1. Introduction
The epidemic of crack-cocaine use in the late 1980’s and early 1990’s has lead researchers to examine the relationship between mothers’ use of cocaine during pregnancy and their children’s development over time. Research on prenatal cocaine effects on child development, particularly intellectual/language development and physical development, is inconsistent, with many studies findings no or small effects [22, 46, 47] (but for exception, see [6]). However, prenatal cocaine exposure may be associated, either through direct teratogenic effects or mediated by associated poor economic and caregiving conditions experienced postnatally, with subtle alterations in children’s attentional and emotional arousal and regulation [13, 22, 31, 44]. Emotional arousal and regulation differences are important to examine in that they may set the stage for children’s development of behavior problems and, possibly, substance use initiation in later childhood and adolescence.
Animal and human studies find that prenatal cocaine exposure may alter developing emotional arousal and regulation systems, including monoaminergic neurotransmitters systems in the mesocortical and mesolimbic areas [24, 31, 38, 41, 45]. Behaviorally, prenatally cocaine exposed human infants are found to be more excitable and irritable with poorer state regulation[11, 25, 32, 34], and greater physiological lability[40] than non-exposed infants, although some research finds reduced arousal in cocaine-exposed infants[4].
In toddlerhood, these emotion-related differences are likely to persist and intensify, given that this is a period in which children develop emotional self-regulation through repeated interactions with caregivers[27]. However, little research has focused on emotional arousal and regulation in cocaine-exposed toddlers. One study found increased irritability/frustration in cocaine-exposed toddlers during a free play[36] as compared to non-exposed toddlers. In addition to free-play, it would be useful to understand toddlers’ emotions in emotionally-arousing situations. The present study examined prenatally cocaine exposed and non-exposed toddlers’ reactions to a frustrating situation, having to wait for an attractive toy for 6 minutes.
In addition to context, gender may be an important factor. Boys tend to display greater levels of anger-related emotions, such as agitation/frustration, in response to challenge than girls[12], and this may be particularly true for cocaine-exposed boys. For example, Dennis, Bendersky, Ramsay, and Lewis[21] found that cocaine exposed preschool-aged boys (but not girls) showed more irritability than non-exposed boys in a problem-solving task. Thus, gender may moderate cocaine exposure associations with emotionality.
Additionally, research is needed to understand the consequences of altered emotionality for cocaine-exposed youth. Increased irritability and difficulties in dampening this arousal may contribute to the later development of behavior problems, particularly as children prepare to enter school and are faced with increasing demands to regulate their emotional arousal[16, 17]. Research has not yet examined links between arousal/regulation patterns and the emergence of behavior problems in cocaine-exposed children, although these children may be at heightened risk for externalizing problems[27], particularly for boys[20].
Along with prenatal cocaine exposure come associated risk factors, complicating research in this area. Cocaine-using mothers typically use other substances during pregnancy, including opiates, alcohol, and tobacco, making it difficult to isolate cocaine-specific effects[42]. The present study addressed this by excluding opiate-using mothers and including a comparison group of demographically-similar children who were exposed to tobacco, alcohol, and/or marijuana prenatally. Also, cocaine exposure is associated with several risk factors, such as low birth weight[8] and compromised caregiving, which may mediate or moderate differences in emotional development[5, 9]. The present study included analyses of obstetric risk factors as a mediator and parent-child relationship quality as a moderator of cocaine-exposure “effects”. Finally, studies of cocaine-exposed children often focus on one outcome time point, although data are often available on repeated time points[42]. We used Hierarchical Linear Modeling (HLM) to model change in externalizing symptoms over three years (six timepoints). HLM is also able to estimate missing data, a common feature in studies with substance-abusing samples [33].
The present study examined links between prenatal cocaine exposure and agitated emotional arousal, self-regulation, and references to caregivers during a frustrating toy wait task in 2 ½ year-old toddlers. We hypothesized that prenatally cocaine (and other drug) exposed (PCE) toddlers would show greater agitated emotional arousal and difficulty with self-regulation than non drug exposed (NDE) toddlers, particularly PCE boys. Non-cocaine but other drug exposed (NCE) toddlers would fall between PCE and NDE toddlers in arousal and regulation. Further, greater agitated arousal and lower self-regulation in toddlerhood (age 2 1/2) would predict increases in externalizing symptoms through preschool age (age 5 1/2). This might be particularly true for PCE children[10]. We conducted these analyses with and without covarying for mothers’ tobacco, alcohol, and marijuana use in pregnancy. We also conducted secondary analyses to examine the hypotheses that: 1. Obstetric complications would mediate links between prenatal drug exposure and emotional arousal/regulation, and 2. Parent-child relationship quality would be a moderator of links between exposure and emotional arousal/regulation, with lower parent-child relationship quality exacerbating negative “effects” of prenatal cocaine exposure on emotional arousal/regulation.
2. Methods
2.1 Participants
Participants were drawn from a larger longitudinal study of emotional and cognitive development of cocaine-exposed and non-exposed children. 370 families participated in the larger longitudinal study (225 Prenatally Cocaine and other drug Exposed [PCE], 49 Non Cocaine but other drug Exposed [NCE], and 97 Non Drug Exposed [NDE]). These families were followed from the pregnancy or child’s birth onward, with biannual assessments. Of the 370 families, 225 attended the 30 month follow up session and completed the toy wait task. These were the sample for the present study. Fifty eight children attended the 30 month session, but did not complete the toy wait task (due to the fact that the toy wait task was not instituted until a few months into the assessments). Eighty seven families were contacted by our team of schedulers, but did not attend the 30 month visit. All of these families later attended the 36 month session. Those who did not come at 30 months missed that session for a number of reasons—child or parent illness, family temporarily away, or multiple cancellations.
The 225 families did not differ significantly from those who were not in the present sample on drug exposure group, child sex, child birth weight or mother’s education level, race, age at delivery, or frequency of cocaine or other substance (tobacco, alcohol, marijuana) use during pregnancy (p’s > .10). Obstetric Complications Scale (OCS[29]) scores tended to be higher (more optimal) for those who did not attend the 30 month session than those who did (t(344)= 1.94, p= .05).
For the longitudinal HLM analyses of child behavior problems, data on behavior problems were available at 2 ½ years (time 1) for 191 children. These children did not differ from the overall sample of 225 on demographic variables or mother drug use during pregnancy. Behavior problem data were available at 3 ½ years for 151, at 4 years for 140, at 4 ½ years for 108, at 5 years for 125, and at 5 ½ years for 133. 177 children had data at at least one follow-up time point and, thus, were included in the HLM model. These 177 were not different from the overall 191 children on any demographic or mother drug use variables except child sex. Girls were more likely than boys to have follow-up data (Chi2= 4.49, p<.05), specifically 90 girls versus 87 boys had follow-up data. These 177 also did not differ from the larger cohort of 370 on demographic or mother drug use variables (p’s > .12), with two exceptions. There was a trend for OCS to be more optimal for those who did not attend the 30 month session and provide behavior problem data than those who did, t(343)= 1.79, p< .10). Mother cocaine use frequency was higher for those who did not attend the 30 month session and provide behavior problem data than those who did (t[357.74]-equal variances not assumed)= 2.13, p< .05).
2.1.1. Present Study Sample
The 225 children in the present study had a mean age of 30.54 months at time 1 (SD=1.1 month). 129 were Prenatally Cocaine and other drug Exposed [PCE], 30 were Non Cocaine but Other Drug Exposed [NCE], and 66 were Non Drug Exposed [NDE]). The children’s caregivers at the 30 month session were mostly biological mothers (87%), with 9% familial foster care parents (aunts and grandmothers), 4% non-familial foster care or adoptive mothers, 3% biological fathers, 1% aunts, and 1% grandmothers. Primary caregivers at the longitudinal follow-up sessions were also mainly biological mothers (76% biological mothers at 42 months, 84% at 48 months, 81% at 54 months, 81% at 60 months, and 78% at 66 months).
2.1.2. Recruitment and Drug Exposure Categorization
Participants’ mothers were recruited over a five-year period from women registering for prenatal care at the Women’s Center of a large urban hospital in the Northeast and, for those who did not receive prenatal care, upon admission to the postpartum ward. The Women’s Center provided care primarily for inner-city women and served a low-income primarily minority population.
Women were screened for substance use by trained research associates. Self-report information was obtained through a detailed interview (the Addiction Severity Index- ASI[35]) that covered lifetime use (number of years using) and frequency and amount of use in the previous 30 days of cocaine, tobacco, alcohol, marijuana, and other drugs (e.g., sedatives and opiates). Interviews were conducted either during the first prenatal visit or (for those not receiving prenatal care) directly after delivery. For all women, regardless of reported drug use, urine samples were obtained for toxicology either several times throughout the pregnancy (for those women attending prenatal visits) and/or at delivery (for those not receiving prenatal care). Every mother and infant had a urine screen at delivery. Urine was screened for metabolites of cocaine (e.g., benzoylecognine), opiods, benzodiazepines, and marijuana, using the Abbott TDx system and the recommended cutoff levels[39]. Meconium screening was also instituted after two years of recruitment into the project. Meconium screening did not identify any additional cocaine users who were both interview and urine toxicology negative.
Mothers were considered to be in the cocaine and other drug using group (PCE) if they reported cocaine use during pregnancy even if in those instances, urine or meconium toxicological results were negative. Also, if mothers reported that they did not use cocaine, but urine toxicological or meconium results were positive for cocaine, infants were considered exposed. Mothers who used opiates were excluded from the study. As cocaine use frequently co-occurs with use of nicotine, alcohol and/or marijuana, mothers in the cocaine using group were not excluded if they used these other substances, and other drug use was included as a covariate in secondary analyses.
Non-cocaine using women were eligible for recruitment into the two comparison groups. If they reported or tested positive in a urine screen for non-cocaine substances, such as alcohol, marijuana, or tobacco, they were assigned to the non cocaine but other drug using group (NCE). If they were negative in self-report and urine toxicology for all substances, they were assigned to the no drug-use prenatally (NDE) group.
2.1.3. Demographic and Birth Status
Demographic, birth status, and caregiver-child relationship information is shown in Table 1. Forty nine percent of the overall sample was male, with no exposure group differences in child gender. There were exposure group differences in race and mother education (summarized in Table 1). Since there were group differences in race and mother education, these variables were covaried in all analyses with exposure status as a predictor variable. Mother education data were missing for 2 participants. Thus, these analyses only included 223 families.
Table 1.
Demographic and birth status information for Prenatally Cocaine Exposed (PCE), Non Cocaine but Other Drug Exposed (NCE), and Non-Drug Exposed (NDE) groups
| Cocaine Exposed Mean (SD) |
Other drug Exposed Mean (SD) |
Non drug Exposed Mean (SD) |
Test of drug group differences | |
|---|---|---|---|---|
| Mother Variables | ||||
| Race: % in each group | χ2(6, n = 225)= 23.09** | |||
| African American | 89.1% | 63.3% | 72.7% | |
| Asian American | .8% | 0% | 0% | |
| Hispanic | .8% | 16.7% | 15.2% | |
| Caucasian | 9.3% | 20.0% | 12.1% | |
| % Completed high school | 59.4% | 80.0% | 83.1 % | χ2(4, n = 223)= 13.53** |
|
| ||||
| Child Variables | ||||
| % male | 52.7% | 30.0% | 51.5% | χ2(2, n = 225)= 5.20 (ns) |
| Obstetric Complications Scale scoresa | 77.73 (16.97) | 96.67 (16.24) | 88.92 (19.86) | F(2, 199)= 16.07*** |
| Caregiving Variable | ||||
| Parent-child dysfunctional interaction (PCDI) score | 20.31 (6.77) | 17.00 (4.87) | 17.91 (7.01) | F(2, 190)= 4.10* |
p<.05,
p<.01,
p<.001;
: Obstetric Complications Scale (OCS) includes 41 pregnancy and birth factors, including birth weight, head circumference, mother age, and parity. Higher OCS scores indicate more optimal birth conditions.
Scores on the Obstetric Complications Scale (OCS[29]) are also listed in Table 1. The OCS is a checklist of the number of favorable conditions (out of 41 conditions) during the pregnancy and delivery, including birth weight, gestation age, parity, mother age, bleeding during pregnancy, and infections or acute medical conditions during pregnancy. Higher scores on the OCS represent more optimal birth factors. The OCS was completed through mother interview and medical chart abstraction. OCS scores were calculated as the percentage of optimal scores and then converted to the “converted raw score”, following Littman and Parmelee[29].
As shown in Table 1, OCS scores differed by drug exposure group. Post-hoc Bonferroni comparisons indicated that PCE children had less optimal conditions than the NCE or the NDE children (p’s <.05), with no significant differences between the NCE and NDE groups. Because PCE children had less optimal birth conditions, we examined whether OCS scores mediated associations between exposure status and emotional arousal/regulation. Data were missing on the OCS variable for 23 children, so these children were not included in the meditational analyses.
Parent-child relationship quality was measured by the Parent-Child Dysfunctional Interaction (PCDI) subscale of the Parenting Stress Index short form[1]. The PCDI scale is a parent-report of dissatisfaction with their interactions with their children and with their children generally. Primary caregivers completed this measure at the 24-month (2 year) assessment point. PCDI information was available on 193 of the 225 children in the sample. As shown in Table 1, PCDI scores differed by exposure group. Post-hoc comparisons indicated a trend for PCE children to have greater parent-child dysfunctional interaction than the NCE or NDE children (p’s <.08), with no differences between the NCE and NDE groups. PCDI scores were examined as a moderator of associations between exposure group and emotional arousal and regulation.
2.1.4. Substance Use During Pregnancy
Mothers’ reported levels of cocaine and other drug use in the previous 30 days was obtained through interviews during pregnancy or at delivery. Substance use information for PCE and NCE mothers is provided in Table 2 (NDE mothers did not report any substance use).
Table 2.
Mothers’ self reported substance use for Prenatally Cocaine Exposed (PCE) and Non Cocaine but Other Drug Exposed (NCE) groups
| Cocaine Exposed Mean (SD) n = 129 |
Other Drug Exposed Mean (SD) n = 30 |
Drug group differences? | |
|---|---|---|---|
| Substance Use in Pregnancy | |||
| Cocaine | |||
| Days used out of 30 | 4.04 (6.12) | 0 | * |
| If used, # grams per day | .71 (1.11) | 0 | * |
| Alcohol | |||
| Days used out of 30 | 2.88 (5.30) | .50 (.51) | * |
|
| |||
| If used, amount per day (drinks) | 2.66 (5.47) (n = 84) | .41 (.53) (n = 15) | * |
| Tobacco | |||
| Days used out of 30 | 1.81 (5.51) | 1.37 (5.43) | * |
| If used, amount per day (packs) | .80 (.55) (n = 85) | .83 (.39) (n = 12) | |
| Marijuana | |||
| Days used out of 30 | 1.26 (3.74) | .13 (.35) | * |
| If used, amount per day (joints) | .88 (1.59) (n = 62) | .05 (04) (n = 4) | * |
indicates statistically significant difference of p<.05 in Mann-Whitney test. The Mann-Whitney test was used due to non-normal distribution of data.
PCE mothers reported using cocaine an average of 4.04 days per month (SD= 6.12 days) and used an average of .71 grams (SD= 1.11 grams) per occasion. PCE mothers reported using cocaine for an average of 5.85 years (SD= 2.99). PCE mothers in this study typically reported that they used cocaine from early in their pregnancy and throughout the pregnancy.
Regarding other substances used during pregnancy, PCE mothers were more likely than NCE mothers to use tobacco, and marijuana. The percentage reporting use of tobacco was 67% for PCE and 43% for NCE (Chi2(1, n= 159)= 5.64, p< .05), of alcohol was 65% for PCE and 50% for NCE (Chi2(1, n= 159)= 2.37, ns), and of marijuana was 48% for PCE and 13% for NCE (Chi2(1, n= 159)= 12.09, p< .01). The PCE group was greater than the NCE group on measures of alcohol, tobacco, and marijuana frequency of use (days per month) and amount used per occasion (see Table 2). Since PCE and NCE women differed in their use of other substances during pregnancy, whenever there was a significant exposure group effect, we conducted a secondary analysis including tobacco, alcohol, and marijuana use as control variables.
2.2. Toy Wait Task Procedure
The toy wait task, a widely-used task with toddlers (e.g., [18]), was used to elicit emotional arousal and regulation behaviors in the children. In this task, the child was shown an attractive new toy by a research assistant. The toy was then taken away and placed on a counter just out of the child’s reach. The child was instructed to wait 6 minutes for the toy and to instead play with other (less attractive) toys that were displayed on the floor. The child’s caregiver was present, but was instructed not to initiate interaction with the child. If the child asked for the toy, the caregiver was instructed only to “remind [child] that there are the other toys for [child] to play with.” The child’s behavior was videotaped and later coded for arousal and regulation.
2.3. Measures
2.3.1. Arousal and Regulation
Child behavior/affect in the task was coded for the frequency of: 1. agitated emotional arousal (e.g., whining, pouting, yelling, aggressive behavior); 2. references to the prohibited toy (e.g., looks to, reaches towards, or holds prohibited object); 3. references to the caregiver (e.g., looks to, approaches/reaches towards, physically contacts caregiver). The references to the caregiver code may reflect an attempt to regulate emotion by drawing in the caregiver[14].
Coders were unaware of children’s drug exposure status. Inter-rater reliability was assessed on 23% of the tapes and Kappas were .89 for agitated arousal and .91 for references to toy and caregiver. Frequencies of each behavior were divided by total time in the task, to control for slight differences in task length across children (usually due to the research assistant coming in slightly early). Mean arousal and regulation levels (and child behavior problem variables) are summarized by exposure group in Table 3.
Table 3.
Raw Means and Standard Deviations for Outcome Variables by Exposure Group
| Cocaine Exposed Mean (SD) |
Other drug Exposed Mean (SD) |
Non drug Exposed Mean (SD) |
Significant group difference (ANCOVAa) |
|
|---|---|---|---|---|
|
Arousal and Regulation (frequency per minute) | ||||
| Agitated emotional arousal | .21 (.39) | .08 (.17) | .13 (.27) | Trend |
| References to toy | .83 (.78) | .92 (.97) | .77 (.67) | |
| References to caregiver | 3.37 (1.72) | 2.81 (1.65) | 2.79 (1.65) | Trend |
|
| ||||
|
Child Behavior Problems (Average Scores) | ||||
| Externalizing at age 2 ½ | .56 (.35) | .57 (.25) | .49 (.33) | |
| Internalizing at age 2 ½ | .40 (.32) | .38 (.22) | .35 (.24) | |
| Externalizing at age 3 1/2 | .54 (.36) | .52 (.26) | .37 (.27) | * |
| Internalizing at age 3 ½ | .42 (.30) | .33 (.20) | .32 (.22) | |
| Externalizing at age 4 | .37 (.27) | .36 (.25) | .30 (.21) | |
| Internalizing at age 4 | .18 (.20) | .14 (.14) | .14 (.13) | |
| Externalizing at age 4 ½ | .29 (.24) | .26 (.15) | .29 (.21) | |
| Internalizing at age 4 ½ | .12 (.11) | .10 (.10) | .13 (.09) | |
| Externalizing at age 5 | .31 (.20) | .31 (.20) | .31 (.23) | |
| Internalizing at age 5 | .15 (.17) | .15 (.11) | .15 (.14) | |
| Externalizing at age 5 ½ | .30 (.22) | .26 (.17) | .25 (.18) | |
| Internalizing at age 5 ½ | .13 (.14) | .12 (.10) | .14 (.15) | |
: Group differences tested with ANCOVAs, controlling for race and mother education level;
p < .05, Trend p < .10.
2.3.2. Child Behavior Problems
Child externalizing and internalizing problems were measured with the caregiver-reported Child Behavior Checklist (CBCL). The CBCL has been used extensively in research and applied settings, shows good psychometric properties, and was normed on a nationally representative sample[2, 3]. CBCL/version 2–3 was used when child was 2 ½ – 3 years old[3] and CBCL/4-18 when child was 4– 5 ½ years old[2]. Analyses used externalizing and internalizing problem raw scores. The CBCL/2-3 had 26 externalizing items and 25 internalizing items, whereas the CBCL/4-18 had 33 externalizing items and 32 internalizing items. Because of the different numbers of items, an average score for each subscale was used.
2.4. Data Analyses
Exposure group differences in arousal and regulation (agitated arousal, references to caregiver, and references to toy) were examined with separate ANCOVAs, controlling for race and maternal education, with gender main effects and exposure status X child gender interactions included in the model. Cohen’s d effect sizes were calculated for ANCOVA findings using the difference (PCE minus NDE, PCE minus NCE, NCE minus NDE) in estimated marginal means. The difference in estimated marginal means was divided by the pooled standard deviation.
Because PCE and NCE/NDE women differed in their other drug use during pregnancy, whenever we found a significant exposure effect, we conducted a secondary analysis including tobacco, alcohol, and marijuana use as control variables. Results were similar (i.e., results for exposure status that were significant remained significant) when including the other drug use variables and so here we present the findings without these covariates.
We also examined whether Obstetric Complications Scale (OCS) scores mediated and whether parent-child dysfunctional interaction (PCDI) scores moderated relationships between prenatal exposure status and arousal/regulation. We conducted these analyses following Baron and Kenny’s[7] procedures for examination of mediators and moderators. For mediation, if Baron and Kenny’s criteria were met, we also conducted a statistical test of mediation using the Sobel test[43]. These analyses also controlled for race and maternal education.
Hierarchical Linear Models with unstructured covariance matrices were conducted (in SPSS 14.0 using the “Mixed” command) to examine predictors of the trajectories of externalizing and internalizing problem scores from 2 ½ to 5 ½ years. Random intercept/random slope models with unstructured covariance matrices were used. The unstructured covariance matrix was chosen based on a comparison of the −2 Restricted Log Likelihood Estimates across various covariance structures. HLMs were first conducted for the prediction of externalizing and internalizing behavior problems from arousal/regulation variables, time, and their interaction. This was to examine the hypothesis that arousal/regulation variables would be associated with change in behavior problems over time. After these were conducted, a second set of HLM models were conducted for the prediction of externalizing/internalizing problems from arousal/regulation variables, exposure status, time, and their interactions, with race and mother education included as control variables. These were to examine the secondary hypothesis that associations between arousal/regulation variables and behavior problems would be greater for cocaine exposed children than non-exposed children.
For the HLM analyses, time (age 2 ½, age 3 ½, age 4, age 4 ½, age 5, age 5 ½) was entered as a random effects coefficient. Either agitated arousal, references to caregiver, or references to toy was entered as the time-invariant covariate. For the equations including exposure status, exposure status was entered as a fixed effects factor. Subjects was entered as a random effects factor.
3. Results
3.1. Data Inspection and Transformations
Measures were examined for normality. The agitated arousal, references to prohibited toy, and externalizing problems variables were positively skewed, so square root transformations for these were used in analyses, although untransformed values were presented in tables and the figure for ease of interpretation (except for Table 4). The internalizing problems variable was also positively skewed and kurtotic and so a log transformation was used in analyses.
Table 4.
Estimates and Standard Errors for HLM Analyses of Square Root of Agitated Emotional Arousal Predicting Externalizing Behavior Problems
| Equations | Estimate | Standard Error | T-statistic | Significance |
|---|---|---|---|---|
| Intercept only model | ||||
| Intercept | 1.15 | .01 | 177.54 | *** |
| Intercept and time model | ||||
| Intercept | 1.33 | .01 | 89.04 | *** |
| Time | −.003 | .00 | −13.36 | *** |
| Intercept, time, and square root of agitated arousal model | ||||
| Intercept | 1.37 | .05 | 26.76 | *** |
| Time | −.003 | .00 | −13.38 | *** |
| SQRT(Agitated Arousal) | −.03 | .05 | −.76 | ns |
| Intercept, time, agitated arousal, and time X agitated arousal model | ||||
| Intercept | 1.11 | .11 | 9.77 | *** |
| Time | .001 | .002 | .77 | ns |
| SQRT(Agitated arousal) | .21 | .11 | 1.95 | p = .05 |
| Time X SQRT(Agitated arousal) | −.004 | .002 | −2.53 | * |
Note. SQRT indicates that the square root of the variable was used in analyses;
p < .05,
p < .001, ns not significant.
3.2. Arousal and Regulation: Exposure Status by Gender Effects
3.2.1. Agitated arousal
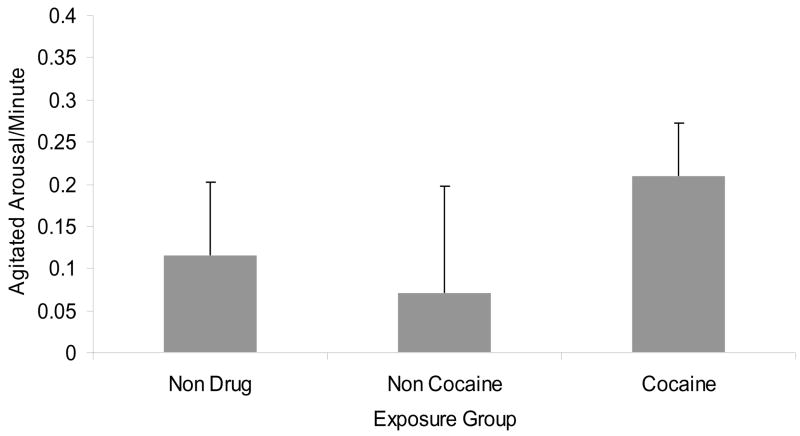
The gender X exposure status interaction was not significant and so was removed from the equation. The main effect of exposure status was a trend (F(2, 218)= 2.58, p< .10), with follow-ups indicating trends for greater arousal in PCE than NCE (F(1, 154)= 2.84, p< .10, Cohen’s d effect size= .26) and NDE toddlers (F(1, 189)= 2.74, p< .10, Cohen’s d effect size= .18), with no differences between NCE and NDE toddlers (see Figure 1).
Figure 1.
Agitated affect frequency by exposure status, controlling race and mother education
3.2.2. References to Prohibited Toy
There were no significant main effects or interactions for references to toy.
3.2.3. References to Caregiver
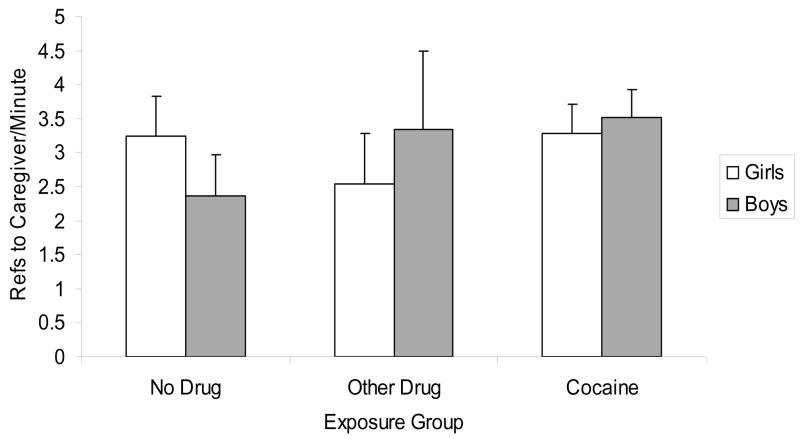
Findings revealed a significant exposure status X gender effect (F(2, 216)= 3.48, p< .05, see Figure 2). Follow-ups indicated that references to caregivers were higher for PCE than NDE boys (F(1, 96)= 7.73, p< .01, Cohen’s d effect size= .44) and for NCE than NDE boys (F(1, 39)= 3.97, p= .05, Cohen’s d effect size=.47). There were no significant exposure group differences for girls. In addition, there was a trend for an exposure status main effect (F(2, 216)= 2.52, p< .10), with the PCE group higher than the NDE group (F(1, 189)= 3.93, p< .05, Cohen’s d effect size= .22).
Figure 2.
References to caregiver frequency by exposure status and gender, controlling race and mother education
3.3. Obstetric Complications and Parent-Child Relationship as Mediator/Moderator
We examined whether Obstetric Complications Scale (OCS) scores mediated the relationship between prenatal cocaine exposure status and arousal/regulation variables, following Baron and Kenny’s[7] procedures. The first condition of mediation (that the predictor variable is associated with the outcome variable) was met for agitated arousal and references to caregiver: PCE children showed greater references to caregiver than NDE children and PCE children tended to show greater agitated arousal than NCE and NDE children. As argued by MacKinnon and colleagues, the first condition may not be necessary for mediation to be present as long as the other conditions are met[30]. As such, we also tested for the other conditions for references to the prohibited toy.
The second condition of mediation (that the predictor variable is associated with the mediator) was met- PCE children were lower than NCE and NDE children in OCS scores (Bonferroni follow-ups p’s < .001). The third condition of mediation (that the mediator is associated with the outcome variable in the presence of the predictor variable) was met for references to the prohibited toy (β for OCS scores= .16, t= 2.11, p< .05), but not for agitated arousal or references to caregiver. The fourth condition (that the predictor variable’s association with the outcome variable decreases or falls to zero when the mediator variable is included) was not met for references to the prohibited toy. The Beta for the relation between exposure status and references to toy actually increased when OCS scores were included in the equation. The sobel test for mediation by references to toy was not significant (Sobel test statistic= −.02, ns). Thus, mediation was not found for any arousal/regulation variable.
We examined whether parent-child relationship quality moderated relations between exposure status and arousal/regulation by including an interaction between parent-child relationship quality and exposure status in equations predicting arousal/regulation. Interactions were not significant, so moderation was not supported.
3.4. Emotional Arousal/Regulation Predicting the Trajectory of Externalizing and Internalizing Problems through Age 5 1/2
Random Slope/Random Intercept HLM models were conducted for the prediction of externalizing and internalizing problems. For internalizing problems, the random slope/random intercept models did not converge, possibly due to a lack of variability in the internalizing scores. Thus, for internalizing problems, a random intercept only model was used.
3.4.1. Arousal/Regulation
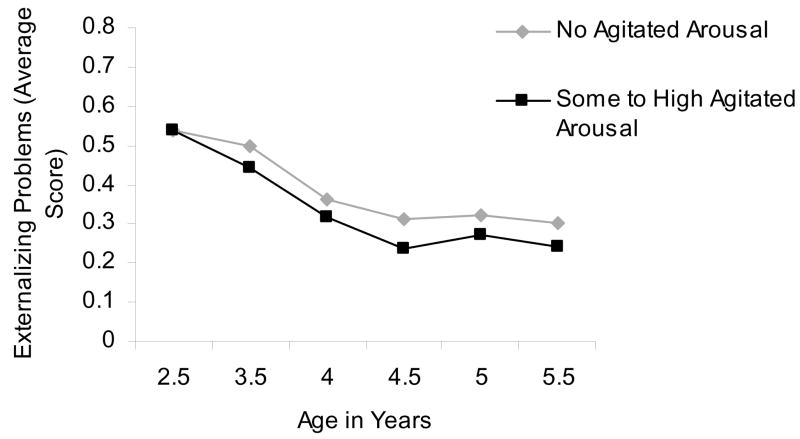
We tested the hypothesis that arousal/regulation variables would predict change in externalizing and internalizing problems over time. For agitated arousal, there was a significant (and negative) time X agitated arousal interaction predicting externalizing problems (Estimate= −.004 [SE= .002], t[192.95]= −2.53, p= .01). This indicated that higher agitated arousal at age 2 ½ predicted a greater decrease in externalizing problems over time (see Table 4, Figure 3). Agitated arousal did not predict change in internalizing symptoms over time.
Figure 3.
Mean level of Externalizing Problems by Agitated Arousal (None/Some to high) from age 2 ½ to 5 ½ years
References to caregiver and to prohibited toy (and their interactions with time) were not associated with externalizing or internalizing symptoms.
3.4.2. Arousal/Regulation by Exposure Status Interactions
We also conducted HLM analyses to test the second hypothesis of an interaction between exposure status and arousal/regulation predicting behavior problems over time (including race and maternal education as covariates). Arousal/regulation X exposure status interactions and Arousal/regulation X exposure status X time interactions were not significant for externalizing or internalizing problems. There was one non-significant trend for an interaction between agitated affect X exposure status X time on externalizing problems, F (190.70)= 2.53, p< .10. Follow up HLMs indicated that agitated arousal X time effects were significant for children in the PCE group (Estimate= −.01, SE= .002, t[111.60]= −2.72, p < .01), but not for the NDE or NCE groups. Thus, the effect of agitated arousal by time on externalizing symptoms may be driven by cocaine exposed children (although the interaction term was only a non-significant trend).
3.4.3. Exposure status
It is also possible to find exposure status effects on change in behavior problems over time. We conducted exploratory HLM analyses to test this. Analyses revealed that exposure status was not a significant predictor of externalizing or internalizing problems or of change in those problems over time. Time was a significant predictor of externalizing problems (F[1, 157.69)= 138.12, p< .0001) and internalizing problems (F[1, 689.20)= 261.35, p< .0001), with both externalizing and internalizing problems decreasing over time.
Mean levels of externalizing and internalizing problems at each time point by exposure status group are summarized in Table 3. Out of 12 comparisons, there was only one significant exposure group difference in symptoms- PCE and NCE children had higher externalizing symptoms than NDE children at age 3 ½ years. This one significant finding out of 12 is little more than would be expected to occur 5% of the time.
4. Discussion
The present study was one of few to examine links between prenatal cocaine and other drug exposure and risk for emotional arousal and regulation difficulties in toddlerhood- a period of rapid growth in emotion regulation capacities. The study found links between drug exposure (both prenatal cocaine and other drug exposure) and children’s greater tendency to reach out to their caregiver in a frustrating task for boys. In addition, there was a trend for prenatal cocaine exposure specifically to be related to greater observed agitated emotional arousal, arousal that was linked to arousal that was linked to a greater decrease in externalizing behavior problems over a three-year follow up. The findings for prenatal drug exposure and emotional arousal and regulation were found controlling for demographic variables (race and maternal education) and were not mediated by birth complications or moderated by caregiver-reported caregiver-child relationship quality.
When confronted with a frustrating task (waiting for an attractive toy for 6 minutes), cocaine exposed and other drug (alcohol, tobacco, and/or marijuana) exposed boys were more likely than non-exposed boys to approach their caregivers. Interestingly, this pattern was not seen for girls, with both exposed and non-exposed girls showing a similar moderate level of attention to their caregivers. Also of note, boys’ references to caregivers were not different between the cocaine exposed group and the non cocaine but other drug exposed group, suggesting that this it is not cocaine-specific effect. Further, the effect was not accounted for by the child’s level of birth complications or by caregiver-reported parent-child relationship quality.
What might explain exposed boys’ greater references to caregivers in the frustrating task? This may signify an adaptive ability of exposed boys to use their caregivers to help them regulate emotion in this frustrating task. During toddlerhood, caregivers often help their children regulate emotion, which leads children to develop independent emotion regulation skills[27]. Thus, exposed boys’ greater references to caregivers may represent a protective factor for their development, particularly if caregivers respond positively to their bids for attention. If caregivers are not responsive or respond negatively, as caregivers with a history of substance use may do[37], this strategy may not be optimally effective. We conducted a follow-up analysis examining whether parent-child relationship quality moderated any relationship between references to caregiver and behavior problems over time. We did not find moderation or prediction from references to caregiver, suggesting that references to caregiver did not predict behavior problems, regardless of whether parenting quality was good or bad. References to caregiver may predict other facets of social/emotional development and this could be examined in future research.
There was a trend for prenatally cocaine exposed toddlers to show greater agitated emotional arousal in the frustrating task than non-exposed or non cocaine but other drug-exposed toddlers. This is consistent with previous findings of greater negative emotional arousal in cocaine-exposed than non-exposed infants.[32] Further, because there was a trend for cocaine exposed toddlers to be higher in agitated arousal than other drug exposed toddlers, changes in emotional arousal levels may be seen as a cocaine-specific outcome rather than one associated with drug exposure in general. Of course, the mechanism by which cocaine exposure might be linked to greater arousal could be a number of things, including direct biological effects of cocaine on developing emotional systems and post-natal environmental conditions experienced by children of cocaine using mothers. We did not find that obstetric complications or caregiver-child relationship quality accounted for this trend-level relationship. However, there are other environmental factors (such as a high number of residence changes) that should be explored in future studies.
Notably, higher levels of agitated arousal in the frustrating task, experienced more often by cocaine-exposed toddlers, predicted greater decreases in externalizing behavior problems from toddlerhood to the preschool years. Normatively, externalizing behaviors decrease from age 2 into school age[15, 23], so it is notable that toddlers with low agitated arousal showed less of a decrease in these problems. The prediction of externalizing problem trajectory was not found for references to caregiver or to the prohibited toy, so may have been specific to emotional arousal in the task. Expressing some amount of negative emotion in a frustrating task may be normative, appropriate behavior for toddlers. A lack of affect expression may reflect overregulation of negative affect.[19] Over-regulated negative emotion has been hypothesized to lead to externalizing behavior problems in early childhood and to internalizing disorders in late childhood and adolescence.[19, 26] Low levels of negative emotion may also reflect a physiological under arousal, or a “blunted” affect, which is linked to conduct problems among youth.[28] Interestingly, there was a trend for cocaine-exposed children to show higher agitated arousal levels than other toddlers, which may represent a protective factor for them from such conduct problems. Indeed, there was a trend-level interaction between cocaine exposure status and agitated arousal in predicting change in externalizing behaviors over time, indicating that the strongest relation between high arousal and lower externalizing problems was for the prenatally cocaine and other drug exposed children. Interestingly, cocaine exposure status on it’s own did not predict the trajectory of behavior problems.
Emotional arousal and regulation did not predict children’s trajectory of internalizing behavior problems through age 5 1/2, however. This may be due to a lack of variability in internalizing symptoms in the present sample. Normatively, internalizing symptoms tend to increase gradually during the preschool years[23], however, in this sample, the internalizing symptoms started out low and decreased slightly over time. It is possible that children from high risk, drug abusing families, show a different trajectory of internalizing symptoms. Further research is needed to test this hypothesis. It is also possible that internalizing problems may be better evaluated using child self-reports, which could be collected in later childhood or adolescence.
4.1. Summary/Limitations
In sum, the present findings showed a trend for an association between prenatal cocaine and other drug exposure and altered emotional arousal and a significant association between cocaine exposure and emotion regulation attempts for boys. The findings are important in that emotional arousal and regulation have implications for risk for or protection from the development of psychopathology. However, the study had limitations that should be addressed in future research. First, we examined children whose mothers consented to study participation when pregnant or at delivery and attended a session 2 ½ years later, mothers who may have lower addiction severity than those who did not do this. Consistent with this, families who did not have behavior problem data through the follow-ups had mothers who used cocaine more frequently during pregnancy than those who were included in behavior problem analyses. Thus, the families studied here may not generalize to the larger population of cocaine and other drug exposed toddlers, who may show more difficulties with emotional arousal and regulation. Nonetheless, even in this less at-risk group, we see a trend for exposure-related differences in emotional arousal and interesting protective links between arousal and future behavior problems.
A second limitation is that the study examined cocaine exposure as a categorical variable and, due to lack of information, we were unable to examine associations between level of cocaine exposure and emotional outcomes. In addition, we did not have information on mothers’ post-partum drug use, which may also affect child development. Third, although we did examine parent-child relationship quality as a moderator, our measure was obtained at age 2 and was a parent-report of their satisfaction with the relationship and their child. Future studies should examine more objective measures, such as a report of specific parenting behaviors. Despite these limitations, the present study showed a trend for a link between prenatal cocaine exposure and greater negative emotional arousal in toddlerhood, arousal that was a protective factor from the development of externalizing problems across the preschool years.
Acknowledgments
Support for this project was provided by the National Institutes of Health (NIH) through Grants R01-DA-06025 (Mayes), DA-017863 (Mayes), KO5 (Mayes), P50-DA-16556 (PI: Sinha), and K01-DA-024759 (PI: Chaplin) and a grant from the Pfeffer Foundation (Mayes). The study sponsors had no involvement in the study design; collection, analysis, and interpretation of data; the writing of the manuscript; or the decision to submit the manuscript for publication.
The authors gratefully acknowledge the sponsors, the participating families, and the research staff that helped to collect and code the data.
Footnotes
Conflict of Interest Statement
The authors have no conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abidin RR. Parenting Stress Index (PSI) Manual. Psychological Assessment Resources, Inc; Odessa, FL: 1990. [Google Scholar]
- 2.Achenbach TM. Manual for the Child Behavior Checklist/4-18 and 1991 Profile. University of Vermont Department of Psychiatry; Burlington, VT: 1991. [Google Scholar]
- 3.Achenbach TM, Edelbrock C, Howell CT. Empirically based assessment of the behavioral/emotional problems of 2-and 3-year-old children. J Abnorm Child Psychol. 1987;15:629–650. doi: 10.1007/BF00917246. [DOI] [PubMed] [Google Scholar]
- 4.Alessandri SM, Sullivan MW, Imaizumi S, Lewis M. Learning and emotional responsivity in cocaine-exposed infants. Dev Psychol. 1993;29:989–997. [Google Scholar]
- 5.Bada HS, Das A, Bauer CR, et al. Impact of Prenatal Cocaine Exposure on Child Behavior Problems Through School Age. Pediatrics. 2007;119:e348–e359. doi: 10.1542/peds.2006-1404. [DOI] [PubMed] [Google Scholar]
- 6.Bandstra ES, Vogel AL, Morrow CE, Xue L, Anthony JC. Severity of prenatal cocaine exposure and child language functioning through age seven years: A longitudinal latent growth curve analysis. Subst Use Misuse. 2004;39:25–59. doi: 10.1081/JA-120027765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 8.Bauer CR, Langer JC, Shankaran S, Bada HS, Lester B, Wright LL, Krause-Steinrauf H, Smeriglio VL, Finnegan LP, Maza PL, Verter J. Acute Neonatal Effects of Cocaine Exposure During Pregnancy. Arch Pediatr Adolesc Med. 2005;159:824–834. doi: 10.1001/archpedi.159.9.824. [DOI] [PubMed] [Google Scholar]
- 9.Behnke M, Eyler FD, Warner TD, Garvan CW, Hou W, Wobie K. Outcome from a prospective, longitudinal study of prenatal cocaine use: preschool development at 3 years of age. J Pediatr Psychol. 2006;31:41–49. doi: 10.1093/jpepsy/jsj027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bendersky M, Bennett D, Lewis M. Aggression at age 5 as a function of prenatal exposure to cocaine, gender, and environmental risk. J Pediatr Psychol. 2006;31:71–84. doi: 10.1093/jpepsy/jsj025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bendersky M, Lewis M. Arousal Modulation in Cocaine-Exposed Infants. Dev Psychol. 1998;34:555–564. [PMC free article] [PubMed] [Google Scholar]
- 12.Brody L, Hall J. Gender, emotion, and expression. In: Lewis M, Haviland-Jones J, editors. Handbook of Emotions. 2. Guilford Press; New York: 2000. pp. 325–414. [Google Scholar]
- 13.Brooks-Gunn J, McCarton C, Hawley T. Effects of in utero drug exposure on children’s development. Review and recommendations. Arch Pediatr Adolesc Med. 1994;148:33–39. doi: 10.1001/archpedi.1994.02170010035007. [DOI] [PubMed] [Google Scholar]
- 14.Calkins SD, Johnson MC. Toddler regulation of distress to frustrating events: temperamental and maternal correlates. Infant Behavior and Development. 1998;21:379–395. [Google Scholar]
- 15.Campbell SB. Behavior problems in preschool children: A review of recent research. J Child Psychol Psychiatry. 1995;36:113–149. doi: 10.1111/j.1469-7610.1995.tb01657.x. [DOI] [PubMed] [Google Scholar]
- 16.Chaplin TM, Cole PM. The role of emotion regulation in the development of psychopathology. In: Hankin BL, Abela JRZ, editors. Development of Psychopathology: A Vulnerability-Stress Perspective. Sage; Thousand Oaks, CA: 2005. pp. 49–74. [Google Scholar]
- 17.Cole PM, Michel MK, Teti LO. The development of emotion regulation and dysregulation: A clinical perspective. The Development of Emotion Regulation: Biological and Behavioral Considerations. In: Fox NA, editor. Monogr Soc Res Child Dev. 2–3 Serial No 240. Vol. 59. 1994. pp. 73–100. [PubMed] [Google Scholar]
- 18.Cole PM, Teti LO, Zahn-Waxler C. Mutual emotion regulation and the stability of conduct problems between preschool and early school age. Devel Psychopathol. 2003;15:1–18. [PubMed] [Google Scholar]
- 19.Cole PM, Zahn–Waxler C, Fox N, Usher BA, Welsh JD. Individual differences in emotion regulation and behavior problems in preschool children. J Ab Psychol. 1996;104:518–529. [PubMed] [Google Scholar]
- 20.Delaney-Black V, Covington C, Nordstrom B, Ager J, Janisse J, Hannigan JH, Chiodo L, Sokol RJ. Prenatal cocaine: Quantity of exposure and gender moderation. J Dev Behav Pediatr. 2004;25:254–263. doi: 10.1097/00004703-200408000-00005. [DOI] [PubMed] [Google Scholar]
- 21.Dennis T, Bendersky M, Ramsay D, Lewis M. Reactivity and regulation in children prenatally exposed to cocaine. Devel Psychol. 2006;42:688–697. doi: 10.1037/0012-1649.42.4.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frank DA, Augustyn M, Knight WG, Pell T, Zuckerman B. Growth, development, and behavior in early childhood following prenatal cocaine exposure: a systematic review. JAMA. 2001;285:1613–1625. doi: 10.1001/jama.285.12.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gilliom M, Shaw DS. Codevelopment of externalizing and internalizing problems In early childhood. Dev Psychopathol. 2004;16:313–333. doi: 10.1017/s0954579404044530. [DOI] [PubMed] [Google Scholar]
- 24.Glatt SJ, Bolanos CA, Trksak GH, Jackson D. Effects of prenatal cocaine exposure on dopamine system development: A meta analysis. Neurotoxicology & Teratology. 2000;22:617–629. doi: 10.1016/s0892-0362(00)00088-x. [DOI] [PubMed] [Google Scholar]
- 25.Karmel BZ, Gardner JM. Prenatal cocaine exposure effects on arousal-modulated attention during the neonatal period. Dev Psychobiol. 1996;29:463–480. doi: 10.1002/(SICI)1098-2302(199607)29:5<463::AID-DEV5>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 26.Keenan K, Hipwell AE. Preadolescent clues to understanding depression in girls. Clin Child Fam Psychol Rev. 2005;8:89–105. doi: 10.1007/s10567-005-4750-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kopp CB. Regulation of Distress and Negative Emotions: A Developmental View. Dev Psychol. 1989;25:343–54. [Google Scholar]
- 28.Lahey BB, Hart EL, Pliszka S, Applegate B, McBurnett K. Neurophysiological correlates of conduct disorder: A rationale and a review of research. J Clin Child Adolesc Psychol. 1993;22:141–153. [Google Scholar]
- 29.Littman B, Parmelee AH. Manual for Obstetric Complications, Infant Studies Project, Department of Pediatrics, School of Medicine. University of California; Los Angeles, CA: 1974. [Google Scholar]
- 30.MacKinnon DP, Fairchild AJ, Fritz MS. Mediation Analysis. Annu Rev Psychol. 2007;58:593–614. doi: 10.1146/annurev.psych.58.110405.085542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mayes L. Developing brain and in utero cocaine exposure: Effects on neural ontogeny. Devel Psychopathol. 1999;11:685–714. doi: 10.1017/s0954579499002278. [DOI] [PubMed] [Google Scholar]
- 32.Mayes LC, Bornstein MH, Chawarska K, Haynes OM. Impaired regulation of arousal in three-month-old infants Exposed prenatally to cocaine and other drugs. Devel Psychopathol. 1996;8:29–42. [Google Scholar]
- 33.Mayes LC, Cicchetti D. Prenatal cocaine exposure and neurobehavioral development: How subjects lost to follow-up bias study results. Child Neuropsychol. 1995;1:128–139. [Google Scholar]
- 34.Mayes LC, Grillon C, Granger R, Shottenfeld R. Regulation of arousal and attention in preschool children exposed to cocaine prenatally. Ann N Y Acad Sci. 1998;846:126–143. [PubMed] [Google Scholar]
- 35.McLellan AT, Luborsky L, O’Brien CP, Woody GE. An improved evaluation instrument for substance abuse patients: The Addiction Severity Index. J Nerv Ment Dis. 1980;168:26–33. doi: 10.1097/00005053-198001000-00006. [DOI] [PubMed] [Google Scholar]
- 36.Metosky P, Vondra J. Prenatal drug exposure and play and coping in toddlers: A comparative study. Infant Behavior and Development. 1995;18:15–25. [Google Scholar]
- 37.Molitor A, Mayes LC, Ward A. Emotion regulation behavior during a separation procedure in 18-month-old children of mothers using cocaine and other drugs. Dev Psychopathol. 2003;15:39–54. doi: 10.1017/s0954579403000038. [DOI] [PubMed] [Google Scholar]
- 38.Needlman R, Zuckerman B, Anderson GM. Cerebrospinal Fluid Monoamine Precursors and Metabolites in Human Neonates Following in Utero Cocaine Exposure: A Preliminary Study. Pediatrics. 1993;92:55–60. [PubMed] [Google Scholar]
- 39.Poklis A. Evaluation of TDx cocaine metabolite assay. J Anal Toxicol. 1987;11:228–230. doi: 10.1093/jat/11.5.228. [DOI] [PubMed] [Google Scholar]
- 40.Regalado MG, Schechtman VL, Khoo MC, et al. Spectral analysis of heart rate variability and respiration during sleep in cocaine-exposed neonates. Clin Physiol. 2001;21:428–436. doi: 10.1046/j.1365-2281.2001.00353.x. [DOI] [PubMed] [Google Scholar]
- 41.Seidler FJ, Slotkin TA. Fetal cocaine exposure causes persistent noradrenergic hyperactivity in rat brain regions: effects on neurotransmitter turnover and receptors. J Pharmacol Exp Ther. 1992;263(2):413–21. [PubMed] [Google Scholar]
- 42.Singer LT. Advances and redirections in understanding effects of fetal drug exposure. Journal of Drug Issues. 1999;29:253–262. doi: 10.1177/002204269902900207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sobel ME. Asymptotic confidence intervals for indirect effects in structural equation models. In: Leinhardt S, editor. Sociological Methodology. American Sociological Association; Washington, DC: 1982. pp. 290–93. [Google Scholar]
- 44.Tronick E, Beeghley M. Prenatal cocaine exposure, child development, and the compromising effects of cumulative risk. Clinics of Perinatology. 1999;26:151–171. [PubMed] [Google Scholar]
- 45.Volpe JJ. Effect of cocaine use on the fetus. N Engl J Med. 1992;327:399–404. doi: 10.1056/NEJM199208063270607. [DOI] [PubMed] [Google Scholar]
- 46.Wasserman GA, Kline JK, Bateman DA, Chiriboga C, Lumey LH, Friedlander H, Melton L, Heagarty MC. Prenatal cocaine exposure and school-age intelligence. Drug Alcohol Depend. 1998;50:203–210. doi: 10.1016/s0376-8716(98)00037-4. [DOI] [PubMed] [Google Scholar]
- 47.Zuckerman B, Frank DA, Mayes L. Cocaine-exposed infants and developmental outcomes: “Crack kids” revisited. JAMA. 2002;287:1990–1991. doi: 10.1001/jama.287.15.1990. [DOI] [PubMed] [Google Scholar]