Summary
Although many similarities in arthropod central nervous systems (CNS) development exist, differences in midline cell formation and ventral nerve cord axonogenesis have been noted in arthropods. It is possible that changes in the expression of axon guidance molecules such as Netrin, which functions during commissural axon guidance in Drosophila and many other organisms, may parallel these differences. In this investigation, we analyze this hypothesis by examining Netrin accumulation during development of the brine shrimp Artemia franciscana, a branchiopod crustacean. An Artemia franciscana netrin (afrnet) orthologue was cloned. An antibody to the afrNet protein was generated and used to examine the pattern of afrNet accumulation during Artemia development. Despite differences between Drosophila and Artemia nerve cord development, examination of afrNet accumulation suggests that this protein functions to regulate commissure formation during Artemia CNS development. However, detection of afrNet at the midline and on commissural axons occurs at a relatively later time point in Artemia as compared with Drosophila. Detection of afrNet in a subset of midline cells that closely resemble Netrin-expressing cells at the Drosophila midline provides evidence for homology of midline cells in arthropods. Expression of Netrins in many other tissues is comparable, suggesting that Netrin proteins may play many conserved roles during arthropod development.
Introduction
Recent studies indicate that neuroblasts and the neurons that they produce are homologous in arthropods. For example, morphological data suggest that various arthropods have homologous neurons bearing similar cell body locations and axonal projections (Thomas et al. 1984; Whitington et al. 1993; reviewed by Whitington 1996). Furthermore, early even-skipped neural and engrailed neural/neuroblast expression is conserved among insects and crustaceans (Duman-Scheel and Patel 1999). Despite these similarities, differences at various stages of arthropod neurogenesis have been noted. For example, pioneering of the longitudinal connective axon tracts of the brine shrimp Artemia franciscana differs from that of the fruit fly Drosophila melanogaster. The purpose of this investigation is to examine the molecular basis for this difference.
Blanchard (1987) found that in Artemia, two pairs of terminally located neurons originating at the anterior pioneer the longitudinal connectives along the entire length of the larval trunk. Many axons join the Artemia longitudinal axon tracts before formation of the commissural axon tracts. Most commissural axons do not cross the Artemia midline until later stages of larval development, after the longitudinals are well established. By contrast, in stage 12 Drosophila, the first longitudinal pathway, the combined MP1/vMP2 pathway, is pioneered separately in each segment by the ascending growth cone of pCC, an interneuron. Although the longitudinals are pioneered during early central nervous systems (CNS) development in flies, the commissures are well-established before completion of the longitudinal connectives; the majority of commissural axons turn rostrally or caudally into one of the longitudinal axon tracts after they have crossed the midline (reviewed by Doe and Goodman 1993). Thus, in flies the longitudinal connectives are completed after commissural axons cross and join them, and in Artemia the longitudinals are well established before commissure formation.
Another difference relates to the anterior–posterior gradient of CNS maturation found in Artemia (Blanchard 1987; Harzsch and Glötzner 2002) and other crustaceans. CNS development in Artemia, an animal in which new segments are generated over time from the posterior growth zone (see Fig. 1 in Copf et al. 2003), occurs in a graded fashion. The most anterior segments, the first segments to form, are therefore the most developmentally advanced. This differs from nerve cord formation in D. melanogaster, a long germ insect in which nerve cord development is synchronized in each segment (reviewed by Doe and Goodman 1993). It is possible that differences in the ways that nerve cords are generated in these organisms may be related to these different mechanisms for generating segments.
Fig. 1.
Artemia franciscana Netrin (afrnet) encodes a netrin orthologue (A). The predicted Artemia franciscana Netrin (afrNet) sequence is aligned to the Drosophila melanogaster NetA (DmNetA) sequence. Regions corresponding to the primers used in this study (FP1, RP6, RP7, and GSP1, see methods for more details) are marked. Conserved amino acid residues are shown in black, whereas nonconserved residues are shown in gray. Two Laminin EGF-like repeats, each containing eight conserved cysteine residues (shaded), are marked by arrows. Aligned sequences of various Net and Laminin proteins are shown in (B). The portion of the afrNet sequence aligned corresponds to the region used to generate the anti-afrNet antibody. Amino acid residue numbers for the proteins used in this study are indicated. This alignment was used to generate a phylogenetic tree in Phylip (C), which supports placement of afrNet among the Net proteins. Bootstrap values for each clade are shown as percentages.
It is also possible that the role of midline cells during nerve cord formation may have diverged in arthropods. Specialized midline cells regulate the axon guidance of both crossing and non crossing axons at the ventral midline of the Drosophila ventral nerve cord, as well as the spinal cord of vertebrate organisms. These cells, the floor plate cells of higher vertebrates and the midline glia in Drosophila, secrete guidance molecules that regulate the growth of commissural axons (reviewed by Tessier-Lavigne and Goodman 1996 and Kaprielian et al. 2001). Although there is evidence for homology of insect and crustacean midline cells (Duman-Scheel and Patel 1999; Gerberding and Scholtz 1999, 2001; Manzanares et al. 1996), these cells form differently in various arthropods (discussed by Gerberding and Scholtz 1999). In Drosophila, the midline is formed from the right and left mesectoderm anlagen, which fuse following gastrulation (reviewed by Doe and Goodman 1993). In the branchiopod crustacean Leptodora kindti, the midline differentiates after germ-band growth from a uniform ectodermal layer (Gerberding 1997). Midline formation in Artemia (Freeman 1989; Manzanares et al. 1996) is fairly comparable with Leptodora (Gerberding 1997), suggesting that the means of generating homologous midline cells is conserved among branchiopods but differs from Drosophila.
Given these differences in arthropod CNS development, it would be interesting to compare the roles of axon guidance molecules in arthropods with divergent nerve cord development. Such an analysis could provide insight into the molecular mechanisms underlying the differences in nerve cord formation noted above and could provide additional markers for midline cells. This study focuses on the role of Netrin (Net) proteins in A. franciscana versus D. melanogaster. Net proteins, laminin-related diffusible molecules that regulate midline axonal guidance, have been identified in many organisms, from nematodes and insects to higher mammals (reviewed by Tessier-Lavigne and Goodman 1996; Kaprielian et al. 2001). The Drosophila NetA and B proteins are expressed at the midline and are required for proper commissure formation. Early studies suggested that deletion of netA and B results in defective guidance of commissural axons in fruit flies (Harris et al. 1996; Mitchell et al. 1996). More recent data suggest that Drosophila Nets act as short-range guidance cues that promote midline crossing (Brankatschk and Dickson 2006).
Receptors that bind Net proteins have been identified in both vertebrates and invertebrates. For example, Drosophila Frazzled (Fra; Kolodziej et al. 1996), deleted in colorectal cancer (DCC) in mice (Keino-Masu et al. 1996; Fazeli et al. 1997), and Unc-40 in Caenorhabditis elegans (Chan et al. 1996) are expressed on commissural axons and their growth cones and function as cell-surface receptors for Net proteins in axon attraction (reviewed by Kaprielian et al. 2001). Mutation of these receptors results in commissural axon defects (Hedgecock et al. 1990; Kolodziej et al. 1996; Fazeli et al. 1997). Thus, binding of Net proteins to their receptors, which are expressed by neurons, promotes growth cone guidance. Redistribution of Net protein by these receptors also seems to create positional information for other axons, even those lacking Net receptor expression (Hiramoto et al. 2000).
In this investigation, a Net homolog was cloned from A. franciscana. Despite differences in arthropod nerve cord formation, analysis of the accumulation pattern of this protein suggests that Net functions in Artemia axon guidance and provides evidence for homology of midline cells in arthropods. Comparable with Drosophila, accumulation of Artemia Net is observed in many other developing tissues, suggesting that Nets may function in many aspects of brine shrimp development.
Materials and Methods
Animal sources and culturing conditions
San Francisco Bay Brand A. franciscana were obtained from Marine Depot. Artemia were hatched in a separatory funnel and then transferred to larger tanks. The specific gravity of the salt water was maintained from 1.025 to 1.050 with a pH of 7.0–8.0. Animals were fed baker's yeast and maintained on a 12 h light:12 h dark cycle. Animals were staged as described previously (Schrehardt 1987; Copf et al. 2003).
PCR and cloning
PCR and cloning were performed generally as described by Duman-Scheel et al. (2002). Total Artemia RNA was isolated with Trizol (Invitrogen Life Technologies, San Jose, CA) from L3 larvae. cDNA was synthesized with the Superscript First Strand Synthesis System for RT-PCR (Invitrogen Life Technologies). Degenerate primers based on the sequences of previously identified Net proteins from a variety of species were used to amplify Artemia cDNA. External primers FP1 5′TgY AAR TgY AAY ggN CAY g3′ and RP6 5′AY Ngg RTg RCA RTC RCA3′ were used in the first amplification step. 1/100 of the product of this reaction was reamplified with FP1 and nested primer RP7 5′RCA RTT YTg RCA NAC NCC3′. The location of each of these primers relative to the Drosophila NetA protein sequence is indicated in Fig. 1A. Each 50-μl PCR reaction contained one-tenth of the cDNA synthesis reaction, 1.5 mM MgCl2, 20 mM Tris-HCl, 50 mM KCl, 10 mM dNTPs, 0.2 μM primers, and 5 U Taq polymerase (Fisher, Springfield, NJ). Following PCR in a MJR PTC-100 thermal cycler (Hercules, CA) (5 min denaturation at 94°C preceding 40 cycles of 30 sec denaturation at 94°C, 30 sec annealing at 50°C, and 1.0 min extension at 72°C), PCR products were gel purified, cloned, and sequenced. A 225 bp PCR product from the second reaction was cloned and sequenced. BLASTx searches indicated that the clone corresponds to a novel net gene, which was named Artemia franciscana netrin (afrnet).
Gene-specific primers (GSP1 in Fig. 1A) corresponding to the most 3′ regions of this clone were used in a 3′ rapid-amplification of cDNA ends (RACE) strategy (Invitrogen Life Technologies). In all, 534 bp of the afrnet gene were cloned. As many organisms possess two net genes, multiple attempts to clone a second afrnet gene were made. A variety of primers, PCR conditions, and DNA templates (genomic, cDNA from multiple larval stages) were used. Although multiple laminin homologues were cloned (M. Duman-Scheel, unpublished), a second afrnet gene was not identified.
Phylogenetic sequence analysis
Protein alignments with members of the Net and Laminin families were generated with ClustalW. Trees based on these alignments were created using maximum parsimony as the optimality criterion and the ProtDist distance matrix program (Phylogeny Inference Package, Phylip, J. Felsenstein 1993), which was analyzed with neighbor joining. One thousand bootstrapped data sets were generated. Trees were also created using the branch swapping algorithm in PAUP v4.0b10 (Swofford 2001). Accession numbers for the other Net and Laminin protein sequences used in this study are as follows: Human Net1 NP_004813, Chick Net1 NP_990750, Mouse Net1 NP_032770, Rat Net1 NP_446183, Fly NetB NP_511155, Fly NetA AAB17533, Leech Net AAC83376, C. elegans Unc-6 NP_509165, Fly LanB2 NP_524006, and Chick Lam Gamma 1 AAK55397.
Expression and purification of afrnet protein and antibody production
The afrnet 3′ RACE cDNA was cloned into the Qiagen pQE31 (Valencia, CA) expression vector. An N-terminally His-tagged version of the afrNet protein was expressed in bacteria and purified according to the method described in the Qiaexpress Type IV Kit (Qiagen). This procedure involved the use of Ni-agarose to purify His-tagged afrNet protein. This method resulted in the purification of a 28kDa afrNet protein, which was used for the production of antibodies.
New Zealand White rabbits (Covance, Kalamazoo, MI, USA) were injected with 0.5 cc containing 50 μg of Net protein (antigen) that was prepared in an equal volume of Complete Freund's Adjuvant. Animals were boosted at 14-day intervals with 50 μg for the first three boosts and 140 μg for the last two boosts. Animals were sacrificed 2 weeks following the fifth boost. Test and final bleeds were assayed through Western blotting and on Artemia. Pre-immune sera were used as controls in these assays.
Immunohistochemistry
Staining was generally completed according to the procedures discussed by Patel et al. (1989) and Patel (1994). Artemia were fixed for 25–30 min in 4% formaldehyde in PEM buffer. Longer fixation times were utilized to improve detection of afrNet, a secreted protein. Following dissection or sonication to promote better penetration of antibodies (Patel et al. 1989; Patel 1994), animals were rinsed briefly in PBS+0.1% Triton-X, blocked in PBS+0.1% Triton X+5% NGS, and stained. Primary and secondary antibody incubations were completed at 4°C overnight. Anti-afrNet antibody was used at a concentration of 1:200. Anti-acetylated tubulin (Zymed, San Francisco, CA) was used at a concentration of 1:100. HRP-conjugated secondary antibodies (Jackson Immuno-histochemicals, Westgrove, PA) were used at a final concentration of 1:200.
In situ hybridization
In situ hybridization was performed with Drosophila netA and netB riboprobes according to the procedure described by Patel (1996).
Results
Cloning and sequence analysis of afrnet
Changes in the expression patterns of axon guidance molecules may underlie differences in CNS development observed among arthropods. In order to examine this possibility, a piece of the afrnet gene (Accession No. DQ832187) was cloned using a degenerate PCR/RACE strategy. The predicted partial protein product of this sequence is aligned to D. NetA in Fig. 1A. The aligned region includes two Laminin EGF-like domains (marked by arrows), each of which includes eight conserved cysteine residues (shaded). A portion of the predicted partial protein product of afrnet cDNA aligned to other known Net and Laminin protein sequences is shown in Fig. 1B. afrNet shows a high degree of similarity to other Net proteins within this alignment. For example, in the aligned region, afrNet and human Net-1 are 89% identical. afrNet also shares regions of homology with Laminin proteins. For example, afrNet shares 40% amino acid identity with Drosophila LamB2 in the region shown in Fig. 1B.
Extensive phylogenetic analyses including net and laminin clones from Artemia and other organisms using both PAUP and Phylip indicate that afrnet is a net orthologue. One such phylogenetic tree (generated in Phylip from the alignment shown in Fig. 1B) is shown in Fig. 1C and supports the placement of afrNet among the Net proteins. During the course of this work, a number of other PCR products with homology to net and laminin genes were also isolated from Artemia. Phylogenetic analyses of these clones suggested that they correspond to Laminin homologues (M. Duman-Scheel, unpublished data). Though it is not currently possible to rule out the existence of a second Artemia net gene, present data suggest that there is a single net gene in Artemia (comparable results were found in the leech by Gan et al. 1999).
Examination of the accumulation pattern of afrnet during early CNS development in Artemia
afrnet mRNA expression data is desirable but was not collected because of the technical difficulty of performing in situ hybridization in larvae (particularly those at older stages) with thick cuticles. Instead, analysis of the accumulation pattern of afrNet protein during Artemia development was performed with a rabbit polyclonal antibody raised to purified afrNet protein. afrNet is detected in a dynamic pattern in many developing brine shrimp tissues. As the roles of Drosophila Nets have been characterized primarily during CNS development, this report will first focus on detection of afrNet during CNS development (Figs. 2 and 3) and then in other tissues (Fig. 4). afrNet can be detected in the brain beginning at stage L1 and continuing throughout larval development (Fig. 2A). It is detected in many regions, including the protocerebrum, deutocerebrum, and labral commissure (Fig. 2A; terminology from Harzsch and Glötzner 2002 is used). Expression of Drosophila Nets has also been detected in the brain (Harris et al. 1996; Mitchell et al. 1996).
Fig. 2.
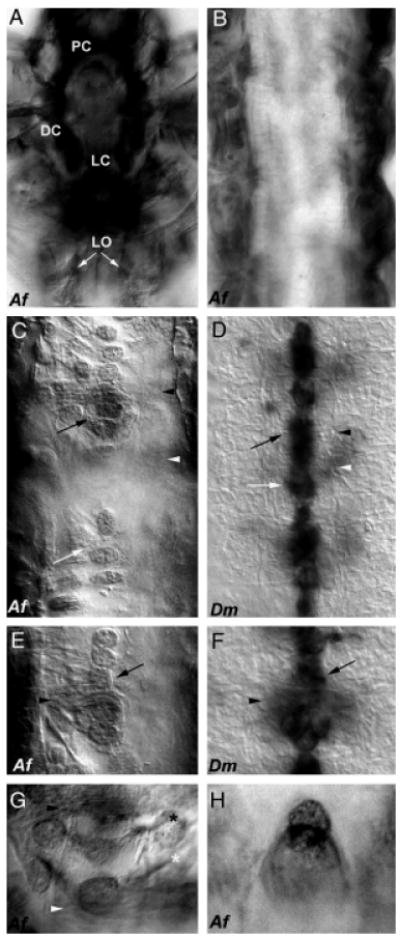
Comparison of Netrin central nervous systems (CNS) expression in Artemia and Drosophila. Comparable with the fly, expression of afrNet is found in the Artemia brain during early development (L3 shown in (A); PC, protocerebrum; DC, deutocerebrum; LC, labral commissure). afrNet can be detected on longitudinal (LO marked by white arrows in (A)) axons before commissural axon formation. By stage L6, the longitudinal axon tracts have thickened, and afrNet expression can be detected on many axons (B). Once all of the segments have been generated, afrNet can still be detected on longitudinal axons (dark staining along both sides of trunk in C and E), but can also be detected in midline cells in thoracic segments (C, E, G, H) and on commissural axons (C, E). This midline and commissural axon staining is much less intense than staining on the longitudinal axons, which is also observed in these preparations. Midline expression is comparable with that of stage 13 Drosophila (D, F), though it occurs at a relatively later time point in Artemia. Note that axons are viewed by Nomarski optics only in Drosophila, which are stained through in situ hybridization with a netA RNA probe that labels only the cell bodies (D, F). By contrast, the afrNet antibody marks both cell bodies and axons. Note that one Artemia segment is shown in (C), but three segments are shown in the more compact Drosophila CNS in (D). Black arrowheads mark the anterior commissures, and white arrowheads mark the posterior commissures in (C–G). Black arrows mark the midline glia in (C–F). White arrows mark clusters of cells posterior to the commissures (C, D). Higher magnification views of midline glia encircling the anterior commissure are shown (Artemia in (E), Drosophila in (F)). Net-positive axons in Artemia (G) may correspond to the VUM (white asterisk) and MP (black asterisk) axons. A higher magnification view of posterior afrNet-expressing cells, which may correspond to the MNB cluster in Artemia, is shown in (H). Anterior is oriented up in all figures. Af, Artemia franciscana; Dm, Drosophila melanogaster.
Fig. 3.
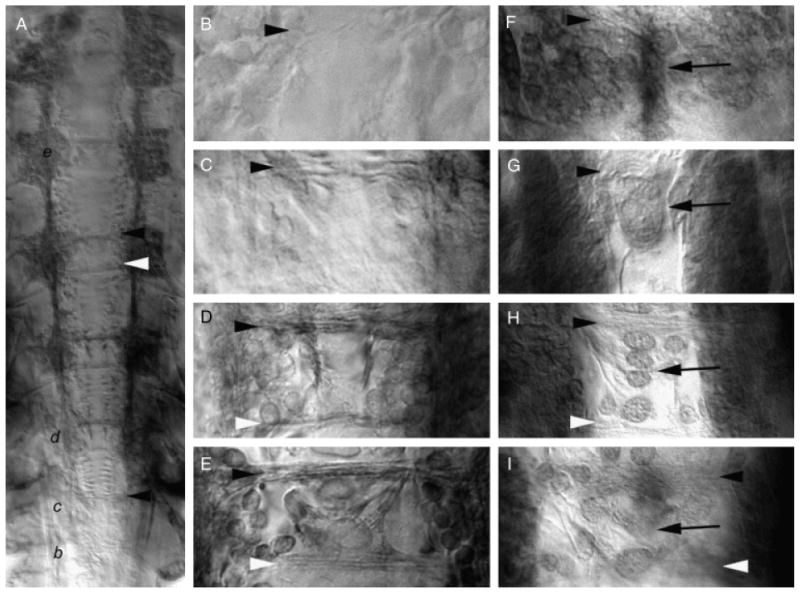
Midline expression of Artemia franciscana netrin (afrNet) coincides with formation of the commissural axon tracts. Axonogenesis in Artemia was followed with anti-acetylated tubulin staining (A–E). The temporal gradient of central nervous systems (CNS) development is evident in the L8 animal shown in (A), where commissure formation is initiating in the most posterior segment (marked with black letter b, which is magnified in panel (B). Commissure formation has progressed further in more anterior segments which are marked in panel (A) by the black italicized letters c, d, and e and magnified in the corresponding panels (C, D, and E). Accumulation of afrNet is shown at corresponding stages of development in (F–I). Segments (B) and (F), (C) and (G, D and H, and E and I) are at comparable stages of development. Midline afrNet-positive cells are detected before and during formation of the anterior and posterior commissures (F–I). At later stages of CNS development, afrNet is detected on commissural axons (H and I). These data support a role for afrNet in commissure formation. In this figure, anterior commissures are marked with black arrowheads, and posterior commissures are marked with white arrowheads in various panels. Midline Net-positive cells are marked by black arrows. Anterior is oriented up in all figures.
Fig. 4.
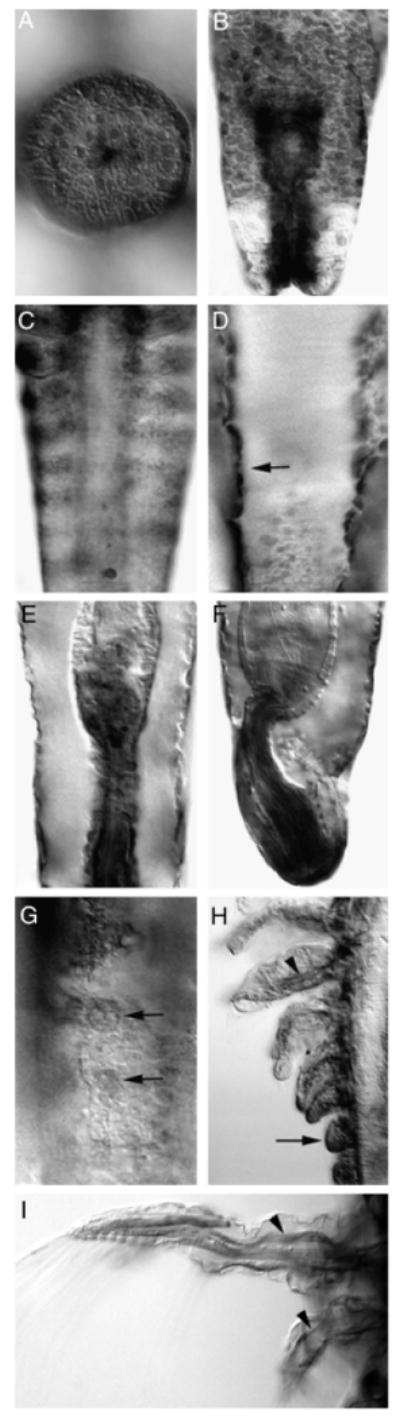
Artemia franciscana netrin (afrNet) is expressed in a variety of tissues. afrNet expression is initially detected in the salt gland (A) and posterior ectoderm (B) in L1 animals (L2 shown in A and B). As segments are generated, afrNet expression is found in the trunk ectoderm (L4 shown in C) and the dorsal mesoderm (marked by arrow in dorsal view of L3 animal shown in D). Expression of afrNet is detected in the hindgut throughout development (stage L4 is shown in ventral view shown in (E) and lateral view shown in (F). Expression is also found throughout limb development. Expression is shown in limb precursors at stage L4 (G) and at later stages of development in L7 (H). Arrows in g and h mark expression in early limbs (G, H), and arrowheads mark expression in the nerves of more mature leg segments (H) as well as in the L4 antennae and mandible (I). Anterior is oriented up in all figures but (A), in which anterior is oriented to the left.
During early larval stages of Artemia development, longitudinal axons originating in the anterior grow along the entire trunk of the animal to the posterior. These longitudinal connective pioneers run parallel to each other, originating in the brain and projecting posteriorly into the trunk (Blanchard 1987; Harzsch and Glötzner 2002). afrNet accumulation is detected on these longitudinal axons (Fig. 2A) during stage L2 and continuing as the axons grow to the posterior. Additional afrNet-positive axons join the connectives (Fig. 2B), which are well established before the crossing of most commissural axons. afrNet accumulation is maintained on longitudinal connectives throughout larval neurogenesis.
Late midline accumulation of Netrin is comparable with that of Drosophila
Expression of Drosophila NetA and NetB is detected in clusters of midline cells. Net function is required for proper nerve cord formation in Drosophila (Harris et al. 1996; Mitchell et al. 1996; Brankatschk and Dickson 2006). Compared with Drosophila, crossing of commissural axons is observed at relatively later stages of Artemia development; in other words, commissural axons cross only after the longitudinal axon tracts are well established. Therefore, a role for Net in midline axon guidance could be conserved from Drosophila to Artemia but at relatively later stages of Artemia development. In order to examine this possibility, afrNet accumulation was examined throughout Artemia CNS development. Midline accumulation of afrNet can be detected in thoracic segments during late larval stages at a time when the longitudinal tracts are well established (Figs. 2, C, E, G and H, and 3). Interestingly, the levels of afrNet midline accumulation are weaker than levels on the longitudinals (Figs. 2, C, E, G and H, and 3, F–I).
The accumulation of afrNet was compared with expression of Drosophila netA and netB (described by Harris et al. 1996; Mitchell et al. 1996). netA mRNA expression is shown here for comparison (Fig. 2, D and F); the expression pattern of netB at the midline is fairly comparable and is not shown. Drosophila Nets are expressed in midline glia, including the MGM and MGA clusters (Fig. 2, D and F). Net expression is detected in cells posterior to the commissures (Fig. 2D); Mitchell et al. (1996) suggested that these cells may correspond to the MNB cluster. Net accumulation is also detected in the Drosophila VUM and MP neurons. The fly MP neurons do not themselves synthesize net mRNA but receive the protein from other sources (Hiramoto et al. 2000). These cells all play roles in guidance of commissural axons (reviewed by Doe and Goodman 1993). In Artemia, afrNet expression is detected in midline glia (Fig. 2, C and E). These cells appear to wrap the commissures (Fig. 2E) as they do in Drosophila (Fig. 2F). afrNet expression is also detected in axons with locations and projections that resemble those of the Drosophila VUM and MP axons (Fig. 2G). Clusters of afrNet-positive cells are located posterior to the commissures (Fig. 2, C and H) and may be homologous to the Drosophila MNB cluster. Thus, many midline Net-positive cells with likely homology to Drosophila midline cells are observed in Artemia.
Given the similar patterns of Net-positive cells observed at the Drosophila and Artemia midlines, a more detailed analysis of the accumulation pattern of afrNet in relation to axon growth over the course of commissure formation in Artemia was performed. In order to follow commissure formation, axons were labeled using an anti-acetylated tubulin antibody. The time course of commissure formation can be observed in an L8 animal in which the anterior–posterior temporal gradient of CNS development is evident (Fig. 3A). In the most posterior segment (marked by black letter b, which is magnified in Fig. 3B), commissural axons of the anterior tract have just begun to grow toward the midline. During this stage of development, afrNet accumulation (analyzed at a similar stage in another embryo pictured in Fig. 3F) is detected at high levels in a cluster of cells along the midline. This accumulation pattern is observed just before the initiation of axon growth toward the midline. It should be noted that afrNet accumulation is also detected in more lateral clusters of neuroectodermal cells during this period (Fig. 3F; this is comparable with Drosophila; Harris et al. 1996; Mitchell et al. 1996). In the next-anterior segment (marked by letter c in Fig. 3A and magnified in Fig. 3C), the anterior commissure has thickened, and a few axons of the posterior commissure have begun to extend toward the midline. At this time, accumulation of afrNet is found in a cluster of relatively large midline cells, as well as in more lateral cells (Fig. 3G). In the segments marked by letters d and e in Fig. 3A and magnified in Fig. 3, D and E, the anterior and posterior axon tracts have thickened. Midline afrNet accumulation is detected at corresponding times (Fig. 3, H and I) in patterns similar to that observed in Drosophila (Fig. 2, D and F). The relative age of the segment shown in 3 h is comparable with that of the fly embryo shown in Fig. 2D and to the Artemia segment shown in Fig. 2C; however, the focus in Fig. 3H is in the plane of the axons, where accumulation of afrNet can be detected. In summary, accumulation of afrNet is observed at the midline and on commissural axons during development of the commissural axon tracts in Artemia.
Expression of afrnet in additional tissues
Although functions of the Drosophila Nets and their receptors have been characterized within the nervous system (Harris et al. 1996; Kolodziej et al. 1996; Mitchell et al. 1996; Gong et al. 1999; Hiramoto et al. 2000; Keleman and Dickson 2001; Brankatschk and Dickson 2006), Drosophila Net expression is also detected in many tissues outside of the nervous system. Likewise, afrNet accumulation is observed in many tissues outside of the brine shrimp CNS. afrNet is detected in the naupliar salt gland (Fig. 4A) beginning during stage L1. afrNet is also found in the L1 and L2 trunk ectoderm, with very high levels being found in the posterior regions (Fig. 4B). As segments are generated, afrNet accumulates in neuroectodermal stripes (Fig. 4C). As in Drosophila (Mitchell et al. 1996), these stripes initiate in lateral patches in the epidermis and subsequently elongate along the anterior edge of each segment (Fig. 4C).
The Drosophila Nets are expressed in several mesodermal derivatives. afrNet is detected in the dosal mesoderm (Fig. 4D) beginning at stage L2 and continuing into late larval development. Similar dorsal mesoderm expression in Drosophila coincides with cardiac and lateral muscle cells (Harris et al. 1996; Mitchell et al. 1996). The Drosophila Nets are also expressed in the visceral mesoderm, including the hindgut. Beginning at early naupliar stages, afrNet is found in the visceral mesoderm, with highest levels being detected in the hindgut (Fig. 4, E and F). Hindgut expression is maintained throughout larval development.
Drosophila Net expression is detected in the embryonic leg primordia (Mitchell et al. 1996). Similar expression (Fig. 4G) is detected in Artemia beginning during stage L2. As limb growth continues, afrNet is found throughout the developing limbs (Fig. 4H) but is eventually restricted to nerves innervating more mature legs (Fig. 4H). Similarly, diffuse afrNet expression is detected in the Artemia antennae and mandible in early naupliar stages, but afrNet accumulation is eventually restricted to nerves in these structures (Fig. 4I).
Discussion
A role for netrins in Artemia nerve cord development
Although arthropods share a significant degree of early CNS homology, differences in the generation of midline cells and ventral nerve cord formation have been observed. Given these differences, one might expect that the expression of Nets may have changed during arthropod evolution. In order to examine this hypothesis, the afrnet gene was cloned from A. franciscana (Fig. 1). An antibody raised to purified afrNet protein revealed a Net accumulation pattern that is consistent with a role for Net during ventral nerve cord formation in Artemia (Figs. 2 and 3).
afrNet is bound to longitudinal axons (Fig. 2, A and B) throughout neurogenesis. Given the detection of afrNet on longitudinal pioneers in early larval stages, afrNet may function in the pioneering of longitudinal axon tracts. As afrNet is detected in the posterior region of Artemia (Fig. 4B) at the time when longitudinals are pioneered, it is possible that this posterior expression may function to guide longitudinal pioneers; however, this would require long-range signaling, and Drosophila Nets were recently shown to act only at short range (Brankatschk and Dickson 2006). Alternatively, a large amount of afrNet is detected in the Artemia brain during this time. The longitudinal axons originate in the brain (Harzsch and Glötzner 2002), so it is possible that the pioneers actually make and secrete their own afrNet protein, which might bind to Net receptors on the surfaces of these axons. Regardless of the source of Net, neurons expressing Net are known to provide guidance cues for other neurons (Hiramoto et al. 2000). Hiramoto and Hiromi (2006) suggest that Drosophila Nets may mediate association or fasciculation with commissural axons, and that Net may mediate axon–axon recognition via a yet unidentified Net receptor expressed by longitudinal pioneer neurons. If this is true, then longitudinal pioneers may use Net signaling to recruit other axons to the longitudinal connectives in Artemia.
At later stages following the pioneering of the longitudinal axon tracts, commissural axon growth is observed in A. franciscana (Fig. 3, A–E). During this time period, midline afrNet-positive cells (Figs. 2, C, E, G and H, and 3, F–I) are detected in Artemia. Furthermore, commissural axons bind and maintain expression of afrNet (Figs. 2, C and E, and 3I). Although commissural axon guidance occurs after the longitudinal connectives are well established in Artemia, these data suggest that Net protein does function during commissure formation in Artemia. Temporal changes in the expression of axon guidance molecules therefore parallel the morphological differences that are observed.
afrNet-positive cells are located at the midline of Artemia before and during the time of commissure formation. Until recently, it was thought that midline expression of chemoat-tractive Nets guides commissural axons at long-range toward the midline (Harris et al. 1996; Mitchell et al. 1996). More recently, however, Brankatschk and Dickson (2006) proposed that Nets act not as long-range chemoattractants, but as short-range cues that promote midline crossing. Without the ability to perform the sophisticated genetic analyses that shape the present understanding of Net function in Drosophila, it is difficult to know the exact role of Nets during Artemia nerve cord formation. However, it is possible that afrNet may also act at short range to promote midline crossing rather than as a long range chemotrophic guidance molecule. Other guidance molecules would then be responsible for guiding axons to the midline. Such reasoning would make it easier to understand how Artemia commissural axons seem to ignore relatively higher levels of afrNet on longitudinal axons, which might be expected to keep these axons in the longitudinal pathways. Alternatively, it is possible that in Artemia, chemorepulsive Net signals from the longitudinal axons might be received by Unc-5 homologues on commissural axons and be repelled to the midline. Identification and analysis of Net receptors in Artemia could thus prove interesting.
Late midline expression of netrin in Artemia provides evidence for homologous midline cells
The data reported here provide molecular marker evidence for homology of midline cells in Artemia and Drosophila. Gerberding and Scholtz (1999, 2001) studied the homology of insect and crustacean midline cells. They suggested that the MNB of Drosophila, the neuroglioblast of the grasshopper Schistocerca americana, and the d0 cell of the amphipod Orchestia cavimana are homologous. Mitchell et al. (1996) located a Net-positive cluster of cells posterior to the commissures in Drosophila (Fig. 2D) and suggested that these cells might correspond to the MNB cluster. A comparable Net-positive cluster of cells (Fig. 2, C and H) is found in Artemia. Gerberding and Scholtz (2001) also indicated that the a0 and c0 midline cells of Orchestia are homologous to the glial precursor of Drosophila. Expression of Net is detected in midline glial cells during formation of the Drosophila (Fig. 2, D and F) and Artemia (Fig. 2, C and E) ventral nerve cords, providing molecular evidence that these cells are homologous. A number of other potential homologies are noted above. Identification of additional molecular markers for all of these midline cells in Artemia in the future will provide further support for midline cell homology.
Divergent developmental mechanisms produce homologous arthropod nervous systems
Despite differences in the ways that the midline cells and ventral nerve cords are formed in Drosophila and Artemia, different developmental paths ultimately lead to the same destination: establishment of a nerve cord with a ladder-like appearance. Although the structures produced are ultimately similar, one could imagine that changing the temporal or spatial expression of axon guidance molecules could also potentially result in different morphologies. Such changes could be responsible for generating divergent neural circuitry patterns.
It is possible that divergent mechanisms of nerve cord formation may be related to the different ways that segments are established in long-germ insects versus crustaceans. Although segments form simultaneously in long germ insects like Drosophila, crustaceans such as Artemia (Manzanares et al. 1996; Copf et al. 2003) and short-germ insects generate segments sequentially over time (reviewed by Davis and Patel 2002). Future research may examine nerve cord formation in additional arthropods in order to address this issue. It will be interesting to determine whether the Artemia mechanism for generating a nerve cord is found in other crustaceans or short-germ insects.
Similar early divergences in the development of homologous cells/structures have been noted in arthropod nervous system development (reviewed by Whitington 1996). For example, insect and malacostracan crustacean neuroblasts differ in several ways. In insects, the neuroblast/epidermoblast fate choice is governed by a lateral inhibition process (reviewed by Campos-Ortega 1993). However, in the malacostracan crustacean ventral neuroectoderm, NB formation is lineage invariant, and a lateral inhibition process does not specify NB cell fate (Dohle 1970; Dohle and Scholtz 1988). Furthermore, unlike insect NBs, malacostracan NBs do not delaminate from the neuroectoderm. Also, after their first division, some malacostracan NBs switch and become epidermoblasts but can later switch back to producing GMCs (Dohle 1970; Dohle and Scholtz 1988), a phenomenon that is not observed in insects. Finally, NBs that remain on the surface, dividing unequally to produce GMCs, have been observed in only one member of the crustacean Class Branchiopoda: the enteromostracan L. kindti (Gerberding 1997). NBs in other branchiopods produce neurons through a general inward proliferation of cells (Weygoldt 1960; Benesch 1969). Despite these differences, expression of neural markers is conserved among arthropods (Duman-Scheel and Patel 1999). Thus, although many early divergences in neurogenesis exist, homologous neurons and ventral nerve cords are ultimately formed.
Conserved netrin expression in a variety of developing tissues
afrNet expression was detected in many developing tissues outside of the nervous system, including the salt gland (Fig. 4A), epidermis (Fig. 4, B and C), dorsal (Fig. 4D) and visceral (Fig. 4E) mesoderm, legs (Fig. 4G), antennae, and mandible (Fig. 4H). Many of these expression domains are conserved between Drosophila and Artemia. For example, Net expression is detected in the Drosophila visceral mesoderm, including the hindgut, where afrNet accumulation is detected. Dorsal mesodermal expression in Drosophila corresponds to the dorsal muscles and cardiac cells, and it is likely that dorsal mesoderm expression of Net in Artemia corresponds to these cell types. Expression of even-skipped in dorsal muscle and cardiac mesoderm is also conserved between insects and crustaceans (Duman-Scheel et al. 2002). Thus expression of two molecular markers is conserved in insect and crustacean dorsal mesodermal derivatives. The role of Net in the development of these tissues is presently unknown.
Accumulation of afrNet in the limbs (Fig. 4, G and H) is interesting in light of the observation that the Drosophila Nets are detected in developing tracheal cells (Harris et al. 1996; Mitchell et al. 1996). Limb expression of several tracheal-inducing genes has recently been detected in crustaceans (Mitchell and Crews 2002; Franch-Marro et al. 2006) and may be indicative of an evolutionary relationship between the insect trachea and the crustacean gill (Franch-Marro et al. 2006). Although detection of another common marker between these structures is notable, a role for Nets in Drosophila tracheal development has not yet been established. However, these observations suggest that Nets may play similar roles in the development of many developing tissues in distantly related arthropods.
Acknowledgments
We thank Nipam Patel, William Browne, Matthias Gerberding, Lisa Nagy, and Paul Beach for their suggestions, and Fletcher for his patient assistance. We are grateful to Jack Duman, Dawn Verleye, and Sandy Sass for help with antibody production, Kevin Mitchell for providing Drosophila net DNA, Ellen Wilch for transporting DNA, and Gary Belovsky for suggestions on rearing brine shrimp. The Michigan State RTSF Facility performed sequencing reactions. The Albion College Developmental Biology 324 Class aided in primer construction and testing. This project was funded by Albion College Faculty Development, Blanchard, and Foundation for Interdisciplinary Study Awards, as well as NIH Award R15 NS 048904-0 to M. D. S. A. H. and B. H. are supported by an NIH Supplement to R15 NS48904-0. The Albion Foundation for Undergraduate Research and Scholarly Activity (FURSCA) funded E. G., S. C., and W. S., NSF Award DUE-0511302 to MDS has supported the incorporation of aspects of this research into the teaching laboratory at Albion College.
References
- Benesch R. Zur ontogenie und morphologie von Artemia salina. L Zool Jb Anat Ont. 1969;86:307–458. [Google Scholar]
- Blanchard CE. Pioneer neurons and the early development of the nervous system in Artemia. In: Sorgeloss P, Bengtson DA, Declair W, Jaspers E, editors. Artemia Research and its Applications. I. Universa Press; Wetteren, Belgium: 1987. p. 122. [Google Scholar]
- Brankatschk M, Dickson BJ. Netrins guide Drosophila commissural axons at short range. Nat Neurosci. 2006;9:188–194. doi: 10.1038/nn1625. [DOI] [PubMed] [Google Scholar]
- Campos-Ortega J. Early neurogenesis in Drosophila melanogaster. In: Martinez Arias A, editor. The Development of Drosophila Melanogaster. Cold Spring Harbor Laboratory Press; Plainview, NY: 1993. pp. 1091–1130. [Google Scholar]
- Chan SS, et al. UNC-40, a C. elegans homolog of DCC (deleted in colorectal cancer), is required in motile cells responding to UNC-6 netrin cues. Cell. 1996;87:187–195. doi: 10.1016/s0092-8674(00)81337-9. [DOI] [PubMed] [Google Scholar]
- Copf T, Rabet N, Celniker SE, Averof M. Posterior patterning genes and the identification of a unique body region in the brine shrimp Artemia franciscana. Development. 2003;130:5915–5927. doi: 10.1242/dev.00835. [DOI] [PubMed] [Google Scholar]
- Davis GK, Patel NH. Short, long and beyond: molecular and embryological approaches to insect segmentation. Ann Rev Entomol. 2002;47:669–699. doi: 10.1146/annurev.ento.47.091201.145251. [DOI] [PubMed] [Google Scholar]
- Doe CQ, Goodman C. Embryonic development of the Drosophila central nervous system. In: Martinez Arias A, editor. The Development of Drosophila Melanogaster. Cold Spring Harbor Laboratory Press; Plainview, NY: 1993. pp. 1091–1130. [Google Scholar]
- Dohle W. Die Bildung und differenzierung des postnauplialen keimtreifs von Diastylis rathkei (Crustacea, Cumacea). I. Die bildung der teloblasten und ihrer derivate. Z Morph Tiere. 1970;67:307–392. [Google Scholar]
- Dohle W, Scholtz G. Clonal analysis of the crustacean segment: the discordance between genealogical and segmental borders. Development. 1988;104(suppl):147–160. [Google Scholar]
- Duman-Scheel M, Patel NH. Analysis of molecular marker expression reveals neuronal homology in distantly related arthropods. Development. 1999;126:2327–2334. doi: 10.1242/dev.126.11.2327. [DOI] [PubMed] [Google Scholar]
- Duman-Scheel M, Pirkl N, Patel NH. Analysis of the expression pattern of Mysidium columbiae wingless provides evidence for conserved mesodermal and retinal patterning processes among insects and crustaceans. Dev Genes Evol. 2002;212:114–123. doi: 10.1007/s00427-002-0215-6. [DOI] [PubMed] [Google Scholar]
- Fazeli A, et al. Phenotype of mice lacking functional Deleted in colorectal cancer (Dcc) gene. Nature. 1997;386:796–804. doi: 10.1038/386796a0. [DOI] [PubMed] [Google Scholar]
- Franch-Marro X, Martin N, Averof M, Casanova J. Association of tracheal placodes with leg primordia in Drosophila and implications for the origin of insect tracheal systems. Development. 2006;133:785–790. doi: 10.1242/dev.02260. [DOI] [PubMed] [Google Scholar]
- Freeman JA. Segment morphogenesis in Artemia larvae. In: Warner AH, Macrae TH, Bagshaw JC, editors. Cell and Molecular Biology of Artemia Development. Plenum Press; New York: 1989. pp. 77–90. [Google Scholar]
- Gan WB, Wong VY, Phillips A, Ma C, Gershon TR, Macagno ER. Cellular expression of a leech netrin suggests roles in the formation of longitudinal nerve tracts and in regional innervation of peripheral targets. J Neurobiol. 1999;40:103–115. [PubMed] [Google Scholar]
- Gerberding M. Germ band formation and early neurogenesis of Leptodora kindti (Cladocera): first evidence for neuroblasts in the entomostracan crustaceans. Invert Reprod Dev. 1997;32:63–73. [Google Scholar]
- Gerberding M, Schotz G. Cell linage of the midline cells in the amphipod crustacean Orchestia cavmana (Crustacea, Malacostraca) during formation and separation of the germ band. Dev Genes Evol. 1999;209:91–102. doi: 10.1007/s004270050231. [DOI] [PubMed] [Google Scholar]
- Gerberding M, Scholtz G. Neurons and glia in the midline of the higher crustacean Orchestia cavimana are generated via an invariant cell lineage that comprises a median neuroblast and glial progenitors. Dev Biol. 2001;235:397–409. doi: 10.1006/dbio.2001.0302. [DOI] [PubMed] [Google Scholar]
- Gong Q, Rangarajan R, Seeger M, Gaul U. The Netrin receptor Frazzled is required in the target for establishment of retinal projections in the Drosophila visual system. Development. 1999;126:1451–1456. doi: 10.1242/dev.126.7.1451. [DOI] [PubMed] [Google Scholar]
- Harris R, Sabatelli LM, Seeger MA. Guidance cues at the Drosophila CNS midline: identification and characterization of two Drosophila Netrin/UNC-6 homologs. Neuron. 1996;17:217–228. doi: 10.1016/s0896-6273(00)80154-3. [DOI] [PubMed] [Google Scholar]
- Harzsch S, Glötzner J. An immunohistochemical study of structure and development of the nervous system in the brine shrimp Artemia salina Linnaueus, 1758 (Brachiopoda, Anostraca) with remarks on the evolution of the arthropod brain. Arthropod Struct Dev. 2002;30:251–270. doi: 10.1016/s1467-8039(02)00012-9. [DOI] [PubMed] [Google Scholar]
- Hedgecock EM, Culotti JG, Hall DH. The unc-5, unc-6, and unc-40 genes guide circumferential migrations of pioneer axons and mesodermal cells on the epidermis in C. elegans. Neuron. 1990;4:61–85. doi: 10.1016/0896-6273(90)90444-k. [DOI] [PubMed] [Google Scholar]
- Hiramoto M, Hiromi Y. Robo directs axon crossing of segmental boundaries by suppressing responsiveness to relocalized Netrin. Nat Neurosci. 2006;9:58–66. doi: 10.1038/nn1612. [DOI] [PubMed] [Google Scholar]
- Hiramoto M, Hiromi Y, Giniger E, Hotta Y. The Drosophila netrin receptor frazzled guides axons by controlling netrin distribution. Nature. 2000;406:886–889. doi: 10.1038/35022571. [DOI] [PubMed] [Google Scholar]
- Kaprielian Z, Runko E, Imondi R. Axon guidance at the midline choice point. Dev Dyn. 2001;221:154–181. doi: 10.1002/dvdy.1143. [DOI] [PubMed] [Google Scholar]
- Keino-Masu K, et al. Deleted in Colorectal Cancer (DCC) encodes a netrin receptor. Cell. 1996;87:175–185. doi: 10.1016/s0092-8674(00)81336-7. [DOI] [PubMed] [Google Scholar]
- Keleman K, Dickson BJ. Short- and long-range repulsion by the Drosophila Unc5 Netrin receptor. Neuron. 2001;32:605–617. doi: 10.1016/s0896-6273(01)00505-0. [DOI] [PubMed] [Google Scholar]
- Kolodziej PA, et al. frazzled encodes a Drosophila member of the DCC immunoglobulin subfamily and is required for CNS and motor axon guidance. Cell. 1996;87:197–204. doi: 10.1016/s0092-8674(00)81338-0. [DOI] [PubMed] [Google Scholar]
- Manzanares M, Williams TA, Marco R, Garesse R. Segmentation in the crustacean Artemia: engrailed staining studied with an antibody raised against the Artemia protein. Roux's Arch Dev Biol. 1996;205:424–431. doi: 10.1007/BF00377222. [DOI] [PubMed] [Google Scholar]
- Mitchell B, Crews S. Expression of Artemia trachealess gene in the salt gland and epipod. Evol Dev. 2002;4:433–353. doi: 10.1046/j.1525-142x.2002.02023.x. [DOI] [PubMed] [Google Scholar]
- Mitchell KJ, et al. Genetic analysis of Netrin genes in Drosophila: Netrins guide CNS commissural axons and peripheral motor axons. Neuron. 1996;17:203–215. doi: 10.1016/s0896-6273(00)80153-1. [DOI] [PubMed] [Google Scholar]
- Patel N. In situ hybridization to whole mount Drosophila embryos. In: Krieg P, editor. A Laboratory Guide to RNA: Isolation, Analysis, and Synthesis. Wiley-Liss; New York: 1996. pp. 357–370. [Google Scholar]
- Patel NH. Imaging neuronal subsets and other cell types in whole-mount Drosophila embryos and larvae using antibody probes. Meth Cell Biol. 1994;44:445–487. doi: 10.1016/s0091-679x(08)60927-9. [DOI] [PubMed] [Google Scholar]
- Patel NH, Kornberg TB, Goodman CS. Expression of engrailed during segmentation in grasshopper and crayfish. Development. 1989;107:201–212. doi: 10.1242/dev.107.2.201. [DOI] [PubMed] [Google Scholar]
- Schrehardt A. A scanning electron-microscope study of the post-embryonic development of Artemia. In: Sorgeloss P, Bengtson DA, Declair W, Jaspers E, editors. Artemia Research and Its Applications. I. Universa Press; Wetteren, Belgium: 1987. pp. 5–32. [Google Scholar]
- Swofford DL. PAUP* Phylogenetic Analysis Using Parsimony (*and Other Methods) Sinauer Associates; Sunderland, MA: 2001. [Google Scholar]
- Tessier-Lavigne M, Goodman CS. The molecular biology of axon guidance. Science. 1996;274:1123–1133. doi: 10.1126/science.274.5290.1123. [DOI] [PubMed] [Google Scholar]
- Thomas JB, Bastiani MJ, Bate M, Goodman CS. From grasshopper to Drosophila: a common plan for neuronal development. Nature. 1984;310:203–207. doi: 10.1038/310203a0. [DOI] [PubMed] [Google Scholar]
- Weygoldt P. Embryologische untersuchungen an ostrakoden: die entwicklung von Cyprideis litoralis. Zool Jb Anat Ont. 1960;78:369–426. [Google Scholar]
- Whitington PM. Evolution of neural development in the arthropods. Semin Cell Dev Biol. 1996;7:605–614. [Google Scholar]
- Whitington PM, Leach D, Sandeman R. Evolutionary change in neural development within the arthropods: axonogenesis in the embryos of two crustaceans. Development. 1993;118:449–461. doi: 10.1242/dev.118.2.449. [DOI] [PubMed] [Google Scholar]



