Abstract
Solid-state two-dimensional refocused INADEQUATE MAS NMR experiments resolve distinct helical and β-sheet conformational environments for both alanine and glycine in Nephila clavipes dragline silk fibers; the fraction of alanine and glycine in β-sheet structures is determined to be 82% ± 4% and 28% ± 5%, respectively.
Silk spun from the major ampullate gland of spiders (dragline silk) has a unique combination of strength and extensibility that renders it one of the toughest materials known.1 The silk’s impressive mechanical properties are related to the primary, secondary and hierarchical structure of the two proteins2,3 that comprise the silk: major ampullate spidroin I (MaSp1) and major ampullate spidroin II (MaSp2).† It has been recognized for more than a decade that the poly(Ala) runs in the amino acid sequence of MaSp1 and MaSp2 are primarily in an ordered β-sheet conformation.4 Double-quantum single-quantum NMR correlation experiments for static samples (DOQSY) showed that Gly is present in a disordered 31 helical structure and β-sheet domains.5 Recently, it was proposed that Ala and Gly, the two most abundant amino acids in spider silk,6 are present in both the β-sheet and 31-helical regions.7 In the present communication, we combine the plasticizing effect8–15 water has on spider silk with two-dimensional (2D) through-bond double-quantum (DQ)/single-quantum (SQ) 13C homonuclear NMR correlation experiments to resolve two distinct carbonyl sites for Gly and Ala. The 13C chemical shifts and relaxation properties of the two sites can be used to characterize Gly and Ala in β-sheet and helical structures. The information extracted from the 2D DQ/SQ experiments is successfully used to fit the carbonyl region in the 13C magic angle spinning (MAS) spectrum of plasticized spider silk to quantify the amount of Gly and Ala present in the two conformations.
The refocused INADEQUATE (incredible natural abundance double quantum transfer experiment) NMR experiment is particularly useful for acquiring 2D DQ/SQ through-bond homonuclear correlation spectra in disordered solids.16,17 Spider dragline silk has a large degree of structural disorder that results in inhomogeneous line broadening, analogous to many amorphous polymers and therefore, will greatly benefit from the refocused approach compared to the earlier solid-state INADEQUATE methods that do not utilize a spin-echo for detection.18–20 In the present work, we have collected refocused INADEQUATE NMR spectra on moderately 13C-enriched spider dragline silk fibers obtained from the spider Nephila clavipes with a modified NMR pulse sequence.‡ This is the first application of INADEQUATE experiments to characterize a silk.
INADEQUATE spectra were collected in the native (Fig. 1) and water plasticized state (Fig. 2). In the native state, the spider silk is completely rigid on the NMR timescale and cross polarization (CP) is utilized, similar to previous INADEQUATE experiments.16 In the water plasticized state, the spider silk exhibits a combination of motional dynamics that range from completely rigid to near isotropic on NMR timescales.11–13 Because CP is inefficient for the mobile regions in water plasticized spider silk, a version of the refocused INADEQUATE experiment that employs 13C direct excitation was implemented. †
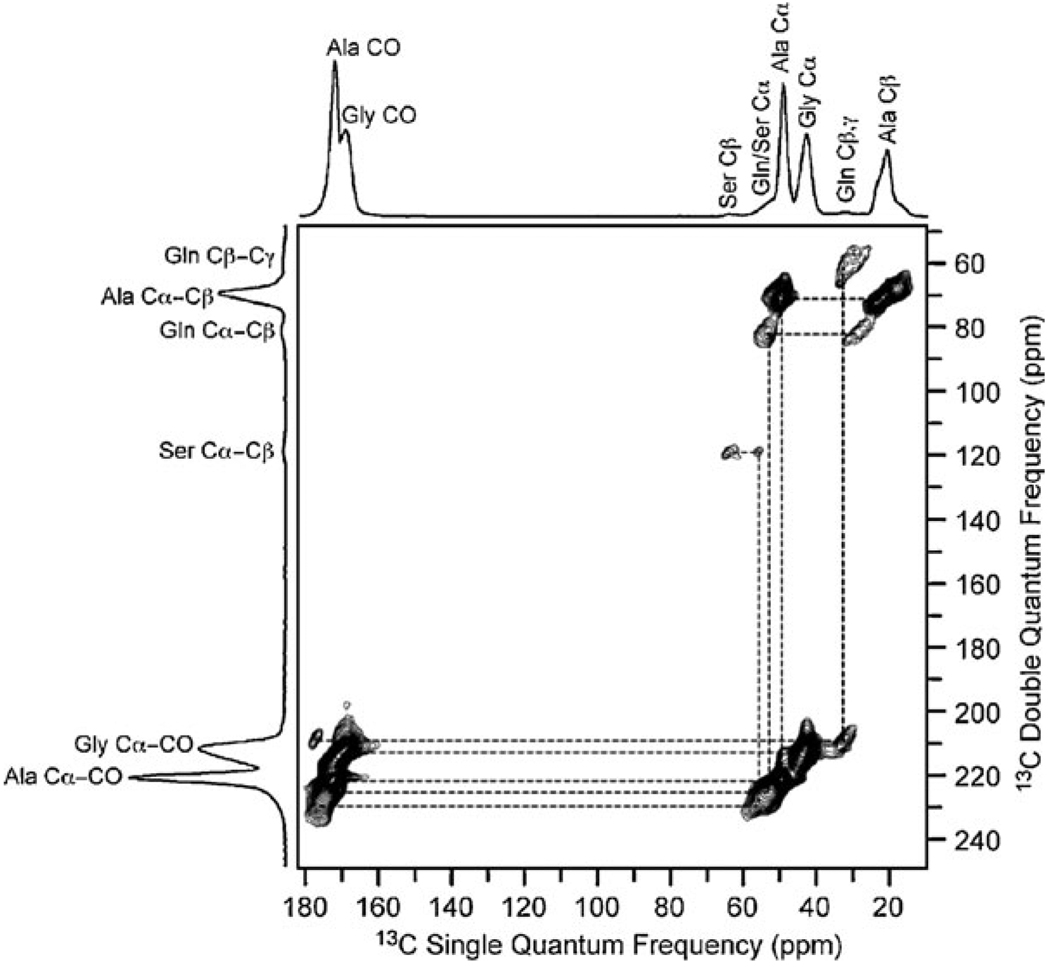
Fig. 1.
The 2D 13C homonuclear through-bond DQ/SQ spectrum of native (dry) Nephila clavipes spider dragline silk obtained with the refocused INADEQUATE pulse sequence. Data were collected on an 800 MHz spectrometer with a 1 ms CP contact time and a 40 kHz MAS frequency. The spectral width was 62.5 kHz in both dimensions and 256 t1 increments were collected in the indirect dimension with a 3 s recycle delay. The connectivity and assignment agrees with previous 13C correlation spectra collected with through-space dipolar recoupling.7
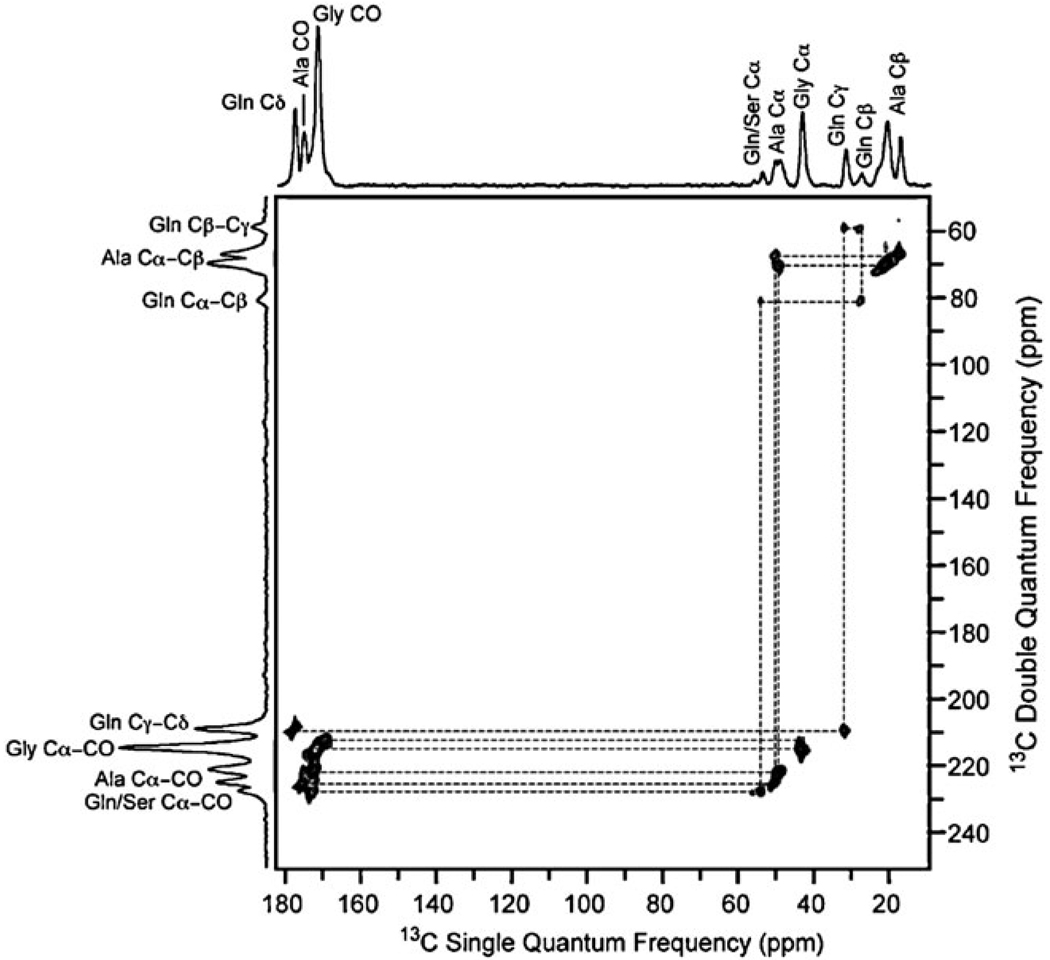
Fig. 2.
The 2D 13C homonuclear through-bond DQ/SQ spectrum of Nephila clavipes spider dragline silk plasticized with water. The refocused INADEQUATE pulse sequence was used with 13C direct excitation. Data were collected on a 400 MHz spectrometer with a 20 kHz MAS frequency. The spectral width was 50 kHz in both dimensions and 288 t1 increments were collected in the indirect dimension with a 1 s recycle delay. The short recycle delay enhances the signal from mobile amino acids.
The impact of water on spider silk has been studied in depth for a number of years.7,8,10–13 On the macroscopic scale, it causes the silk fiber to shrink ~50% in length and swell in diameter; a process known as supercontraction.10 The presence of water has a plasticizing effect on the material, with an associated increase in chain dynamics for those amino acids present in disordered helical regions. The 13C resonances of these amino acids sharpen considerably compared to the native silk, greatly improving spectral resolution (see Fig. 2). These sites also exhibit enhanced 13C T1 relaxation, indicative of mobility on the nanosecond timescale.11,12 The β-sheet domains remain completely rigid in the presence of water and the resonances display similar line-widths to the native silk and long T1 relaxation times.8,11–13
The resolution enhancement and variation in T1 relaxation in wet spider silk can be exploited to resolve two distinct carbonyl resonances for both glycine and alanine for the first time (Fig. 3). The 13C carbonyl chemical shifts for Gly (δ = 169.1 and 171.7 ppm) and Ala (δ = 172.3 and 175.4 ppm) indicate β-sheet and helical structures, respectively.21 The relaxation behavior of the different carbonyl sites was probed by collecting spectra with different recycle delays. As expected, the carbonyl resonances assigned to rigid β-sheets are accentuated when longer recycle delays are utilized, confirming the assignment (Fig. 3b).
Fig. 3.
The carbonyl region of the refocused INADEQUATE spectrum of Nephila clavipes spider dragline silk plasticized with water. The recycle delay was (a) 1 s and (b) 3 s. Mobile helical regions are enhanced in (a), while the rigid β-sheet domains are apparent in (b).
The information extracted from these 13C through-bond correlation spectra (chemicals shifts and linewidths) can be used to fit the carbonyl resonance of a fully relaxed 13C MAS spectrum of wet spider silk to extract the fraction of Ala and Gly in β-sheet and helical structures. † The results of the fit are 28% ± 5% and 82% ± 4% for the fraction of Gly and Ala in a β-sheet conformation, respectively. The helical fraction (72% for Gly and 18% for Ala) is loosely defined as helical here. Further work will be required to determine the exact type of helix and/or if there is a possibility for loop or turn like structures. Nonetheless, if one assumes that all the Gly and Ala present in the poly(Ala) runs and the poly(Gly–Ala) regions that flank the poly(Ala) runs in the amino acid sequence form a β-sheet structure, the expected result would be 26% and 86% for Gly and Ala, respectively. This is in excellent agreement with the β-sheet fraction determined experimentally for the two amino acids.
In conclusion, through-bond DQ/SQ 13C homonuclear correlation NMR experiments have resolved distinct sites for Gly and Ala that represent β-sheet and helical structures in plasticized spider silk. These sites can be quantified and correlated with the amino acid sequence of MaSp1 and MaSp2. Since, the β-sheet domains are thought to provide the spider silk its strength and helical structures its elasticity, an ability to resolve and quantify these two conformations is imperative to understanding the mechanical properties of natural spider silk and guide the production of synthetic silks.
Acknowledgments
This work was supported by the NSF (CHE-0612553 and DMR-0805197) and the NIH (NIBIB-5R01EB000490-05). We thank Dr Brian Cherry for help with NMR instrumentation, student training and scientific discussion.
Footnotes
Electronic supplementary information (ESI) available: Consensus amino acid sequence of spider silk proteins, INADEQUATE pulse sequences and fit of the carbonyl resonance.
‡ The 13C-enrichment is 16–45%, see ref. 7 for sample details.
Notes and references
- 1.Lewis RV. Chem. Rev. 2006;106:3762–3774. doi: 10.1021/cr010194g. [DOI] [PubMed] [Google Scholar]
- 2.Xu M, Lewis R. Proc. Natl. Acad. Sci. U. S. A. 1990;87:7120–7124. doi: 10.1073/pnas.87.18.7120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hinman M, Lewis RV. J. Biol. Chem. 1992;267:19320–19324. [PubMed] [Google Scholar]
- 4.Simmons A, Ray E, Jelinski LW. Macromolecules. 1994;27:5235–5237. [Google Scholar]
- 5.van Beek JD, Hess S, Vollrath F, Meier B. Proc. Natl. Acad. Sci. U. S. A. 2002;99:10266–10271. doi: 10.1073/pnas.152162299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Work RW, Young CT. J. Arachnol. 1987;15:65–80. [Google Scholar]
- 7.Holland GP, Creager MS, Jenkins JE, Lewis RV, Yarger JL. J. Am. Chem. Soc. 2008;130:9871–9877. doi: 10.1021/ja8021208. [DOI] [PubMed] [Google Scholar]
- 8.Simmons AH, Michal CA, Jelinski LW. Science. 1996;271:84–87. doi: 10.1126/science.271.5245.84. [DOI] [PubMed] [Google Scholar]
- 9.Work RW, Morosoff N. Text. Res. J. 1982;52:349–356. [Google Scholar]
- 10.Work RW. J. Exp. Biol. 1985;118:379–404. [Google Scholar]
- 11.Yang Z, Liivak O, Seidel A, LaVerde G, Zax DB, Jelinski LW. J. Am. Chem. Soc. 2000;122:9019–9025. [Google Scholar]
- 12.Holland GP, Jenkins JE, Creager M, Lewis RV, Yarger JL. Biomacromolecules. 2008;9:651–657. doi: 10.1021/bm700950u. [DOI] [PubMed] [Google Scholar]
- 13.Holland GP, Lewis RV, Yarger JL. J. Am. Chem. Soc. 2004;126:5867–5872. doi: 10.1021/ja031930w. [DOI] [PubMed] [Google Scholar]
- 14.Gosline JM, Denny MW, DeMont ME. Nature. 1984;309:551–552. [Google Scholar]
- 15.Eles PT, Michal CA. Macromolecules. 2004;37:1342–1345. [Google Scholar]
- 16.Lesage A, Bardet M, Emsley L. J. Am. Chem. Soc. 1999;121:10987–10993. [Google Scholar]
- 17.Sakellariou D, Brown SP, Lesage A, Hediger S, Bardet M, Meriles CA, Pines A, Emsley A. J. Am. Chem. Soc. 2003;125:4376–4380. doi: 10.1021/ja0292389. [DOI] [PubMed] [Google Scholar]
- 18.Early TA, John BK, Johnson LF. J. Magn. Reson. 1987;75:134–138. [Google Scholar]
- 19.Lesage A, Auger C, Caldarelli S, Emsley L. J. Am. Chem. Soc. 1997;119:7867–7868. [Google Scholar]
- 20.Benn R, Grondey H, Brevard C, Pagelot A. J. Chem. Soc., Chem. Commun. 1988:102–103. [Google Scholar]
- 21.Saitô H. Magn. Reson. Chem. 1986;24:835–852. [Google Scholar]