Abstract
Background and Purpose
The Los Angeles Motor Scale (LAMS) is a brief 3-item stroke severity assessment measure designed for prehospital and Emergency Department use.
Methods
The LAMS and NIHSS were scored in under-12-hour acute anterior circulation ischemic stroke patients. Stroke severity ratings were correlated with cervicocerebral vascular occlusion on CTA, MRA, and catheter angiography. Receiver operating curves, c statistics, and likelihood ratios were used to evaluate the predictive value for vascular occlusion of stroke severity ratings.
Results
Among 119 patients, mean age was 67 (±18), 45% were male. Time from onset to ED arrival was mean 190 minutes (range 10 to 660). Persisting large vessel occlusions (PLVOs) were present in 62% of patients. LAMS stroke severity scores were higher in patients harboring a vascular occlusion, median 5 (IQR 4 to 5) versus 2 (IQR 1 to 3). Similarly, NIHSS stroke severity scores were higher in PLVO patients, 19 (14 to 24) versus 5 (3 to 7). ROC curves demonstrated that the LAMS was highly effective in identifying patients with PLVOs, c statistic 0.854. At the optimal threshold of 4 or higher, LAMS scores showed sensitivity 0.81, specificity 0.89, and overall accuracy 0.85. LAMS performance was comparable to NIHSS performance (c statistic 0.933). The positive likelihood ratio associated with a LAMS score ≥4 was 7.36 and the negative likelihood ratio 0.21.
Conclusions
Stroke severity assessed by the LAMS predicts presence of large artery anterior circulation occlusion with high sensitivity and specificity. The LAMS is a promising instrument for use by prehospital personnel to identify select stroke patients for direct transport to Comprehensive Stroke Centers capable of endovascular interventions. (Stroke.2008;39:2264-2267.)
Keywords: acute stroke, cerebral infarct, scales, LAMS (Los Angeles Motor Scale), NIHSS
A two-tier regional stroke system of care, comprising Primary and Comprehensive Stroke Centers (CSCs), is being increasingly adopted throughout the United States and the world for efficient emergent management of patients with acute strokes.1 Primary Stroke Centers provide prompt intravenous therapies, while more complex endovascular therapies are available at Comprehensive Centers, including endovascular recanalization interventions for select patients with persisting large vessel occlusions (PLVOs) beyond the 3-hour time window for intravenous tissue plasminogen activator.2,3 Paramedic identification of >3-hour patients likely harboring PLVOs might improve prehospital management, as routing >3-hour PLVO patients directly to CSCs by Emergency Medical Services would accelerate endovascular treatment intervention.
Standard prehospital stroke recognition instruments identify patients likely having a stroke, but do not demarcate the subgroup of stroke patients harboring persisting large vessel occlusions who are candidates for endovascular intervention. Stroke deficit severity scales have been proposed for this purpose. Several studies have demonstrated a strong correlation between stroke deficit severity and the presence of persisting large vessel occlusion (PLVO) among acute ischemic stroke patients.4–6 Based on this association, stroke deficit severity assessed with the NIH Stroke Scale in the Emergency Department (ED) has been used to select patients for entry into clinical trials of intraarterial thrombolysis and is used in regular clinical practice at some sites for initial triage for acute endovascular therapy.7 However, the full 42-point NIHSS is too time consuming and unwieldy for routine prehospital use.
The Los Angeles Motor Scale (LAMS) is a validated, 3-item, 0- to 5-point motor stroke deficit scale, developed for prehospital and ED use, that takes 20 to 30 seconds to perform.8 As the LAMS score is derived from the motor examination portion of the Los Angeles Prehospital Stroke Screen (LAPSS), a validated and widely used stroke identification instrument that is a part of the international training curriculum for prehospital personnel,9 the LAMS does not add any time to standard prehospital stroke assessments. The LAMS has good interrater reliability, correlates strongly with the full NIHSS (concurrent validity), and predicts final stroke functional outcomes as well as the NIHSS (predictive validity).10,11
This study was undertaken to determine whether LAMS scores can predict the presence of large vessel occlusions in acute cerebral ischemia patients.
Methods
The patient sample was drawn from 2 database sources: (1) Consecutive patients enrolled in acute stroke clinical trials at the UCLA Stroke Center from 1996 to 2006, and (2) patients entered into the UCLA Get with the Guidelines Stroke Registry in calendar year 2005. Inclusion criteria for this study were: (1) Last known well time within 12 hours of initial ED examination, and (2) Final diagnosis of acute ischemic stroke in the anterior circulation.
Los Angeles Motor Scale (Table) scores at entry were derived from (1) the face and arm strength item of the entry NIHSS, and (2) the grip examination as documented in the charted admission pretreatment physical examination. For facial strength, a LAMS score of 0 (normal) was assigned if the NIHSS facial palsy item was scored 0 (normal) or 1 (minor asymmetry), and a LAMS score of 1 (weak) was assigned if the NIHSS facial palsy item was scored 2 (partial) or 3 (complete). For arm strength, a LAMS score of 0 (normal) was assigned if the NINHS motor arm item was scored 0 (normal); a LAMS score of 1 (drifts down) was assigned if the NIHSS motor arm item was scored 1 (drift); and a LAMS score of 2 (falls rapidly) was assigned if NIHSS motor arm item was scored 2 (some effort against gravity) or 3 (no effort against gravity). For grip strength, a LAMS score of 0 (normal) was assigned if the admission neurological examination rated grip strength as 5 (normal); a LAMS score of 1 (weak) was assigned if the neurological examination rated grip strength as 4 (weak), 3 (some movement against gravity), or 2 (some movement, but not against gravity); a LAMS score of 2 (no grip) was assigned if the neurological examination rated grip strength as 1 (muscle contraction but no movement) or 0 (no muscle contraction). All examiners were certified in the NIH Stroke Scale.
Table 1.
The Los Angeles Motor Scale (LAMS)
| Facial droop | |
| Absent | 0 |
| Present | 1 |
| Arm drift | |
| Absent | 0 |
| Drifts down | 1 |
| Falls rapidly | 2 |
| Grip strength | |
| Normal | 0 |
| Weak grip | 1 |
| No grip | 2 |
During the study period, standard practice was to obtain arrival intracranial vascular imaging in all patients with magnetic resonance angiography, computed tomography angiography, or catheter angiography, and arrival cervical vascular imaging with magnetic resonance angiography, computed tomography angiography, carotid ultrasound, or catheter angiography. Findings on the first vessel imaging study performed were used to classify patients in 1 to 6 groups according to the location of their arterial occlusion.
Internal carotid artery (ICA)
Middle cerebral artery main stem (M1)
Middle cerebral artery division (M2)
Middle cerebral artery branches (M3-M4)
Anterior cerebral artery (ACA)
No visible occlusion (No occlusion)
Patients with 2 or more tandem occluded arteries were classified in the group of the largest occluded artery.6
Receiver operating curves, c statistics, and likelihood ratios were used to evaluate the predictive value for vascular occlusion of stroke severity ratings.
Results
Among the 119 patients, 45% were male and mean (±SD) age was 67±18. The median NIHSS score was 14, range 1 to 40. Mode of transport to the ED was ambulance in 67 (56%), air transport in 12 (10%), private vehicle in 20 (16%), and not documented in 15 (12%). Five patients (4%) had in-hospital onset of stroke.
The time interval from last known well to ED arrival was mean 190± 128 minutes, range 10 to 660. The time interval from last known well to LAMS and NIHSS examination was mean 254±137 minutes, last known well to first intracranial vascular imaging 324±171 minutes, and last known well to first cervical vascular imaging 400±297 minutes. The modality used for first intracranial vascular imaging was MRA in 80 (67%), catheter angiography in 33 (27%), and CTA in 6 (5%). The modality used for first cervical vascular imaging was catheter angiography in 58 (48%), MRA in 45 (37%), CTA in 6 (5%), and carotid ultrasound in 2 (2%).
At vascular imaging, 74 patients (62%) had arterial occlusions and 45 no arterial occlusions. Among the patients with vascular occlusions, sites of occlusion were ICA in 21, M1 MCA in 48, M2 MCA in 4, and M3/4 MCA in 1. LAMS stroke severity scores were higher in patients harboring vascular occlusion, median 5 (IQR 4 to 5) versus 2 (IQR 1 to 3), P<0.001. Similarly, NIHSS stroke severity scores were higher in PLVO patients, median 19 (IQR 14 to 24) versus 5 (3 to 7), P<0.001. Site of PLVO affected both LAMS scores and NIHSS scores. For the LAMS, the median score for an ICA PLVO was 5 (IQR 5- to 5); M1 MCA 5 (4 to 5); M2 1.5 (0.5- to 3.5) For the NIHSS, the median score for an ICA PLVO was 24 (IQR 19- to 26); M1 MCA 17 (14- to 22.5); M2 6 (4- to 13.5).
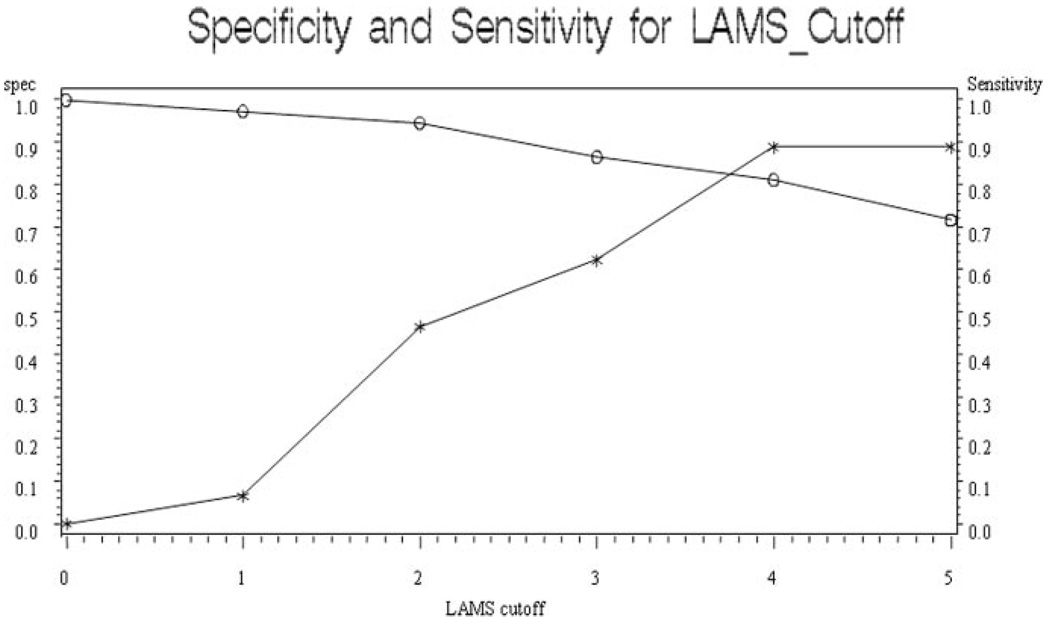
ROC curves (Figure) demonstrated that the LAMS was highly effective in identifying patients with PLVOs, c statistic 0.854. At the optimal threshold of 4 or higher, LAMS scores showed sensitivity 0.81, specificity 0.89, and overall accuracy 0.85. The positive likelihood ratio associated with a LAMS score ≥4 was 7.36 and the negative likelihood ratio 0.21. LAMS performance was comparable to NIHSS performance (c statistic 0.933). At the optimal cut point of 11 or higher, NIHSS scores showed sensitivity 0.91, specificity 0.87, and overall accuracy 0.89.
Figure 1.
Receiver operating curve showing specificity (asterisks) and sensitivity (open circles) of LAMS Scores in predicting persisting large vessel occlusion.
Discussion
This study demonstrates that stroke severity rapidly assessed by the Los Angeles Motor Scale predicts the presence of large vessel occlusion with high sensitivity and specificity. A LAMS score ≥4 increases by 7-fold the likelihood that a stroke patient harbors a persisting large vessel vascular occlusion. During the hyperacute phase of stroke, knowledge of the persistence of arterial occlusion can help guide many aspects of management, particularly regarding endovascular recanalization interventions. Patients with acute deficits and without visible vessel occlusions tend to have less severe clinical deficits and better functional outcomes with supportive management alone. These patients typically have already spontaneously recanalized, have occlusions of small, deep penetrators, or have occlusions of far distal superficial branch arteries.12 They generally harbor less tissue at risk and have no vascular lesion target appropriate for endovascular intervention.
The LAMS already has demonstrated several advantageous properties as an instrument for prehospital rating of stroke severity. It has been validated as an accurate assessment of stroke severity in both clinical trial and broad typical clinical practice populations.8,11 Compared with other instruments proposed for prehospital stroke severity assessment,13,14 the LAMS has advantages of greater simplicity, more rapid administration, and of being immediately derivable from a validated stroke recognition instrument, rather than requiring separate examinations for stroke diagnosis and stroke severity assessment. This study’s demonstration that the LAMS can additionally identify patients likely harboring persistent large vessel occlusions that are appropriate targets for endovascular therapy adds to the value of this instrument.
In addition to demonstrating the utility of the LAMS in predicting arterial occlusion, this study confirms and extends the finding of prior studies that the NIHSS has substantial discriminating value in predicting PLVO. The optimal cutoff NIHSS score of 11 in our cohort is consonant with prior studies that have found NIHSS thresholds of 10 to 12 to have the greatest discriminatory value.4–6,15 Our study expands on prior studies confined to patients undergoing catheter angiography4–6 to demonstrate the predictive utility of stroke severity assessments in a broad range of ischemic stroke patients.
This study does have several limitations. LAMS exams were performed by physicians on hospital arrival rather than by prehospital personnel in the field. However, a prior study has demonstrated good concordance between LAMS scores generated by physicians and prehospital personnel.10 Vascular occlusions were identified in most patients on the basis of noninvasive MRA and CTA studies, rather than catheter angiograms. But MRA and CTA have been repeatedly shown to correlate well with invasive angiography in acute stroke patients, especially for the proximal occlusions that are the appropriate targets for endovascular intervention. This was a retrospective study of 2 well-characterized convenience samples (1 year of consecutive admissions and 11 years of patients enrolled in acute clinical trials). Prospective studies of a fully unselected, consecutive sample are needed to fully validate the findings.
Stroke severity scores on the Los Angeles Motor Scale predict the presence of persisting large vessel occlusions. Performing the LAMS/LAPSS is a highly expeditious strategy to identify stroke patients in the field, assess their severity, and determine the likelihood that they are candidates for endovascular therapies. For individual hospitals performing endovascular interventions, once paramedics identify a patient meeting with a LAMS score of ≥4, prearrival notification could accelerate endovascular interventions by permitting early mobilization of the endovascular team and readying of the angiographic suite. For regional systems of care in which EMS routes patients directly to the most appropriate receiving hospitals, a LAMS score ≥4 may be used to route select patients directly to Comprehensive Stroke Centers, avoiding delays introduced by initial delivery to and secondary transfer from nonendovascular sites.
Acknowledgments
Sources of Funding
This work was supported in part by a Visiting Scholar Award from TUBITAK, the Scientific and Technological Research Council of Turkey (B.N.), and NIH-NINDS Award P50 NS044378 (J.L.S.).
Footnotes
Reprints: Information about reprints can be found online at http://www.lww.com/reprints
Disclosures
None.
References
- 1.Schwamm LH, Pancioli A, Acker JE, III, Goldstein LB, Zorowitz RD, Shephard TJ, Moyer P, Gorman M, Johonston SC, Duncan PW, Gorelick P, Frank J, Stranne SK, Smith R, Federspiel W, Horton KB, Magnis E, Adams RJ. American Stroke Association’s Task Force on the Development of Stroke Systems. Recommendations for the establishment of stroke systems of care: recommendations from the American Stroke Association’s Task Force on the Development of Stroke Systems. Stroke. 2005;36:690–703. doi: 10.1161/01.STR.0000158165.42884.4F. [DOI] [PubMed] [Google Scholar]
- 2.Alberts MJ, Latchaw RE, Selman WR, Shephard T, Hadley MN, Brass LM, Koroshetz W, Marler JR, Booss J, Zorowitz RD, Croft JB, Magnis E, Mulligan D, Jagoda A, O’Connor R, Cowley CM, Connors JJ, Rose-DeRenzy JA, Emr M, Warren M, Welker MD. Brain Attack Coalition. Recommendations for comprehensive stroke centers: a consensus statement from the Brain Attack Coalition. Stroke. 2005;36:1597–1616. doi: 10.1161/01.STR.0000170622.07210.b4. [DOI] [PubMed] [Google Scholar]
- 3.Adams HP, Jr, del Zoppo G, Alberts MJ, Bhatt DL, Brass L, Furlan A, Grubb RL, Higashida RT, Jaunch EC, Kidwell C, Lyden PD, Morgenstern LB, Qureshi AI, Rosenwasser RH, Scott PA, Wijdicks EF. American Heart Association, American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisiplinary Working Group. Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardio-vascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: the American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Stroke. 2007;38:1655–1711. doi: 10.1161/STROKEAHA.107.181486. [DOI] [PubMed] [Google Scholar]
- 4.Lewandowski CA, Frankel M, Tomsick TA, Broderick J, Frey J, Clark W, Starkman S, Grotta J, Spielker J, Khoury J, Brott T. Combined intravenous and intra-arterial r-TPA versus intra-arterial therapy of acute ischemic stroke: Emergency Management of Stroke (EMS) Bridging Trial. Stroke. 1999;30:2598–2605. doi: 10.1161/01.str.30.12.2598. [DOI] [PubMed] [Google Scholar]
- 5.Nakajima M, Kimura K, Ogata T, Takada T, Uchino M, Minematsu K. Relationships between angiographic findings and National Institutes of Health stroke scale score in cases of hyperacute carotid ischemic stroke. AJNR Am J Neuroradiol. 2004;25:238–241. [PMC free article] [PubMed] [Google Scholar]
- 6.Fischer U, Arnold M, Nedeltchev K, Brekenfeld C, Ballinari P, Remondo L, Schroth G, Mattle HP. NIHSS score and arteriographic findings in acute ischemic stroke. Stroke. 2005;36:2121–2125. doi: 10.1161/01.STR.0000182099.04994.fc. [DOI] [PubMed] [Google Scholar]
- 7.Combined intravenous and intra-arterial recanalization for acute ischemic stroke: the Interventional Management of Stroke Study. Stroke. 2004;35:904–911. doi: 10.1161/01.STR.0000121641.77121.98. [DOI] [PubMed] [Google Scholar]
- 8.Llanes JN, Kidwell CS, Starkman S, Leary MC, Eckstein M, Saver JL. The Los Angeles Motor Scale (LAMS): a new measure to characterize stroke severity in the field. Prehosp Emerg Care. 2004;8:46–50. doi: 10.1080/312703002806. [DOI] [PubMed] [Google Scholar]
- 9.2005 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care, Part 9: Adult Stroke. Circulation. 2005;112:IV-111–IV-120. doi: 10.1161/CIRCULATIONAHA.105.166550. [DOI] [PubMed] [Google Scholar]
- 10.Ferguson K, Kidwell CS, Starkman S, Eckstein M, Saver JL. Inter-rater and intra-rater reliability of the Los Angeles Motor Scale (LAMS), a prehospital measure of stroke severity. Stroke. 2002;33:384. Abstract. [Google Scholar]
- 11.Saver JL, Starkman S, Hills NK, Johnston SC. CASPR Investigators. Prospective, multicenter validation of a rapid stroke severity measure for prehospital use. Stroke. 2006;37:655. Abstract. [Google Scholar]
- 12.Rajajee V, Kidwell C, Starkman S, Ovbiagale B, Alger JR, Villablanca P, Vinuela F, Duckwiler G, Jahan R, Fredieu A, Suzuki S, Saver JL. Early MRI and outcomes of untreated patients with mild or improving ischemic stroke. Neurology. 2006;67:980–984. doi: 10.1212/01.wnl.0000237520.88777.71. [DOI] [PubMed] [Google Scholar]
- 13.Smith WS, Corry MD, Fazackerley J, Isaacs M. Paramedic accuracy in the application of the NIH Stroke Scale to victims of stroke. Acad Emerg Med. 1997;4:379–380. [Google Scholar]
- 14.Tirschwell DL, Longstreth WT, Jr, Becker KJ, Gammans RE, Sr, Sabounjian LH, Hamilton S, Morgenstern LB. Shortening the NIH Stroke Scale for use in the prehospital setting. Stroke. 2002;33:2801–2806. doi: 10.1161/01.str.0000044166.28481.bc. [DOI] [PubMed] [Google Scholar]
- 15.Derex L, Nighoghossian N, Hermier M, Adeleine P, Froment JC, Trouillas P. Early detection of cerebral arterial occlusion on magnetic resonance angiography: predictive value of the baseline NIHSS score and impact on neurological outcome. Cerebrovasc Dis. 2002;13:225–229. doi: 10.1159/000057847. [DOI] [PubMed] [Google Scholar]