Abstract
We studied the growth of transgenic adenocarcinoma of mouse prostate (TRAMP)-C1 tumor cells expressing human prostate specific antigen (PSA) in HLA-DRB1*1501 (DR2b) transgenic mice. TRAMP-PSA tumors were frequently rejected by HLA-DR2b- mice but had increased incidence in HLA-DR2b+ littermates. The levels of PSA-specific CD8 T cell responses were significantly higher in HLA-DR2b- mice that rejected TRAMP-PSA tumors compared to HLA-DR2b+ tumor-bearing littermates. In contrast, antibody responses to PSA were strong in HLA-DR2b+ mice bearing TRAMP-PSA tumors and were virtually undetectable in HLA-DR2b- littermates. The analysis of CD4 T cell responses to PSA revealed the presence of several CD4 T cell epitopes in HLA-DR2b+ mice, but failed to identify strong I-Ab-restricted epitopes in HLA-DR2b- mice. Our data demonstrate that the expression of a permissive HLA class II allele can change the pattern of the immune response to a tumor antigen resulting in the failure of tumor rejection.
Keywords: antibodies, MHC, transgenic mice, tumor immunity
Introduction
Numerous attempts to develop immunotherapy for cancer have been shown to induce a measurable immune response but, with few exceptions, evidence of significant anti-tumor effects has been notably absent. While vaccination strategies directed to enhance anti-tumor CD8 T cell response have been shown to be unlikely, by itself, to represent an effective treatment for cancer (1), the use of MHC class II vaccination strategies is currently being exploited by many investigators (2). In our initial studies, we attempted to explore MHC class II-restricted epitopes as means to improve MHC class I peptide-based vaccines to prevent and treat prostate cancer. We focused on prostate-specific antigen (PSA) in these studies because it has highly restricted tissue distribution (3) and it is expressed in the majority of prostate cancer tissues (4). We also demonstrated that men with granulomatous prostatitis, a destructive inflammatory process in the prostate gland, showed the strongest recognition of PSA by CD4 and CD8 T cells, and that CD4 T cell-mediated recognition occurred in the context of HLA-DRB1*1501 (5). Using transgenic (tg) mice engineered to express HLA-DRB1*1501 (DR2b in old nomenclature) we have also demonstrated that HLA-DRB1*1501 can accommodate a CD4 T cell response to PSA and identified two dominant peptides of PSA restricted by this allele (6).
Transgenic adenocarcinoma of mouse prostate (TRAMP)-C1 tumor cell line derived from TRAMP mice is tumorigenic when grafted into syngeneic C57BL/6 hosts, and is widely used to test various therapeutic approaches for the treatment of prostate cancer (7). In order to understand the role of MHC class II alleles in the development of the anti-tumor immune response, we studied the growth of TRAMP-C1 tumor cells engineered to express PSA (8) in a HLA-DR2b transgenic mouse model. The experiments were carried out in HLA-DR2bxC57BL/6J (B6) F1 mice that accommodate transplantable tumors of B6 origin. In the F1 mice we found that the HLA-DRB1*1501 chimeric transgene was not always expressed in offspring; this allowed us to examine the influence of the MHC class II gene on the growth of the tumor in transgene positive and negative littermates. We observed an unexpected and striking difference in the pattern of immunological response and the overall outcome of tumor growth in HLA-DR2b+ and HLA-DR2b- F1 mice. HLA-DR2b+ mice demonstrated low levels of CD8 T cell responses and high levels of PSA-specific antibody responses, and this pattern of the immune response was accompanied by the failure to reject PSA-expressing tumors. In contrast, HLA-DR2b- F1 littermates demonstrated strong PSA-specific CD8 T cell responses in the absence of Ab to PSA, and rejected PSA-expressing tumors. The simple presence of a “permissive” MHC class II allele was all that was required in this model for the suppression of CD8 T cell responses and enhanced growth of the tumor. This is potentially a fundamental observation about the anti-tumor immune response. In the polymorphic human population it is likely that most tumors contain antigens that are presented by the patient’s HLA class II alleles.
Materials and Methods
Cell lines
TRAMP tumor cell line engineered to express human PSA (TRAMP-PSA) was provided by Dr. J. Medin (University of Toronto, Canada) (8). Parental TRAMP-C1 line was obtained from American Type Culture Collection (Manassas, VA). PSA secretion in the culture supernatants was confirmed by sandwich ELISA (Supplemental Figure 1A) as described (6). TRAMP-PSA and parental TRAMP-C1 tumor cells did not express detectable MHC molecules on the cell surface, but up-regulated both MHC class I and class II molecules upon treatment with recombinant mouse IFN-γ (Supplemental Figure 1B), consistent with previously published data (9).
Mice
All animal studies have been approved by the University of Maryland Institutional Animal Care and Use Committee. DR2b tg mice bearing chimeric MHC class II molecules (DRa1*0101:I-Eα and DRb1*1501:I-Eβ) were provided by Dr. L. Fugger (Aarhus University, Denmark). These mice were engineered on a murine MHC class II knockout B6 background (10), and did not express mouse MHC class II molecules. Tumors of B6 origin were rejected by these mice, probably due to the MHC class II mismatch between the host and the tumor cell line (data not shown). To establish a tumor model based on TRAMP-C1 tumor cells, HLA-DR2b tg mice were crossed to the wild type C57BL/6J (B6) mice (Jackson Laboratories, Bar Harbor, ME), and tumor experiments were carried out using F1 male offspring. The expression of HLA-DR transgene and I-A was analyzed by flow cytometry as described (6). The majority of F1 mice co-expressed both I-A and HLA-DR, while some littermates lost transgene expression for unknown reasons, and displayed a phenotype similar to the wild type B6 mice (Supplemental Figure 2A). The distribution of HLA-DR transgene was similar to the distribution of I-A molecule on all major professional APC populations, including B220+, CD11b+, and CD11c+ cells (Supplemental Figure 2B).
Peptides
H-2Db-restricted immunodominant peptide PSA65-73 (HCIRNKSVI) has been described by Pavlenko et al (11). H-2Db-restricted peptide encoded by the cryptic open reading frame that maps to the non-coding strand of the ampicillin resistance gene (NEO49-59, SSPVNSLRNVV) (12) was used as an irrelevant control peptide. HLA-DRB1*1501-restricted peptides PSA171-190 and PSA221-240 have been identified in our laboratory (6). The peptides were synthesized by NeoMPS Inc. (San Diego, CA), and were of >95% purity. A peptide library consisting of 20-mers that overlap by 10 amino acids and span the mature human PSA amino acid sequence was synthesized at the Biopolymer Core Facility, University of Maryland (Baltimore, MD) and purified to >90% by reversed-phase HPLC. The sequences of 20-mer peptides were described previously (6).
Tumor experiments
HLA-DR2bxB6 F1 male mice between 8 and 12 weeks of age were inoculated s.c. in the dorsal neck area with either wild type TRAMP-C1 (TRAMP-WT) or PSA-expressing TRAMP-C1 (TRAMP-PSA) tumor cells (3×106 cells per mouse in 100 μl PBS). Tumor growth was monitored weekly for 14 weeks. Tumor base area was calculated by measuring two bisecting diameters of the tumor and multiplying these two values. Since TRAMP tumors have a tendency to ulcerate at relatively early stages, we chose time when a tumor base area had reached 100 mm2 as a surrogate end point for the survival analysis, which was calculated by linear regression analysis using Excel software (R-squared values > 0.95). Time-to-event (survival) curves were generated using SigmaPlot software. Comparison of survival curves by log rank test was performed using MedCalc Software.
In some experiments, tumor cells were injected in Basement Membrane Matrix solution (BD Bioscience, final concentration 7-10 mg/ml). Two weeks later, the implants were harvested and fixed in 10% buffered formaldehyde. Paraffin-embedded tissue sections were stained with goat anti-PSA Ab (R&D Systems Inc., Minneapolis, MN) followed by development using goat-specific DAB detection kit (R&D Systems). Images were acquired on an Axioscope Zeiss microscope using AxioVision software.
Immunological assays
IFN-γ ELISPOT assay, PSA Ab ELISA and in vivo CTL assay are described online in “Extended methods” section.
Results and Discussion
To determine the effect of the HLA-DR2b transgene on the development of the anti-tumor immune response, we studied the growth and immune response to wild type TRAMP-C1 and TRAMP-PSA tumor cells in both HLA-DR2b+ and HLA-DR2b- F1 male littermates. We found that wild type TRAMP-C1 tumors were equally tumorigenic in both groups (Figure 1A, left panel); tumor incidence was 93% (10/11 mice) and 90% (9/10 mice) respectively. In contrast, TRAMP-PSA tumors were frequently rejected by HLA-DR2b- F1 mice (tumor incidence 3 of 11 mice, 27%) but grew in HLA-DR2b+ F1 littermates (tumor incidence 13 of 14 animals, 93%, 2-tailed Fisher exact test, p=0.0012) as shown in Figure 1A, right panel. Time-to-event (time when a tumor base area had reached 100 mm2) analysis was also performed. Statistically significant differences in the growth of TRAMP-PSA tumors were observed between HLA-DR2b+ and HLA-DR2b- F1 littermates as shown in Figure 1A. Wild type B6 male mice also rejected TRAMP-PSA tumor cells with a rate similar to HLA-DR2b- F1 mice (Supplemental Figure 3).
Figure 1. TRAMP tumor cell growth in HLA-DR2bxB6 F1 mice.
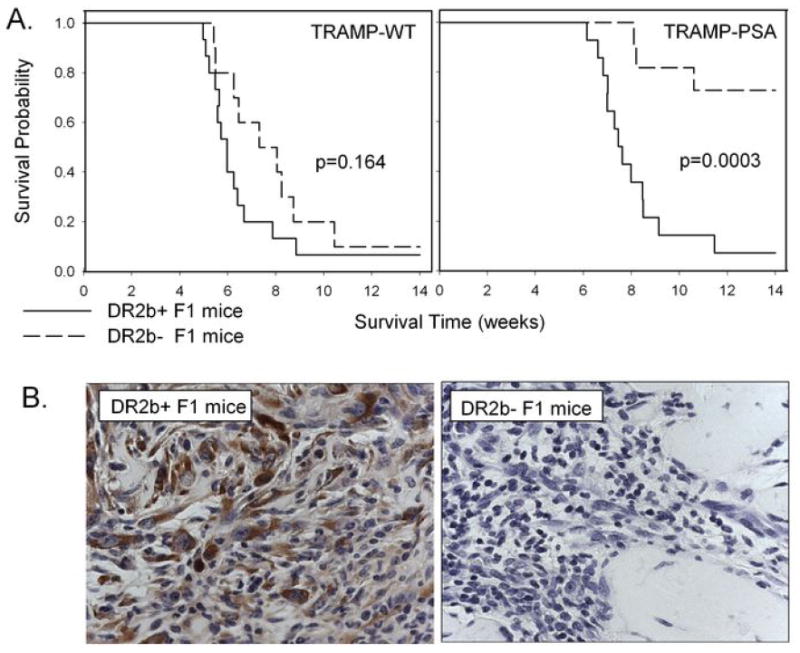
(A) TRAMP-C1 (left panel) or TRAMP-PSA (right panel) tumor cells were inoculated s.c. into HLA-DR2b+ or HLA-DR2b- F1 male mice. Tumor growth was monitored as described in Materials and Methods, p values (log rank test) are displayed on the graphs. (B) TRAMP-PSA tumor cells were inoculated s. c. into HLA-DR2b+ (left panel) and HLA-DR2b- (right panel) F1 male mice in Matrigel. The implants were harvested two weeks later, and PSA expression in the tissue sections was detected as described in Materials and Methods. Magnification 200x.
To facilitate histochemical studies of TRAMP-PSA tumors at early stages of tumor development, we implanted tumor cells into animals in Matrigel Basement Membrane Matrix solution. We found that PSA-expressing tumor cells were abundant in Matrigel implants two weeks after inoculation in HLA-DR2b+ F1 mice (Figure 1B, left panel), but were virtually undetectable in HLA-DR2b- F1 littermates (Figure 1B, right panel). PSA-negative cells with tumor-like morphology were detected in DR2b- F1 mice suggesting that tumor escape by loss of antigen may be one of the major mechanisms of tumor growth in DR2b- F1 mice in our model. Numerous tumor-infiltrating CD45+ leukocytes were detected in both groups (data not shown). These results suggest that an immune response against tumor cells did take place in both groups, but with different outcomes. In HLA-DR2b- F1 mice, an immune response against PSA resulted in the elimination of the PSA-expressing tumor cells, whereas the immune response in HLA-DR2b+ F1 mice did not facilitate tumor rejection.
We hypothesized that differences in the CTL response to the tumor antigen PSA might explain the differences in tumorigenicity of TRAMP-PSA in the F1 mice. CD8 T cells secreting IFN-γ in response to TRAMP-PSA tumor cells as well as the peptide PSA65-73 were significantly more frequent in splenocytes from HLA-DR2b- F1 mice inoculated with TRAMP-PSA tumors compared to HLA-DR2b+ tumor-bearing F1 littermates (Figure 2A). This suggests that the presence of the DR2b transgene in the mice somehow led to inhibition of the CTL response to PSA expressed by the tumor cells. These results were confirmed using an in vivo CTL assay. As shown in Figure 2B, the loss of CFSEhigh labeled cells pulsed with PSA65-73 peptide resulted in the increase of CFSElow/CFSEhigh ratios that was observed in both HLA-DR2b+ and HLA-DR2b- tumor-bearing mice compared to naïve syngeneic controls indicating the presence of specific cytotoxicity and immunity in both groups. However, the magnitude of the cytotoxic response was significantly higher in DR2b- tumor-bearing mice, where CFSEhigh labeled cells virtually disappeared in almost all recipients.
Figure 2. PSA-specific CD8 T cell response to PSA-expressing TRAMP tumor cells in HLA-DR2bxB6 F1 mice.
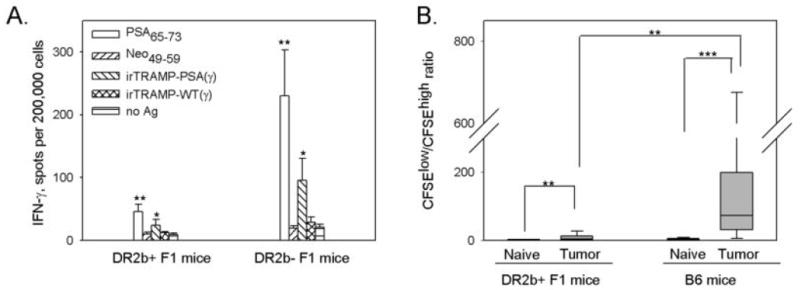
(A) HLA-DR2b+ or HLA-DR2b- F1 mice were inoculated s.c. with TRAMP-PSA tumor cells. Spleens were harvested two weeks later, and frequencies of IFN-γ secreting cells were estimated by ELISPOT assay as described in Materials and Methods. Lymphocytes from 5 mice per group were tested individually in triplicates, mean ± SE was calculated for each group. Combined data from two independent experiments with similar trend are shown. (B) HLA-DR2b+ F1 or wild type B6 mice were inoculated s.c. with TRAMP-PSA tumor cells. Two weeks later, tumor-bearing and syngeneic naïve animals were injected i.v. with CFSE-labeled target cells. A mixture consisted of equal numbers (10×106 each per mouse) of CFSEhigh cells pulsed with PSA65-73 peptide and CFSElow untreated cells. Spleens were removed 16 hr later, and analyzed for the loss of CFSEhigh population by flow cytometry. CFSElow/CFSEhigh ratios are presented as a box plot, the boundaries of the box show the 5th/95th percentile, a line within the box marks the median. Combined data from three independent experiments with similar trend are shown (total 9 mice per group).
* p<0.05, ** p<0.01, *** p<0.001 (two-tailed Mann-Whitney U-test).
In contrast to CD8 T cell responses, antibody responses to PSA were strong in HLA-DR2b+ F1 mice bearing TRAMP-PSA tumors and were virtually undetectable in HLA-DR2b- F1 littermates (Figure 3). The antibodies were predominantly of IgG1 subtype (data not shown). The data suggest that the presence of the HLA-DR2b allele in the F1 animals was sufficient to induce a humoral immune response to the tumor antigen PSA and this immune response was associated with suppression of the otherwise robust CTL response to PSA.
Figure 3. PSA-specific humoral immune response to the PSA-expressing TRAMP tumor cells in HLA-DR2bxB6 F1 mice.
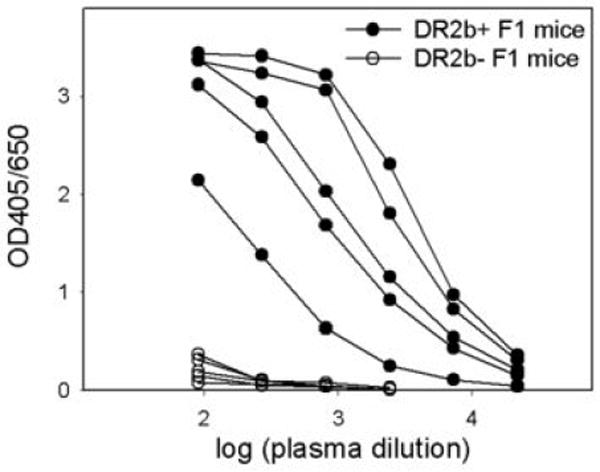
Mice were inoculated with TRAMP-PSA tumor cells as described in Figure 2. Blood was collected two weeks after tumor inoculation. PSA Ab response was measured by ELISA. The titration curves are shown for the individual mice (5 mice per group, same animals as depicted in Figure 2A).
To explain the differences in the levels of humoral immune response between HLA-DR2b+ and HLA-DR2b- F1 mice, both groups were immunized with soluble PSA in CFA. HLA-DR2b+ F1 mice demonstrated strong IFN-γ response to soluble PSA, while the response in HLA-DR2b- F1 mice was negligible (Figure 4). Screening of a 20-mer overlapping PSA peptide library in these mice revealed the presence of several CD4 T cell epitopes in HLA-DR2b+ F1 mice (PSA171-190, PSA221-240, and PSA241-261) but failed to identify a strong I-Ab-restricted epitope in HLA-DR2b- F1 mice (Figure 4) as well as in B6 wild type mice (Supplemental Figure 4). These data indicate that HLA-DRB1*1501 is a permissive allele for PSA, while the I-Ab allele could not accommodate a strong CD4 T cell response to PSA.
Figure 4. CD4 T cell response to soluble PSA in HLA-DR2bxB6 F1 mice.
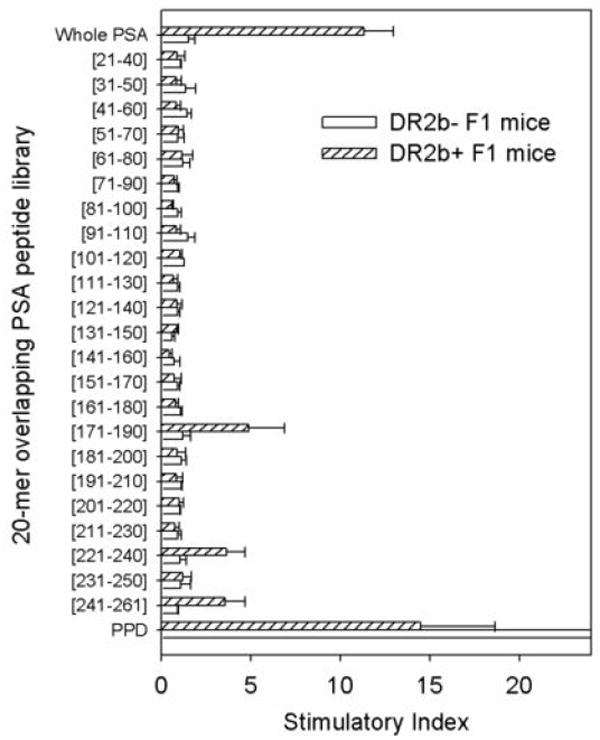
HLA-DR2b+ or HLA-DR2b- F1 mice were immunized s.c. with human PSA in CFA. Spleens were harvested two weeks later, and lymphocytes were cultured in the presence of soluble PSA or a series of overlapping 20-mer peptides derived from the primary amino acid sequence of human PSA. The response to the purified protein derivate (PPD) of tuberculin served as a positive control. IFN-γ secretion was measured by ELISPOT. Stimulatory index was calculated by equation: [Spot number (sample)] / [Spot number (no Ag)]. Data are means ± SD of triplicates.
We show here that the presence of human PSA in the TRAMP tumor line confers strong immunogenicity to the tumor (as expected for a xenogeneic protein) that results in a robust CD8 T cell response to PSA and leads to the rejection of PSA-expressing tumor cells in B6 mice and in HLA-DR2b- F1 mice. In these animals, a CD4 T cell response to PSA does not apparently occur, most likely because there are no epitopes in PSA that are presented by I-Ab. In contrast, the presence of the HLA-DRB1*1501 chimeric transgene in HLA-DR2b+ F1 mice increased the tumorigenicity of TRAMP-PSA tumor cells compared to littermates not expressing the transgene. Since TRAMP-PSA tumor cells did not express HLA-DR transgene, the MHC class II-restricted immune response to PSA in DR2b+ F1 mice in our model occurred most likely via the indirect pathway of antigen presentation by host’s APC.
We hypothesize that, in human malignancies, the diversity of available MHC class II haplotypes in the human population may manifest a similar effect on tumor antigen recognition as we identified in these experiments. Our data would support, therefore, the notion that the general immune suppression attributed to human tumors is a feature of the immune response to the tumor in a patient with an appropriate HLA class II haplotype. Whether this phenomenon is a specific feature of either the HLA-DRB1*1501 allele or may be associated with antigenic or biochemical properties of PSA remains to be investigated. One of the major mechanisms by which the presence of CD4 T cell epitope in PSA can negatively affect anti-tumor immunity may involve a classical pathway of humoral immunity (antibodies/immune complexes, B cells and Th2 type cytokines). The other major mechanism may include CD4+CD25+ regulatory T cells. We could not detect any differences in the proportion of CD4+CD25+ T cells or foxp3-expressing T cells between DR2b+ and DR2b- F1 mice (Supplemental Table I). However, Treg cells can be selectively activated systemically or at the tumor site in an antigen-specific manner in HLA-DR2b+ mice.
Inflammation is one of the critical factors that may contribute to prostatic carcinogenesis, however the cause of prostatic inflammation is unclear (13). There is considerable evidence for an association between prostate cancer development, inflammation and autoantibody generation against tumor proteins (14). The presence of tumor antigen-specific antibodies in serum and/or deposition of immune complexes at the tumor site has been shown to be correlated with poor patient survival in various epithelial cancers, including prostate cancer (15-17). Despite the accumulating evidence, the presence of humoral immune response in patients with advanced prostate cancer is often interpreted as an indicator of a beneficial immune response (14,16,17). Based on our observations in the HLA-DR2b transgenic mouse model of prostate cancer, we hypothesize that the presence of naturally-occurring antibody responses to tumor antigen(s) in advanced prostate cancer is an indicator of an immune response that is ineffective at rejecting the tumor. In the polymorphic human population, we suggest that many tumors may contain antigens that are presented by the patient’s HLA class II alleles in this fashion and affect the ability of the host to mount an effective response against the tumor.
Supplementary Material
Acknowledgments
The authors thank Dr. Masaki Terabe and Dr. Suzanne Ostrand-Rosenberg for helpful advice and discussion.
Footnotes
Supported by NIH grant DK65268, an Intramural Pilot grant from the University of Maryland Greenebaum Cancer Center, American Cancer Society Institutional Research Grant #97-153-04 and by a grant from the US Department of Veterans Affairs.
Abbreviations: TRAMP - transgenic adenocarcinoma of mouse prostate; PSA - prostate specific antigen; tg – transgenic; CFSE - 5- (and 6-) carboxyfluorescein diacetate succinimidyl ester.
References
- 1.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10:909–915. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ostrand-Rosenberg S. CD4+ T lymphocytes: Critical components of anti-tumor immunity. Cancer Invest. 2005;23:413–419. [PubMed] [Google Scholar]
- 3.Cunha AC, Weigle B, Kiessling A, Bachmann M, Rieber EP. Tissue-specificity of prostate specific antigens: comparative analysis of transcript levels in prostate and non-prostatic tissues. Cancer Lett. 2006;236:229–238. doi: 10.1016/j.canlet.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 4.Lilja H, Ulmert D, Vickers AJ. Prostate-specific antigen and prostate cancer: prediction, detection and monitoring. Nat Rev Cancer. 2008;8:268–278. doi: 10.1038/nrc2351. [DOI] [PubMed] [Google Scholar]
- 5.Klyushnenkova EN, Ponniah S, Rodriguez A, Kodak J, Mann DL, Langerman A, Nishimura MI, Alexander RB. CD4 and CD8 T-lymphocyte recognition of prostate specific antigen in granulomatous prostatitis. J Immunother. 2004;27:136–146. doi: 10.1097/00002371-200403000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Klyushnenkova EN, Link J, Oberle WT, Kodak J, Rich C, Vandenbark AA, Alexander RB. Identification of HLA-DRB1*1501-restricted T-cell epitopes from prostate-specific antigen. Clin Cancer Res. 2005;11:2853–2861. doi: 10.1158/1078-0432.CCR-04-1927. [DOI] [PubMed] [Google Scholar]
- 7.Foster BA, Gingrich JR, Kwon ED, Madias C, Greenberg NM. Characterization of prostatic epithelial cell lines derived from transgenic adenocarcinoma of the mouse prostate (TRAMP) model. Cancer Res. 1997;57:3325–3330. [PubMed] [Google Scholar]
- 8.Medin JA, Liang SB, Hou JW, Kelley LS, Peace DJ, Fowler DH. Efficient transfer of PSA and PSMA cDNAs into DCs generates antibody and T cell antitumor responses in vivo. Cancer Gene Ther. 2005;12:540–551. doi: 10.1038/sj.cgt.7700810. [DOI] [PubMed] [Google Scholar]
- 9.Grossmann ME, Wood M, Celis E. Expression, specificity and immunotherapy potential of prostate-associated genes in murine cell lines. World J Urol. 2001;19:365–370. doi: 10.1007/pl00007104. [DOI] [PubMed] [Google Scholar]
- 10.Madsen LS, Andersson EC, Jansson L, Krogsgaard M, Andersen CB, Engberg J, Strominger JL, Svejgaard A, Hjorth JP, Holmdahl R, Wucherpfennig KW, Fugger L. A humanized model for multiple sclerosis using HLA-DR2 and a human T-cell receptor. Nat Genet. 1999;23:343–347. doi: 10.1038/15525. [DOI] [PubMed] [Google Scholar]
- 11.Pavlenko M, Leder C, Roos AK, Levitsky V, Pisa P. Identification of an immunodominant H-2D(b)-restricted CTL epitope of human PSA. Prostate. 2005;64:50–59. doi: 10.1002/pros.20221. [DOI] [PubMed] [Google Scholar]
- 12.van Hall T, van de Rhee NE, Schoenberger SP, Vierboom MP, Verreck FA, Melief CJ, Offringa R. Cryptic open reading frames in plasmid vector backbone sequences can provide highly immunogenic cytotoxic T-lymphocyte epitopes. Cancer Res. 1998;58:3087–3093. [PubMed] [Google Scholar]
- 13.Nelson WG, De Marzo AM, Isaacs WB. Prostate cancer. N Engl J Med. 2003;349:366–381. doi: 10.1056/NEJMra021562. [DOI] [PubMed] [Google Scholar]
- 14.Taylor BS, Pal M, Yu J, Laxman B, Kalyana-Sundaram S, Zhao R, Menon A, Wei JT, Nesvizhskii AI, Ghosh D, Omenn GS, Lubman DM, Chinnaiyan AM, Sreekumar A. Humoral response profiling reveals pathways to prostate cancer progression. Mol Cell Proteomics. 2008;7:600–611. doi: 10.1074/mcp.M700263-MCP200. [DOI] [PubMed] [Google Scholar]
- 15.Tan TT, Coussens LM. Humoral immunity, inflammation and cancer. Curr Opin Immunol. 2007;19:209–216. doi: 10.1016/j.coi.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 16.McNeel DG, Nguyen LD, Storer BE, Vessella R, Lange PH, Disis ML. Antibody immunity to prostate cancer associated antigens can be detected in the serum of patients with prostate cancer. J Urol. 2000;164:1825–1829. [PubMed] [Google Scholar]
- 17.Fossa A, Berner A, Fossa SD, Hernes E, Gaudernack G, Smeland EB. NY-ESO-1 protein expression and humoral immune responses in prostate cancer. Prostate. 2004;59:440–447. doi: 10.1002/pros.20025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


