Abstract
Background and Objectives
The posterior approach to the brachial plexus—or cervical paravertebral block—has advantages over the anterolateral interscalene approach, but concerns regarding “blind” needle placement near the neuraxis have limited the acceptance of this useful technique. We present a technique to place an interscalene perineural catheter that potentially decreases neuraxial involvement with the use of ultrasound guidance.
Methods
A 55-year-old man scheduled for total shoulder arthroplasty underwent placement of an interscalene perineural catheter. The posterior approach was selected to avoid the external jugular vein and anticipated sterile surgical field. Under in-plane ultrasound guidance, a 17-gauge insulated Tuohy-tip needle was inserted between the levator scapulae and trapezius muscles, and guided through the middle scalene muscle, remaining less than 2 cm below the skin throughout. Deltoid and biceps contractions were elicited at a current of 0.6 mA, and a 19-gauge stimulating catheter was advanced 5 cm beyond the needle tip, without a concomitant decrease in motor response.
Results
The initial 40 mL 0.5% ropivacaine bolus via the catheter resulted in unilateral anesthesia typical of an interscalene block; and subsequent perineural infusion of 0.2% ropivacaine was delivered via portable infusion pump through postoperative day 4.
Conclusions
Continuous interscalene block using an ultrasound-guided posterior approach is an alternative technique that retains the benefits of posterior catheter insertion, but potentially reduces the risk of complications that may result from blind needle insertion.
Keywords: continuous peripheral nerve block, perineural catheter, perineural local anesthetic infusion, patient-controlled regional analgesia, ultrasound-guided regional anesthesia
Continuous interscalene nerve blocks using the anterolateral approach have demonstrated efficacy in reducing pain, decreasing supplemental opioid requirements and side effects, improving sleep quality and range-of-motion, as well as shortening the time until discharge-readiness following moderate to severely painful shoulder surgery.1–3 However, placing a perineural catheter can be challenging using this approach, with catheter placement failure rates up to 20% even among experienced practitioners.2,4 Studies using stimulating catheters have reported high success rates in placement and retention,3,5,6 but the time required for placement may be greatly increased—in some cases in excess of 30 minutes.6,7 Furthermore, other factors may limit or complicate the application of the anterolateral approach, such as the external jugular vein overlying the brachial plexus, catheter dislodgment due to superficial placement, and inclusion of the catheter site in the surgical field.
Using a posterior approach to the brachial plexus may decrease the incidence of these problems.8,9 The cervical paravertebral block introduces the needle between the levator scapulae and trapezius muscles and directs the catheter anteriorly to lie along the brachial plexus.10–12 In the “blind” technique previously described,10 12 the needle is advanced in an anterior direction until it contacts the transverse process of the cervical vertebra, then “walked” laterally along the transverse process, and advanced further anteriorly until the brachial plexus is located.9,11,12 However, the proximity of the needle to the neuraxis has led to complications related to the “blind” technique including epidural,13 intrathecal,14 and intracord injection,15 leading some practitioners to question the acceptability of the risk-benefit ratio.16,17 Proponents of the posterior approach maintain that complications may be avoided with anatomic familiarity, proper equipment, and improvements in technique.18
We describe an ultrasound-guided interscalene perineural catheter technique that retains the multiple benefits of the posterior approach, by using real-time imaging to accurately place the needle into the interscalene groove, combined with a stimulating perineural catheter to select the distribution of anesthesia.
METHODS
Technique Description
A 55-year-old man presented for total shoulder arthroplasty. The patient desired perineural catheter placement for postoperative analgesia, and the posterior approach to the brachial plexus was selected to avoid the external jugular vein and anticipated sterile surgical field by the surgeon’s request. Of note, the University of California San Diego Institutional Review Board specifically does not require review of medical case reports (personal communication, Noel Myers, November 2007).
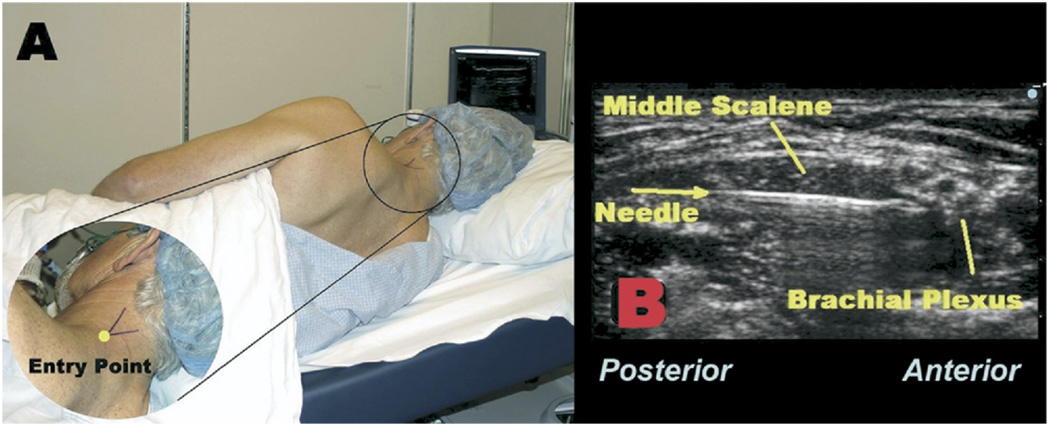
Standard American Society of Anesthesiologists (ASA) monitors and oxygen via facemask were applied, the patient was positioned right lateral decubitus with the head of the bed slightly raised, and the skin was prepared with antiseptic solution prior to application of a sterile drape (Fig. 1A). Intravenous midazolam (2 mg) and fentanyl (100 µg) were titrated for patient comfort. Using a 6 to 13 MHz linear ultrasound probe (HFL38 MicroMaxx, SonoSite, Bothell, WA), the brachial plexus trunks were identified between the left anterior and middle scalene muscles at the cephalad-caudad level of the cricothyroid membrane. At the junction of the levator scapulae and trapezius muscles (Fig. 1A), 1% lidocaine was injected to anesthetize skin, and the track into the middle scalene muscle under ultrasound guidance. With the bevel directed caudad and lateral, an 8.89 cm, 17-gauge, insulated Tuohy-tip needle (Stimucath, Arrow International, Reading, PA) was inserted through the lidocaine skin wheal. The needle was connected to a nerve stimulator (Stimuplex-DIG; B. Braun Medical, Bethlehem, PA) initially set at 1.2 mA, 0.1 ms, and 2 Hz.
FIGURE 1.
A, The patient is placed in the right lateral decubitus position. The junction of the levator scapulae and trapezius muscles is identified by the “V.” B, A 1 7-gauge Tuohy-tip needle is directed under in-plane ultrasound guidance through the middle scalene muscle toward the brachial plexus.
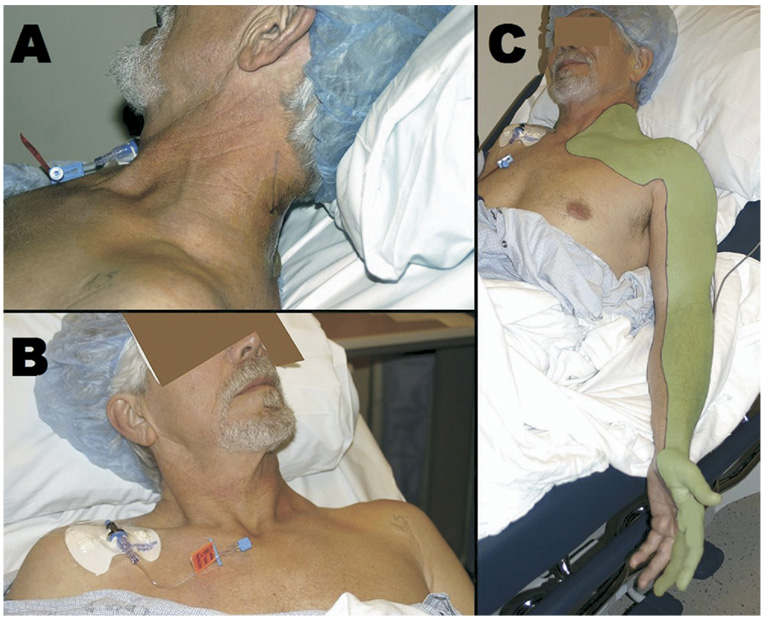
Under continuous in-plane ultrasound guidance (probe held in the operator’s left hand), the needle (in the operator’s right hand) was directed anteriorly toward the brachial plexus, passing lateral to the posterior scalene and through the middle scalene muscles (Fig. 1B). Deltoid and biceps motion were sought and elicited at a current of 0.6 mA on the first attempt. A 19-gauge catheter was then placed through the length of the needle, and the nerve stimulator lead transferred from the needle to the catheter, which has a conducting wire through its length delivering current to its tip. The stimulating current remained at 0.6 mA and the catheter was advanced 5 cm beyond the needle tip on the first insertion, without a decrease in motor response, or need for additional needle manipulation. The needle was withdrawn over the catheter and the catheter stylet was removed. Using the 17-gauge Tuohy needle, the catheter was tunneled subcutaneously below the hairline (Fig. 2A) toward the contralateral side to avoid the surgical field, and affixed to the contralateral shoulder using liquid adhesive and clear occlusive dressings (Fig. 2B).
FIGURE 2.
A, The perineural catheter has been tunneled posteriorly beneath the hairline. The initial catheter placement site has been covered by liquid adhesive and a clear occlusive dressing. B, The anchoring device has been secured on the contralateral shoulder to avoid the anticipated surgical field. C, Following a 40 mL bolus injection of 0.5% ropivacaine, unilateral upper extremity anesthesia has been achieved in a distribution typical of the interscalene block (shaded area).
An initial 40 mL bolus of ropivacaine (0.5%) with epinephrine (2.5 µg/mL), injected under ultrasound visualization (Fig. 2C), produced topical anesthesia to cold and light touch in the distribution typical of an anterolateral interscalene single-injection block within 15 minutes (Fig. 2C). The patient underwent an uncomplicated surgical procedure under general anesthesia (provided for patient comfort during the 4 hour procedure), receiving 150 µg of fentanyl for induction without subsequent opioid administration. A perineural infusion of ropivacaine (0.2%, 8 mL/h basal rate, 4 mL patient-controlled bolus dose, 30-minute lockout) was initiated intraoperatively using a portable infusion pump (Pain Pump 2 BlockAid, Stryker Instruments, Kalamazoo, MI).
RESULTS
The patient emerged from general anesthesia pain-free and was discharged from the recovery room after 1 hour without requiring additional analgesics.
The patient was discharged home the morning of postoperative day 1, with a full infusion pump (400 mL reservoir of 0.2% ropivacaine), a prescription for oral oxycodone tablets (5 mg) for breakthrough pain, detailed oral and written catheter-related instructions, and Acute Pain Service contact information. During the perineural infusion, the patient reported a pain score of 0 to 1 using a 0 to 10 numeric rating scale (0 equivalent to no pain), without supplemental analgesics required in the hospital or at home. The patient was contacted daily by telephone until home catheter removal by the patient’s caretaker in the afternoon of postoperative day 4 (also with real-time physician guidance).
DISCUSSION
While continuous interscalene nerve blocks provide dramatic postoperative patient benefits, perineural catheter placement at this level of the brachial plexus may be technically challenging and carry potential risks. The ultrasound-guided technique presented in this report is easily mastered and, in our experience, has resulted in a high success rate with both residents and fellows performing procedures under attending supervision.
The needle trajectory of this technique is similar to the recently described single-injection “transscalene brachial plexus block” that uses a posterior needle insertion along the lateral border of the middle scalene muscle (trapezius when the middle scalene is not identifiable).19 The technique described in the present report differs in 2 important respects: (1) ultrasound guidance permits rapid identification of the brachial plexus, allowing a single needle pass in the overwhelming majority of cases, along with confirmation of adequate perineural local anesthetic distribution of the initial surgical block; and (2) the perineural catheter placement allows extended duration of postoperative analgesia with a local anesthetic infusion.
In addition, tracking the course of the Tuohy needle under direct visualization allows a relatively superficial trajectory and possibly decreases the risk of neuraxial complications to near 0.20–22 Unlike traditional “blind” paravertebral approaches that suggest contacting the vertebral transverse process to gauge depth,9,11,12 the ultrasound-guided technique employs sonography to locate the brachial plexus, maintain a needle trajectory which is lateral to the transverse process, and minimize the number of needle redirections that can lead to needle misplacement.13–15 Surface ultrasound allows anesthesiologists to study anatomy in real time and adjust the needle trajectory based on visual feedback. For example, blood vessels in the projected path of the needle may be avoided. In addition, the longer length of catheter insertion using an in-plane ultrasound-guided posterior approach may improve catheter retention rate.
The use of an electric current via both the insulated needle and stimulating catheter provides electrophysiologic information in addition to the anatomic data conveyed by surface ultrasound. While interscalene catheters may be placed under ultrasound guidance without the concurrent use of nerve stimulation,23 stimulation via the needle and catheter suggests appropriate catheter tip position at the desired brachial plexus level, in addition to visual confirmation by ultrasound. In the current case of catheter placement for shoulder surgery, eliciting a deltoid and/or biceps motor response from the tip of the perineural catheter at the C5 to C6 nerve root level helped to confirm ideal placement for shoulder surgery. Extension at the elbow or activation of the intrinsic hand muscles would indicate the need for catheter repositioning.
Although the ultrasound-guided posterior approach has multiple potential advantages, there are limitations as well. As described in this report, an ultrasound machine is required with its associated training and cost.24–26 The benefits conferred by ultrasound guidance are dependent on the practitioner’s ability to correctly identify anatomic structures, and visualize the needle in-plane. In addition, some may question the need for a new interscalene catheter-placement technique since the well-described anterior approach has demonstrated efficacy, and a relatively high safety margin.27–29 However, it should be noted that the anterolateral approach is associated with multiple potential complications as well,30–34 and the ultrasound-guided posterior approach may help to avoid these. As in the modified cervical paravertebral block previously mentioned,9,12 we still recommend inserting the needle between the trapezius and levator scapulae muscles with the ultrasound-guided technique, to minimize the risk of neck pain.
In summary, we present a technique that, compared with the anterolateral approach, displaces the catheter insertion site further away from the surgeon’s sterile field without concern of external jugular vein location and, in our experience, may be easily placed in a relatively short period of time, with a very high rate of success. Confirmation of these proposed benefits requires prospective study in a randomized, controlled trial.
Acknowledgments
Dr. Ilfeld is supported by National Institutes of Health grant GM077026 from the National Institute of General Medical Sciences (Bethesda, MD). The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of this entity. Drs. Mariano and Loland conduct continuous peripheral nerve block workshops for Stryker Instruments (Kalamazoo, MI). Drs. Mariano and Ilfeld have received research material and funding for clinical investigations from Arrow International (Reading, PA) and Stryker Instruments. These two companies had no input into any aspect of this manuscript or its preparation.
REFERENCES
- 1.Klein SM, Grant SA, Greengrass RA, et al. Interscalene brachial plexus block with a continuous catheter insertion system and a disposable infusion pump. Anesth Analg. 2000;91:1473–1478. doi: 10.1097/00000539-200012000-00033. [DOI] [PubMed] [Google Scholar]
- 2.Ilfeld BM, Morey TE, Wright TW, et al. Continuous interscalene brachial plexus block for postoperative pain control at home: a randomized, double-blinded, placebo-controlled study. Anesth Analg. 2003;96:1089–1095. doi: 10.1213/01.ANE.0000049824.51036.EF. [DOI] [PubMed] [Google Scholar]
- 3.Ilfeld BM, Vandenborne K, Duncan PW, et al. Ambulatory continuous interscalene nerve blocks decrease the time to discharge readiness after total shoulder arthroplasty: a randomized, triple-masked, placebo-controlled study. Anesthesiology. 2006;105:999–1007. doi: 10.1097/00000542-200611000-00022. [DOI] [PubMed] [Google Scholar]
- 4.Grant SA, Nielsen KC, Greengrass RA, et al. Continuous peripheral nerve block for ambulatory surgery. Reg Anesth Pain Med. 2001;26:209–214. doi: 10.1053/rapm.2001.22256. [DOI] [PubMed] [Google Scholar]
- 5.Stevens MF, Werdehausen R, Golla E, et al. Does interscalene catheter placement with stimulating catheters improve postoperative pain or functional outcome after shoulder surgery? A prospective, randomized and double-blinded trial. Anesth Analg. 2007;104:442–447. doi: 10.1213/01.ane.0000253513.15336.25. [DOI] [PubMed] [Google Scholar]
- 6.Ilfeld BM, Morey TE, Wright TW, et al. Interscalene perineural ropivacaine infusion: a comparison of two dosing regimens for postoperative analgesia. Reg Anesth Pain Med. 2004;29:9–16. doi: 10.1016/j.rapm.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 7.Ilfeld BM, Morey TE, Thannikary LJ, et al. Clonidine added to a continuous interscalene ropivacaine perineural infusion to improve postoperative analgesia: a randomized, double-blind, controlled study. Anesth Analg. 2005;100:1172–1178. doi: 10.1097/01.ASN.0000145571.41015.D5. [DOI] [PubMed] [Google Scholar]
- 8.Vranken JH, van der Vegt MH, Zuurmond WW, et al. Continuous brachial plexus block at the cervical level using a posterior approach in the management of neuropathic cancer pain. Reg Anesth Pain Med. 2001;26:572–575. doi: 10.1053/rapm.2001.26488. [DOI] [PubMed] [Google Scholar]
- 9.Borene SC, Rosenquist RW, Koorn R, et al. An indication for continuous cervical paravertebral block (posterior approach to the interscalene space) Anesth Analg. 2003;97:898–900. doi: 10.1213/01.ANE.0000072702.79692.17. [DOI] [PubMed] [Google Scholar]
- 10.Pippa P, Cominelli E, Marinelli C, et al. Brachial plexus block using the posterior approach. Eur J Anaesthesiol. 1990;7:411–420. [PubMed] [Google Scholar]
- 11.Boezaart AP, De Beer JF, Nell ML. Early experience with continuous cervical paravertebral block using a stimulating catheter. Reg Anesth Pain Med. 2003;28:406–413. doi: 10.1016/s1098-7339(03)00221-9. [DOI] [PubMed] [Google Scholar]
- 12.Boezaart AP, Koorn R, Rosenquist RW. Paravertebral approach to the brachial plexus: an anatomic improvement in technique. Reg Anesth Pain Med. 2003;28:241–244. doi: 10.1053/rapm.2003.50049. [DOI] [PubMed] [Google Scholar]
- 13.Frohm RM, Raw RM, Haider N, et al. Epidural spread after continuous cervical paravertebral block: a case report. Reg Anesth Pain Med. 2006;31:279–281. doi: 10.1016/j.rapm.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 14.Aramideh M, van den Oever HL, Walstra GJ, et al. Spinal anesthesia as a complication of brachial plexus block using the posterior approach. Anesth Analg. 2002;94:1338–1339. doi: 10.1097/00000539-200205000-00054. [DOI] [PubMed] [Google Scholar]
- 15.Voermans NC, Crul BJ, de Bondt B, et al. Permanent loss of cervical spinal cord function associated with the posterior approach. Anesth Analg. 2006;102:330–331. doi: 10.1213/01.ANE.0000190722.55703.61. [DOI] [PubMed] [Google Scholar]
- 16.Blumenthal S, Ekatodramis G, Nadig M, et al. Paravertebral approach to the brachial plexus: an anatomic improvement in technique–is it really advantageous to come from behind? Reg Anesth Pain Med. 2003;28:582–583. doi: 10.1016/j.rapm.2003.10.003. author reply 583–585. [DOI] [PubMed] [Google Scholar]
- 17.Jack NT, Slappendel R, Gielen MM. Don’t make an easy block more difficult! Reg Anesth Pain Med. 2003;28:580–581. doi: 10.1016/s1098-7339(03)00389-4. author reply 583–585. [DOI] [PubMed] [Google Scholar]
- 18.Boezaart AP. Please don’t blame the block. Anesth Analg. 2007;104:211–212. doi: 10.1213/01.ane.0000247714.67531.77. author reply 212. [DOI] [PubMed] [Google Scholar]
- 19.Nguyen HC, Fath E, Wirtz S, et al. Transscalene brachial plexus block: a new posterolateral approach for brachial plexus block. Anesth Analg. 2007;105:872–875. doi: 10.1213/01.ane.0000271916.26357.8d. [DOI] [PubMed] [Google Scholar]
- 20.Benumof JL. Permanent loss of cervical spinal cord function associated with interscalene block performed under general anesthesia. Anesthesiology. 2000;93:1541–1544. doi: 10.1097/00000542-200012000-00033. [DOI] [PubMed] [Google Scholar]
- 21.Baker R, Dreyfuss P, Mercer S, et al. Cervical transforaminal injection of corticosteroids into a radicular artery: a possible mechanism for spinal cord injury. Pain. 2003;103:211–215. doi: 10.1016/s0304-3959(02)00343-3. [DOI] [PubMed] [Google Scholar]
- 22.Brouwers PJ, Kottink EJ, Simon MA, et al. A cervical anterior spinal artery syndrome after diagnostic blockade of the right C6-nerve root. Pain. 2001;91:397–399. doi: 10.1016/S0304-3959(00)00437-1. [DOI] [PubMed] [Google Scholar]
- 23.Bryan NA, Swenson JD, Greis PE, et al. Indwelling interscalene catheter use in an outpatient setting for shoulder surgery: technique, efficacy, and complications. J Shoulder Elbow Surg. 2007;16:388–395. doi: 10.1016/j.jse.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 24.Sandhu NS, Sidhu DS, Capan LM. The cost comparison of infraclavicular brachial plexus block by nerve stimulator and ultrasound guidance. Anesth Analg. 2004;98:267–268. doi: 10.1213/01.ANE.0000077685.55641.7C. [DOI] [PubMed] [Google Scholar]
- 25.Tsui B. Ultrasound-guidance and nerve stimulation: implications for the future practice of regional anesthesia. Can J Anaesth. 2007;54:165–170. doi: 10.1007/BF03022635. [DOI] [PubMed] [Google Scholar]
- 26.Sites BD, Gallagher JD, Cravero J, et al. The learning curve associated with a simulated ultrasound-guided interventional task by inexperienced anesthesia residents. Reg Anesth Pain Med. 2004;29:544–548. doi: 10.1016/j.rapm.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 27.Capdevila X, Dadure C, Bringuier S, et al. Effect of patient-controlled perineural analgesia on rehabilitation and pain after ambulatory orthopedic surgery: a multicenter randomized trial. Anesthesiology. 2006;105:566–573. doi: 10.1097/00000542-200609000-00022. [DOI] [PubMed] [Google Scholar]
- 28.Borgeat A, Dullenkopf A, Ekatodramis G, et al. Evaluation of the lateral modified approach for continuous interscalene block after shoulder surgery. Anesthesiology. 2003;99:436–442. doi: 10.1097/00000542-200308000-00026. [DOI] [PubMed] [Google Scholar]
- 29.Borgeat A. All roads do not lead to Rome. Anesthesiology. 2006;105:1–2. doi: 10.1097/00000542-200607000-00002. [DOI] [PubMed] [Google Scholar]
- 30.Ekatodramis G, Macaire P, Borgeat A. Prolonged Horner syndrome due to neck hematoma after continuous interscalene block. Anesthesiology. 2001;95:801–803. doi: 10.1097/00000542-200109000-00040. [DOI] [PubMed] [Google Scholar]
- 31.Tuominen MK, Pere P, Rosenberg PH. Unintentional arterial catheterization and bupivacaine toxicity associated with continuous interscalene brachial plexus block. Anesthesiology. 1991;75:356–358. doi: 10.1097/00000542-199108000-00026. [DOI] [PubMed] [Google Scholar]
- 32.Cook LB. Unsuspected extradural catheterization in an interscalene block. Br J Anaesth. 1991;67:473–475. doi: 10.1093/bja/67.4.473. [DOI] [PubMed] [Google Scholar]
- 33.Souron V, Reiland Y, De Traverse A, et al. Interpleural migration of an interscalene catheter. Anesth Analg. 2003;97:1200–1201. doi: 10.1213/01.ANE.0000077651.77618.A2. [DOI] [PubMed] [Google Scholar]
- 34.Ribeiro FC, Georgousis H, Bertram R. Plexus irritation caused by interscalene brachial plexus catheter for shoulder surgery. Anesth Analg. 1996;82:870–872. doi: 10.1097/00000539-199604000-00035. [DOI] [PubMed] [Google Scholar]