Abstract
Background and purpose:
Strontium ranelate reduces fracture risk in postmenopausal women with osteoporosis. Evidence from non-clinical studies and analyses of bone markers in phase III trials indicate that this is due to an increase in osteoblast formation and a decrease of osteoclastic resorption. The aim of this work was to investigate, in human cells, the mechanisms by which strontium ranelate is able to influence the activities of osteoblasts and osteoclasts.
Experimental approach:
Human primary osteoblasts were used to examine effects of strontium ranelate on replication (thymidine incorporation), differentiation (Runx2 and alkaline phosphatase) and cell survival (cell counts and caspase activity). Osteoprotegerin (OPG) was measured by quantitative reverse transcription PCR (qRT-PCR) and elisa and receptor activator of NFκB ligand (RANKL) by qRT-PCR and Western blot. As strontium ranelate has been proposed as an agonist of the calcium-sensing receptor (CaSR), the involvement of CaSR in the effects of strontium ranelate on OPG and RANKL expression, and cell replication was examined using siRNA.
Key results:
Strontium ranelate increased mRNA and protein levels of OPG and suppressed those of RANKL. Strontium ranelate also stimulated osteoblast replication and differentiation and increased cell survival under stress. Knocking down CaSR suppressed strontium ranelate-induced stimulation of OPG mRNA, reduction of RANKL mRNA, and increase in replication, indicating the involvement of CaSR in these responses.
Conclusions and implications:
Our results demonstrate that osteoblasts play a key role in the mechanism of action of the anti-fracture agent, strontium ranelate by mediating both its anabolic and anti-resorptive actions, at least in part, via activation of CaSR.
Keywords: strontium ranelate, human osteoblasts, OPG, RANKL, alkaline phosphatase, Runx2, calcium-sensing receptor
Introduction
Postmenopausal osteoporosis is a debilitating disease of skeletal fragility whereby bone strength is compromised, leading to increased fracture risk. Many anti-osteoporotic drugs target either bone resorption (anti-resorptives), or bone formation (anabolics) (Häuselmann and Rizzoli, 2003). These therapies have some limitations as the down-regulation of bone resorption induced by anti-resorptives results in a similar effect on bone formation at the tissue level, whereas the up-regulation of bone formation induced by anabolics increases bone resorption. A more recent approach has been adopted with the development of strontium ranelate which appears to affect both resorption and formation. Clinical trials have demonstrated the efficacy of strontium ranelate to reduce fracture risk (Meunier et al., 2002a,b; 2004a; Reginster et al., 2002; 2005;). Furthermore, analysis of bone markers showed strontium ranelate reduced the urinary excretion of the bone resorption marker, type I collagen N-telopeptide and increased the levels of a serum marker of osteoblast differentiation, bone-specific alkaline phosphatase in the strontium ranelate group as compared with placebo (Meunier et al., 2002a). Examinations of bone biopsies from patients who were treated with strontium ranelate (2 g per day) showed significantly increased mineral apposition rates and endosteal osteoblast surfaces compared with placebo, while other formation parameters were maintained (Arlot et al., 2008).
Further evidence for increases in formation and decreases in resorption have been found in a number of in vivo animal models (Marie et al., 1985; Buehler et al., 2001; Delannoy et al., 2002; Ammann et al., 2004; 2007;). At the macroscopic level, a recent study has reported that the increase in bone strength induced by strontium ranelate in rats can be explained by an improvement of microarchitecture as well as intrinsic bone tissue quality measured by hardness and dissipated energy upon nanoindentation (Ammann et al., 2007). Furthermore, studies in ovariectomized rats have strengthened the evidence that strontium ranelate decreases bone resorption (Marie et al., 1993).
In vitro studies show that strontium ranelate stimulates osteoblastic differentiation markers such as ALP, bone sialoprotein and osteocalcin in primary murine osteoblasts while also inhibiting the formation of osteoclasts and number of differentiated osteoclasts (Bonnelye et al., 2008). Further studies have reported an induction of apoptosis of mature osteoclasts (Mentaverri et al., 2005; Hurtel et al., 2007; 2008;).
The calcium-sensing receptor (CaSR) is critical for bone development (Chang et al., 2008) and there is some evidence that the CaSR is involved in the actions of strontium ranelate (Brown et al., 1990; Ruat et al., 1996; Kifor et al., 1997; Mailland et al., 1997; Brown and MacLeod, 2001; McLarnon et al., 2002; Coulombe et al., 2004). In bone-resorbing osteoclasts, strontium ranelate exerted pro-apoptotic effects in vitro via the CaSR (Mentaverri et al., 2005; Hurtel et al., 2007; 2008;) and in osteoblasts, a recent study showed that strontium ranelate-induced stimulation of replication was inhibited by transfection of a dominant negative CaSR (Brown, 2003; Chattopadhyay et al., 2007).
While these findings all help to explain the reduction in fracture risk seen in patients taking strontium ranelate, there is a missing link in the explanation of how this agent is able to concurrently affect resorption and positively affect formation. It is possible that osteoblasts play a key role in both the promotion of bone formation and indirectly by modulating osteoclast differentiation through receptor activator of NFκB ligand (RANKL) and osteoprotegerin (OPG), which are known to regulate osteoclast formation and activity (Lacey et al., 1998; Khosla, 2001).
We first reported that strontium ranelate induced increases in mRNA levels of OPG and decreased RANKL mRNA (Brennan et al., 2007) in human primary osteoblasts, which has now been confirmed (Atkins et al., 2009) albeit at supraphysiological concentrations. However, it is yet to be shown whether the protein levels of these cytokines are affected in the same manner. In the current study, therefore, we assessed both parameters that could account for the benefits of strontium ranelate on bone formation, that is, replication, differentiation and lifespan, and the possible down-regulation of osteoblast-induced osteoclast differentiation via mRNA and, importantly for the first time, protein levels of RANKL and OPG using concentrations ranging from 0.1 to 10 times the plasma concentrations found in patients treated with strontium ranelate 2 g per day. Furthermore, for the first time, we assessed the involvement of the CaSR in the modulation of OPG and RANKL by strontium, as well as cell replication by knocking down the CaSR with the use of siRNA technology.
Methods
Culture conditions
Human osteoblasts (HOBs) were grown from the minced trabecular ends of fetal long bone in accordance with the National Health and Medical Research Council guidelines and with the approval of the University of Sydney Human Ethics Committee (approval number: 01/02/40), as previously described (Slater et al., 1994a). HOBs were trypsinized from 75 cm2 flasks and plated at a density of 5 × 105 cells per well in 10% DMEM in 6-well plates or 1.5 × 104 cells per well in 10% DMEM in 96-well plates. Cells were incubated for a 24-h period in serum-reduced OptiMEM media before treatments were added. The culture medium used contains 1 mM calcium. Experiments were performed independently at least three times and triplicate wells were used in all experiments. Cells were treated for different periods of time depending on the parameter tested as follows: 48 h for replication, 72 h for ALP activity, 10 days for Runx2 mRNA expression, 24 h for OPG and RANKL mRNA expression, or 48 h for OPG and RANKL protein expression.
Composition of treatment media
For the purposes of this study, strontium ranelate consisted of a 1:100 molar ratio of Sr2+, derived from SrCl2 and ranelate, sourced from sodium ranelate. This ratio reflects the relative concentrations of strontium and ranelic acid in the serum of patients treated with strontium ranelate 2 g per day for 3 years. Concentrations of strontium ranelate used in this study are expressed in terms of Sr2+ (mM).
mRNA expression assays (real-time reverse transcription PCR)
RNA was extracted from HOBs plated in 6-well plates using a QIAGEN RNeasy Mini extraction kit according to the manufacturer's instructions. Total RNA (1.5 µg) was converted to cDNA using Superscript III reverse transcriptase and random hexamer primers as previously described (Sivagurunathan et al., 2005) for OPG, RANKL and Runx2/Cbfa1.
For RANKL and OPG mRNA expression analysis: the cDNA solution created in the reverse transcription step (1 µL) was added to a final volume of 20 µL of PCR reaction mixture containing 1× buffer, 2.5 mM MgCl2, 0.2 mM dNTP, SYBR Green I dye, 0.025 U·µL−1 Taq polymerase and 200 nM of each of forward and reverse primers for RANKL or OPG. Primers for OPG were newly designed for this study as follows: Fwd: 5′ GTCATCTAAAGCACCCTGTAG and Rev: 5′ TGAAGAATGCCTCCTCACAC. Primers for RANKL have been previously described (Sivagurunathan et al., 2005). Serial dilutions of a known concentration (1 × 108 copies µL−1) of a purified form of OPG or RANKL as required were used as standards. The purified target gene was obtained as reported previously (Sivagurunathan et al., 2005). Tubes were placed in a Rotor Gene real-time PCR cycler. mRNA expression for OPG and RANKL were determined as copy number per microgram of total RNA (Sivagurunathan et al., 2005) and expressed as percentages of the respective controls. This method has recently been documented as the most valid reference for data normalization in real-time reverse transcription PCR (RT-PCR) (Bustin, 2002; Tricarico et al., 2002; Anderson, 2003), while the use of house-keeping genes has increasingly been seen as problematic (Ke et al., 2000; Zhu et al., 2001; Bustin, 2002). The expression of Runx2/Cbfa1 mRNA was quantified by real-time RT-PCR, using TaqMan fluorogenic probes and an ABI PRISM 7700 sequence detector system as a standard curve for this gene was not established in the laboratory. Human Gus β was used to normalize expression of Runx2/Cbfa1. mRNA from vehicle-treated cells was chosen as the calibrator sample (i.e. target expression = 1).
Osteoprotegerin protein assays (elisa)
Human osteoblasts were plated in 6-well plates and cell culture supernatants were collected after 48-h treatments. The Biomedica elisa test kits were enzyme immunoassays designed to determine OPG directly in biological fluids, in this case cell culture supernatants. Serial dilutions (standards) were made from the 500 pM stock solution of protein provided in the kit in order to create a standard/calibration curve. Samples were processed according to manufacturer's instructions and placed in an elisa plate reader to determine the absorbance at 450 nm against 690 nm (as reference). Protein loading was corrected for using a BCA assay according to manufacturer's instructions.
RANKL and CaSR protein assays (Western blotting)
Human osteoblasts were plated in 6-well plates (105 cells per well for CaSR and 5 × 105 cells per well for RANKL expression). For RANKL, at the end of the 48-h culture period, and for CaSR expression, at the end of the transfection period, HOB cells were lysed in ice cold lysis buffer (50 mM Tris, pH 8, 150 mM NaCl, 1% (v/v) NP-40, 0.25% (w/v) sodium deoxycholate, 1 mM PMSF, 1 µM bestatin-HCl, 1 µM pepstatin A and 10% (v/v) glycerol). Protein concentrations were determined by BCA assay and equal amounts of protein were subjected to electrophoresis on 12% SDS-PAGE for RANKL determinations and 10% SDS-PAGE for CaSR, and then transferred electrophoretically onto a PVDF membrane and incubated in 1% (w/v) heat-denatured casein in PBS containing 0.04% (w/v) thymol (HDC) for 1 h at room temperature. Membranes were then exposed to primary antibody (1 µg·mL−1 anti-human RANKL antibody or 1 µg·mL−1 anti-human CaSR (ADD antibody)) in HDC for 1 h at room temperature, washed with 0.05% (v/v) Tween-20 in PBS and incubated with secondary anti-IgG horseradish peroxidase-conjugated antibody for 1 h at room temperature. Membranes were washed extensively and an enhanced chemiluminescence detection assay (from Millipore) was performed according to manufacturer's instructions. Band densities were measured using an Alpha Innotech Digital Imaging System.
CaSR siRNA knock-down assays
Cells were plated at a density of 105 cells per well in 6-well (PCR experiments) or 1.5 × 104 cells per well in 96-well (thymidine incorporation) plates with 10% DMEM. Twenty-four hours later, cells were transfected with complexes of 60 nM siRNA directed at CaSR, scrambled sequence and 1% (v/v) Invitrogen Lipofectamine RNAiMAX transfection reagent contained in OptiMEM medium. Transfection occurred over 4–24 h, after which time transfection medium was aspirated and replaced with 10% OptiMEM for a further 2–24 h. Medium was then changed to serum-free OptiMEM and incubated at 37°C for 2–24 h and then treated for 24 h with strontium ranelate treatments made up in the serum-free medium.
Cell replication studies (thymidine incorporation)
Human osteoblasts were plated in 96-well plates. Serum-reduced medium was supplemented with strontium ranelate (0.01–2 mM Sr2+, all in vehicle). A final volume of 100 µL was used. Cells were then cultured for 48 h prior to the addition of [3H]-thymidine. [3H]-thymidine incorporation was determined as previously described (Namkung-Matthai et al., 1998). Scintillation vials were counted for 5 min in a Tri-Carb 1900CA liquid β-scintillation counter.
Detection of osteoblast differentiation (alkaline phosphatase assay)
Human osteoblasts were plated in 96-well plates. Serum-reduced medium was supplemented with strontium ranelate (0.01–2 mM Sr2+) and the cells were cultured for a further 72 h. Alkaline phosphatase was determined as previously described (Namkung-Matthai et al., 1998) using a modification of the method of Lowry (1955), which uses p-nitrophenyl phosphate as the substrate of alkaline phosphatase. Plates were read at 405 nm to detect the alkaline phosphatase product (p-nitrophenolate). Alkaline phosphatase measurements were corrected for changes in cell number using total cellular protein. Total cellular protein from HOBs was determined using a Bio-rad kit based on the method of Bradford (1976) according to the manufacturer's instructions.
Osteoblast lifespan – H2O2-induced oxidative stress
Human osteoblasts were plated in 96-well plates. Serum-reduced medium was supplemented with strontium ranelate (0.01–2 mM Sr2+) and the cells were cultured for a further 48 h. Apoptosis was then induced by the addition of 0.2 mM H2O2 for 1 h. The cells were subsequently treated with strontium, in the absence of H2O2 for a further 3 h. After this time, the degree of apoptosis in the cell populations was measured by live cell counts using Trypan blue exclusion. Trypan blue dye added to the wells remains excluded from live cells, while non-viable cells incorporate the dye.
Osteoblast lifespan – serum deprivation
For serum-deprivation studies, HOBs were plated in 96-well plates. After 24-h adaptation to serum-reduced conditions in OptiMEM, the cells were treated with vehicle or strontium ranelate (0.01–1 mM Sr2+) for a further 7 days under serum-reduced conditions. At the end of the culture, the degree of apoptosis in the cell populations were measured using the CaspaseGlo assay kit according to manufacturer's instructions.
Statistics and data analysis
Experiments were routinely performed in triplicate or quintuplicate. Each experiment was repeated at least three times. Grouped data are presented as means ± SEM. Where single, representative results are presented, data are given as means ± SD. ANOVA, with Newman-Keuls post test, was used to determine significant differences between treatments. Where the data have been expressed as ratios, the coefficients of variation were calculated using the method of Colquhoun (1971).
Materials
Culture media were purchased from ThermoTrace Biosciences (Melbourne, VIC, Australia), and serum-reduced OptiMEM was from Invitrogen (San Diego, CA, USA). FBS was from CSL Limited (Parkville, VIC, Australia). Methyl, 1′,2′-[3H]-thymidine was purchased from Amersham Biosciences (Little Chalfont, Buckinghamshire, UK). The RNA extraction kit (RNeasy kit) was obtained from QIAGEN (Hilden, Germany). DNA molecular weight markers (0.07–12.2 kb) were from Roche Molecular Biochemicals (Mannheim, Germany). Superscript III reverse transcriptase and Lipofectamine RNAiMAX transfection reagent were obtained from Invitrogen (San Diego, CA, USA). TaqMan fluorogenic probes were purchased from Applied Biosystems (Courtaboeuf, France). The OPG ELISA kit for OPG determinations was obtained from Biomedica (Vienna, Austria). Porcine trypsin for passaging cultured cells was from JRH Biosciences (Lenexa, KS, USA). Bradford assay kit was from Bio-Rad Laboratories (Carlsbad, CA, USA). BCA assay from Pierce (Rockford, IL, USA). CaspaseGlo assay kit was from Promega (Madison, WI, USA). Anti-human RANKL antibody was from Abcam (Cambridge, MA, USA). Secondary IgG horseradish peroxidase-conjugated antibody was obtained from Chemicion (Temecula, CA, USA). Calcium chloride and strontium chloride were from Sigma Chemical Co. (St Louis, MO, USA). Sodium ranelate came from Les Laboratoires Servier (Neuilly, France). All other chemicals were obtained from Sigma Chemical Co. (St Louis, MO, USA). The Tri-Carb 1900CA liquid β-scintillation counter was purchased from Packard Instruments Co. (Downers Grove, IL, USA), the fluorescence plate reader was a FLUOstar Optima from BMG LABTECH GmbH (Offenburg, Germany), the Corbett Rotor Gene 3000 thermocycler was purchased from QIAGEN (Hilden, Germany) and the ABI PRISM 7700 sequence detector system was from Applied Biosystems (Courtaboeuf, France). The chemiluminescence detection assay was from Millipore (Billerica, MA, USA) and the Digital Imaging System was purchased from Alpha Innotech (San Leandro, CA, USA).
Results
Strontium ranelate increases the secretion of OPG protein as well as mRNA levels in human primary osteoblasts
After 24 h, strontium ranelate treatments from 0.01 to 2 mM induced concentration-dependent increases in OPG mRNA expression, determined by quantitative RT-PCR (Figure 1A). OPG mRNA expression increased by around 50% and 200% in response to 1 and 2 mM strontium ranelate respectively (P < 0.05, P < 0.001). Strontium ranelate significantly increased the secretion of OPG measured by elisa from HOBs; this increase in protein paralleled the increase in OPG mRNA expression. Strontium ranelate (1 and 2 mM Sr2+) increased OPG protein by approximately sevenfold and 8.5-fold respectively (Figure 1B).
Figure 1.
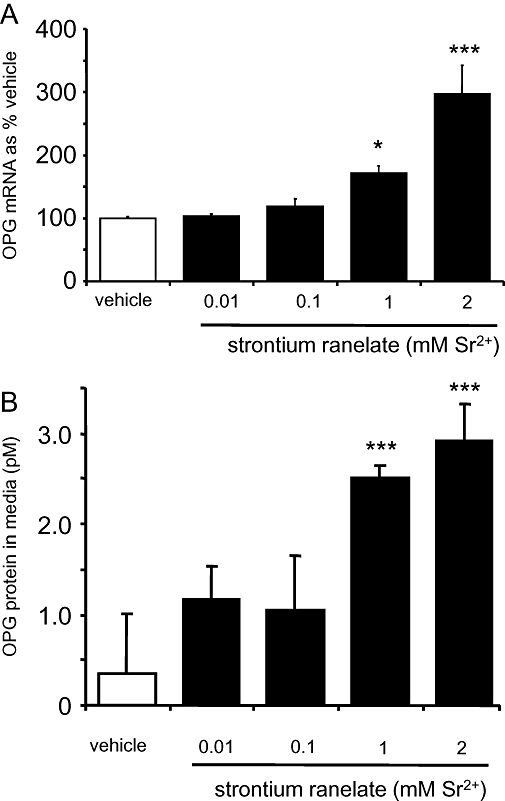
Strontium ranelate increases osteoprotegerin (OPG) mRNA and protein expression in human osteoblasts (HOBs). Cells were exposed for 24 h to vehicle or: 0.01, 0.1, 1 and 2 mM strontium ranelate, before RNA was extracted, converted to cDNA and then analysed by real-time reverse transcription PCR. (A) OPG mRNA expression was quantified and expressed as % of the value after treatment with vehicle (% vehicle). The results are presented as means ± SEM from four experiments. (B) OPG protein was measured by elisa according to manufacturer's instructions. The results are representative of three experiments ± SD each with triplicates. Concentrations are expressed as added to the vehicle, which contains 1 mM Ca2+. ***P < 0.001 compared with vehicle; *P < 0.05 compared with vehicle.
Strontium ranelate down-regulates both RANKL protein and mRNA expression in human primary osteoblasts
Treatment with strontium ranelate resulted in concentration-dependent decreases in RANKL mRNA expression measured by qRT-PCR. Concentrations of strontium ranelate as low as 0.1 mM, markedly reduced the expression of RANKL mRNA to around 20 % of the value after treatment with vehicle (Figure 2A; P < 0.001). As seen on the Western blot, the down-regulation of RANKL expression by strontium ranelate induced a similar decrease in cell-associated RANKL protein after 48-h treatment with strontium ranelate (Figure 2B).
Figure 2.
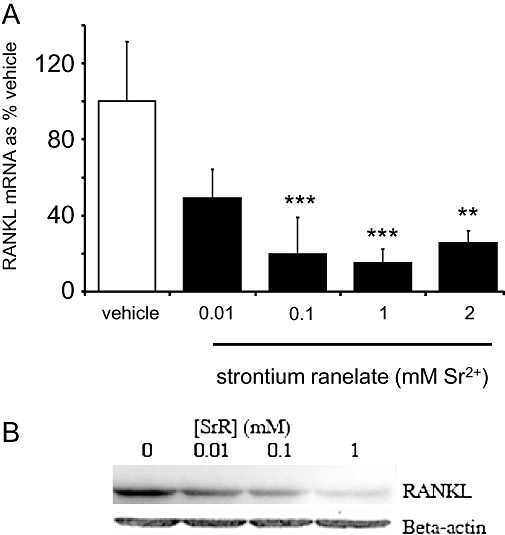
Strontium ranelate decreases receptor activator of NFκB ligand (RANKL) mRNA and protein expression in human osteoblasts. Cells were exposed for 24 h to vehicle or: 0.01, 0.1, 1 and 2 mM strontium ranelate, before RNA was extracted, converted to cDNA and then analysed by real-time reverse transcription PCR. (A) RANKL mRNA expression was quantified and expressed as % of the value after treatment with vehicle (% vehicle). The results are presented as means ± SEM from three experiments, each with triplicates. (B) RANKL protein was detected by Western blotting after cells were exposed to treatments for 24 h. One blot is shown, representative of two experiments. Concentrations are expressed as added to the vehicle, which contains 1 mM Ca2+. ***P < 0.001 compared with vehicle; **P < 0.01 compared with vehicle.
The CaSR is involved in strontium ranelate-induced OPG mRNA expression and suppression of RANKL mRNA
The extent of the knockdown of the CaSR in HOBs using siRNA technology was tested by Western blot analysis of the CaSR compared with HOBs which were exposed to the transfection reagent in the absence of siRNA, and HOBs which were transfected with scrambled siRNA. siRNA directed against the CaSR inhibited the expression of the CaSR in HOBs by around 50% compared with both HOBs transfected with siScrambled and without transfection (Figure 3A); β-actin was used as the control.
Figure 3.
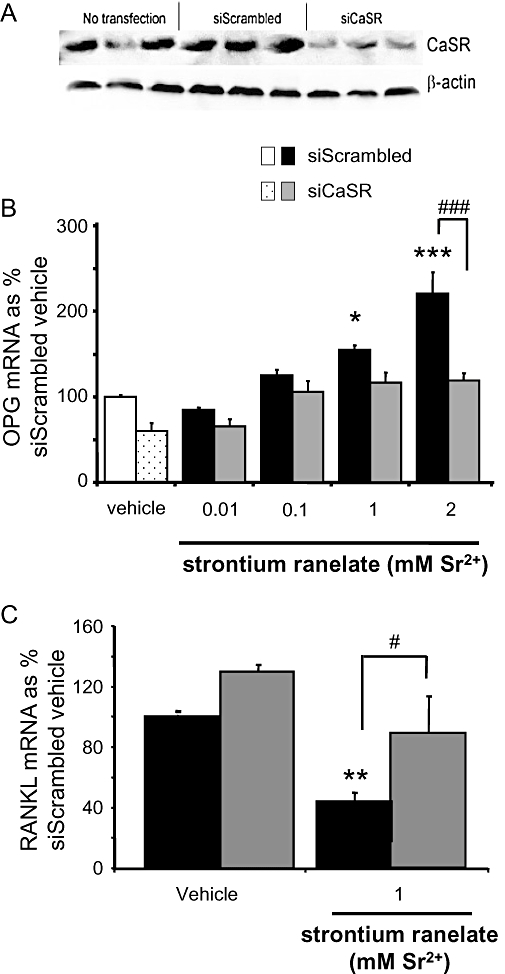
Calcium-sensing receptor (CaSR) is involved in strontium ranelate-induced changes in osteoprotegerin (OPG) and receptor activator of NFκB ligand (RANKL) mRNA expression. (A) Protein blots from human osteoblasts (HOBs) transfected with siRNA directed at the CaSR (siCaSR), scrambled siRNA (siScrambled) and without transfection. siCaSR inhibited the expression of the CaSR in HOBs by around 50% compared with both HOBs transfected with siScrambled and without transfection. β-Actin was used as the loading control. (B and C) siRNA directed against the CaSR was transfected into cells prior to the addition of strontium ranelate. Total RNA was extracted, converted to cDNA and then analysed by real-time reverse transcription PCR (A: OPG, B: RANKL). The results are presented as means ± SEM from three experiments, each with triplicates. Concentrations are expressed as added to the vehicle, which contains 1 mM Ca2+. ***P < 0.001 compared with vehicle; **P < 0.01 compared with vehicle; *P < 0.05 compared with vehicle; #P < 0.05 compared with same treatment with addition of siRNA; ###P < 0.001 compared with same treatment with addition of siRNA.
Transfection of HOBs with siRNA directed against CaSR non-significantly suppressed OPG mRNA expression in vehicle-treated cells (Figure 3B). In the presence of scrambled siRNA, OPG mRNA expression was significantly stimulated more than twofold by 2 mM strontium ranelate. Knocking down the CaSR abolished this stimulation. In the presence of scrambled siRNA, RANKL mRNA expression was significantly reduced (by more than 50%) with 1 mM strontium ranelate. Knocking down the CaSR abolished this inhibition of RANKL expression (Figure 3C).
Strontium ranelate increases cell replication via activation of the CaSR
Strontium ranelate (0.01–2 mM) dose-dependently stimulated [3H]-thymidine incorporation by 2–3-fold at 0.1 and 1 mM, and by approximately fivefold at 2 mM (P < 0.001) after a 48-h culture (Figure 4A). In the presence of scrambled siRNA, [3H]-thymidine incorporation was significantly increased by strontium ranelate, whereas in the presence of siRNA directed against the CaSR this effect was eliminated (Figure 4B).
Figure 4.
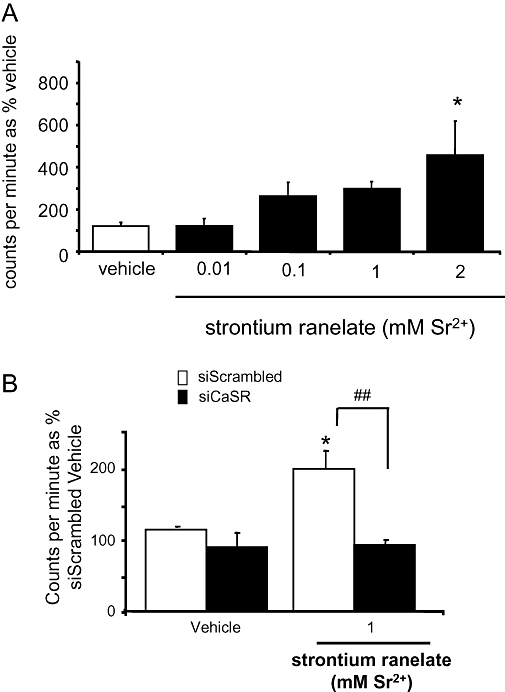
Strontium ranelate increases human osteoblast (HOB) cell replication via the calcium-sensing receptor (CaSR) as measured by [3H] thymidine incorporation. [3H] thymidine incorporation was measured in HOBs treated for 24 h with vehicle or: 0.01, 0.1, 1 and 2 mM strontium ranelate (A). siRNA directed against the CaSR was transfected into cells prior to the addition of strontium ranelate for 24 h and the increased [3H] thymidine incorporation seen with strontium was blunted in cells transfected with siRNA directed towards the CaSR (B). *P < 0.05 compared with vehicle.
Strontium ranelate increases bone cell differentiation
The effects of strontium ranelate on the differentiation of human primary osteoblasts were investigated in parallel via the assessment of two indirect and independent markers. Alkaline phosphatase activity, corrected for total cell protein, was assessed after a culture of 72 h in presence of the different treatments. Strontium ranelate (1 and 2 mM) increased alkaline phosphatase activity by twofold (P < 0.01, Figure 5A).
Figure 5.
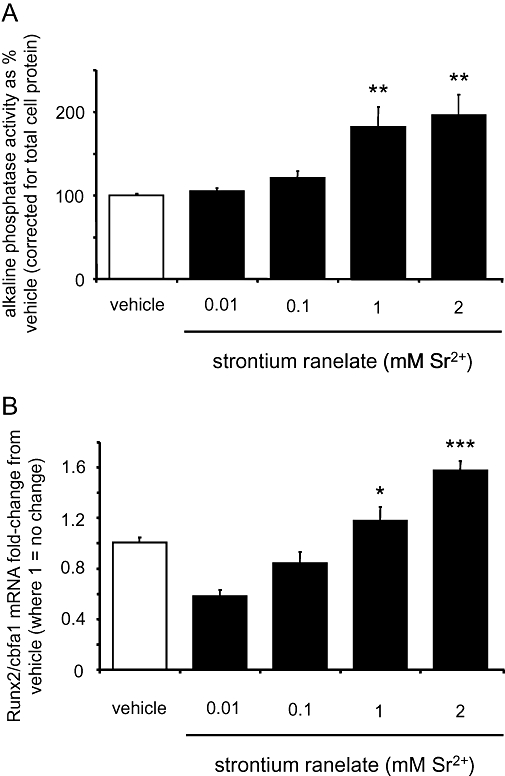
Strontium ranelate increases human osteoblast cell differentiation assessed by Runx2/Cbfa1 mRNA expression and alkaline phosphatase activity. Alkaline phosphatase activity was measured after 72-h treatments of 0.01, 0.1, 1 and 2 mM strontium ranelate and corrected for total cellular protein (A). Cells were exposed for 10 days to vehicle or: 0.01, 0.1, 1 and 2 mM strontium ranelate, before RNA was extracted, converted to cDNA and then analysed by real-time reverse transcription PCR. Runx2/Cbfa1 expression was quantified and expressed as fold-change from the value after treatment with vehicle (B). Concentrations are expressed as added to the vehicle, which contains 1 mM Ca2+. ***P < 0.001 compared with vehicle; **P < 0.01 compared with vehicle; *P < 0.05 compared with vehicle.
A longer culture duration was necessary to assess the effect of strontium ranelate on the expression of the osteoblast-specific transcription factor, Runx2/Cbfa1, which is an early sign of commitment to the osteoblast lineage. After 10-days culture, strontium ranelate dose-dependently increased the expression of Runx2/Cbfa1 with a maximum around 1.3-fold and 1.5-fold, with the concentrations of 1 and 2 mM respectively (P < 0.05 and P < 0.001, Figure 5B).
Strontium ranelate increases bone cell survival
As a decrease in the lifespan of osteoblasts has been shown to be involved in osteoporosis, we assessed the capability of strontium ranelate to counteract this deleterious effect. Two approaches were used to mimic the decrease in osteoblast lifespan observed under conditions of stress, namely oxidative stress induced by H2O2 and the deprivation of serum. Strontium ranelate dose-dependently increased HOB survival under oxidative stress conditions induced by the addition of peroxide (Figure 6A). Strontium ranelate also decreased apoptosis induced by prolonged serum deprivation as measured by caspase 3 and caspase 7 activities at concentrations as low as 0.01 mM (P < 0.05 for 0.01 and 0.1 mM, P < 0.001 for 1 mM; Figure 6B).
Figure 6.
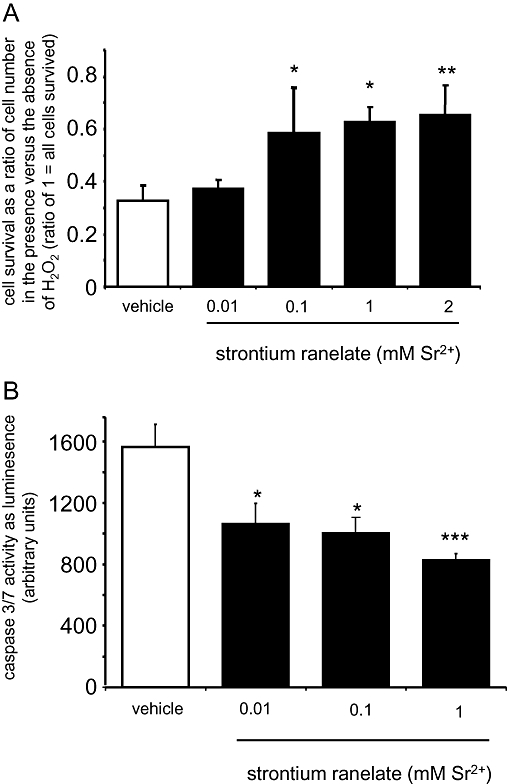
Strontium ranelate increases human osteoblast survival. Cell survival was measured as live cell counts post H2O2-induced oxidative stress after 48-h strontium ranelate treatment (A) or caspase 3/7 was measured with a commercial kit after 6 days' serum deprivation (B). Concentrations are expressed as added to the vehicle, which contains 1 mM Ca2+. ***P < 0.001 compared with vehicle; **P < 0.01 compared with vehicle; *P < 0.05 compared with vehicle.
Discussion and conclusions
Concentrations of OPG and RANKL protein are key determinants of osteoclast production with increased secretion of OPG and diminished RANKL availability down-regulating osteoclastogenesis. Here we show that strontium ranelate, at concentrations spanning those found in the serum of patients taking 2 g per day, significantly enhanced OPG mRNA expression in HOBs and for the first time, induced OPG protein secretion from these cells as measured by elisa. In addition, strontium ranelate suppressed RANKL mRNA expression in HOBs and decreased the membrane-associated protein levels of RANKL, as detected by Western blotting, which has not been previously reported. Apart from an earlier abstract (Brennan et al., 2007) a recent report indicated that strontium ranelate influences RANKL and OPG mRNA levels in human cells in vitro, although the concentrations of strontium ranelate tested were supraphysiological and it is difficult, therefore, to relate these results to the clinical setting (Atkins et al., 2009). The ability of strontium ranelate to modulate these cytokines suggests it is able to down-regulate osteoclastogenesis through an indirect stimulation of osteoblasts. In vivo histomorphometry has revealed a decrease in bone resorption after treatment with strontium ranelate in monkeys (Buehler et al., 2001), rats (Marie et al., 1985) and mice (Marie and Hott, 1986), and a decrease in bone resorption markers in humans (Meunier et al., 2002a,b;), inducing a trend to lower osteoclast surfaces in human biopsies (Arlot et al., 2008). The results from the current study provide a potential explanation for these effects via a strontium ranelate-induced increase in the osteoblast-produced OPG and a concurrent reduction in RANKL.
Furthermore, we show for the first time that these specific effects of strontium are mediated by the CaSR. It has been recently reported that strontium ranelate-induced increases in bone cell replication are CaSR-dependent, with the overexpression of a dominant negative version of the CaSR leading to a loss of response (Brown, 2003; Chattopadhyay et al., 2007). We noted a similar effect on replication using siRNA. In the current study, a 50% reduction in the CaSR protein expression also blunted the increased production of OPG mRNA levels usually induced by strontium ranelate. RANKL mRNA levels were likewise unaffected in cells with a silenced CaSR, whereas RANKL levels were depressed following treatment with strontium ranelate in cells transfected with scrambled siRNA. This does not, however, completely exclude the involvement of another receptor or mechanism that might be sensitive to strontium ranelate. Interesting recent data from rabbit osteoclasts suggest that the downstream signalling mechanism by which strontium ranelate acts to enhance osteoclast apoptosis is distinct from calcium, yet both activate the CaSR (Hurtel et al., 2008). From the results presented here, it is likely that the effects of strontium ranelate on both osteoblasts and osteoclasts are mediated, at least in part, by the CaSR.
In the current study statistically significant effects were observed with strontium ranelate, at concentrations between 0.1 and 2 mM, depending on the variables measured. These concentrations are in the range of the plasma concentrations of strontium ranelate in patients included in the phase III clinical trials and what can be expected in the bone microenvironment (Boivin et al., 1996; Dahl et al., 2001). It is thus likely that the current findings may help explain some of the mechanisms of action by which strontium ranelate decreases fracture risk in postmenopausal women with osteoporosis (Meunier et al., 2004b; Reginster et al., 2005).
The HOB cells used here have been extensively characterized in previous studies (Slater et al., 1994a,b;). Strontium ranelate increased the replication of HOBs, confirming previous observations made on osteoblasts from rodent species or osteoblastic cell lines (Marie et al., 1985; Marie and Hott, 1986; Canalis et al., 1996; Ammann et al., 2004). Moreover, strontium ranelate promoted the differentiation of human primary osteoblasts as detected by changes both in Runx2 mRNA expression and in alkaline phosphatase activity corrected for total cellular protein. Runx2 is a key transcription factor expressed by committed osteoblasts (Karsenty et al., 1999; Karsenty and Wagner, 2002; Marie 2008) which has not previously been reported to be induced by strontium and is similarly affected by the natural ligand of the CaSR, calcium (Dvorak et al., 2004). It should be noted that cells which grow from the ends of long bones are a mixed population of cells already committed to the osteoblast lineage and uncommitted osteoblast precursors. Strontium ranelate induced significant increases in alkaline phosphatase activity, an early marker of differentiation in cells already committed to the osteoblast lineage in around 72 h, with non-consistent increases seen before that time (data not shown). A similar time course for the induction of alkaline phosphatase by strontium ranelate is also seen in the relatively differentiated MG63 cell line (Brennan et al., 2005) and an even longer time course of 7 days for induction of alkaline phosphatase and for another functional marker, osteocalcin, by strontium ranelate has been reported in mouse bone marrow stromal cells (Choudhary et al., 2007). The time course for induction of Runx2 in uncommitted cells is much longer according to the literature (Igarashi et al., 2004), and we also did not see consistent increases in Runx2 in the presence of strontium ranelate at earlier time points tested (data not shown). It was not possible to directly test the involvement of the CaSR in the strontium ranelate induction of Runx2 in HOBs, as it is technically difficult to maintain siRNA knockdown and keep a population of primary cells healthy over the 10 days required. Nevertheless, the study of Chang et al. (2008) showed that Runx2 expression was markedly reduced in CaSR knockout chondrocytes, indicating an important role for the CaSR in Runx2 induction. Stimulation of cellular replication and differentiation may underlie the positive effects of strontium ranelate on bone formation observed during in vivo and clinical studies (Arlot et al., 2008).
The decreased lifespan of osteoblasts following menopause is considered to play a role in the decrease in bone mineral density and increase in fracture risk (Bonewald, 2004; Almeida et al., 2007), and it is well established that the protective mechanism of oestrogen to reduce fracture risk in patients with glucocorticoid-induced osteoporosis includes an increased osteoblast and osteocyte lifespan (Weinstein et al., 1998; Dennison, 1999). The protective effects of strontium ranelate on HOB cell survival under hydrogen peroxide or long-term serum-reduced stress mimic the protective effects seen with both oestrogen (Kousteni et al., 2001) and intermittent parathyroid hormone (Jilka et al., 1999) and are in contrast to the increased osteoblastic cell death induced by glucocorticoids (Sambrook et al., 2003). It has also been postulated that reduced osteoblast/osteocyte death contributes to reduced resorption (Gu et al., 2005). It is plausible, therefore, that the increase in osteoblast lifespan we observe in the presence of strontium ranelate could contribute to anti-fracture efficacy through improved bone quality.
In conclusion, strontium ranelate significantly down-regulates both mRNA and protein levels of the osteoblast-induced signals for osteoclastogenesis through the CaSR and has positive effects on osteoblastic replication, differentiation and lifespan. These findings in human primary osteoblasts provide new insights into the mechanism of action of strontium ranelate in the bone microenvironment.
Acknowledgments
These studies were supported by Servier International.
Glossary
Abbreviations:
- CaSR
calcium sensing receptor
- HOBs
human primary osteoblasts
- OPG
osteoprotegerin
- RANKL
receptor activator of NFκB ligand
- SR
strontium ranelate
Conflict of interest
This work was funded by Servier International.
References
- Almeida M, Han L, Martin-Millan M, Plotkin LI, Stewart SA, Roberson PK, et al. Skeletal involution by age-associated oxidative stress and its acceleration by loss of sex steroids. J Biol Chem. 2007;282:27285–27297. doi: 10.1074/jbc.M702810200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammann P, Shen V, Robin B, Mauras Y, Bonjour JP, Rizzoli R. Strontium ranelate improves bone resistance by increasing bone mass and improving architecture in intact female rats. J Bone Miner Res. 2004;20:548. doi: 10.1359/JBMR.040906. [DOI] [PubMed] [Google Scholar]
- Ammann P, Badoud I, Barraud S, Dayer R, Rizzoli R. Strontium ranelate treatment improves trabecular and cortical intrinsic bone tissue quality, a determinant of bone strength. J Bone Miner Res. 2007;22:1419–1425. doi: 10.1359/jbmr.070607. [DOI] [PubMed] [Google Scholar]
- Anderson P, O'Loughlin P, May B, Morris H. Quantification of mRNA for the vitamin D metabolizing enzymes CYP27B1 and CYP24 and vitamin D receptor in kidney using real-time reverse transcriptase-polymerase chain reaction. J Mol Endocrinol. 2003;31:123–132. doi: 10.1677/jme.0.0310123. [DOI] [PubMed] [Google Scholar]
- Arlot ME, Jiang Y, Genant HK, Zhao J, Burt-Pichat B, Roux J-P, et al. Histomorphometric and microCT analysis of bone biopsies from postmenopausal osteoporotic women treated with strontium ranelate. J Bone Miner Res. 2008;23:215–222. doi: 10.1359/jbmr.071012. [DOI] [PubMed] [Google Scholar]
- Atkins GJ, Welldon KJ, Halbout P, Findlay DM. Strontium ranelate treatment of human primary osteoblasts promotes an osteocyte-like phenotype while eliciting an osteoprotegerin response. Osteoporosis Int. 2009;20:653–664. doi: 10.1007/s00198-008-0728-6. [DOI] [PubMed] [Google Scholar]
- Boivin G, Deloffre P, Perrat B, Panczer G, Boudeulle M, Mauras Y, et al. Strontium distribution and interactions with bone mineral in monkey iliac bone after strontium salt (S 12911) administration. J Bone Miner Res. 1996;11:1302–1311. doi: 10.1002/jbmr.5650110915. [DOI] [PubMed] [Google Scholar]
- Bonewald LF. Osteocyte biology: its implications for osteoporosis. J Musculoskelet Neuronal Interact. 2004;4:101–104. [PubMed] [Google Scholar]
- Bonnelye E, Chabadel A, Saltel F, Jurdic P. Dual effect of strontium ranelate: stimulation of osteoblast differentiation and inhibition of osteoclast formation and resorption in vitro. Bone. 2008;42:129–138. doi: 10.1016/j.bone.2007.08.043. [DOI] [PubMed] [Google Scholar]
- Bradford M. A rapid and sensitive for the quantitation of microgram quantitites of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brennan TC, Muir MM, Conigrave AD, Mason RS. Functional responses of osteoblastic cells to stimulation by strontium. J Bone Miner Res. 2005;20:M196. p S359. [Google Scholar]
- Brennan TC, Rybchyn MS, Halbout P, Conigrave AD, Mason RS. Strontium ranelate effects in human osteoblasts support its uncoupling effect on bone formation and bone resorption. J Bone Miner Res. 2007;22:M014. [Google Scholar]
- Brown E, Fuleihan Gel-H, Chen C, Kifor O. A comparison of the effects of divalent and trivalent cations on parathyroid hormone release, 3′,5′-cyclic-adenosine monophosphate accumulation, and the levels of inositol phosphates in bovine parathyroid cells. Endocrinology. 1990;127:1064–1071. doi: 10.1210/endo-127-3-1064. Published erratum appears in Endocrinology 1992 Aug. 131 (2): 862. [DOI] [PubMed] [Google Scholar]
- Brown EM. Is the calcium receptor a molecular target for the actions of strontium on bone. Osteoporos Int. 2003;14:S25–S34. doi: 10.1007/s00198-002-1343-6. [DOI] [PubMed] [Google Scholar]
- Brown EM, MacLeod RJ. Extracellular calcium sensing and extracellular calcium signaling. Physiol Rev. 2001;81:239–297. doi: 10.1152/physrev.2001.81.1.239. [DOI] [PubMed] [Google Scholar]
- Buehler J, Chappuis P, Saffar JL, Tsouderos Y, Vignery A. Strontium ranelate inhibits bone resorption while maintaining bone formation in alveolar bone in monkeys (Macaca fascicularis) Bone. 2001;29:176–179. doi: 10.1016/s8756-3282(01)00484-7. [DOI] [PubMed] [Google Scholar]
- Bustin SA. Quantification of mRNA using real-time reverse transcription PCR (RT-PCR): trends and problems. J Endocrinol. 2002;29:23–39. doi: 10.1677/jme.0.0290023. [DOI] [PubMed] [Google Scholar]
- Canalis E, Hott M, Deloffre P, Tsouderos Y, Marie JP. The divalent strontium salt S12911 enhances bone cell replication and bone formation in vitro. Bone. 1996;18:517–523. doi: 10.1016/8756-3282(96)00080-4. [DOI] [PubMed] [Google Scholar]
- Chang W, Tu C, Chen T-H, Bikle D, Shoback D. the extracellular calcium-sensing receptor (CaSR) is a critical modulator of skeletal development. Sci Signal. 2008;1:ra1. doi: 10.1126/scisignal.1159945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay N, Quinn SJ, Kifor O, Ye C, Brown EM. The calcium-sensing receptor (CaR) is involved in strontium ranelate-induced osteoblast proliferation. Biochem Pharmacol. 2007;74:438–447. doi: 10.1016/j.bcp.2007.04.020. [DOI] [PubMed] [Google Scholar]
- Choudhary S, Halbout P, Alander C, Raisz L, Pilbeam C. Strontium ranelate promotes osteoblastic differentiation and mineralization of murine bone marrow stromal cells: involvement of prostaglandins. J Bone Miner Res. 2007;22:1002–1010. doi: 10.1359/jbmr.070321. [DOI] [PubMed] [Google Scholar]
- Colquhoun D. Lectures on Biostatistics – An Introduction to Statistics with Applications in Biology and Medicine. Oxford: Clarendon Press; 1971. [Google Scholar]
- Coulombe J, Faure H, Robin B, Ruat M. In vitro effects of strontium ranelate on the extracellular calcium-sensing receptor. Biochem Biophys Res Commun. 2004;323:1184–1190. doi: 10.1016/j.bbrc.2004.08.209. [DOI] [PubMed] [Google Scholar]
- Dahl SG, Allain P, Marie PJ, Mauras Y, Boivin G, Ammann P, et al. Incorporation and distribution of strontium in bone. Bone. 2001;28:446–453. doi: 10.1016/s8756-3282(01)00419-7. [DOI] [PubMed] [Google Scholar]
- Delannoy P, Bazot D, Marie PJ. Long-term treatment with strontium ranelate increases vertebral bone mass without deleterious effect in mice. Metabolism. 2002;51:906–911. doi: 10.1053/meta.2002.33360. [DOI] [PubMed] [Google Scholar]
- Dennison E. Epidemiology of glucocorticoid-induced osteoporosis. Osteoporos Int. 1999;9:S16. doi: 10.1159/000061078. [DOI] [PubMed] [Google Scholar]
- Dvorak MM, Siddiqua A, Ward DT, Carter DH, Dallas SL, Nemeth EF, et al. Physiological changes in extracellular calcium concentration directly control osteoblast function in the absence of calciotropic hormones. Proc Natl Acad Sci. 2004;101:5140–5145. doi: 10.1073/pnas.0306141101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu G, Mulari M, Peng Z, Hentunen TA, Väänänen HK. Death of osteocytes turns off the inhibition of osteoclasts and triggers local bone resorption. Biochem Biophys Res Commun. 2005;335:1095–1101. doi: 10.1016/j.bbrc.2005.06.211. [DOI] [PubMed] [Google Scholar]
- Häuselmann HJ, Rizzoli R. A comprehensive review of treatments for postmenopausal osteoporosis. Osteoporos Int. 2003;14:2–12. doi: 10.1007/s00198-002-1301-3. [DOI] [PubMed] [Google Scholar]
- Hurtel AS, Mentaverri R, Wattel A, Kamel S, Brazier M. Strontium ranelate and calcium exert cumulative effects on osteoclasts by activation of different intracellular signalling pathways, downstream of the calcium-sensing receptor. Calcif Tissue Int. 2007;(Suppl 1):P352-M. [Google Scholar]
- Hurtel AS, Mentaverri R, Caudrillier A, Cournarie F, Wattel A, Kamel S, et al. The calcium-sensing receptor is involved in strontium ranelate-induced osteoclast apoptosis: new insights into the associated signalling pathways. J Biol Chem. 2008 doi: 10.1074/jbc.M801668200. M801668200. [DOI] [PubMed] [Google Scholar]
- Igarashi M, Kamiya N, Hasegawa M, Kasuya T, Takahashi T, Takagi M. Inductive effects of dexamethasone on the gene expression of Cbfa1, osterix and bone matrix proteins during differentiation of cultured primary rat osteoblasts. J Mol Histol. 2004;35:3–10. doi: 10.1023/b:hijo.0000020883.33256.fe. [DOI] [PubMed] [Google Scholar]
- Jilka RL, Weinstein RS, Bellido T, Roberson P, Parfitt AM, Manolagas SC. Increased bone formation by prevention of osteoblast apoptosis with parathyroid hormone. J Clin Invest. 1999;104:439–446. doi: 10.1172/JCI6610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karsenty G, Wagner EF. Reaching a genetic and molecular understanding of skeletal development. Dev Cell. 2002;2:389–406. doi: 10.1016/s1534-5807(02)00157-0. [DOI] [PubMed] [Google Scholar]
- Karsenty G, Ducy P, Starbuck M, Priemel M, Shen J, Geoffroy V, et al. Cbfa1 as a regulator of osteoblast differentiation and function. Bone. 1999;25:107–108. doi: 10.1016/s8756-3282(99)00111-8. [DOI] [PubMed] [Google Scholar]
- Ke L, Chen Z, Yung W. A reliability test of standard-based quantitative PCR: exogenous vs endogenous standards. Mol Cell Probes. 2000;14:127–135. doi: 10.1006/mcpr.2000.0288. [DOI] [PubMed] [Google Scholar]
- Khosla S. Minireview: the OPG/RANKL/RANK system. Endocrinology. 2001;142:5050–5055. doi: 10.1210/endo.142.12.8536. [DOI] [PubMed] [Google Scholar]
- Kifor O, Diaz R, Butters R, Brown EM. The Ca2+-Sensing receptor (CaR) activates phospholipases C, A(2) and Din bovine parathyroid and CaR-transfected, human embryonic kidney (HEK293) cells. J Bone Miner Res. 1997;12:715–725. doi: 10.1359/jbmr.1997.12.5.715. [DOI] [PubMed] [Google Scholar]
- Kousteni S, Bellido T, Plotkin LI, O'Brien CA, Bodenner DL, Han L, et al. Nongenotropic, sex-nonspecific signaling through the estrogen or androgen receptors: dissociation from transcriptional activity. Cell. 2001;104:719–730. [PubMed] [Google Scholar]
- Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165–176. doi: 10.1016/s0092-8674(00)81569-x. [DOI] [PubMed] [Google Scholar]
- Lowry OH. Specific procedures. alkaline phosphatase. In: Pastan IH, editor. Micromethods for the Assay of Enymes. New York: Academic Press; 1955. pp. 265–371. [Google Scholar]
- McLarnon SJ, Holden D, Ward DT, Jones MN, Elliott AC, Riccardi D. Aminoglycoside antibiotics induce pH-sensitive activation of the calcium-sensing receptor. Biochem Biophys Res Commun. 2002;297:71–77. doi: 10.1016/s0006-291x(02)02133-2. [DOI] [PubMed] [Google Scholar]
- Mailland M, Waelchli R, Ruat M, Boddeke HGWM, Seuwen K. Stimulation of cell proliferation by calcium and a calcimimetic compound. Endocrinology. 1997;138:3601–3605. doi: 10.1210/endo.138.9.5417. [DOI] [PubMed] [Google Scholar]
- Marie P, Hott M. Short-term effects of flouride and strontium on bone formation and resoption in the mouse. Metabolism. 1986;35:547–551. doi: 10.1016/0026-0495(86)90013-2. [DOI] [PubMed] [Google Scholar]
- Marie P, Hott M, Modrowski D, De Pollak C, Guillemain J, Deloffre P, et al. An uncoupling agent containing strontium prevents bone loss by depressing bone resorption and maintaining bone formation in estrogen-deficient rats. J Bone Miner Res. 1993;8:607–615. doi: 10.1002/jbmr.5650080512. [DOI] [PubMed] [Google Scholar]
- Marie PJ. Transcription factors controlling osteoblastogenesis. Arch Biochem Biophys. 2008;473:98–105. doi: 10.1016/j.abb.2008.02.030. [DOI] [PubMed] [Google Scholar]
- Marie PJ, Hott M. Short-term effects of fluoride and strontium on bone formation and resorption in the mouse. Metabolism. 1986;35:547–551. doi: 10.1016/0026-0495(86)90013-2. [DOI] [PubMed] [Google Scholar]
- Marie PJ, Garber MT, Hott M, Miravet L. Effect of low doses of stable strontium on bone metabolism in rats. Miner Electrolyte Metab. 1985;11:5–13. [PubMed] [Google Scholar]
- Mentaverri R, Chattopadhyay N, Lemaire-Hurtel AS, Kamel S, Brazier M, Brown EM. The effects of strontium ranelate on osteoclasts are calcium-sensing receptor dependent. J Bone Miner Res. 2005;20:S309. [Google Scholar]
- Meunier PJ, Slosman DO, Delmas PD, Sebert JL, Brandi ML, Albanese C, et al. Strontium ranelate: dose-dependent effects in established postmenopausal vertebral osteoporosis–a 2-year randomized placebo controlled trial. J Clin Endocrinol Metab. 2002a;87:2060–2066. doi: 10.1210/jcem.87.5.8507. [DOI] [PubMed] [Google Scholar]
- Meunier PJ, Roux C, Ortolani S, Badurski J, Kaufman JM, Spector T, et al. Strontium ranelate reduces the vertebral fracture risk in women with postmenopausal osteoporosis. Osteoporos Int. 2002b;13:521–522. [Google Scholar]
- Meunier PJ, Roux C, Seeman E, Ortolani S, Badurski JE, Spector TD, et al. The effects of strontium ranelate on the risk of vertebral fracture in women with postmenopausal osteoporosis. N Engl J Med. 2004a;350:459–468. doi: 10.1056/NEJMoa022436. [DOI] [PubMed] [Google Scholar]
- Meunier PJ, Roux C, Seeman E, Ortolani S, Badurski JE, Spector TD, et al. The effects of strontium ranelate on the risk of vertebral fracture in women with postmenopausal osteoporosis. N Engl J Med. 2004b;350:459–468. doi: 10.1056/NEJMoa022436. [DOI] [PubMed] [Google Scholar]
- Namkung-Matthai H, Seale JP, Brown K, Mason RS. Comparative effects of anti-inflammatory corticosteroids in human bone-derived osteoblast-like cells. Eur Respir J. 1998;12:1327–1333. doi: 10.1183/09031936.98.12061327. [DOI] [PubMed] [Google Scholar]
- Reginster JY, Deroisy R, Dougados M, Jupsin I, Colette J, Roux C. Prevention of early postmenopausal bone loss by strontium ranelate: the randomized, two-year, double-masked, dose-ranging, placebo-controlled PREVOS trial. Osteoporos Int. 2002;13:925–931. doi: 10.1007/s001980200129. [DOI] [PubMed] [Google Scholar]
- Reginster JY, Seeman E, De Vernejoul MC, Adami S, Compston J, Phenekos C, et al. Strontium ranelate reduces the risk of nonvertebral fractures in postmenopausal women with osteoporosis: treatment of peripheral psteoporosis (TROPOS) study. J Clin Endocrinol Metab. 2005;90:2816–2822. doi: 10.1210/jc.2004-1774. [DOI] [PubMed] [Google Scholar]
- Ruat M, Snowman AM, Hester LD, Snyder SH. Cloned and expressed rat Ca2+-sensing receptor. J Biol Chem. 1996;271:5972–5975. doi: 10.1074/jbc.271.11.5972. [DOI] [PubMed] [Google Scholar]
- Sambrook PN, Hughes DR, Nelson AE, Robinson BG, Mason RS. Osteocyte viability with glucocorticoid treatment: relation to histomorphometry. Ann Rheum Dis. 2003;62:1215–1217. doi: 10.1136/ard.2003.008839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivagurunathan S, Muir M, Brennan TC, Seale JP, Mason RS. Influence of glucocorticoids on human osteoclast generation and activity. J Bone Miner Res. 2005;20:390–398. doi: 10.1359/JBMR.041233. [DOI] [PubMed] [Google Scholar]
- Slater M, Patava J, Mason RS. Role of chondroitin sulfate glycosaminoglycans in mineralizing osteoblast-like cells: effects of hormonal manipulation. J Bone Miner Res. 1994a;9:161–169. doi: 10.1002/jbmr.5650090205. [DOI] [PubMed] [Google Scholar]
- Slater M, Patava J, Kingham K, Mason RS. Modulation of growth factor incorporation into ECM of human osteoblast-like cells in vitro by 17 beta-estradiol. Am J Physiol. 1994b;267:E990–1001. doi: 10.1152/ajpendo.1994.267.6.E990. [DOI] [PubMed] [Google Scholar]
- Tricarico C, Pinzani P, Bianchi S, Paglierani M, Distante V, Pazzagli M, et al. Quantitative real-time reverse transcription polymerase chain reaction: normalization to rRNA or single housekeeping genes is inappropriate for human tissue biopsies. Anal Biochem. 2002;309:293–300. doi: 10.1016/s0003-2697(02)00311-1. [DOI] [PubMed] [Google Scholar]
- Weinstein RS, Jilka RL, Parfitt AM, Manolagas SC. Inhibition of osteoblastogenesis and promotion of apoptosis of osteoblasts and osteocytes by glucocorticoids. Potential mechanisms of their deleterious effects on bone. J Clin Invest. 1998;102:274–282. doi: 10.1172/JCI2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu G, Chang Y, Zuo J, Dong X, Zhang M, Hu G, et al. Fundine, a C-terminal truncated rat homologue of mouse prominin, is blood glucose-regulated and can up-regulate the expression of GAPDH. Biochem Biophys Res Commun. 2001;281:951–956. doi: 10.1006/bbrc.2001.4439. [DOI] [PubMed] [Google Scholar]


