Abstract
Cardiovascular magnetic resonance (CMR) has become the primary tool for non-invasive assessment of myocardial inflammation in patients with suspected myocarditis. The International Consensus Group on CMR Diagnosis of Myocarditis was founded in 2006 to achieve consensus among CMR experts and develop recommendations on the current state-of-the-art use of CMR for myocarditis. The recommendations include indications for CMR in patients with suspected myocarditis, CMR protocol standards, terminology for reporting CMR findings, and diagnostic CMR criteria for myocarditis (“Lake Louise Criteria”).
Keywords: Cardiovascular Magnetic Resonance, Myocarditis, Consensus
Background: Myocarditis
Incidence and Etiology
In this paper, myocarditis is defined as inflammation of myocardial tissue.
Myocarditis has been reported in up to 12% of young adults presenting with sudden death (1) (2) (3) (4) and is an important underlying etiology of other myocardial diseases such as dilated (5) and arrhythmogenic right ventricular (6) cardiomyopathy. The incidence of non-fatal myocarditis is likely higher than actually diagnosed, mostly due to the challenges of establishing the diagnosis in standard clinical settings.
Infectious disease accounts for the majority of cases, in previously healthy patients typically due to either a direct viral infection or post-viral immune-mediated reaction. Myocardial inflammation, however, may also be triggered by reversible and/or irreversible toxic, ischemic or mechanical injury, drug-related inflammation, transplant rejection or other immune reactions.
Pathogenesis and pathology
Pathogenetic features of myocarditis are reviewed in detail elsewhere (7). Following the initial injury, local and systemic immune responses activate cytokines and B cells with subsequent edema, additional myocyte injury, and autoantibody production. Although the molecular and cellular pathophysiology may differ between different etiologies, cellular infiltration, edema, necrosis and (in later stages) fibrotic scars are common features.
Diagnostic approaches to myocarditis and their limitations
Currently, no single clinical or imaging finding confirms the diagnosis of myocarditis with absolute certainty. Rather, an integrated, synopsis including history, clinical assessment and noninvasive test results should be used to diagnose the disease and guide treatment.
History and physical exam
Although of limited specificity, a careful history and thorough clinical assessment have to precede further diagnostic tests. Patients may appear almost normal, may have non-specific symptoms, but may also present with features of acute myocardial infarction, or heart failure with hemodynamic compromise. Physical exams of patients with myocarditis are often normal.
Ventricular functional analysis
Although many patients with myocarditis have regional or global wall motion abnormalities (8) (9) (10), dysfunction is not specific to inflammation, and its sensitivity is limited (9) (11) (12) (13). Biventricular dysfunction in myocarditis, however, was found to be the main predictor of death and transplantation (14).
ECG
ECG findings associated with myocarditis may include ST segment and T wave changes, Q waves, AV block, and bundle-branch block. Arrhythmias such as ventricular tachycardia and ventricular fibrillation occur. The diagnostic value of the ECG in myocarditis, however, is limited. Aside from a low specificity, the presence of either ST elevation or T inversion as the most sensitive ECG criterion is present in less than 50% of patients, even during the first weeks of the disease (15).
Biomarkers
Depending on the severity and time of testing during the course of disease, serum biomarkers of myocardial injury such as creatine kinase, CK-MB and troponin may be elevated. When present, the magnitude of rise as well as the time to clearance is similar to that of a small to medium sized myocardial infarction and indicates more severe disease. The prevalence of an increased troponin T in biopsy-proven myocarditis, however, is only 35–45% (16).
Biopsy
Endomyocardial biopsy (EMB) is a widely accepted method for diagnosing myocarditis, based upon histopathology, immunohistology and molecular techniques to identify viral genomes. A Joint Scientific Statement of several professional societies on its use in various clinical scenarios has been published (17).
Some limitations of EMB have to be considered:
- The sensitivity of EMB is limited due to so-called sampling error (18) (19) (20) (21).
- Severe complications (perforation, tamponade) occur in 0.1 to 0.5%, the overall complication rate is 6% (17).
- There is substantial debate about diagnostic criteria for analyzing myocardial tissue specimens. (22) The utility of the Dallas criteria (23), with inflammatory infiltration and associated myocyte necrosis uncharacteristic for an ischemic event as disease markers is limited by poor inter-observer agreement (24) (25).
Immunohistochemistry has a higher sensitivity than standard histopathology for the diagnosis of myocarditis (26) (27) and immunohistology protocols and evaluation criteria have been proposed (28) (10). Cost, availability, and limited standardization, however, have limited the widespread use of immunohistology and viral genome analysis.
- In adults, the recommended indications for endomyocardial biopsy are confined to patients with heart failure (17) and therefore EMB is not recommended in many patients with myocarditis.
In summary, history, clinical exam, ECG, and serology have an unsatisfactory diagnostic accuracy in myocarditis. Biopsy including immunohistochemistry remains the widely accepted standard, which may however not be appropriate for many patients, especially those with less severe disease.
Imaging Modalities Other than CMR
A detailed review of non-invasive imaging in myocarditis can be found elsewhere (29). Ultrasound studies of the heart in myocarditis typically are performed to visualize associated functional abnormalities, wall thickness and pericardial effusion (8) (30). The diagnostic value of echocardiography is limited by the fact that many patients with less severe myocarditis have a normal echocardiogram and the highly variable echocardiographic findings lack specificity (8). 111Indium antimyosin antibody and 67gallium nuclear imaging have been used to diagnose myocarditis (31). The specificity of these approaches, however, is very limited (32). Nuclear medicine techniques are also hampered by limited availability of tracers mentioned above, poor spatial resolution and radiation issues. In current clinical practice, nuclear medicine is only rarely used to diagnose myocarditis.
Cardiovascular Magnetic Resonance (CMR) in Myocarditis
Published Data
CMR imaging offers a unique combination of safety, clarity of anatomical visualization, inter-observer consistency, and quantitative accuracy. Furthermore, it allows for the comprehensive use of a wide spectrum of diagnostic targets, especially using the modifiable inherent tissue contrast. CMR has become a standard tool in many medical centers and currently is considered by many the most versatile and powerful cardiovascular imaging modality.
Since the first description of T2-weighted CMR findings in children with myocarditis by Gagliardi et al. in 1991 (33) and the first controlled clinical study using contrast-enhanced CMR in 1998 (9), numerous studies have investigated the diagnostic utility of non-contrast (11) (13) (34) and contrast-enhanced (35) (11) (36) (12) (13) (37) (38) (39) (40) (41) (42) (34) (43) CMR in patients with myocarditis. Results have consistently shown the clinical feasibility and high diagnostic accuracy with different single-technique or combined CMR protocols. Tables 1 to 4 show a list of published controlled trials on CMR in myocarditis (table 1), and data on the diagnostic accuracy of LV dysfunction (table 2) and of CMR criteria for myocarditis (table 3: individual criteria; table 4: combined criteria).
Table 1.
Published controlled studies on CMR in myocarditis
| Validation | n patients | n controls | |
|---|---|---|---|
| Friedrich et al., Circulation 1998 (9) | Clinical | 19 | 18 |
| Laissy et al., Chest 2002 (11) | Clinical | 20 | 7 |
| Rieker et al., RoFo 2002 (36) | Clinical | 11 | 10 |
| Laissy et al., Radiology 2005 (37)* | Clinical | 24 | 31 |
| Abdel-Aty et al., J Am Coll Cardiol 2005 (13) | Clinical | 25 | 22 |
| Mahrholdt et al., Circulation 2006 (40) | Histology | 87 | 26 |
| Gutberlet et al., Radiology 2008 (34)** | Histology | 48 | 35 |
| Yilmaz et al., Heart 2008 (43) ** | Histology | 55 | 30 |
| 289 | 179 |
Comparison to patients with acute myocardial infarction.
Comparison to patients with clinical evidence, but lack of immunohistologic evidence for chronic myocarditis
Table 4.
Overview of the diagnostic accuracy of several combinations of tissue criteria.
| T2+LGE | Validation | Sens | Spec | Acc | PPV | NPV |
|---|---|---|---|---|---|---|
| Abdel-Aty et al., J Am Coll Cardiol 2005 (13) | Clinical | 40% | 100% | 69% | 100% | 61% |
| Gutberlet et al., Radiology 2008 (34) | Histology | 17% | 91% | 48% | 73% | 44% |
| Pooled data (n=130) | 25% | 95% | 56% | 86% | 50% | |
| T2 and/or LGE | Validation | Sens | Spec | Acc | PPV | NPV |
| Abdel-Aty et al., J Am Coll Cardiol 2005 (13) | Clinical | 88% | 74% | 81% | 100% | 85% |
| Gutberlet et al., Radiology 2008 (34) | Histology | 50% | 57% | 52% | 80% | 25% |
| Pooled data (n=130) | 60% | 66% | 62% | 79% | 43% | |
| Any one of three | Validation | Sens | Spec | Acc | PPV | NPV |
| Abdel-Aty et al., J Am Coll Cardiol 2005 (13) | Clinical | 100% | 48% | 75% | 68% | 100% |
| Gutberlet et al., Radiology 2008(42) | Histology | 81% | 49% | 67% | 68% | 65% |
| Pooled data (n=130) | 88% | 48% | 70% | 68% | 76% | |
| Any two of three | Validation | Sens | Spec | Acc | PPV | NPV |
| Abdel-Aty et al., J Am Coll Cardiol 2005 (13) | Clinical | 76% | 96% | 85% | 95% | 79% |
| Gutberlet et al., Radiology 2008 (34) | Histology | 63% | 89% | 73% | 88% | 63% |
| Pooled data (n=130) | 67% | 91% | 78% | 91% | 69% |
Sens: Sensitivity; Spec: Specificity; Acc: Accuracy; PPV: Positive predictive value; NPV: Negative predictive value
Table 2.
Diagnostic accuracy of LV dysfunction as assessed in controlled trials.
| LV dysfunction (EF<55%) | Validation | Sens | Spec | Acc | PPV | NPV |
|---|---|---|---|---|---|---|
| Friedrich et al., Circulation 1998 (9) | Clinical | 100% | 100% | 100% | 100% | 100% |
| Laissy et al., Chest 2002 (11) | Clinical | 62% | 100% | 75% | 100% | 58% |
| Laissy et al., Radiology 2005 (37)* | Clinical | 46% | 62% | 57% | 37% | 70% |
| Abdel-Aty et al., J Am Coll Cardiol 2005 (13) | Clinical | 38% | 100% | 61% | 100% | 49% |
| Gutberlet et al., Radiology 2008 (34) | Histology | 50% | 63% | 55% | 65% | 48% |
| Pooled data (n=276) | 54% | 76% | 64% | 71% | 60% | |
Sens: Sensitivity; Spec: Specificity; Acc: Accuracy; PPV: Positive predictive value; NPV: Negative predictive value.
Table 3.
Overview of the diagnostic accuracy of individual tissue criteria as assessed in controlled trials.
| Early myocardial gadolinium enhancement | Validation | Sens | Spec | Acc | PPV | NPV |
|---|---|---|---|---|---|---|
| Friedrich et al., Circulation 1998 (9) | Clinical | 84% | 89% | 86% | 89% | 84% |
| Laissy et al., Chest 2002 (11) | Clinical | 85% | 100% | 89% | 100% | 70% |
| Abdel-Aty et al., J Am Coll Cardiol 2005 (13) | Clinical | 80% | 68% | 74% | 74% | 75% |
| Gutberlet et al., Radiology 2008 (34) | Histology | 63% | 86% | 72% | 86% | 63% |
| Pooled data (n=194) | 74% | 83% | 78% | 86% | 70% | |
| T2 | Validation | Sens | Spec | Acc | PPV | NPV |
| Rieker et al., RoFo 2002 (36) | Clinical | 100% | 50% | 76% | 69% | 100% |
| Laissy et al., Chest 2002 (11) | Clinical | 45% | 100% | 59% | 100% | 39% |
| Abdel-Aty et al., J Am Coll Cardiol 2005 (13) | Clinical | 84% | 74% | 79% | 78% | 81% |
| Gutberlet et al., Radiology 2008 (34) | Histology | 67% | 69% | 67% | 74% | 60% |
| Pooled data (n=178) | 70% | 71% | 70% | 77% | 63% | |
| Late enhancement | Validation | Sens | Spec | Acc | PPV | NPV |
| Rieker et al., RoFo 2002 (36) | Clinical | 45% | 60% | 52% | 56% | 50% |
| Abdel-Aty et al., J Am Coll Cardiol 2005(18) | Clinical | 44% | 100% | 71% | 78% | 62% |
| Mahrholdt et al., Circulation 2006 (40) | Histology | 95% | 96% | 96% | 99% | 81% |
| Gutberlet et al., Radiology 2008 (34) | Histology | 27% | 80% | 49% | 65% | 44% |
| Yilmaz et al., Heart 2008 (43) | Histology | 35% | 83% | 51% | 81% | 38% |
| Pooled data (n=336) | 59% | 86% | 68% | 89% | 53% | |
Sens: Sensitivity; Spec: Specificity; Acc: Accuracy; PPV: Positive predictive value; NPV: Negative predictive value
Although published data on diagnostic accuracy provide solid evidence for the use of CMR in clinical settings, it is important to emphasize that most of these studies were single-center reports, had a small sample size, variable inclusion criteria, and non-uniform patient populations. Furthermore, CMR studies were performed at variable time points after disease onset, used different imaging diagnostic criteria, and mostly did not include biopsy for confirmation.
Furthermore, the specificity was mostly compared to normal controls or myocardial infarction, and not to other heart diseases with similar clinical presentation such as acute coronary syndrome or other secondary cardiomyopathies. Current data do not allow for a clear definition of the diagnostic accuracy of CMR in various clinical, histological and immunohistochemical subgroups, and data from larger (multi-center) trials with standardized protocols comparing comprehensive CMR studies to biopsy-derived criteria are lacking.
The prognostic value of CMR criteria for myocarditis remains to be defined. In a small study, increased myocardial early gadolinium enhancement ratio at 4 weeks after clinical onset of the disease was associated with an impaired prognosis regarding functional recovery and symptoms after a 3-year follow-up (44). Confirmative studies on the prognostic value of the various parameters are required.
Diagnostic Targets of CMR in Myocarditis
Different from other diagnostic modalities, targets for CMR not only include functional and morphological abnormalities but also tissue pathology as diagnostic features of myocardial inflammation.
Functional abnormalities
CMR assessment of right and left ventricular (LV) function is very reproducible and thus allows for identifying, quantifying, and following even mild functional abnormalities, if present. In patients with more severe myocarditis, global LV dysfunction is frequent. It is, however, reemphasized that regional or less severe LV wall motion abnormalities have a low specificity for the underlying pathophysiology.
Pericardial effusion
Pericardial effusion has been reported in 32% to 57% of patients with myocarditis (45) (46) (47). Although not specific for myocarditis, its presence is supportive evidence for active inflammation.
Regional distribution, extent and hemodynamic significance of pericardial effusion can be assessed in standard short and long axis steady-state free precession (SSFP) images acquired for morphology and function. This sequence type has an inherent T2 sensitivity, rendering pericardial fluid bright signal intensity (figure 1A). The differentiation from epicardial fat (which also appears bright) is straightforward: The latter is found around coronary vessels (which are embedded in the epicardial fat layer) or in the AV groove and - in SSFP images - typically separated from effusion by a (single-pixel) thin chemical shift artifact layer, i.e. a fine line without signal. Furthermore, fat mostly appears with a slightly lower signal intensity, and effusion may have a more “deformable” appearance through the cardiac cycle. In T1-weighted images, e.g. spin-echo images, fluid has low signal intensity. In phase-sensitive inversion-recovery sequences, however, may be black or white, depending on the inversion time settings. Small, physiological accumulations of pericardial fluid are not circumferential and may not be considered pathologic. A fluid layer that contains non-fluid components (fibrinous deposits, thrombus) is pathologic.
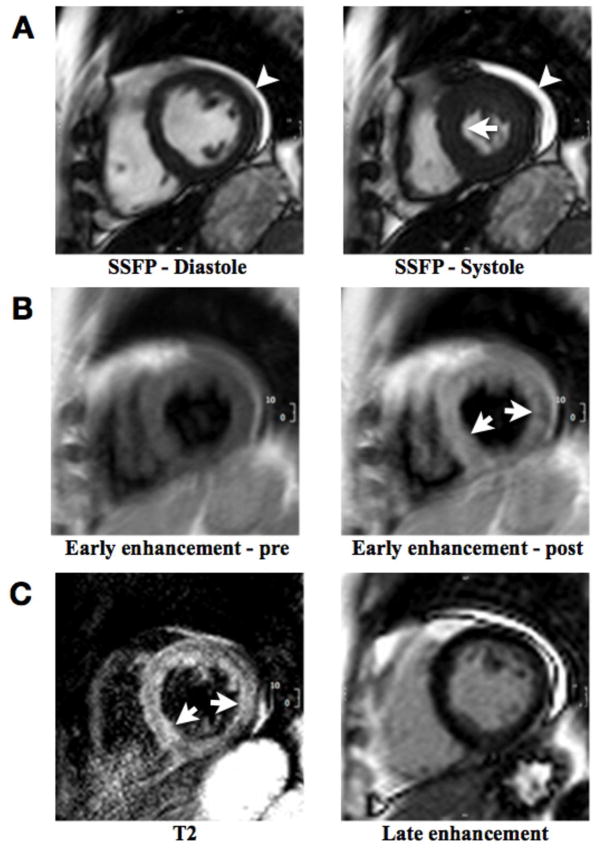
Figure 1. Short axis CMR views in a patient with clinically acute myocarditis.
A: Still frames from a cine series at end-diastole (left) and end-systole (right), showing only very mild septal hypokinesis (arrow) with preserved ejection fraction. Small pericardial effusion is present along the lateral segments (arrowhead). These findings represent two supportive criteria for myocarditis.
B: T1-weighted spin echo images before (left) and shortly after (right) gadolinium administration with early gadolinium accumulation in the septum (arrows). Quantitative evaluation of the signal enhancement (skeletal-muscle normalized myocardial enhancement ratio of equal to or greater than 4.0 or an absolute enhancement of equal to or greater than 45%) is required to use information from this pulse sequence as a positive criterion.
C: Left: T2-weighted spin echo image with high signal intensity of the septum and lateral wall (arrows). Evidence for regional edema, or a signal intensity ratio of equal to or greater than 2.0 (signal intensity normalized to skeletal muscle in the same slice) renders T2 findings positive. Right: Late enhancement image without evidence for significant delay of gadolinium washout. The thin subepicardial layer of high signal intensity in the inferolateral region represents fat.
Morphological abnormalities
A transient increase of wall thickness during myocarditis was first described in echocardiography studies (48) and may serve as a supportive finding during follow-up. A decrease of LV mass during the course of uncomplicated myocarditis was found to be associated with edema as assessed by T2-weighted CMR (49). A transient increase of LV volumes has been observed in the course of myocarditis (9) and may also serve as retrospective, supportive evidence for recent myocarditis.
CMR Tissue Characterization
Given the unique potential of CMR to visualize tissue changes, this area is of special interest. As outlined above, expected tissue pathology in active myocarditis includes intracellular and interstitial edema, capillary leakage, hyperemia and – in more severe cases - cellular necrosis and subsequent fibrosis (50).
Edema
An important hallmark of inflammatory cell injury is the increased permeability of cellular membranes. Whereas initial membrane defects are of functional nature, leading to Na+ influx and subsequent intracellular edema, a more severe injury allows for a net efflux of water and transmembranous leakage of larger molecules such as troponin, eventually leading to loss of cellular functions.
T2-weighted imaging sensitively detects tissue edema using the long T2 of water-bound protons as the contrast-generating mechanism resulting in a high signal intensity of edematous tissue (figure 1C). Triple inversion recovery turbo spin echo sequences with inversion pulses for fat and blood suppression (51) provide excellent contrast between regional edema and normal myocardium due to the dual suppression of the fat and flowing blood signal. Double inversion recovery sequences may provide a higher SNR and be used alternatively. Importantly, edema in patients with myocarditis may have a global myocardial distribution and thus a quantitative signal intensity analysis of the entire myocardium may be necessary. A high diagnostic accuracy has been shown for this approach in acute inflammatory or ischemic injury (52) (13) (34).
Regional edema visible on T2-weighted CMR images was not observed in “borderline myocarditis”, but could be seen in 36% of patients with histologically “active myocarditis” as defined by the Dallas criteria (39). Thus, regional edema may have a limited sensitivity in less severe inflammation. Short axis views typically provide a more robust image quality than long axis images, although apical slices may have to be discarded because of artifacts related to intraventricular blood signal.
Signal-to-noise ratio of T2-weighted images strongly depends on sequence parameters. Particularly in patients with arrhythmia and other motion artifacts, image quality may not allow for reliable visualization or quantification of edema. Newly developed sequences may yield a more consistent image quality and better diagnostic accuracy than currently used fast spin echo triple inversion recovery prepared protocols (53) (54).
Hyperemia and capillary leak (“myocardial early gadolinium enhancement”)
Regional vasodilatation is an integral feature of tissue inflammation. The increased blood volume in the inflamed area leads to an increased uptake of contrast agents during the early vascular phase. Because gadolinium-based contrast agents distribute quickly into the interstitial space after administration, this phase lasts for the first minutes after the contrast bolus. Contrast-enhanced fast spin echo T1-weighted MR during this time can be used to assess experimentally induced myocardial hyperemia (55) and to detect muscular inflammation (56). Accordingly, the purpose of myocardial early gadolinium enhancement ratio (EGEr) is to detect an overall increased volume of gadolinium distribution into the intravascular and interstitial space during the early washout period.
The diagnostic utility of contrast-enhanced T1-weighted imaging in patients with clinically acute and chronic myocarditis has been shown in several studies (9) (35) (13) (34).
Currently, fast spin-echo sequences are used, which are vulnerable to inconsistent image quality in patients with varying heart rate and irregular breathing patterns. New sequences to assess the early phase of gadolinium kinetics may overcome existing limitations of image quality.
Necrosis and fibrosis (“late gadolinium enhancement”)
Myocardial late gadolinium enhancement (LGE) specifically reflects irreversible myocardial injury, i.e. necrosis and fibrosis. LGE imaging uses an inversion pulse to decrease the signal response from normal myocardium, thereby highlighting areas with increased accumulation of gadolinium as bright regions.
In earlier stages of necrosis, gadolinium enters the cells through acutely injured cell membranes (7). Hence, the volume of distribution of gadolinium is increased (12) and visualizes myocarditis-related necrosis (figure 2). After inflammatory clearance of necrotic regions, a mesh of fibrocytes with a large interstitial component replaces formerly viable tissue, again increasing the volume of distribution for gadolinium into this extracellular space during the late washout period. Thus, the late sequelae of inflammatory tissue damage can also be observed by LGE.
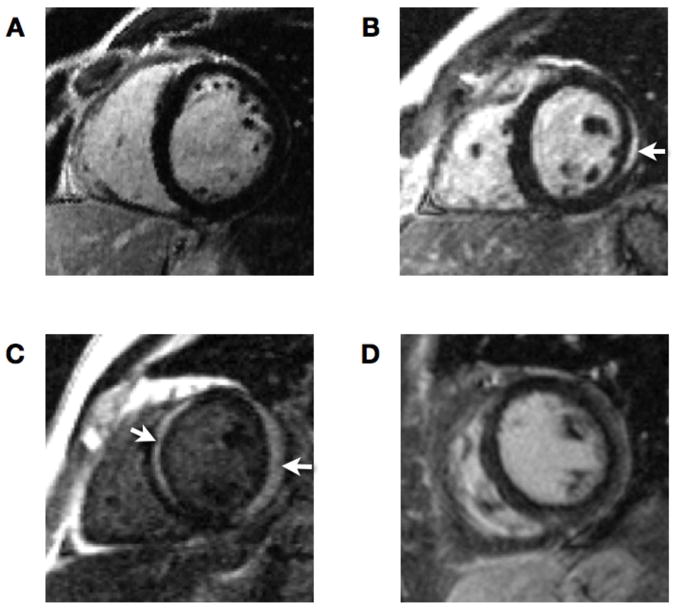
Figure 2. Late enhancement patterns in myocarditis.
A: Normal myocardium with no evidence of irreversible myocyte injury
B: Regional sub-epicardial enhancement of the lateral wall (arrow)
C: Subepicardial enhancement of lateral and midwall enhancement of the septal wall (arrows)
D: Diffuse sub-epicardial enhancement
Both, microscopic (57), animal (58) and clinical (59) studies have confirmed the role of LGE imaging as s gold standard for in vivo detection of irreversible myocardial injury associated with myocardial infarction. In patients with myocarditis, several studies have demonstrated a high specificity of LGE for the detection of such injury in myocarditis (12) (13) (37) (38) (40). The regional distribution of injury as defined by LGE not only allows differentiating ischemic (with mandatory subendocardial involvement) from non-ischemic injury (60), but also may indicate the underlying etiology of the non-ischemic insult (61).
As a potential limitation, LGE showed a variable sensitivity to detect active or chronic inflammation, depending on the selection of patients (12) (13) (40) (39) (34) (43). Using the Dallas criteria, De Cobelli et al. found LGE to be less sensitive in “borderline” myocarditis (44%) than in “active” myocarditis (84%) (39).
One reason may be that active myocarditis may not always lead to large enough regions of necrotic myocytes to be visually detectable given the pixel size in CMR images. This contrasts with the situation in ischemic necrosis for which LGE has been shown to be highly sensitive. Therefore, LGE may be insensitive for the detection of symptomatic myocarditis with limited or non-focal irreversible injury. More studies are needed to address this issue.
Combined use of tissue pathology markers
Two studies have compared all three tissue-based markers as well as various combinations of these. Abdel-Aty et al. used combined clinical criteria for active myocarditis (13), whereas Gutberlet et al. assessed patients with chronic myocarditis, validated against histopathological criteria of myocardial inflammation (34). In both studies, the approach with the best overall diagnostic accuracy was found by the combined use of all three tissue-based CMR parameters, with the presence of at least two positive criteria defining the CMR study as positive for myocarditis (see tables 3 and 4).
CMR Indications, Procedure and Protocol
Indications for CMR
A CMR study should only be performed if patients are symptomatic, if there is sufficient clinical evidence for myocarditis and if the CMR result will likely affect clinical management. Thus, it is generally indicated in patients with current or persisting symptoms, evidence for significant myocardial injury and suspected viral etiology. CMR is of potential use in patients with chest pain, elevated troponin and normal coronary arteries, where it was shown to identify myocarditis in more than 30% of patients (62).
Additional indications may exist for subjects with possible myocarditis being exposed to strenuous physical exercise, e.g. professional athletes or for patients with otherwise unexplained new ECG findings consistent with myocarditis, even in the absence of symptoms suggestive of myocarditis.
Table 5 lists recommended criteria for requesting a CMR study in patients with suspected myocarditis.
Table 5.
Indications for CMR in patients with suspected myocarditis.
| New onset or persisting symptoms suggestive of myocarditis | plus | Evidence for recent/ongoing myocardial injury | plus | Suspected viral etiology |
|---|---|---|---|---|
| Dyspnea or orthopnea or palpitations or effort intolerance/malaise or chest pain |
Ventricular dysfunction or new or persisting ECG abnormalities or elevated troponin |
History of recent systemic viral disease or previous myocarditis or absence of risk factors for coronary artery disease or age of <35y or symptoms not explained by coronary stenosis on coronary angiogram or recent negative ischemic stress test |
CMR procedure
The patient should be monitored throughout the session including ECG, blood pressure, breathing, and O2 saturation. Furthermore, communication to the patient should be ensured using intercom devices. A physician trained in cardiac resuscitation should be available. As for all cardiac diagnostic modalities, drugs and equipment for immediate interventions should be within reach.
Typically, patients are examined in a supine position. A dedicated cardiac phased-array surface coil should be used to acquire functional images. It is very important to emphasize that for all sequences used to analyze signal intensity (qualitatively or quantitatively), either a signal intensity correction algorithm or the body coil should be used. The inhomogeneous sensitivity field of surface coils may otherwise lead to false negative (inferolateral wall) or false positive (septum) results.
The coverage of the heart should allow for assessing all 17 LV segments according to published recommendations (63). Images of the apex may be of insufficient image quality and may have to be excluded.
Published data on contrast-enhanced CMR in myocarditis mostly have been obtained using gadolinium gadopentetate dimeglumine (gadolinium-DTPA) and thus recommendations are only valid for this substance or compounds with an equivalent pharmacokinetic profile.
CMR protocol
Recommended imaging parameters and detailed protocol recommendations are provided in the supplemental material of this article.
CMR sequences generally will be ECG-gated and performed using breath-hold protocols.
These recommendations are based on the current evidence as published in peer-reviewed literature as of January 2009. Some of the currently recommended sequences have distinct limitations. Images obtained by T1-weighted spin-echo sequences during free breathing may have limited diagnostic quality, and T2-weighted spin-echo images suffer from an inherently low signal-to-noise ratio. Although new sequences are being tested for these purposes, their value and clinical role remains to be defined.
Evaluation of CMR images in suspected myocarditis
The versatility, accuracy and reproducibility of CMR and the generally high expectations of referring physicians call for a careful, responsible evaluation of all available parameters. Table 6 summarizes CMR findings and proposed terminology in patients with suspected myocarditis.
Table 6.
Proposed terminologies for describing CMR findings
| normal | CMR findings consistent with myocardial inflammation | ||||
|---|---|---|---|---|---|
| Edema | Lack of evidence for myocardial edema | Patchy areas or regions of high T2 signal intensity indicating focal or regional edema* | Subepicardial or septal layer of high T2 signal intensity indicating regional edema | Transmural high T2 signal intensity indicating regional edema, consistent with but not specific for myocardial inflammation | Global high T2 signal intensity indicating global edema† |
| Hyperemia Capillary leak | Lack of evidence for increased myocardial early gadolinium enhancement ratio | Increased myocardial early gadolinium enhancement ratio‡ | |||
| Irreversible cell injury | Lack of evidence for regional late gadolinium enhancement | Patchy areas of late gadolinium enhancement indicating focal injury | Subepicardial or septal layer of late gadolinium enhancement indicating regional injury | Transmural late gadolinium enhancement, consistent with but not specific for myocardial inflammation | |
| Supportive CMR findings | |||||
| LV dysfunction | Normal LV function | Regional systolic dysfunction | Global systolic dysfunction | ||
| Pericardial effusion | Lack of evidence for pericardial effusion | Small pericardial effusion | Moderately large pericardial effusion | Large pericardial effusion without hemodynamic relevance | Large pericardial effusion with hemodynamic relevance |
To avoid misinterpretation of artifacts, areas with abnormal SI should consist of at least 10 adjacent pixels to be regarded as relevant.
Global high T2 signal is defined by an SI ratio between myocardium and skeletal muscle of ≥2.0
An increased myocardial early gadolinium enhancement ratio is defined by either an SI enhancement ratio between myocardium and skeletal muscle of 4.0 or an absolute myocardial enhancement of ≥45%
Edema
Myocardial edema appears as an area of high signal intensity in T2-weighted images (figure 1C/left panel). In myocarditis, it may be regional or global. It is important to keep in mind that, in the absence of LGE, edema reflects reversible myocardial injury (52) (64).
Regional edema can be identified visually (see fig. 1C), although a quantitative assessment of the signal abnormality seems appropriate. Evaluation software allows for verifying edema as regions with signal intensity more than 2 standard deviations above the mean value of normal tissue. The lower signal-to-noise of T2-weighted images should be considered, limiting the ability to correctly identify small regions of signal inhomogeneity. Thus, it is recommended to consider only areas of at least 10 adjacent pixels with high signal intensity as relevant. Areas with abnormally low signal in T2-weighted images (e.g. fibrotic scars) should not be used for normalization.
In myocarditis, edema may be global and thus not recognizable to the naked eye. A quantitative analysis by normalizing the signal intensity of the myocardium to that of skeletal muscle has been shown to allow for the detection of a global T2 signal abnormality. Values for the T2 ratio (for calculation see appendix) of more than 1.9 indicate myocarditis (13).
Involvement of skeletal muscle in systemic inflammation may limit the sensitivity of a signal intensity analysis normalized to skeletal muscle (11). This should be taken into consideration in patients with evidence for ongoing myositis. Future studies will have to address the diagnostic accuracy in different scenarios.
When analyzing signal intensity, great care should be taken to exclude high signal of inadequately suppressed slowly flowing cavitary blood. This should not be a problem in visual analysis because slow flow signal would have an apparent ‘subendocardial’ location whereas the T2 signal hyperintensity of myocarditis is almost always subepicardial or transmural. The identification of skeletal muscle to calculate myocardium to skeletal muscle ratio in the same slice may be difficult with a fat-suppressed sequence. Viewing the T2 images side by side with co-localized SSFP or T1-weighted images is recommended to correctly identify skeletal muscle and differentiate it from subcutaneous fat.
Hyperemia and capillary leakage (myocardial early gadolinium enhancement)
EGEr is defined as an increased normalized gadolinium-DTPA accumulation in the myocardium during the early washout period. Although sometimes visually appreciated (see fig. 1B), quantitative evaluation of myocardial EGEr is required. Normalization of the signal intensity in T1-weighted images to that of skeletal muscle may be hampered by co-existing myositis. In patients with evidence for skeletal muscle involvement as indicated by a skeletal muscle signal intensity increase of 20% or higher or by a recent history of muscular pain, an absolute myocardial signal intensity increase between pre-gadolinium and post-gadolinium images of more than 45% should be used as a threshold consistent with myocarditis instead of the normalized myocardial early gadolinium enhancement ratio (11).
In patients with irregular breathing patterns or significant arrhythmia, image quality may be reduced or even be non-diagnostic.
Necrosis and fibrosis (late gadolinium enhancement/LGE)
Several patterns of LGE may be seen in patients with active myocarditis (fig. 2). Focal signal increases are typically localized to the sub-epicardial regions of the left ventricle and extend to a variable extent through the ventricular wall. LGE may be localized in inferolateral and less frequently anteroseptal segments (Figure 1B). However, LGE may be multi-focal or diffuse in distribution (Figure 1C.D). As a rule, the sub-endocardium typically is not involved in an isolated fashion, clearly distinguishing this injury pattern from ischemia-mediated injury. In the basal septum, the left ventricular outflow tract and the membraneous septum may mimic septal LGE in short axis images and lead to false positive results. Also, a line of increased signal intensity may appear in the basal septum on transverse, long axis or short axis images which may not represent pathologic LGE but may be related to the fusion of the right ventricular moderator band to the right ventricular portion of the interventricular septum.
Comprehensive use of CMR criteria (“Lake Louise Criteria”)
Due of the lack of large-scale multi-center data, current recommendations can only reflect the experts’ best achievable consensus based on currently available literature. It is important to reemphasize that rigorous test data of the pulse sequences evaluated against the gold standard of myocardial biopsy in clearly defined clinical subsets of patients are still scarce. The sensitivity and specificity as compared to endomyocardial biopsy for the pulse sequences recommended in this paper are based on the limited number of patients in controlled trials. At the current time, this needs to be kept in mind when employing CMR for making the diagnosis of myocarditis.
The authors recommend the combined use of all three tissue markers. If all sequences can be performed and two or more of the three tissue-based criteria are positive, myocardial inflammation can be predicted or ruled out with a diagnostic accuracy of 78% (pooled data, table 4); if only LGE imaging is performed, the diagnostic accuracy is 68% (pooled data, table 3).
The authors acknowledge that there may be clinical settings which require a higher sensitivity, even if this comes with a reduced specificity, or vice versa. One example may be the use of CMR to assess patients with a high pre-test probability, or children with suspected inflammation after cardiac transplantation. It is re-emphasized that both referring physicians and CMR readers should use the reported criteria as part of a comprehensive diagnostic approach, which also includes clinical, functional and other information.
Table 7 summarizes the recommended diagnostic CMR criteria for myocardial inflammation.
Table 7.
Proposed diagnostic CMR criteria (Lake Louise Consensus Criteria) for myocarditis
In the setting of clinically suspected myocarditisa, CMR findings are consistent with myocardial inflammation, if at least two of the following criteria are present:
|
A repeat CMR study between 1 and 2 weeks after the initial CMR study is recommended, if
|
| The presence of LV dysfunction or pericardial effusion provides additional, supportive evidence for myocarditis. |
The clinical suspicion for active myocarditis should be based on the criteria listed in table 5.
Images should be obtained using a body coil or a surface coil with an effective surface coil intensity correction algorithm; global SI increase has to be quantified by an SI ratio of myocardium over skeletal muscle of ≥2.0). If the edema is more subendocardial or transmural in combination with a co-localized ischemic (including the subendocardial layer) pattern of late gadolinium enhancement, acute myocardial infarction is more likely and should be reported.
Images should be obtained using a body coil or a surface coil with an effective surface coil intensity correction algorithm; a global SI enhancement ratio of myocardium over skeletal muscle of ≥4.0 or an absolute myocardial enhancement of ≥45% is consistent with myocarditis.
Images should be obtained at least 5 minutes after gadolinium injection; foci typically exclude the subendocardial layer, are often multi-focal, and involve the subepicardium. If the late gadolinium enhancement pattern clearly indicates myocardial infarction and is co-localized with a transmural regional edema, acute myocardial infarction is more likely and should be reported.
Follow-up of myocarditis by CMR
The decision regarding follow-up of patients with active myocarditis depends on the individual scenario. Anecdotal evidence suggests that CMR studies during the first days of myocarditis may be less sensitive than those obtained 7 days after clinical onset of the disease (65). This may be due to the focal nature of early stages of the disease. Thus, in a patient with strong clinical evidence for myocarditis yet negative criteria in the initial CMR study, a repeat scan may be needed to establish the diagnosis. A follow-up at least 4 weeks after the onset of disease may be useful to differentiate uncomplicated involvement of the myocardium in a systemic viral illness from a complicated course with viral persistence or autoimmunologic disease, as viral clearance usually is completed within the first days after infection and tissue inflammation should not take more than 2 to 3 weeks. Indeed, pilot data indicate a prognostic relevance of persisting CMR markers for inflammation at 4 weeks after onset (44).
Reporting of CMR results
The report for a CMR study should address the specific questions raised by the referring physician. In suspected myocarditis, this will usually include the inflammatory activity, left ventricular function and other information such as pericardial effusion, cardiac index and extent of scarring.
There was consensus that for the time being the presence or absence of the 3 criteria, if acquired, should be reported. The report summary should include components as listed in table 8. The report should relate quantitative values to published reference values. References may be cited as deemed appropriate.
Table 8.
Summary of recommended components for the CMR study report
| Recommended CMR reports components | |
|---|---|
| LV volume and function | LV end-diastolic volume and volume index |
| LV end-systolic volume and volume index | |
| Ejection fraction | |
| Cardiac index | |
| LV mass and mass index | |
| Presence or absence of markers for inflammatory activity and injury |
|
| Conclusion | Based on the presence or absence of 2 or more criteria, considering additional evidence by the presence of LV dysfunction and/or pericardial effusion |
| Recommendation for follow-up | Based on clinical setting A follow-up >4 weeks after the onset of symptoms may have prognostic implications and thus is recommended. |
It is important to be aware that CMR, like myocardial biopsy, depicts the patient’s status at one point in time and cannot characterize acute, chronic or relapsing forms. These attributes are based on the clinical course rather than imaging (or biopsy) findings. The consensus group therefore recommends against using the terms acute, chronic etc. with respect to CMR findings, but rather to comment on the presence or absence of “active” or “ongoing” inflammation.
Future Developments of CMR for Myocarditis
CMR methodology is evolving at a rapid pace. Among numerous interesting developments many can be expected to be useful for application in myocarditis. As hardware and coil technology are improving, image quality and thus diagnostic yield will be more consistent. But more importantly, novel approaches for characterizing tissue such as time-resolved assessment of gadolinium wash-out, T1 mapping, T2 mapping, parametric imaging and combination of imaging criteria with seromarkers likely will further increase the utility of CMR.
Summary
This paper provides recommendations on the use of cardiovascular magnetic resonance as part of a comprehensive diagnostic approach in patients with suspected myocardial inflammation.
CMR appears suitable to identify patients with significant ongoing inflammation. This may be especially important for patients with recurrent or persisting symptoms, and in patients with new onset heart failure.
Based on published data, we propose a comprehensive CMR protocol to determine extent and regional distribution of reversible and irreversible myocardial injury, as well as to detect functional and other abnormalities.
Furthermore, we suggest consensus criteria providing evidence for or against myocardial inflammation based on CMR findings.
We are aware that these recommendations are based on limited data and that not all centers will be able to apply all components of the suggested protocol. New hardware, software and contrast agent techniques may become available to further improve diagnostic and procedural efficiency of CMR in myocarditis.
Supplementary Material
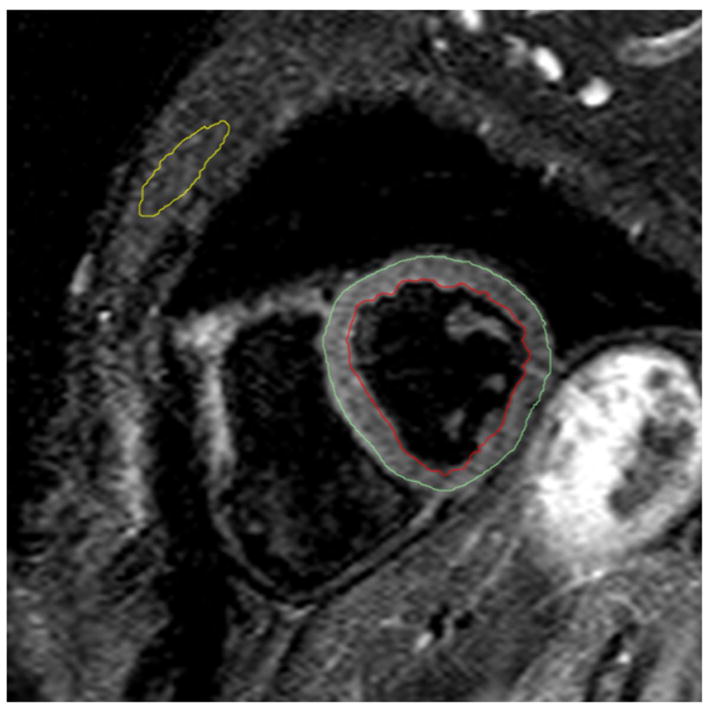
Figure 3. Signal intensity analysis contours for tissue characterization.
T2-weighted image in a short axis orientation with example contours for skeletal muscle (yellow), subepicardial border (green) and subendocardial border (red). The contour for the skeletal muscle was copied from a mid-diastolic SSFP still frame in the same slice position.
Table 9.
Recommended sequence parameters for CMR in suspected myocardial inflammation.
| Sequence | Orientation | Slice thickness | Repetition time | Echo time | Flip angle | |
|---|---|---|---|---|---|---|
| Function, pericardial effusion (SSFP images) | Steady-state free precession (SSFP) | Multiple short axis or multiple long axis |
8mm (+2mm gap) or 10mm (no gap) | <5ms | <2ms | 45 to 65° |
| Edema (T2-weighted images) | Triple-inversion recovery, black-blood fast/turbo spin echo (STIR) For assessing regional edema, the following sequences can be used instead: - ACUT2E TSE SSFP - T2-prepared SSFP - Double-inversion recovery fast/turbo spin echo (DIR) |
Multiple short axis and long axis | 10 to 15mm | >2000ms | 60–70ms | 90° |
| Hyperemia Capillary leak (myocardial early gadolinium enhancement ratio) | Non-breath-hold black-blood fast/turbo (FSE/TSE) spin echo | Multiple short axis or axial |
10mm | 1 R-R interval | <20m s | |
| Irreversible cell injury (myocardial late gadolinium enhancement) | T1-weighted, inversion-recovery prepared gradient echo with fat-sat prepulse, if available | Multiple short axis and long axis | 6 to 10mm | ≥ 2 R-R intervals | <4ms |
SSFP: Steady-state free precession; STIR: Short-TI inversion recovery; ACUT2E TSE: Acquisition for Cardiac Unified T2 Edema.
Table 10.
International Consensus Group on Cardiovascular MR in Myocarditis (in alphabetical order)
| Name | Institution | Email address |
|---|---|---|
| Hassan Abdel-Aty | Franz-Volhard-Klinik, Charité University Hospital, Berlin, Germany | hassan.abdel-aty@charite.de |
| Pauline Alakija | Dept. of Pathology & Laboratory Medicine, University of Calgary, AB, Canada | pauline.alakija@cls.ab.ca |
| Anthony Aletras | NHLBI, Bethesda, MD | aletrasa@mail.nih.gov |
| Leslie T. Cooper | Mayo Clinic College of Medicine, Rochester, MN, USA | cooper.leslie@mayo.edu |
| Neil Filipchuk | Nuclear Cardiology and Stephenson Cardiovascular MR Centre, Calgary, AB, Canada | filipchn@shaw.ca |
| Matthias G. Friedrich | Stephenson Cardiovascular MR Centre, Calgary and Departments of Cardiac Sciences and Radiology, University of Calgary, Calgary, AB, Canada | matthias.friedrich@ucalgary.ca |
| Matthias Gutberlet | Dept. of Diagnostic and Interventional Radiology, University Leipzig | matthias.gutberlet@herzzentrum-leipzig.de |
| Godtfred Holmvang | Massachusetts General Hospital, Harvard Medical Medical School, Boston, USA | gholmvang@partners.org |
| Debra Isaac | Dept. of Cardiac Sciences, University of Calgary, Calgary, AB, Canada | dlisaac@ucalgary.ca |
| Reinhard Kandolf | Dept. of Molecular Pathology, University of Tuebingen, Tuebingen, Germany | reinhard.kandolf@med.uni-tuebingen.de |
| Andreas Kumar | Stephenson Cardiovascular MR Centre, Calgary, AB, Canada | akumar@ucalgary.ca |
| Jean-Pierre Laissy | Dept. of Radiology, Hopital Bichat, Paris, France | jean-pierre.laissy@bch.ap-hop-paris.fr |
| Peter Liu | Toronto General Hospital, Max Bell Research Centre, Toronto, ON, Canada | peter.liu@utoronto.ca |
| Heiko Mahrholdt | Robert-Bosch-Krankenhaus, Stuttgart, Germany | heiko.mahrholdt@rbk.de |
| Bernhard Maisch | Dept. of Internal Medicine and Cardiology, Philipps University, Marburg, Germany | maisch@mailer.uni-marburg.de |
| Sabine Pankuweit | Dept. of Internal Medicine and Cardiology, Philipps University, Marburg, Germany | pankuwei@med.uni-marburg.de |
| Ian Paterson | Division of Cardiology, University of Alberta, Edmonton, Canada | ian.Paterson@capitalhealth.ca |
| Matthias Pauschinger | Klinikum Süd, Nürnberg, Germany | matthias.pauschinger@klinikum-nuernberg.de |
| Sanjay Prasad | Royal Brompton Hospital, London, UK | s.prasad@rbht.nhs.uk |
| Jeanette Schulz-Menger | Franz-Volhard-Klinik, Charité University Hospital, Berlin, Germany | jeanette.schulz-menger@charite.de |
| Udo Sechtem | Robert-Bosch-Krankenhaus, Stuttgart, Germany | udo.sechtem@rbk.de |
| James White | London Health Sciences Centre and Division of Cardiology, University of Western Ontario,, London, ON, Canada | jwhite@imaging.robarts.ca |
Acknowledgments
We want to express our thanks to Dr. Debra Isaac, Dr. Sabine Pankuweit, and Dr. Oliver Strohm for their careful review of the manuscript and helpful suggestions.
One of the meetings of the Consensus Group was in part supported by non-restricted grants from Siemens Medical Solutions Canada and from Berlex Canada Inc. None of the sponsors was involved in the writing process.
Footnotes
There are no severe potential conflicts of interest.
Dr. Friedrich is a shareholder and advisor for Circle Cardiovascular Imaging, Inc, Calgary Canada.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fabre A, Sheppard MN. Sudden adult death syndrome and other non-ischaemic causes of sudden cardiac death. Heart. 2006;92:316–20. doi: 10.1136/hrt.2004.045518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Doolan A, Langlois N, Semsarian C. Causes of sudden cardiac death in young Australians. Med J Aust. 2004;180:110–2. doi: 10.5694/j.1326-5377.2004.tb05830.x. [DOI] [PubMed] [Google Scholar]
- 3.Puranik R, Chow CK, Duflou JA, Kilborn MJ, McGuire MA. Sudden death in the young. Heart Rhythm. 2005;2:1277–82. doi: 10.1016/j.hrthm.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 4.Virmani R, Burke AP, Farb A. Sudden cardiac death. Cardiovasc Pathol. 2001;10:211–8. doi: 10.1016/s1054-8807(01)00091-6. [DOI] [PubMed] [Google Scholar]
- 5.Kawai C. From myocarditis to cardiomyopathy: mechanisms of inflammation and cell death: learning from the past for the future. Circulation. 1999;99:1091–100. doi: 10.1161/01.cir.99.8.1091. [DOI] [PubMed] [Google Scholar]
- 6.Calabrese F, Basso C, Carturan E, Valente M, Thiene G. Arrhythmogenic right ventricular cardiomyopathy/dysplasia: is there a role for viruses? Cardiovasc Pathol. 2006;15:11–7. doi: 10.1016/j.carpath.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 7.Liu PP, Mason JW. Advances in the understanding of myocarditis. Circulation. 2001;104:1076–82. doi: 10.1161/hc3401.095198. [DOI] [PubMed] [Google Scholar]
- 8.Pinamonti B, Alberti E, Cigalotto A, Dreas L, Salvi A, Silvestri F, Camerini F. Echocardiographic findings in myocarditis. Am-J-Cardiol. 1988;62:285–91. doi: 10.1016/0002-9149(88)90226-3. [DOI] [PubMed] [Google Scholar]
- 9.Friedrich MG, Strohm O, SchulzMenger J, Marciniak H, Luft FC, Dietz R. Contrast media-enhanced magnetic resonance imaging visualizes myocardial changes in the course of viral myocarditis. Circulation. 1998 May;97:1802–9. doi: 10.1161/01.cir.97.18.1802. [DOI] [PubMed] [Google Scholar]
- 10.Maisch B, Portig I, Ristic A, Hufnagel G, Pankuweit S. Definition of inflammatory cardiomyopathy (myocarditis): on the way to consensus. A status report. Herz. 2000;25:200–9. doi: 10.1007/s000590050007. [DOI] [PubMed] [Google Scholar]
- 11.Laissy JP, Messin B, Varenne O, Iung B, Karila-Cohen D, Schouman-Claeys E, Steg PG. MRI of acute myocarditis: a comprehensive approach based on various imaging sequences. Chest. 2002;122:1638–48. doi: 10.1378/chest.122.5.1638. [DOI] [PubMed] [Google Scholar]
- 12.Mahrholdt H, Goedecke C, Wagner A, Meinhardt G, Athanasiadis A, Vogelsberg H, Fritz P, Klingel K, Kandolf R, Sechtem U. Cardiovascular magnetic resonance assessment of human myocarditis: a comparison to histology and molecular pathology. Circulation. 2004;109:1250–8. doi: 10.1161/01.CIR.0000118493.13323.81. [DOI] [PubMed] [Google Scholar]
- 13.Abdel-Aty H, Boye P, Zagrosek A, Wassmuth R, Kumar A, Messroghli D, Bock P, Dietz R, Friedrich MG, Schulz-Menger J. Diagnostic performance of cardiovascular magnetic resonance in patients with suspected acute myocarditis: comparison of different approaches. J Am Coll Cardiol. 2005;45:1815–22. doi: 10.1016/j.jacc.2004.11.069. [DOI] [PubMed] [Google Scholar]
- 14.Caforio AL, Calabrese F, Angelini A, Tona F, Vinci A, Bottaro S, Ramondo A, Carturan E, Iliceto S, Thiene G, Daliento L. A prospective study of biopsy-proven myocarditis: prognostic relevance of clinical and aetiopathogenetic features at diagnosis. Eur Heart J. 2007;28:1326–33. doi: 10.1093/eurheartj/ehm076. [DOI] [PubMed] [Google Scholar]
- 15.Morgera T, Di Lenarda A, Dreas L, Pinamonti B, Humar F, Bussani R, Silvestri F, Chersevani D, Camerini F. Electrocardiography of myocarditis revisited: clinical and prognostic significance of electrocardiographic changes. Am Heart J. 1992;124:455–67. doi: 10.1016/0002-8703(92)90613-z. [DOI] [PubMed] [Google Scholar]
- 16.Lauer B, Niederau C, Kuhl U, Schannwell M, Pauschinger M, Strauer BE, Schultheiss HP. Cardiac troponin T in patients with clinically suspected myocarditis. J Am Coll Cardiol. 1997;30:1354–9. doi: 10.1016/s0735-1097(97)00317-3. [DOI] [PubMed] [Google Scholar]
- 17.Cooper LT, Baughman KL, Feldman AM, Frustaci A, Jessup M, Kuhl U, Levine GN, Narula J, Starling RC, Towbin J, Virmani R American Heart Association, American College of Cardiology, European Society of Cardiology, Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology. The role of endomyocardial biopsy in the management of cardiovascular disease: a scientific statement from the American Heart Association, the American College of Cardiology, and the European Society of Cardiology. Endorsed by the Heart Failure Society of America and the Heart Failure Association of the European Society of Cardiology. J Am Coll Cardiol. 2007;50:1914–31. doi: 10.1016/j.jacc.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 18.Chow LH, Radio SJ, Sears TD, McManus BM. Insensitivity of right ventricular endomyocardial biopsy in the diagnosis of myocarditis. J Am Coll Cardiol. 1989;14:915–20. doi: 10.1016/0735-1097(89)90465-8. [DOI] [PubMed] [Google Scholar]
- 19.Hauck AJ, Kearney DL, Edwards WD. Evaluation of postmortem endomyocardial biopsy specimens from 38 patients with lymphocytic myocarditis: implications for role of sampling error. Mayo Clin Proc. 1989;64:1235–45. doi: 10.1016/s0025-6196(12)61286-5. [DOI] [PubMed] [Google Scholar]
- 20.Shirani J, Freant LJ, Roberts WC. Gross and semiquantitative histologic findings in mononuclear cell myocarditis causing sudden death, and implications for endomyocardial biopsy. Am J Cardiol. 1993;72:952–7. doi: 10.1016/0002-9149(93)91113-v. [DOI] [PubMed] [Google Scholar]
- 21.Feldman AM, McNamara D. Medical Progress: Myocarditis. N Engl J Med. 2000;343:1388–1398. doi: 10.1056/NEJM200011093431908. [DOI] [PubMed] [Google Scholar]
- 22.Baughman KL. Diagnosis of myocarditis: death of Dallas criteria. Circulation. 2006;113:593–5. doi: 10.1161/CIRCULATIONAHA.105.589663. [DOI] [PubMed] [Google Scholar]
- 23.Aretz HT, Billingham ME, Edwards WD, Factor SM, Fallon JT, Fenoglio JJJ, Olsen EG, Schoen FJ. Myocarditis. A histopathologic definition and classification. Am J Cardiovasc Pathol. 1987;1:3–14. [PubMed] [Google Scholar]
- 24.Hahn EA, Hartz VL, Moon TE, O’Connell JB, Herskowitz A, McManus BM, Mason JW. The Myocarditis Treatment Trial: design, methods and patients enrollment. Eur Heart J. 1995;16(Suppl O):162–7. doi: 10.1093/eurheartj/16.suppl_o.162. [DOI] [PubMed] [Google Scholar]
- 25.Shanes JG, Ghali J, Billingham ME, Ferrans VJ, Fenoglio JJ, Edwards WD, Tsai CC, Saffitz JE, Isner J, Furner S, et al. Interobserver variability in the pathologic interpretation of endomyocardial biopsy results. Circulation. 1987;75:401–5. doi: 10.1161/01.cir.75.2.401. [DOI] [PubMed] [Google Scholar]
- 26.Herskowitz A, Ahmed-Ansari A, Neumann DA, Beschorner WE, Rose NR, Soule LM, Burek CL, Sell KW, Baughman KL. Induction of major histocompatibility complex antigens within the myocardium of patients with active myocarditis: a nonhistologic marker of myocarditis. J Am Coll Cardiol. 1990;15:624–32. doi: 10.1016/0735-1097(90)90637-5. [DOI] [PubMed] [Google Scholar]
- 27.Angelini A, Crosato M, Boffa GM, Calabrese F, Calzolari V, Chioin R, Daliento L, Thiene G. Active versus borderline myocarditis: clinicopathological correlates and prognostic implications. Heart. 2002;87:210–5. doi: 10.1136/heart.87.3.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Richardson P, McKenna W, Bristow M, Maisch B, Mautner B, O’Connell J, Olsen E, Thiene G, Goodwin J, Gyarfas I, Martin I, Nordet P. Report of the 1995 World Health Organization/International Society and Federation of Cardiology Task Force on the Definition and Classification of cardiomyopathies [news] [see comments] Circulation. 1996;93:841–2. doi: 10.1161/01.cir.93.5.841. [DOI] [PubMed] [Google Scholar]
- 29.Skouri HN, Dec GW, Friedrich MG, Cooper LT. Noninvasive imaging in myocarditis. J Am Coll Cardiol. 2006;48:2085–93. doi: 10.1016/j.jacc.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 30.Felker GM, Boehmer JP, Hruban RH, Hutchins GM, Kasper EK, Baughman KL, Hare JM. Echocardiographic findings in fulminant and acute myocarditis. J Am Coll Cardiol. 2000;36:227–32. doi: 10.1016/s0735-1097(00)00690-2. [DOI] [PubMed] [Google Scholar]
- 31.Sarda L, Colin P, Boccara F, Daou D, Lebtahi R, Faraggi M, Nguyen C, Cohen A, Slama MS, Steg PG, Le Guludec D. Myocarditis in patients with clinical presentation of myocardial infarction and normal coronary angiograms. J Am Coll Cardiol. 2001;37:786–92. doi: 10.1016/s0735-1097(00)01201-8. [DOI] [PubMed] [Google Scholar]
- 32.Dec GW, Palacios I, Yasuda T, Fallon JT, Khaw BA, Strauss HW, Haber E. Antimyosin antibody cardiac imaging: its role in the diagnosis of myocarditis [see comments] J Am Coll Cardiol. 1990;16:97–104. doi: 10.1016/0735-1097(90)90463-y. [DOI] [PubMed] [Google Scholar]
- 33.Gagliardi MG, Bevilacqua M, Di Renzi P, Picardo S, Passariello R, Marcelletti C. Usefulness of magnetic resonance imaging for diagnosis of acute myocarditis in infants and children, and comparison with endomyocardial biopsy. Am J Cardiol. 1991;68:1089–91. doi: 10.1016/0002-9149(91)90501-b. [DOI] [PubMed] [Google Scholar]
- 34.Gutberlet M, Spors B, Thoma T, Bertram H, Denecke T, Felix R, Noutsias M, Schultheiss HP, Kuhl U. Suspected chronic myocarditis at cardiac MR: diagnostic accuracy and association with immunohistologically detected inflammation and viral persistence. Radiology. 2008;246:401–9. doi: 10.1148/radiol.2461062179. [DOI] [PubMed] [Google Scholar]
- 35.Roditi GH, Hartnell GC. MC. C. MRI changes in myocarditis – evaluation with spin echo, cine MR angiography and contrast enhanced spin echo imaging. Clin Radiol. 2000;55:752–758. doi: 10.1053/crad.2000.0519. [DOI] [PubMed] [Google Scholar]
- 36.Rieker O, Mohrs O, Oberholzer K, Kreitner KF, Thelen M. Cardiac MRI in suspected myocarditis. Rofo Fortschr Geb Rontgenstr Neuen Bildgeb Verfahr. 2002;174:1530–6. doi: 10.1055/s-2002-35999. [DOI] [PubMed] [Google Scholar]
- 37.Laissy JP, Hyafil F, Feldman LJ, Juliard JM, Schouman-Claeys E, Steg PG, Faraggi M. Differentiating Acute Myocardial Infarction from Myocarditis: Diagnostic Value of Early- and Delayed-Perfusion Cardiac MR Imaging. Radiology. 2005;237:75–82. doi: 10.1148/radiol.2371041322. [DOI] [PubMed] [Google Scholar]
- 38.Ingkanisorn WP, Paterson DI, Calvo KR, Rosing DR, Schwartzentruber DJ, Fuisz AR, Arai AE. Cardiac magnetic resonance appearance of myocarditis caused by high dose IL-2: similarities to community-acquired myocarditis. J Cardiovasc Magn Reson. 2006;8:353–60. doi: 10.1080/10976640500452000. [DOI] [PubMed] [Google Scholar]
- 39.De Cobelli F, Pieroni M, Esposito A, Chimenti C, Belloni E, Mellone R, Canu T, Perseghin G, Gaudio C, Maseri A, Frustaci A, Del Maschio A. Delayed gadolinium-enhanced cardiac magnetic resonance in patients with chronic myocarditis presenting with heart failure or recurrent arrhythmias. J Am Coll Cardiol. 2006;47:1649–54. doi: 10.1016/j.jacc.2005.11.067. [DOI] [PubMed] [Google Scholar]
- 40.Mahrholdt H, Wagner A, Deluigi CC, Kispert E, Hager S, Meinhardt G, Vogelsberg H, Fritz P, Dippon J, Bock CT, Klingel K, Kandolf R, Sechtem U. Presentation, patterns of myocardial damage, and clinical course of viral myocarditis. Circulation. 2006;114:1581–90. doi: 10.1161/CIRCULATIONAHA.105.606509. [DOI] [PubMed] [Google Scholar]
- 41.Schulz-Menger J, Wassmuth R, Abdel-Aty H, Siegel I, Franke A, Dietz R, Friedrich MG. Patterns of myocardial inflammation and scarring in sarcoidosis as assessed by cardiovascular magnetic resonance. Heart. 2006;92:399–400. doi: 10.1136/hrt.2004.058016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yelgec NS, Dymarkowski S, Ganame J, Bogaert J. Value of MRI in patients with a clinical suspicion of acute myocarditis. Eur Radiol. 2007;17:2211–7. doi: 10.1007/s00330-007-0612-3. [DOI] [PubMed] [Google Scholar]
- 43.Yilmaz A, Mahrholdt H, Athanasiadis A, Vogelsberg H, Meinhardt G, Voehringer M, Kispert EM, Deluigi C, Baccouche H, Spodarev E, Klingel K, Kandolf R, Sechtem U. Coronary vasospasm as the underlying cause for chest pain in patients with PVB19-myocarditis. Heart. 2008:1456–1463. doi: 10.1136/hrt.2007.131383. [DOI] [PubMed] [Google Scholar]
- 44.Wagner A, Schulz-Menger J, Dietz R, Friedrich MG. Longterm follow-up of patients with acute myocarditis by magnetic resonance imaging. Magma. 2003;16:17–20. doi: 10.1007/s10334-003-0007-7. [DOI] [PubMed] [Google Scholar]
- 45.Ammann P, Naegeli B, Schuiki E, Straumann E, Frielingsdorf J, Rickli H, Bertel O. Long-term outcome of acute myocarditis is independent of cardiac enzyme release. Int J Cardiol. 2003;89:217–22. doi: 10.1016/s0167-5273(02)00478-3. [DOI] [PubMed] [Google Scholar]
- 46.Carniel E, Sinagra G, Bussani R, Di Lenarda A, Pinamonti B, Lardieri G, Silvestri F. Fatal myocarditis: morphologic and clinical features. Ital Heart J. 2004;5:702–6. [PubMed] [Google Scholar]
- 47.Karjalainen J, Heikkila J. “Acute pericarditis”: myocardial enzyme release as evidence for myocarditis. Am Heart J. 1986;111:546–52. doi: 10.1016/0002-8703(86)90062-1. [DOI] [PubMed] [Google Scholar]
- 48.Hiramitsu S, Morimoto S, Kato S, Uemura A, Kubo N, Kimura K, Sugiura A, Itoh T, Hishida H. Transient ventricular wall thickening in acute myocarditis: a serial echocardiographic and histopathologic study. Jpn Circ J. 2001;65:863–6. doi: 10.1253/jcj.65.863. [DOI] [PubMed] [Google Scholar]
- 49.Zagrosek A, Wassmuth R, Abdel-Aty H, Rudolph A, Dietz R, Schulz-Menger J. Relation between myocardial edema and myocardial mass during the acute and convalescent phase of myocarditis - a CMR study. J Cardiovasc Magn Reson. 2008;10:19. doi: 10.1186/1532-429X-10-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kishimoto C, Hiraoka Y. Clinical and experimental studies in myocarditis. Curr Opin Cardiol. 1994;9:349–56. doi: 10.1097/00001573-199405000-00014. [DOI] [PubMed] [Google Scholar]
- 51.Simonetti OP, Kim RJ, Fieno DS, Hillenbrand HB, Wu E, Bundy JM, Finn JP, Judd RM. An improved mr imaging technique for the visualization of myocardial infarction. Radiology. 2001;218:215–3. doi: 10.1148/radiology.218.1.r01ja50215. [DOI] [PubMed] [Google Scholar]
- 52.Abdel-Aty H, Zagrosek A, Schulz-Menger J, Taylor AJ, Messroghli D, Kumar A, Gross M, Dietz R, Friedrich MG. Delayed enhancement and T2-weighted cardiovascular magnetic resonance imaging differentiate acute from chronic myocardial infarction. Circulation. 2004;109:2411–6. doi: 10.1161/01.CIR.0000127428.10985.C6. [DOI] [PubMed] [Google Scholar]
- 53.Kellman P, Aletras AH, Mancini C, McVeigh ER, Arai AE. T2-prepared SSFP improves diagnostic confidence in edema imaging in acute myocardial infarction compared to turbo spin echo. Magn Reson Med. 2007;57:891–7. doi: 10.1002/mrm.21215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Aletras AH, Kellman P, Derbyshire JA, Arai AE. ACUT2E TSE-SSFP: a hybrid method for T2-weighted imaging of edema in the heart. Magn Reson Med. 2008;59:229–35. doi: 10.1002/mrm.21490. [DOI] [PubMed] [Google Scholar]
- 55.Miller DD, Holmvang G, Gill JB, Dragotakes D, Kantor HL, Okada RD, Brady TJ. MRI detection of myocardial perfusion changes by gadolinium-DTPA infusion during dipyridamole hyperemia. Magn Reson Med. 1989;10:246–55. doi: 10.1002/mrm.1910100209. [DOI] [PubMed] [Google Scholar]
- 56.Paajanen H, Brasch RC, Schmiedl U, Ogan M. Magnetic resonance imaging of local soft tissue inflammation using gadolinium-DTPA. Acta Radiol. 1987;28:79–83. [PubMed] [Google Scholar]
- 57.Rehwald WG, Fieno DS, Chen EL, Kim RJ, Judd RM. Myocardial magnetic resonance imaging contrast agent concentrations after reversible and irreversible ischemic injury. Circulation. 2002;105:224–9. doi: 10.1161/hc0202.102016. [DOI] [PubMed] [Google Scholar]
- 58.Kim RJ, Fieno DS, Parrish TB, Harris K, Chen EL, Simonetti O, Bundy J, Finn JP, Klocke FJ, Judd RM. Relationship of MRI delayed contrast enhancement to irreversible injury, infarct age, and contractile function. Circulation. 1999;100:1992–2002. doi: 10.1161/01.cir.100.19.1992. [DOI] [PubMed] [Google Scholar]
- 59.Kim RJ, Wu E, Rafael A, Chen EL, Parker MA, Simonetti O, Klocke FJ, Bonow RO, Judd RM. The Use of Contrast-Enhanced Magnetic Resonance Imaging to Identify Reversible Myocardial Dysfunction. N Engl J Med. 2000;343:1445–53. doi: 10.1056/NEJM200011163432003. [DOI] [PubMed] [Google Scholar]
- 60.Codreanu A, Djaballah W, Angioi M, Ethevenot G, Moulin F, Felblinger J, Sadoul N, Karcher G, Aliot E, Marie PY. Detection of myocarditis by contrast-enhanced MRI in patients presenting with acute coronary syndrome but no coronary stenosis. J Magn Reson Imaging. 2007;25:957–64. doi: 10.1002/jmri.20897. [DOI] [PubMed] [Google Scholar]
- 61.Mahrholdt H, Wagner A, Judd RM, Sechtem U, Kim RJ. Delayed enhancement cardiovascular magnetic resonance assessment of non-ischaemic cardiomyopathies. Eur Heart J. 2005;26:1461–74. doi: 10.1093/eurheartj/ehi258. [DOI] [PubMed] [Google Scholar]
- 62.Assomull RG, Lyne JC, Keenan N, Gulati A, Bunce NH, Davies SW, Pennell DJ, Prasad SK. The role of cardiovascular magnetic resonance in patients presenting with chest pain, raised troponin, and unobstructed coronary arteries. Eur Heart J. 2007;28:1242–9. doi: 10.1093/eurheartj/ehm113. [DOI] [PubMed] [Google Scholar]
- 63.Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, Pennell DJ, Rumberger JA, Ryan T, Verani MS. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: a statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002;105:539–42. doi: 10.1161/hc0402.102975. [DOI] [PubMed] [Google Scholar]
- 64.Aletras AH, Tilak GS, Natanzon A, Hsu LY, Gonzalez FM, Hoyt RFJ, Arai AE. Retrospective determination of the area at risk for reperfused acute myocardial infarction with T2-weighted cardiac magnetic resonance imaging: histopathological and displacement encoding with stimulated echoes (DENSE) functional validations. Circulation. 2006;113:1865–70. doi: 10.1161/CIRCULATIONAHA.105.576025. [DOI] [PubMed] [Google Scholar]
- 65.Friedrich MG, Strohm O, Schulz-Menger J, Marciniak H, Luft FC, Dietz R. Noninvasive diagnosis of acute myocarditis by contrast-enhanced magnetic resonance imaging - response to the author. Circulation. 1999;99:459–460. (letter) [Google Scholar]
- 66.Hudsmith LE, Petersen SE, Francis JM, Robson MD, Neubauer S. Normal human left and right ventricular and left atrial dimensions using steady state free precession magnetic resonance imaging. J Cardiovasc Magn Reson. 2005;7:775–82. doi: 10.1080/10976640500295516. [DOI] [PubMed] [Google Scholar]
- 67.Castillo E, Osman NF, Rosen BD, El-Shehaby I, Pan L, Jerosch-Herold M, Lai S, Bluemke DA, Lima JA. Quantitative assessment of regional myocardial function with MR-tagging in a multi-center study: interobserver and intraobserver agreement of fast strain analysis with Harmonic Phase (HARP) MRI. J Cardiovasc Magn Reson. 2005;7:783–91. doi: 10.1080/10976640500295417. [DOI] [PubMed] [Google Scholar]
- 68.Maceira AM, Prasad SK, Khan M, Pennell DJ. Normalized left ventricular systolic and diastolic function by steady state free precession cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2006;8:417–26. doi: 10.1080/10976640600572889. [DOI] [PubMed] [Google Scholar]
- 69.Friedrich MG, Schulz-Menger J, Strohm O, Dick AJ, Dietz R. The diagnostic impact of 2D- versus 3D left ventricular volumetry by MRI in patients with suspected heart failure. Magma. 2000;11:16–9. doi: 10.1007/BF02678483. [DOI] [PubMed] [Google Scholar]
- 70.Bloomer TN, Plein S, Radjenovic A, Higgins DM, Jones TR, Ridgway JP, Sivananthan MU. Cine MRI using steady state free precession in the radial long axis orientation is a fast accurate method for obtaining volumetric data of the left ventricle. J Magn Reson Imaging. 2001;14:685–92. doi: 10.1002/jmri.10019. [DOI] [PubMed] [Google Scholar]
- 71.Kim RJ, Shah DJ, Judd RM. How we perform delayed enhancement imaging. J Cardiovasc Magn Reson. 2003;5:505–14. doi: 10.1081/jcmr-120022267. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.