Abstract
Background
Impaired olfactory function leads to a decrease in the quality of life for many patients. Surgical treatment options are limited, especially for those suffering from hyposmia or anosmia following post-traumatic injury to the olfactory nerves. Stem cells located in the olfactory epithelium have the capacity to grow new neurons, making the olfactory epithelium an ideal candidate for restorative tissue grafting.
Objective
To determine if strips of olfactory epithelium survive transplantation directly to the olfactory bulb.
Methods
Transgenic mice, expressing a green fluorescent protein (GFP), were used to obtain the donor graft tissue. Strips of olfactory epithelium from GFP donor mice were transplanted directly to sites in the olfactory bulb and cerebral cortex (control sites) of wild type mice. Graft survival rates at 30 days were determined for transplant sites in the olfactory bulb and cerebral cortex.
Results
Strips of olfactory epithelium from transgenic mice survived transplantation to the olfactory bulb and continued to express the GFP marker protein. The 30 day survival rate in the olfactory bulb (83%, 5 out of 6 grafts) was the same as in the cerebral cortex (10 out of 12 grafts). The morphology of the graft revealed characteristics found in normal olfactory epithelium
Conclusion
We demonstrated that strips of olfactory epithelium can be successfully grafted to both the olfactory bulb and cerebral cortex. Grafts of the olfactory epithelium, if strategically positioned on the ventral surface of the bulb and given access to the nasal cavity, could provide the basis for new surgical treatments to restore olfactory function.
Keywords: Olfactory epithelium, Grafts, Anosmia, Hyposmia, Olfactory bulb, GFP, Stem cells, Olfactory impairment, Surgical treatment
INTRODUCTION
Olfactory dysfunction often results from neural damage caused by etiologies such as head injury, upper respiratory infection and sinonasal disease. Olfactory function plays an important role in day to day activities and if impaired can alter the quality of life 1 or put patients at risk for experiencing life threatening events 2. Clinicians currently have few treatment options to offer patients with impaired olfactory function. Spontaneous recovery rates from causes such as head trauma and upper respiratory tract infections remain low 3,4. A new approach for repairing damage throughout the central nervous system has been the transplantation of olfactory cells harvested from the olfactory mucosa. Cells located within the basal layers of the olfactory epithelium (OE) are considered to be an important source of multi potential stem cells 5. Preliminary studies suggest that olfactory transplants may be helpful in the treatment of spinal cord injury 6, facial nerve injury 7 and Parkinson’s disease 8.
We previously reported that cells from the olfactory epithelium can be successfully transplanted into different regions of cerebral cortex 9. The use of OE transplants for the repair of olfactory nerves requires further investigation. The restoration of connections between sensory cells in the OE and the olfactory bulb would be required for recovery of olfactory function. This study investigates the success rates for transplantation of strips of olfactory epithelium grafted directly onto the olfactory bulb (OB) and represents an important step towards the long term goal of developing new surgical methods to restore sensory function following olfactory nerve injury.
MATERIALS AND METHODS
Transplantation Procedure
Strips of olfactory epithelium were obtained from the nasal cavities of transgenic mice (C57Bl/6-Tg(UBC-GFP)30Scha/J) in which all tissues express GFP, a green fluorescent protein (stock number 004353, The Jackson Laboratory, Bar Harbor, ME,). Mice 1–4 weeks in age were euthanized, the olfactory epithelium was excised from the nasal septum and then cut into small strips approximately 1 mm in length. Epithelial strips were immediately transplanted to the brains of recipient mice.
Prior to surgery recipient mice, adult C57BL/6 wild-type (stock number 00664, The Jackson Laboratory, Bar Harbor, ME), were anesthetized with sodium pentobarbital (60 mg/kg, intraperitoneal). The surface of the skull was surgically exposed, and then a dental drill was used to remove small sections of bone over the left olfactory bulb (OB) and the left and right somatosensory area of cerebral cortex (CC). Using a number 11 scalpel blade inserted diagonally, tissue flaps were made at the surface of the OB and CC to retain the transplant tissue. An ultra thin Dumont jeweler’s forceps (World Precision Instruments, Sarasota, FL) was used to insert the olfactory epithelium diagonally into the brain directly under the tissue flap (Fig. 1A). To prevent immediate extrusion of the transplant tissue, a small blunt-tipped glass rod was applied to the surface of the brain to hold the flap in place for about 15 seconds following the withdrawal of the tissue insertion forceps. Next the bone was replaced covering the transplant site and the skin margins over the skull were reapproximated with 5-0 sutures. Recipient mice were allowed to recover under a heat lamp until the anesthetic was fully metabolized and then were returned to their home cage. In this study donor tissue was transplanted in two different locations: the OB and CC. Based on our previous studies we predicted a relatively high success rate for the CC transplants. For the CC transplants (controls) we used sites in both the left (LCC) and right (RCC) cerebral cortex (2 CC transplants per mouse), however for the OB transplants we only used the left bulb (1 OB per mouse). We limited the OB transplants to the left olfactory bulb (LOB) to avoid the possibility of causing bilateral disruption of olfactory function. A 30 day recovery time point was used to determine the survival rates and morphological characteristics for OB and CC transplants in 6 recipient mice (M1-M6). All surgical procedures and animal care protocols used in this study were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of Virginia Commonwealth University.
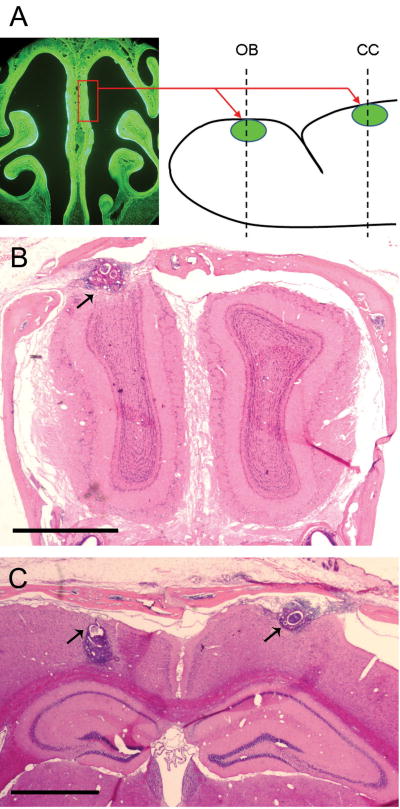
Figure 1.
Diagram illustrating the method used for transplanting olfactory tissue grafts (A). Strips of olfactory epithelium harvested from the nasal septum (rectangle) of mice expressing a green fluorescent protein (GFP) were transplanted to sites in the olfactory bulb (OB) and cerebral cortex (CC) of wild type mice. H&E stained coronal sections through the OB (B) and CC (C) show the size and location of the transplants (arrows) after a survival period of 30 days. Tissue section shown in B is from mouse M1, C is from M4. Scale Bars = 1mm.
Tissue Processing
Recipient mice were deeply anesthetized with sodium pentobarbital (90 mg/kg intraperitoneal) and perfuse with 4% paraformaldehyde in phosphate buffer preceded by a saline rinse through an intracardiac route. The heads were then removed and postfixed by immersion under vacuum for 1 hour. Then the skin was removed and the skull placed in a decalcification solution of 0.3M ethylenediamine tetraacetic acid at pH 7.4 for 7 days. The tissue was cryoprotected with a 30% sucrose solution for 2 to 3 days, immersed in optimal cutting temperature (OCT) embedding compound (Tissue-Tek, Sakura Finetek, Torrance, CA) for 1 hour and then snap-frozen in liquid nitrogen. Coronal sections (10um) were cut using a cryostat at a temperature of −20 degrees C. Examination of brain sections using a fluorescence microscopy was employed to confirm the presence of GFP positive cells and differentiate the transplant cells from surrounding OB and CC brain tissue. A successful transplant was defined as the retention of GFP positive donor cells within the OB or CC. After GFP observations, Hematoxylin and Eosin (H&E) staining was performed on the histological sections to examine the morphological characteristics of the transplant tissue. All histological sections were examined using a Nikon Eclipse E600 microscope (Nikon, Tokyo, Japan) fitted with a fluorescent lamp. Fluorescent GFP positive cells were visualized using a B-2E/C filter, and photographic images were obtained using a high-resolution digital camera (DXM 1200, Nikon).
RESULTS
The location of OE grafts was first confirmed in both the OB and CC by detecting GFP positive cells in unstained histological sections (Fig 2A&B). Subsequent H&E staining revealed a characteristic morphological rearrangement of the OE strips after transplantation (Fig 2, C&D). Most of the OE grafts consisted of epithelial cell layers, of varying thickness, that surrounded small cavities or spaces often filled with cellular debris. The OE grafts were typically found just below or near the surface of the brain. Table 1 gives the 30 day survival data for OE graft retention in both the OB and CC.
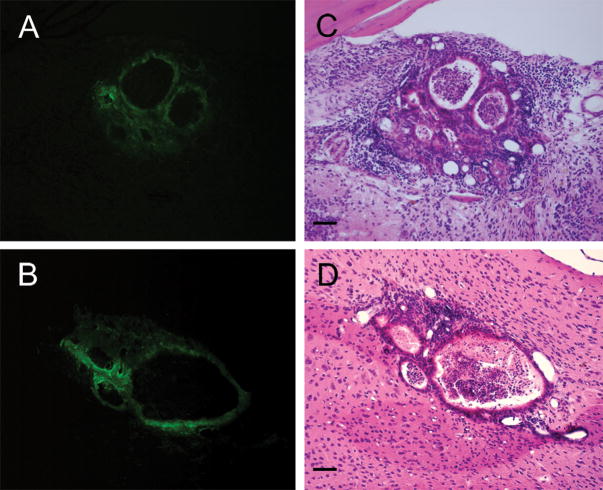
Figure 2.
(A, B) Examples of fluorescent photomicrographs used to confirm the location and identify GFP positive graft tissue in unstained sections of the OB (A) and CC (B) after 30 days survival. Subsequent H&E staining (C, D) of the same sections show in A and B reveal the morphological characteristics of surviving transplants including the formation of multiple vesicles surrounded by olfactory epithelial cells observed in both the OB (C) and CC (D) sites. Tissue sections shown in A&C are from M5, in B&C from M3. Scale bars = 50um.
Table 1.
30 Day Survival of OE grafts
| LOCATION OF GRAFT |
|||
|---|---|---|---|
| (Mouse #) | LOB | LCC | RCC |
| M1 | Yes | No | Yes |
| M2 | Yes | Yes | Yes |
| M3 | Yes | Yes | Yes |
| M4 | No | Yes | Yes |
| M5 | Yes | Yes | Yes |
| M6 | Yes | No | Yes |
| Survival | |||
| Ratio | 5/6 | 4/6 | 6/6 |
| Percent | 83% | 67% | 100% |
LOB, Left Olfactory Bulb; LCC, Left Cerebral Cortex; RCC Right Cerebral Cortex
Only 1 of the 6 OB grafts was rejected and did not survive transplantation. A low rejection rate, 2 out of 12, was also observed for the CC graft sites. The graft survival rate (5 out of 6, 83%) for sites in the OB was no different than that observed for control sites in the cerebral cortex (10 out of 12, 83%). There results confirm the feasibility of grafting olfactory epithelium directly to the olfactory bulb. At 30 days the OE grafts showed morphological characteristics similar to that found in normal olfactory epithelium. One of the OB grafts (M5) is shown in three figures (Fig. 1B, 2A&C and 4A-D) at different magnification levels. The remaining 4 successful OB grafts are shown in Figure 3. In all OB grafts, layers of epithelial cells were seen surrounding small cavities within the graft site. At high magnification (Figures 3 and 4) ciliated cells could be seen at the surface of the epithelium. The epithelium consisted of several layers of cells including elongated cells that are typically seen adjacent to a the basement membrane in histological sections of normal olfactory epithelium (Figure 4, C&D). In addition, cells that appear to have dendritic processes similar to those seen extending from olfactory sensory neurons were observed in both H&E and OMP stained sections (see Figure 4, E&D). This degree of epithelial organization observed within the graft tissue demonstrates the unique potential of OE grafts for use in surgical repair and restoration.
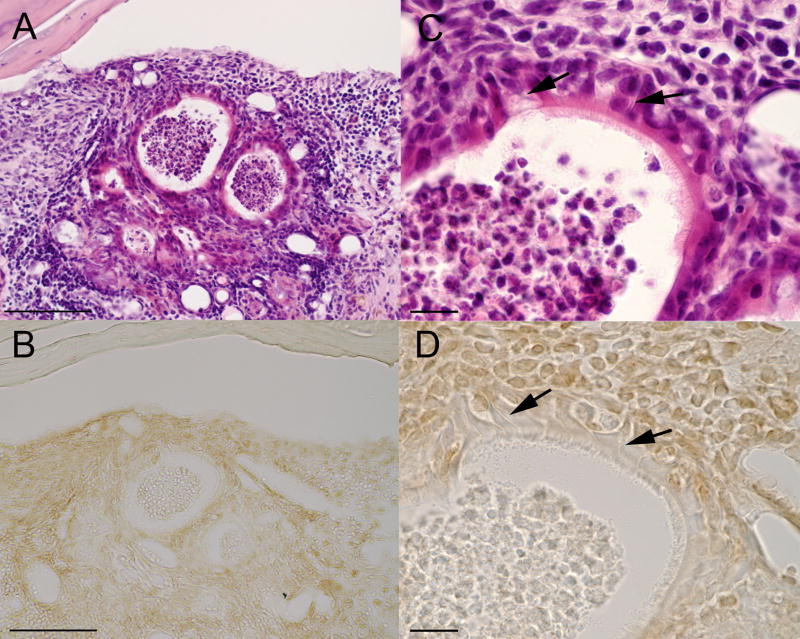
Figure 4.
Histological sections from OB graft site showing the location and morphology of ciliated and neuronal cell types similar to that found in the normal olfactory epithelium. All sections are from the same OB graft site for M5. (A&C) low and high magnification of the same H&E section (also shown in Figures 1B and 2A&B). (B&D) Section cut from same tissue block and immunostained for the Olfactory Marker Protein (OMP). At high magnification (C&D) ciliated epithelial cells can been seen along the surface of the epithelium. Cells with elongated processes (arrows) are similar in morphology to the bipolar olfactory neurons with dendrites extending to the epithelial surface. Scale bar = 100um (A&B), 15um (C&D).
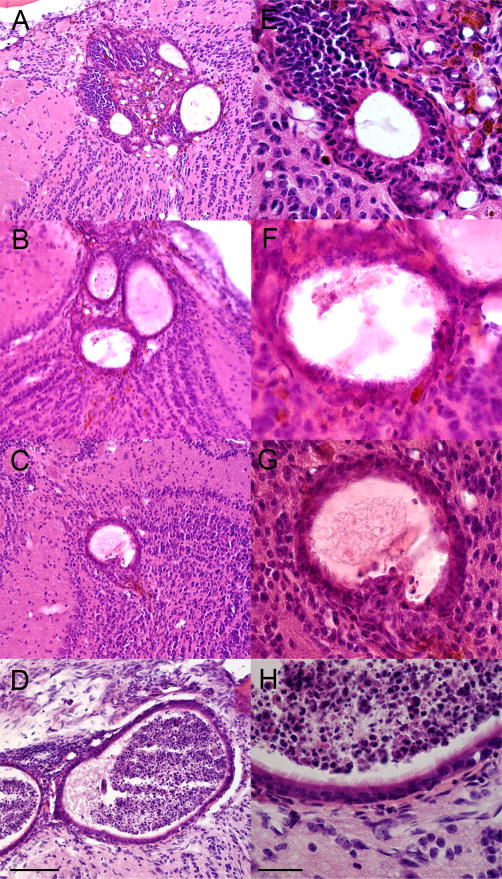
Figure 3.
H&E stained sections showing the morphology characteristics of OB grafts at 30 days. At low magnification (A–D) the formation of multiple vesicles can be observed. At higher magnification (E–H) a multilayered epithelium surrounds the vesicles and ciliated cells can be seen at the surface. Sections shown in A&E are from M1; B&F, M2; C&G, M3; D&H, M6. The OB graft for M5 is shown in Figures 2&4. Scale bar =100um (A–D), 30um (E–H).
DISCUSSION
This study demonstrates that OE grafts can be successfully transplanted to both the OB and CC. Using donor tissue harvested from GFP mice we were able to identify the transplanted cells and differentiate them from other cells that may have migrated into the graft site. In previous transplant studies it was necessary to use histological staining methods in fixed tissue to verify the presence and survival of donor tissue. The success of this GFP model provides a new tool for future transplant studies making it possible to visualize and locate OE grafts as well as assess the functional properties of the grafts in living tissue. It has been reported that GFP does not affect the integrity of the olfactory epithelium 10. Endogenous GFP fluorescence can also be maintained in cultured epithelial tissue. Skin grafts using GFP labeled cells have been shown to survive in mice for up to six months 11.
In our study, OE grafts formed multiple vesicles or cavities lined with epithelial cells similar to that reported in other transplant study 12,13,14. It is likely that the formation of these cavities is an intrinsic property of the olfactory epithelium. At 30 days the number of epithelium cell layers in the transplants varied. Cells maturity may be dependant on the survival time, including the extent of cilia outgrowth from the surface of the epithelium as well as the growth of neuronal dendritic processes. There were no major differences detected in the morphology between transplants in the OB and CC at the 30 day survival time point.
In one of the OB grafts (Figure 4, M5) we observed cells in the epithelium that looked like olfactory sensory neurons with dendritic processes extending to the surface. Attempts to label cells with antibodies to the olfactory marker protein were inconclusive due to high levels of background staining. Limited tissue sections prevented additional testing with other molecular cell markers. However, based on the characteristic morphology of these cells it is likely that they are olfactory sensory neurons (Figure 4, arrows). In a previous study 9 we found OMP positive neurons present in 34% of grafts that were transplanted into the cerebral cortex. However, in that study graft recovery times were much longer, up to 120 days. In that study OMP positive neurons were confirmed at 60 and 100 days after transplantation. Longer recovery times may be required in future studies of OB grafts to obtain a higher yield of mature cells and confirm the presence of OMP positive neurons. Having demonstrated that the olfactory epithelium can be successfully transplanted to the olfactory bulb, new experiments are being planned to assess the ability of graft cells to respond to electrical current pulses as well as odor stimulation.
Findings from this and future studies may lead to a new therapeutic approach for the treatment of olfactory dysfunction. The restoration of olfactory function requires that there be functional connections between the olfactory epithelium and the olfactory bulb. Olfactory tissue grafts may provide an effective method for restoring connections in cases of post traumatic olfactory nerve injury. Obtaining tissue grafts from the olfactory epithelium is technically feasible. Future studies are needed to determine the properties of OE graft at longer survival times to verify if neural cell types are present and if axon outgrowth from sensory neurons in the donor epithelium can establish functional synapses with cells in the olfactory bulb.
CONCLUSION
GFP labeled grafts were successfully transplanted to the olfactory bulb in mice with a survival rate of 83%. The application of GFP labeled graft tissue provides a new experimental model for evaluating grafting methods. The results of this study have important implications for the development of new treatment strategies to restore olfactory function. Grafts of the olfactory epithelium, if strategically positioned on the ventral surface of the bulb and given access to the nasal cavity, could provide the basis for new surgical treatments to restore olfactory function.
Acknowledgments
This study was supported in part by a grant from the Richmond Eye and Ear Healthcare Alliance.
References
- 1.Miwa T, Furukawa M, Tsukatani T, et al. Impact of olfactory impairment on quality of life and disability. Arch Otolaryngol Head Neck Surg. 2001;127:497–503. doi: 10.1001/archotol.127.5.497. [DOI] [PubMed] [Google Scholar]
- 2.Santos DV, Reiter ER, DiNardo LJ, et al. Hazardous events associated with impaired olfactory function. Arch Otolaryngol Head Neck Surg. 2004;130:317–319. doi: 10.1001/archotol.130.3.317. [DOI] [PubMed] [Google Scholar]
- 3.Reden J, Mueller A, Mueller C, et al. Recovery of olfactory function following closed head injury or infections of the upper respiratory tract. Arch Otolaryngol Head Neck Surg. 2006;132:265–269. doi: 10.1001/archotol.132.3.265. [DOI] [PubMed] [Google Scholar]
- 4.Doty RL. Office procedures for quantitative assessment of olfactory function. Am J Rhinol. 2007;21:460–473. doi: 10.2500/ajr.2007.21.3043. [DOI] [PubMed] [Google Scholar]
- 5.Goldstein BJ, Fang H, Youngentob SL, et al. Transplantation of multipotent progenitors from the adult olfactory epithelium. Neuroreport. 1998;9:1611–1617. doi: 10.1097/00001756-199805110-00065. [DOI] [PubMed] [Google Scholar]
- 6.Iwatsuki K, Yoshimine T, Kishima H, et al. Transplantation of olfactory mucosa following spinal cord injury promotes recovery in rats. Neuroreport. 2008;19:1249–1252. doi: 10.1097/WNR.0b013e328305b70b. [DOI] [PubMed] [Google Scholar]
- 7.Guntinas-Lichius O, Wewetzer K, Tomov TL, et al. Transplantation of olfactory mucosa minimizes axonal branching and promotes the recovery of vibrissae motor performance after facial nerve repair in rats. J Neurosci. 2002;22:7121–7131. doi: 10.1523/JNEUROSCI.22-16-07121.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murrell W, Wetzig A, Donnellan M, et al. Olfactory mucosa is a potential source for autologous stem cell therapy for Parkinson's disease. Stem Cells. 2008;26:2183–2192. doi: 10.1634/stemcells.2008-0074. [DOI] [PubMed] [Google Scholar]
- 9.Holbrook EH, DiNardo LJ, Costanzo RM. Olfactory epithelium grafts in the cerebral cortex: an immunohistochemical analysis. Laryngoscope. 2001;111:1964–1969. doi: 10.1097/00005537-200111000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Othman MM, Klueber KM, Roisen FJ. Identification and culture of olfactory neural progenitors from GFP mice. Biotech Histochem. 2003;78:57–70. doi: 10.1080/10520290310001593801. [DOI] [PubMed] [Google Scholar]
- 11.Matsuo S, Kurisaki A, Sugino H, et al. Analysis of skin graft survival using green fluorescent protein transgenic mice. J Med Invest. 2007;54:267–275. doi: 10.2152/jmi.54.267. [DOI] [PubMed] [Google Scholar]
- 12.Monti Graziadei AG, Morrison EE. Experimental studies on the olfactory marker protein. IV. Olfactory marker protein in the olfactory neurons transplanted within the brain. Brain Res. 1988;455:401–406. doi: 10.1016/0006-8993(88)90103-5. [DOI] [PubMed] [Google Scholar]
- 13.Monti Graziadei AG, Graziadei PP. Experimental studies on the olfactory marker protein. V. Olfactory marker protein in the olfactory neurons transplanted within the olfactory bulb. Brain Res. 1989;484:157–167. doi: 10.1016/0006-8993(89)90358-2. [DOI] [PubMed] [Google Scholar]
- 14.Morrison EE, Graziadei PP. Transplants of olfactory mucosa in the rat brain I. A light microscopic study of transplant organization. Brain Res. 1983;279:241–245. doi: 10.1016/0006-8993(83)90184-1. [DOI] [PubMed] [Google Scholar]