium supplemented with as above but without mercaptoethanol.
Effect on cytokine secretion
Cytokine secretions were determined in THP-1 cells cultured in the presence of BGMP, bovine serum albumin or casoplatelin for 24 h. After 24 h cells were collected and cytokine concentration was measured by ELISA (Biosource Europe, Nivelles, Belgium and BD Biosciences, Erembodegem, Belgium), following the protocols recommended by the manufacturer. In some experiments the serine protease inhibitor Pefabloc® SC [4-(2-aminoethyl)-benzenesulphonyl fluoride, hydrochloride, 0.1 mmol·L−1, Roche Applied Science, Mannheim, Germany] or a protease inhibitor cocktail (1:200 v·v−1, Sigma) – containing 4-(2-aminoethyl)-benzenesulphonyl fluoride, aprotinin, leupeptin, bestatin, pepstatin A and E-64 – was added to the cell medium together with BGMP (1 mg·mL−1), the cells were incubated for 24 h and cytokine concentration was measured as described above.
Cell proliferation assay
THP-1 cells were incubated for 24 h with different concentrations of BGMP and then cells were pulsed for 6 h with [3H] thymidine (0.6 µCi·mL−1; GE Healthcare, Spain). The cells were subsequently harvested, washed three times with trichloroacetic acid (10% v·v−1), resuspended in lysis buffer (1% w·v−1 SDS, 0.3 N NaOH) for 30 min at room temperature and collected into plastic vials. Then 4 mL of scintillation liquid (Beckman Coulter, Madrid, Spain) per vial was added and the amount of incorporated [3H] thymidine was determined on a Tri-Carb liquid scintillation analyser (Packard Instrument, Meriden, CT).
Lactate dehydrogenase assay
Cellular toxicity was measured as the release of lactate dehydrogenase. Cells were cultured with different concentrations of BGMP for 24 h and lactate dehydrogenase activity in supernatants was measured spectrophotometrically using sodium pyruvate (25 mmol·L−1) as substrate in 50 mmol·L−1 sodium phosphate buffer (pH = 7.5) (Halprin and Ohkawara, 1966).
NF-κB and MAPK inhibitors assay
In order to explore signalling pathways, the kinase inhibitors [PD98059 for the mitogen-activated protein kinase MAPK ERK1/2, SB203580 for p38 MAPK, SP600125 for c-Jun N-terminal kinase (JNK) and Bay11-7082 for NF-κB] were added to the cell culture medium (10 µmol·L−1 in all cases) 1 h before the addition of the BGMP (1 mg·mL−1). Then the cells were incubated for 24 h and the supernatants were used to determine cytokine concentrations as described above.
Western blot
For the detection of phosphorylated IκB-α, cells were homogenized in lysis buffer (0.1% w·v−1 SDS, 0.1% w·v−1 sodium deoxycholate, 1% v·v−1 Triton X-100 in PBS) with protease inhibitor cocktail 1:100 (v·v−1). Then homogenates were sonicated and centrifuged at 7000× g for 5 min at 4°C. For the detection of nuclear NF-κB p50 and p65 subunits, nuclear extracts were obtained using the Nuclear Extract kit (Active Motif Europe, Rixensart, Belgium) following the kit instructions. Protein concentrations in cell and nuclear extracts were determined by the bicinchoninic acid assay (Smith et al., 1985).
Samples were boiled for 5 min in Laemmli buffer, separated by SDS-PAGE, electroblotted to PVDF membranes (Millipore, Madrid, Spain), and probed with the corresponding antibodies. The bands were detected by enhanced chemiluminescence (PerkinElmer, Waltham, MA) and quantified with NIH software (Scion Image). The composition of the Laemmli buffer (5×) was: 312 nmol·L−1 SDS, 50% v·v−1 glycerol, 1% v·v−1 2-mercaptoethanol, 22.5 mmol·L−1 EDTA trisodium salt, 220 mmol·L−1 Tris and traces of bromphenol blue (pH = 6.8).
Statistical analysis
All results are expressed as mean ± SEM. Differences among means were tested for statistical significance by one-way anova and a posteriori least significance tests. All analyses were carried out with the SigmaStat 2.03 program (Jandel Corporation, San Rafael, CA). Concentration–response curves were fitted to a logistic curve when possible with Origin 7.0 (OriginLab Corporation, Northampton, MA). Differences were considered significant at P < 0.05.
Materials
Except where indicated, all reagents were obtained from Sigma (Barcelona, Spain). The NF-κB p65 and p60 antibodies were purchased from Santa Cruz Biotechnology, Inc. (Heidelberg, Germany); the phospho-IκB-a (Ser32) antibody was purchased from Cell Signaling Technology (Boston, MA, USA); the JLA20 antibody against actin developed by Dr Lin (Lin, 1981) was obtained from the Development Studies Hybridoma Bank developed under the auspices of the National Institute of Child Health and Human Development and maintained by the University of Iowa, Department of Biological Sciences (Iowa City, IA). BGMP (BioPURE-GMP™) was the kind gift of Davisco Foods International (Eden Prairie, MN). Product certificate of analysis indicated that BGMP content was 93% (97% of dry weight) while fat and lactose contents were 0.5% and less that 1% respectively. The BGMP product also contained small amounts of β-lactoglobulin and α-lactalbumin, which were <1% based on Western blot analysis (not shown), and 4.0% minerals. Casoplatelin was synthesized with a purity >95% by Innovagen (Lund, Sweden).
Results
Effect of BGMP on cytokine secretion in THP-1 cells
To test the hypothesis that BGMP modifies the secretion of cytokines in monocytes/macrophages, THP-1 cells were cultured with different concentrations of BGMP for 24 h and TNF, IL-1β and IL-8 concentrations were determined in the cell culture medium. The addition of BGMP to THP-1 cells increased the concentration of TNF, IL-1β and IL-8 in the cell culture medium in a concentration-dependent fashion (Figure 1). This effect was obtained consistently at concentrations of 1 gl−1 or higher. The resulting curves appear sigmoidal, but they could not be completed because of the solubility limits of BGMP and thus a EC50 could not be calculated. The effect of bovine serum albumin was also studied to determine whether the action of the BGMP was specific or simply the consequence of the addition of protein (Figure 2). Bovine serum albumin had no effect on cytokine secretion at 1 mg·mL−1, although a certain tendency for increase was noted. However, these experiments were all carried out with complete culture medium, which contains FBS and therefore bovine serum albumin. Thus, we repeated the experiments in FBS-free medium, finding in this case a robust induction of TNF, IL-1β and IL-8 that was comparable to that evoked by BGMP at the same concentration.
Figure 1.
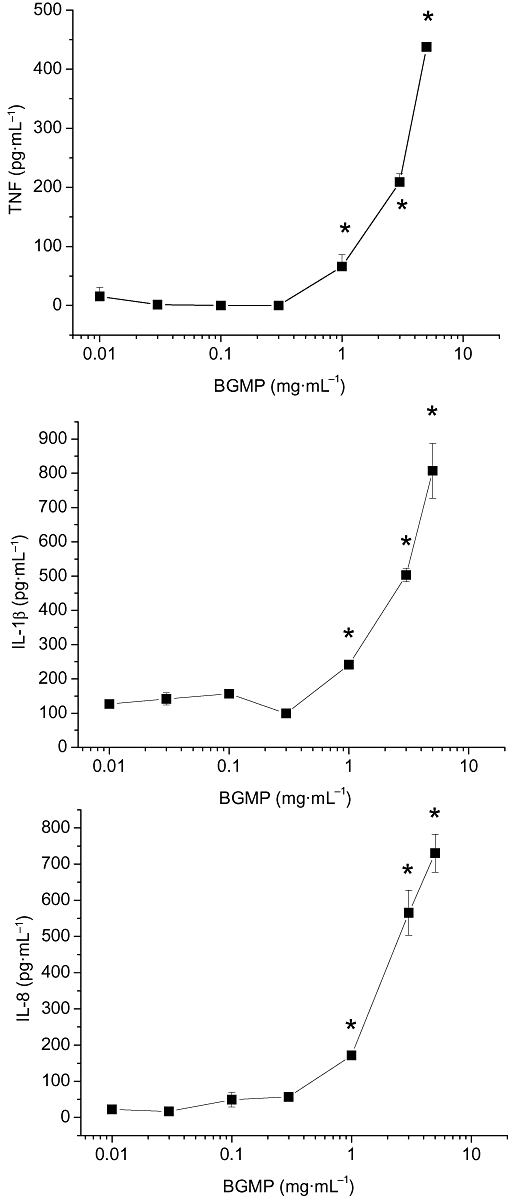
Concentration–response curves for the production of tumour necrosis factor (TNF), interleukin (IL)-1β and IL-8 by THP-1 cells in the presence of bovine glycomacropeptide (BGMP). After a 24 h incubation in the presence of the products, secretion of cytokines was measured in foetal bovine serum-containing culture medium by ELISA. Results are expressed as mean ± SEM of three different experiments (n= 3 in each experiment). *P < 0.05 versus control.
Figure 2.
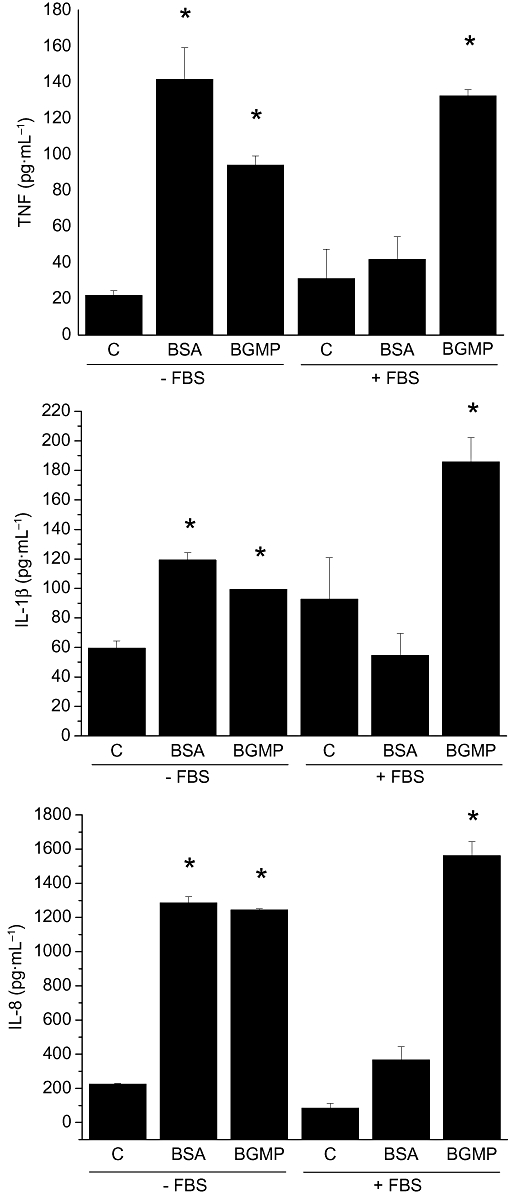
Effect of bovine serum albumin (BSA) and bovine glycomacropeptide (BGMP) on the production of tumour necrosis factor (TNF), interleukin (IL)-1β and IL-8 by THP-1 cells. After a 24 h incubation with either peptide (1 mg·mL−1) the secretion of cytokines was measured in foetal bovine serum (FBS)-containing or FBS-free culture medium by ELISA. Results are expressed as mean ± SEM of three different experiments (n= 3 in each experiment). *P < 0.05 versus control (C).
Effect of BGMP on THP-1 cell viability and proliferation
As a routine procedure we assessed the possible effect of BGMP on cell viability and proliferation. The level of lactate dehydrogenase activity in the culture medium was similar in the presence and absence of the peptide, indicating that BGMP was not toxic to the cells (data not shown). Cell proliferation (studied by [3H] thymidine incorporation) was slightly (∼22%), but significantly lowered in the presence of BGMP at 1 mg·mL−1, but not at lower concentrations (data not shown).
Effect of protein hydrolysis on BGMP activity
As several peptides derived from milk proteins by digestive proteolysis have been shown to exert biological actions, we next studied whether intact BGMP or hydrolytic peptide fragments account for the observed activity. We used a serine protease inhibitor and a cocktail of protease inhibitors to block the possible hydrolysis of BGMP by cell proteases. As shown in Figure 3, blocking protease activity in THP-1 cells did not alter BGMP-stimulated cytokine production. In addition, as casoplatelin is a bioactive peptide (Bal dit Sollier et al., 1996) whose sequence comprises the first 11 amino acids of BGMP (106–116 of the κ-casein), we tested the hypothesis that casoplatelin could be responsible for the effects of BGMP. The addition of casoplatelin to the cell culture medium in a concentration equivalent, after proteolysis, to 1 mg·mL−1 of BGMP produced no effect on cytokine production (not shown).
Figure 3.
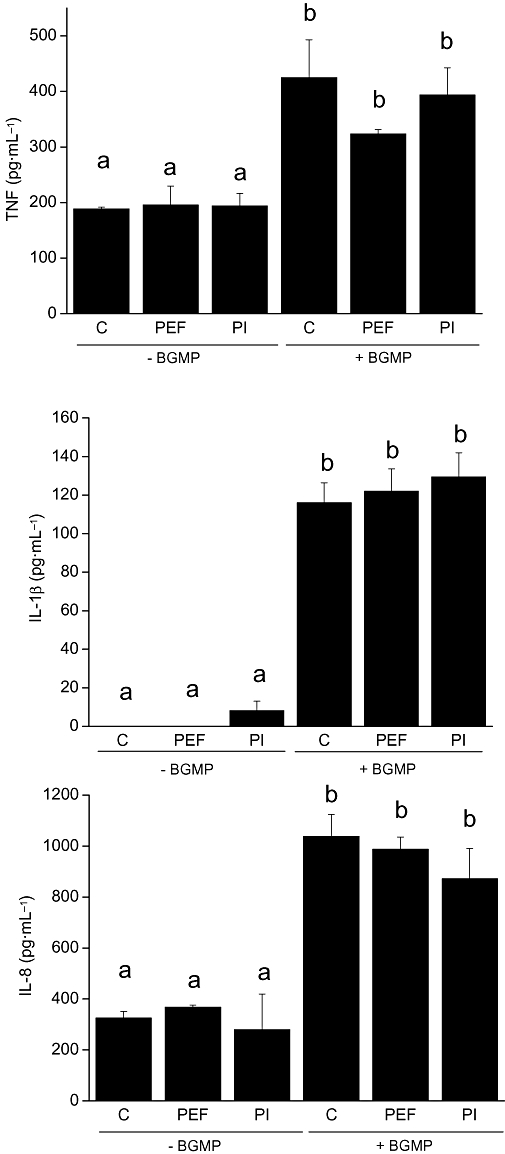
Effect of protease inhibitors on the production of tumour necrosis factor (TNF), interleukin (IL)-1β and IL-8 by THP-1 cells stimulated with bovine glycomacropeptide (BGMP). After 1 h of incubation with the serine protease inhibitor Pefabloc® (PEF, 0.1 mmol·L−1) or a protease inhibitor cocktail (PI, 1:200 v·v−1) the cells were exposed to BGMP (1 mg·mL−1). After a 24 h incubation, the secretion of cytokines was measured in the culture medium by ELISA. Results are expressed as mean ± SEM of three different experiments (n= 3 in each experiment). Means marked with the same letter were not different from each other, but were different from means marked with a different letter; P < 0.05. C, control group.
Signal transduction pathways
Both the MAPK and the NF-κB signalling pathways have been shown to be implicated in the production of TNF, IL-1β and IL-8 in monocytes/macrophages (Beinke and Ley, 2004). The stimulation of the MAPK signal transduction pathways by BGMP was studied using inhibitors for ERK1/2 (PD98059), p38 MAPK (SB203580) and JNK (SP600125) that were added to the culture medium of the cells for 1 h. The BGMP was then added, cells were incubated for 24 h and cytokines were measured in the culture medium by ELISA. The addition of either PD98059 or SP600125 to the cell culture medium prevented the increase in the expression of TNF and IL-1β almost completely, while a substantial but significantly lower inhibitory effect was achieved with SB203580 (Figure 4). Inhibition of IL-8 secretion was less effective, and it only reached statistical significance in the case of PD98059 or SP600125 (Figure 4).
Figure 4.
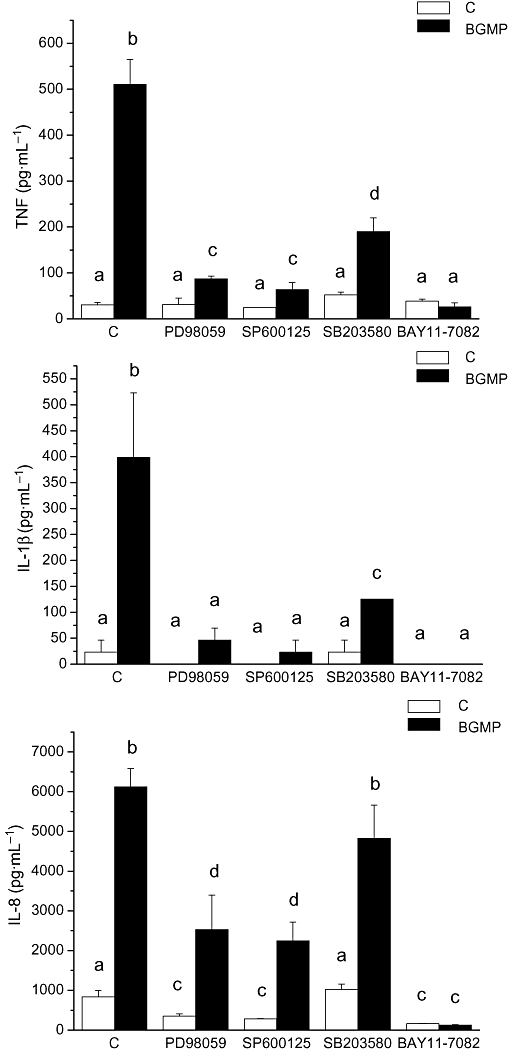
Effect of MAP kinase and NF-κB inhibitors on tumour necrosis factor (TNF), interleukin (IL)-1β and IL-8 secretion by THP-1 cells stimulated with bovine glycomacropeptide (BGMP). Cytokine concentrations were determined by ELISA in the supernatants of cells pre-incubated for 1 h with the signal transduction inhibitors [MAP kinase inhibitors PD98059 (ERK1/2 inhibitor), SB203580 (ERK1/2 inhibitor), SP600125 (JNK inhibitor) or the NF-κB inhibitor Bay 11-7082] followed by a 24 h incubation with BGMP. Data are mean ± SEM from at least three independent experiments. Means marked with the same letter were not different from each other, but were different from means marked with a different letter; P < 0.05. C, control group.
The role of the NF-κB signalling pathway in the stimulation of THP-1 by BGMP was also studied. One of the ways to activate NF-κB by extracellular stimuli involves the rapid degradation of IκB-α following phosphorylation of Ser32 of IκB-α by IκB kinase (IKK, the so-called canonical pathway). Bay11-7082 inhibits this phosphorylation step and therefore blocks the NF-κB canonical activation pathway. Complete inhibition of the secretion of all the cytokines assayed was observed when cells were cultured in the presence of Bay11-7082, indicating that this pathway plays a pivotal role in the response to BGMP (Figure 5). To further confirm this point, we studied the effect of BGMP on the phosphorylation of IκB-α, adding BGMP to cells for 5, 10 or 20 min and 1, 6 or 24 h and carrying out a Western blot to detect Ser32-phosphorylated IκB-α. As expected, an increase in specific Ser32-phosphorylated IκB-α immunoreactivity was observed, with a maximum expression 1 h after BGMP addition, confirming that BGMP operates chiefly via the canonical pathway (Figure 5A). As the phosphorylation of IκB-α is necessary but not sufficient for the activation of the NF-κB pathway, nuclear levels of NF-κB p65 and p50 were also determined by Western blot in nuclear extracts from THP-1 cells cultured with BGMP for 1 and 3 h. Both p65 and p50 were present in the cell nucleus 1 h after the addition of BGMP. As a control, this effect was fully prevented by Bay11-7082 (Figure 5B).
Figure 5.
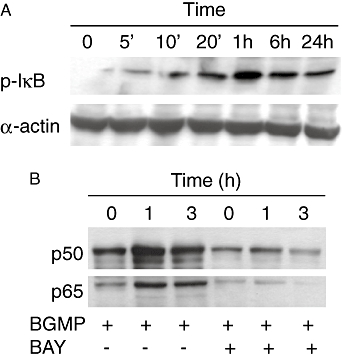
Effect of bovine glycomacropeptide (BGMP) on the phosphorylation of IκB-α (A) and on the translocation of p50 and p65 to the nucleus of THP-1 cells (B). (A) Cells were cultured with BGMP for different periods of time and Western blots were carried out with cell extracts. α-Actin was used as a loading control. (B) Cells were cultured for different periods with BGMP or BGMP plus the NF-κB inhibitor Bay 11-7082 (BAY). Western blot experiments were carried out with nuclear extracts.
BGMP activates cytokine secretion in primary monocytes
Finally, to validate the data obtained and to make sure that the effect described was not specific for this cell line, human monocytes were obtained from blood and cultured with BGMP. As shown in Figure 6, our results indicate that BGMP also induced the production of TNF, IL-1β and IL-8 in human blood monocytes while casoplatelin had no effect on the production of these cytokines (not shown). Of note, the potency of BGMP for IL-8 induction was higher than that for TNF or IL-1β in primary monocytes but not in THP-1 cells.
Figure 6.
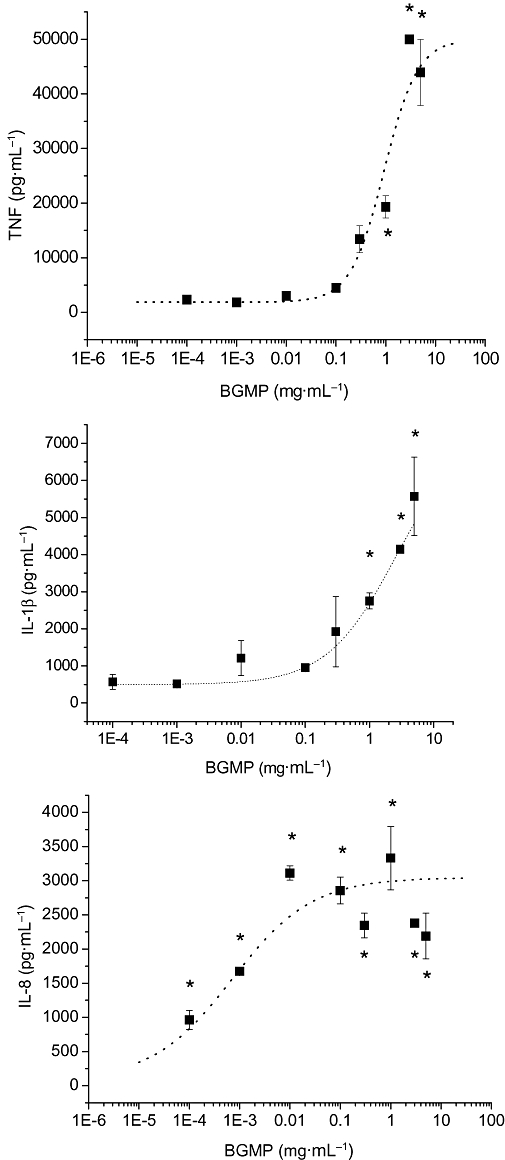
Concentration–response curves for the production of tumour necrosis factor (TNF), interleukin (IL)-1β and IL-8 by primary human monocytes in the presence of BGMP. After a 24 h incubation in the presence of the products, secretion of cytokines was measured in the culture medium by ELISA. Logistic sigmoidal curves could be fitted in the cases of TNF and IL-8 (EC50 0.92 ± 0.09 mg·mL−1 and 0.72 ± 0.40 µg·mL−1 respectively). Results are expressed as mean ± SEM of two different experiments (n= 3 in each experiment). *P < 0.05 versus control.
Discussion
The present study was undertaken with the aim to assess the biological activity of BGMP on monocytes, on the basis of its immunomodulatory effects, including intestinal anti-inflammatory activity. BGMP has been previously shown to increase IL-1ra but not IL-1β secretion in the P388D1 mouse monocyte cell line (Monnai and Otani, 1997) at a maximal concentration of 0.1 mg·mL−1, which in our hands had no effect in THP-1 cells and only evoked IL-8 secretion in primary monocytes. IL-1ra is a cytokine that behaves as an antagonist of IL-1 receptors, thereby limiting IL-1α and IL-1β effects. From a physiological point of view, IL-1ra production is a normal counterpart of IL-1α/β induction and its role is to limit the pro-inflammatory response of this cytokine. Similar mechanisms operate for TNF. To the best of our knowledge, IL-1ra is not normally induced on its own, that is, without parallel IL-1α/β increase. Thus, the predominant effect of BGMP on monocytes seems to be an activating one, as supported by the induction of pro-inflammatory cytokines and inhibited proliferation in BGMP-treated THP-1 cells (activated monocytes/macrophages are characterized by slowed rather than hastened proliferation). This is also consistent with the previous study by Li and Mine (2004), showing increased phagocytic activity of U937 cells after BGMP treatment. However, we cannot exclude a possible disproportionate increase in IL-1ra levels by BGMP.
The concentration–response curves obtained in THP-1 cells indicate that the potency of BGMP for the induction of the three cytokines was comparable, with reliable increase of production being observed starting at 1 mg·mL−1 (lower amounts were active, although slightly, in some experiments). Because of the limits in solubility, the curves could not be completed; that is, the secretory response was not saturated even at 5 mg·mL−1 of BGMP. Naturally, this circumstance prevents us from calculating EC50 values, at least in THP-1 cells. Interestingly, while these effects were largely reproduced in primary human monocytes, used for validation purposes here, the potency for IL-8 induction was clearly higher than that for TNF or IL-1β in these cells. This discrepancy may be explained by the differences of the two cell populations (tumour vs. normal cells), but it should be noted that the magnitude of cytokine secretion was increased dramatically in primary monocytes compared with THP-1 cells despite the fact that identical cell culture conditions were used. This robust response of primary cells may explain why maximal secretion was achieved only in these cells (with the exception of IL-1β). In addition, these data may be interpreted to indicate a predominant effect of BGMP on the signalling pathways leading to IL-8 versus TNF/IL-1β production, probably NF-κB versus MAPK activation. The biological implications of this preference are unclear, but the effects of IL-8 are generally considered more restricted and less pronounced than those of TNF/IL-1β. Thus, this may represent a ‘scalated’ or gradual biological response of monocytes to BGMP.
Next we addressed the issue of whether BGMP exerts these effects itself or by way of hydrolytic peptide fragments. Our data strongly indicate that native rather than hydrolysed BGMP is required to affect monocyte activity, based on the fact that BGMP effects are unchanged in the presence of protease inhibitors and that casoplatelin, a biologically active undecapeptide comprising the BGMP terminal sequence that features a trypsin cleaving site, does not elicit cytokine production. We have additionally delineated the signalling pathway involved in BGMP effects. All the cytokines studied were clearly induced via JNK and ERK, while they were less sensitive to the inhibition of p38 MAPK. In addition, IL-8 secretion was relatively resistant to inhibition compared with that of TNF or IL-1β (specially with SB203580), indicating that it is less dependent on MAPK signalling. On the other hand, NF-κB was essential for the induction of the three cytokines, as Bay 11-7082 showed the highest degree of inhibition, resulting in abolition of TNF, IL-1β and IL-8 release. Furthermore, p50/p65 nuclear translocation was shown to be elicited by BGMP and blocked by Bay 11-7082. The dual involvement of MAPK and NF-κB is not unexpected because they are both activated by the IKK complex in monocytes, for instance, in response to LPS (Beinke and Ley, 2004). Our data show that the NF-κB pathway was absolutely required for the induction of TNF, IL-1β and IL-8, while MAPK pathways appeared to play a secondary role, possibly enhancing the effect of NF-κB. It is likely also that the MAPK may exert additional regulatory functions with regard to other genes such as cyclooxygenase 2, as previously suggested (Beinke and Ley, 2004). It should be noted that these conclusions are based largely on pharmacological assays, which have their well-recognized limitations in terms of specificity, and therefore they should be considered with caution. An additional question is how BGMP activates these signalling pathways. Although we initially used bovine serum albumin as a control in order to assess the specificity of BGMP effects, finding that it did not elicit cytokine secretion, this was later found to be due to continuous exposure to the protein, which is contained in cell culture medium as part of FBS. The question of whether similar tolerance to BGMP develops cannot be answered with our present data, but clearly there is no cross-tolerance with bovine serum albumin because BGMP stimulates cytokine secretion in the presence or absence of FBS. Previous evidence suggests that BGMP is not immunogenic by itself (Mikkelsen et al., 2006). Hence, BGMP may exert its effects by interaction with cell receptors. In this regard, it is interesting to note that bovine β-casein has been shown recently to act as a TLR4 ligand in mouse splenocytes (Tobita et al., 2006). It is possible that human monocytes do not respond to human albumin, but if they do tolerance makes sense because albumin is present in plasma at concentrations well over those activating monocytes in the present study. We are currently exploring the receptor responsible for BGMP effects and the ligand specificity compared with human and bovine albumin and other peptides.
We have previously demonstrated that BGMP has intestinal anti-inflammatory effects in preclinical models of colitis and ileitis, displaying a degree of efficacy similar to that of sulphasalazine, a drug widely used in the therapy of inflammatory bowel disease (Daddaoua et al., 2005; Baumgart and Sandborn, 2007; Requena et al., 2008). Of note, in both cases BGMP treatment resulted in normalization of the expression of IL-1β, whose main cellular sources are monocytes/macrophages. Clearly, BGMP does not reproduce this effect in vitro. There are two possible explanations for this discrepancy. First, it is possible that BGMP may act by other mechanisms, for instance, via effects on epithelial cells or lymphocytes (studies are underway to test these hypotheses), and that the activation of monocytes does not play a role in its intestinal anti-inflammatory activity or even opposes it. Second, monocyte activation may be involved in anti-inflammatory activity, a mechanism that certainly appears in the context of intestinal inflammation. However, the immune regulation of the intestine is especially complex in this regard. For instance, one of the few genes positively identified to affect the incidence of inflammatory bowel disease is NOD2, a member of the Nod1/Apaf-1 family (Peyrin-Biroulet and Chamaillard, 2007). The protein product is expressed intracellularly and recognizes LPS-derived muramyl dipeptide. The polymorphic alleles linked to increased risk of inflammatory bowel disease have been reported to be associated with reduced rather than augmented activation of leukocytes via NF-κB mechanisms, although this issue remains controversial (Bamias and Cominelli, 2007; Cho and Weaver, 2007; Peyrin-Biroulet and Chamaillard, 2007). In line with this concept, Nenci et al. (2007) used a smart in vivo mouse model in which the intestinal epithelial expression of IKKγ (also known as NEMO), IKKα and IKKβ was suppressed conditionally, resulting in reduced activation of the NF-κB pathway. The consequence was spontaneous severe colonic inflammation. Cytosine-phosphate-guanosine oligonucleotides, which are ligands for TLR9 and produce epithelial and immune cell activation, have anti-inflammatory activity when administered as a pre-treatment in experimental colitis, but exacerbate the inflammatory response when given as a post-treatment (Obermeier et al., 2003). Similarly, granulocyte-macrophage-colony stimulating factor (GM-CSF) has anti-inflammatory effects, which have been linked to the expansion of dendritic cells (Sainathan et al., 2008). Other studies have shown that the absence of monocytes and dendritic cells aggravates rather than ameliorates experimental colitis (Qualls et al., 2006). Taken together, these data suggest that a defective response to pro-inflammatory stimuli may actually worsen the outcome, at least in some cases. It has been well established that experimental colitis is strongly dependent on the presence of non-pathogenic bacteria, probably acting as a source of antigens that fuel the intestinal immune reaction, ultimately potentiating inflammation (Seksik et al., 2006). Thus, defects in immune function may impair a prompt resolution of intestinal injury, triggering a more robust reaction to a normally trivial challenge. If so, monocyte stimulation would result in a more efficient and prompt response to luminal antigens as they gain access to the subepithelial milieu. In this regard, it is tempting to speculate that BGMP may exert monocyte/macrophage-stimulating functions in breastfed infants. It is well known that the intestine of newborns is immature, exhibiting an increased permeability to macromolecules, and it is also prone to developing intense inflammatory responses (necrotizing enterocolitis) (Martinez-Augustin et al., 1997; Goldman, 2000). Additional experiments will be required to confirm the exact role of monocyte activation by BGMP in intestinal inflammation.
Acknowledgments
CIBEREHD is funded by the Instituto de Salud Carlos III. This study was supported by grants from the Instituto de Investigación Carlos III to F.S.M.L.H. and O.M.A. (PI051651 and PI051625). Additional funding was provided by the Junta de Andalucía. Pilar Requena was funded by the Spanish Ministry of Science and Technology.
Glossary
Abbreviations:
- BGMP
bovine glycomacropeptide
- FBS
foetal bovine serum
- IKK
IκB kinase
Conflicts of interest
None.
References
- Aimutis WR. Bioactive properties of milk proteins with particular focus on anticariogenesis. J Nutr. 2004;134:989S–995S. doi: 10.1093/jn/134.4.989S. [DOI] [PubMed] [Google Scholar]
- Bal dit Sollier C, Drouet L, Pignaud G, Chevallier C, Caen J, Fiat AM, et al. Effect of kappa-casein split peptides on platelet aggregation and on thrombus formation in the guinea-pig. Thromb Res. 1996;81:427–437. doi: 10.1016/0049-3848(96)00015-1. [DOI] [PubMed] [Google Scholar]
- Bamias G, Cominelli F. Immunopathogenesis of inflammatory bowel disease: current concepts. Curr Opin Gastroenterol. 2007;23:365–359. doi: 10.1097/MOG.0b013e3281c55eb2. [DOI] [PubMed] [Google Scholar]
- Baumgart DC, Sandborn WJ. Inflammatory bowel disease: clinical aspects and established and evolving therapies. Lancet. 2007;369:1641–1657. doi: 10.1016/S0140-6736(07)60751-X. [DOI] [PubMed] [Google Scholar]
- Beinke S, Ley SC. Functions of NF-kappaB1 and NF-kappaB2 in immune cell biology. Biochem J. 2004;382:393–409. doi: 10.1042/BJ20040544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody EP. Biological activities of bovine glycomacropeptide. Br J Nutr. 2000;84:S39–S46. doi: 10.1017/s0007114500002233. [DOI] [PubMed] [Google Scholar]
- Bruck WM, Kelleher SL, Gibson GR, Graverholt G, Lonnerdal BL. The effects of alpha-lactalbumin and glycomacropeptide on the association of CaCo-2 cells by enteropathogenic Escherichia coli, Salmonella typhimurium and Shigella flexneri. FEMS Microbiol Lett. 2006;259:158–162. doi: 10.1111/j.1574-6968.2006.00268.x. [DOI] [PubMed] [Google Scholar]
- Cho JH, Weaver CT. The genetics of inflammatory bowel disease. Gastroenterology. 2007;133:1327–1339. doi: 10.1053/j.gastro.2007.08.032. [DOI] [PubMed] [Google Scholar]
- Clare DA, Catignani GL, Swaisgood HE. Biodefense properties of milk: the role of antimicrobial proteins and peptides. Curr Pharm Des. 2003;9:1239–1255. doi: 10.2174/1381612033454874. [DOI] [PubMed] [Google Scholar]
- Cross ML, Gill HS. Immunomodulatory properties of milk. Br J Nutr. 2000;84(Suppl. 1):S81–S89. doi: 10.1017/s0007114500002294. [DOI] [PubMed] [Google Scholar]
- Daddaoua A, Puerta V, Zarzuelo A, Suarez MD, Sanchez de Medina F, Martinez-Augustin O. Bovine glycomacropeptide is anti-inflammatory in rats with hapten-induced colitis. J Nutr. 2005;135:1164–1170. doi: 10.1093/jn/135.5.1164. [DOI] [PubMed] [Google Scholar]
- Florisa R, Recio I, Berkhout B, Visser S. Antibacterial and antiviral effects of milk proteins and derivatives thereof. Curr Pharm Des. 2003;9:1257–1275. doi: 10.2174/1381612033454810. [DOI] [PubMed] [Google Scholar]
- Goldman AS. Modulation of the gastrointestinal tract of infants by human milk. Interfaces and interactions. An evolutionary perspective. J Nutr. 2000;130:426S–431S. doi: 10.1093/jn/130.2.426S. [DOI] [PubMed] [Google Scholar]
- Halprin KM, Ohkawara A. Lactate production and lactate dehydrogenase in the human epidermis. J Invest Dermatol. 1966;47:222–229. doi: 10.1038/jid.1966.133. [DOI] [PubMed] [Google Scholar]
- Li EW, Mine Y. Immunoenhancing effects of bovine glycomacropeptide and its derivatives on the proliferative response and phagocytic activities of human macrophagelike cells, U937. J Agric Food Chem. 2004;52:2704–2708. doi: 10.1021/jf0355102. [DOI] [PubMed] [Google Scholar]
- Lim K, van Calcar SC, Nelson KL, Gleason ST, Ney DM. Acceptable low-phenylalanine foods and beverages can be made with glycomacropeptide from cheese whey for individuals with PKU. Mol Genet Metab. 2007;92:176–178. doi: 10.1016/j.ymgme.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JJ. Monoclonal antibodies against myofibrillar components of rat skeletal muscle decorate the intermediate filaments of cultured cells. Proc Natl Acad Sci U S A. 1981;78:2335–2339. doi: 10.1073/pnas.78.4.2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Augustin O, Boza JJ, Navarro J, Martinez-Valverde A, Araya M, Gil A. Dietary nucleotides may influence the humoral immunity in immunocompromised children. Nutrition. 1997;13:465–469. doi: 10.1016/s0899-9007(97)00012-9. [DOI] [PubMed] [Google Scholar]
- Mikkelsen TL, Bakman S, Sorensen ES, Barkholt V, Frokiaer H. Sialic acid-containing milk proteins show differential immunomodulatory activities independent of sialic acid. J Agric Food Chem. 2005;53:7673–7680. doi: 10.1021/jf050398o. [DOI] [PubMed] [Google Scholar]
- Mikkelsen TL, Rasmussen E, Olsen A, Barkholt V, Frokiaer H. Immunogenicity of kappa-casein and glycomacropeptide. J Dairy Sci. 2006;89:824–830. doi: 10.3168/jds.S0022-0302(06)72145-2. [DOI] [PubMed] [Google Scholar]
- Monnai M, Otani H. Effect of bovine k-caseinoglycopeptide on secretion of interleukin-1 family cytokines by P388D1 cells, a line derived from mouse monocyte/macrophage. Milchwissenschaft. 1997;52:192–196. [Google Scholar]
- Monnai M, Horimoto Y, Otani H. Immunomodificatory effect of dietary bovine kappa-caseinoglycopeptide on serum antibody levels and proliferative responses of lymphocytes in mice. Milchwissenschaft. 1998;53:129–132. [Google Scholar]
- Nakajima K, Tamura N, Kobayashi-Hattori K, Yoshida T, Hara-Kudo Y, Ikedo M, et al. Prevention of intestinal infection by glycomacropeptide. Biosci Biotechnol Biochem. 2005;69:2294–2301. doi: 10.1271/bbb.69.2294. [DOI] [PubMed] [Google Scholar]
- Nenci A, Becker C, Wullaert A, Gareus R, van Loo G, Danese S, et al. Epithelial NEMO links innate immunity to chronic intestinal inflammation. Nature. 2007;446:557–561. doi: 10.1038/nature05698. [DOI] [PubMed] [Google Scholar]
- Newby TJ, Stokes CR, Bourne FJ. Immunological activity of milk. Vet Immunol Immunopathol. 1982;3:67–94. doi: 10.1016/0165-2427(82)90032-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obermeier F, Dunger N, Strauch UG, Grunwald N, Herfarth H, Scholmerich J, et al. Contrasting activity of cytosin-guanosin dinucleotide oligonucleotides in mice with experimental colitis. Clin Exp Immunol. 2003;134:217–224. doi: 10.1046/j.1365-2249.2003.02288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otani H, Monnai M. Inhibition of proliferative responses of mouse spleen lymphocytes by bovine milk k-casein digests. Food Agric Immunol. 1993;5:219–229. [Google Scholar]
- Otani H, Horimoto Y, Monnai M. Suppression of interleukin-2 receptor expression on mouse CD4+ T cells by bovine kappa-caseinoglycopeptide. Biosci Biotechnol Biochem. 1996;60:1017–1019. doi: 10.1271/bbb.60.1017. [DOI] [PubMed] [Google Scholar]
- Peyrin-Biroulet L, Chamaillard M. NOD2 and defensins: translating innate to adaptive immunity in Crohn's disease. J Endotoxin Res. 2007;13:135–139. doi: 10.1177/0968051907080429. [DOI] [PubMed] [Google Scholar]
- Qualls JE, Kaplan AM, van Rooijen N, Cohen DA. Suppression of experimental colitis by intestinal mononuclear phagocytes. J Leukoc Biol. 2006;80:802–815. doi: 10.1189/jlb.1205734. [DOI] [PubMed] [Google Scholar]
- Requena P, Daddaoua A, Martínez-Plata E, González M, Zarzuelo A, Suárez MD, et al. Bovine glycomacropeptide ameliorates experimental rat ileitis by mechanisms involving downregulation of interleukin 17. Br J Pharmacol. 2008;154:825–832. doi: 10.1038/bjp.2008.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainathan SK, Hanna EM, Gong Q, Bishnupuri KS, Luo Q, Colonna M, et al. Granulocyte macrophage colony-stimulating factor ameliorates DSS-induced experimental colitis. Inflamm Bowel Dis. 2008;14:88–99. doi: 10.1002/ibd.20279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seksik P, Sokol H, Lepage P, Vasquez N, Manichanh C, Mangin I, et al. Review article: the role of bacteria in onset and perpetuation of inflammatory bowel disease. Aliment Pharmacol Ther. 2006;24(Suppl. 3):11–18. doi: 10.1111/j.1365-2036.2006.03053.x. [DOI] [PubMed] [Google Scholar]
- Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, et al. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Tobita K, Kawahara T, Otani H. Bovine beta-casein (1-28), a casein phosphopeptide, enhances proliferation and IL-6 expression of mouse CD19+ cells via Toll-like receptor 4. J Agric Food Chem. 2006;54:8013–8017. doi: 10.1021/jf0610864. [DOI] [PubMed] [Google Scholar]
- Zimecki M, Kruzel ML. Milk-derived proteins and peptides of potential therapeutic and nutritive value. J Exp Ther Oncol. 2007;6:89–106. [PubMed] [Google Scholar]


