Abstract
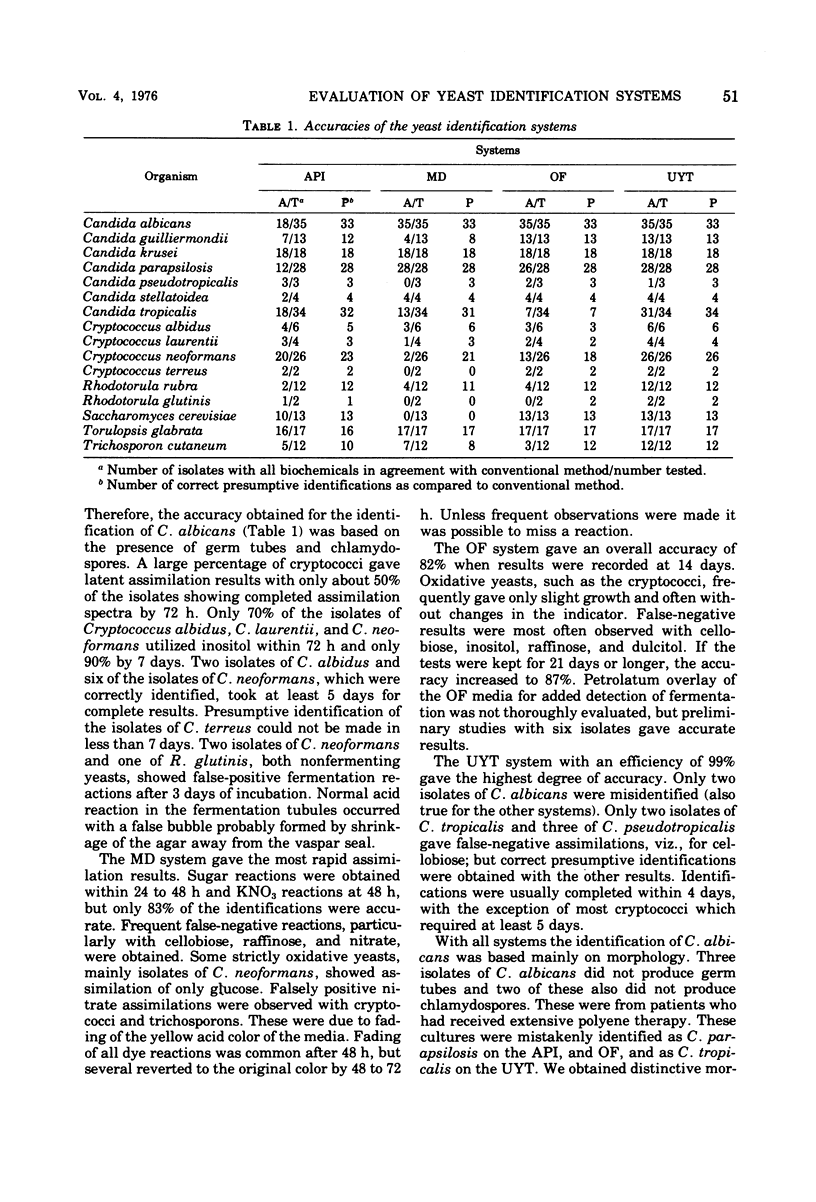
The Analytab Products Inc. (API), Micro-Drop (MD), and Uni-Yeast-Tek (UYT) systems for the presumptive identification of common clinical yeast isolates were compared with the oxidation-fermentation (OF) and a conventional procedure. With 229 coded isolates, the identification accuracies were API 94, MD 83, OF 82, and UYT 99%. The API system required the greatest technical ability. The MD materials were prone to malfunction. OF media, if incubated beyond 14 days, gave an accuracy of 87%, but this offered no advantage over the conventional procedure. The UYT system was the easiest to use.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams E. D., Jr, Cooper B. H. Evaluation of a modified Wickerham medium for identifying medically important yeasts. Am J Med Technol. 1974 Sep;40(9):377–388. [PubMed] [Google Scholar]
- Bowman P. I., Ahearn D. G. Evaluation of the Uni-Yeast-Tek kit for the identification of medically important yeasts. J Clin Microbiol. 1975 Oct;2(4):354–358. doi: 10.1128/jcm.2.4.354-358.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]



