Abstract
Objective
This study examines the association of hormone use and lung cancer among women.
Methods
This is a prospective study of 2,861 women aged 31 to 79 years from the Rancho Bernardo cohort. After enrollment in 1972 to 1974, women were followed up for 31 years for morbidity and mortality. Incident lung cancer was based on self-report or death certificates. Diagnosis was validated from the California Cancer Registry for cases that occurred after 1988. Cox proportional hazard models were used to estimate the adjusted association of hormone use and lung cancer.
Results
During the 31-year follow-up, 87 women developed lung cancer. There was no association between hormone use and lung cancer (hazard ratio, 1.13; 95% CI, 0.73-1.73). Stratification by age 55 years (proxy for menopause status) showed divergent results. In women 55 years older, lung cancer risk was 1.58 (95% CI, 0.95-2.53), whereas in women younger than 55 years, lung cancer risk was 0.44 (95% CI, 0.16-1.23). The confidence intervals for both groups contained the null value.
Conclusions
Although not statistically significant, our results from a long follow-up suggest that postmenopausal women on hormone therapy may have an increased risk of lung cancer, whereas younger women do not.
Keywords: Hormones, Lung Cancer, Menopause
Lung cancer is the second most common cancer in women and the leading cause of cancer deaths.1 Other than tobacco smoking, few risk factors are known for lung cancer among women. Although 84% of male lung cancer cases worldwide can be attributed to smoking, it is estimated that only 46% of cases in women can be attributed to the same exposure.2 Thus, identifying additional lung cancer causes for women is warranted.
Women use supplemental hormones to prevent pregnancy or replace the natural hormones that decline during menopause. Approximately 24% of women older man 40 years are current hormone users in the United States.3 Although hormone use has decreased in recent decades, many European and American physicians remain confident that the benefits of hormone therapy outweigh the risks in suitable patients.4
It has been suggested that female sex hormones can regulate tumor development by stimulating or inhibiting cell proliferation.5 Previous studies examined the association of these hormones with specific cancers, including breast cancer,6-9 colorectal cancer,8, 10 and endometrial cancer8, 11, 12 However, existing studies of hormone use and lung cancer8, 13-23 report inconsistent results. Two studies reported an increased risk of lung cancer in those taking hormones13, 21 whereas another three studies reported a decreased risk.17, 20, 23
Cigarette smoking is the major known risk factor of lung cancer.24 Therefore, detailed smoking information is needed for lung cancer studies. Lung cancer latency is estimated to be 18 to 30 years from the time of exposure,25-27 underscoring the need for a follow-up period of appropriate length. The purpose of this study is to examine the potential effects of supplemental female hormones on incident lung cancer in a population-based sample of women followed for 31 years, while controlling for smoking and other confounders.
Methods
Study population
Between 1972 and 1974, 82% (n = 6,339) of adult residents of Rancho Bernardo, a middle-class community in Southern California, enrolled in a heart disease risk factor study the Lipid Research Clinics Prevalence Study. Participants received annual surveys to ascertain vital status (99% obtained). The 2,861 women aged 31 to 79 years at enrollment for whom follow-up information on incident lung cancer was available formed the basis of this report.
Baseline
At enrollment, participants had a standardized interview, which included questions about cigarette smoking, with response choices of “never smoked,” “quit smoking >2 years ago,” “quit smoking <2 years ago,” “smokes <1 cigarette a day.” “smokes 1-10 cigarettes a day,” “smokes 11-20 cigarettes a day,” “smokes 21-40 cigarettes a day,” and “smokes >40 cigarettes per day.” Women were also asked “are you taking oral contraceptives, estrogens or pills for hot flashes or to regulate periods?” Nine women were excluded because they did not answer this question. Self-reported hormone use was validated by an examination of pills and prescriptions brought to the clinic at a second visit usually within a month of baseline. Other information collected during this interview included date of birth, education level and marital status. Height and weight were measured with the participant wearing light clothing and no shoes to calculate body mass index (BMI; weight in kilograms per height in meters squared), as an estimate of obesity.
All individuals gave written informed consent before their participation. This study was approved by the Human Research Protections Program at the University of California San Diego.
Follow-up
Contact with participants included subsequent clinic visits, generally occurring approximately every 4 years, and annual mailers to determine vital status (>99% ascertainment rate) through the end of 2002. An individual was considered to have incident lung cancer if it was self-reported (n = 9) at any of the research clinic visits or annual mailers or if lung cancer appeared anywhere on the death certificate (n = 78). Death certificates were obtained and coded for cause of death by a certified nosologist according to the International Classification of Diseases, Ninth Revision, Clinical Modification. All lung cancer deaths that occurred after 1988 (when the California Cancer Registry began collecting data) were confirmed by the California Cancer Registry. All women with lung cancer who had a history of breast cancer (94% validation from cancer registry) were excluded from analysis, providing reasonable evidence against any lung cancer cases being secondary metastasis to a primary breast cancer.
Statistical analysis
For continuous variables, mean values were compared using t tests. For categorical variables, frequencies were compared using a χ2 test. Cox proportional hazards models were used to calculate crude and adjusted hazard ratios (HRs) of hormone use with incident lung cancer, after adjustment for confounders. These confounders were used in previous studies and included age, BMI, education, and smoking. Age and BMI were continuous variables; education level was categorized as high school graduate or less, some college, and college graduate or more. Smoking status was collapsed into five categories from the original eight levels (never, former, 1-10 cigarettes/d, 11-20 cigarettes/d, and 21 or more cigarettes/d). Current marital status was categorized as currently married and not currently married (which included never married, divorced, and widowed).
As a proxy for menopause status (unavailable from enrollment data), women were stratified by age (<55 and >55 y), and the survival analysis was repeated separately for each age group. Although this information was not collected directly, it is assumed that premenopausal women were taking hormones for birth control and postmenopausal women were doing so for hormone therapy.
Survival was measured in days from the date of the participant's initial visit until the date of lung cancer outcome (date of death if participant died; date of diagnosis other-wise). death from other causes, or the end of the follow-up period, whichever occurred first. The follow-up period for survival analysis concluded at the end of the 2002 calendar year. All analyses were two-tailed, with P ≤ 0.05 considered significant. Statistical analyses were performed using the SAS statistical package.
Results
The 2,861 participants were aged 31 to 79 years at baseline, with 35.8% reporting current use of hormones at enrollment As shown in Table 1, hormone users were similar in age to nonusers (mean ± SD, 58.5 ± 10.8 y vs 58.9 ± 14.4 y; P = 037) but had a significantly lower BMI (23.1 ± 2.9 kg/m2 vs 24.0 ± 3.8 kg/m2; P < 0.0001), had more education (P = 0.03), and were more likely to be married (P = 0.003) than those not taking hormones. Hormone users were also more likely to be a current or former smoker titan those who were not taking hormones (56.3% vs 48.5%; P= 0.001).
Table 1. Characteristics of women who are using hormones versus nonusers in a population living in Rancho Bernardo, California, from 1972 to 1974 (N = 2,861).
| No hormone use (n = 1,837) |
Current hormone use (n = 1,024) |
||
|---|---|---|---|
| Mean (SD) | Pa | ||
| Age, y | 58.9 (14.4) | 58.5 (10.8) | 0.37 |
| BMI, kg/m2 | 24.0 (3.8) | 23.1 (2.9) | <0.0001 |
| Frequency (%) | |||
| Education | 0.033 | ||
| College graduate or more | 449 (24.5) | 289 (28.4) | |
| Some college | 620 (33.8) | 347 (34.1) | |
| High school graduate or less | 764 (41.7) | 381 (37.5) | |
| Smoking category | 0.001 | ||
| Never smoked | 943 (51.5) | 447 (43.7) | |
| Former smoker | 435 (23.7) | 276 (27.0) | |
| 1-10 cigarettes/d | 159 (8.7) | 90 (8.8) | |
| 11-20 cigarettes/d | 179 (9.8) | 123 (12.0) | |
| 21 or more cigarettes/d | 116 (6.3) | 86 (8.4) | |
| Marital status | 0.003 | ||
| Not marriedb | 341 (18.6) | 146 (14.3) | |
| Married | 1496 (81.4) | 878 (85.7) | |
BMI, body mass index.
P value for t test for means and χ2 test for categories.
”Not married“ includes those who have never been married or are separated, divorced, or widowed.
During the 31-year follow-up (average of 22.4 y), there were 87 incident cases of lung cancer. Women who developed lung cancer were significantly older than those without lung cancer (mean ± SD, 62.7 ± 10.3 y vs 58.7 ± 13.3 y, respectively; P = 0.0055) but did not differ with regard to baseline BMI, education, or marital status.
Table 2 shows the univariate and adjusted Cox proportional hazards for lung cancer incidence. Hormone use was positively but not significantly related to lung cancer in the multivariate survival analysis (HR, 1.13; 95% CI, 0.73-1.73). Each 1-year increase in age raised the risk of lung cancer by 1.08 (95% CI, 1.06-1.11) after controlling for the other variables in the model. The risk of lung cancer increased with each escalating category of smoking status in comparison to the reference group of “never smoked” (Ptrend for smoking <0.000l). There were 17 lung cancer cases among those women who had never smoked, and the odds of lung cancer for hormone users among ”never smokers“ was 0.75 (95% CI, 0.23-2.38). BMI, education, and marital status were not significantly associated with lung cancer risk in the adjusted model.
Table 2. Univariate and adjusted Cox proportional hazards for incident lung cancer in women living in Rancho Bernardo, California, from 1972 to 2002 (N = 2,861).
| Univariate hazards | Adjusted hazardsa | |||||
|---|---|---|---|---|---|---|
| Variable | Hazard ratio | 95% CI | Hazard ratio | 95% CI | ||
| Hormone status (yes vs no) | 1.23 | 0.80-1.87 | 1.13 | 0.73-1.73 | ||
| Age | 1.05 | 1.03-1.08 | 1.08 | 1.06-1.11 | ||
| BMI | 0.96 | 0.90-1.03 | 0.96 | 0.90-1.03 | ||
| Education category | ||||||
| College graduate or moreb | 1.0 | 1.0 | ||||
| Some college | 1.58 | 0.90-2.77 | 1.51 | 0.86-2.67 | ||
| High school graduate or less | 1.17 | 0.66-2.10 | 1.11 | 0 62-1.99 | ||
| Marital status (married vs other) | 0.47 | 0.29-0.76 | 0.66 | 0.40-1.10 | ||
| Smoking status | ||||||
| Never smokedb | 1.0 | 1.0 | ||||
| Former smoker | 1.98 | 1.01-3.88 | 2.17 | 1.10-4.26 | ||
| 1-10 cigarettes/d | 2.77 | 1.19-6.42 | 3.67 | 1.57-8.58 | ||
| 11-20 cigarettes/d | 5.37 | 2.81-10.27 | 7.69 | 3.94-15.00 | ||
| 21 or more cigarettes/d | 9.69 | 5.23-17.95 | 16.22 | 8.47-31.06 | ||
| Pc | <0.0001 | <0.0001 | ||||
BMI, body mass index.
Adjusted for all other variables in the table.
Reference category.
P trend for smoking status.
Figure 1 shows adjusted HRs for incident lung cancer after stratification by age as a proxy for menopause status (<55 and ≥55 y). Compared with the overall survival analysis in Table 2, there was a divergence of risk between the two age groups. In younger women, hormone users had a reduced lung cancer risk compared with nonusers (HR, 0.44; 95% CI, 0.16-1.23). In contrast, older women who were taking hormones at baseline had an increased lung cancer risk compared with nonusers (HR, 1.58; 95% CI, 0.95-2.53). Although the association was in opposite directions, the CI contained the null value in both groups. The P value for interaction by “age 55” (proxy for menopause status) was not significant at 0.27.
Fig 1.
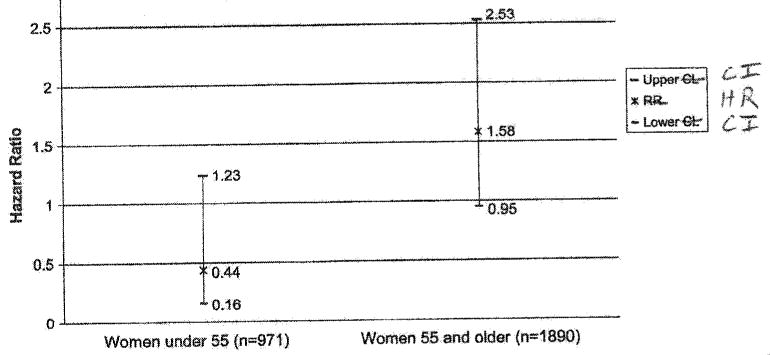
Cox proportional hazards for incident lung cancer in women living in Rancho Bernardo, California, from 1972 to 2002 (N = 2861), stratified by age (>55 and <55 y) as a proxy for menopause status. Adjusted for age, body mass index, education, marital status, and smoking status; P value for interaction = 0.27. HR, Hazard ratio; CI, confidence interval.
Discussion
The present study found no overall association between the use of female hormones and lung cancer. However, the data do suggest that diverging trends may exist whereby premenopausal women taking hormones may have a reduced risk of lung cancer, whereas older postmenopausal women taking hormones may be at increased risk of lung cancer. Although it did not reach statistical significance, both of these associations were independent of the stepwise increase in lung cancer associated with smoking. It is not possible to determine if this divergence is due to age, menopause status, different hormone doses by menopause status, or chance.
The overall null results are similar to those from a much larger but shorter prospective study of estrogen use in Swedish women13 (23,244 women with 6.4 y of follow-up; odds ratio [OR], 1.3; 95% CI, 0.9-1.7). Although the authors report an increased risk of lung cancer, the CI in that study was similar to that in our study and included the null value. The large Women's Health Initiative randomized controlled clinical trial of postmenopausal hormone therapy also found no association between postmenopausal hormone therapy and lung cancer (HR, 1.04; 95% CI, 0.71-1.53), but this trial only had an average of 5.2 years of follow-up per person.8 One case-control study found that women with lung cancer were more likely than controls to be hormone users21 (OR, 1.7; 95% CI, 1.0-2.8). Several other retrospective or prospective studies with 8 to 16.4 (average) years of follow-up found no hormone-lung cancer association.14-16,18,19,22
A recent prospective study of the association between hormone therapy and lung cancer from the Cancer Prevention Study Π Nutrition Cohort23 (72,772 women; 659 lung cancer cases) used an analysis that stratified the entire population by hormone therapy use and smoking status and found a decreased lung cancer risk associated with hormone therapy for postmenopausal women. That study had 12 years of follow-up for lung cancer incidence compared with 31 years in our study. Protection against lung cancer for ever-use of female hormones was also suggested by a case control study in the United States20 with 499 lung cancer cases and 519 controls (OR, 0.66; 95% CI, 0.51-0.89) and a German study with 811 lung cancer cases and 912 controls (OR, 0.69; 95% CI, 0.57-0.92) that examined users of hormone therapy.17 In the latter study that included women of any age, the association became nonsignificant after controlling for duration of hormone use.
Several limitations of the present study should be considered. With 87 incident cases of lung cancer during the 31 -year follow up, this study may not be sufficiently powered to detect significant risk differences if they truly exist. Women were asked about their menopause status at follow-up visits but not at the enrollment visit from which this study draws the study population. Therefore, age was used as a proxy for menopause status. Among Rancho Bernardo women, the average age at natural menopause using data from the two later visits (1984-1987 and 1988-1991) was 49 years, similar to the average age of spontaneous menopause (51.4 y) reported by The North American Menopause Society.28 We intentionally chose a higher age (55 y) as a proxy for menopause to introduce a conservative bias and more confident results in the older group. Consequently, misclassification of menopause status may be higher in the younger group. Women were not asked about duration of hormone use, and this lack of information detailing the exposure of interest might be expected to attenuate the association toward nullity. Because most women begin hormone replacement at the time of menopause, the use was probably much longer in the oldest women, possibly explaining the unfavorable hormone-lung cancer association only in postmenopausal women. Although we do not have information on duration of smoking and duration of hormone use from the initial 1972 visit, this information was collected at a later visit in 1984. We found that hormone duration was similar in cases (9.7 ± 12.8 y) and noncases (10.0 ± 11.6 y) from the 1984 data. More detailed smoking data from the 1984 visit showed that the distribution of smoking duration among hormone users and nonusers in 1984 was comparable to the distribution of cigarettes per day (hormone users were slightly heavier smokers and with longer duration of smoking), both of which were similar to the distribution of cigarettes per day among hormone users and nonusers in the original 1972 data. If we assume that this similarity between smoking duration and cigarettes per day among hormone users and nonusers was consistent from 1972, residual uncontrolled confounding by smoking duration may be negligible but cannot be completely ruled out. It was not possible to assess the influence of these variables on lung cancer risk from the later visit due to small number of cases. The Rancho Bernardo cohort is predominantly white, well educated, and middle-class and has good access to health care. This should have reduced confounding by social class, access to mammography or treatment, and environmental tobacco smoke. These results are likely to be generalizable to other typical white suburbs of the 1970s.
There are several strengths to this study. It is prospective with a sample of 2,861 women who were followed up for 31 years, much longer than any other reported prospective studies on this topic. Loss to follow-up was very low with 99% ascertainment of vital status. Furthermore, our analysis adjusted for smoking exposure level in a more quantitative manner than other studies that simply describe never/former/current smokers.
Our study results did not show an overall association between hormone use and lung cancer but suggests a potential divergence of effects in younger versus older women. The possible role of postmenopausal hormones in lung cancer incidence observed here may further help women and their physicians make informed decisions on therapy. Future studies with a more accurate quantification of hormone use and smoking could lead to a more precise characterization of the role hormone use plays on lung cancer incidence.
Acknowledgments
Funding/support: This study was supported by grants AG007181 and AG028507 from the National Institutes of Health/National Institute on Aging and by grant DK31801 from the National Institute of Diabetes and Digestive and Kidney Diseases.
Footnotes
Financial disclosure/conflicts of interest: None reported.
References
- 1.U.S. Cancer Statistics Working Group. United States Cancer Statistics; 2002 Incidence and Mortality. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute; 2005. [Google Scholar]
- 2.Parkin DM, Sasco AJ. Lung cancer: worldwide variation in occurrence and proportion attributable to tobacco use. Lung Cancer. 1993;9:1–16. [Google Scholar]
- 3.Brett KM, Reuben CA. Prevalence of estrogen or estrogen-progestin hormone therapy use. Obstet Gynecol. 2003;102:1240–1249. doi: 10.1016/j.obstetgynecol.2003.09.024. [DOI] [PubMed] [Google Scholar]
- 4.Birkhauser MH, Reinecke I. Current trends in hormone replacement therapy: perceptions and usage. Climacteric. 2008;11:192–200. doi: 10.1080/13697130802060455. [DOI] [PubMed] [Google Scholar]
- 5.Genazzani AR, Gadducci A, Gambacciani M. Controversial issues in climacteric medicine II. Hormone replacement therapy and cancer. Maturitas. 2001;40:117–130. doi: 10.1016/s0378-5122(01)00282-1. [DOI] [PubMed] [Google Scholar]
- 6.Coombs NJ, Taylor R, Wilcken N, Fiorica J, Boyages J. Hormone replacement therapy and breast cancer risk in California. Breast J. 2005;11:410–415. doi: 10.1111/j.1075-122X.2005.00132.x. [DOI] [PubMed] [Google Scholar]
- 7.Lund E, Bakken K, Dumeaux V, Andersen V, Kumle M. Hormone replacement therapy and breast cancer in former users of oral contraceptives—The Norwegian Women and Cancer study. Int J Cancer. 2007;121:645–648. doi: 10.1002/ijc.22699. [DOI] [PubMed] [Google Scholar]
- 8.Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 9.Shah NR, Wong T. Current breast cancer risks of hormone replacement therapy in postmenopausal women. Expert Opin Pharmacother. 2006;7:2455–2463. doi: 10.1517/14656566.7.18.2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.La Vecchia C, Gallus S, Fernandez E. Hormone replacement therapy and colorectal cancer: an update. J Br Menopause Soc. 2005;11:166–172. doi: 10.1258/136218005775544264. [DOI] [PubMed] [Google Scholar]
- 11.Beral V, Bull D, Reeves G. Endometrial cancer and hormone replacement therapy in the Million Women Study. Lancet. 2005;365:1543–1551. doi: 10.1016/S0140-6736(05)66455-0. [DOI] [PubMed] [Google Scholar]
- 12.Strom BL, Schinnar R, Weber AL, et al. Case-control study of postmenopausal hormone replacement therapy and endometrial cancer. Am J Epidemiol. 2006;164:775–786. doi: 10.1093/aje/kwj316. [DOI] [PubMed] [Google Scholar]
- 13.Adami HO, Persson I, Hoover R, Schairer C, Bergkvist L. Risk of cancer in women receiving hormone replacement therapy. Int J Cancer. 1989;44:833–839. doi: 10.1002/ijc.2910440515. [DOI] [PubMed] [Google Scholar]
- 14.Blackman JA, Coogan PF, Rosenberg L, et al. Estrogen replacement therapy and risk of lung cancer. Pharmacoepidemiol Drug Saf. 2002;11:561–567. doi: 10.1002/pds.733. [DOI] [PubMed] [Google Scholar]
- 15.Elliott AM, Hannaford PC. Use of exogenous hormones by women and lung cancer: evidence from the Royal College of General Practitioners' Oral Contraception Study. Contraception. 2006;73:331–335. doi: 10.1016/j.contraception.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 16.Kabat GC, Miller AB, Rohan TE. Reproductive and hormonal factors and risk of lung cancer in women: a prospective cohort study. Int J Cancer. 2007;120:2214–2220. doi: 10.1002/ijc.22543. [DOI] [PubMed] [Google Scholar]
- 17.Kreuzer M, Gerken M, Heinrich J, Kreienbrock L, Wichmann HE. Hormonal factors and risk of lung cancer among women? Int J Epidemiol. 2003;32:263–271. doi: 10.1093/ije/dyg064. [DOI] [PubMed] [Google Scholar]
- 18.Liu Y, Inoue M, Sobue T, Tsugane S. Reproductive factors, hormone use and the risk of lung cancer among middle-aged never-smoking Japanese women: a large-scale population-based cohort study. Int J Cancer. 2005;117:662–666. doi: 10.1002/ijc.21229. [DOI] [PubMed] [Google Scholar]
- 19.Olsson H, Bladstrom A, Ingvar C. Are smoking-associated cancers prevented or postponed in women using hormone replacement therapy? Obstet Gynecol. 2003;102:565–570. doi: 10.1016/s0029-7844(03)00564-7. [DOI] [PubMed] [Google Scholar]
- 20.Schabath MB, Wu X, Vassilopoulou-Sellin R, Vaporciyan AA, Spitz MR. Hormone replacement therapy and lung cancer risk: a case-control analysis. Clin Cancer Res. 2004;10:113–123. doi: 10.1158/1078-0432.ccr-0911-3. [DOI] [PubMed] [Google Scholar]
- 21.Taioli E, Wynder EL. Re: Endocrine factors and adenocarcinoma of the lung in women. J Natl Cancer Inst. 1994;86:869–870. doi: 10.1093/jnci/86.11.869. [DOI] [PubMed] [Google Scholar]
- 22.Wu AH, Yu MC, Thomas DC, Pike MC, Henderson BE. Personal and family history of lung disease as risk factors for adenocarcinoma of the lung. Cancer Res. 1988;48:7279–7284. [PubMed] [Google Scholar]
- 23.Rodriguez C, Spencer Feigelson H, Deka A, et al. Postmenopausal hormone therapy and lung cancer risk in the cancer prevention study II nutrition cohort. Cancer Epidemiol Biomarkers Prev. 2008;17:655–660. doi: 10.1158/1055-9965.EPI-07-2683. [DOI] [PubMed] [Google Scholar]
- 24.Fontana RS. Smoking and lung cancer: a review. Bull Schweiz Akad Med Wiss. 1979;35:25–31. [PubMed] [Google Scholar]
- 25.Archer VE, Coons T, Saccomanno G, Hong DY. Latency and the lung cancer epidemic among United States uranium miners. Health Phys. 2004;87:480–489. doi: 10.1097/01.hp.0000133216.72557.ab. [DOI] [PubMed] [Google Scholar]
- 26.Finkelstein MM. Use of “time windows” to investigate lung cancer latency intervals at an Ontario steel plant. Am J Ind Med. 1991;19:229–235. doi: 10.1002/ajim.4700190210. [DOI] [PubMed] [Google Scholar]
- 27.Hu JF, Liu YY, Yu YK. Estimation of latency period of lung cancer. Zhonghua Zhang Liu Za Zhi. 1994;16:18–21. [PubMed] [Google Scholar]
- 28. [March 1, 2007];Overview of Menopause and Aging. Available at: http://www.menopause.org/Portals/0/Content/PDF/A.pdf.


