Abstract
Background
Fish is an important source of nutrition worldwide. Fish contain both the neurotoxin methyl mercury (MeHg) and nutrients important for brain development. The developing brain appears to be most sensitive to MeHg toxicity and mothers who consume fish during pregnancy expose their fetus prenatally. Although brain development is most dramatic during fetal life, it continues for years postnatally and additional exposure can occur when a mother breast feeds or the child consumes fish. This raises the possibility that MeHg might influence brain development after birth and thus adversely affect children’s developmental outcomes. We reviewed postnatal MeHg exposure and the associations that have been published to determine the issues associated with it and then carried out a series of analyses involving alternative metrics of postnatal MeHg exposure in the Seychelles Child Development Study (SCDS) Main Cohort.
Methods
The SCDS is a prospective longitudinal evaluation of prenatal MeHg exposure from fish consumption. The Main Cohort includes 779 subjects on whom recent postnatal exposure data were collected at the 6, 19, 29, 66, and 107 month evaluations. We examined the association of recent postnatal MeHg exposure with multiple 66 and 107-month outcomes and then used three types of alternative postnatal exposure metrics to examine their association with the children’s intelligence quotient (IQ) at 107 months of age.
Results
Recent postnatal exposure at 107 months of age was adversely associated with four endpoints, three in females only. One alternative postnatal metric was beneficially associated with 9-year IQ in males only.
Conclusions
We found several associations between postnatal MeHg biomarkers and children’s developmental endpoints. However, as has been the case with prenatal MeHg exposure in the SCDS Main Cohort study, no consistent pattern of associations emerged to support a causal relationship.
Keywords: Methylmercury, prenatal exposure, postnatal exposure, child development, neurodevelopment, fish, Seychelles Child Development Study
INTRODUCTION
Mercury is naturally present in the earth’s crust and is widespread in the environment (WHO, 1990). Common bacteria in aquatic environments methylate part of the environmental inorganic mercury to the organic form of MeHg. After entering the aquatic food web, MeHg is bioaccumulated and bioconcentrated and all fish acquire it to varying degrees. All fish consumption leads to some degree of MeHg exposure.
Several outbreaks of poisoning following exposure to high concentrations of MeHg took place during the twentieth century. Pathological studies of subjects poisoned during those episodes indicated that MeHg poisoning affects the brain differently depending upon the age at which exposure occurs (Takeuchi, 1968; Choi & Lapham). Prenatal poisoning in Japan and Iraq was reported to cause diffuse brain damage while adult poisoning initially caused more focal damage affecting the visual cortex, motor area and cerebellum. Poisoning during childhood produced a pattern of damage with features of both prenatal and adult exposure. However, the pathological findings were closer to those of prenatal exposure. One study of prenatal MeHg exposure at the levels achieved by fish consumption found no pathological changes in the brain using routine histopathology (Lapham et al., 1995). Children prenatally poisoned by MeHg presented clinically with severe cognitive and motor deficits, seizures, and microcephaly (Harada 1968). Adults who were poisoned presented initially with paresthesias, visual loss, and ataxia reflecting more focal impairment of the brain. Clinical outcomes when poisoning occurred during childhood were less well characterized but included motor and cognitive deficits (Amin-Zaki, 1976; Englesson & Herner, 1952). These poisoning outbreaks confirmed that MeHg appears to have its greatest neurotoxic effect on the developing brain.
Fish consumption and consequently exposure to MeHg is common. The Food and Agriculture Organization of the United Nations (FAO) estimates that one billion people worldwide depend upon fish for daily nutrition (FAO, 2000). Methyl mercury poisoning with clinical symptoms resulting from fish consumption has been reported on two occasions, both from Japan. Methyl mercury poisoning from other types of exposure has been reported on several occasions. The possibility of more subtle adverse associations that might be difficult to detect has led to widespread public concern (Myers & Davidson, 2006). From a public health perspective it is important to know if prenatal or postnatal exposure to MeHg at the levels achieved by fish consumption causes neurotoxic consequences that can be detected in children. This question has been the focus of several epidemiologic studies. These studies have primarily focused on prenatal exposure to MeHg and examined its association with the children’s developmental outcomes using epidemiological methods.
The rate of brain growth peaks during gestation, but it continues to progress rapidly during the first two years of life and at a slower pace through adolescence and beyond. There are no specific anatomical or physiologic events that occur in the brain at birth to mark the transition from fetal life to infancy. Exposure to MeHg can continue following birth if mothers consume fish and breast feed (Bakir et al., 1973; Amin-Zaki, et al., 1974, 1981; Grandjean et al., 1994, 1995; Chien et al, 2006). In addition, as children grow and fish is introduced into their diets, further exposure can occur.
In this paper we provide a background on brain development and review the literature on postnatal MeHg exposure in children and its association with children’s development. We then describe the association of recent postnatal MeHg exposure and developmental testing present in the primary Seychelles Child Development Study (SCDS) analyses. Next we examine three alternative metrics for measuring postnatal exposure based on the recent postnatal MeHg measurements at multiple time points, and report on their association with the children’s full scale IQ measured by the WISC III at 107 months of age. We present our results in two sections, first for the associations of postnatal exposure from the primary SCDS main cohort analyses that focused on prenatal MeHg exposure when the children were 66 (Davidson et al, 1998) and 107 months of age (Myers et al, 2003) and second for the alternative postnatal MeHg exposure metrics.
Postnatal Brain Development
The normal neonatal brain weighs about 350–400 grams at birth and triples in size during the first 18 years of life. This rapid brain development is most apparent in the enlarging postnatal head circumference (HC). The average HC of a child at birth is about 35 cm, but by age 2 years it increases to about 50 cm and by 18 years to 56 cm. The increase in HC and in corresponding brain weight is associated with a host of anatomic and physiological changes that occur continuously from birth through adolescence and beyond (Rice & Barone, 2000; Volpe, 2001). Nearly all neurons in the cortex develop in the germinal matrix near the ventricles prior to birth and then migrate to their final location in the cerebral cortex before establishing connections. Neurons in the cerebellum in contrast develop following birth. At birth some cortical neurons are still migrating and many have not started to mature or differentiate. Those that have reached their final destination are still in the process of extending axons and dendrites, developing dendritic spines, and forming synapses. The establishment of the myriad connections that characterize the mature brain is a continuing postnatal process that continues for many years following birth. Synapses begin to form in the third trimester and develop actively during the first two years of life. The maximum number of synapses is achieved at about age two years and they are subsequently pruned and reduced by approximately 40% by adolescence. Myelination is minimal at birth and continues well into adulthood. The numerous neurotransmitters present in the brain have individual patterns of development and some develop after birth (Rice & Barone, 2000). For example nicotinic receptors in the reticular formation develop during mid gestation while GABA receptors in the neocortex develop predominantly during the first year of life. The blood brain barrier does not completely form until after birth (Rodier, 2004). A significant number of the cortical neurons present at birth subsequently undergo apoptosis, but the factors that determine which neurons will die and which synapses will be retained are largely unknown. Neurotoxins might conceivably influence this process.
Effects of MeHg on the Developing Brain
Several mechanisms have been proposed by which MeHg might damage the developing brain. Among them are MeHg induced alterations in microtubules, oxidative damage to neurons, impairment of neuronal and glial calcium homeostasis, and the potentiation of glutamatergic neurotransmission (Castoldi et al., 2003). The effect of disrupting microtubules and consequently mitosis, migration, and cortical organization of neurons is especially serious prenatally, although these processes still continue postnatally (Rodier, 2004; Vogel et al, 1985; Clarkson, 1987; Choi et al., 1978). Chemically, MeHg has a strong affinity for sulfhydryl groups that are present on proteins and glutathione. When MeHg complexes with these compounds it can adversely affect anabolic processes and protein synthesis (Bondy, 1994; Slikker, 1994; Syversen, 1982). By inactivating sulfhydryl enzymes MeHg can interfere with cellular metabolism and function. Methyl mercury is also known to catalyze the formation of excess reactive oxygen species and the regional distribution of this activity parallels the sites of known neuropathological changes (Bondy, 1994). Methylmercury rapidly binds to reduced glutathione (GSH) which is present in most cells in millimolar concentrations (Clarkson & Magos, 2006). This binding may serve to protect intracellular proteins. There are also adverse effects of MeHg on the synthesis of fetal DNA in astrocytes, and on the growth cones of neurons (Marsh, 1994). Additionally, it can induce structural chromosomal aberrations in experimental animals (Ehrenstein et al., 2002). Most of these physiological processes are continuously active in the brain following birth.
Takeuchi (1968) noted a number of developmental brain deviations present in prenatal MeHg poisoning (fetal Minamata disease) that were not found with postnatal or infantile exposure. These included the presence of nerve cells in the cerebral medulla, columnar grouping of nerve cells in the cerebral cortex, abnormal cytoarchitecture of nerve cells in the cerebral and cerebellar cortices, and dysplasia of nerve cells with poor myelination (Choi, 1989).
In cell cultures, low dose exposure to MeHg has been shown to cause physiological disturbances such as cell cycle inhibition without cytotoxicity (Gribble et al., 2005). In experimental animals such as the rat, low dose exposure over long time periods has been reported to result in alteration of brain neurotransmitters (Slikker, 1994).
Postnatal MeHg Exposure and Developmental Outcomes
Reports of postnatal MeHg exposure fall into two general categories. Some are of children with overt clinical poisoning and others are from epidemiology studies looking for subtle population differences between normal children with varying levels of chronic MeHg exposure. In cases of overt poisoning such as occurred in Iraq, the Hg exposure level that correlated best with clinical outcomes was the peak hair value (Bakir et al., 1973). However, most human exposure is to small amounts of MeHg present in dietary sources such as fish and seafood where no significant peaks are usually present.
Postnatal Clinical Poisoning by MeHg
A small number of children have been reported in the literature with postnatal MeHg poisoning (Englesen & Herner; 1952; Harada 1968; Amin-Zaki et al., 1976; Davis et.al., 1994). Among these reports, individuals where exposure was from fish consumption were reported only from Minamata and Niigata Japan. No cases of poisoning from fish consumption have been reported elsewhere. The first child reported in the literature consumed MeHg treated grain and subsequently was diagnosed with a developmental delay (Englesen & Herner, 1952). In Japan the children poisoned at Minamata where exposed to MeHg along with several other neurotoxicants (Harada, 1968; Takeuchi & Eto, 1977). Several cases were reported from Iraq where exposure was to MeHg treated seed grain (Amin-Zaki et al., 1976, 1978, 1980, 1981). Table 1 outlines the cases of postnatal MeHg poisoning that have been reported in children.
Table 1.
Reported cases of childhood postnatal MeHg poisoning with clinical symptoms.
| Country/Reference | Postnatal Hg Exposure index | Age exposed | Age tested | Reported Symptoms/Signs |
|---|---|---|---|---|
|
Sweden Engleson & Herner, 1952 |
62 gamma/L urine | 9m M | 3y. | Mental retardation |
|
United States Snyder, 1972 Pierce et al., 1972 Brenner & Snyder, 1980 Davis et al., 1994 |
1,397 ppm hair 0.20 ppm urine 1.92 ppm serum | 8y F | 8y | Ataxia & agitation then dementia, blind & paralysis, died at 29y |
| 1,910 ppb blood 0.21 ppm urine 3.3 ppm csf | 13y M | 35y | Verbal IQ 86, only central vision, poor coordination | |
| 329 ppm hair | 16y F | 38y | Normal | |
|
Japan * Harada, 1968 Takeuchi et al., 1977 |
4–165 ppm (average 43 ppm) hair 0.04 – 21.4 ppm in autopsies | Chronic infancy | 1–14 y N=30 |
All had dysarthria, ataxia, and mental impairment |
|
Iraq* Amin-Zaki et al., 1976 Amin Zaki et al., 1978 Elhassani SB et al., 1978 Amin-Zaki et al., 1980 Amin-Zaki et al., 1981 |
30–1,500 ppb blood | Subacute infancy | 1–5 y N=50 |
Ataxia, dysarthria, impaired vision, & hearing, weakness, hyperreflexia |
| 1,600 ng/ml (ppb) blood | 1y F | 5.5y | 5.5y normal motor & cognitive | |
| 1,734 ng/ml (ppb) blood | 10y M | 15y | 15y MR & CP |
= It is unclear if there is overlap of patients reported in these papers
M= male
F = female
There is one report of children poisoned by MeHg in the United States (Snyder 1972; Brenner & Snyder 1980; Pierce et al., 1972; Davis et al., 1994). A family in New Mexico consumed a hog that had been fed MeHg treated seed grain and had a MeHg level of 35 ppm. The family included four children under the age of 18 years. Two of the children had severe neurological damage. An 8-year old girl with a hair Hg level of 1,398 ppm had cognitive impairment, choreoathetosis, seizures and quadriparesis. Her 13-year old brother also had severe neurological impairment, but his exposure was not measured. Two sisters ages 9 and 16 years at exposure had no symptoms. The older girl had a hair Hg level of 329 ppm following exposure. These two girls were examined over 20 years later and reported to be neurologically normal (Davis et al., 1994).
Epidemiology Studies that include Postnatal MeHg Exposure
Two longitudinal and three cross sectional epidemiology studies have included an index of postnatal MeHg exposure. Both longitudinal studies obtained children’s hair samples for postnatal exposure when they were undergoing clinical assessments to determine the children’s development. Table 2 lists the reports and references from these studies. The Faeroe Islands study measured prenatal MeHg exposure in cord blood and maternal hair during pregnancy, and postnatal exposure in the children’s blood at ages 7 and 14 years, and in children’s hair at ages 1, 7, and 14 years. The SCDS measured prenatal MeHg exposure in maternal hair growing during pregnancy, and postnatal exposure in children’s hair at ages 6, 19, 29, 66, and 107 months.
Table 2.
Epidemiology studies reporting associations between postnatal MeHg exposure and developmental endpoints
| Reference | Postnatal index | N | Age of testing | Tests Associated with Postnatal exposure measured in child hair |
|---|---|---|---|---|
| Grandjean et al., 1995 | Hair Hg 1 y | 581 | 1 yr | Beneficial association with Developmental milestones (sitting, creeping, standing): |
| Grandjean et al., 1997 | Hair Hg 1 and 7 y | 917 | 7 yr | Child’s hair at 1 year “…was a significant predictor for Finger Tapping with both hands and CPT reaction time…” Child’s hair at 7 years “…was significantly associated with CPT reaction time, Block Designs, and Bender Visual Motor Gestalt errors.” However, “…after adjustment for the cord blood mercury concentration, the hair mercury measures were not significantly related to any dysfunction.” |
| Grandjean et al., 1999a | Hair Hg 7 y Blood Hg | 903 | 7 yr | Bender Gestalt: significant adverse association with both biomarkers |
| Debes et al., 2006 | Hair Hg 14 y | 878 | 14 yr | “Postnatal methylmercury exposure had no discernible effect.” |
| Cordier et al. 2002 | Hair Hg 0.5–6 y | 378 | 0.5–6 y | Child hair Hg associated with bead memory score and digit span score in girls only |
| Grandjean et al., 1999b | Hair Hg 7–12 y | 351 | 7–12 y | “Neuropsychological tests of motor function, attention, and visuospatial performance showed decrements associated with the [child] hair-mercury concentrations.” |
| Murata et al. 1999 | Hair Hg 7 y | 149 | 7 y | “No…relationships were seen with the child’s own hair-mercury concentration…” |
| Davidson et al., 1998 | Hair Hg 5.5 y | 711 | 5.5 yr | Bender-Gestalt: THg by Sex interaction significant. Males significantly worse as exposure increases PLS: No interaction. Significant improvement with increasing exposure W-J Applied Problems: No interaction. Significant improvement with increasing exposure |
| Myers et al., 2000 | Hair Hg 5.5 y | 711 | 5.5 yr | CBCL: No significant associations with subscales |
| Axtell et al., 2000 | Hair Hg 5.5 y | 711 | 5.5 yr | MSCD GCI: Nonlinear models showed an association. Scores improved below 10 ppm and worsened above 10 ppm |
| Huang et al., 2003 | Hair Hg 5.5 y | 711 | 5.5 y | MSCD-GCI, W-J subtests, and Bender Gestalt only for females: using measurement error models a beneficial association present |
| Myers et al., 2004 | Hair Hg 9 y | 643 | 9 y | CBCL - Thought Problems subscale: An adverse association |
CBCL = Child Behavior Checklist
MSCD = McCarthy Scales of Child Development
CPT = Connor’s Continuous Performance Test
Hg = mercury
W-J = Woodcock-Johnson
PLS = Preschool Language Scale
GCI = General Cognitive Index
THg = Total mercury
The Faeroe Islands investigators reported that at age 12 months longer periods of breastfeeding were associated with early achievement of developmental milestones assessed by history (Grandjean et al., 1995). At ages 7 and 14 years cohort children were administered an extensive battery of tests. The investigators reported significant adverse associations between children’s hair Hg levels measured at 12 months of age and two endpoints measured at age 7 years (Finger Tapping with both hands and the Reaction Time from the Continuous Performance Test) (Grandjean et al., 1997). They also reported adverse associations between the children’s hair Hg level measured at 7 years of age and several endpoints (the Continuous Performance Test, Reaction Time [CPT-RT], the Block Design subtest from the Wechsler Intelligence Scale for Children –Revised [WISC R], and the Bender Visual Motor Gestalt Copying Errors score [BG-ES]). None of the associations with postnatal MeHg exposure were significant when the analyses were adjusted for prenatal exposure measured in cord blood. At the 14 year evaluations they reported that “postnatal methylmercury exposure had no discernable effect” (Debes et al., 2006).
In the SCDS main cohort primary analyses, recent postnatal exposure was first included as a covariate in the 66 month evaluation since children were then actively consuming fish. At age 66 months in the primary analysis increasing postnatal total Hg (THg) exposure was associated with improving performance on the Preschool Language Scale –Total Score (PLS-TS), the Woodcock-Johnson Applied Problems (WJ-AP), and the BG-ES (Davidson et al., 1998). For the BG-ES the interaction of postnatal Hg with sex was significant (p=0.004) (Davidson et al., 1998). For males their performance improved (regression coefficient = −0.16 ppm; p=0.009). For females the slope was slightly positive indicating poorer performance, but it was not significant (p=0.14). The association of THg exposure with improved performance on some tests was not expected since MeHg is toxic and has no known function in the human body. However, MeHg exposure is mainly from consuming fish, and they also contain nutrients. These results raised the intriguing possibility the nutrients present in fish might be having a significant beneficial influence on outcomes.
Methods
The SCDS is a longitudinal, prospective, double-blind epidemiological evaluation examining the association between prenatal MeHg exposure from maternal fish consumption and developmental outcomes in children. The main cohort consists of 779 maternal child pairs enrolled in 1989–1990 and followed longitudinally. Cohort children were administered a battery of developmental tests at ages 6, 19, 29, 66, and 107 months of age. The test batteries included global and domain specific tests including nearly all of the tests administered in other epidemiological studies. Study methods and the results of analyses examining the association between prenatal MeHg exposure and outcomes have been described extensively in the peer reviewed literature (Shamlaye et al., 1995; Davidson et al., 1998; Myers et al., 2003).
Measuring postnatal exposure
To determine recent postnatal MeHg exposure, samples of the children’s hair were taken at evaluations. We measured postnatal MeHg exposure as THg in the 1 cm of hair closest to the child’s scalp using cold vapor atomic absorption spectroscopy. This metric represents about one month of exposure. Total mercury in hair correlates well with blood mercury and has been used in most previous studies. Concentrations of mercury in newborn hair are known to correlate with those in the child’s brain and in their mother’s hair and this is thought to be the case for older children as well (Cernichiari et al., 1995). This metric was selected because many of the children’s hair samples were relatively short and it provided a comparable measure of postnatal exposure for all the subjects. Maternal hair samples cannot serve as a proxy for postnatal exposure since there is no reason to suspect a biological relationship. A continuous measure of postnatal exposure would be preferable, but logistics and resources precluded more frequent sampling. The SCDS was designed to examine prenatal exposure and there are gaps in the postnatal exposure data. To address this discontinuity, we used the available data to develop alternative postnatal metrics based upon theoretical considerations. Each alternative metric combines postnatal THg measurements from multiple time points into a single postnatal exposure metric. The postnatal exposure metrics except for the High-Low are continuous.
Alternative metrics
To study postnatal exposure, we used three alternative postnatal metrics based on different biological postulates and the children’s hair values measured at multiple time points. We examined the association of each metric with the full scale IQ measured at 9 years of age. We selected the children’s IQ because one of the main clinical findings in reported human cases of postnatal MeHg poisoning is cognitive delay (Englesson & Herner, 1952; Amin-Zaki et al., 1980; Davis et al., 1994). Each of the alternative metrics was determined first using postnatal hair values from 19 and 66 months and then using hair values from 6, 19, and 66 months. These alternative metrics did not make use of hair samples taken at 29 or 107 months because of the limited number of samples available at these time points (Davidson et al., 2006).
Cumulative Exposure, Measured by the Area under the Exposure Curve (AUC)
The AUC metric is based on the hypothesis that MeHg entering the brain is cumulative and that damage occurs as exposure increases over time. The AUC is defined as the area under the curve of exposure over a specific time interval, i.e. cumulative exposure during a specified time interval. A cumulative exposure metric is commonly used with other toxins such as tobacco (Thurston et al., 2005). We determined AUC by assuming a linear exposure trajectory between the Hg values at observed time points and then calculating the area under the curve between adjacent time points. We used two AUC values: Cumulative exposure between 19 and 66 months, and cumulative exposure between 6 and 66 month. The latter makes use of postnatal measurements at 6, 19, and 66 months.
High-Low Exposure (HL)
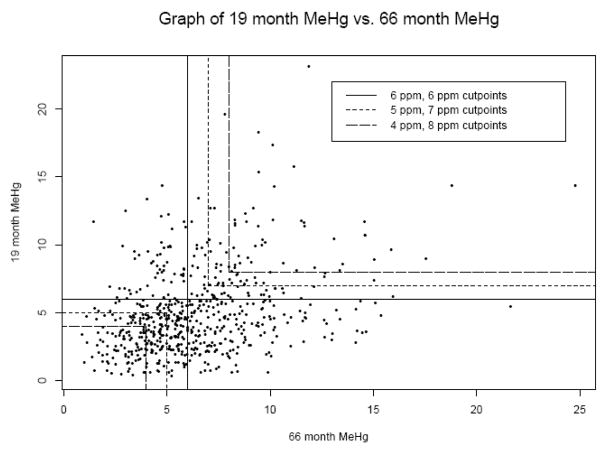
The HL metric is based on the biological premise that the greatest toxicity results from exposure levels that are consistently high. It compares children who had consistently high Hg levels at multiple time points, to children who had consistently low levels. Different versions of HL used different cutoff points to distinguish high and low exposures. We started with a cutoff of 6.0 ppm, which is approximately the average postnatal MeHg exposure in the SCDS. Individuals with postnatal MeHg concentrations below 6.0 ppm at a particular time point were considered in a “low” category at that time point, and those above were in a “high” category. Categorizations using the concentration threshold pairs of 5.0 and 7.0 ppm (where “low” was considered below 5.0 ppm and “high” was considered above 7.0 ppm), and also 4.0 and 8.0 ppm were used in separate metrics. Children who had concentration measures between the threshold pair values for the 5.0 and 7.0 ppm and 4.0 and 8.0 ppm pairs were not considered in this portion of the analysis. Each threshold (6.0 ppm) or threshold pair (5.0 and 7.0 ppm, then 4.0 and 8.0 ppm) was applied to the 19 and 66 month time points. If an individual’s Hg levels were below the lower threshold at both time points, his or her HL was deemed “low” (coded as 0), and conversely if exposure was above the higher threshold at both times, the HL was deemed “high” (coded as 1). Children who were “low” at one time point and “high” at another were not included in the analysis. Due to sample size constraints, when applying this metric to all three time points we only used the 6.0 ppm cutoff to distinguish “high” from “low”. In this case a child’s HL was “high” if his or her exposure was above 6.0 ppm at all three time points and “low” if his or her exposure was below 6.0 ppm at all three time points.
Brain Growth Weighted Exposure (BGW)
The BGW postnatal metric is based on the observation that MeHg is most toxic to the developing brain and that postnatal brain development is most rapid during the early months and years of life. This metric weights exposure at a given age based on the relative estimated increase in brain size at that age. We reasoned that the MeHg toxicity affects the child’s brain in proportion to the rate of neurodevelopment ongoing at the time of exposure and that the rate of neurodevelopment can be approximated by the rate of brain growth. We used two methods to estimate brain growth rate, one based on change in head volume and one based on change in head area. Both relied on first estimating head circumference at 6, 19, and 66 months and were calculated for boys and girls separately using standard head circumference growth charts available through the Center for Disease Control (CDC) website (CDC 2008) and the Nellhaus charts (Nellhaus G, 1968). For the first BGW metric the relative growth rates were converted into relative changes in head volume, assuming the head is approximately spherical, and then converted into weights summing to one. We then multiplied each weight by the THg levels at the corresponding time point and summed these to obtain the BGW THg exposure. However, the 6 month value accounted for approximately 98% of the weight, due to the very rapid change in head circumference at 6 months as compared to 19 and 66 months. Because of the disproportionate weighting on one time point and for simplicity reasons, we replaced this particular metric by the 6 month THg value.
The second BGW metric approximates the rate of brain growth at a particular age by the change in head area at that age. Like head volume, the estimated change in head circumference was calculated from standard growth charts, converted to changes in head area assuming the head area is approximately circular, and converted into weights summing to one. As before, the metric multiplies the estimated brain growth weight at a particular age by the THg exposure at that age, and this is then summed over the time points. We developed this metric first using 19 and 66 month data, and then using 6, 19, and 66 month data. For BGW based on area changes using 19 and 66 month growth rates, the estimated weights were 0.83 and 0.17 respectively for boys, and were 0.86 and 0.14 for girls. Using all three time points of 6, 19, and 66 months to determine area changes, the circumference weights were 0.78, 0.18 and 0.04 months respectively for boys, and 0.74, 0.23 and 0.04 for girls. In all cases the weights were then multiplied by the THg hair level at the corresponding time point to give the BGW THg metric.
Statistical analysis
We used linear regression as in all the earlier SCDS analyses (Davidson et al., 1998, Myers et al., 2003). We first considered models that included the postnatal THg by sex interaction, and report results from this model if the interaction for postnatal THg was significant at p<0.05. Otherwise we report results from the corresponding model without this interaction. We did not include prenatal MeHg by sex interactions for any new analyses reported in this paper, because our primary interest was in the postnatal effects, and how these differed when postnatal exposure was defined in different ways.
The covariates used in each model, and their definitions, are presented in Table 3. The covariates used for the alternate postnatal THg metrics are those adjusted for in the earlier SCDS analyses, with the following exceptions: (i) we did not adjust for hearing group, (ii) we collapsed family status into two groups rather than three, and (iii) we treated both HOME score and SES as continuous variables rather than categorizing them. The first two decisions were made because the reduced sample size for testing the H-L alternative metric did not support inclusion of indicator variables with a very small number of observations in a cell. The decision not to categorize continuous variables was made based on earlier analyses which suggested approximate linear relationships between 107-month IQ and both HOME score and SES. We did not exclude outliers in any of the models using alternative postnatal metrics.
Table 3.
Covariates used in postnatal analyses and their definitions.
| Covariate | Definition | 66 Month Endpoint | 107 Month Primary analysis | 107 Month IQ Alternative metrics |
|---|---|---|---|---|
| Prenatal MeHg exposure | Average of maternal hair during pregnancy | Continuous | Continuous | Continuous |
| Postnatal MeHg exposure | Measured in 1 cm of hair taken at evaluation | Continuous | Continuous | Various Metrics |
| Maternal | ||||
| Age | In years | Continuous | Continuous | Continuous |
| Mother IQ* | Measured with Ravens Progressive Matrices | 3 categories | - | - |
| Measured with K-Bit | - | Continuous | Continuous | |
| Socioeconomic status | Measured using the Hollingshead Socio- economic scale at 107 months | 4 categories | 4 categories | Continuous |
| Child | ||||
| Sex | Male/female | Male/female | Male/female | |
| Medical history | Positive if head circumference >2 SD from norm or SGA | Yes/No | Yes/No | Yes/No |
| Hearing status | Measured by audiogram at evaluation | 3 categories | 3 categories | - |
| Birth Weight | Child Weight | Continuous | - | |
| Age at testing | Continuous | Continuous | Continuous | |
| Family | ||||
| HOME score | Measured at 4.5 years of age during a home visit | 3 categories | 3 categories | Continuous |
| Family resource scale | Continuous | Continuous | ||
| Family status | Positive if lives with 0 or 1 biological parent | - | 3 categories | 2 categories |
| HELPS score | Henderson Early Learning Process scale | Continuous | Continuous | |
| Tester | Indicator variables to distinguish between 3 testers | - | 3 categories | 3 categories |
2% of care givers were not mothers
HOME = Home Observation for Measurement of the Environment
HELPS = Henderson Early Learning Process
Results
Correlations between Exposure Measures
We first examined the association between prenatal and postnatal metrics measured at different time points. These correlations are presented in Table 4 and were all 0.30 or below. The correlation between prenatal and postnatal exposure was greatest at 6 months of age and decreased as the children matured. The correlation between postnatal exposures measured at different ages was greatest between the 66 and 107 month values. This suggests that by about age 5 years the children’s hair THg values and presumably their dietary patterns of fish consumption have stabilized.
Table 4.
Correlations and mean (SD) values of prenatal and postnatal hair Hg in ppm from maternal and child samples in the SCDS main cohort.
| 6 m Mean = 6.6 (4.4) n = 699 |
19 m Mean = 4.8 (3.1) n = 739 |
66 m Mean = 6.5 (3.3) n = 694 |
107 m Mean = 6.1 (3.6) n = 537 |
|
|---|---|---|---|---|
|
Prenatal Mean = 6.8 (4.5) n = 711 |
0.303 n = 641 |
0.178 n = 687 |
0.152 n = 694 | 0.065 n = 511 |
| 6 m | 0.199 n = 671 |
0.218 n = 626 |
0.158 n = 479 |
|
| 19 m | 0.366 n = 678 |
0.131 n = 517 |
||
| 66 m | 0.443 n =508 |
Postnatal Associations in the 66 and 107 Month Primary Linear Models
in the primary and secondary analyses of prenatal exposure starting at 66 months of age recent postnatal exposure was included as a covariate (Davidson, et al, 1998; Myers, et al., 2003; Myers et al., 2000; Axtell et al., 2000; Davidson et al., 2004; Myers et al., 2004; Huang et al., 2003; Huang et al., 2005). The results specific to postnatal THg exposure in the primary analysis were reported at 66 months, but space limitations precluded reporting them at 107 months. Those results are presented here. At the 107 month evaluations there were 143 males for whom a hair sample could not be obtained because shaving the head had become a popular fad. For those individuals, the missing values were replaced with the most recent postnatal THg value available, usually that from 66 months (Davidson et al, 2006). However for 14 subjects, 66 month values were also not available, and their 48-month THg hair values were used. Results from the primary 66-month analyses (Davidson et al 1998) also used the 48-month values when the 66-month values were not available. We followed the same procedure when analyzing additional models at 66 months.
The primary analyses at 66 months (Davidson et al, 1998) and at 107 months (Myers et al, 2003) reported on results using a reduced set of covariates, and from models fit excluding outliers. For consistency, our alternative models for the 66-month outcomes used the same covariates and excluded outliers. The contribution of this paper to the 66-month outcomes is to compare the results reported in Davidson et al. (1998), which used 66-month postnatal THg values, to results from models in which the 66-month postnatal THg value is replaced first by 19-month postnatal MeHg, and then by the sum of the 19 and 66 month values.
66 Month Outcomes
The recent postnatal THg associations present in the primary analysis for the 66 month endpoints have been previously reported (Davidson et al., 1998), and for convenience, are repeated in the top row of Table 5. We report slopes and associated p-values for the new analyses, whereas Davidson et al. reported slopes and associated standard errors. When 19 month postnatal exposure was included in the primary analysis in place of the 66-month postnatal exposure there was an association with enhanced performance on two test outcomes (PLS-TS for boys only and WJ-AP for both sexes). When the sum of the 19-month and 66-month postnatal values was used as the postnatal metric in the primary analysis, postnatal exposure was significantly associated with improved performance on three outcomes for both sexes (PLS-TS, WJ-AP, and MSCA-GCI).
Table 5.
Results of primary linear analyses examining the association between 66 month endpoints and pre and postnatal THg, with postnatal THg exposure coded as 66 month exposure, 19 month exposure, or their sum.
| Hg Exposure |
MSCI–GCI Coefficient (p-value) |
PLS-TS Coefficient (p-value) |
WJ-LW Coefficient (p-value) |
WJ-AP Coefficient (p-value) |
CBCL –TS Coefficient (p-value) |
BG-ES Coefficient (p-value) |
|---|---|---|---|---|---|---|
| Model including 66 month postnatal exposure * | ||||||
| N | 644 | 606 | 641 | 641 | 645 | 631 |
| Prenatal | −0.06 (0.59) | 0.13 (0.02) | 0.02 (0.84) | 0.11 (0.41) | −0.11 (0.22) | −0.02 (0.64) |
| Postnatal 66 months | 0.26 (0.06) | 0.18 (0.02) | 0.15 (0.20) | 0.36 (0.0501) | −0.02 (0.85) | --- |
| Postnatal × Sex p-value | (0.009) | |||||
| Postnatal: Female | 0.08 (0.20) | |||||
| Postnatal: Male | −0.15 (0.01) | |||||
| Model including 19 month postnatal exposure | ||||||
| N | 626 | 588 | 623 | 624 | 627 | 615 |
| Prenatal | −0.06 (0.56) | 0.14 (0.02) | 0.02 (0.83) | 0.10 (0.49) | −0.11(0.20) | −0.02 (0.52) |
| Postnatal 19 months | 0.26 (0.09) | --- | 0.22 (0.10) | 0.52 (0.01) | −0.07 (0.60) | −0.08 (0.12) |
| Postnatal × Sex p-value | (0.02) | |||||
| Postnatal: Female | 0.03 (0.79) | |||||
| Postnatal: Male | 0.42 (< 0.001) | |||||
| Model including 19 and 29 month postnatal exposure | ||||||
| N | 624 | 586 | 621 | 622 | 625 | 613 |
| Prenatal | −0.07 (0.5) | 0.13 (0.03) | 0.01 (0.91) | 0.08 (0.55) | −0.11 (0.21) | −0.02 (0.55) |
| Postnatal sum: 19 and 66 months | 0.20 (0.03) | 0.15 (0.003) | 0.13 (0.09) | 0.36 (0.003) | −0.03 (0.73) | −0.05 (0.07) |
Data published previously (Davidson et al., 1998)
MSCI-GCI = McCarthy Scales of Children’s Intelligence General Cognitive Index score
PLS-TL = Preschool Language Scale Total Language score
WJ-LW = Woodcock Johnson Letter Word subtest
WJ-AP = Woodcock Johnson Applied Problems subtest
CBCL-TS = Child Behavior Checklist (Achenbach) Total score
BG-ES = Bender Gestalt Error score
107 Month Outcomes
There were 21 endpoints evaluated at 107 months and here we report on the coefficients for postnatal THg from the primary analysis (Table 6). Each model was fit using data from all subjects with complete data on model covariates and the outcome reported is after deleting statistical outliers. Approximately half the subjects were boys. The model for the WISC III FS IQ used data from 506 subjects and sample sizes for other models were similar. There were four adverse associations with recent postnatal THg exposures in one or both sexes. Postnatal THg was adversely associated with the Connor’s Teacher Rating Scale ADHD Index in both sexes. Models for the WISC III FS IQ, the Grooved Pegboard with the non dominant hand, and the Connor’s Continuous Performance Task Risk Taking had significant postnatal THg by sex interactions and were adversely associated with females only. The grooved pegboard is a test of fine motor coordination. Two other tests that are influenced by fine motor coordination (Finger Tapping and Trail Making A and B) showed no association with recent postnatal exposure.
Table 6.
Associations of recent postnatal exposure to MeHg with test outcomes measured at 107 months from the primary linear regression models.
| Test | Postnatal MeHg p-value | Postnatal x sex p-value | Female Slope | Male Slope |
|---|---|---|---|---|
| WISC III FS IQ | - | 0.02 | −0.48 (0.01) | 0.14 (0.45) |
| B-0 Test of Motor Development – Total score | −0.02(0.75) | - | - | - |
| Beery-Buktenica VMI - Total score | −0.12 (0.45) | - | - | - |
| W-J Achievement Test – Letter- Word | 0.85 (0.11) | - | - | - |
| W J Achievement Test – Applied problems | 0.11 (0.57) | - | - | - |
| CBCL - Total score | 0.05 (0.71) | - | - | - |
| CTRS – ADHD index * | 0.01 (< 0.0001) | - | - | - |
| CVLT – Short delay recall | 0.01 (0.62) | - | - | - |
| CVLT – Long delay recall | 0.01 (0.66) | - | - | - |
| WRAML – Design memory subtest | −0.08 (0.06) | - | - | - |
| Trail Making – A * | 0.003 (0.59) | - | - | - |
| Trail Making – B * | −0.001 (0.92) | - | - | - |
| Grooved Pegboard – Preferred hand * | 1.5E-6 (0.95) | - | - | - |
| Grooved Pegboard – Non- preferred hand * | - | 0.003 | <0.00001 (0.01) | −0.00005 (0.10) |
| BNT – Total correct no cues | −0.07 (0.25) | - | - | - |
| Haptic free forms solid discrimination test – Total correct | −0.03 (0.16) | - | - | - |
| CCPT – Hit reaction time | −0.01 (0.98) | - | - | - |
| CCPT – Attentiveness | 0.13 (0.36) | - | - | - |
| CCPT – Risk taking | - | 0.03 | 1.04 (0.01) | −0.12 (0.75) |
| Finger Tapping preferred hand | −0.03 (0.63) | - | - | - |
| Finger Tapping – non-preferred hand | −0.01 (0.90) | - | - | - |
WISC III FS IQ = Wechsler Intelligence Scale for Children II Full Scale IQ
B-O = Brunincks-Oseretsky
W-J = Woodcock Johnson
CTRS = Connor’s Teacher Rating Scale
CCPT = Connor’s Continuous Performance Task
WRAML = Wide Range Assessment of Memory and Learning
BNT – Boston Naming Test
CBCL = Child Behavior Checklist
CVLT = California Verbal Learning Test
= test score was transformed for analysis
Postnatal Associations of the Alternative Metrics with the 107-month IQ
All of the models for 107-month IQ using the alternative postnatal metrics yielded significant overall model F statistics. The mean of the different postnatal metrics varied widely, resulting in very different magnitudes for the postnatal metric regression coefficients. However p-values should be approximately comparable for pairs of models that contain the same set of subjects.
In the models in which 19 and 66 month THg values formed the basis of the alternate metrics, the postnatal metric was not significant if it did not include the THg by sex interactions (top part of Table 7). The AUC and BGW models were each based on 483 subjects, and thus these results are directly comparable. Prenatal THg was of borderline significance for both the AUC and BGW models. HOME score, care giver IQ, and SES (Hollingshead at 9 years) were each strong predictors of 107-month IQ for both AUC and BGW. The interaction of the postnatal metric by gender was significant (p=0.039) for the BGW metric. When this interaction was reparameterized into separate slopes by sex, the sign of the postnatal metric was positive for boys and negative for girls, but neither slope was significantly different from 0. As a check to see whether adjustment for postnatal THg was obscuring a prenatal THg effect, we fit a post-hoc model using the AUC alternative metric incorporating 19 and 66 month data. The model is identical to that reported in Table 7, but without adjustment for postnatal THg. The coefficient (−0.19) and p-value (P=0.060) for prenatal THg were almost identical in the two models, suggesting that not adjusting for postnatal THg has little effect on the estimated prenatal THg effect.
Table 7.
Analyses of the relationship between 107-month IQ and alternative metrics based on 19 and 66 month recent postnatal hair Hg levels with and without THg by sex interaction.
| 19 and 66 month data |
|||||
|---|---|---|---|---|---|
| Hi/Low |
AUC n =483 | Brain Growth Weighting(using brain area) n =483 | |||
| 6/6 cuts n =300 | 5/7 cuts n =193 | 4/8 cuts n =99 | |||
| Variable | Coefficient (p-value) | Coefficient (p-value) | Coefficient (p-value) | Coefficient (p-value) | Coefficient (p-value) |
|
Prenatal Mercury |
−0.26 (0.053) | −0.18 (0.35) | −0.20 (0.42) | −0.19 (0.066) | −0.19 (0.074) |
|
Postnatal Metric |
−0.42 (0.75) | −0.44 (0.81) | --- | −0.0001 (0.99) | --- |
|
Sex (male) |
0.078 (0.95) | 0.79 (0.62) | −4.27 (0.14) | 0.17 (0.85) | −3.18 (0.085) |
|
Postnatal Metric × Sex p-value |
--- | --- | (0.012) | --- | (0.039) |
| Postnatal Slope: | |||||
| Male | --- | --- | 8.95 (0.0091) | --- | 0.28 (0.22) |
| Female | --- | --- | −3.42 (0.37) | --- | −0.36 (0.10) |
|
Family status |
0.66 (0.59) | 0.71 (0.67) | 4.45 (0.057) | 0.058 (0.95) | 0.019 (0.98) |
|
HELPS |
0.023 (0.74) | −0.0004 (0.90) | 0.091 (0.42) | 0.049 (0.36) | 0.048 (0.37) |
|
child med history |
−0.098 (0.95) | 0.90 (0.68) | 0.035 (0.99) | −0.21(0.87) | −0.32 (0.80) |
|
maternal age |
0.21 (0.053) | 0.15 (0.30) | 0.60 (0.006) | 0.17 (0.034) | 0.18 (0.029) |
|
HOME score |
0.59 (< 0.001) | 0.75 (< 0.001) | 0.88 (0.002) | 0.44 (<0.001) | 0.44 (< 0.001) |
|
Care giver IQ |
0.13(0.006) | 0.13 (0.048) | 0.15 (0.082) | 0.12 (<0.001) | 0.12 (< 0.001) |
|
SES |
0.12 (0.066) | 0.097 (0.24) | 0.088 (0.44) | 0.18 (<0.001) | 0.18 (< 0.001) |
|
Family Resource scale |
0.029 (0.34) | 0.052 (0.16) | 0.035 (0.51) | 0.018 (0.43) | 0.016 (0.46) |
|
Child age at testing |
−0.55 (0.78) | 0.57 (0.82) | 3.62 (0.34) | −0.69 (0.65) | −0.44(.077) |
|
Tester = 2 |
1.049 (0.41) | 1.84 (0.27) | 2.17 (0.37) | 0.59 (0.55) | 0.72 (0.46) |
| Tester = 3 | 1.79 (0.34) | 3.76 (0.14) | 1.11 (0.75) | 0.91 (0.54) | 1.13 (0.44) |
SES = Socioeconomic status measured as the Hollingshead score at age 9 years
HOME = Home observation of
HELPS = Henderson early learning process scale
The sample sizes for H-L using 19 and 66 month THg values were considerably smaller than for models using other metrics, ranging from n=300 (for cut points at 6 ppm) to n=99 (for cut points at 4 and 8 ppm). H-L was not a significant predictor of 107-month IQ in the H-L models based on 19 and 66 month hair values using cut points at 6 ppm and at 5 and 7 ppm. However, there was a significant postnatal by gender interaction (p=0.012) using cut points of 4 and 8 ppm. When the interaction was reparametrized into separate slopes by sex, the H-L slope for males was 8.95 (p=0.009), suggesting that boys who had consistently very high levels of postnatal Hg had an IQ of 9 points higher on average than boys who consistently had low postnatal Hg, after adjusting for covariates. It should be noted that among the 99 subjects used for this analysis, only 34 were boys (17 in each H-L group). Prenatal Hg was of borderline significance (p=0.053) in the H-L model using a 6 ppm cutoff, but was not significant in the other H-L models based on more extreme cut points and a smaller number of subjects. HOME score and care-giver IQ continued to be significant predictors of 107-month IQ, even in the H-L model using cut points of 4 and 8 ppm.
Results using metrics based on 3 time points (6, 19 and 66 month values (Table 8) were similar to results based on 2 time points. The postnatal MeHg metric was not a significant predictor of 107-month IQ in any of these models, although for the H-L model (6/6 cut points) the magnitude of the coefficient was much farther from zero and the p-value was much smaller than for the H-L model based on only two time points. As was the case for models already reported, HOME score was a significant predictor of 107-month IQ in all models, and maternal IQ and SES were significant predictors in the AUC and BWG models. Using the AUC postnatal metric, the AUC by gender interaction term was of borderline significance (p=0.065, not shown in Table 8). When these interactions were reparameterized, the postnatal slope for each sex separately was not significantly different from zero.
Table 8.
Analyses of the association between 107-month IQ and alternative metrics based on recent postnatal hair THg values at 6, 19, and 66 months with and without MeHg by sex interaction.
| 6, 19, and 66 month data |
|||
|---|---|---|---|
| Hi/Low 6/6 cuts n = 178 | AUC n = 400 | Brain Growth Weighting (using brain area) n = 400 | |
| Variable | Coefficient (p-value) | Coefficient (p-value) | Coefficient (p-value) |
|
Prenatal Mercury |
−0.19 (0.28) | −0.14 (0.21) | −0.14 (0.22) |
|
Postnatal Metric |
−2.75 (0.13) | −0.002 (0.60) | −0.054 (0.68) |
|
Sex (male) |
2.25 (0.13) | 0.70 (0.46) | 0.74 (0.43) |
|
Postnatal Metric × Sex p-value |
--- | --- | --- |
|
Family status |
−0.52 (0.74) | −0.39 (0.69) | −0.36 (0.72) |
|
HELPS |
−0.047 (0.60) | 0.051 (0.37) | 0.049 (0.39) |
|
child med history |
0.014 (0.99) | −0.34 (0.794) | −0.34 (0.79) |
|
maternal age |
0.070 (0.61) | 0.17 (0.047) | 0.17 (0.040) |
|
HOME score |
0.60 (< 0.001) | 0.45 (< 0.001) | 0.45 (< 0.001) |
|
care giver IQ |
0.11 (0.061) | 0.13 (< 0.001) | 0.13 (< 0.001) |
|
SES |
0.13 (0.096) | 0.17 (< 0.001) | 0.17 (< 0.001) |
|
Family Resource scale |
−0.003 (0.94) | 0.004 (0.88) | 0.004 (0.87) |
|
Child age at testing |
−2.84 (0.24) | −0.44 (0.79) | −0.56 (0.72) |
|
Tester = 2 |
−0.96 (0.55) | 0.98 (0.34) | 0.97 (0.35) |
| Tester = 3 | 1.92 (0.42) | 0.66 (0.67) | 0.64 (0.68) |
SES = Socioeconomic status measured as the Hollingshead score at age 9 years
HOME = Home observation of
HELPS = Henderson early learning process scale
Finally, the results from the BGW model using weights based on change in estimated brain volume (essentially equivalent to using 6-month THg values, see Table 9) were similar to the results from the BGW model with weights based on changes in estimated brain area, as reported in Tables 7 and 8. The postnatal metric did not predict 107-month IQ, whereas HOME score, care-giver IQ, and SES were important predictors. One difference between the results of the two BGW models is that in the model using 6-month THg values, prenatal THg was not of even borderline significance. This is likely due to the fact that 6-month THg values were more strongly correlated with prenatal THg levels than were the later postnatal exposures.
Table 9.
Analyses of relationship between 107-month IQ and Brain Growth Weighting for brain volume (equivalent to 6 month postnatal Hg) with and without THg by sex interaction.
| 6 month data |
|
|---|---|
| Brain Growth Weighting (using brain volume) n = 460 | |
| Variable | Coefficient (p-value) |
|
Prenatal Mercury |
−0.11 (0.33) |
|
Postnatal Metric |
−0.02 (0.84) |
|
Male Sex |
0.70 (0.39) |
|
Postnatal Metric * Male Interaction |
--- |
|
Family status |
−0.59 (0.54) |
|
HELPS |
0.040 (0.48) |
|
child med history |
−0.08 (0.95) |
|
maternal age |
0.19 (0.022) |
|
HOME score |
0.44 (< 0.001) |
|
care giver IQ |
0.13 (< 0.001) |
|
SES (Hollingshead 9 yr) |
0.17 (0.001) |
|
Family Resource scale |
−0.0002 (0.99) |
|
Childs age at testing |
−1.06 (0.49) |
|
Tester = 2 |
1.069 (0.30) |
| Tester = 3 | 0.25 (0.87) |
SES = Socioeconomic status measured as the Hollingshead score at age 9 years
HOME = Home observation of
HELPS = Henderson early learning process scale
Discussion
Using several different metrics for recent postnatal MeHg exposure we evaluated the SCDS main cohort for associations with children’s developmental outcomes. We found a number of associations present at the 66 and 107 month evaluations in the primary linear analyses that examined the covariate adjusted association between prenatal exposure and outcomes. Some of the associations in the primary analyses were in the direction of declining performance as postnatal exposure increased and with others the performance improved. Some of these associations were sex specific. One alternative postnatal metric we evaluated showed an association between postnatal THg exposure and IQ present in males only. The associations we found were intriguing, but we were not able to discern a recognizable pattern of associations between the postnatal MeHg exposure metrics we studied and children‘s development.
In the primary analysis at 66 months there were three postnatal associations present. All associations indicated improved performance as exposure increased (Davidson et al., 1998). For the BG-ES, the improved performance was present in males only. When instead of the 66-month THg level we substituted the19-month THg or the sum of the 19-month and 66-month THg, we also found improved performance on the MSCI-GCI, the PLS-TS, and the WJ-AP as exposure increased.
In the primary analysis at 107 months there were four postnatal associations present. All were in the direction of declining performance as exposure increased. Three were present only in females, a finding we are unable to explain. There is limited evidence regarding postnatal exposure, but prenatal MeHg exposure is generally thought to affect males more than females (Marsh, 1995). The postnatal associations seen here were not consistent across psychological domains since some tests that measured similar cognitive domains showed no association with exposure. If THg affects a psychological domain, it would seem likely that all the tests examining that function would be affected. The absence of consistent findings across ages and psychological domains and concern about continuing brain development and exposure postnatally were factors that led us to explore postnatal exposure and develop alternative metrics.
The reversal of associations between postnatal exposure and endpoints from improved performance to deteriorating performance between the 66 and 107 month exams has not been previously reported, was not expected, and its significance is not clear. The adverse association with the Conner’s Teacher Rating Scale ADHD index is especially intriguing given the prevalence of ADHD and the reports of behavioral changes with other toxicants such as lead (Bellinger, 2008). However, we are cautious in interpreting this finding since there is no clear evidence that behavioral changes should be expected with MeHg. In addition, we have found some associations with both improving and deteriorating performance on varying endpoints in the past, but no consistent or clear pattern of associations has emerged. A consistent pattern of associations would support a causal relationship. However, inconsistent findings could occur if there is an exposure threshold and if some of the cohort subjects being studied were at or above that level. Presently it is not known if there is a threshold for postnatal MeHg exposure. Varying findings might also occur if the interplay between Hg exposure and the nutrients present in fish such as long chain polyunsaturated fatty acids, iodine, selenium, or other factors were more complex than we presently understand or more important earlier in development (Davidson et al., 2008; Strain et al., 2008).
We examined the association of the alternative postnatal metrics only with the IQ measured at 107 months. A significant association was present only when the model included a postnatal THg by sex interaction, only for males, and only for the H-L metric with the most extreme cut points. This model suggests that boys who are consistently exposed to higher levels of THg postnatally did better on IQ testing than boys consistently exposed to lower THg within the range we are studying in Seychelles. However due to the small sample size used for this model, this suggestion should be interpreted with caution. The models using the alternative metrics did show the expected positive association between maternal IQ and 9-year child IQ (p<0.005). The HOME score in all models was a significant predictor of 9 year IQ (p<0.005). Other covariates known to be significant predictors of 9 year IQ such as SES and maternal age were also significant in some models. These finding suggest that the data are robust enough to detect associations known to be associated with children’s IQ and might have detected an association with postnatal THg exposure if its effect size was similar to that of these covariates.
Each of the alternative postnatal metrics could be constructed using recent THg measured at multiple time points. Unfortunately, we had only samples obtained at the time of evaluations for postnatal analysis and there were significant amounts of missing data. Consequently we were limited to using at most only 3 time points from which to calculate each metric. Two of the alternate postnatal metrics (AUC and BGW) are weighted averages of the recent postnatal THg values at each time point. These two metrics are scaled in different ways and weight the time of exposure differently. The AUC metric weights the THg measurement at each time point in proportion to the period of time covered by the measurement (which is determined by how close in time other THg measurements were taken). However the AUC metric does not treat any time period as being more important than any other time period. In contrast, the BGW metric assumes that THg exposure that occurs during periods of rapid brain development are the most detrimental to the child. When determining weights for the BGW metric based on changes in brain volume, the 6-month THg level accounted for nearly 100% of the weight, suggesting that THg exposure that occurs much later is less important in comparison to the 6-month exposure. Had we extended this thinking to derive a BGW metric that included the prenatal period, it seems likely the prenatal exposure would have had a much greater weight than even the 6-month THg exposure. The rationale for the BGW metric is therefore consistent with the theory that prenatal THg exposure is more detrimental to neurodevelopment than postnatal exposure, because the prenatal period is the time of most rapid brain growth. Our BGW metrics assume that the changes in head area or volume are reasonable proxies for overall brain development, but this may not be entirely accurate. The metrics we used might not account for some developmental brain processes such as myelination that can continue into early adulthood.
An advantage of the AUC and BGW metrics as compared to the H-L metric is that all the data on postnatal THg levels could be included as well as all subjects with postnatal data. The H-L metric differs in that an increasing number of subjects were excluded as the “high” and “low” categories moved farther apart. This reduction in sample size for the H-L models is one reason we might expect results using this metric to differ from the other models. However, if consistent high exposure to THg is detrimental, this metric may be better than the other metrics to detect an association. Indeed, at the highest cut points (4 and 8) there was a significant postnatal THg by sex interaction and a significant increase in IQ present in males.
This study has a number of strengths. The cohort size was large and over 500 children had postnatal exposure measured at the time points we evaluated. The population studied consumes fish daily and does not consume marine mammals. The average postnatal exposure for the cohort ranged from 6.6 ppm at 6 months of age to 4.8 ppm at 66 months. The analyses did show the expected effects of covariates known to influence child development such as maternal IQ, HOME, SES, and maternal age, suggesting they might have detected an association with postnatal THg if one was present.
The study also has limitations. The primary goal of the SCDS was to study prenatal MeHg exposure and there was incomplete collection of postnatal hair samples at some ages. Measuring continuous postnatal exposure would have been preferable to the one centimeter recent exposure that we measured, but we had neither the hair samples nor the resources to recapitulate more continuous postnatal exposure. The optimum postnatal metric that most closely reflects the brain exposure is not presently known. Although we selected three postnatal metrics with biological rationales, other metrics or combinations of metrics might have resulted in different associations. Similarly, if we had examined the association of postnatal exposure with other outcomes, the results might have been different. Our metrics that recapitulated exposure over time assumed that the exposures measured at the three time points were representative of other unobserved exposure over this time period. However, this assumption would not be warranted if exposure were episodic as might occur in societies where the source of THg exposure is different than daily consumption of fish. There may also be differences in exposure effects on development at different ages related to different events in the developing central nervous system, even if the exposure is not episodic. The mean THg exposure present in the SCDS may have been too low to find more than inconsistent associations with the endpoints measured. Postnatal exposure could also have been influenced by nutritional variables that were not available for these studies. Recent evidence suggests that long chain polyunsaturated fatty acids and other nutrients present in fish may significantly modify the influence of exposure (Davidson et al., 2008; Strain et al., 2008).
In summary, there are biological reasons to believe that postnatal exposure to MeHg might influence children’s development. We measured recent postnatal exposure to MeHg at several ages in the SCDS main cohort and examined the association of several different postnatal metrics with some of the subjects developmental test scores. We found a number of associations between postnatal exposure metrics and test outcomes, but the results varied across ages and psychological domains. These findings are consistent with our earlier findings in the SCDS and do not provide clear evidence for an adverse association between the levels of THg exposure studied in this cohort and the children’s development. However, the findings do raise intriguing possibilities and suggest that postnatal exposure should be studied prospectively.
Figure 1.
High/Low graph showing cutoff lines used for postnatal analysis of 19 and 66 month hair mercury.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amin-Zaki L, Elhassani S, Majeed MA, Clarkson TW, Doherty RA, Greenwood MR. Studies of infants postnatally exposed to methylmercury. J Pediatrics. 1974;85 (1):81–84. doi: 10.1016/s0022-3476(74)80291-x. [DOI] [PubMed] [Google Scholar]
- Amin-Zaki L, Elhassani S, Majeed MA, Clarkson TW, Doherty RA, Greenwood MR, Giovanoli-Jakubczak T. Perinatal methylmercury poisoning in Iraq. Amer J Dis Child. 1976;130:1070–1076. doi: 10.1001/archpedi.1976.02120110032004. [DOI] [PubMed] [Google Scholar]
- Amin-Zaki L, Majeed MA, Clarkson TW, Greenwood MR. Methylmercury poisoning in Iraqi children: clinical observations over two years. British Med J. 1978;1:613–616. doi: 10.1136/bmj.1.6113.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin-Zaki L, Elhassani SB, Majeed MA, Clarkson TW, Doherty RA, Greenwood MR. Methylmercury poisoning in mothers and their suckling infants. In: Holmstedt B, Lauwerys R, Mercier M, Roberfroid M, editors. Mechanisms of Toxicity and Hazard Evaluation. Elsevier: North-Holland Biomedical Press; 1980. pp. 75–78. [PubMed] [Google Scholar]
- Amin-Zaki L, Majeed MA, Greenwood MR, Elhassani SB, Clarkson TW, Doherty RA. Methylmercury poisoning in the Iraqi suckling infant: A longitudinal study over five years. J Applied Toxicology. 1981;1(4):210–214. doi: 10.1002/jat.2550010405. [DOI] [PubMed] [Google Scholar]
- Axtell CD, Cox C, Mywers GJ, Davidson PW, Choi AL, Cernichiari E, Sloane-Reeves J, Shamlaye CF, Clarkson TW. Association between methylmercury exposure from fish consumption and child development at five and a half year sof age in the Seychelles Child Development Study: An evaluation of nonlinear relationships. Environ Res. 2000;84:71–80. doi: 10.1006/enrs.2000.4082. [DOI] [PubMed] [Google Scholar]
- Bakir F, Damluji SF, Amin-Zaki M, Murtadha M, Khalidi A, Al-Rawi NY, Tikriti S, Dhahir HI, Clarkson TW, Smith JC, Doherty RA. Methylmercury poisoning in Iraq. Science. 1973;181:230–241. doi: 10.1126/science.181.4096.230. [DOI] [PubMed] [Google Scholar]
- Bellinger DC. Very low lead exposures and children’s neurodevelopment. Curr Opin Pediatr. 2008;20:172–177. doi: 10.1097/MOP.0b013e3282f4f97b. [DOI] [PubMed] [Google Scholar]
- Bondy SC. Chapter 20: Induction of oxidative stress in the brain by neurotoxic agents. In: Chang LW, editor. Principles of Neurotoxicology. Marcel Dekker, Inc; New York: 1994. pp. 563–582. [Google Scholar]
- Brenner RP, Snyder RD. Late EEG findings and clinical status after organic mercury poisoning. Arch Neurol. 1980;37:282–284. doi: 10.1001/archneur.1980.00500540060006. [DOI] [PubMed] [Google Scholar]
- Castoldi AF, Coccini T, Manzo L. Neurotoxic and molecular effects of methylmercury in humans. Rev Environ Health. 2003;18 (1):19–31. doi: 10.1515/reveh.2003.18.1.19. [DOI] [PubMed] [Google Scholar]
- CDC (Center for Disease Control) growth charts accessed 4/25/08 . http://www.cdc.gov/nchs/about/major/nhanes/growthcharts/charts.htm.
- Cernichiari E, Brewer R, Myers GJ, Marsh DO, Lapham LW, Cox C, Shamlaye CF, Berlin M, Davidson PW, Clarkson TW. Monitoring methylmercury during pregnancy: maternal hair predicts fetal brain exposure. NeuroToxicology. 1995;16 (4):705–710. [PubMed] [Google Scholar]
- Chien LC, Han BC, Hsu CS, Jiang CB, You HJ, Shieh MJ, Yeh CY. Analysis of the health risk of exposure to breast milk mercury in infants in Taiwan. Chemosphere. 2006;64 (1):79–85. doi: 10.1016/j.chemosphere.2005.11.059. [DOI] [PubMed] [Google Scholar]
- Choi BH, Lapham LW, Amin-Zaki L, Saleem T. Abnormal neuronal migration, deranged cerebral cortical organization, and diffuse white matter astrocytosis of human fetal brain: a majaor effect of methylmercury poisoning in utero. J Neuropath Experimental Neurology. 1978;37 (6):719–733. doi: 10.1097/00005072-197811000-00001. [DOI] [PubMed] [Google Scholar]
- Choi BH. The effects of methylmercury on the developing brain. Progress Neurobiology. 1989;32:447–470. doi: 10.1016/0301-0082(89)90018-x. [DOI] [PubMed] [Google Scholar]
- Clarkson TW. Metal toxicity in the central nervous system. Environ Health Perspect. 1987;75:59–64. doi: 10.1289/ehp.877559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson TW, Magos L. The toxicology of mercury and its chemical compounds. Critical Reviews Toxicology. 2006;36:609–662. doi: 10.1080/10408440600845619. [DOI] [PubMed] [Google Scholar]
- Cordier S, Garel M, Mandereau L, Morcel H, Doineau P, Gosme-Seguret S, Josse D, White R, Amiel-Tison C. Neurodevelopmental investigations among methylmercury-exposed children in French Guiana. Environ Research. 2002;89:1–11. doi: 10.1006/enrs.2002.4349. [DOI] [PubMed] [Google Scholar]
- Davidson PW, Myers GJ, Cox C, Axtell CD, Shamlaye CF, Sloane-Reeves J, Cernichiari E, Needham L, Choi A, Wang Y, Berlin M, Clarkson TW. Effects of prenatal and postnatal methylmercury exposure from fish consumption at 66 months of age: The Seychelles Child Development Study. J Amer Med Assoc. 1998;280:701–707. doi: 10.1001/jama.280.8.701. [DOI] [PubMed] [Google Scholar]
- Davidson PW, Myers GJ, Shamlaye C, Cox C, Wilding G. Prenatal exposure to methylmercury and child development: influence of social factors. Neurotox Teratology. 2004;26:553–559. doi: 10.1016/j.ntt.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Davidson PW, Myers GJ, Cox C, Wilding GE, Shamlaye CF, Huang L, Cernichiari E, Sloane-Reeves J, Palumbo D, Clarkson TW. Methylmercury and development: longitudinal analysis of the Seychelles child development cohort. Neurotox Teratology. 2006;28:529–535. doi: 10.1016/j.ntt.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Davidson PW, Strain JJ, Myers GJ, Thurston SW, Bonham MP, Shamlaye CF, Stokes-Riner A, Wallace JMW, Robson PJ, Duffy EM, Georger LA, Sloane-Reeves J, Cernichiari E, Canfield RL, Cox C, Huang LS, Janciuras J, Clarkson TW. Neurodevelopmental Effects of Maternal Nutritional Status and Exposure to Methylmercury from Eating Fish during Pregnancy. NeuroToxicology. 2008;29:767–775. doi: 10.1016/j.neuro.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis LE, Kornfeld M, Mooney HS, Fiedler KJ, Haaland KY, Orrison WW, Cernichiari E, Clarkson TW. Methylmerucry poisoning: Long-term clinical, radiological, toxicological, and pathological studies of an affected family. Ann Neurology. 1994;34 (6):680–688. doi: 10.1002/ana.410350608. [DOI] [PubMed] [Google Scholar]
- Debes F, Budtz-Jorgensen E, Weihe P, White RF, Grandjean P. Impact of prenatal methylmercury exposure at age 14 years. Neurotox Teratology. 2006;28:363–375. doi: 10.1016/j.ntt.2006.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenstein C, Shu P, Wickenheiser EB, Hirner AV, Dolfen M, Emons H, Obe G. Methyl mercury uptake and associations with the induction of chromosomal aberrations in Chinese hamster ovary (CHO) cells. Chemico-Biolological Interactions. 2002;141 (3):259–274. doi: 10.1016/s0009-2797(02)00079-0. [DOI] [PubMed] [Google Scholar]
- Elhassani SB, Amin-Zaki L, Majeed MA, Clarkson TW, Doherty RA, Greenwood M, Klipper RW. Exchange transfusion treatment of methylmercury-poisoned children. J Environm Science Health-Part C. 1978;13(1):63–80. [PubMed] [Google Scholar]
- Engleson E, Herner T. Alkyl mercury poisoning. Acta Paediatrica. 1952;41:289–294. doi: 10.1111/j.1651-2227.1952.tb17033.x. [DOI] [PubMed] [Google Scholar]
- FAO (Food and Agriculture Organization of the United Nations) [accessed 5/1/08];The state of the world fisheries and aquaculture. 2000 ftp://ftp.fao.org/docrep/fao/003/x8002e.
- Grandjean P, Jorgensen PJ, Weihe P. Human milk as a source of methylmercury exposure in infants. Environ Health Perspectives. 1994;102:74–77. doi: 10.1289/ehp.9410274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean P, Weihe P, White RF. Milestone development in infants exposed to methylmercury from human milk. Neurotoxicology. 1995;16 (1):27–34. [PubMed] [Google Scholar]
- Grandjean P, Weihe P, White RF, Debes F, Araki S, Yokoyama K, Murata K, Sorensen N, Dahl R, Jorgensen PJ. Cognitive deficit in 7-year-old children with prenatal exposure to methylmercury. Neurotox Teratology. 1997;19 (6):417–428. doi: 10.1016/s0892-0362(97)00097-4. [DOI] [PubMed] [Google Scholar]
- Grandjean P, Budtz-Jorgensen E, White RF, Jorgensen PJ, Weihe P, Debes F, Keiding N. Methylmercury exposure biomarkers as indicators of neurotoxicity in children aged 7 years. Amer J Epidemiology. 1999a;150 (3):301–305. doi: 10.1093/oxfordjournals.aje.a010002. [DOI] [PubMed] [Google Scholar]
- Grandjean P, White RF, Nielsen A, Cleary D, de Oliveira Santos EC. Methylmercury neurotoxicty in Amazonian children downstream from gold mining. Environ Health Perspect. 1999b;107:587–591. doi: 10.1289/ehp.99107587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gribble EJ, Hong SW, Faustman EM. The magnitude of methylmercury-induced cytotoxicity and cell cycle arrest is p53-dependent. Birth Defects Research (Part A) 2005;73:29–38. doi: 10.1002/bdra.20104. [DOI] [PubMed] [Google Scholar]
- Harada Y. Minamata disease: Study group of Minamata disease. Kumamoto University; Japan: 1968. Infantile Minamata disease; pp. 73–92. [Google Scholar]
- Huang L, Cox C, Wilding GE, Myers GJ, Davidson PW, Shamlaye CF, Cernichiari E, Sloane-Reeves J, Clarkson TW. Using measurement error models to assess effects of prenatal and postnatal methylmerucyr exposure in the Seychelles Child Development Study. Environ Research. 2003;93:115–122. doi: 10.1016/s0013-9351(03)00089-6. [DOI] [PubMed] [Google Scholar]
- Huang L, Cox C, Myers GJ, Davidson PW, Cernichiari E, Shamlaye CF, Sloane-Reeves J, Clarkson TW. Exploring nonlinear association between prenatal methylmerucy exposure from fish consumption and child development: evaluation of the Seychelles Child Development Study nine-year data using semiparametric additive models. Environ Research. 2005;97:100–108. doi: 10.1016/j.envres.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Lapham LW, Cernichiari E, Cox C, Myers GJ, Baggs RB, Brewer R, Shamlaye CF, Davidson PW, Clarkson TW. An analyis of autopsy brain tissue from infants prenatally exposed to methylmercury. NeuroToxicology. 1995;16(4):689–704. [PubMed] [Google Scholar]
- Marsh DO. Chapter 26: Organic mercury: clinical and neurotoxicological aspects. In: de Wolff FA, editor. Handbook of Clinical Neurology. 64. Vol. 20. Elsevier; New York: 1994. pp. 413–429. [Google Scholar]
- Murata K, Weihe P, Renzoni A, Debes F, Vasconcelos R, Zino F, Araki S, Jorgensen PJ, White RF, Grandjean P. Delayed evoked potentials in children exposed to methylmercury from seafood. Neurotox Teratology. 1999;21 (4):343–348. doi: 10.1016/s0892-0362(99)00011-2. [DOI] [PubMed] [Google Scholar]
- Myers GJ, Davidson PW, Palumbo D, Shamlaye C, Cox C, Cernichiari E, Clarkson TW. Secondary analysis from the Seychelles Child Development Study: The Child Behavior Checklist. Environ Res. 2000;84:12–19. doi: 10.1006/enrs.2000.4085. [DOI] [PubMed] [Google Scholar]
- Myers GJ, Davidson PW, Cox C, Shamlaye CF, Palumbo D, Cernichiari E, Sloane-Reeves J, Wilding GE, Kost J, Huang L, Clarkson TW. Prenatal methylmercury exposure from ocean fish consumption in the Seychelles child development study. The Lancet. 2003;361:1686–1692. doi: 10.1016/S0140-6736(03)13371-5. [DOI] [PubMed] [Google Scholar]
- Myers GJ, Davidson PW, Shamlaye C, Cox C, Kost J, Beck C, Huang L, Weiss B. The Seychelles Child Development Study of methyl mercury from fish consumption: analysis of subscales from the Child Behavior Checklist at age 107 months in the main cohort. Seychelles Medical Dental Journal. 2004;7:107–114. doi: 10.1016/j.neuro.2020.09.025. [DOI] [PubMed] [Google Scholar]
- Myers GJ, Davidson PW, Shamlaye C. Developmental Disabilities following prenatal exposure to methyl mercury from maternal fish consumption: a review of the evidence. In: Davidson PW, Myers GJ, Weiss B, editors. Neurotoxicity and Developmental Disabilities. Volume 30 of the International Review of Research in Mental Retardation. Elsevier; New York: 2006. pp. 141–170. [Google Scholar]
- Nellhaus G. Composite International and Interracial Graphs. Pediatrics. 1968;41:106–114. [PubMed] [Google Scholar]
- Pierce PE, Thompson JF, Likosky WH, Nickey LN, Barthel WF, Hinman AR. Alkyl mercury poisoning in humans: report of an outbreak. J Amer Med Assoc. 1972;220 (11):1439–1442. [PubMed] [Google Scholar]
- Rice D, Barone S., Jr Critical periods of vulnerability for the developing nervous system: Evidence from human and animal models. Environ Health Perspect. 2000;108 (Suppl 3):511–533. doi: 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodier PM, Aschner M, Sager PR. Mitotic arrest in the developing CNS after prenatal exposure to methylmercury. Neurobehavioral Toxicology Teratology. 1984;6:379–385. [PubMed] [Google Scholar]
- Rodier PM. Environmental causes of central nervous system maldevelopment. Pediatrics. 2004;113:1076–1083. [PubMed] [Google Scholar]
- Shamlaye CF, Marsh DO, Myers GJ, Cox C, Davidson PW, Choisy O, Cernichiari E, Choi A, Tanner MA, Clarkson TW. The Seychelles Child Development Study on neurodevelopmental outcomes in children following in utero exposure to methylmerucyr from a maternal fish diet: background and demographics. NeuroToxicology. 1995;16 (4):597–612. [PubMed] [Google Scholar]
- Slikker W., Jr . Chapter 23: Placental transfer and pharmacokinetics of developmental neurotoxicant. In: Chang LW, editor. Principles of Neurotoxicology. Marcel Dekker, Inc; New York: 1994. pp. 659–680. [Google Scholar]
- Snyder RD. The involuntary movements of chronic mercury poisoning. Arch Neurol. 1972;26:379–381. doi: 10.1001/archneur.1972.00490100109013. [DOI] [PubMed] [Google Scholar]
- Strain JJ, Davidson PW, Bonham MP, Duffy EM, Stokes-Riner A, Thurston SW, Wallace JMW, Robson PJ, Shamlaye CF, Georger LA, Sloane-Reeves J, Cernichiari E, Canfield RL, Cox C, Huang LS, Janciuras J, Myers GJ, Clarkson TW. Associations of maternal long chain polyunsaturated fatty acids, methyl mercury, and infant development in the Seychelles Child Development Nutrition Study. NeuroToxicology. 2008;29:776–782. doi: 10.1016/j.neuro.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syversen TL. Effects of repeated dosing of methylmercury on in vivo protein synthesis in isolated neurons. Acta Pharmacol Toxicol (Copenhagen) 1982;50:391–397. doi: 10.1111/j.1600-0773.1982.tb00993.x. [DOI] [PubMed] [Google Scholar]
- Takeuchi T. Minamata disease: Study group of Minamata disease. Kumamoto University; Japan: 1968. Pathology of Minamata disease; pp. 141–228. [Google Scholar]
- Thurston SW, Liu G, Miller DP, Christiani DC. Modeling lung cancer risk in case-control studies using a new dose metric of smoking. Cancer Epidemiology, Biomarkers Prevention. 2005;14:2296–2302. doi: 10.1158/1055-9965.EPI-04-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi T, Eto K. Chapter 2: Pathology and pathogenesis of Minamata disease. In: Tsubaki T, Irukayama K, editors. Minamata disease: Methylmercury poisoning in Minamata and Niigata, Japan. Kodansha Ltd; Tokyo: Elsevier Scientific Publishing Company; New York: 1977. pp. 103–141. [Google Scholar]
- Vogel DG, Margolis RL, Mottet NK. The effects of methyl mercury binding to microtubules. Toxicology Applied Pharmacology. 1985;80:473–486. doi: 10.1016/0041-008x(85)90392-8. [DOI] [PubMed] [Google Scholar]
- Volpe JJ. Neurology of the newborn. 4. WB Saunders Co; Philadelphia: 2001. [Google Scholar]
- WHO (World Health Organization) International Programme on Chemical Safety Geneva: Environmental Health Criteria 101 Methylmercury. 1990 [Google Scholar]