Abstract
In this study, we assessed the distribution of cortical neurons immunoreactive for tyrosine hydroxylase (TH) in prefrontal cortical regions of humans and nonhuman primate species. Immunohistochemical methods were used to visualize TH-immunoreactive (TH-ir) neurons in areas 9 (dorsolateral prefrontal cortex) and 32 (anterior paracingulate cortex). The study sample included humans, great apes (chimpanzee, bonobo, gorilla, orangutan), one lesser ape (siamang), and Old World monkeys (golden guenon, patas monkey, olive baboon, moor macaque, black and white colobus, and François' langur). The percentage of neurons within the cortex expressing TH was quantified using computer-assisted stereology. TH-ir neurons were present in layers V and VI and the subjacent white matter in each of the Old World monkey species, the siamang, and in humans. TH-ir cells were also occasionally observed in layer III of human, siamang, baboon, colobus, and François' langur cortex. Cortical cells expressing TH were notably absent in each of the great ape species. Quantitative analyses did not reveal a phylogenetic trend for percentage of TH-ir neurons in these cortical areas among species. Interestingly, humans and monkey species exhibited a bilaminar pattern of TH-ir axon distributions within prefrontal regions, with layers I-II and layers V-VI having the densest contingent of axons. In contrast, the great apes had a different pattern of laminar innervation, with a remarkably denser distribution of TH-ir axons within layer III. It is possible that the catecholaminergic afferent input to layer III in chimpanzees and other great apes covaries with loss of TH-ir cells within the cortical mantle.
Keywords: dopamine, area 9, area 32, human evolution
Tyrosine hydroxylase (TH) is the rate-limiting enzyme in catecholamine synthesis. The major CNS catecholamines (dopamine and norepinephrine) are each involved in supporting certain aspects of higher cognition by modulating prefrontal cortex function (von Bohlen und Halbach and Dermietzel, 2006). For example, the dopaminergic system is well known for its role in working memory (Abi-Dargham, 2004; Goldman-Rakic, 1998; Kulisevsky, 2000), and norepinephrine supports attention and memory retrieval in the absence of a stress response (Kodama et al., 2002; Murchison et al., 2004).
The prefrontal cortex receives catecholaminergic innervation from long projection neurons that originate in the ventral tegmental area and locus coeruleus. However, there also exists an intrinsic source of catecholamines in the form of tyrosine hydroxylase-immunoreactive (TH-ir) neurons within the neocortex itself. These cortical neurons have been classified as aspiny non-pyramidal cells (Benavides-Piccione and DeFelipe, 2003) and demonstrate considerable species-specific variation in their location and concentration in the cortical mantle (for review see Smeets and González, 2000). For example, TH-ir neurons were reported in the lower portion of layer I in two cetacean species (Hof et al., 1995), whereas TH-ir cells in rats have been found in all layers of the cortex, with the highest density occurring in layers II and III (Kosaka et al., 1987a). In the human neocortex, TH-ir interneurons have been found almost exclusively in the infragranular layers and subjacent white matter (Benavides-Piccione and DeFelipe, 2003; Gaspar et al., 1987; Hornung et al., 1989).
Recently, Benavides-Piccione and DeFelipe (2007) reported that humans had a higher density and more extensive distribution of cortical TH-ir neurons relative to other species studied to date. Humans were the only species to possess TH-ir neurons in every cortical area, whereas macaque monkeys lacked TH-ir neurons in temporal cortex (Benavides-Piccione and DeFelipe, 2007; Kohler et al., 1983). Moreover, the highest densities of TH-ir neurons were found in the dorsolateral prefrontal and anterior cingulate cortex in humans, both areas critical to higher cognitive functions (Benavides-Piccione and DeFelipe, 2007). In addition, recent studies have found that there is a significant decrease in cortical TH-ir neurons in the brains of patients affected by dementia with Lewy bodies (Marui et al., 2003) and by Parkinson's disease (Fukuda et al., 1999). This evidence suggests that this population of cortical catecholaminergic neurons is functionally significant for intrinsic local circuits. Because humans have a dense and ubiquitous distribution of TH-ir neurons, it is tempting to speculate that these cells might have played a role in the evolution of the human brain and its associated cognitive and behavioral advances. However, the presence of TH-ir neurons in the cortex of most primate species, including the great apes, is unknown. These comparative data are necessary for evaluating potential human-specific neuroanatomical specializations. The main objective of the present research was to examine the distribution of TH-ir neurons in prefrontal cortical areas, specifically Brodmann areas 9 and 32, among a wide range of catarrhine primate species, including great apes, lesser apes, and Old World monkeys.
EXPERIMENTAL PROCEDURES
Specimens
The nonhuman brain specimens for this research included 27 individuals representing 11 anthropoid species. All specimens with the exception of one (François' langur) were from adult individuals and all brain samples were free of gross neuropathology on routine examination. The postmortem nonhuman brains were acquired from zoological or research institutions and the animals were housed according to each institution's guidelines. Specifically, all animals had been maintained in AALAC- or AZA-accredited facilities and died natural deaths or were humanely euthanized as part of unrelated experiments to minimize the number of animals used for study. The golden guenon brain was provided by the Office Rwandais du Tourisme et des Parcs Nationaux and the Mountain Gorilla Veterinary Project in compliance with CITES regulations. Adult, non-geriatric human brain specimens were provided by Northwestern University Alzheimer's Disease Center Brain Bank. The human subjects showed no evidence of dementia before death and all individuals received a score of zero for the CERAD senile plaque grade (Mirra et al., 1991) and the Braak and Braak (1991) neurofibrillary tangle stage. The age, sex, and method of fixation for each specimen can be found in Table 1.
Table 1.
Sample used in this study
| Group | Species | Common name | Sex | Age | Fixation |
|---|---|---|---|---|---|
| Humans | Homo sapiens | Human | F | 40 | I |
| Homo sapiens | Human | F | 43 | I | |
| Homo sapiens | Human | F | 53 | I | |
| Homo sapiens | Human | M | 35 | I | |
| Homo sapiens | Human | M | 48 | I | |
| Homo sapiens | Human | M | 54 | I | |
| Great apes | Pan troglodytes | Chimpanzee | F | 19 | I |
| Pan troglodytes | Chimpanzee | F | 27 | I | |
| Pan troglodytes | Chimpanzee | F | 35 | I | |
| Pan troglodytes | Chimpanzee | M | 17 | I | |
| Pan troglodytes | Chimpanzee | M | 19 | I | |
| Pan troglodytes | Chimpanzee | M | 41 | I | |
| Pan paniscus | Bonobo | F | 25 | I | |
| Gorilla gorilla | Gorilla | F | 50 | I | |
| Gorilla gorilla | Gorilla | M | 13 | I | |
| Pongo pygmaeus | Orangutan | M | 11.5 | I | |
| Pongo pygmaeus | Orangutan | M | 33.7 | I | |
| Pongo pygmaeus | Orangutan | M | 39 | I | |
| Lesser ape | Symphalangus syndactylus | Siamang | M | 33 | I |
| Cercopithecines | Papio anubis | Olive baboon | M | 10 | P |
| Papio anubis | Olive baboon | M | 11 | P | |
| Macaca maura | Moor macaque | F | 5 | P | |
| Macaca maura | Moor macaque | F | 7 | P | |
| Macaca maura | Moor macaque | F | 7 | P | |
| Macaca maura | Moor macaque | F | 8 | P | |
| Macaca maura | Moor macaque | M | 8 | P | |
| Macaca maura | Moor macaque | M | 10 | P | |
| Cercopithecus kanti | Golden guenon | M | Adult | I | |
| Erythrocebus patas | Patas monkey | M | 12 | P | |
| Erythrocebus patas | Patas monkey | M | 12 | P | |
| Colobines | Trachypithecus francoisi | François' langur | M | 1 | I |
| Trachypithecus francoisi | François' langur | M | 16 | I | |
| Colobus angolensis | Black and white colobus | M | 18 | I |
Group reflects major taxonomic group. Age is in years.
Abbreviations: I, immersion-fixed; P, perfused.
Fixation
Baboons, moor macaques, and patas monkeys were perfused transcardially with 4% paraformaldehyde as part of unrelated experiments following methods described previously (Hof and Nimchinsky, 1992; Hof et al., 1996). All other brains were collected postmortem and fixed by immersion in 10% buffered formalin for at least 10 days, then transferred to a 0.1 M phosphate-buffered saline (PBS, pH 7.4) solution containing 0.1% sodium azide and stored at 4 °C to prevent further tissue shrinkage and antigen blockade. The postmortem interval (PMI) prior to fixation for immersion-fixed brains never exceeded 14 h. For human cases, the PMI ranged from 6 to 17 h.
Sample processing
All samples derived from the left hemisphere. Prior to sectioning, samples were cryoprotected by immersion in a series of sucrose solutions (10%, 20%, and 30%).
For the nonhuman primate brains, the entire frontal lobe was removed as a coronal slab. Human brain specimens were dissected out of the cortical areas of interest by the donating institution.
Brain specimens were frozen on dry ice and cut to 40 μm-thick sections using a sliding microtome. As each block of tissue was cut, sections were placed into individual microcentrifuge tubes containing freezer storage solution (30% each distilled water, ethylene glycol, and glycerol and 10% 0.244 M PBS) and numbered sequentially. Sections were stored at -20 °C until immunohistochemical processing.
A 1-in-10 series for all samples was stained for Nissl substance with a solution of 0.5% Cresyl Violet to reveal cell somata. Nissl-stained sections were used to identify cytoarchitectural boundaries and to obtain neuron densities. Cortical regions of interest were identified based on topological location and distinctive regional cytoarchitecture recognizable on Nissl-stained sections. Cytoarchitectural features were relied upon for identification of cortical regions due to individual variation in their gross anatomical location (e.g. Amunts et al., 1996; Petrides and Pandya, 1999; Rademacher et al., 2001; Zilles et al., 1996). Area 9 is located in the dorsolateral prefrontal cortex, extending medially to the paracingulate sulcus of humans and the cingulate sulcus of macaque monkeys (Paxinos et al., 2000; Petrides and Pandya, 1999). This cortical area is expanded in anthropoids (i.e. monkeys, apes, and humans) with no obvious homologue in other mammals (Preuss and Goldman-Rakic, 1991; Aboitiz and Garcia, 1997). For this study, the part of area 9 sampled was located on the dorsal portion of the superior frontal gyrus in humans and chimpanzees, and corresponded to area 9L in macaque monkeys as designated by Paxinos et al. (2000). This area has been identified in numerous primate species, including macaques (Walker, 1940; von Bonin and Bailey, 1947; Petrides and Pandya, 1999), hamadryas baboons (Watanabe-Sawaguchi et al., 1991), chimpanzees (Bailey et al., 1950), and humans (Rajkowska and Goldman-Rakic, 1995; Petrides and Pandya, 1999). Additionally, area 9L was outlined in many of the individuals used in this study as part of unrelated projects (Raghanti et al., 2008; Sherwood et al., 2006).
Area 32 is defined as the portion of the paracingulate cortex anterior to the genu of the corpus callosum (Gallagher and Frith, 2003; Öngür et al., 2003). In humans, area 32 has been implicated in `theory of mind,' the ability to infer the mental states of others (Adolphs, 2001; Gallagher et al., 2000; Gallagher and Frith, 2003; Johnson et al., 2002; Vogeley et al., 2001). Old World monkeys do not possess a strict homologue to the portion of area 32 that is activated in human theory of mind studies (i.e. anterior cingulate area 32) (Öngür et al., 2003), thus we were limited to the most similar anatomical territory of the medial prefrontal cortex of monkeys, defined as prelimbic cortex (i.e. prelimbic area 32) (Öngür et al., 2003). In chimpanzees, the cytoarchitecture of cortex within the anterior paracingulate gyrus was described by Bailey et al. (1950) closely to resemble area FDL in humans (von Economo and Koskinas, 1925), suggesting that they are homologous in structure.
Immunohistochemistry
A minimum of two sections that contained the cortical areas of interest per individual were used for immunohistochemical studies. Floating tissue sections were stained using the avidin-biotin-peroxidase method, as described previously (Raghanti et al., 2008). Briefly, sections were pretreated for antigen retrieval by incubating in 10 mM sodium citrate buffer (pH 3.5) at 37 °C for 30 min. Sections were then rinsed and endogenous peroxidase was quenched using a solution of 75% methanol, 2.5% hydrogen peroxide (30%), and 22.5% distilled water for 20 min at room temperature. Sections were preblocked in a solution of PBS with 2% normal goat serum and 0.3% Triton X-100 detergent. Following this, sections were incubated in primary antibody (rabbit anti-TH polyclonal antibody, AB152, Millipore, Billerica, MA, USA) diluted to 1:1000 in PBS for 48 h at 4 °C. After incubation in primary antibody, the tissue was incubated in biotinylated secondary antibody (1:200) in a solution of PBS and 2% normal goat serum for 1 h at room temperature. Sections were then incubated in avidin-peroxidase complex (PK-6100, Vector Laboratories, Burlingame, CA, USA) for 1 h at room temperature. A 3,3′-diaminobenzidine-peroxidase substrate with nickel solution enhancement was used as the chromogen (SK-4100, Vector Laboratories). Negative controls omitted the primary antibody and omitted the secondary antibody. Omission of the primary or secondary antibody resulted in a complete absence of labeled neurons and axons.
Data collection and analysis
Immunohistochemically stained sections were qualitatively and quantitatively analyzed using an Olympus BX-51 photomicro-scope equipped with a Ludl XY motorized stage, Heidenhain z-axis encoder, StereoInvestigator software (MBF Bioscience, Williston, VT, USA, version 8), and a digital camera that projects images onto a 24-inch LCD flat panel monitor. The presence or absence and the laminar distribution of TH-ir neurons were recorded for each section. Character state evolution was mapped on to a phylogeny of primates from Goodman et al. (1998). The evolution of TH-ir cell distribution was reconstructed using maximum parsimony analysis with character state transformations unordered as implemented in Mesquite software version 1.06 (Maddison and Maddison, 2005). Characters were coded based on direct qualitative observation for the species examined in the current study.
TH-ir neuron densities were obtained by exhaustively sampling the cortical areas of interest from pia to white matter (i.e. cortex) and subjacent white matter with the aid of an optical disector. The cortex and white matter were outlined separately at low magnification (4×), and the optical disector was performed at 40×. A guard zone of at least 2 μm was employed at the top and bottom of each section, and section thickness was measured at five random sites per area of interest (i.e. cortex and white matter). TH-ir neuron density (Nv) was calculated as the total number of TH-ir neurons divided by the product of surface area (x, y), the tissue sampling fraction, and the sectioned thickness (40 μm). The tissue sampling fraction was the ratio of the optical disector height to mean measured section thickness.
Nissl-stained sections were used to obtain overall Nv (neuron Nv) measures using optical disector probes combined with a fractionator sampling scheme (see Raghanti et al., 2008). The cortex from layer II to the white matter was outlined at low magnification. The optical disector probes were performed under Koehler illumination at 63×. A guard zone of at least 2 μm was employed at the top and bottom of each section, and section thickness was measured at every 5th sampling site. Neuron densities were calculated as the sum of neurons counted with the optical disectors divided by the product of the disectors and the volume of the disector (see Sherwood et al., 2005).
The ratio of TH-ir Nv to total Nv represents the percentage of neurons that expressed TH immunoreactivity. The percentage of TH-ir neurons was used for comparisons among species because overall cell density per unit volume varies with differences in brain size (Haug, 1987; Sherwood et al., 2007). Thus, the percentage of TH-ir neurons functions to standardize the data for the evaluation of TH-ir Nv in the context of species differences in overall Nv. Variation in the number of individuals available to represent each species and small sample size precluded the use of a fully factorial statistical design for data analysis. However, to determine if the percentage of TH-ir cortical neurons was correlated to brain weight, Spearman's rho correlation coefficients were calculated for each cortical area. Brain weight data used for this analysis were taken from Sherwood et al. (2006) and Raghanti et al. (2008). Statistical significance was set at α=0.05, two-tailed.
Original photomicrographs were processed using Adobe Photoshop, v. 7.0 (San Jose, CA, USA). Brightness, contrast, and sharpness were adjusted to obtain images that most closely resembled the appearance of histological features as seen through the microscope. Additional figures were produced by obtaining montage images of the cortical areas at low magnification and tracing the TH-ir neurons using Adobe Photoshop software.
RESULTS
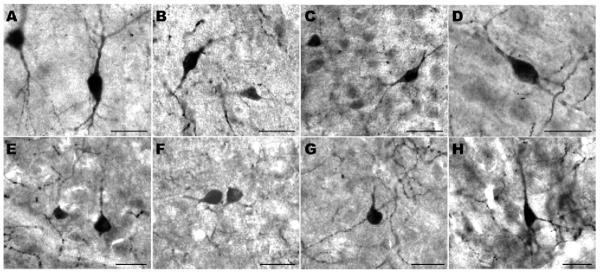
Previous morphological characterization of cortical TH-ir neurons in humans and other species indicated that most are bipolar, with other cell types being single-tufted, bitufted, tripolar, and multipolar (e.g. Benavides-Piccione and DeFelipe, 2003, 2007). Our present findings among catarrhine primates are in accordance with these earlier assessments in that neuron types conformed in morphological appearance to inhibitory interneurons with multiple subtypes represented and a predominance of bipolar neurons (Fig. 1). Fig. 2A-D shows the distributions of TH-ir neurons for each species within each cortical area.
Fig. 1.
Examples of TH-ir neurons in the investigated species. Panels show neurons in (A) human, (B) siamang, (C) baboon, (D) macaque, (E) golden guenon, (F) patas monkey, (G) François' langur, and (H) colobus. Scale bars=25 μm
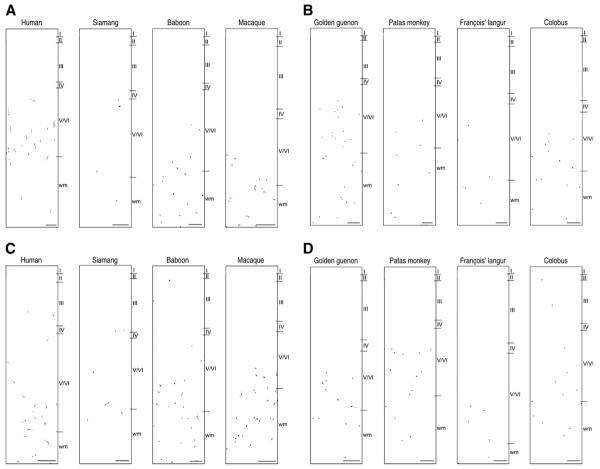
Fig. 2.
TH-ir neuron tracings in area 9 (A, B) and area 32 (C, D). Scale bars=250 μm
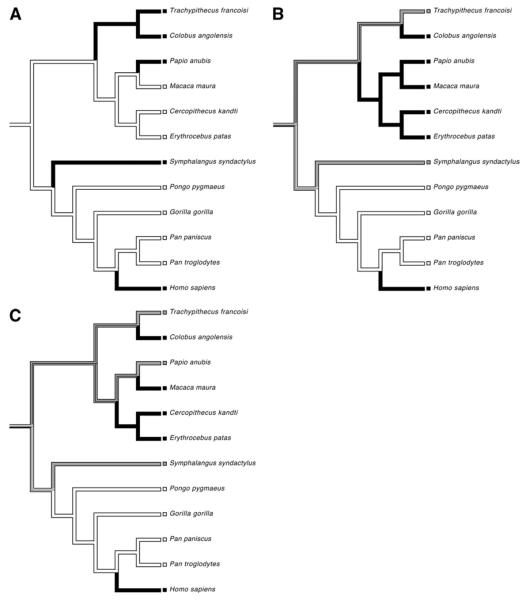
The results of the maximum parsimony analysis of character state evolution are depicted in Fig. 3A-C. Among the Old World monkeys, the cercopithecines (cheek-pouched monkeys; golden guenon, patas monkey, moor macaque and baboon) and the colobines (leaf-eating monkeys; François' langur and colobus) are each distinct subfamilies (Goodman et al., 1998). African apes (gorilla, chimpanzee, bonobo) are more closely related to humans than the Asian apes (orangutan, siamang), with chimpanzees and bonobos representing our closest living relatives.
Fig. 3.
Phylogenetic trees demonstrating the occurrence of TH-ir cells in (A) layer III, (B) layers V-VI, and (C) white matter. In (A) black bars indicate the presence of TH-ir cells and white bars indicate their absence. In both (B) and (C), white bars represent an absence of TH-ir cells, gray bars indicate sparse presence, and black bars are used to indicate a denser distribution of cells
The presence of TH-ir neurons in the supragranular layers was restricted to humans, siamangs, baboons, and the leaf monkeys (i.e. black and white colobus and François' langur) (Fig. 3A). In each of these cases, TH-ir neurons were sparsely distributed and most frequently located in layer III. TH-ir neurons were also distributed within the infragranular layers (Fig. 3B) and subjacent white matter (Fig. 3C) of all species studied with the exception of the great apes. However, their density was not uniform across species. Rather, the degree of layer-specific concentration was variable from one species to the next, with no obvious phylogenetic trend. TH-ir neurons were notably absent in chimpanzee, bonobo, gorilla, and orangutan cortex. Considering the phylogenetic relationships among these species, it is most parsimonious to infer that the last common ancestor of catarrhines possessed TH-ir neurons in the neocortex. This trait was subsequently lost in the ancestor of the great ape-human clade. Remarkably, there was a reversal of this character state during human evolution. Thus, humans and Old World monkeys share TH-ir neocortical neurons due to homoplasy, or the independent evolution of a similar phenotype.
The results of quantitative analyses are listed in Tables 2 and 3. For the species that had TH-ir neurons, the percentage of cortical neurons immunoreactive for TH was not correlated with brain weight in area 9 (Spearman's rho=-/-0.38, P=0.35, n=8) or area 32 (Spearman's rho=-0.31, P=0.45, n=8). Furthermore, neither TH-ir Nv nor the percentage of cortical neurons expressing TH showed a relationship to phylogeny.
Table 2.
TH-ir neuron density per mm3
| Area 9 |
Area 32 |
|||
|---|---|---|---|---|
| Species | wm | Cortex | wm | Cortex |
| François' langur | 274.33±86.36 | 90.94±34.52 | 172.05±32.94 | 86.82±18.72 |
| Colobus | 405.22 | 272.11 | 712.42 | 266.37 |
| Baboon | 866.43±30.30 | 334.89±99.57 | 977.35±580.29 | 520.14±211.33 |
| Macaque | 864.47±302.11 | 209.09±122.18 | 896.68±355.34 | 294.96±125.02 |
| Golden guenon | 669.70 | 435.35 | 468.76 | 607.04 |
| Patas monkey | 927.34±601.44 | 272.84±219.01 | 972.12±255.00 | 479.91±84.93 |
| Siamang | 258.73 | 75.02 | 312.18 | 113.24 |
| Human | 177.41±28.80 | 121.04±5.11 | 198.05±60.69 | 174.04±25.88 |
The mean is reported for each species ±S.D. for species represented by two or more individuals.
Table 3.
Percentage of cortical neurons expressing TH for each species ± S.D. where two or more individuals are represented
| Species | Area 9 | Area 32 |
|---|---|---|
| François' langur | 0.0014±0.0003 | 0.0014±0.0002 |
| Colobus | 0.0041 | 0.0032 |
| Baboon | 0.0052±0.0008 | 0.0090±0.0012 |
| Macaque | 0.0020±0.0011 | 0.0034±0.0011 |
| Golden guenon | 0.0058 | 0.0093 |
| Patas monkey | 0.0023±0.0018 | 0.0034±0.0007 |
| Siamang | 0.0018 | 0.0016 |
| Human | 0.0019±0.0002 | 0.0026±0.0006 |
DISCUSSION
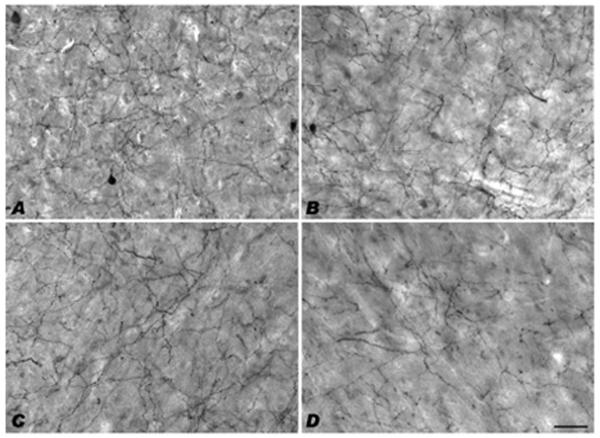
The present results represent the first large-scale comparative analysis of TH-ir neuron distributions among primate species. Cortical TH-ir neurons were observed in 8 of the 12 species assessed here, with TH-ir neurons notably absent in the great ape cortical regions examined (chimpanzee, bonobo, gorilla, and orangutan). These results are not likely to have been affected by differences in fixation because several immersion-fixed monkey and human brain specimens displayed TH-ir neurons, while they were consistently absent in the immersion-fixed great apes. Moreover, while the great apes lacked TH-ir neurons, staining of TH-ir axons was present and robust (Fig. 4). Among the Old World monkeys, the laminar distributions and densities were species-specific without a clear concordance to phylogenetic relationships. For example, while both representatives of the subfamily Colobinae (François' langur and black and white colobus) exhibited TH-ir neurons in layers III, V-VI, and in the white matter, the langur had fewer neurons overall relative to the colobus (see Fig. 2B, D and Table 3). When examining the density of TH-ir neurons within area 9, the colobus, golden guenon, and baboon had a greater percentage of TH-ir neurons relative to humans (see Table 3). Further, when compared with humans, both the baboon and golden guenon have a higher percentage of TH-ir neurons within area 32.
Fig. 4.
Examples of TH immunostaining in monkeys and great apes. Panels show brightfield photomicrographs taken of the infragranular layers of area 9 for (A) François' langur, (B) colobus, (C) chimpanzee, and (D) bonobo. Scale bar=50 μm (D)
TH-ir neurons within the human neocortex were initially thought to be dopaminergic, as they do not express dopamine-ß-hydroxylase, the enzyme required for norepinephrine synthesis (Gaspar et al., 1987; Fukuda et al., 1999). However, TH-ir neurons in the human anterior cingulate cortex also do not colocalize aromatic amino acid decarboxylase (AADC), an enzyme requisite for dopamine synthesis (Ikemoto et al., 1999). Whereas these findings suggest that TH-ir neurons are neither dopaminergic nor norepinephrinergic, it is possible that the sensitivity of current immunohistochemical detection methods precludes accurate characterization (Benavides-Piccione and DeFelipe, 2007). Although TH-ir neurons have been noted in several species, little is known regarding their function, particularly as the end-product is yet to be defined. Colocalization studies in humans revealed that only half of the cortical TH-ir neurons also express GABA (Trottier et al., 1989), and 24% express nitric oxide synthase (Benavides-Piccione and DeFelipe, 2003). In contrast, most TH-ir neurons of the rat cortex are also immunoreactive for either GABA or glutamic acid decarboxylase (GAD), the enzyme that catalyzes the reaction required for GABA production (Kosaka et al., 1987b), and the detection of phosphorylated TH in the majority of rat TH-ir cortical neurons suggests L-DOPA as a potential end-product for this species (Asmus et al., 2008). Weihe and colleagues (2006) recently identified two TH-ir neuron types in the rhesus macaque cortex in addition to the traditional dopamine- and norepinephrine-producing neurons. Their classification was based on colocalization studies with the vesicular uptake system necessary for concentration and exocytotic release (VMAT2) and AADC. Interestingly, Weihe et al. (2006) found TH-ir neurons that lacked VMAT2 and others that lacked both VMAT2 and AADC. This indicates that specific populations of TH-ir neurons have the ability to produce dopamine without a capacity for exocytotic release and others that appear to have L-DOPA as an end-product, potentially to supply monoenzymatic AADC-containing neurons for the synthesis of dopamine.
The unusual phylogenetic pattern of TH-ir neuron distribution is difficult to interpret, particularly in light of the fact that the end-product of these cells is yet to be determined. Neurons that express TH may produce L-DOPA, dopamine, or norepinephrine, depending on the complex of enzymes present within the cell. Although progress has been made toward the identification of end-products and phenotypic characterizations for cortical TH-ir cells in rats (Asmus et al., 2008) and rhesus macaques (Weihe et al., 2006), the reported differences between rodents and primates in colocalization studies as well as the present findings indicate that definitive studies may not be applicable across different species. This is especially important in the context of our current results, suggesting that cortical TH-ir neurons have evolved independently multiple times in the course of primate diversification.
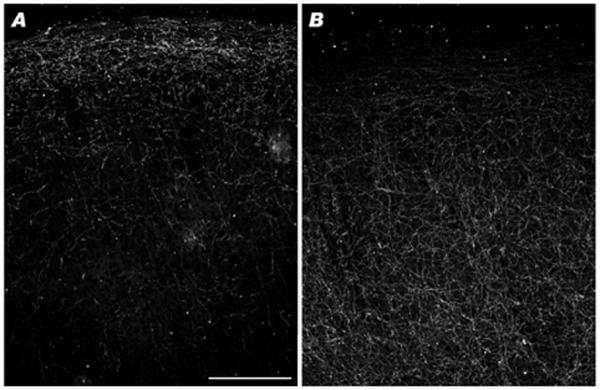
The absence of TH-ir neurons within the cortex of the great apes is intriguing. Our reconstruction of character state evolution indicates that TH-ir neurons were lost in the ancestor of the great ape-human clade. Of note, Raghanti et al. (2008) reported that the chimpanzee ratio of TH-ir axon length density to Nv in layer III of areas 9 and 32 was twice that of humans or moor macaques (Fig. 5). Although the limited sample available for the present study precluded robust quantitative analyses, it is possible that all of the great apes share an increase in TH-ir axons originating from extrinsic cortical sources. If so, then great apes may compensate for the loss of the intrinsic cortical source of TH by increasing TH supplied by extrinsic afferents. TH-ir axons in human and long-tailed macaque cerebral cortex are thought to be dopaminergic as TH and the dopamine transporter are extensively colocalized (Ciliax et al., 1999; Lewis et al., 2001). In addition, TH and the enzyme for norepinephrine synthesis, dopamine-β-hydroxylase, do not colocalize in the cortex of long-tailed macaques or the New World squirrel monkey (Lewis et al., 1987), also supporting the contention that cortical TH-ir axons are dopaminergic in primates. However, comparable studies have not been completed for the great apes and it is possible that a contingent of the cortical TH-ir axons would colocalize TH and dopamine-β-hydroxylase rather than the dopamine transporter. For example, cortical TH-ir axons are primarily noradrenergic in rabbits (Wang et al., 1996), demonstrating that there is not uniformity in the biochemistry of TH-ir neurons among species.
Fig. 5.
Darkfield images showing layers I-III of area 32 in (A) macaque and (B) chimpanzee, illustrating the differences in TH-ir axon densities in layer III. Scale bar=250 μm (A)
Benavides-Piccione and DeFelipe (2007) speculated that cortical TH-ir neurons within the human cortex may represent an evolutionary adaptation, facilitating specialized cortical circuits. In this context, the present findings raise questions regarding the absence of cortical TH-ir neurons within the cortex of our closest living relatives, the great apes, and the abundance of these cells within the cortex of some Old World monkeys. Further work is necessary to characterize the molecular biology of TH-ir neurons among different species and to determine their functional role within cortical circuits. In particular, it will be important to examine extrinsic cortical catecholaminergic sources in addition to a full characterization of mono- and multienzymatic interneurons that may facilitate the role of the TH-ir neurons in the cerebral cortex. Finally, distributions of neocortical cell types are not uniform across phylogeny. In the current study, we demonstrated variation in the expression of TH in prefrontal neurons among closely related taxa. Such findings highlight the importance of evaluating potential neuroanatomical features that may have predisposed the human brain to increasing cognitive complexity within an evolutionary framework.
Acknowledgments
This work was supported by the National Science Foundation (BCS-0515484 and BCS-0549117), National Institutes of Health (NS42867), the Wenner-Gren Foundation for Anthropological Research, and the James S. McDonnell Foundation (22002078). Brain material used in this study was loaned by the Great Ape Aging Project (USPHS/NIH grant AG14308, “A Comparative Neurobiology of Aging Resource,” J. Erwin, PI), the Foundation for Comparative and Conservation Biology, Office Rwandais du Tourisme et des Parcs Nationaux (Dr. Antoine Mudakikwa), the Mountain Gorilla Veterinary Project (Dr. Mike Cranfield), and the Northwestern University Alzheimer's Disease Center Brain Bank. This study was supported in part by an Alzheimer's Disease Core Center grant (P30 AG13854) from the National Institute of Aging to Northwestern University, Chicago, Illinois. We gratefully acknowledge the assistance of the Neuropathology Core.
Abbreviations
- AADC
amino acid decarboxylase
- Nv
neuron density
- PBS
phosphate-buffered saline
- PMI
postmortem interval
- TH
tyrosine hydroxylase
- TH-ir
tyrosine hydroxylase-immunoreactive
REFERENCES
- Abi-Dargham A. Do we still believe in the dopamine hypothesis? New data bring new evidence. Int J Neuropsychopharmacol. 2004;7:S1–S5. doi: 10.1017/S1461145704004110. [DOI] [PubMed] [Google Scholar]
- Aboitiz F, Garcia GL. The evolutionary origin of language areas in the human brain. Brain Res Rev. 1997;25:381–396. doi: 10.1016/s0165-0173(97)00053-2. [DOI] [PubMed] [Google Scholar]
- Adolphs R. The neurobiology of social cognition. Curr Opin Neurobiol. 2001;11:231–239. doi: 10.1016/s0959-4388(00)00202-6. [DOI] [PubMed] [Google Scholar]
- Amunts K, Schlaug G, Schleicher A, Steinmetz H, Dabringhaus A, Roland PE, Zilles K. Asymmetry in the human motor cortex and handedness. Neuroimage. 1996;4:216–222. doi: 10.1006/nimg.1996.0073. [DOI] [PubMed] [Google Scholar]
- Asmus S, Anderson E, Ball M, Barnes B, Bohnen A, Brown A, Hartley L, Lally M, Lundblad T, Martin J, Moss B, Phelps K, Phillips L, Quilligan C, Steed R, Terrell S, Warner A. Neurochemical characterization of tyrosine-hydroxylase-immunoreactive interneurons in the developing rat cerebral cortex. Brain Res. 2008;1222:95–105. doi: 10.1016/j.brainres.2008.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey P, von Bonin G, McCulloch WS. The isocortex of the chimpanzee. University of Illinois Press; Urbana, IL: 1950. [Google Scholar]
- Benavides-Piccione R, DeFelipe J. Different populations of tyrosine-hydroxylase-immunoreactive neurons defined by differential expression of nitric oxide synthase in the human temporal cortex. Cereb Cortex. 2003;13:297–307. doi: 10.1093/cercor/13.3.297. [DOI] [PubMed] [Google Scholar]
- Benavides-Piccione R, DeFelipe J. Distribution of neurons expressing tyrosine hydroxylase in the human cerebral cortex. J Anat. 2007;211:212–222. doi: 10.1111/j.1469-7580.2007.00760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological staging of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Ciliax BJ, Drash GW, Staley JK, Haber S, Mobley CJ, Miller GW, Mufson EJ, Mash DC, Levey AI. Immunocytochemical localization of the dopamine transporter in human brain. J Comp Neurol. 1999;409:38–56. doi: 10.1002/(sici)1096-9861(19990621)409:1<38::aid-cne4>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Fukuda T, Takahashi J, Tanaka J. Tyrosine hydroxylase-immunoreactive neurons are decreased in number in the cerebral cortex of Parkinson's disease. Neuropathology. 1999;19:10–13. doi: 10.1046/j.1440-1789.1999.00196.x. [DOI] [PubMed] [Google Scholar]
- Gallagher H, Happé F, Brunswick N, Fletcher P, Frith U, Frith C. Reading the mind in cartoons and stories: an fMRI study of `theory of mind' in verbal and nonverbal tasks. Neuropsychologia. 2000;38:11–21. doi: 10.1016/s0028-3932(99)00053-6. [DOI] [PubMed] [Google Scholar]
- Gallagher HL, Frith CD. Functional imaging of `theory of mind.'. Trends Cogn Neurosci. 2003;7:77–83. doi: 10.1016/s1364-6613(02)00025-6. [DOI] [PubMed] [Google Scholar]
- Gaspar P, Berger B, Febvret A, Vigny A, Krieger-Poulet M, Borri-Voltattorni C. Tyrosine hydroxylase-immunoreactive neurons in the human cerebral cortex: a novel catecholaminergic group? Neurosci Lett. 1987;80:257–262. doi: 10.1016/0304-3940(87)90464-2. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. The cortical dopamine system: Role in memory and cognition. Adv Pharmacol. 1998;42:707–711. doi: 10.1016/s1054-3589(08)60846-7. [DOI] [PubMed] [Google Scholar]
- Goodman M, Porter CA, Czelusniak J, Page SL, Schneider H, Shoshani J, Gunnell G, Groves CP. Toward a phylogenetic classification of primates based on DNA evidence complemented by fossil evidence. Mol Phylogenet Evol. 1998;9:585–598. doi: 10.1006/mpev.1998.0495. [DOI] [PubMed] [Google Scholar]
- Haug H. Brain sizes, surfaces, and neuronal sizes of the cortex cerebri: a stereological investigation of man and his variability and a comparison with some mammals (primates, whales, marsupials, insectivores, and one elephant) Am J Anat. 1987;180:126–142. doi: 10.1002/aja.1001800203. [DOI] [PubMed] [Google Scholar]
- Hof PR, Glezer II, Revishchin AV, Bouras C, Charnay Y, Morgane PJ. Distribution of dopaminergic fibers and neurons in visual and auditory cortices of the harbor porpoise and pilot whale. Brain Res Bull. 1995;36:275–284. doi: 10.1016/0361-9230(94)00202-c. [DOI] [PubMed] [Google Scholar]
- Hof PR, Nimchinsky EA. Regional distribution of neurofilament and calcium-binding proteins in the cingulate cortex of the macaque monkey. Cereb Cortex. 1992;2:456–467. doi: 10.1093/cercor/2.6.456. [DOI] [PubMed] [Google Scholar]
- Hof PR, Ungerleider LG, Webster MJ, Gattass R, Adams MM, Sailstad CA, Morrison JH. Neurofilament protein is differentially distributed in subpopulations of corticocortical projection neurons in the macaque monkey visual pathways. J Comp Neurol. 1996;376:112–127. doi: 10.1002/(SICI)1096-9861(19961202)376:1<112::AID-CNE7>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Hornung JP, Tork I, De Tribolett N. Morphology of tyrosine hydroxylase-immunoreactive neurons in the human cerebral cortex. Exp Brain Res. 1989;76:12–20. doi: 10.1007/BF00253618. [DOI] [PubMed] [Google Scholar]
- Ikemoto K, Kitahama K, Nishimura A, Jouvet A, Nishi K, Arai R, Jouvet M, Natgatsu I. Tyrosine hydroxylase and aromatic 1-amino acid decarboxylase do not coexist in neurons in the human anterior cingulate cortex. Neurosci Lett. 1999;269:37–40. doi: 10.1016/s0304-3940(99)00409-7. [DOI] [PubMed] [Google Scholar]
- Johnson SC, Baxter LC, Wilder LS, Pipe JG, Heiserman JE, Prigatano GP. Neural correlates of self-reflection. Brain. 2002;125:1808–1814. doi: 10.1093/brain/awf181. [DOI] [PubMed] [Google Scholar]
- Kodama T, Honda Y, Watanabe M, Hikosaka K. Release of neurotransmitters in the monkey frontal cortex is related to level of attention. Psychiatry Clin Neurosci. 2002;56:341–342. doi: 10.1046/j.1440-1819.2002.00977.x. [DOI] [PubMed] [Google Scholar]
- Kohler C, Everitt BJ, Pearson J, Goldstein M. Immunohistochemical evidence for a new group of catecholamine-containing neurons in the basal forebrain of the monkey. Neurosci Lett. 1983;37:161–166. doi: 10.1016/0304-3940(83)90147-7. [DOI] [PubMed] [Google Scholar]
- Kosaka T, Hama K, Nagatsu I. Tyrosine hydroxylase-immunoreactive intrinsic neurons in the rat cerebral cortex. Exp Brain Res. 1987a;68:393–405. doi: 10.1007/BF00248804. [DOI] [PubMed] [Google Scholar]
- Kosaka T, Kosaka K, Hataguchi Y, Nagatsu I, Wu J, Ottersen O, Storm-Mathisen J, Hama K. Catecholaminergic neurons containing GABA-like and/or glutamic acid decarboxylase-like immunoreactivities in various regions of the rat. Exp Brain Res. 1987b;66:191–210. doi: 10.1007/BF00236215. [DOI] [PubMed] [Google Scholar]
- Kulisevsky J. Role of dopamine in learning and memory: Implications for the treatment of cognitive dysfunction in patients with Parkinson's disease. Drugs Aging. 2000;16:365–379. doi: 10.2165/00002512-200016050-00006. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Campbell MJ, Foote SL, Goldstein M, Morrison JH. The distribution of tyrosine hydroxylase-immunoreactive fibers in primate neocortex is widespread but regionally specific. J Neurosci. 1987;7:279–290. doi: 10.1523/JNEUROSCI.07-01-00279.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Melchitzky DS, Sesack SR, Whitehead RE, Auh S, Sampson A. Dopamine transporter immunoreactivity in monkey cerebral cortex: regional, laminar, and ultrastructural localization. J Comp Neurol. 2001;432:119–136. doi: 10.1002/cne.1092. [DOI] [PubMed] [Google Scholar]
- Maddison WP, Maddison DR. Mesquite: A modular system for evolutionary analysis. Version 1.06. 2005. http://mesquiteproject.org. [Google Scholar]
- Marui W, Iseki E, Kato M, Kosaka K. Degeneration of tyrosine hydroxylase-immunoreactive neurons in the cerebral cortex and hippocampus of patients with dementia with Lewy bodies. Neurosci Lett. 2003;340:185–188. doi: 10.1016/s0304-3940(03)00101-0. [DOI] [PubMed] [Google Scholar]
- Mirra S, Heyman A, McKeel S, Sumi S, Crain B, Brownlee L, Vogel F, Hughes J, van Belle G, Berg L. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD) Neurology. 1991;41:479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- Murchison CF, Zhang XY, Zhang WP, Ouyang M, Lee A, Thomas SA. A distinct role for norepinephrine in memory retrieval. Cell. 2004;117:131–143. doi: 10.1016/s0092-8674(04)00259-4. [DOI] [PubMed] [Google Scholar]
- Öngür D, Ferry AT, Price JL. Architectonic subdivision of the human orbital and medial prefrontal cortex. J Comp Neurol. 2003;460:425–449. doi: 10.1002/cne.10609. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Huang XF, Toga AW. The rhesus monkey brain in stereotaxic coordinates. Academic Press; San Diego, CA: 2000. [Google Scholar]
- Petrides M, Pandya DN. Dorsolateral prefrontal cortex: comparative cytoarchitectonic analysis in the human and the macaque brain and corticocortical connection patterns. Eur J Neurosci. 1999;11:1011–1036. doi: 10.1046/j.1460-9568.1999.00518.x. [DOI] [PubMed] [Google Scholar]
- Preuss TM, Goldman-Rakic PS. Architectonics of the parietal and temporal association cortex in the strepsirhine primate Galago compared to the anthropoid primate Macaca. J Comp Neurol. 1991;310:475–506. doi: 10.1002/cne.903100403. [DOI] [PubMed] [Google Scholar]
- Rademacher J, Burgel U, Geyer S, Schormann T, Schleicher A, Freund HJ, Zilles K. Variability and asymmetry in the human precentral motor system. A cytoarchitectonic and myeloarchitectonic brain mapping study. Brain. 2001;124:2232–2258. doi: 10.1093/brain/124.11.2232. [DOI] [PubMed] [Google Scholar]
- Raghanti MA, Stimpson CD, Marcinkiewicz JL, Erwin JM, Hof PR, Sherwood CC. Cortical dopaminergic innervation among humans, chimpanzees, and macaque monkeys: A comparative study. Neuroscience. 2008;155:203–220. doi: 10.1016/j.neuroscience.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkowska G, Goldman-Rakic PS. Cytoarchitectonic definition of prefrontal areas in the normal human cortex: I. Remapping of areas 9 and 46 using quantitative criteria. Cereb Cortex. 1995;5:307–322. doi: 10.1093/cercor/5.4.307. [DOI] [PubMed] [Google Scholar]
- Sherwood CC, Raghanti MA, Stimpson CD, Bonar CJ, de Sousa AJ, Preuss TM, Hof PR. Scaling of inhibitory interneurons in areas V1 and V2 of anthropoid primates as revealed by calcium-binding protein immunohistochemistry. Brain Behav Evol. 2007;69:176–195. doi: 10.1159/000096986. [DOI] [PubMed] [Google Scholar]
- Sherwood CC, Raghanti MA, Wenstrup JJ. Is humanlike cyto-architectural asymmetry present in another species with complex social vocalization? A stereologic analysis of mustached bat auditory cortex. Brain Res. 2005;1045:164–174. doi: 10.1016/j.brainres.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Sherwood CC, Stimpson CD, Raghanti MA, Wildman DE, Uddin M, Grossman LI, Goodman M, Redmond JC, Bonar CJ, Erwin JM, Hof PR. Evolution of increased glia-neuron ratios in the human frontal cortex. Proc Natl Acad Sci U S A. 2006;103:13606–13611. doi: 10.1073/pnas.0605843103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeets WJAJ, González A. Catecholamine systems in the brain of vertebrates: new perspectives through a comparative approach. Brain Res Rev. 2000;33:308–379. doi: 10.1016/s0165-0173(00)00034-5. [DOI] [PubMed] [Google Scholar]
- Trottier S, Geffard M, Evrard B. Co-localization of tyrosine hydroxylase and GABA immunoreactivities in human cortical neurons. Neurosci Lett. 1989;106:76–82. doi: 10.1016/0304-3940(89)90205-x. [DOI] [PubMed] [Google Scholar]
- Vogeley K, Bussfeld P, Newen A, Herrmann S, Happé F, Falkai P, Maier W, Shah N, Fink G, Zilles K. Mind reading: Neural mechanisms of theory of mind and self-perspective. Neuroimage. 2001;14:170–181. doi: 10.1006/nimg.2001.0789. [DOI] [PubMed] [Google Scholar]
- von Bohlen und Halbach O, Dermietzel R. 2nd edition Wiley-VCH Verlag GmbH & Co.; Weinheim, Germany: 2006. Neurotransmitters and neuromodulators. Handbook of receptors and biological effects. [Google Scholar]
- von Bonin G, Bailey P. The neocortex of Macaca mulatta. University of Illinois Press; Urbana, IL: 1947. [Google Scholar]
- von Economo C, Koskinas GN. Die Cytoarchitektonik der Hirnrinde des erwachsenen Menschen. J. Springer; Wien: 1925. [Google Scholar]
- Walker AE. A cytoarchitectural study of the prefrontal area of the macaque monkey. J Comp Neurol. 1940;73:59–86. [Google Scholar]
- Wang X-H, Levitt P, O'Brien Jenkins A, Murphy EH. Normal development of tyrosine hydroxylase and serotonin immunoreactive fibers innervating anterior cingulate cortex and visual cortex in rabbits exposed prenatally to cocaine. Brain Res. 1996;715:221–224. doi: 10.1016/0006-8993(96)00012-1. [DOI] [PubMed] [Google Scholar]
- Watanabe-Sawaguchi K, Kubota K, Arikuni T. Cytoarchitecture and intrafrontal connections of the frontal cortex of the brain of the hamadryas baboon (Papio hamadryas) J Comp Neurol. 1991;311:108–133. doi: 10.1002/cne.903110109. [DOI] [PubMed] [Google Scholar]
- Weihe E, Depboylu C, Schütz B, Schäfer MK-H, Eiden LE. Three types of tyrosine hydroxylase-positive CNS neurons distinguished by dopa decarboxylase and VMAT2 co-expression. Cell Mol Neurobiol. 2006;26:659–678. doi: 10.1007/s10571-006-9053-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilles K, Dabringhaus A, Geyer S, Amunts K, Qu M, Schleicher A, Gilissen E, Schlaug G, Steinmetz H. Structural asymmetries in the human forebrain and the forebrain of non-human primates and rats. Neurosci Biobehav Rev. 1996;20:593–605. doi: 10.1016/0149-7634(95)00072-0. [DOI] [PubMed] [Google Scholar]