Abstract
Translational Relevance
Clear cell carcinoma (CCC) of the ovary is a distinctive subtype of epithelial ovarian cancer associated with a poorer sensitivity to platinum-based chemotherapy and a worse prognosis than the more common serous adenocarcinoma (SAC). To improve survival, the development of new treatment strategies that target CCC more effectively is necessary. Our results show that mTOR is more frequently activated in CCCs than in SACs. Our data have relevance for the design of future clinical studies of first-line treatment for patients with CCC of the ovary. Moreover, the finding of increased expression of phospho-mTOR and greater sensitivity to RAD001 in cisplatin-resistant CCC cells than in cisplatin-sensitive cells suggests a novel treatment option for patients with recurrent disease after cisplatin-based first-line chemotherapy.
Purpose
mTOR (mammalian target of rapamycin) plays a central role in cell proliferation and is regarded as a promising target in cancer therapy including for ovarian cancer. This study aims to examine the role of mTOR as a therapeutic target in clear cell carcinoma (CCC) of the ovary which is regarded as aggressive, chemo-resistant histological subtype.
Experimental Design
Using tissue microarrays of 98 primary ovarian cancers (52 clear cell carcinomas and 46 serous adenocarcinomas), the expression of phospho-mTOR was assessed by immunohistochemistry. Then, the growth-inhibitory effect of mTOR inhibition by RAD001 (everolimus) was examined using 2 pairs of cisplatin-sensitive parental (RMG1 and KOC7C) and cisplatin-resistant human CCC cell lines (RMG1-CR and KOC7C-CR) both in vitro and in vivo.
Results
Immunohistochemical analysis demonstrated mTOR was more frequently activated in CCCs than in serous adenocarcinomas (86.6% vs 50%). Treatment with RAD001 markedly inhibited the growth of both RMG1 and KOC7C cells both in vitro and in vivo. Increased expression of phospho-mTOR was observed in cisplatin-resistant RMG1-CR and KOC7C-CR cells, compared to the respective parental cells. This increased expression of phospho-mTOR in cisplatin-resistant cells was associated with increased activation of AKT. RMG1-CR and KOC7C-CR cells showed greater sensitivity to RAD001 than parental RMG1 and KOC7C cells, respectively, in vitro and in vivo.
Conclusion
mTOR is frequently activated in CCC and can be a promising therapeutic target in the management of CCC. Moreover, mTOR inhibition by RAD001 may be efficacious as a second-line treatment of recurrent disease in patients previously treated with cisplatin.
Keywords: mTOR, AKT, RAD001, cisplatin, clear cell carcinoma
Introduction
Ovarian carcinoma is the fourth most common cause of cancer death among women in the United States, with more than 21,000 new cases each year and an estimated 15,520deaths in 2008 (1). Cytoreductive surgery followed by platinum-based chemotherapy usually combined with paclitaxel is the standard initial treatment and has improved survival in patients with epithelial ovarian cancer (2). However, there still exists many clinical problems in the treatment of epithelial ovarian cancer. One of the most important problems that needs to be resolved is the management of clear cell carcinoma (CCC) of the ovary, which was first recognized by the World Health Organization as a distinct histological subtype in 1973 (3). The precise incidence of CCC is unknown, but it is reported to be 3.7~12.1% of all histological subtypes among epithelial ovarian cancer (4).
There have been two major clinical problems in the clinical management of CCC. First is its poor sensitivity to first-line platinum-based chemotherapy and the association with a worse prognosis than the more common serous adenocarcinomas (SAC). In the setting of front-line chemotherapy, the response rate to conventional platinum-based chemotherapy, platinum agent alone or in combination with cyclophosphamide and adriamycin, was reported to be only 11% in CCC. In contrast, patients with SAC had a response rate of 72% (5). The response to carboplatin-paclitaxel, a current standard regimen, was also reported to be relatively low, ranging from 22% to 56% (6-8). When analyzed by clinical stage, worse clinical outcome in patients with CCC has been more evident in advanced (stage III-IV) than in early stage disease (stage I-II). In a retrospective analysis (5), a statistically significant difference in overall survival between CCC and SAC was observed in patients with stage III disease (12.7 months vs 26.8 months, respectively; p=0.0015). However, the difference was not significant in stage I-II disease (31.8 months vs 42.3 months, respectively; p=0.2761). Similar results were reported by several groups of investigators (9-11). A more recent retrospective review of six randomized phase III clinical trials also demonstrated that patients with stage III CCC treated with carboplatin-paclitaxel had a shorter survival compared to those with other histological subtypes of epithelial ovarian cancer (12).
The second important clinical problem in the management of CCC is the lack of effective chemotherapy for recurrent CCCs after front-line treatment with platinum-based chemotherapy. A recent report demonstrated that the response rate for various regimens in the setting of second-line chemotherapy for recurrent CCC was only 1% (13). Therefore, to improve survival of patients with CCC, a better understanding of the mechanism of platinum-resistance and the identification of effective treatment strategies especially for both advanced and recurrent disease are needed.
The sensitivity of cancer cells to chemotherapeutic drug-induced apoptosis depends on the balance between pro-apoptotic and anti-apoptotic signals. Therefore, inhibition of anti-apoptotic signals, such as those mediated by the AKT pathway, has been proposed as a promising strategy to enhance the efficacy of conventional chemotherapeutic agents (14). Among the numerous AKT substrates, mTOR is thought to be one of the major targets of relevance to cancer therapy (15, 16). mTOR phosphorylates p70 S6 kinase (p70S6K) and the 4E-BP1 translational repressor, leading to translation of proteins required for cell proliferation (17). It has been reported that AKT-mTOR signaling is frequently activated in epithelial ovarian cancer (18). Recently, an orally bioavailable derivative of rapamycin, everolimus (RAD001), has been shown to inhibit the proliferation of ovarian cancer cells and enhance sensitivity to cisplatin in vitro and in vivo (19-22). However, no reports have addressed the impact of mTOR inhibitors on ovarian cancer cells that have acquired resistance after the exposure to platinum agents. Moreover, since most tumor specimens and tumor-derived cell lines used in these investigations have been ovarian SACs (19-21), the role of mTOR in CCC remains largely unknown.
It has been reported that loss of PTEN expression is common in CCC of the ovary (23). It also has been reported that ovarian endometriosis, from which CCC is thought to arise, is characterized by hyperactivation of the AKT-mTOR pathway (24). Since it is well known that loss of PTEN expression and consequent activation of AKT signaling result in hypersensitivity to mTOR inhibition (20, 25, 26), CCC may be a good candidate for therapy with a mTOR inhibitor.
In the current investigation, we examined the activation status of mTOR both in early stage and advanced stage CCC, and we determined whether RAD001 has anti-neoplastic efficacy in both in vitro and in vivo models of CCC. Moreover, we investigated the role of AKT/mTOR signaling in the acquired resistance to cisplatin in CCC cells.
Materials and methods
Reagents/Antibodies
RAD001 was obtained from Novartis Pharma AG (Basel, Switzerland). ECL Western blotting detection reagents were from Perkin Elmer (Boston, MA). Antibodies recognizing p70S6K, phospho-p70S6K (Thr389), mTOR, phospho-mTOR (Ser2448), AKT, phospho-AKT (Ser473), PARP, LC3B and β-actin were obtained from Cell Signaling Technology (Beverly, MA). The Cell Titer 96-well proliferation assay kit was obtained from Promega (Madison, WI). Cisplatin was purchased from Sigma (St. Louis, MO).
Drug Preparation
RAD001 was formulated at 2% (w/v) in a microemulsion vehicle (Novartis Pharma AG). RAD001 was prepared according to the manufacturer's protocols. Thus, for animal studies, RAD001 was diluted to the appropriate concentration in double-distilled water just before administration by gavage. For in vitro analyses, RAD001 was prepared in DMSO before addition to cell cultures.
Clinical samples
All surgical specimens were collected and archived according to protocols approved by the institutional review boards (IRBs) of the parent institutions. Appropriate informed consent was obtained from each patient. The tumors included 46 SACs and 52 CCCs. Based on criteria of the International Federation of Gynecology and Obstetrics (FIGO) criteria, 22 SACs were stage I-II tumors and 24 were stage III-IV tumors. Among CCCs, 27 were stage I-II tumors and 25 were stage III-IV tumors.
Immunohistochemistry
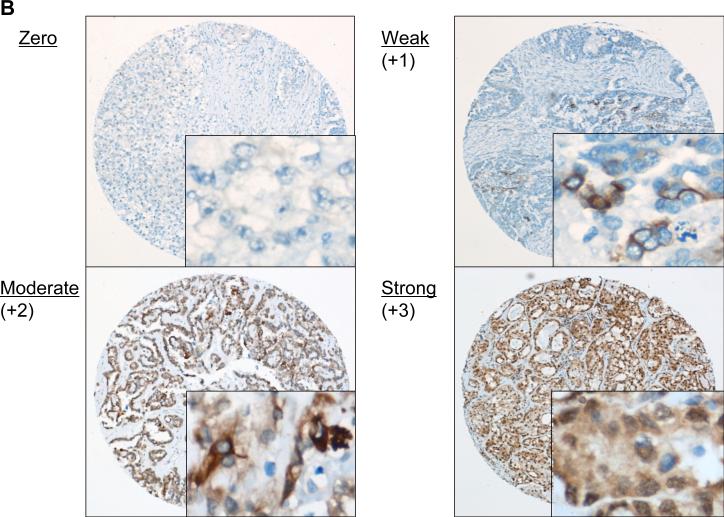
Tumor samples were fixed in 10% neutral buffered formalin (10% formaldehyde, phosphate-buffered) overnight and then embedded in paraffin. In all patients, the diagnosis was based on a light microscopy examination using conventional hematoxylin and eosin (H&E) stain. Ovarian cancer tissue microarrays consisting of two cores from each tumor sample were prepared by the Tumor Bank Facility at Fox Chase Cancer Center, as described previously (18, 19). Tissue sections were cut at 4 μm, mounted on slides, and processed for either H&E or immunohistochemical staining. For immunohistochemical studies, sections were incubated with the primary antibody, followed by the appropriate peroxidase-conjugated secondary antibody, as reported previously (19). The primary antibody used was anti-phospho-mTOR (Ser 2448) at 1:50 dilution. Negative controls were incubated with primary antibody preabsorbed with blocking peptide (Cell Signaling Technology). Surrounding non-neoplastic stroma served as an internal negative control for each slide. The slides were scored semiquantitatively by a pathologist who was blinded to the clinical outcome. A score of 0 indicated no staining, +0.5 was weak focal staining (less than 10% of the cells were stained), +1 was indicative of focal staining (10-50% of the cells were stained), +2 indicated clearly positive staining (more than 50% of the cells were stained), and a score of +3 was intensely positive, as described in detail elsewhere (18). The slides were examined under a bright field microscope. Tumors with staining of +2 or +3 were grouped as strong staining-group, whereas tumors with staining of +0.5 or +1 were grouped as a weak staining-group. When the two cores from the same tumor sample showed different positivity results, then the lower score was considered valid.
Cell Culture
Human ovarian CCC cell lines RMG1, RMG2, KOC7C, and HAC2 were kindly provided by Dr. H. Itamochi (Tottori University, Tottori, Japan). These cells were cultured in phenol red free Dulbecco's Modified Eagles Medium (DMEM Ham's F-12, Gibco Ltd, Paisley, Strathclyde, UK) with 10% FBS, as reported previously (27-29).
Establishment of cisplatin-resistant cell lines
Cisplatin-resistant sublines from RMG1 and KOC7C were developed in our laboratory by continuous exposure to cisplatin, as described previously (30). Briefly, cells of both lines were exposed to stepwise increases in cisplatin concentrations. Initial cisplatin exposure was at a concentration of 10nM. After the cells had regained their exponential growth rate, the cisplatin concentration was doubled and then the procedure was repeated until selection at 10μM was attained. The resulting cisplatin-resistant sublines, called RMG1-CR and KOC7C-CR were subcultured weekly and treated monthly with 10 μM cisplatin to maintain a high level of chemoresistance.
Cell Proliferation Assay
An MTS assay was used to analyze the effect of RAD001 on cell viability as described (31). Cells were cultured overnight in 96-well plates (1 × 104 cells/well). Cell viability was assessed after addition of RAD001 and/or cisplatin at the indicated concentrations for 48h. The number of surviving cells was assessed by determination of the A490 nm of the dissolved formazan product after addition of MTS for 1 h as described by the manufacturer (Promega, Madison, WI). Cell viability is expressed as follows: Aexp group/Acontrol × 100.
Western Blot Analysis
Cells were treated with either DMSO (vehicle) or 10 nM RAD001 for 6h. Cells were washed twice with ice-cold PBS and lysed in lysis buffer (20 mM Tris-HCl, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM Na3VO4, 1 mM β-glycerophosphate, 2.5 mM sodium pyrophosphate, 1 mM 4-(2-aminoethyl) benzenesulfonyl fluoride hydrochloride, 10 μg/ml aprotinin, 1 μg/ml leupeptin, and 1% Triton X-100) for 10 min at 4°C. Lysates were centrifuged at 12,000 × g at 4°C for 15 min, and protein concentrations of the supernatants were determined using Bio-Rad protein assay reagent. Equal amounts of proteins were separated by SDS-PAGE and transferred to nitrocellulose membranes. Blocking was done in 5% nonfat milk in 1X Tris-buffered saline. Western blot analyses were performed with various specific primary antibodies. Immunoblots were visualized with horseradish peroxidase-coupled goat anti-rabbit or anti-mouse immunoglobulin by using the enhanced chemiluminescence Western blotting system (Perkin Elmer, Boston MA).
Cell Cycle Analysis
Cells were incubated with or without 20 nM RAD001 for 2 days. After the cells were washed with PBS, they were fixed with 75% ethanol overnight at 4°C. The cells were then washed twice with PBS and stained with propidium iodide (50 μg/ml) in the presence of RNase A (100 μg/ml; Roth, Karlsruhe, Germany) for 20 min at 4°C. Cell cycle distribution was determined by analyzing 10,000 cells using a FACScan flow cytometer and Cell Quest software (Becton Dickinson, San Jose, CA)
Immunofluorescence Microscopy
Cells were incubated with or without 20 nM RAD001 for 2 days. Cells were washed with ice-cold phosphate-buffered saline (PBS), fixed in 4% paraformaldehyde in PBS for 10 min, and then blocked and incubated with anti-LC3B antibody overnight at 4C. After washing with PBS, the coverslips were incubated with FITC-conjugated secondary antibody (Alexab Fluor 488 anti-rabbit IgG, Molevular Probes, Eugene, OR) for 1 h, followed by 10 min of incubation with 4',6-diamidino-2-phenylindole (DAPI). Slides were washed with PBS, mounted with Vectashield hardest mounting medium (Vector Laboratories). Images were acquired with a fluorescence microscope and processed using Photoshop software.
Subcutaneous Xenograft Model
All procedures involving animals and their care were approved by the Institutional Animal Care and Usage Committee of Osaka University, in accordance with institutional and NIH guidelines. 5-7-week-old nude mice (n=40) were inoculated s.c. into the right flank either with 5×106 RMG1, RMG1-CR, KOC7C, or KOC7C-CR cells in 200 μl of PBS, with 10 mice in each group. When tumors reached about 50 mm3, mice were assigned into two treatment groups, with 10 mice in each group. The first group was treated with placebo twice a week. The second group was treated with RAD001 (2.5 mg/kg) twice a week. RAD001 was administered intragastrically using an animal-feeding needle. Body weight was measured weekly. Caliper measurements of the longest perpendicular tumor diameters were performed every week to estimate tumor volume using the following formula: V = L × W × D × π/6, where V is the volume, L is the length, W is the width, and D is the depth.
Statistical Analysis
Cell proliferation was analyzed by Wilcoxon exact test. Tumor volume of RAD001-treated mice was compared with that of placebo-treated mice and analyzed by Wilcoxon exact test. Immunoreactivity was analysed using Fisher's exact test. A p-value of <0.05 was considered significant.
Results
Difference in phospho-mTOR expression between CCCs and SACs
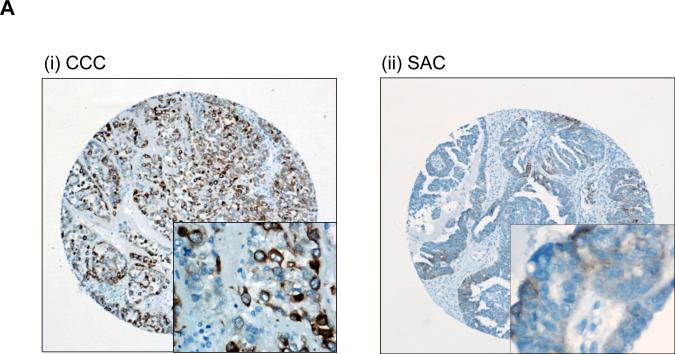
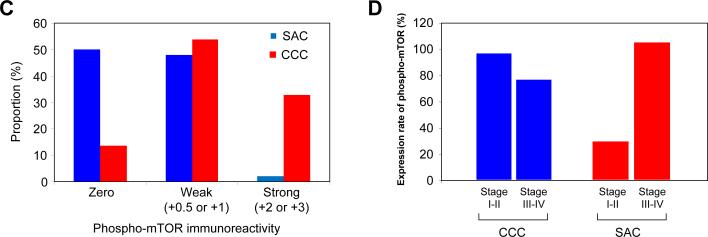
Immunohistochemical analysis of ovarian cancer tissue microarrays for phospho-mTOR expression was performed using 52 CCCs of the ovary and 46 ovarian SACs as described in “Material and Methods.” Representative photographs of CCC and SAC are shown in Fig. 1A. Phospho-mTOR immunoreactivity was scored semiquantitatively (Fig. 1B), and stronger immunoreactivity for phospho-mTOR was observed in CCCs than in SACs. Among the 46 SAC, 23 (50%) showed negative staining, 7 (15.2%) were scored as +0.5, 15 (32.6%) were +1, and 1 (2.1%) was scored as +2. In contrast, among the 52 CCCs, 7 tumors (13.4%) had negative staining. Five (9.6%) were scored as +0.5, 23 (44.2%) were +1, and 15 (28.8%) were +2, and 2 (3.8%) were scored as +3. The frequency of strong phospho-mTOR immunoreactivity was significantly higher, and frequency of tumors with no immunoreactivity was significantly lower in CCCs than in SACs (Fig. 1C). These results indicate that CCCs may be more strongly dependent on mTOR for tumor progression than SACs.
Figure 1. mTOR is frequently activated in ovarian clear cell carcinomas.
Ovarian cancer tissue microarrays were stained with anti-phospho-mTOR antibody. A, representative photographs of ovarian tissue microarray cores of (i) clear cell carcinoma (CCC), and (ii) serous adenocarcinoma (SAC). Magnifications: ×100, and ×400 (inset). B, phospho-mTOR immunoreactivity was scored semiquantitatively from zero to strong, as described in “Materials and Methods.” Representative photos of weak, moderate, and strong staining are shown. Magnifications: ×100, and ×400 (inset). C, histogram indicating immunostaining profile. Proportion indicates proportion of tumors examined. Phospho-mTOR expression was more frequent and stronger in intensity in CCCs than in SACs. D, histogram indicating the expression of phospho-mTOR according to clinical stage.
When analyzed by clinical stage, phospho-mTOR expression was observed in 76% of advanced stage CCCs and in 96% of early stage CCCs (Fig. 1D). Thus, most patients with CCC may be candidates for therapy with a mTOR inhibitor. In contrast, in SACs, phospho-mTOR expression was uncommon in early stage tumors, although it was significantly increased in advanced stage tumors. Therefore, in SACs, mTOR inhibition may be a therapeutic option only in advanced stage disease.
Collectively, these results indicate that pharmacologic inhibition of mTOR may be a promising therapeutic strategy in the management of CCCs, both in early stage and in advanced stage disease.
In vitro growth-inhibitory effect of RAD001 on cisplatin-sensitive CCC cell lines
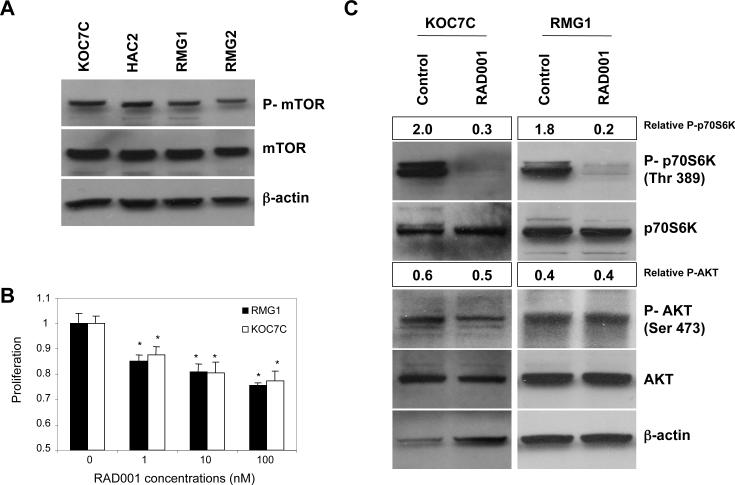
Given the frequent mTOR activation found in human CCC tumor specimens (Fig. 1), we evaluated the expression of phospho-mTOR in four human CCC cell lines by western blotting. As shown in Fig. 2A, under serum-starvation conditions, mTOR was phosphorylated in all CCC cell lines tested, which is consistent with immunohistochemical results observed with tumor samples. We next examined the efficacy of mTOR pathway inhibition by RAD001 on the proliferation of CCC cells in vitro. For this purpose, we performed a MTS assay using two of these CCC cell lines with activated AKT/mTOR signaling. As shown in Fig. 2B, RAD001 inhibited the proliferation of RMG1 and KOC7C cells in vitro, with ~25% inhibition at the highest drug concentration tested (Fig. 2B).
Figure 2. RAD001 inhibits mTOR activity and proliferation of cisplatin-sensitive CCC cells.
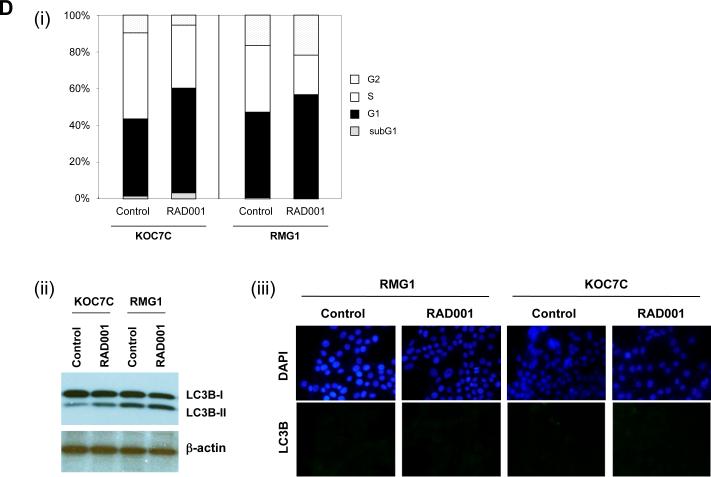
A, analysis of phospho-mTOR status in four ovarian CCC cell lines. CCC cells were serum-starved overnight, after which the expression of phospho-mTOR was determined by Western blot analysis. Actin expression was used as a loading control. B, sensitivity of CCC cells to RAD001. CCC cells were treated with the indicated concentrations of RAD001 in the presence of 5% FBS for 72 h. Cell viability was assessed by MTS assay. Points, mean; bars, SD (*, significantly different from control; p<0.01). C, RAD001 attenuates the phosphorylation of p70S6K in vitro. KOC7C and RMG1 cells were treated with 10 nM of RAD001 for 6 h in thepresence of 5% FBS. Cells were harvested, and equivalent amounts (30 μg) of protein were subjected to SDS-PAGE and blotted with anti-phospho-p70S6K (Thr389), anti-p70S6K, anti-phospho-AKT (Ser473), anti-AKT, or anti-b-actin antibodies. Blots were scanned and quantified by densitometry. The density of phospho-p70S6K or phospho-AKT was normalized to the corresponding density of bands for total p70S6K or AKT, respectively, and the results are depicted as “Relative P-AKT” or “Relative P-TOR” values. D.(i), RAD001 induced an increase in the percentage of cells in G1 phase. RMG1 and KOC7C cells were treated with or without 10 nM RAD001 for 48 h. Cell cycle analyses were performed with a fluorescence activated cell sorter (FACS)can as described in the “Materials and Methods.” (ii, iii), RAD001 does not induce autophagy. KOC7C and RMG1 cells were treated with 10 nM of RAD001 for 48 h in the presence of 5% FBS. (i) Cells were harvested, and equivalent amounts (30 μg) of protein were subjected to SDS-PAGE and blotted with anti-LC3B or anti-β-actin antibodies. (ii), Cells were fixed, stained with anti-LC3B antibodies, and viewed with a fluorescence microscope.
RAD001 attenuates phosphorylation of p70S6K in vitro
To determine if the anti-proliferative effects of RAD001 result from inhibition of mTOR signaling, we examined the effect of RAD001 on the phosphorylation of downstream p70S6K in RMG1 and KOC7C cells. As shown in Fig. 2C, AKT, mTOR and p70S6K were phosphorylated in both cell lines, indicative of the hyperactivation of the AKT/mTOR pathway. As expected, phosphorylation of the downstream effector p70S6K was significantly decreased in both cell lines by treatment with RAD001, indicating that RAD001 effectively inhibits mTOR signaling in CCC cells. Although previous studies have shown that mTOR inhibition is associated with a feedback activation of AKT which may result in resistance to mTOR inhibition (32-35), no significant increase in the phosphorylation of AKT was observed in response to RAD001 in these CCC cell lines. Rapamycin and its derivatives are generally regarded as having cytostatic effects; however, in some tumor cells, these agents have also been reported to induce apoptosis (36). To determine the mechanism by which RAD001 inhibits cell proliferation, we first examined the effect of RAD001 on cell cycle progression by flow cytometry. As shown in Fig. 2D (i), the percentage of cells in G1 phase was significantly increased in both RMG1 and KOC7C cells after 2-day treatment with 10 nM RAD001. In both cell lines, the percentage of apoptotic cells in the sub-G1 peak did not change after treatment with RAD001. Moreover, as shown in Fig. 4B, treatment with 10 nM RAD001 did not induce cleavage of PARP in these cells. We also examined whether treatment with RAD001 induces autophagic cell death in CCC cells. It has been reported that LC3B-I is converted to LC3B-II during autophagy (37). However, as shown in Fig. 2D (ii), the conversion of LC3B-I to the lower migrating form LC3B-II was not induced in response to treatment with RAD001 in RMG1 or KOC7C cells. Furthermore, as shown in Fig. 2D (iii), treatment with 10 nM of RAD001 did not induce punctate staining for LC3B, an indicator of authophagy associated with the concentration of LC3 in autophagosomalvacuoles (37). Collectively, these results suggest that RAD001 most likely affects CCC cells by inducing cell cycle arrest (17).
Figure 4. Phospho-mTOR expression and its role as a therapeutic target in cisplatin-resistant CCC cells.
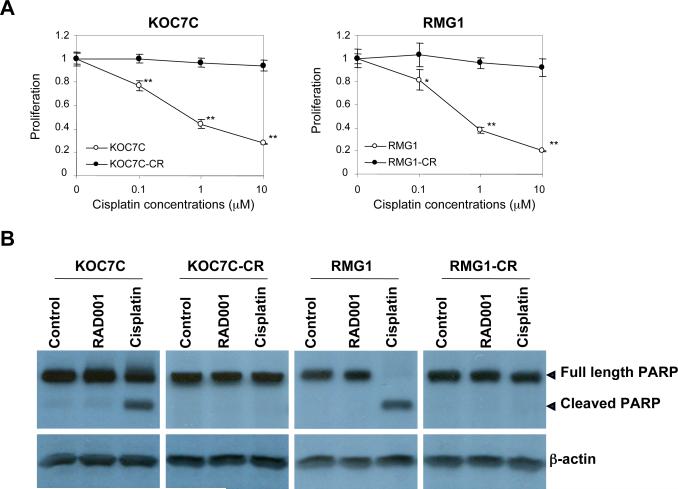
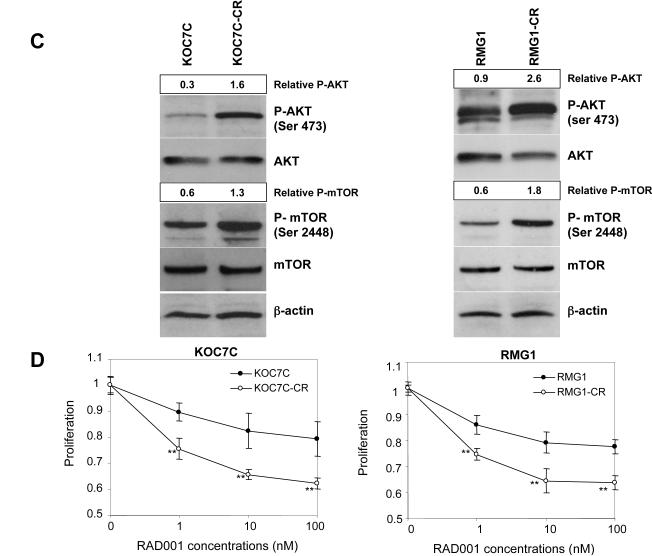
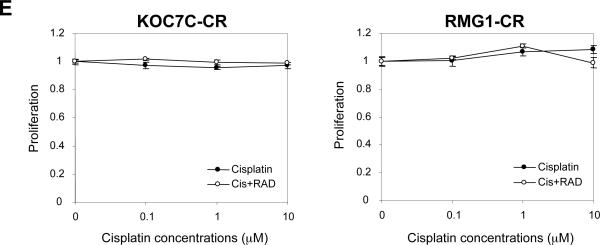
A and B, establishment of cisplatin-resistant variant cell lines. Cisplatin-resistant sublines were established as described in “Materials and Methods.” A, cisplatin-sensitive parental (KOC7C and RMG1) and cisplatin-resistant variant (KOC7C-CR and RMG1-CR) cells were treated with the indicated concentrations of cisplatin in the presence of 5% FBS for 72 h. Cell viability was assessed by MTS assay. Points, mean; bars, SD (*, p< 0.05, **, p<0.01). B, effect of cisplatin on the cleavage of PARP in cisplatin-sensitive parental and cisplatin-resistant variant cell lines. KOC7C, KOC7C-CR, RMG1 and RMG1-CR treated with 10 μM cisplatin or 10 nM RAD001 for 24 h. Cells were harvested, and then lysates were subjected to Western blotting using anti-PARP or anti-b-actin antibody. C, activation of AKT/mTOR signaling in cisplatin-sensitive parental and cisplatin-resistant variant cells in vitro. KOC7C, KOC7C-CR, RMG1 and RMG1-CR cells were serum starved overnight. Cells were harvested, and equivalent amounts (30 μg) of protein were subjected to SDS-PAGE and blotted with anti-phospho-mTOR (Ser2448), anti-mTOR, anti-phospho-AKT (Ser473), anti-AKT or anti-b-actin antibodies. AKT, phosphor-AKT and β-actin are from the same membrane and 8% gel, mTOR and phosphor-mTOR were from a separate 6% gel.Blots were scanned and quantified by densitometry. The density of phospho-AKT or phospho-mTOR bands was normalized to the corresponding density of total AKT or mTOR bands, respectively, and the results are shown as “Relative P-AKT” or “Relative P-TOR” values. D, enhanced sensitivity to RAD001 in cisplatin-resistant CCC cells in vitro. Cisplatin-sensitive parental (KOC7C and RMG1) and cisplatin-resistant variant (KOC7C-CR and RMG1-CR) cells were treated with the indicated concentrations of RAD001 in the presence of 5% FBS for 72 h. Cell viability was assessed by MTS assay. Points, mean; bars, SD (**, p<0.01). E, cisplatin-resistant variant (KOC7C-CR and RMG1-CR) cells were treated with the indicated concentrations of cisplatin in the presence or absence of 10 nM of RAD001 for 72 h. Cell viability was assessed by MTS assay.
Effect of RAD001 on the growth of ovarian CCC
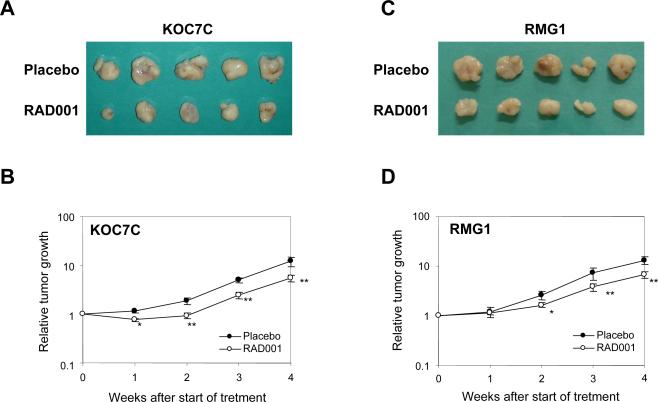
To further examine the in vivo growth-inhibitory effect of RAD001, we employed a subcutaneous (s.c.) xenograft model in which athymic mice were inoculated s.c. with RMG1 or KOC7C cells. When tumors reached ~50 mm3, the mice were randomized into two treatment groups receiving placebo or RAD001, as described in “Material and Methods.” Drug treatment was well tolerated, with no apparent toxicity throughout the study. Tumor volume was measured weekly after the start of treatments (Fig. 3B and 3D). The appearance of tumors four weeks from the first day of treatment is also shown in Fig. 3A and 3C. Histologically, these subcutaneous tumors were CCCs (data not shown). Mean RMG1-derived tumor burden in mice treated with RAD001 was 332.5 mm3 compared to 652.5 mm3 in placebo-treated mice, and mean KOC7C-derived tumor burden in animals treated with RAD001 was 276 mm3 compared to 605.5 mm3 in placebo-treated mice. Overall, treatment with RAD001 decreased RMG1 derived- and KOC7C derived-tumor burden by 49% and 55%, respectively, compared to placebo. These results indicate that RAD001 hassignificant anti-tumor effects as a single agent in CCC.
Figure 3. Effect of RAD001 on the growth of cisplatin-sensitive CCC-derived tumor cells in vivo.
Athymic nude mice were inoculated s.c. with KOC7C cells or RMG1 cells. When the tumors reached an average size of about 50 mm3, mice were treated with placebo or 2.5 mg/kg RAD001 twice a week for 4 wks. A and C, appearance of subcutaneous tumors.B and D, graphs depicting weekly tumor volumes (mm3) for each treatment group. Points, mean; bars, SD. *, p< 0.05, significantly different from placebo-treated mice. **, p< 0.01, significantly different from placebo-treated mice.
Increased mTOR activation and the sensitivity to RAD001 in cisplatin-resistant cell lines
Cisplatin-resistance is regarded as a major clinical problem in the management of CCC of the ovary (11, 38). It has been previously reported that AKT is involved in the resistance of ovarian SAC cells to cisplatin (39, 40). To examine whether AKT/mTOR signaling is involved in cisplatin-resistance in CCC, we established cisplatin-resistant sublines from RMG1 and KOC7C cells, as described in “Material and Methods.” To examine whether these sublines had acquired resistance to cisplatin, we first evaluated the sensitivity of these cell lines to cisplatin by MTS assay. As shown in Fig. 4A, clear differential sensitivity to cisplatin was observed between cisplatin-sensitive parental and respective cisplatin-resistant sublines. We next examined cisplatin-induced apoptosis in these cell lines. Treatment with cisplatin induced cleavage of PARP in parental cells, but not in cisplatin-resistant sublines (Fig. 4B). Using these cell lines, we have investigated the activity of AKT/mTOR in both cisplatin-resistant sublines and parental chemosensitive cells by western blotting. As shown in Fig. 4C, higher phospho-AKT and phospho-mTOR expression was observed in both chemoresistant cell lines compared with their respective parental cell lines. Increased activation of AKT/mTOR signaling was also observed in another cisplatin-resistant subline, HAC2-CR, which was established from parental HAC2 cells (data not shown). The increased phosphorylation of AKT and mTOR was inhibited by treatment with a PI3K inhibitor,LY294002 (data not shown).
Since it is well known that loss of PTEN expression and consequent activation of AKT result in hypersensitivity to mTOR inhibition (20, 25), we considered chemoresistant sublines to be good candidates for treatment with RAD001. Thus, we next examined the inhibitory effect of RAD001 on chemoresistant and parental chemosensitive CCC cell lines by MTS assay (Fig. 4D). A clear differential effect was demonstrated depending on the cell sensitivity to cisplatin. Cisplatin-resistant RMG1-CR and KOC7C-CR cells are significantly more sensitive to RAD001 than their respective parental cell lines RMG1 and KOC7C. We also confirmed that treatment with RAD001 effectively inhibited the phosphorylation of p70S6K in vitro, without inducing negative feedback activation of AKT (data not shown). Moreover, using RMG1-CR and KOC7C-CR cells, we next determined whether the treatment with RAD001 enhances the efficacy of cisplatin. As shown in Fig. 4E, in the presence of 10 nM of RAD001, the ability of cisplatin (0-10μM) to inhibit cell proliferation was not enhanced in these cisplatin-resistant cell lines. These results suggest that RAD001 may have efficacy as a single agent for cisplatin-resistant CCCs.
Effect of RAD001 on the cisplatin-resistant CCC in vivo
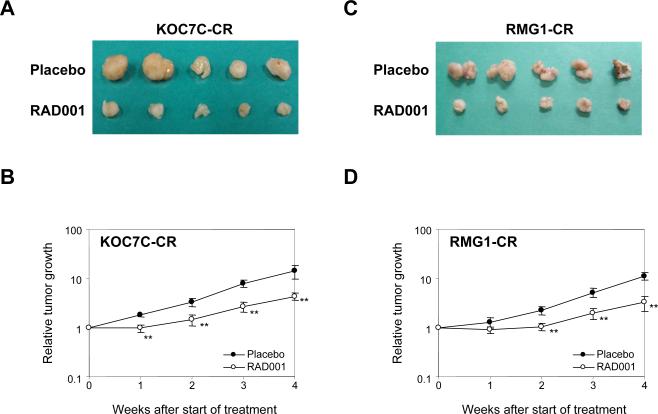
To further examine the in vivo effect of RAD001 on cisplatin-resistant sublines, athymic mice were inoculated s.c. with RMG1-CR or KOC7C-CR cells, and were randomized into two treatment groups receiving placebo or RAD001, as described in “Material and Methods. The appearance of the tumors four weeks from the first day of treatment is shown in Fig. 5A, C. Moreover, corresponding graphs depicting diminished tumor volumes for RAD001-treated mice relative to placebo-treated mice are presented in Fig. 5B, D. Mean RMG1-CR-derived tumor burden in mice treated with RAD001 was 163 mm3 compared to 553 mm3 in placebo-treated mice, and mean KOC7C-CR-derived tumor burden in animals treated with RAD001 was 218.5 mm3 compared to 710 mm3 in placebo-treated mice. Treatment with RAD001 decreased RMG1-CR-derived tumors burden by 72% compared to only 49% reduction in RMG1-derived tumors (Fig. 3C, D). Similar results were obtained in mice inoculated with KOC7C-CR cells. Treatment with RAD001 decreased KOC7C-CR derived tumor burden by 69% compared to a 55% reduction in RAD001-treated KOC7C-derived tumors (Fig. 3A, B). Collectively, these in vitro and in vivo data suggest that the anti-tumor effect of RAD001 is greater in cisplatin-resistant CCC than in cisplatin-sensitive CCC.
Figure 5. Effect of RAD001 on the growth of cisplatin-resistant CCC-derived tumors in vivo.
Athymic nude mice were inoculated s.c. with KOC7C-CR cells or RMG1-CR cells. When the tumors reached an average size of about 50 mm3, mice were treated with placebo or 2.5 mg/kg RAD001 twice a week for 4 wks. A and C, appearance of subcutaneous tumors. B and D, graphs depicting weekly tumor volumes (mm3) for each treatment group. Points, mean; bars, SD. **, p< 0.01, significantly different from placebo-treated mice.
Discussion
Despite recent developments in platinum-based combination chemotherapy, patients with CCC of the ovary, especially in advanced stage or recurrent disease, have a worse progression free survival and overall survival when compared with patients with a serous histology (5-11). Therefore, to improve survival, new strategies are necessary to more effectively treat CCC.
In the present study, we observed activation of mTOR in 86.6% of CCC of the ovary (Fig. 1). Importantly, the frequency of strong phospho-mTOR immunoreactivity in CCCs was significantly higher than that found in SACs, indicating that CCCs are more strongly dependent on mTOR signaling for tumor progression than are SACs. In addition, mTOR was frequently activated in both stage III-IV CCCs (76%) and stage I-II CCCs (96%). Therefore, mTOR appears to be a promising target for the treatment of patients with both early and advanced stage CCC. In contrast, phospho-mTOR expression was uncommon in early stage SACs but was significantly increased in advanced stage SACs. The very high frequency of mTOR activation observed in early stage CCCs suggests that hyperactivation of mTOR kinase is an early event in the development of CCCs. This is noteworthy in light of the fact that activated AKT/mTOR signaling has been reported in ovarian endometriosis, from which CCC is thought to arise (24). We have recently demonstrated that the mTOR inhibitor RAD001 markedly inhibited tumor onset and progression in a transgenic mouse model of ovarian cancer that develops ovarian SACs with activated AKT/mTOR signaling. Thus, mTOR might be a reasonable target for the chemoprevention of CCC in patients with ovarian endometriosis.
Our data demonstrate that treatment with RAD001 effectively attenuates the phosphorylation of p70S6K in vitro and markedly inhibits the proliferation of ovarian CCC cells. There exists a concern in inhibiting mTOR, in that mTOR inhibition may trigger a feedback mechanism that activates AKT to potentially promote tumor growth and may consequently reduce the anti-tumor effect of mTOR inhibitors (32-35). Although such a feedback has been observed in several cancer cell types including breast cancer (32), rhabdomyosarcoma (33), non-small cell lung cancer (34), and multiple myeloma (35), in the current study treatment with RAD001 did not induce activation of AKT in ovarian CCC cells (Fig. 2D).
We also evaluated the efficacy of RAD001 in vivo, employing s.c. xenograft models (Fig 3). In mice inoculated s.c. with RMG1 or KOC7C cells, treatment with RAD001 significantly inhibited tumor growth. Moreover, orally administrated RAD001 in our treatment schedule was well tolerated. Taken together, these findings indicate that RAD001 could have significant anti-tumor effects as a single agent for CCC in a setting of front-line therapy.
An additional important finding in our study is the anti-tumor activity of RAD001 in cisplatin-resistant CCC. In general, patients with platinum-resistant recurrent epithelial ovarian cancer have been treated with anti-neoplastic agents that do not exhibit cross-resistance with platinum-agents. However, these patients have dismal prognosis, with overall response rate ranging from 9% to 33% (41). Unfortunately, the prognosis of patients with cisplatin-resistant CCCs is even worse. For example, in one study, the response rate for salvage chemotherapy for cisplatin-resistant CCC was only 1% (13), indicative of the urgent need of new treatment strategies for recurrent CCC of the ovary.
In this study, we found that cisplatin-resistant CCC cell lines exhibit enhanced phospho-mTOR expression compared to the corresponding cisplatin-sensitive parental cell lines (Fig. 4B). The increased phospho-mTOR expression was associated with increased activation of AKT. The involvement of AKT in the resistance to cisplatin has been reported previously (39, 42). Although we and others have previously reported that inhibition of AKT activity sensitizes human ovarian cancer cells to conventional anticancer agents such as cisplatin (39) and paclitaxel (43), there are concerns associated with inhibiting AKT, because AKT also mediates certain biologically important cell processes such as glucose metabolism (44). Thus, a safer approach may be to target downstream therapeutic effectors such as mTOR. Interestingly, our cisplatin-resistant CCC cells showed significantly higher sensitivity to RAD001 in vitro, compared with the respective cisplatin-sensitive parental cell lines. Furthermore, the in vivo anti-tumor effect of RAD001 was also greater in cisplatin resistant cell-derived tumors than in cisplatin sensitive cell-derived tumors (Fig. 5). It has been previously reported that AKT activation may be a biomarker to predict the sensitivity to mTOR inhibitors (20, 25, 26). Although AKT activation is not the sole determinant of sensitivity to mTOR inhibition (26), our results indicate that enhanced sensitivity to mTOR inhibitors in cisplatin-resistant CCC cells is associated with, at least in part, the activation of AKT/mTOR signaling. Since the RMG1-CR and KOC7C-CR cells used in this study mimic the clinical situation of resistance development in cisplatin-treated patients, our results may suggest that a mTOR inhibitor might have efficacy for the clinical management of cisplatin-resistant CCCs.
We should note, however, that a potential limitation of our experimental design is the use of a subcutaneous xenograft model. Peritoneal dissemination is the main process involved in the progression in human ovarian cancer. Thus, intra-peritoneal injection of cancer cells would more accurately model advanced disease. Therefore, further investigation using an intra-peritoneal model or a genetically engineered mouse model of ovarian cancer would be helpful.
Our results indicate that RAD001 is a promising agent for the treatment of CCC of the ovary both as a front-line treatment and as a salvage treatment for recurrence after platinum-based chemotherapy. A recent phase III study demonstrated that RAD001 had significant activity in some patients with advanced renal cell carcinoma (45). For patients with recurrent ovarian cancer, the Southwest Oncology Group (SWOG) will soon initiate a randomized phase II trial of carboplatin and paclitaxel with or without everolimus in patients with ovarian cancer in first relapse. We believe that our data support the scientific justification or this and future clinical trials with RAD001 in patients with CCC of the ovary, a chemoresistant histological subtype characterized by frequent hyperactivation of mTOR pathway.
Acknowledgements
The following Fox Chase Cancer Center/Ovarian SPORE shared facilities were used in the course of this work: Tumor Bank and Histopathology. We would like to thank Dr. Min Huang and Dr. Yulan Gong in the Tumor Bank Facility for preparing tissue microarrays. We also thank Dr. Y. Nishio (Dept of OBGYN, Osaka Police Hospital), Dr. Y. Terai (Dept of OBGYN, Osaka Medical College), Dr. Y Tsubota (Dept of Pathology, Wakayama Rosai Hospital), Dr. K. Ito (Dept of OBGYN, Kansai Rosai Hospital), Dr. Y. Hoshida (Dept of Pathology, Kansai Rosai Hospital), and Dr. H. Miwa (Dept of Pathology, Sakai Municipal Hospital) for providing us with tumor specimens and clinical information.
This work was supported by National Cancer Institute Grants CA83638 (SPORE in Ovarian Cancer), CA77429 and CA06927, Grant-in-aid for Young Scientists, No 19890125 from the Ministry of Education, Culture, Sports, Science and Technology of Japan. D.A.A. is supported in part by the Liz Tilberis Scholar Program, funded by the Ovarian Cancer Research Found, Inc.
Abbreviations
- CCC
clear cell carcinoma
- SAC
serous adenocarcinoma
- mTOR
mammalian target of rapamycin
- p70S6K
p70 S6 kinase
- 4E-BP1
elF4E binding protein 1
- PI3K
phosphatidylinositol 3-kinase
- PAGE
polyacrylamide gel electrophoresis
- cisplatin
cis-diaminodichloroplatinum
- TBS
tris-buffered saline
- IHC
immunohistochemistry, MTS, 3-[4,5,dimethylthiazol-2-yl]-5-[3-carboxymethoxy-phenyl]-2-[4-sulfophenyl]-2H-tetrazolium, inner salt
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Ozols RF, Bundy BN, Greer BE, et al. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2003;21:3194–3200. doi: 10.1200/JCO.2003.02.153. [DOI] [PubMed] [Google Scholar]
- 3.Serov SF, Scully RE, Sobin LH. Histologic Typing of Ovarian Tumors. Vol. 9. World Health Organization; Geneva: 1973. International histologic classification of tumors. [Google Scholar]
- 4.Orezzoli JP, Russell AH, Oliva E, Del Carmen MG, Eichhorn J, Fuller AF. Prognostic implication of endometriosis in clear cell carcinoma of the ovary. Gynecol Oncol. 2008;110:336–344. doi: 10.1016/j.ygyno.2008.05.025. [DOI] [PubMed] [Google Scholar]
- 5.Sugiyama T, Kamura T, Kigawa J, et al. Clinical characteristics of clear cell carcinoma of the ovary: a distinct histologic type with poor prognosis and resistance to platinum-based chemotherapy. Cancer. 2000;88:2584–2589. [PubMed] [Google Scholar]
- 6.Enomoto T, Kuragaki C, Yamasaki M, et al. Is clear cell carcinoma and mucinous carcinoma of the ovary sensitive to combination chemotherapy with paclitaxel and carboplatin? Proc Am Soc Clin Oncol. 2003;22:447. (abstract 1797) [Google Scholar]
- 7.Ho CM, Huang YJ, Chen TC, et al. Pure-type clear cell carcinoma of the ovary as a distinct histological type and improved survival in patients treated with paclitaxel-platinum-based chemotherapy in pure-type advanced disease. Gynecol Oncol. 2004;94:197–203. doi: 10.1016/j.ygyno.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 8.Utsunomiya H, Akahira J, Tanno S, Moriya T, Toyoshima M, Niikura H, Ito K, Morimura Y, Watanabe Y, Yaegashi N. Paclitaxel-platinum combination chemotherapy for advanced or recurrent ovarian clear cell adenocarcinoma: a multicenter trial. Int J Gynecol Cancer. 2006;16:52–56. doi: 10.1111/j.1525-1438.2006.00289.x. [DOI] [PubMed] [Google Scholar]
- 9.Mizuno M, Kikkawa F, Shibata K, et al. Long-term follow-up and prognostic factor analysis in clear cell adenocarcinoma of the ovary. J Surg Oncol. 2006;94:138–143. doi: 10.1002/jso.20251. [DOI] [PubMed] [Google Scholar]
- 10.Takano M, Kikuchi Y, Yaegashi N, et al. Clear cell carcinoma of the ovary: a retrospective multicentre experience of 254 patients with complete surgical staging. Br J Cancer. 2006;94:1369–1374. doi: 10.1038/sj.bjc.6603116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pectasides D, Fountzilas G, Aravantinos G, et al. Advanced stage clear-cell epithelial ovarian cancer: the Hellenic Cooperative Oncology Group experience. Gynecol Oncol. 2006;102:285–291. doi: 10.1016/j.ygyno.2005.12.038. [DOI] [PubMed] [Google Scholar]
- 12.Winter WE, 3rd, Maxwell GL, Tian C, et al. Prognostic factors for stage III epithelial ovarian cancer: a Gynecologic Oncology Group Study. J Clin Oncol. 2007;25:3621–3627. doi: 10.1200/JCO.2006.10.2517. [DOI] [PubMed] [Google Scholar]
- 13.Crotzer DR, Sun CC, Coleman RL, Wolf JK, Levenback CF, Gershenson DM. Lack of effective systemic therapy for recurrent clear cell carcinoma of the ovary. Gynecol Oncol. 2007;105:404–408. doi: 10.1016/j.ygyno.2006.12.024. [DOI] [PubMed] [Google Scholar]
- 14.Vivanco I, Sawyers CL. The phosphatidylinositol 3-kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 15.Majumder PK, Febbo PG, Bikoff R, et al. mTOR inhibition reverses Akt-dependent prostate intraepithelial neoplasia through regulation of apoptotic and HIF-1-dependent pathways. Nat Med. 2004;10:594–601. doi: 10.1038/nm1052. [DOI] [PubMed] [Google Scholar]
- 16.Boulay A, Zumstein-Mecker S, Stephan C, et al. Antitumor efficacy of intermittent treatment schedules with the rapamycin derivative RAD001 correlates with prolonged inactivation of ribosomal protein S6 kinase 1 in peripheral blood mononuclear cells. Cancer Res. 2004;64:252–261. doi: 10.1158/0008-5472.can-3554-2. [DOI] [PubMed] [Google Scholar]
- 17.Schmelzle T, Hall MN. TOR, a central controller of cell growth. Cell. 2000;103:253–262. doi: 10.1016/s0092-8674(00)00117-3. [DOI] [PubMed] [Google Scholar]
- 18.Altomare DA, Wang HQ, Skele KL, et al. AKT and mTOR phosphorylation is frequently detected in ovarian cancer and can be targeted to disrupt ovarian tumor cell growth. Oncogene. 2004;23:5853–5857. doi: 10.1038/sj.onc.1207721. [DOI] [PubMed] [Google Scholar]
- 19.Mabuchi S, Altomare DA, Connolly DC, et al. RAD001 (Everolimus) delays tumor onset and progression in a transgenic mouse model of ovarian cancer. Cancer Res. 2007;67:2408–2413. doi: 10.1158/0008-5472.CAN-06-4490. [DOI] [PubMed] [Google Scholar]
- 20.Mabuchi S, Altomare DA, Cheung M, et al. RAD001 inhibits human ovarian cancer cell proliferation, enhances cisplatin-induced apoptosis, and prolongs survival in an ovarian cancer model. Clin Cancer Res. 2007;13:4261–4270. doi: 10.1158/1078-0432.CCR-06-2770. [DOI] [PubMed] [Google Scholar]
- 21.Treeck O, Wackwitz B, Haus U, Ortmann O. Effects of a combined treatment with mTOR inhibitor RAD001 and tamoxifen in vitro on growth and apoptosis of human cancer cells. Gynecol Oncol. 2006;102:292–299. doi: 10.1016/j.ygyno.2005.12.019. [DOI] [PubMed] [Google Scholar]
- 22.Beuvink I, Boulay A, Fumagalli S, et al. The mTOR inhibitor RAD001 sensitizes tumor cells to DNA damaged induce apoptosis through inhibition of p21 translation. Cell. 2005;120:747–59. doi: 10.1016/j.cell.2004.12.040. [DOI] [PubMed] [Google Scholar]
- 23.Hashiguchi Y, Tsuda H, Inoue T, Berkowitz RS, Mok SC. PTEN expression in clear cell adenocarcinoma of the ovary. Gynecol Oncol. 2006;101:71–75. doi: 10.1016/j.ygyno.2005.09.047. [DOI] [PubMed] [Google Scholar]
- 24.Yagyu T, Tsuji Y, Haruta S, et al. Activation of mammalian target of rapamycin in postmenopausal ovarian endometriosis. Int J Gynecol Cancer. 2006;16:1545–1551. doi: 10.1111/j.1525-1438.2006.00625.x. [DOI] [PubMed] [Google Scholar]
- 25.Neshat MS, Mellinghoff IK, Tran C, et al. Enhanced sensitivity of PTEN-deficient tumors to inhibition of FRAP/mTOR. Proc Natl Acad Sci U S A. 2001;98:10031–10033. doi: 10.1073/pnas.171076798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Noh WC, Mondesire WH, Peng J, et al. Determinants of rapamycin sensitivity in breast cancer cells. Clin Cancer Res. 2004;10:1013–1023. doi: 10.1158/1078-0432.ccr-03-0043. [DOI] [PubMed] [Google Scholar]
- 27.Fujimura M, Hidaka T, Saito S. Selective inhibition of the epidermal growth factor receptor by ZD1839 decreases the growth and invasion of ovarian clear cell adenocarcinoma cells. Clin Cancer Res. 2002;8:2448–2454. [PubMed] [Google Scholar]
- 28.Suzuki N, Aoki D, Oie S, et al. A novel retinoid, 4-[3,5-bis (trimethylsilyl) benzamido] benzoic acid (TAC-101), induces apoptosis of human ovarian carcinoma cells and shows potential as a new antitumor agent for clear cell adenocarcinoma. Gynecol Oncol. 2004;94:643–649. doi: 10.1016/j.ygyno.2004.06.026. [DOI] [PubMed] [Google Scholar]
- 29.Takemoto Y, Yano H, Momosaki S, et al. Antiproliferative effects of interferon-alphaCon1 on ovarian clear cell adenocarcinoma in vitro and in vivo. Clin Cancer Res. 2004;10:7418–7426. doi: 10.1158/1078-0432.CCR-04-0279. [DOI] [PubMed] [Google Scholar]
- 30.Behrens BC, Hamilton TC, Masuda H, et al. Characterization of a cis-diamminedichloroplatinum(II)-resistant human ovarian cancer cell line and its use in evaluation of platinum analogues. Cancer Res. 1987;47:414–418. [PubMed] [Google Scholar]
- 31.Mabuchi S, Ohmichi M, Nishio Y, et al. Inhibition of NF kappaB increases the efficacy of cisplatin in in vitro and in vivo ovarian cancer models. J Biol Chem. 2004;279:23477–85. doi: 10.1074/jbc.M313709200. [DOI] [PubMed] [Google Scholar]
- 32.O'Reilly KE, Rojo F, She QB, et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 2006;66:1500–1508. doi: 10.1158/0008-5472.CAN-05-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wan X, Harkavy B, Shen N, Grohar P, Helman LJ. Rapamycin induces feedback activation of Akt signaling through an IGF-1R-dependent mechanism. Oncogene. 2007;26:1932–1940. doi: 10.1038/sj.onc.1209990. [DOI] [PubMed] [Google Scholar]
- 34.Sun SY, Rosenberg LM, Wang X, et al. Activation of Akt and eIF4E survival pathways by rapamycin-mediated mammalian target of rapamycin inhibition. Cancer Res. 2005;65:7052–7058. doi: 10.1158/0008-5472.CAN-05-0917. [DOI] [PubMed] [Google Scholar]
- 35.Shi Y, Yan H, Frost P, Gera J, Lichtenstein A. Mammalian target of rapamycin inhibitors activate the AKT kinase in multiple myeloma cells by up-regulating the insulin-like growth factor receptor/insulinreceptor substrate-1/phosphatidylinositol 3-kinase cascade. Mol Cancer Ther. 2005;4:1533–1540. doi: 10.1158/1535-7163.MCT-05-0068. [DOI] [PubMed] [Google Scholar]
- 36.Huang S, Shu L, Dilling MB, et al. Sustained activation of the JNK cascade and rapamycin-induced apoptosis are suppressed by p53/p21(Cip1) Mol Cell. 2003;11:1491–501. doi: 10.1016/s1097-2765(03)00180-1. [DOI] [PubMed] [Google Scholar]
- 37.Aoki H, Kondo Y, Aldape K, Yamamoto A, Iwado E, Yokoyama T, Hollingsworth EF, Kobayashi R, Hess K, Shinojima N, Shingu T, Tamada Y, Zhang L, Conrad C, Bogler O, Mills G, Sawaya R, Kondo S. Monitoring autophagy in glioblastoma with antibody against isoform B of human microtubule-associated protein 1 light chain 3. Autophagy. 2008;4:467–475. doi: 10.4161/auto.5668. [DOI] [PubMed] [Google Scholar]
- 38.Takano M, Kikuchi Y, Yaegashi N, et al. Clear cell carcinoma of the ovary: a retrospective multicentre experience of 254 patients with complete surgical staging. Br J Cancer. 2006;94:1369–1374. doi: 10.1038/sj.bjc.6603116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ohta T, Ohmichi M, Hayasaka T, et al. Inhibition of phosphatidylinositol 3-kinase increases efficacy of cisplatin in in vivo ovarian cancer models. Endocrinology. 2006;147:1761–9. doi: 10.1210/en.2005-1450. [DOI] [PubMed] [Google Scholar]
- 40.Asselin E, Mills GB, Tsang BK. XIAP regulates Akt activity and caspase-3-dependent cleavage during cisplatin-induced apoptosis in human ovarian epithelial cancer cells. Cancer Res. 2001;61:1862–1868. [PubMed] [Google Scholar]
- 41.Leitao MM, Jr, Hummer A, Dizon DS, et al. Platinum retreatment of platinum-resistant ovarian cancer after nonplatinum therapy. Gynecol Oncol. 2003;91:123–129. doi: 10.1016/s0090-8258(03)00464-5. [DOI] [PubMed] [Google Scholar]
- 42.Hayakawa J, Ohmichi M, Kurachi H, et al. Inhibition of BAD phosphorylation either at serine 112 via extracellular signal-regulated protein kinase cascade or at serine 136 via Akt cascade sensitizes human ovarian cancer cells to cisplatin. Cancer Res. 2000;60:5988–5994. [PubMed] [Google Scholar]
- 43.Mabuchi S, Ohmichi M, Kimura A, et al. Inhibition of phosphorylation of BAD and Raf-1 by Akt sensitizes human ovarian cancer cells to paclitaxel. J Biol Chem. 2002;277:33490–33500. doi: 10.1074/jbc.M204042200. [DOI] [PubMed] [Google Scholar]
- 44.Ueki K, Yamamoto-Honda R, Kaburagi Y, et al. Potential role of protein kinase B in insulin-induced glucose transport, glycogen synthesis, and protein synthesis. J Biol Chem. 1998;273:5315–5322. doi: 10.1074/jbc.273.9.5315. [DOI] [PubMed] [Google Scholar]
- 45.Motzer RJ, Escudier B, Oudard S, et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008;372:449–456. doi: 10.1016/S0140-6736(08)61039-9. [DOI] [PubMed] [Google Scholar]