Abstract
The investigation of methamphetamine exposure during neonatal development in rats has demonstrated that long-term spatial learning deficits are induced. A previous dose–response study showed that administration of 5 mg/kg methamphetamine, four times daily from postnatal days 11 to 20 produced these deficits, although the effects were not as severe as at higher doses of 10 or 15 mg/kg. This study examined concentrations of methamphetamine at or below 5 mg/kg given over the same period of time. Five different concentrations of methamphetamine (i.e., 5, 2.5, 1.25, 0.625, or 0) were administered every 2 h four times daily from postnatal days 11 to 20. Body weights, zero maze performance, and Morris water maze learning were examined. A dose-dependent decrease in body weight was observed during the period of methamphetamine administration and these lower weights continued throughout adulthood for the 5, 2.5, and 1.25 mg/kg concentrations, although the adult decreases were negligible. No differences were noted in the zero maze. In the Morris water maze during the acquisition period, dose-dependent differences in spatial orientation were seen, however non-dose related deficits were observed for other parameters. During the shifted platform phase (“reversal”), a similar dose-dependent difference in spatial orientation was observed, although no other effects were noted during this phase. Females performed worse than males regardless of treatment or the phase of learning in the Morris water maze. These data suggest that even lower doses of methamphetamine can alter learning and memory in adulthood, although with less consistent results than with doses higher than 5 mg/kg/dose. These data would caution against even casual use of methamphetamine by women during pregnancy since even low doses could alter the ability of the child to learn.
Keywords: Dose response, Learning and memory, Morris water maze, Sex differences, Straight channel, Substituted amphetamines, Swimming, Zero maze
1. Introduction
The effects of substituted amphetamines during pregnancy have not been well characterized in humans even though the use of some of these drugs, for example, methamphetamine (MA), increased dramatically during the 1990s (Johnston et al., 2002a,b). It has been suggested that in humans long-term cognitive deficits may result following exposure to amphetamine in utero (Cernerud et al., 1996), although no retrospective studies exist for MA. There have been several reports of short-term effects of in utero MA exposure in humans and these include: decreased body weights at birth, smaller head circumferences, increased morbidity, and reduced performance in a visual recognition test during the first year of life (Dixon and Bejar, 1989; Hansen et al., 1993; Little et al., 1988; Oro and Dixon, 1987; Struthers and Hansen, 1992). It has also been shown that women who use MA throughout all of gestation have smaller babies than those who use during only the first trimester or the first and second trimesters (Smith et al., 2003). MRI spectroscopy performed on children exposed to MA during gestation identified an increase in creatine and glutamate/glutamine concentrations in the striatum, suggesting increased cellular metabolic activity that continued years after the in utero exposure (Smith et al., 2001). The volume of neurons and glia in the MA exposed children did not appear to be altered in that study. Therefore, in humans, in utero MA exposure alters cellular function, while leaving the volume of cells relatively intact. Taken together, these data suggest that MA causes prolonged central nervous system changes that may alter cognitive ability. Nonetheless, it remains to be proven whether MA exposure during human gestation definitively results in long-term cognitive deficits.
Animal models offer the ability to examine the potential for long-term cognitive deficits following MA. Since neurodevelopment in rats spans both prenatal and post-natal periods, modeling the effects of stimulants during various periods of human pregnancy requires a range of exposure periods. For example, we have been using the neonatal rat as a model of human third trimester exposure since analogous development of the granule cells of the dentate gyrus occurs between the rat and human during these respective time points (Bayer et al., 1993; Rice and Barone Jr., 2000). In our initial study demonstrating cognitive deficits following neonatal MA, we showed that multiple exposures to 30 mg/kg × 2 per day MA from postnatal days (P)11 to 20, but not from P1 to P10, results in spatial learning and memory deficits in the Morris water maze (MWM), but spared sequential learning and memory in the Cincinnati water maze (Vorhees et al., 1994a). Furthermore, regardless of the dosing period (i.e., P1–10 or P11–20), animals showed increased acoustic startle and decreased locomotor behavior in the open-field, although these effects were somewhat sex and age dependent (Vorhees et al., 1994a,b). In these initial experiments MA was delivered twice daily, although more recently we have demonstrated that four daily doses of MA (10 mg/kg) produced more pronounced deficits than two daily doses of MA (20 mg/kg) even though the daily concentrations of MA were identical (Vorhees et al., 2000a). The spatial deficits in the MWM resulting from neonatal MA are neither the result of working memory deficits (Williams et al., 2003d) nor the inability to learn the basic parameters of the task such as swimming away from the wall of the tank or recognizing and climbing on the platform (Williams et al., 2002). It does appear that in combination with the spatial deficits, the MA-exposed animals also have different behavioral strategies to learn the MWM (Williams et al., 2002). The amount of MA delivered appears to influence these results since examination of MA administration at concentrations of 5, 10, or 15 mg/kg four times daily from P11 to P20 resulted in some dose-related differences in spatial learning among these animals, with the 5 mg/kg group being the least affected on some measures, especially in learning a new platform position (Williams et al., 2003d).
There is a wide range of dosages consumed by human users of MA. For example, it has been shown that MA use can range from 150 to 15 000 mg per day (Cho, 1990; Derlet and Heischober, 1990). With such a large range of usage, it may be that incidental or low MA consumption during pregnancy may not be as deleterious to cognitive ability in the children of these users. Therefore, the purpose of the present study was to determine if administration of MA at or below 5 mg/kg, a model of the low-end human use, would produce impairments in the MWM when the animals were tested as adults. Male and female rats within a litter were administered MA from P11 to P20 in a dose–response paradigm that ranged from 0 to 5 mg/kg in order to determine a possible no effect limit for MA-induced spatial learning deficits. A total of five different doses were used: 0, 0.625, 1.25, 2.5 or 5 mg/kg MA. As adults, the animals were tested for anxiety in the zero maze and in the MWM for spatial learning ability. A small platform was used throughout all of the MWM testing.
2. Methods
2.1. Animals
Female (151–175 g) and male (251–275 g) Sprague–Dawley CD IGS rats (Charles River, Raleigh, NC, USA) were allowed at least 2 weeks to acclimate to the housing (2 females/polycarbonate cage) and lighting (14:10 (light:dark), lights on at 06:00 h) conditions in the lab prior to being mated. Females were placed with a male in a hanging wire cage for a period of 2 weeks, after which time the females were singly housed in polycarbonate cages. The day a sperm plug was detected was designated embryonic day 0 (E0). Beginning on E22, litters were checked in the morning and afternoon for the presence of a litter and the day of birth was designated postnatal day 0 (P0). The offspring were used as the subjects for this experiment. On P1, litters were reduced to 10 with equal numbers of males and females. On P11, the beginning of drug administration, pups were uniquely identified with an ear punch. Dams were allowed to wean their offspring (Blass and Teicher, 1980; Redman and Sweney, 1976) and offspring were then separated on P28 and housed in same sex groups until P42 when the animals were randomly housed two to three per cage in polycarbonate cages. Because of weight limitations for animal housing, some males that had been housed in groups of three were randomly selected and singly housed prior to behavioral testing. The body weights of offspring were collected on a weekly basis. The vivarium was temperature and humidity controlled and accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care and in compliance with all Federal animal care and use guidelines. The Institutional Animal Care and Use Committee approved all procedures prior to the experiments, and the guidelines outlined in the “Principles of Laboratory Care” (NIH Publication No. 85-23, revised 1985) were followed.
2.2. Treatments
D-Methamphetamine HCl (MA; expressed as the free-base) was delivered at doses of 0.625, 1.25, 2.5, or 5 mg/kg body weight (MA-0.625, MA-1.25, MA-2.5, and MA-5, respectively) or the saline vehicle (SAL) was administered to a randomly selected male and female within each litter (n = 16/dose). From P11 to P20, MA or SAL was administered four times daily at 2 h intervals. Prior to each injection the animals were weighed, and a subcutaneous route of administration in the dorsum was used. MA and SAL were delivered in a volume of 3 ml/kg per injection with injection sites rotated to minimize irritation. Necrosis does not occur using this procedure.
2.3. Behavioral methods
2.3.1. Zero maze
On approximately P60, animals were tested in the elevated zero maze for response to an anxiety provoking task (Shepherd et al., 1994) as described previously (Williams et al., 2003b). Briefly, animals were placed in the center of one of the closed areas of the ring-shaped apparatus and behavior was recorded for 5 min with an overhead camera connected to a video recorder. Overhead fluorescent lighting illuminated the maze and in between animals the maze was cleaned with 70% ethanol. The dependent measures for this task were scored from the video recordings and included the number of head dips, stretch-attends, and time in the open area. Time in the open was considered when animals had both front paws past the boundary of the closed area and extending into an open area.
2.3.2. Straight channel
One day after the zero maze, animals were examined for swimming ability in a water-filled straight channel as described previously (Williams et al., 2003d). Each rat received four timed consecutive trials (maximum time = 2 min/trial) and was placed in the channel at one end facing away from an escape ladder positioned at the opposite end. The water temperature was 22 ± 1 °C. This procedure allows the animals to be exposed to swimming prior to the Morris water maze and determines if pre-existing motor deficits or different motivational states are present.
2.3.3. Morris water maze
The Morris water maze apparatus was 210 cm in diameter and filled with room temperature water (22 ± 1 °C) as described previously (Williams et al., 2003b). Briefly, a 5 cm × 5 cm wide goal platform was used during each phase of testing. A camera attached to a computer and monitor automatically tracked the performance of each rat using a video tracking system (San Diego Instruments, Polytrack System, San Diego, CA, USA). The maze was arbitrarily divided at four cardinal points designated N, S, E, W, where N was defined as the position farthest from the experimenter. During each phase of testing, the platform was either located in the SW or NE quadrant of the apparatus, counterbalanced among the litters. The start positions, defined previously (Williams et al., 2003b) were quasi-randomized with the stipulation that no position could be used more than once a day.
Training began on the third day after straight channel swimming. The procedure consisted of an acquisition phase and a shifted-platform phase (“reversal”). During both phases, the rat received four trials per day for 5 days with a two-minute trial limit and an ITI of 15 s spent on the platform. If a rat failed to locate the platform, it was removed from the water and placed on the platform. On the day following the learning trials a 30 s probe trial was administered. During the probe trial, the platform was removed and the animal was started from a novel position, 180° from the platform and allowed 30 s to search the tank. The dependent measures for the learning trials during the acquisition and shifted platform phase were first bearing to the platform, latency, path length, and cumulative distance from the platform, as well as the distance swum in the peripheral portion of the tank. For memory (probe) trials, the dependent measures were first bearing and average distance from the previous platform site. First bearing was determined based on the animal’s average heading during the first 13 cm of tracking at the beginning of each trial relative to a direct line to the goal and is therefore, a measure of spatial orienting ability. Cumulative and average distance parameters were recorded every 55 ms. The periphery of the pool included the outer annulus of the pool that did not contain the platform.
2.4. Statistics
Body weight, zero maze, and straight channel data were analyzed with a split-plot analyses of variance (ANOVA) utilizing the general linear modeling procedure (SAS, SAS Institute Inc., Cary, NC, USA). Main effects were treatment group (MA concentration), sex, trial, and time of dose (first or last body weights during daily dosing) and these were all treated as within-subjects factors. The experimental unit was the litter (n = 16). We have repeatedly demonstrated impaired learning in the Morris water maze following neonatal MA exposure from P11 to P20 (Vorhees et al., 1994a, 1998, 1999, 2000a; Williams et al., 2002, 2003b,d). Therefore, since deficits were expected, planned comparisons (one-tailed) were used so that each MA-treated group was compared to the SAL group for first bearing, latency to the platform, path length, cumulative distance, and average distance in the Morris water maze. In order to determine if any sex differences or interactions with sex or MA treatment were apparent, mixed-model split-plot ANOVAs were used with the platform position as a between variable and treatment, sex, and days as within factors. Main effects for these analyses were further analyzed using the step down F-test procedure (Kirk, 1995). Interactions were analyzed by simple effect ANOVA and then with the step-down F-test. Interactions that did not involve treatment have only P-values given in the text. When non-spherical matrices occurred, the Huynh–Feldt correction was used. Significance was set at P = 0.05 and trends were noted at P = 0.10.
3. Results
3.1. Body weights
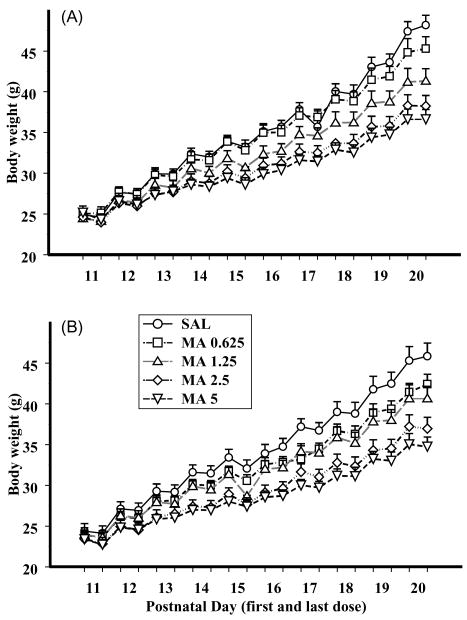
Body weights were analyzed using within-litter ANOVAs at three different stages of development: dosing (P11–20), pre-weaning (P21 and 28), and post-weaning (P35–77). During the period of MA administration a MA treatment × sex × days ANOVA demonstrated that all animals gained weight over days, F(9, 135) = 177.4, P < 0.0001, and males (Fig. 1A) weighed more than females (Fig. 1B), F(1, 15) = 37.9, P < 0.0001. As can be seen in Fig. 1, MA treatment produced a decrease in body weight during dosing, F(4, 60) = 34.3, P < 0.0001, although this effect was dependent upon treatment × day, F(36, 540) = 49.7, P < 0.0001. No other interactions were observed. Further analysis of the interaction showed that beginning on P12 and continuing until the end of treatment the animals treated with 1.25, 2.5, or 5 mg/kg MA weighed less than the SAL controls. For the MA-0.625 treated-animals, body weights were less on P14–16 and then again from P18–20.
Fig. 1.
Body weights are shown for males (A) and females (B) during methamphetamine (MA) administration from P11 to P20. Dose and sex dependent differences, were noted for the various doses of MA. Only a treatment by day interaction was significant. Refer to text for details.
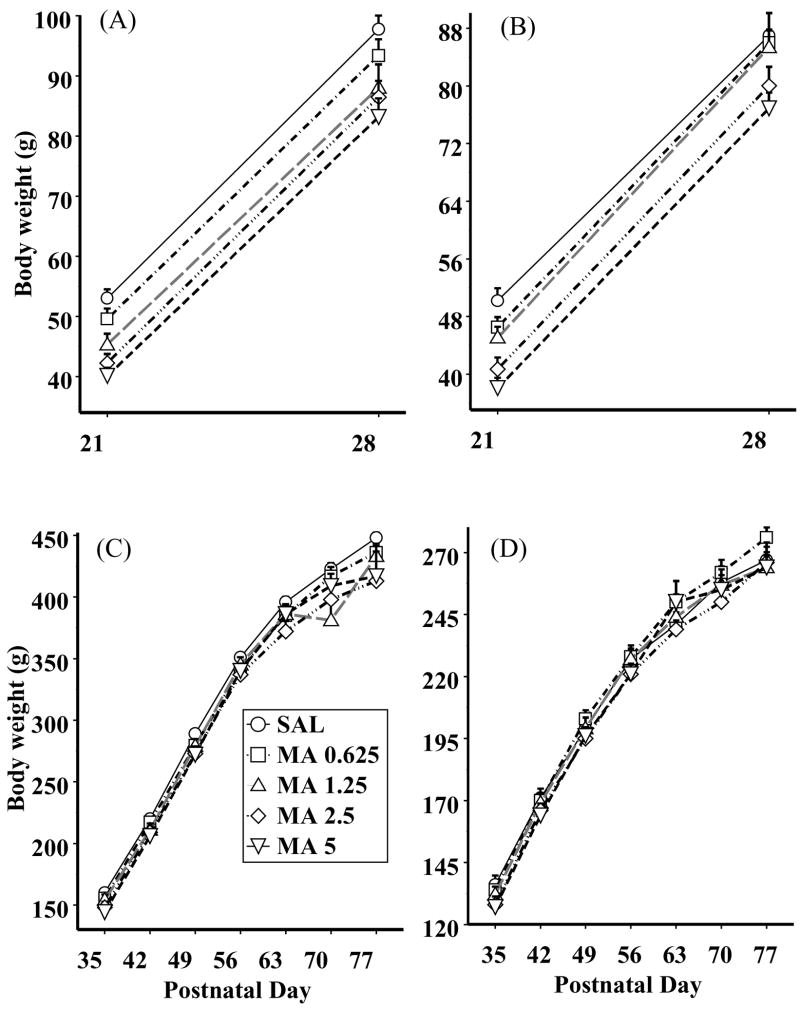
For pre-weaning weights, a MA treatment × sex × week ANOVA revealed similar results obtained during dosing (Fig. 2A and B). That is, the animals gained weight over the week, F(1, 15) = 950.9, P < 0.0001, and males (Fig. 2A) weighed more than females (Fig. 2B), F(1, 15) = 38.8, P < 0.0001. MA treatment had a significant impact on body weight, F(4, 60) = 53.8, P < 0.0001, although this was dependent upon MA treatment × sex × week, F(4, 60) = 2.8, P < 0.05. For males, the MA-treated-animals, regardless of MA concentration, continued to weigh less than the SAL-treated-animals on both P21 and 28 (Fig. 2A). As seen in Fig. 2, panel B, MA-treated-females continued to have lower body weights on P21 regardless of dose relative to SAL animals, but on P28, only the 2.5 and 5 mg/kg MA treatments differed from SAL animals (Fig. 2B). The sex × week interaction was significant, P < 0.0001, although no other interactions were significant.
Fig. 2.
Body weights are shown for males (A and C) and females (B and D) immediately following methamphetamine (MA) administration in the pre-weaning period (panels A and B) as well as the post-weaning period (panels C and D). Dose-dependent differences were noted only in the pre-weaning period. Sex differences were noted at all times. Refer to text for details.
For post-weaning weights, an MA treatment × sex × week ANOVA was employed. Males (Fig. 2C) continued to weigh more than the females (Fig 2D), F(1, 15) = 711.6, P < 0.0001, and the animals gained weight over weeks as expected, F(6, 90) = 1140.7, P < 0.0001. The interaction between sex and week was significant, P < 0.0001, as well. Treatment with neonatal MA continued to influence body weight in adulthood, F(4, 60) = 4.8, P < 0.002. Regardless of sex, the MA-1.25, 2.5, and 5-treated-animals weighed less than the SAL-treated-animals, although the differences were small after P35.
3.2. Behavior
3.2.1. Zero maze
Three litters were not scored for the zero maze because of equipment malfunction, therefore, n = 13. No differences among treatment groups or between sexes were noted in the zero maze for head dips, stretch attends, time in the open (Table 1), or the number of open arm entries. There was a tendency for females to enter the open arms more than males, P < 0.10.
Table 1.
Zero maze (ZM) open area times and Morris water maze probe trial data during acquisition (Acq) and shifted-platform (SP) phases for animals treated with either 0.625, 1.25, 2.5, 5 mg/kg methamphetamine (MA-0.625, MA-1.25, MA-2.5, MA-5, respectively) or saline-treated-animals
| Measures | Treatment |
||||
|---|---|---|---|---|---|
| SAL | MA-0.625 | MA-1.25 | MA-2.5 | MA-5 | |
| ZM open time (s) | 47.67 ± 8.89 | 37.83 ± 5.75 | 38.53 ± 5.45 | 51.80 ± 9.33 | 44.22 ± 5.95 |
| Straight channel (s) | 16.04 ± 1.06 | 18.54 ± 1.13 | 18.20 ± 0.87 | 20.20 ± 2.07 | 17.46 ± 1.23 |
| Acq probe first bearing (°) | 64.75 ± 5.96 | 66.84 ± 5.52 | 62.58 ± 5.27 | 68.28 ± 4.97 | 64.26 ± 5.20 |
| Acq probe average distance (cm) | 83.58 ± 2.30 | 89.11 ± 4.04 | 83.94 ± 2.81 | 89.23 ± 3.39 | 86.70 ± 3.38 |
| SP probe first bearing (°) | 53.96 ± 3.84 | 59.50 ± 5.46 | 57.53 ± 3.25 | 65.38 ± 3.85a | 70.91 ± 2.66a |
| SP probe average distance (cm) | 80.51 ± 3.39 | 81.52 ± 4.41 | 84.38 ± 3.68 | 86.13 ± 4.62 | 84.64 ± 2.44 |
P < 0.01 relative to SAL.
3.2.2. Straight channel
Prior to learning in the MWM, animals were exposed to a test of swimming ability in a straight channel. No differences were noted between any of the MA-treated-animals and SAL-treated-animals (Table 1) or between males and females.
3.2.3. Morris water maze acquisition
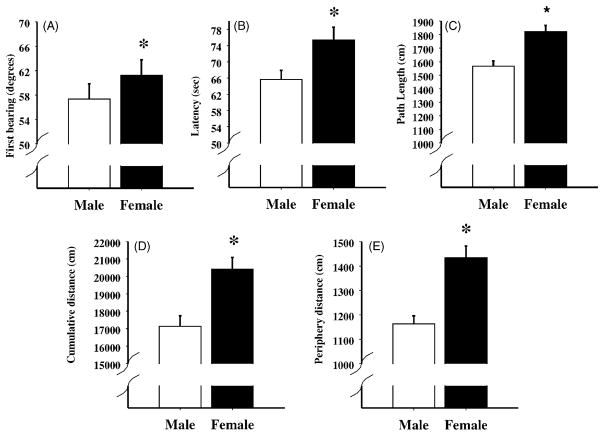
For first bearing, the contrasts between MA-treated animals and SAL-treated animals revealed that the MA-2.5 and MA-5 treated-animals did not spatially orient towards the platform position as well as the SAL-treated-animals, F(1, 15) = 6.5 and 3.4, P < 0.05, respectively (Fig. 3A). A similar tendency was also observed in the MA-1.25 treated-animals, P < 0.06. Males had a more direct orientation to the platform than females, F(1, 14) = 8.1, P = 0.01 (Fig. 4A).
Fig. 3.
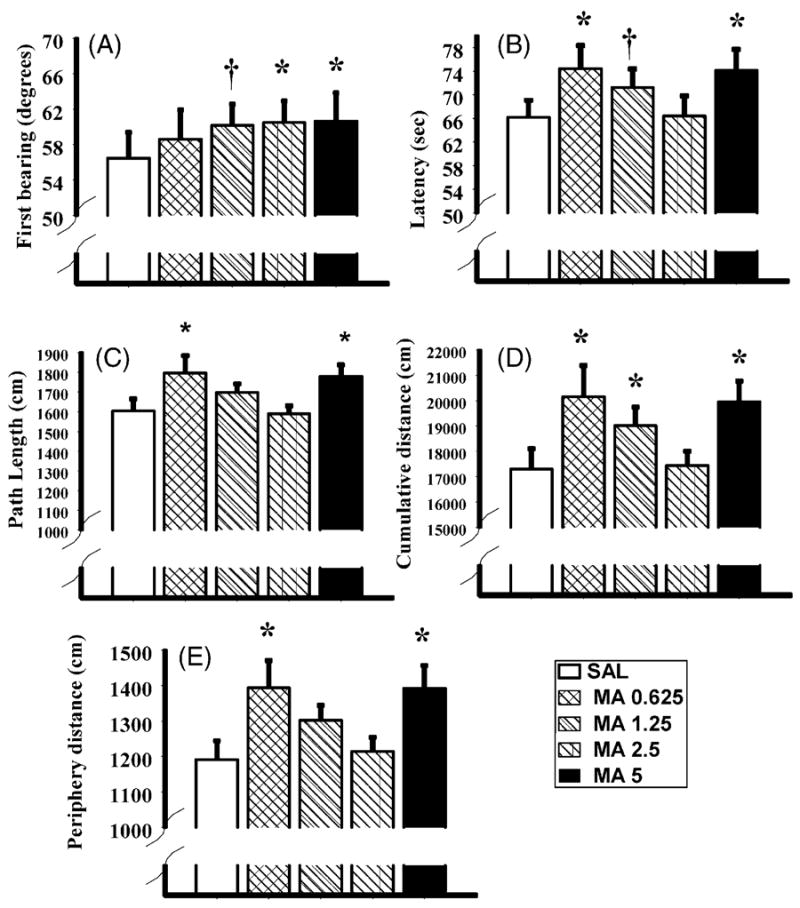
Acquisition phase data for the Morris water maze following methamphetamine (MA) administration at various doses or saline (SAL) administered from P11 to P20. The first bearing (panel A), latency to the platform (panel B), path length (panel C), cumulative distance (panel D), and distance swum in the periphery (panel E) are depicted. As can be seen, dose responsiveness was noted for the first bearing, whereas the MA-0.625 and MA-5 animals consistently performed worse on the other measures compared to SAL animals. *P < 0.05 and †P < 0.1.
Fig. 4.
Acquisition phase data for the Morris water maze comparing male and female performance for first bearing (panel A), latency to the platform (panel B), path length (panel C), cumulative distance (panel D), and distance swum in the periphery (panel E). As can be seen, females performed worse than males on all measures. *P < 0.05.
Different patterns of results emerged for latency to the platform, path length, and cumulative distance from the platform during acquisition. Contrasts showed that the MA-0.625 and MA-5 animals took longer to reach the platform, F(1, 15) = 4.3 and 5.9, respectively, P < 0.03; (Fig. 3B), had longer path lengths, F(1, 15) = 5.3 and 10.6, P < 0.02 and 0.003, respectively; (Fig. 3C), and cumulative distances, F(1, 15) = 5.9 and 14.1, P < 0.03 and 0.001, respectively; (Fig. 3D) relative to the SAL animals. The MA-1.25 animals had greater cumulative distances, F(1, 15) = 2.9, P = 0.05, and tended to have longer latencies, P < 0.10. No differences were noted for the MA-2.5 animals compared to the SAL animals for latency, path length, or cumulative distance. Sex differences were noted for these three measures. Regardless of treatment, females took longer to locate the platform (Fig. 4B), had longer path lengths (Fig. 4C), and greater cumulative distances (Fig. 4D) compared to males, F(1, 14) = 7.9, 22.7, and 17.6, P < 0.02, 0.0004, and 0.0009, respectively. No interactions with sex and treatment were significant for these measures.
For the distance swum in the periphery, there were significant effects of treatment, F(4, 56) = 3.13, P < 0.03 (Fig. 3E) and sex, F(1, 14) = 26.7, P < 0.0001 (Fig. 4E). Step-down post hoc analysis showed that the MA-0.625 and MA-5 animals swam longer distances in the periphery compared to SAL animals. The females also demonstrated more swimming in the periphery relative to males (Fig. 4E). No significant interactions were noted between treatment and sex. As expected, animals swam less in the periphery as testing continued over days, F(4, 56) = 92.3, P < 0.0001 (not shown).
During the probe trial, no differences were noted for first bearing or the average distance from the former platform site (Table 1). The average distance from the platform was greater for the females compared to males, F(1, 14) = 6.3, P < 0.03, however, no sex differences were noted for the first bearing (not shown). No interactions with treatment and sex were found.
3.2.4. Morris water maze shifted platform (reversal)
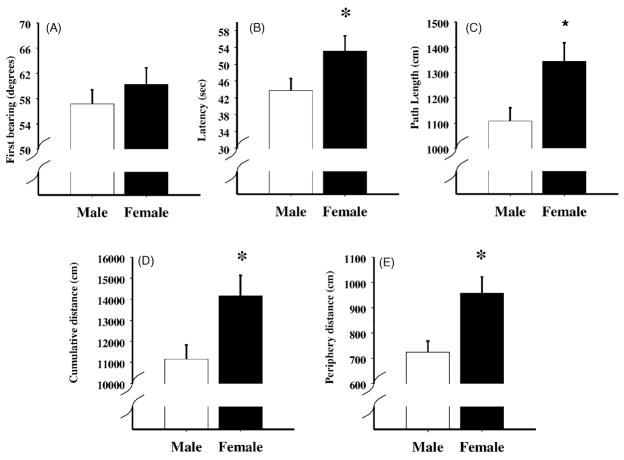
During learning of the shifted platform phase, the first bearing of the MA-5 animals was further from a direct path to the platform relative to the SAL animals, F(1, 15) = 14.21, P < 0.0001. A similar tendency was observed in the MA-1.25 and MA-2.5 animals compared to SAL animals, P < 0.10 and 0.07, respectively. No difference was observed for the MA-0.625 relative to SAL (Fig. 5A). The females tended to have greater discrepancy from a direct path compared to males (Fig. 6A), P < 0.07, and this was influenced, but not significantly, by treatment, P < 0.06 (not shown).
Fig. 5.
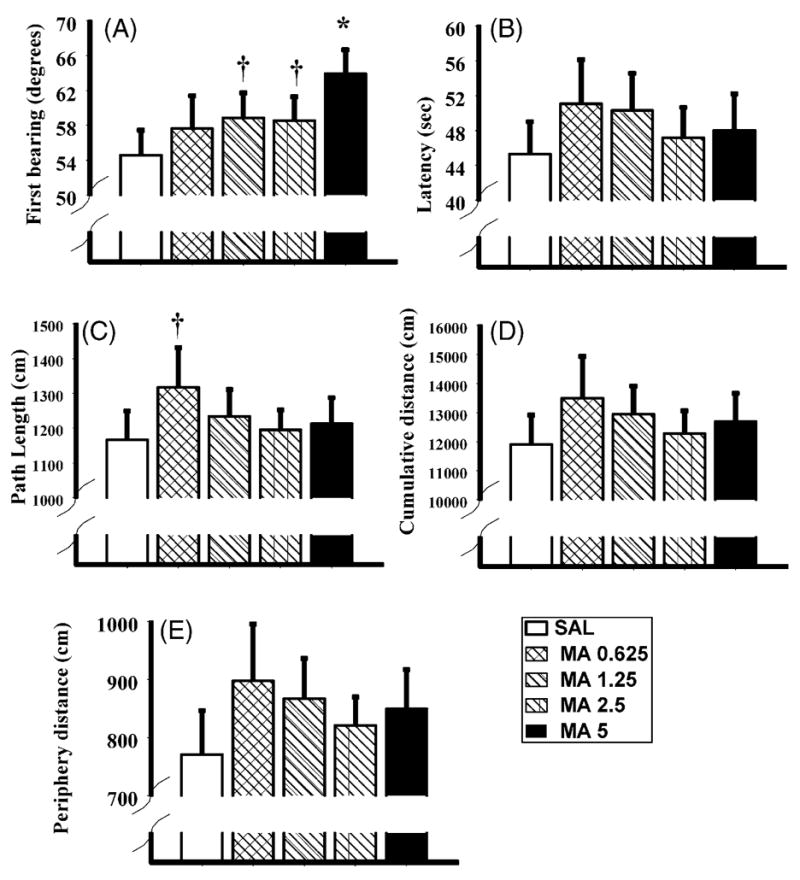
The shifted platform phase data for the Morris water maze following methamphetamine (MA) administration at various doses or saline (SAL) administration from P11 to P20. The first bearing (panel A), latency to the platform (panel B), path length (panel C), cumulative distance (panel D), and distance swum in the periphery (panel E) are depicted. Similar to the acquisition phase, dose responsiveness was noted for the first bearing, however with the exception for a tendency in the MA-1.25 animals on path length, no other differences were noted. *P < 0.05 and †P < 0.1.
Fig. 6.
Shifted platform phase data for the Morris water maze comparing male and female performance for first bearing (panel A), latency to the platform (panel B), path length (panel C), cumulative distance (panel D), and distance swum in the periphery (panel E). As can be seen, with the exception of first bearing the females performed worse than males on all other measures *P < 0.05.
For the latency to the platform, path length, and cumulative distances, no significant effects of treatment were observed during the shifted platform phase, although the MA-0.625 treated-animals tended to have longer path lengths than the SAL treated-animals, P = 0.10 (Fig. 5C). Similar to the acquisition phase, females had greater difficulty solving the task compared to males. This is shown by the greater latencies to the platform, longer path lengths, and increased cumulative distances for the females, F(1, 14) = 16.4, 14.2, and 14.9, P < 0.002, respectively (Fig. 6B–D). For latency, path length, and cumulative distance, the treatment × sex × day × platform position was also significant, F(16, 224) = 2.1, 1.9, and 2.2, P < 0.01, 0.02, and 0.01, respectively. For latency, no differences were detected with post hoc analysis of the interaction and increases for path lengths and cumulative distance on day 1 of testing in the MA-0.625 males that were learning the NE platform position were found (not shown).
No treatment differences were detected for the distance swum in the periphery (Fig. 5E), although the treatment × sex × day × platform position was significant, F(16, 224) = 2.5, P < 0.002, and showed a similar pattern for path length and cumulative distance. Females spent more time in the periphery compared to males, F(1, 14) = 17.3, P < 0.001 (Fig. 6E).
During the probe trial both the MA-2.5 and MA-5 treated-animals had a greater deviation from the platform site trajectory compared to SAL-treated-animals, F(1, 15) = 9.5 and 17.1, P < 0.004 and 0.0005 (Table 1). No differences for average distance were noted (Table 1) and no differences between males and females were seen for either first bearing or average distance (not shown).
4. Discussion
Similar to body weight differences noted in the human literature following prenatal MA exposure (Dixon and Bejar, 1989; Little et al., 1988; Oro and Dixon, 1987; Smith et al., 2003), we have demonstrated that even relatively low doses of MA (2.5 mg/kg per day) administered during a period of brain development that is analogous to third trimester human development (Bayer et al., 1993; Rice and Barone, Jr., 2000) can produce reductions in body weight. These reductions were dose-dependent with the 0.625 dose having the smallest effect and the 2.5 and 5 mg/kg doses producing the largest decrements. The decreased body weights continued throughout life for the MA-1.25, 2.5, and 5 mg/kg treated groups, although the effect was diminished compared to body weight differences during drug administration (see Fig. 1A versus Fig. 2C or Fig. 1B versus Fig. 2D), demonstrating that the MA-treated-animals actually gained more weight relative to their immediate post-drug administration body weights. Taken together these data demonstrate that body weight change is a sensitive indicator of MA exposure.
Because lower body weights might be an indication of malnutrition or more likely undernutrition, there is concern that any changes in learning and memory ability may be the result of the undernutrition rather than the drug exposure per se. MA and other drugs of abuse consistently produce decreased body weights in neonates (Vorhees et al., 2000a,b; Williams et al., 2003c,d). The premise that undernutrition does not induce spatial learning deficits has been supported by previous studies (Levitsky and Strupp, 1995; Strupp and Levitsky, 1995; Williams et al., 2003c) although see (Fukuda et al., 2002). The results from this study suggest that following MA exposure body weight decrements during the period of drug administration and even the long-lasting effects on body weight in adulthood are not predictive of learning deficits. This is especially apparent since a non-dose-dependent relationship was found for various parameters of learning in the MWM, although the decreases in body weight were dose-dependent.
During the acquisition phase of Morris water maze learning, all the MA-treated groups displayed some form of impairment in learning, although this was not consistent across parameters. For first bearing, the ability of an animal to initially orient to the platform, there was a dose-dependent effect such that the 5 and 2.5 mg/kg MA-treated groups were impaired the greatest with intermediate levels in the MA-1.25 treated-animals and no differences noted for the MA-0.625 group. With the exception of the MA-2.5 treated-animals, the other MA-treated groups showed increased cumulative distances from the platform compared to SAL animals during acquisition. Cumulative distance is thought to be a more accurate measure of spatial learning (Gallagher et al., 1993) and generally is consistent at distinguishing neonatal drug-induced spatial learning deficits better than other measures (Williams et al., 2003c). Concurrent with the cumulative distance deficits, a similar pattern of deficits was observed for the MA-0.625 and MA-5 treated-animals for latency, path length, and distance swum in the periphery; however, these measures were not significant for the MA-1.25 animals, although latency approached significance. Why the MA-2.5 treated-animals did not demonstrate any deficits other than first bearing is unknown. Whether or not these animals were influenced differentially within the litter or by the dams or whether the randomized selection of animals included more non-responders to MA than in the other groups will have to be explored in other studies. It is also unlikely that any change in housing would have influenced these results. For example, only males reached the maximum weight limit for group housing in our cages, however this did not occur until approximately P60 and redistribution was completed prior to behavioral testing. Secondly, a single male was randomly removed from a cage that contained three males and housed singly and there were no differences among groups for the number of males that were singly housed. Finally, the females never were separated. Therefore, if housing were an issue it may have been expected that there would be a greater likelihood of a treatment × sex interaction, although this never occurred during the acquisition phase of MWM testing. Taken together, the acquisition data indicate that an animal’s ability to initially orient to a spatial location (first bearing) is more likely to be influenced by early MA exposure and may indicate subtle differences in spatial ability. By contrast, the other measures of spatial learning (latency, path length, and cumulative distance) may be “corrected” while navigating to the platform and therefore not produce a similar pattern of results when the effect of the drug is subtle. In a previous study, we showed greater differences in first bearing in animals treated neonatally with MA when they were learning a new platform location, however these animals did not display differences in other parameters of learning even though underlying differences in spatial learning were present. These underlying differences became apparent when the animals were again tested to find a new location of the platform, except this time the platform was reduced in size (Vorhees et al., 2000a).
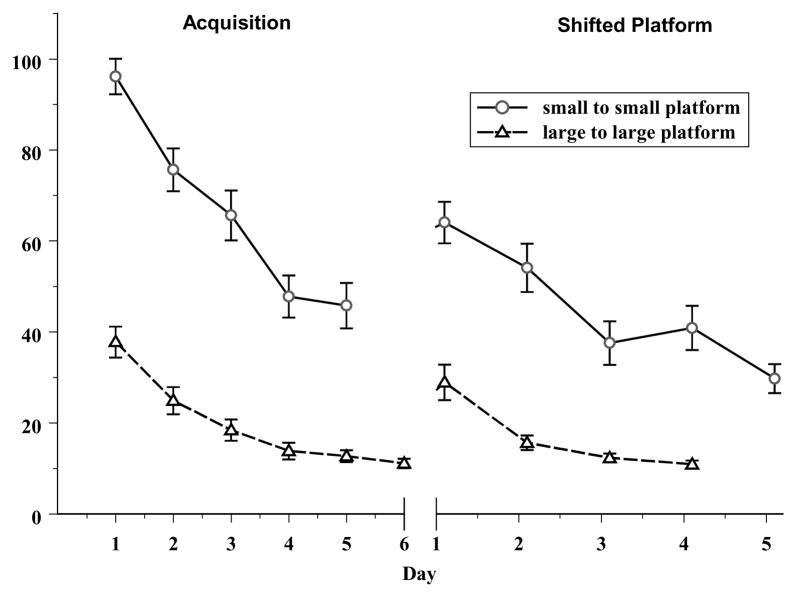
Because we showed that using a reduced platform size can reveal learning and memory deficits even when these deficits may not be apparent using other task parameters (i.e., shifted platform with a large platform), (Broening et al., 2001; Morford et al., 2002; Vorhees et al., 2000a; Williams et al., 2003d), we reasoned that using a smaller platform from the onset, during acquisition, would make the Morris water maze an even more sensitive test of spatial learning ability. It should be noted that the overall latencies to reach the small platform in previous studies when it was introduced during a third phase of testing were comparable to those seen with the large platform during acquisition or reversal. Therefore, in the current study we used a small platform (5 cm × 5 cm) throughout testing, rather than the larger platform (10 cm × 10 cm) we have used in the past for acquisition and reversal. As can be seen in Fig. 7, a comparison of control animals (SAL) from one previous study with an identical tank dimension (Williams et al., 2003d) and those in the current study demonstrates that the learning curves are dramatically shifted upward during acquisition when using a small platform. Furthermore, asymptotic performance appears to occur on day 4 of learning, although animals learning the small platform took almost four times longer to locate the platform. Animals that had a large platform for both acquisition and the shifted platform phases learned the new platform position quickly and in the shifted platform phase were demonstrating asymptotic performance by day 2 of testing. On the other hand, animals that had the smaller platform throughout were still demonstrating improvement in locating the platform on the last day of testing in the shifted platform phase. These data suggest several possibilities. First, when a small platform is used, the animals may have incomplete spatial learning during acquisition, therefore interference from previous learning during the shifted platform phase would be minimal. This might help explain why no deficits in MA-treated-animals were apparent in the shifted platform phase on latency, path length or cumulative distance. Second, the animals with a small platform may develop a different strategy for locating the platform. Others have demonstrated that when the task parameters are increasingly more difficult (e.g., a fixed platform with no extra maze cues, or extramaze cues with a random platform location but equidistant from the tank wall), animals can learn the task, but never do as well as animals with a large platform in a fixed position with many extramaze cues present (Baldi et al., 2003). Although the animals in this study were afforded extramaze cues, a fixed platform position, and located the platform above the level of chance, they did not perform as efficiently as controls in the previous study with a larger platform (Fig. 7). This suggests that a different strategy to locate the platform was used. It has been shown that the distance from the wall is an important factor for animals to locate a hidden platform (Baldi et al., 2003; Maurer and Derivaz, 2000). Therefore, animals in this study may have concentrated on the distance from the tank wall more than on landmarks outside of the maze. It is interesting to note that peripheral swimming during acquisition was greater for the MA-0.625 and MA-5-treated-animals, and these animals showed the most consistent deficits. The third most salient comparison between small and large platform conditions is that there is a dramatic increase in variability (see error bars in Fig. 7). Increased variability has the effect of decreasing detection sensitivity and could obscure treatment effects. Lastly, a general phenomenon may be at work in the Morris water maze regardless of the initial platform size: spatial deficits may be more readily observed during acquisition compared to the shifted platform phase when the same size platform is used in both phases. Pretraining in the Morris water maze is known to attenuate deficits in spatial learning (Cain et al., 1996; Williams et al., 2002). Acquisition might be considered a form of pretraining for the shifted platform phase, in that it teaches the animal to move away from the periphery because the platform is located a fixed distance from the wall. Once this is learned during acquisition it may attenuate deficits observed during the shifted platform phase. However, if a second parameter is manipulated during the shifted platform phase, then the deficits may re-emerge. Perhaps, if we had tried another shifted platform phase with a smaller platform, we might have observed the spatial deficits again as in previous studies (Broening et al., 2001; Morford et al., 2002; Vorhees et al., 2000a; Williams et al., 2003d).
Fig. 7.
Saline control animals in this study (open circles) using a small platform (5 cm × 5 cm) during both acquisition and the shifted platform phases compared to saline control animals from a previous study (open triangles) with a large platform (10 cm × 10 cm) during both phases (Williams et al., 2003d). The tank diameter and extramaze cues were identical between experiments, however as can be seen the small platform disrupts the learning ability of animals during both acquisition and the shifted platform phase.
Males have previously been shown to perform better than females in locating the hidden platform in the Morris water maze and this has been supported here (Roof and Stein, 1999; Williams et al., 2003c,d; Perrot-Sinal et al., 1996; Beiko et al., 2004), however differences between males and females may depend upon the learning requirements. For example, no sex differences were noted when the start position and platform position were held constant (Roof and Stein, 1999) or if females were pretrained prior to spatial learning (Beiko et al., 2004). Interestingly, the difference between males and females, regardless of treatment, was stable using the current method of Morris water maze testing. Females had longer latencies to the platform, increased path lengths, and increased cumulative distances during acquisition and shifted platform phases. Unlike the apparent change in strategies by the MA-treated-animals during the shifted platform phase (i.e., decreased swimming in the periphery), the females continued to spend more time in the periphery than the males. This is somewhat at odds with a recent study demonstrating that pretraining alleviates the peripheral swimming in females (Beiko et al., 2004), however this may be the result of the more difficult task parameters (larger pool with a smaller platform) in the present study. It appears that the differences in females may be related to the level of circulating corticosterone (CORT) present during the swimming task since pretraining reduced CORT levels as well.
In previous studies we have demonstrated that MA administration on P11 produces protracted increases in CORT that were apparent hours after drug administration (Williams et al., 2000). Furthermore, continued daily administration of MA altered this protracted pattern, such that on P15 and P20 only transient increases in CORT were observed. More recently, we demonstrated that the adrenal response to 15 min of forced swim was smaller in animals exposed to MA (5 mg/kg × 4 doses) from P11 to P20 (Williams et al., 2003a). These data suggest that the response to stressful situations in neonatal MA exposed animals may be permanently altered. A potential explanation for the lack of effect in the zero maze might be that the decreased adrenal response in the pervious study was observed following a combination of physical exertion and presentation to a novel environment, whereas with the zero maze, only a novel environment is present. Determination of the adrenal response following a “psychological” (zero maze) stressor may produce a different pattern of response.
In relation to the human usage of MA the doses used in this study increase the range of doses modeled for MA users, i.e., 150–15 000 mg per day (Cho, 1990; Derlet and Heischober, 1990). In addition, it has been reported that the amount of MA used in a single “hit” may be as high as 250 mg (McCann et al., 1998), whereas casual users may only take between 60–100 mg per “hit” (see (Burchfield et al., 1991)). Therefore, on a non-scaled mg/kg basis, a 60 kg women who consumed 60 or 250 mg of MA would have used 1 or 4.2 mg/kg, respectively, in a single “hit.” In this study we administered doses that ranged from 3.125 to 20 mg/kg per day. The lowest dose in this study is well within the range of even a single “hit” of MA for some human users and the upper dose would also fall in the range of human use since many users take several hits during the day (Cho, 1990). It has been argued that rather than using a straight mg/kg dosage for comparison, there should be some scaling to account for the differences in metabolic rate and rate of elimination of drug between humans and other species (for review see Green et al., 2003). Using the Mordenti and Chappell scaling formula of Dosehuman = Doseanimal (Weighthuman/Weightanimal)0.7 (Green et al., 2003) and assuming a 60 kg women, a 60 mg “hit,” and a 300 g animal, the equivalent dose would be 1.47 mg or 4.9 mg/kg. If we used the 250 mg dose then it would be 6.1 mg or 20 mg/kg. In either case, the doses used in this study are comparable to what estimated human exposures are. It should be realized that identifying a comparable dose to model third trimester exposure in a fetus is difficult especially considering that there are several unknowns, such as the amount of drug to which the fetus is exposed or the body weight of the fetus. One study in sheep suggested that the dam had greater peak concentrations of MA present after intravenous administration, but that the fetus had a longer elimination high-life (Burchfield et al., 1991). These authors demonstrated that if one considers the area under the time/concentration curve for the ewe and the fetus, there was very little difference in regard to total exposure to MA. This suggests that fetal concentrations may mimic the concentrations found in the mother. The data in the present study model casual use (i.e., a single hit per day) of MA in humans rather than just heavy users, making the findings applicable to a larger range of MA users.
Overall, these data suggest that MA administration even at very small doses produces lasting effects on spatial learning and memory in rats. These effects may be near the threshold for MA effectiveness since the 2.5 mg/kg treated-animals only displayed subtle differences in learning. Together with the reductions in body weight and the learning deficits, these data would caution against even low dose exposures to MA during the third trimester of pregnancy.
Acknowledgments
This work was supported by National Institutes of Health grants DA14269 (MTW) and DA06733 (CVV).
References
- Baldi E, Lorenzini CA, Corrado B. Task solving by procedural strategies in the Morris water maze. Physiol Behav. 2003;78:785–793. doi: 10.1016/s0031-9384(03)00064-7. [DOI] [PubMed] [Google Scholar]
- Bayer SA, Altman J, Russo RJ, Zhang X. Timetables of neurogenesis in the human brain based on experimentally determined patterns in the rat. Neurotoxicology. 1993;14:83–144. [PubMed] [Google Scholar]
- Beiko J, Lander R, Hampson E, Boon F, Cain DP. Contribution of sex differences in the acute stress response to sex differences in water maze performance in the rat. Behav Brain Res. 2004;151:239–253. doi: 10.1016/j.bbr.2003.08.019. [DOI] [PubMed] [Google Scholar]
- Blass EM, Teicher MH. Suckling. Science. 1980;210:15–22. doi: 10.1126/science.6997992. [DOI] [PubMed] [Google Scholar]
- Broening HW, Morford LL, Inman-Wood SL, Fukumura M, Vorhees CV. 3,4-methylenedioxymethamphetamine (Ecstasy)-induced learning and memory impairments depend on the age of exposure during early development. J Neurosci. 2001;21:3228–3235. doi: 10.1523/JNEUROSCI.21-09-03228.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burchfield DJ, Lucas VW, Abrams RM, Miller RL, DeVane CL. Disposition and pharmacodynamics of methamphetamine in pregnant sheep. J Am Med Assoc. 1991;265:1968–1973. [PubMed] [Google Scholar]
- Cain DP, Saucier D, Hall J, Hargreaves EL, Boon F. Detailed behavioral analysis of water maze acquisition under APV or CNQX: contribution of sensorimotor disturbances to drug-induced acquisition deficits. Behav Neurosci. 1996;110:86–102. doi: 10.1037//0735-7044.110.1.86. [DOI] [PubMed] [Google Scholar]
- Cernerud L, Eriksson M, Jonsson B, Steneroth G, Zetterström R. Amphetamine addiction during pregnancy: 14-year follow-up of growth and school performance. Acta Paediatr. 1996;85:204–208. doi: 10.1111/j.1651-2227.1996.tb13993.x. [DOI] [PubMed] [Google Scholar]
- Cho AK. Ice: a new dosage form of an old drug. Science. 1990;249:631–634. doi: 10.1126/science.249.4969.631. [DOI] [PubMed] [Google Scholar]
- Derlet RW, Heischober B. Methamphetamine: stimulant of the 1990s? West J Med. 1990;153:625–628. [PMC free article] [PubMed] [Google Scholar]
- Dixon SD, Bejar R. Echoencephalographic findings in neonates associated with maternal cocaine and methamphetamine use: incidence and clinical correlates. J Pediatr. 1989;115:770–778. doi: 10.1016/s0022-3476(89)80661-4. [DOI] [PubMed] [Google Scholar]
- Fukuda MTH, Françolin-Silva AL, Almeida SS. Early post-natal protein malnutrition affects learning and memory in the distal but not in the proximal cue version of the Morris water maze. Behav Brain Res. 2002;133:271–277. doi: 10.1016/s0166-4328(02)00010-4. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Burwell R, Burchinal M. Severity of spatial learning impairment in aging: development of a learning index for performance in the Morris water maze. Behav Neurosci. 1993;107:618–626. doi: 10.1037//0735-7044.107.4.618. [DOI] [PubMed] [Google Scholar]
- Green AR, Mechan AO, Elliott JM, O’Shea E, Colado MI. The pharmacology and clinical pharmacology of 3,4-methylenedioxy-methamphetamine (MDMA, “ecstasy”) Pharmacol Rev. 2003;55:463–508. doi: 10.1124/pr.55.3.3. [DOI] [PubMed] [Google Scholar]
- Hansen RL, Struthers JM, Gospe SM., Jr Visual evoked potentials and visual processing in stimulant drug-exposed infants. Dev Med Child Neurol. 1993;35:798–805. doi: 10.1111/j.1469-8749.1993.tb11731.x. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG. Secondary School Students (NIH Publication No. 02-5106) I. National Institute on Drug Abuse; Bethesda, MD: 2002a. Monitoring the Future National Survey Results on Drug Abuse, 1975–2001. [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG. College Students and Adult Ages 19–40 (NIH Publication No. 02-5107) II. National Institute on Drug Abuse; Bethesda, MD: 2002b. Monitoring the Future National Survey Results on Drug Abuse, 1975–2001. [Google Scholar]
- Kirk RE. Experimental Design: Procedures for the Behavioral Sciences. Brooks/Cole Publishing Co; Pacific Grove: 1995. [Google Scholar]
- Levitsky DA, Strupp BJ. Malnutrition and the brain: changing concepts, changing concerns. J Nutrit. 1995;125:2212S–2220S. doi: 10.1093/jn/125.suppl_8.2212S. [DOI] [PubMed] [Google Scholar]
- Little BB, Snell LM, Gilstrap LC., III Methamphetamine abuse during pregnancy: outcome and fetal effects. Obstet Gynecol. 1988;72:541–544. [PubMed] [Google Scholar]
- Maurer R, Derivaz V. Rats in a transparent Morris water maze use elemental and configural geometry of landmarks as well as distance to the pool wall. Spatial Cognit Computat. 2000;2:135–156. [Google Scholar]
- McCann UD, Wong DF, Yokoi F, Villemagne V, Dannals RF, Ricaurte GA. Reduced striatal dopamine transporter density in abstinent methamphetamine and methcathinone users: evidence from positron emission tomography studies with [11C]WIN-35,428. J Neurosci. 1998;18:8417–8422. doi: 10.1523/JNEUROSCI.18-20-08417.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morford LL, Inman-Wood SL, Gudelsky GA, Williams MT, Vorhees CV. Impaired spatial and sequential learning in rats treated neonatally with d-fenfluramine. Eur J Neurosci. 2002;16:491–500. doi: 10.1046/j.1460-9568.2002.02100.x. [DOI] [PubMed] [Google Scholar]
- Oro AS, Dixon SD. Perinatal cocaine and methamphetamine exposure: maternal and neonatal correlates. J Pediatr. 1987;111:571–578. doi: 10.1016/s0022-3476(87)80125-7. [DOI] [PubMed] [Google Scholar]
- Perrot-Sinal TS, Kostenuik MA, Ossenkopp KP, Kavaliers M. Sex differences in performance in the Morris water maze and the effects of initial nonstationary hidden platform training. Behav Neurosci. 1996;110:1309–1320. doi: 10.1037//0735-7044.110.6.1309. [DOI] [PubMed] [Google Scholar]
- Redman RS, Sweney LR. Changes in diet and patterns of feeding activity in developing rats. J Nutrit. 1976;106:615–626. doi: 10.1093/jn/106.5.615. [DOI] [PubMed] [Google Scholar]
- Rice D, Barone S., Jr Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect. 2000;108:511–533. doi: 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roof RL, Stein DG. Gender differences in Morris water maze performance depend on task parameters. Physiol Behav. 1999;68:81–86. doi: 10.1016/s0031-9384(99)00162-6. [DOI] [PubMed] [Google Scholar]
- Shepherd JK, Grewal SS, Fletcher A, Bill DJ, Dourish CT. Behavioural and pharmacological characterisation of the elevated “zero-maze” as an animal model of anxiety. Psychopharmacology. 1994;116:56–64. doi: 10.1007/BF02244871. [DOI] [PubMed] [Google Scholar]
- Smith LM, Chang L, Yonekura ML, Grob C, Osborn D, Ernst T. Brain proton magnetic resonance spectroscopy in children exposed to methamphetamine in utero. Neurology. 2001;57:255–260. doi: 10.1212/wnl.57.2.255. [DOI] [PubMed] [Google Scholar]
- Smith L, Yonekura ML, Wallace T, Berman N, Kuo J, Berkowitz C. Effects of prenatal methamphetamine exposure on fetal growth and drug withdrawal symptoms in infants born at term. J Dev Behav Pediatr. 2003;24:17–23. doi: 10.1097/00004703-200302000-00006. [DOI] [PubMed] [Google Scholar]
- Strupp BJ, Levitsky DA. Enduring cognitive effects of early malnutrition: a theoretical reappraisal. J Nutrit. 1995;125:2221S–2232S. doi: 10.1093/jn/125.suppl_8.2221S. [DOI] [PubMed] [Google Scholar]
- Struthers JM, Hansen RL. Visual recognition memory in drug-exposed infants. J Dev Behav Pediatr. 1992;13:108–111. doi: 10.1097/00004703-199204000-00005. [DOI] [PubMed] [Google Scholar]
- Vorhees CV, Ahrens KG, Acuff-Smith KD, Schilling MA, Fisher JE. Methamphetamine exposure during early post-natal development in rats: I., acoustic startle augmentaion and spatial learning deficits. Psychopharmacology. 1994a;114:392–401. doi: 10.1007/BF02249328. [DOI] [PubMed] [Google Scholar]
- Vorhees CV, Ahrens KG, Acuff-Smith KD, Schilling MA, Fisher JE. Methamphetamine exposure during early post-natal development in rats: II. Hypoactivity and altered responses to pharmacological challenge. Psychopharmacology. 1994b;114:402–408. doi: 10.1007/BF02249329. [DOI] [PubMed] [Google Scholar]
- Vorhees CV, Inman-Wood SL, Morford LL, Broening HW, Fukumura M, Moran MS. Adult learning deficits after neonatal exposure to D-methamphetamine: selective effects on spatial navigation and memory. J Neurosci. 2000a;20:4732–4739. doi: 10.1523/JNEUROSCI.20-12-04732.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorhees CV, Inman-Wood SL, Morford LL, Reed TM, Moran MS, Pu C, Cappon GD. Evaluation of neonatal exposure to cocaine on learning, activity, startle, scent marking, immobility, and plasma cocaine concentrations. Neurotoxicol Teratol. 2000b;22:255–265. doi: 10.1016/s0892-0362(99)00071-9. [DOI] [PubMed] [Google Scholar]
- Vorhees CV, Morford LL, Inman SL, Reed TM, Schilling MA, Cappon GD, Moran MS, Nebert DW. Genetic differences in spatial learning between Dark Agouti and Sprague–Dawley strains: possible correlation with the CYP2D2 polymorphism in rats treated neonatally with methamphetamine. Pharmacogenetics. 1999;9:171–181. [PubMed] [Google Scholar]
- Vorhees CV, Reed TM, Schilling MA, Fisher JE, Moran MS, Cappon GD, Nebert DW. CYP2D1 polymorphism in methamphetamine-treated rats: genetic differences in neonatal mortality and effects on spatial learning and acoustic startle. Neurotoxicol Teratol. 1998;20:265–273. doi: 10.1016/s0892-0362(97)00129-3. [DOI] [PubMed] [Google Scholar]
- Williams MT, Blankenmeyer TL, Schaefer TL, Brown CA, Gudelsky GA, Vorhees CV. Long-term effects of neonatal methamphetamine exposure in rats on spatial learning in the Barnes maze and on cliff avoidance, corticosterone release, and neurotoxicity in adulthood. Dev Brain Res. 2003a;147:163–175. doi: 10.1016/j.devbrainres.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Williams MT, Inman-Wood SL, Morford LL, McCrea AE, Ruttle AM, Moran MS, Rock SL, Vorhees CV. Preweaning treatment with methamphetamine induces increases in both corticosterone and ACTH in rats. Neurotoxicol Teratol. 2000;22:751–759. doi: 10.1016/s0892-0362(00)00091-x. [DOI] [PubMed] [Google Scholar]
- Williams MT, Moran MS, Vorhees CV. Refining the critical period for methamphetamine-induced spatial deficits in the Morris water maze. Psychopharmacology. 2003b;168:329–338. doi: 10.1007/s00213-003-1433-y. [DOI] [PubMed] [Google Scholar]
- Williams MT, Morford LL, Wood SL, Rock SL, McCrea AE, Fukumura M, Wallace TL, Broening HW, Moran MS, Vorhees CV. Developmental 3,4-methylenedioxymethamphetamine (MDMA)-induced learning deficits are not related to undernutrition or litter effects: novel use of litter size to control for MDMA-induced growth decrements. Brain Res. 2003c;968:89–101. doi: 10.1016/s0006-8993(02)04278-6. [DOI] [PubMed] [Google Scholar]
- Williams MT, Morford LL, Wood SL, Wallace TL, Fukumura M, Broening HW, Vorhees CV. Developmental d-methamphetamine treatment selectively induces spatial navigation impairments in reference memory in the Morris water maze while sparing working memory. Synapse. 2003d;48:138–148. doi: 10.1002/syn.10159. [DOI] [PubMed] [Google Scholar]
- Williams MT, Vorhees CV, Boon F, Saber AJ, Cain DP. Methamphetamine exposure from post-natal day 11–20 causes impairments in both behavioral strategies and spatial learning in adult rats. Brain Res. 2002;958:312–321. doi: 10.1016/s0006-8993(02)03620-x. [DOI] [PubMed] [Google Scholar]