Abstract
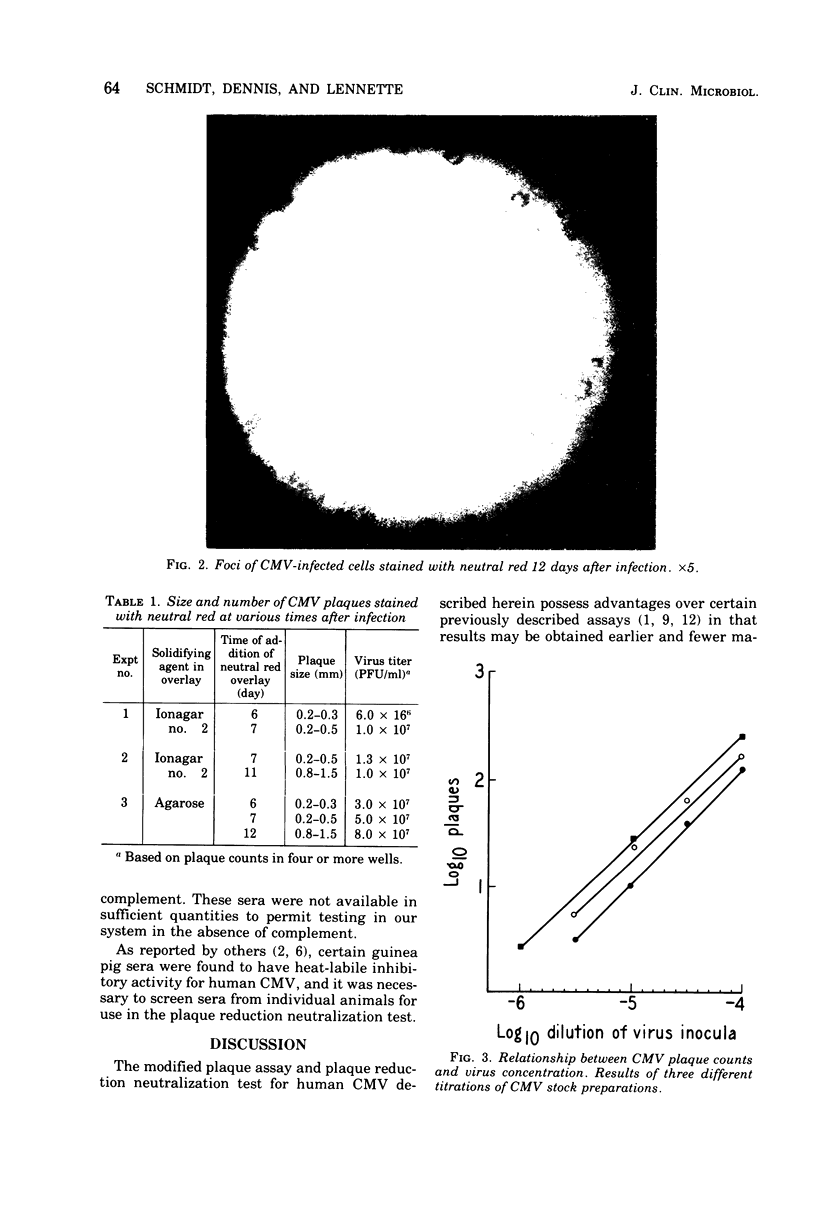
Foci of cells infected with human cytomegalovirus were noted to stain more intensely than uninfected cells with neutral red, and this provided the basis for development of a plaque assay and plaque reduction neutralization test for cytomegalovirus. Plaques demonstrable by neutral red staining could be counted at 8 days after infection; thus, results could be obtained earlier than for plaque assay systems based upon the viral cytopathic effect, a fewer manipulations were required for staining cell monolayers to demonstrate plaques. Certain variables affecting plaque size and numbers and antibody titers were defined. Addition of fresh guinea pig complement to the reaction mixtures markedly enhanced cytomegalovirus-neutralizing antibody titers of hyperimmune animal sera, but titers of human sera were enhanced only two-or fourfold.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen H. K. Cytomegalovirus neutralization by plaque reduction. Arch Gesamte Virusforsch. 1971;35(2):143–151. doi: 10.1007/BF01249705. [DOI] [PubMed] [Google Scholar]
- Andersen H. K. The influence of complement on cytomegalovirus neutralization by antibodies. Arch Gesamte Virusforsch. 1972;36(1):133–140. doi: 10.1007/BF01250303. [DOI] [PubMed] [Google Scholar]
- Chambers R. W., Rose J. A., Rabson A. S., Bond H. E., Hall W. T. Propagation and purification of high-titer human cytomegalovirus. Appl Microbiol. 1971 Nov;22(5):914–918. doi: 10.1128/am.22.5.914-918.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa T., Fioretti A., Plotkin S. Growth characteristics of cytomegalovirus in human fibroblasts with demonstration of protein synthesis early in viral replication. J Virol. 1973 Jun;11(6):991–997. doi: 10.1128/jvi.11.6.991-997.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham B. J., Minamishima Y., Dresman G. R., Haines H. G., Benyesh-Melnick M. Complement-requiring neutralizing antibodies in hyperimmune sera to human cytomegaloviruses. J Immunol. 1971 Dec;107(6):1618–1630. [PubMed] [Google Scholar]
- Huang E. S., Chen S. T., Pagano J. S. Human cytomegalovirus. I. Purification and characterization of viral DNA. J Virol. 1973 Dec;12(6):1473–1481. doi: 10.1128/jvi.12.6.1473-1481.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minamishima Y., Graham B. J., Benyesh-Melnick M. Neutralizing antibodies to cytomegaloviruses in normal simian and human sera. Infect Immun. 1971 Oct;4(4):368–373. doi: 10.1128/iai.4.4.368-373.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PLUMMER G., BENYESH-MELNICK M. A PLAQUE REDUCTION NEUTRALIZATION TEST FOR HUMAN CYTOMEGALOVIRUS. Proc Soc Exp Biol Med. 1964 Oct;117:145–150. doi: 10.3181/00379727-117-29520. [DOI] [PubMed] [Google Scholar]
- Vonka V., Benyeshmelnick M. Thermoinactivation of human cytomegalovirus. J Bacteriol. 1966 Jan;91(1):221–226. doi: 10.1128/jb.91.1.221-226.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waner J. L., Budnick J. E. Three-day assay for human cytomegalovirus applicable to serum neutralization tests. Appl Microbiol. 1973 Jan;25(1):37–39. doi: 10.1128/am.25.1.37-39.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wentworth B. B., French L. Plaque assay of cytomegalovirus strains of human origin. Proc Soc Exp Biol Med. 1970 Nov;135(2):253–258. doi: 10.3181/00379727-135-35031. [DOI] [PubMed] [Google Scholar]




