SUMMARY
Group I metabotropic glutamate receptors (mGluR) induce long-term depression (LTD) that requires protein synthesis. Here, we demonstrate that Arc/Arg3.1 is translationally induced within 5 min of mGluR activation, and this response is essential for mGluR-dependent LTD. The increase in Arc/Arg3.1 translation requires eEF2K, a Ca2+/calmodulin-dependent kinase that binds mGluR and dissociates upon mGluR activation, whereupon it phosphorylates eEF2. Phospho-eEF2 acts to slow the elongation step of translation and inhibits general protein synthesis but simultaneously increases Arc/Arg3.1 translation. Genetic deletion of eEF2K results in a selective deficit of rapid mGluR-dependent Arc/Arg3.1 translation and mGluR-LTD. This rapid translational mechanism is disrupted in the fragile X disease mouse (Fmr1 KO) in which mGluR-LTD does not require de novo protein synthesis but does require Arc/Arg3.1. We propose a model in which eEF2K-eEF2 and FMRP coordinately control the dynamic translation of Arc/Arg3.1 mRNA in dendrites that is critical for synapse-specific LTD.
INTRODUCTION
Long-lasting forms of synaptic plasticity require de novo protein synthesis (Kelleher et al., 2004; Wang and Tiedge, 2004). N-methyl-D-aspartate (NMDA) receptor-dependent long-term potentiation (LTP) of the Schaffer collateral-CA1 synapse lasting longer than ∼60 min is blocked by agents that halt translation. Late-phase NMDA-dependent LTD in hippocampal slice cultures is also protein synthesis dependent (Kauderer and Kandel, 2000). De novo protein synthesis is also required for forms of long-term depression (LTD) that are induced by group I metabotropic glutamate receptor (mGluR) activation or by paired-pulse low-frequency stimulation (PP-LFS) (Huber et al., 2000). In contrast to NMDA receptor-dependent LTP and LTD, where the requirement for protein synthesis is delayed, mGluR-LTD requires de novo protein synthesis within 5–10 min. (Huber et al., 2000).
The products of de novo protein synthesis are hypothesized to be “captured” at active synapses (Frey and Morris, 1997), but their identity remains unknown. Arc/Arg3.1 is an immediate-early gene (IEG) that is induced by NMDA receptor activation in vivo (Link et al., 1995; Lyford et al., 1995) and mediates a postsynaptic endocytic pathway by interacting with endophilin 2/3 and dynamin that selectively traffics AMPA receptors (AMPAR) (Chowdhury et al., 2006). The activity-dependent expression of Arc/Arg3.1 mRNA and protein underlie a homeostatic mechanism that maintains a precise level of AMPAR-dependent excitability in conditions of persistently increased or decreased synaptic input (Shepherd et al., 2006). Studies presented here demonstrate that Arc/Arg3.1 is also required for mGluR and PP-LFS LTD. In contrast to changes in Arc/Arg3.1 expression that occur over hours to days in homeostatic plasticity, mGluR activation results in increases in Arc/Arg3.1 protein within 3–5 min. Using biochemical and genetic approaches, we demonstrate that mGluR evokes rapid Arc/Arg3.1 translation via a signaling pathway that involves eukaryotic elongation factor 2 kinase (eEF2K) and eukaryotic elongation factor 2 (eEF2). eEF2 is required for the elongation step of translation (Ryazanov et al., 1988), while phospho-eEF2 acts as a potent inhibitor of the elongation step in a manner that is similar to chemical protein synthesis inhibitors, such as cycloheximide (Begueret et al., 1977; Obrig et al., 1971). Our data support a model in which rapid translational upregulation of Arc/Arg3.1 is required for mGluR-dependent LTD, and this translational induction is generated as a consequence of local and transient inhibition of the translation of other mRNAs via phospho-eEF2.
Our studies also provide insights into the molecular basis of altered synaptic plasticity in fragile X mental retardation syndrome. Fragile X syndrome, which is the most common inherited cause of mental retardation and autism (O’Donnell and Warren, 2002), is caused by an expansion of CGG in the 5′ untranslated region of the fragile X mental retardation protein (FMRP) gene (Fmr1) that reduces its expression. FMRP binds to G-quartet-containing RNAs through the RGG box (Darnell et al., 2001; Schaeffer et al., 2001). FMRP functions as a translational repressor of specific synaptic mRNAs by a proposed mechanism that may involve BC1 RNA, a nontranslatable message abundant in dendrites (Zalfa et al., 2003; but see also Iacoangeli et al., 2008). FMRP is also reported to function as part of a RISC nuclease complex that represses translation by RNA interference (Caudy et al., 2002; Ishizuka et al., 2002; Jin et al., 2004). In Fmr1 knockout (KO) mice, Arc/Arg3.1, α-CaMKII, and MAP1B proteins are reported to be elevated both in total brain and synaptosomal fractions (Zalfa et al., 2003). Notably, an increased association of Arc/Arg3.1 mRNA with polyribosomes suggests that a greater fraction of Arc/Arg3.1 mRNA is being actively translated in Fmr1 KO. The notion that misregulated expression of these proteins might underlie cognitive deficits in fragile X syndrome is supported by physiological studies that implicate FMRP in altered synaptic plasticity. Fmr1 KO mice show robust mGluR-LTD (Huber et al., 2002) that is insensitive to protein synthesis inhibitors (Hou et al., 2006; Nosyreva and Huber, 2006), suggesting that, when translation is derepressed due to the absence of FMRP, “LTD proteins” that are normally produced in a stimulus-dependent manner are now continuously synthesized and are sufficient to sustain mGluR-LTD without the requirement for de novo protein synthesis. Insight into the molecular basis of mGluR and FMRP-dependent translation is provided by the observations that the efficacy of FMRP to inhibit translation is regulated by phosphorylation (Ceman et al., 2003) and that mGluR activation results in dephosphorylation of FMRP by PP2A within 1 min that is linked to rapid translational upregulation of a target protein, SAPAP3 (Narayanan et al., 2007). A similar rapid mGluR- and FMRP-dependent increase in α-CaMKII, PSD-95 (Muddashetty et al., 2007), and MAP1B (Davidkova and Carroll, 2007) has been reported, and MAP1B is suggested to play a role in glutamate receptor trafficking in cultured neurons. In the present study, we find that rapid, de novo Arc/Arg3.1 expression is disrupted in Fmr1 KO neurons, and Arc/Arg3.1/Fmr1 double KO mice show reduced mGluR-LTD. These observations suggest that eEF2K/eEF2 and FMRP-dependent translational pathways are cofunctional in controlling a rapid and transient switch of the translational machinery to mRNAs that are essential for mGluR-dependent synaptic depression.
RESULTS
mGluR-LTD and PP-LFS LTD Require Arc/Arg3.1
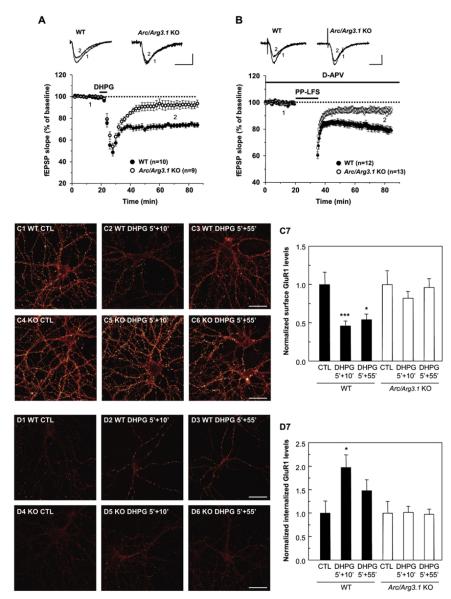
To examine the role of Arc/Arg3.1 in mGluR-LTD, we monitored Schaffer collateral-CA1 synapses in acute hippocampal slices prepared from wild-type (WT) and Arc/Arg3.1 knockout (KO) mice. Baseline synaptic properties, including the fiber volley-fEPSP relationship (an index of basal synaptic strength) and paired-pulse facilitation ratio were normal in Arc/Arg3.1 KO mice (see Figures S1A and S1B available online), confirming a previous report (Plath et al., 2006). In WT slices, treatment with the group I mGluR agonist DHPG (50 μM) for 5 min followed by washout produced a stable reduction of synaptic strength (72.8% ± 2.0% of baseline, mean ± SEM) (Figure 1A). Synaptic stimulation using the paired-pulse low-frequency stimulation (PP-LFS) protocol in the presence of the NMDA receptor antagonist D-APV (50 μM) resulted in a similar stable reduction of synaptic strength to 79.9% ± 2.1% of baseline (Figure 1B). In Arc/Arg3.1 KO slices, treatment with DHPG (92.1% ± 3.7% of baseline, p < 0.001 compared to littermate WT controls by unpaired two-tailed Student’s t test) or PP-LFS (94.3% ± 2.1% of baseline, p < 0.0001) failed to evoke robust LTD, albeit there is a slight residual LTD in Arc/Arg3.1 KO slices (p = 0.09 for DHPG-LTD and p = 0.03 for PP-LFS LTD by paired t test). The residual LTD suggests that an Arc/Arg3.1-independent pathway also contributes to mGluR-LTD. The immediate short-term synaptic depression during the induction period with DHPG and immediately following the PP-LFS protocol was not significantly different between WT and KO mice (Figures 1A and 1B). Furthermore, mGluR-LTD induced by higher concentration of DHPG (100 μM) was also impaired in Arc/Arg3.1 KO slices (88.5% ± 7.4% of baseline for Arc/Arg3.1 KO slices; 64.4% ± 2.6% of baseline for WT slices, p < 0.01), indicating that the requirement for Arc/Arg3.1 does not depend on a specific range of mGluR activation (Figure S1C).
Figure 1. Arc/Arg3.1 Is Required for Hippocampal mGluR-LTD.
Field excitatory postsynaptic potentials (fEPSPs) were recorded in the hippocampal Schaffer collateral-CA1 synapses derived from Arc/Arg3.1 KO mice and compared to WT littermate controls.
(A) Average time course of the change in fEPSP slope induced by the group I mGluR agonist (R,S)-DHPG (50 μM, for 5 min). LTD of WT mice was 72.8% ± 2.0% of baseline at t = 70 min (n = 10). In Arc/Arg3.1 KO, fEPSPs were 92.1% ± 3.7% of the baseline at t = 70 min (n = 9). p < 0.001 when compared to littermate WT. Error bars indicate the standard error of the mean. Measurements correspond to the time points indicated on the time course graph in this and all subsequent figures.
(B) Time course of the change in fEPSP slope produced by paired-pulse low-frequency stimulation (PP-LFS: at 1 Hz, 50 ms interstimulus interval, for 15 min) in the presence of the NMDA receptor antagonist D-APV (50 μM). LTD of WT mice was 79.9% ± 2.1% of baseline at t = 80 min (n = 12). In Arc/Arg3.1 KO mice, fEPSPs were 94.3% ± 2.1% of the baseline at t = 80 min (n = 13). p < 0.0001. Scale bars, 0.5 mV/10 ms.
(C) Five minutes of DHPG application resulted in a loss of surface GluR1 at 15 min (n = 20, ***p < 0.005) and 60 min (n = 19, *p < 0.05) after DHPG application, compared to untreated controls in WT hippocampal cultures. Arc/Arg3.1 KO neurons did not exhibit any changes in surface GluR1 levels after DHPG treatment. Representative pictures of cultures are shown using an LUT scale where white is high intensity and dark red is low intensity. CTL, control.
(D) Five minutes of DHPG application resulted in an increase of internalized GluR1 at 15 min (n = 20, *p < 0.05) compared with untreated cultures. Arc/Arg3.1 KO neurons did not exhibit changes in internalized GluR1 levels after DHPG treatment. Error bars indicate SEM in this and all subsequent figures.
mGluR-Dependent AMPA Receptor Endocytosis Requires Arc/Arg3.1
mGluR1/5 activation results in a rapid and sustained loss of surface AMPARs that underlies synaptic depression (Snyder et al., 2001). Since Arc/Arg3.1 KO mice have deficient mGluR-LTD, we directly tested whether Arc/Arg3.1 is required for mGluR-dependent AMPAR endocytosis. DHPG (50 μM) was applied to DIV 14–21 primary hippocampal neurons for 5 min followed by washout, and surface and internalized AMPARs were measured 15 or 60 min after DHPG application. In WT cultures, DHPG resulted in a significant loss of surface GluR1 at 15 and 60 min as compared with untreated cultures (Figures 1C1–1C3 and 1C7). However, GluR1 surface levels were unchanged after DHPG application in Arc/Arg3.1 KO neurons (Figures 1C4–1C6 and 1C7). WT cultures exhibited a significant increase in internalized GluR1 at 15 min (Figures 1D1–1D3 and 1D7). Arc/Arg3.1 KO neurons did not exhibit an increase in internalized receptors after DHPG application (Figures 1D4–1D6 and 1D7). Thus, we conclude that Arc/Arg3.1 is required for rapid, mGluR-dependent AMPAR endocytosis.
mGluR Induces Rapid Translation of Preexisting Arc/Arg3.1 mRNA
If Arc/Arg3.1 plays a direct role in mGluR-LTD, we anticipated that its protein level should be acutely regulated in dendrites. Therefore, Arc/Arg3.1 protein expression was examined by immunocytochemistry in DIV14 hippocampal cultures. The basal level of Arc/Arg3.1 protein was low but increased significantly in both the soma and dendrites during the 5 min incubation with DHPG (50 μM) (Figure 2A). The increase of Arc/Arg3.1 protein was blocked by the protein synthesis inhibitor emetine, indicating a role for de novo translation. The induced Arc/Arg3.1 immunoreactivity in both proximal and distal dendrites was detected within 5 min of mGluR activation, and there was no evidence of a concentration gradient that might occur with rapid transport of Arc/Arg3.1 from the soma. The rapidity and distribution of the response suggests that Arc/Arg3.1 is synthesized locally in dendrites and is consistent with the observation that mGluR-LTD is expressed in isolated dendrites (Huber et al., 2000). Similar levels of Arc/Arg3.1 induction during 5 min incubation of DHPG were observed by western blot analysis using forebrain cultures (Figure S2A). Treatment with BDNF (10 ng/ml) also increased Arc/Arg3.1 protein expression, but, in contrast to DHPG, this was evident only after 40 min (Figure S2A).
Figure 2. Arc/Arg3.1 Protein Is Rapidly Synthesized by Group I mGluR Activation.
(A) Stimulation of hippocampal neurons with DHPG (50 μM) for 5 min increased Arc/Arg3.1 immunoreactivity in both cell body (1.34 ± 0.063 of untreated soma, n = 13) and dendrites (1.58 ± 0.095 of untreated dendrites, n = 38). The rapid increase of Arc/Arg3.1 was blocked by the protein synthesis inhibitor emetine (10 ng/ml, 10 min).
(B) High-dose cycloheximide (CHX, 50 μM, total 10 min: 5 min pretreatment and 5 min with or without DHPG) blocked the induction of Arc/Arg3.1 by DHPG (5 min).
(C) Transcription inhibitor actinomycin D (ActD: 10 μM, 5 min pretreatment and 5 min with or without DHPG) did not block the induction of Arc/Arg3.1 by DHPG (5 min).
(D) Low-dose CHX increased the level of Arc/Arg3.1 protein. Neurons were treated with vehicle or various doses of CHX for 10 min. Total protein synthesis was measured by counting the incorporation of 35S methionine and cysteine in TCA precipitant.
(E) Statistical analysis of western blots. Five minute treatment of DHPG significantly increased the level of Arc/Arg3.1. Inhibition of new protein synthesis by high dose of cycloheximide not only blocked the induction of Arc/Arg3.1 protein but also slightly decreased the level of Arc/Arg3.1 upon stimulation with DHPG. Inhibition of transcription by actinomycin D did not affect the level of Arc/Arg3.1. Low-dose CHX (50–100 nM, 5 min pretreatment and 5 min with or without DHPG) increased the level of Arc/Arg3.1, which was not further induced by DHPG. *p < 0.05, **p < 0.01.
(F) The level of Arc/Arg3.1 mRNA was measured using real-time RT-PCR. Stimulation of neurons with BDNF (10 ng/ml) and forskolin (50 μM) induced the level of Arc/Arg3.1 mRNA 40 min and 20 min after stimulation, respectively. DHPG slightly increased the level of Arc/Arg3.1 mRNA at 20 and 40 min after stimulation.
*p < 0.05, **p < 0.01, ***p < 0.005.
The rapid increase of Arc/Arg3.1 protein could be mediated by an enhanced rate of translation or by a stable level of translation together with reduced degradation. As reported previously (Rao et al., 2006), the proteosome inhibitor MG132 increased Arc/Arg3.1 protein but did not block the ability of DHPG to further increase Arc/Arg3.1 (Figure S2B). Induction of Arc/Arg3.1 by DHPG at 5 min was blocked by 5 min pretreatment of emetine or cycloheximide (Figures 2A7 and 2B). These data support the notion that Arc/Arg3.1 induction following DHPG treatment involves an increase in the rate of de novo protein translation.
To examine the possible role of de novo transcription of Arc/Arg3.1 mRNA, we monitored the effect of the transcription blocker actinomycin D. Actinomycin D (10 μM, 5 min pretreatment and 5 min with or without DHPG) did not alter the DHPG-induced increase of Arc/Arg3.1 protein (Figures 2C and 2E). DHPG did evoke a modest increase of Arc/Arg3.1 mRNA, but this was detected only after 20 min (Figure 2F). The time course of the delayed Arc/Arg3.1 protein expression by DHPG or BDNF correlated with the mRNA induction, and actinomycin D blocked this response (data not shown). These observations suggest that the rapid increase in de novo translation requires Arc/Arg3.1 mRNA that is present in neurons prior to DHPG stimulation, while the delayed Arc/Arg3.1 expression is coupled to de novo transcription. We note that Arc/Arg3.1 mRNA is detected in dendrites of unstimulated cultured neurons (Giorgi et al., 2007), and we detect Arc/Arg3.1 mRNA in stratum radiatum of the hippocampal CA1 region from home-caged mice (Figure S3).
Low Dose Cycloheximide Can Increase Arc/Arg3.1 Protein Expression
In examining the dose dependence of cycloheximide’s actions, we noted that the level of Arc/Arg3.1 protein rapidly increased when neurons are treated with low doses (Figure 2D). For example, 100 nM cycloheximide increased Arc/Arg3.1 protein within 10 min. Even at these low doses, cycloheximide effectively reduced general protein synthesis. 100 nM cycloheximide reduced the total incorporation of 35S-labeled methionine and cysteine into TCA precipitant to ∼60%. Previous studies have noted the paradoxical action of low-dose cycloheximide to increase the synthesis of specific proteins and rationalized this action by suggesting that global reduction of elongation can increase the availability of factors that are required for translation initiation of specific transcripts that are poorly initiated under control conditions (Fernandez et al., 2005; Gupta and Ono, 1997; Perlman and Feldman, 1982; Scheetz et al., 2000; Walden and Thach, 1986). This notion contributed to our analysis of the eEF2 pathway (below), since activated eEF2 inhibits elongation and can paradoxically increase translation of certain mRNAs (Chotiner et al., 2003; Scheetz et al., 2000).
eEF2K Physically Associates with Homer and Group I mGluRs
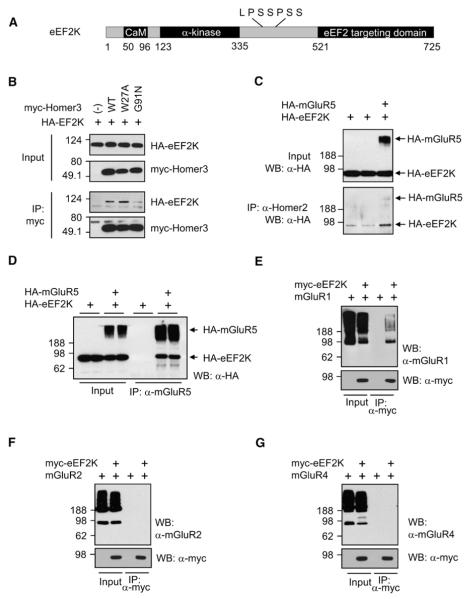
Homer proteins bind group I mGluRs and play a role in their signaling by also binding signaling partners, including IP3R (Tu et al., 1998). Homer proteins bind two known sequence motifs: PPxxF (type 1) and LPSSPSS (type 2) (Yuan et al., 2003). When we searched for candidate Homer-binding molecules (http://us.expasy.org/cgi-bin/scanprosite), we found that eEF2K possesses a type 2 Homer-binding motif (Figure 3A). eEF2K is a highly conserved enzyme that phosphorylates and regulates its only known substrate, eEF2 (Ryazanov et al., 1988). The N-terminal half of eEF2K contains a Ca2+-calmodulin (CaM) binding site, which is required for its activation, and an α kinase domain. The C-terminal half of eEF2K functions as a targeting domain that is required for it to phosphorylate eEF2. A linker region between the N and C terminus includes the putative Homer-binding site and is phosphorylated by multiple signaling kinases, including PI3K/mTOR/S6K, MAPK, and PKA (Browne and Proud, 2002).
Figure 3. eEF2K Binds Homer and mGluR1/5.
(A) Schematic diagram of eEF2K. The N terminus of eEF2K contains a Ca2+/calmodulin (CaM) binding motif and an α-kinase domain. The C-terminal eEF2 targeting domain, which recruits the substrate, eEF2, is linked to the hyperphosphorylated internal region. Putative Homer binding site is shown above the diagram.
(B) Coimmunoprecipitation (co-IP) of eEF2K and Homer. HA-tagged (HA-) eEF2K was coexpressed with myc-tagged WT, W27A, or G91N Homer3 in HEK293T cells, and IP was performed with antimyc antibody. HA-eEF2K co-IPed with WT or W27A Homer 3 was coexpressed, but not with G91N Homer.
(C) mGluR5 increases the interaction of eEF2K and Homer. HA-eEF2K was transfected with or without HA-mGluR5. IP was performed by anti-Homer2 antibody, which IPed endogenous Homer2 protein. Western blot was performed using anti-HA antibody. Co-IP of HA-eEF2K was increased when mGluR5 was coexpressed.
(D) eEF2K co-IPs with mGluR5. HEK293T cells were transfected with HA-eEF2K with or without HA-mGluR5, and lysates were IPed with anti-mGluR5 antibody and blotted with anti-HA antibody. eEF2K co-IPed only when mGluR was coexpressed. Samples were boiled before loading to aggregate and separate mGluR5 monomer from eEF2K.
(E) mGluR1 co-IPs with eEF2K. HEK293T cells were transfected with mGluR1 and eEF2K, and lysates were IPed with mycAb. Samples were not boiled to show the monomer of mGluRs.
(F and G) mGluR2 and mGluR4 do not co-IP with eEF2K.
eEF2K and Homer coimmunoprecipitated (co-IP) from HEK293T cells (Figure 3B). The EVH1 domain of Homer is required to bind eEF2K, and mutation of a critical binding surface for polyproline ligands (Homer3 G91N) disrupted binding. As anticipated by the conservation of their EVH1 domains, Homer 1, -2, and -3 bind eEF2K (data not shown).
We examined conditions that might regulate Homer-eEF2K binding and found that coexpression of mGluR5 strongly enhanced binding (Figure 3C). Moreover, eEF2K was found to interact with group I mGluRs independently of Homer. The interaction of eEF2K and group I mGluRs was observed even when Homer was not coexpressed (Figure 3D and 3E), and eEF2K bound to mGluR5 mutants that do not bind Homer (Figures S4A and S4B). eEF2K also co-IPed with mGluR1 (Figure 3E), another member of group I mGluRs, but not with other mGluRs, including mGluR2 and mGluR4 (Figure 3F and 3G).
To identify regions of eEF2K that are critical for binding Homer and mGluR, we preformed a deletion analysis of eEF2K (Figures S4C and S4D). The linker region of eEF2K, which includes the type 2 Homer ligand, appears essential for binding Homer, since N-terminal fragments that include this region bind, while C-terminal fragments or N-terminal fragments that do not include the linker region do not bind. eEF2K binding to mGluR5 appears to be more complex, since both N- and C-terminal fragments of eEF2K bind mGluR5 (Figure S4C). These data suggest that mGluR, Homer, and eEF2K assemble by multiple interactions into a tertiary complex.
The Interaction of eEF2K with mGluR Is Dynamic and Is Modulated by Ca2+ and mGluR Activity
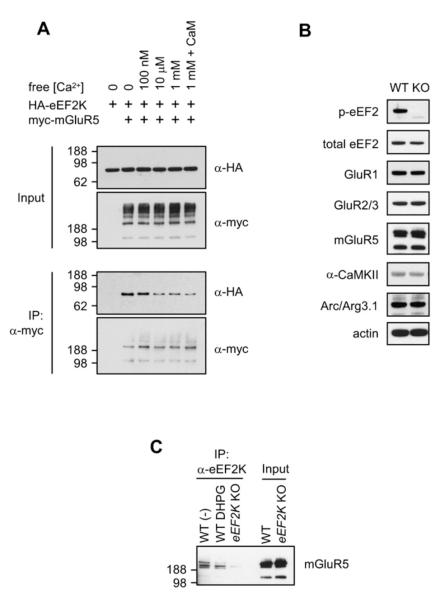
The kinase activity of eEF2K is known to be regulated by Ca2+ via its Ca2+-CaM-binding domain (Nairn and Palfrey, 1987; Ryazanov, 1987). To test whether Ca2+ modulates the mGluR5-eEF2K binding, co-IP experiments were performed using lysates from cotransfected HEK293T cells in the presence of defined concentrations of free Ca2+ (Figure 4A). Co-IP was robust at [Ca2+] less than 1 μM but markedly decreased at concentrations >10 μM. mGluR5 binding to a C-terminal fragment of eEF2K that lacks the CaM-binding domain but retains binding to mGluR5 was not inhibited by [Ca2+] (Figure S4E). These results indicate that [Ca2+] can modulate the interaction of group I mGluRs with eEF2K and suggest a role for CaM binding to eEF2K.
Figure 4. Dynamic Interaction of eEF2K and mGluR5.
(A) Calcium dissociates eEF2K from mGluR5. HEK293T cells were transfected with HA-eEF2K with or without myc-mGluR5, and cells were harvested with lysis buffer without calcium or containing various concentrations of free calcium. Calmodulin (CaM) (25 μg/ml) was also added to the lysis buffer as indicated. Binding was decreased at [Ca2+] higher than 10 μM.
(B) Phospho-eEF2 was not detected in the hippocampus of eEF2K KO, while the level of total eEF2, GluR1, Glur2/3, mGluR5, α-CaMKII, Arc/Arg3.1, and actin was not altered in eEF2K KO mice compared to WT littermate controls.
(C) Synaptoneurosomes, prepared from the forebrain of eEF2K KO and WT mice, were stimulated with vehicle or DHPG for 20 min. Synaptoneurosomes were then lysed and immunoprecipitated with anti-eEF2K antibody. mGluR5 co-IPed with eEF2K only in WT samples. Stimulation of synaptoneurosomes with DHPG decreased the co-IP of mGluR5.
We used eEF2K KO mice in our analysis of mGluR-eEF2K binding. eEF2K KO mice were viable and fertile (Ryazanov, 2002) and showed the anticipated absence of phosphorylated eEF2 (Thr56) (Figure 4B). The levels of several synaptic proteins were not altered in the hippocampus of KO mice (Figure 4B). Synaptoneurosomes from forebrains of WT and eEF2K KO mice were prepared and stimulated with DHPG for 20 min. Co-IP experiments using anti-eEF2K antibody confirmed that native mGluR5 associated with eEF2K (Figure 4C). The co-IP of mGluR5 was reduced when synaptoneurosomes were stimulated with DHPG. Interaction of endogenous mGluR5 and eEF2K was also reduced upon DHPG stimulation of cultured neurons (Figure S4F). We conclude that mGluR and eEF2K associate in vivo and that their interaction is reduced by mGluR activation.
Group I mGluRs Dynamically Regulate the Phosphorylation of eEF2
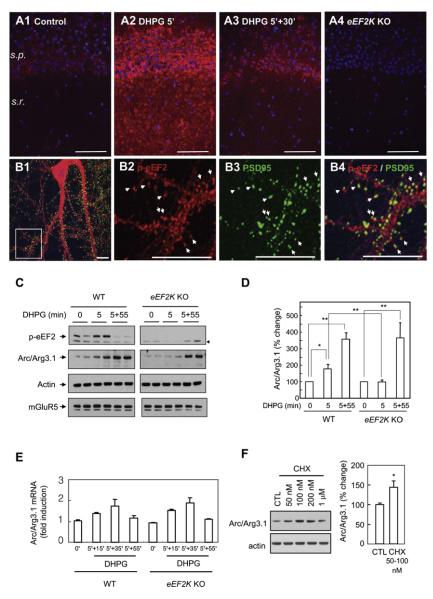
Activated eEF2K selectively phosphorylates eEF2 (Ryazanov et al., 1988). To assess whether mGluR activates this pathway in conditions that evoke LTD, we monitored the level of phospho-eEF2 in hippocampal slices of either WT or eEF2K KO mice using the same stimulus parameters that induce mGluR-LTD. Activation of mGluR increased the phosphorylation of eEF2 in the stratum pyramidal (s.p.), and stratum radiatum (s.r.) of the hippocampal CA1 region within 5 min (Figure 5A). By 30 min after washout of DHPG, the level of phospho-eEF2 was reduced to prestimulation level. No phosphorylation of eEF2 was detected in eEF2K KO slices. The transient induction of phospho-eEF2 by DHPG was confirmed by western blot analysis in hippocampal slices (Figure S5A).
Figure 5. Rapid Induction of Arc/Arg3.1 by Group I mGluRs Is Dependent on eEF2K.
(A) Hippocampal slices were prepared from WT and eEF2K KO mice and were stimulated with DHPG for 5 min. phospho-eEF2 (p-eEF2, red) in area CA1 was increased by DHPG within 5 min and declined by 30 min following washout. Specificity of phospho-eEF2 was confirmed by staining of eEF2K KO slices. s.p., stratum pyramidal; s.r., stratum radiatum.
(B) Cultured hippocampal neurons were treated with DHPG for 5 min and stained with phospho-eEF2 (red) and PSD95 (green) antibodies on DIV14. phospho-eEF2 showed punctal distribution in dendritic spines and dendritic shafts. phospho-eEF2 in spines colocalized with PSD95 (arrows). (B2), (B3), and (B4) are enlarged images of the rectangular region of (B1).
(C and D) mGluR-dependent rapid synthesis of Arc/Arg3.1 is absent in eEF2K KO neurons. Neurons from the forebrains of WT or eEF2K KO mice were cultured for DIV14 and treated with DHPG (50 μM, 5 min). Phosphorylation of eEF2 was undetectable in eEF2K KO neurons. No difference in the level of mGluR5 was observed between WT and eEF2K KO neurons. An arrowhead indicates a non-specific band. p values were obtained by paired t test comparing basal and drug-treated levels. p values for comparison of WT and eEF2K KO mice were obtained by Student’s t test. *p < 0.05, **p < 0.01, n = 8. Error bars are SEM.
(E) Arc/Arg3.1 mRNA expression is not altered in eEF2K KO neurons. The level of Arc/Arg3.1 mRNA was measured in WT and eEF2K KO neurons following the stimulation with DHPG.
(F) Low-dose cycloheximide (CHX) increases Arc/Arg3.1 protein expression. Cultured eEF2K KO neurons were treated with indicated doses of CHX for 10 min. *p < 0.05, n = 8.
To further examine dendritic localization of eEF2K activity, DIV14 neurons were stimulated with DHPG for 5 min and stained with phospho-eEF2 and PSD95, a marker for excitatory synapses (Figure 5B). Phospho-eEF2 was present in dendritic shafts and the cell body. Phospho-eEF2 also showed a distinct punctal distribution in spines that colocalized with PSD95. Staining was absent in eEF2K KO cultures (data not shown). This result is consistent with a previous report that translational regulators, including eEF2K, are enriched in synaptic fractions (Asaki et al., 2003).
Phosphorylation of eEF2 is known to inhibit translational elongation. Therefore, we examined the prediction that global protein translation might be transiently reduced co-incident with the transient increase of phospho-eEF2. Stimulation of neurons with DHPG for 5 min transiently decreased the incorporation of 35S amino acids into TCA precipitants, and this effect was reversed 20 min after washout of DHPG (Figure S5B). A previous study reported that DHPG rapidly increased protein synthesis in synaptoneurosomes (Weiler et al., 2004). We observed that DHPG did not induce p-eEF2 in synaptoneurosomes (data not shown), and it is possible that the eEF2-dependent translational mechanism is not maintained in broken cell preparations.
Rapid De Novo Arc/Arg3.1 Translation Is Selectively Absent in eEF2K KO Neurons
Arc/Arg3.1 expression was examined in DIV14 forebrain neuronal cultures prepared from WT and eEF2K KO mice. The steady-state expression of Arc/Arg3.1 protein was identical in WT and eEF2K KO neurons; however, the increase in Arc/Arg3.1 protein 5 min after DHPG in WT neurons was absent in eEF2K KO neurons in both biochemical (Figures 5C and 5D) and immunocytochemical assays (Figure S6). By contrast, Arc/Arg3.1 protein was induced to the same extent in WT and eEF2K KO neurons 60 min after DHPG stimulation. Arc/Arg3.1 mRNA was identical in WT and eEF2K KO neurons prior to application of DHPG and increased identically at 40 min after stimulation in both WT and eEF2K KO neurons (Figure 5E). Accordingly, the lack of rapid induction of Arc/Arg3.1 protein in the eEF2K KO neurons is not due to reduced Arc/Arg3.1 mRNA expression. We also note that mGluR signaling that is required for induction of Arc/Arg3.1 mRNA and the delayed increase of Arc/Arg3.1 protein are intact in eEF2K KO neurons. Moreover, Arc/Arg3.1 protein expression is identical in the hippocampus of WT and eEF2K KO mice (Figure 4B), indicating that eEF2K is not required for basal expression of Arc/Arg3.1 protein in vivo.
If the failure of DHPG to induce rapid synthesis of Arc/Arg3.1 protein in the eEF2K KO neurons is due to a selective interruption of the action of phospho-eEF2, we predicted that a low dose of cycloheximide, which does not require eEF2K or phopho-eEF2 to inhibit the elongation step, should induce the synthesis of Arc/Arg3.1 protein in eEF2K KO neurons. Treatment of DIV14 eEF2K KO neurons with low-dose cycloheximide (50 nM and 100 nM) increased the level of Arc/Arg3.1 protein in eEF2K KO neurons (Figure 5F), similar to WT neurons (Figure 2D). High-dose cycloheximide (>1 uM) did not induce Arc/Arg3.1 in either WT and eEF2K KO neurons. The ability of low-dose cycloheximide to rescue rapid Arc/Arg3.1 induction indicates that mechanisms that mediate rapid Arc/Arg3.1 translation subsequent to inhibition of elongation are intact in eEF2K KO neurons.
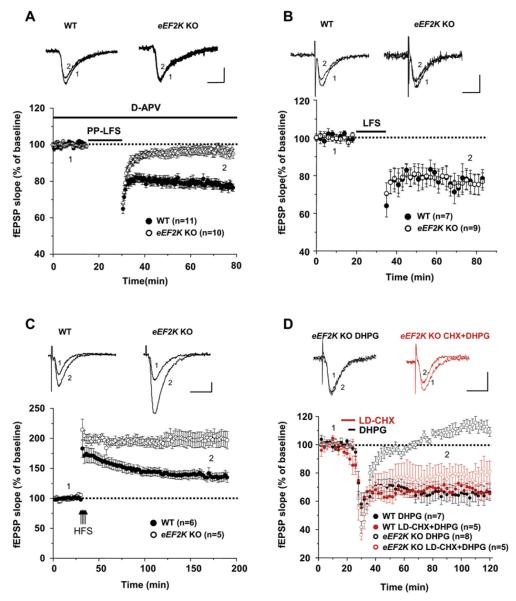
mGluR-LTD and PP-LFS LTD Are Selectively Absent in eEF2K KO Hippocampal Slices
The role of eEF2K in plasticity of the Schaffer collateral-CA1 synapse was tested using acute hippocampal slices. Baseline measures of synaptic strength and presynaptic function were not altered in the eEF2K KO slices (Figure S7). However, LTD induced by either PP-LFS (97.5% ± 2.4% of baseline) or DHPG (108.7% ± 3.6% of baseline) was impaired in the eEF2K KO slices (Figures 6A and 6D). The immediate short-term synaptic depression following DHPG stimulation was identical in WT and eEF2K KO slices; however, synaptic strength returned to near baseline levels in the eEF2K KO slices. Similarly, synaptic transmission returned to near baseline levels within 10 min of completion of the PP-LFS protocol. In contrast to the marked deficit of mGluR-dependent LTD, NMDAR-dependent LTD was identical in time course and stability in slices derived from eEF2K KO mice (72.7% ± 2.2% of baseline) compared to WT mice (73.1% ± 3.4% of baseline) (Figure 6B). LTP was also preserved (Figure 6C). LTP of Schaffer collateral-CA1 synapses was induced by four trains of high-frequency stimulation with an intertrain interval of 3 s. In WT slices, fEPSP was increased to 171.5% ± 13.4% of baseline immediately after stimulation (t = 30 min) and sustained at the level of 138.4% ± 7.7% of baseline at t = 175 min. These stimulus parameters are reported to evoke a form of synaptic plasticity that requires de novo protein synthesis for maintenance longer than ∼60 min and is referred to as late LTP (L-LTP) (Kelleher et al., 2004). In slices prepared from eEF2K KO mice, the initial induction was 204.6% ± 8.9% of baseline at t = 30 min, and this was sustained for 3 hr after stimulation (200.1% ± 11.9% of baseline at t = 175 min) (Figure 6C). The magnitude of LTP was significantly greater in eEF2K KO than WT after 30 min of induction (p < 0.005). These results indicate that eEF2K KO disrupts mGluR-LTD but does not alter NMDAR-dependent LTD or early LTP. The apparent enhancement of latephase LTP deserves further study.
Figure 6. mGluR-LTD Is Impaired in Hippocampal Slices Derived from eEF2K KO Mice.
fEPSPs were recorded in the hippocampal CA1 region of slices derived from eEF2K KO mice and compared to WT littermate controls.
(A) Time course of the change in fEPSP slope produced by paired-pulse low-frequency stimulation (PP-LFS: at 1 Hz, 50 ms interstimulus interval, for 15 min) in the presence of D-APV (50 μM). LTD of WT mice was 77.0% ± 2.1% of baseline at t = 75 min (n = 13). In eEF2K KO mice, fEPSPs were 97.5% ± 2.4% of baseline t = 75 min (n = 15) (p < 0.0001).
(B) Time course of the change in fEPSP slope by low-frequency stimulation (LFS: 1 Hz for 15 min). This form of NMDAR-dependent LTD was not altered in eEF2K KO hippocampal slices (72.7% ± 2.2% of baseline at t = 75 min, n = 9) compared to WT (73.1% ± 3.4% of baseline at t = 75 min, n = 7) (p > 0.5).
(C) Late-phase of LTP was induced by four stimulus trains (100 Hz each) with an intertrain interval of 3 s. In WT, fEPSPs were increased to 171.5% ± 13.4% of baseline immediately after stimulation (t = 30 min) and were sustained at the level of 138.4% ± 7.7% of baseline at t = 175 min (n = 6). However, in eEF2K KO, the initial LTP (204.6% ± 8.9% of baseline at t = 30 min) was maintained for 3 hr after stimulation (200.1% ± 11.9% of baseline at t = 175 min, n = 5). LTP was significantly greater in slices derived from eEF2K KO mice compared to those from WT mice at this time point (p < 0.005).
(D) Average time course of the change in fEPSP slope induced by DHPG (50 μM, for 5 min). LTD of WT mice was 64.7% ± 5.2% of baseline at t = 90 min (n = 7). In eEF2K KO mice, LTD was significantly impaired (108.7% ± 3.6% of baseline at t = 90 min, n = 8). Treatment with low-dose cycloheximide (LD-CHX, 50–75 nM) for 10 min starting from 5 min prior to DHPG restored DHPG-LTD in eEF2K KO (75.7% ± 7.4%, n = 5). In WT mice, treatment with LD-CHX did not alter the expression of LTD (69.0% ± 2.6%, n = 5). p < 0.001 when eEF2K KO DHPG only was compared to eEF2K KO LD-CHX + DHPG, WT DHPG only, or WT LD-CHX + DHPG. Scale bars, 0.5 mV/10 ms.
Our proposed mechanism for the mGluR-LTD deficit in the eEF2K KO slices is linked to failure to rapidly translate Arc/Arg3.1. Since low-dose cycloheximide induced Arc/Arg3.1 synthesis and did not depend on phospho-eEF2, we examined the possibility that cycloheximide could rescue mGluR-LTD in slices from eEF2K KO mice. We found that a 10 min exposure to 50–75 nM cycloheximide (low-dose CHX: LD-CHX) beginning 5 min prior to addition of DHPG rescued mGluR-dependent LTD in the eEF2K KO slice (75.7% ± 7.4% of baseline, p < 0.001, compared to DHPG only in eEF2K KO slices) (Figure 6D). The same treatment of WT slices did not substantially alter the time course of mGluR-LTD (69.0% ± 2.6% of baseline, p > 0.5, compared to DHPG only in WT slices). Low-dose cycloheximide had no effect on baseline synaptic transmission in the absence of mGluR stimulation (101.2% ± 2.0% for WT slices; 100.4% ± 4.6% for eEF2K KO slices). These observations confirm that mGluR signaling required for mGluR-LTD is selectively impaired in eEF2K KO in a manner that can be rescued by transient application of low-dose cycloheximide.
mGluR-LTD, but Not Homeostatic Plasticity, Is Disrupted in eEF2K KO Neurons in Culture
To further assess the selectivity of the eEF2K KO effect on neuronal function, we examined two forms of neuronal plasticity that can be assayed in primary neuronal cultures. Treatment of cultures with DHPG for 5 min to evoke mGluR-LTD reduced the ratio of surface to total GluR2/3 by ∼30% in WT neurons but did not significantly reduce this measure in eEF2K KO neurons (Figures S8A and S8B). This result parallels the deficit of mGluR-LTD seen in acute slices. Cultures were also assayed for homeostatic adaptations of surface AMPA receptors since this response is markedly altered in Arc/Arg3.1 KO neurons (Shepherd et al., 2006). Treatment of eEF2K KO cortical cultures for 2 days with either tetrodotoxin (TTX) or bicuculline evoked homeostatic increases and decreases of surface GluR1 that were identical to WT neurons (Figure S8C). Thus, eEF2K KO results in a selective disruption of mGluR-dependent LTD.
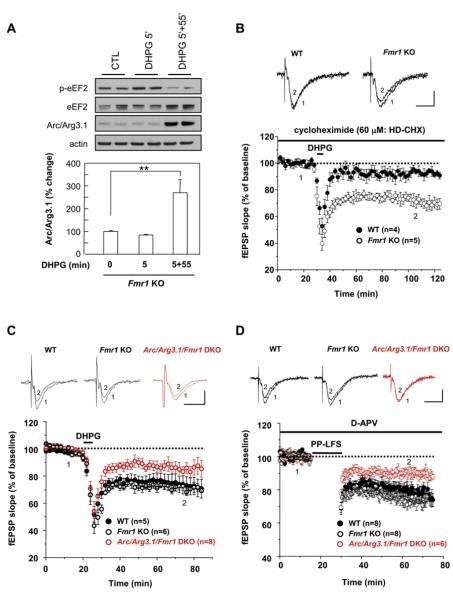
Fmr1 KO Disrupts Rapid, but Not Delayed, Induction of Arc/Arg3.1 Protein
We examined the role of the eEF2K/eEF2/Arc mechanism in the aberrant plasticity described in Fmr1 KO mice. FMRP has been reported to bind Arc/Arg3.1 mRNA (Iacoangeli et al., 2008; Zalfa et al., 2003) and is hypothesized to inhibit translation prior to mGluR stimulation (Bear et al., 2004; Narayanan et al., 2007). To assess whether FMRP might be critical for either rapid or delayed induction of Arc/Arg3.1 protein following mGluR stimulation, we prepared primary neuronal cultures from Fmr1 KO mice and stimulated with DHPG. Arc/Arg3.1 expression in unstimulated cultures was not consistently different between WT and Fmr1 KO neurons. Moreover, Arc/Arg3.1 protein increased 60 min after DHPG stimulation in Fmr1 KO neurons identically as in WT neurons (Figure 7A). However, the rapid increase of Arc/Arg3.1 protein following DHPG stimulation was absent in Fmr1 KO neurons (Figure 7A). DHPG activated mGluR/eEF2K signaling in the Fmr1 KO neurons since phospho-eEF2 was identically induced as in WT neurons (Figure 7A). Assays of Arc/Arg3.1 protein stability and induction following proteasome inhibition with MG132 did not reveal differences between WT and Fmr1 KO neurons (Figures S9B and S9C). Biochemical experiments to monitor Arc/Arg3.1 expression using acute hippocampal slices revealed that basal Arc/Arg3.1 expression was highly variable even when normalized to total protein or actin, indicating a limitation of this preparation (Taubenfeld et al., 2002). When examined histochemically, basal Arc/Arg3.1 varied through the thickness of the slice (data not shown). We conclude that Fmr1 KO neurons selectively lack the ability to rapidly upregulate Arc/Arg3.1 expression. The reported increase of Arc/Arg3.1 mRNA in polysome fractions from Fmr1 KO mice (Zalfa et al., 2003) suggests that failure to detect a DHPG-evoked rapid increase of Arc/Arg3.1 protein is linked to elevated constitutive expression.
Figure 7. LTD Is Impaired in Hippocampal Slices Derived from Arc/Arg3.1/Fmr1 Double KO Mice.
(A) DIV14 Fmr1 KO neurons were treated with DHPG as indicated in Figure 5C. Rapid but not delayed synthesis of Arc/Arg3.1 was absent in Fmr1 KO. The regulation of phospho-eEF2 was intact in Fmr1 KO neurons.
(B) High-dose cycloheximide (60 μM: HD-CHX) did not block DHPG-LTD of Fmr1 KO slices. In the presence of a high dose of cycloheximide, DHPG-LTD of Fmr1 KO was 72.3% ± 4.8% of baseline at t = 105 min (n = 5), while DHPG-LTD in WT (FVB) slices was blocked (fEPSP was 95.5% ± 2.9% of baseline at t = 105 min (n = 4); p < 0.01 when Fmr1 KO was compared to FVB WT).
(C) Average time course of fEPSP slope of Arc/Arg3.1/Fmr1 double KO (DKO) mice. mGluR-LTD was induced by DHPG (50 μM, for 5 min). DHPG-LTD of Arc/Arg3.1/Fmr1 DKO was 85.9% ± 4.1% of baseline at t = 75 min (n = 8). In Fmr1 KO, DHPG-LTD was 68.2% ± 2.6% of baseline at t = 75 min (n = 6). In WT, DHPG-LTD was 73.0% ± 6.6% of baseline at t = 75 min (n = 5). p < 0.01 when Arc/Arg3.1/Fmr1 DKO was compared to either WT or Fmr1 KO. fEPSPs of post-DHPG in Fmr1 KO were not significantly different from those in FVB WT.
(D) Time course of the change in fEPSP slope by PP-LFS. PP-LFS LTD of Arc/Arg3.1/Fmr1 DKO was 88.3% ± 2.1% of baseline at t = 65 min (n = 6). In Fmr1 KO, PP-LFS LTD was 75.5% ± 3.7% of baseline at t = 65 min (n = 8). In FVB WT, PP-LFS LTD was 80.5% ± 2.6% of baseline at t = 65 min (n = 8). p < 0.05 when Arc/Arg3.1/Fmr1 DKO was compared to either WT or Fmr1 KO. fEPSPs of post-DHPG in Fmr1 KO were not significantly different from those in FVB WT (p = 0.4).
Scale bars, 0.5 mV/10 ms.
Arc/Arg3.1 Is Required for mGluR-LTD and PP-LFS LTD in Fmr1 KO Mice
In anticipation of physiological studies to assess the role of Arc/Arg3.1 in synaptic plasticity of Fmr1 KO mice, we examined Arc/Arg3.1 protein expression in hippocampus. Arc/Arg3.1 protein has previously been reported to be modestly upregulated in both total brain and synaptosomal fractions of Fmr1 KO mice (Zalfa et al., 2003). However, in our assays, Arc/Arg3.1 protein was not consistently different in hippocampus (either in vivo or in acute slices) or cortex when care was taken to sacrifice mice without behavioral activation. We generated mice in which both Fmr1 (in FVB background) and Arc/Arg3.1 (in B6 background) were deleted. Double Arc/Arg3.1/Fmr1 KO (DKO) mice are viable, fertile, and not different from WT mice in size or postnatal survival. Indices of basal synaptic transmission were normal in Fmr1 KO and Arc/Arg3.1/Fmr1 DKO (Figures S9D and S9E). As reported previously (Nosyreva and Huber, 2006), DHPG evoked a sustained reduction of synaptic strength (68.2% ± 2.6% of baseline for Fmr1 single KO slices; 73.0% ± 6.6% of baseline for FVB WT slices; Figure 7C). Jackson laboratory provides Fmr1 KO mice in the FVB background, and the magnitude of LTD was not significantly different from FVB WT mice. As reported previously in studies of Fmr1 KO in the B6 background, mGluR-LTD was not inhibited by high-dose cycloheximide (60 μM) (Figure 7B). In Arc/Arg3.1/Fmr1 DKO (in B6/FVB), DHPG evoked an initial reduction of synaptic strength that was not different from WT, Arc/Arg3.1 KO, or Fmr1 KO. However, expression of DHPG-evoked LTD was significantly impaired in Arc/Arg3.1/Fmr1 DKO (85.9% ± 4.1%, p < 0.01 compared to Fmr1 single KO or FVB WT). PP-LFS LTD was also impaired in Arc/Arg3.1/Fmr1 DKO (88.3% ± 2.1% of baseline for Arc/Arg3.1/Fmr1 DKO slices; 75.5% ± 3.7% of baseline for Fmr1 single KO; 80.5% ± 2.6% of baseline for FVB WT slices, p < 0.05 when Arc/Arg3.1/Fmr1 DKO was compared to Fmr1 single KO or FVB WT; Figure 7D). These results indicate that Arc/Arg3.1 is required for mGluR-LTD in both WT and Fmr1 KO neurons. Deletion of Arc/Arg3.1 does not entirely prevent DHPG or PP-LFS LTD, suggesting that additional mechanisms contribute to the aberrant LTD in Fmr1 KO mice.
DISCUSSION
Arc/Arg3.1 Plays a Central Role in mGluR-Dependent LTD
The present study identifies Arc/Arg3.1 as a molecule that is required for certain forms of LTD that are known to be dependent on rapid, de novo protein synthesis, including mGluR-LTD and PP-LFS-LTD. Upon activation of group I mGluRs, Arc/Arg3.1 protein is rapidly upregulated in a process that requires de novo translation. Arc/Arg3.1 protein functions as a regulatory factor for a postsynaptic endocytic signaling pathway that includes endophilins 2 and -3 and dynamin, which together mediate selective endocytosis of AMPA type glutamate receptors (Chowdhury et al., 2006). Accordingly, the local and rapid increase of Arc/Arg3.1 protein can be linked to the selective downregulation of AMPAR at specific synapses during LTD (Figure 8).
Figure 8. eEF2K, FMRP, and Rapid De Novo Translation of Arc/Arg3.1 Protein in mGluR-LTD.
Group I mGluRs activate eEF2K via calcium-calmodulin (CaM). eEF2K phosphorylates eEF2, which inhibits elongation generally but increases Arc/Arg3.1 translation. Arc/Arg3.1 forms a complex with endophilin2/3 (Endo) and dynamin (Dyn) and induces the internalization of AMPAR (Chowdhury et al., 2006). FMRP inhibits the translation of Arc/Arg3.1 at the basal state. Arc/Arg3.1 induction alone is not sufficient for mGluR-LTD, indicating that mGluR activates another pathway that is required to internalize AMPAR (Cho et al., 2008). In Fmr1 KO mice, the synthesis of Arc/Arg3.1 protein is constitutively derepressed, and de novo synthesis of Arc/Arg3.1 is not required for mGluR-LTD.
While group I mGluR activation results in a modest rise of Arc/Arg3.1 mRNA, the translation that underlies mGluR-LTD utilizes Arc/Arg3.1 mRNA that is present in neurons prior to stimulation. Arc/Arg3.1 is regulated as an immediate-early gene that is strongly induced during learning-related behaviors (Guzowski et al., 1999) and expressed in discrete populations of neurons that are part of behaviorally activated networks even when rodents are resting in home cage (Marrone et al., 2008). Arc/Arg3.1 mRNA is present in dendritic regions of CA1 neurons of mice sacrificed immediately from their home cage (Figure S3), indicating that basal Arc/Arg3.1 mRNA can be either activity independent or related to prior neuronal activity. The rapid translation of dendritic Arc/Arg3.1 mRNA rationalizes the known dependence of mGluR-LTD on rapid de novo protein synthesis and independence of de novo RNA synthesis (Huber et al., 2000). It also suggests that prior neuronal activity that drives the expression of Arc/Arg3.1 mRNA could modify subsequent mGluR-dependent plasticity.
In further support for the notion that Arc/Arg3.1 is an essential LTD molecule, we find that mGluR-LTD in Fmr1 KO mice is also dependent on Arc/Arg3.1. This is notable because mGluR-LTD in Fmr1 KO mice is distinctly different from mGluR-LTD in WT mice in several ways, including insensitivity to proteosome inhibitors, protein synthesis inhibitors, and inhibitors of ERK signaling (Hou et al., 2006). Disruption of Arc/Arg3.1 function is the first experimental manipulation shown to reduce mGluR-LTD in Fmr1 KO slices, but it is likely that additional molecular mechanisms contribute to the response since mGluR-LTD is not entirely absent in the Arc/Arg3.1/Fmr1 DKO. Since FMRP functions to repress translation of specific mRNAs, the dependence mGluR-LTD in the Fmr1 KO mouse suggests that Arc/Arg3.1 protein that is present prior to mGluR stimulation can mediate this response and thereby confers resistance to protein synthesis inhibitors.
Group I mGluRs and Rapid De Novo Translation; Convergence of eEF2K/eEF2 and FMRP Pathways
mGluR activation induces the rapid translation of Arc/Arg3.1 and requires eEF2K. eEF2K binds Homer and group I mGluRs and dissociates from this complex in a Ca2+-dependent manner. Phospho-eEF2 is present at excitatory synapses and dendrites and is rapidly upregulated in cultured neurons and hippocampal slices by stimulation of group I mGluRs with DHPG. The ability of phospho-eEF2 to inhibit translation is thought to be general for all mRNAs and appears central to its role in regulating Arc/Arg3.1 protein translation since low-dose cycloheximide, which mimics phospho-eEF2 by inhibiting translation elongation, can also induce Arc/Arg3.1 expression. Low-dose cycloheximide has been reported to increase the translation of mRNAs that are poorly initiated in basal conditions, including α-CaMKII (Fernandez et al., 2005; Gupta and Ono, 1997; Perlman and Feldman, 1982; Scheetz et al., 2000; Walden and Thach, 1986). Both α-CaMKII and Arc/Arg3.1 mRNAs are localized to dendrites (Mori et al., 2000; Steward et al., 1998) and contain internal ribosome entry site (IRES) sequences in their 5′ untranslated regions (Pinkstaff et al., 2001), which may allow the initiation of translation by both Cap-dependent and Cap-independent mechanisms (Pestova et al., 2001). Cap-dependent initiation of translation is tightly controlled by initiation factors such as eukaryotic initiation factor 4E (eIF4E) and eIF4E-binding protein (4EBP). By contrast, Capindependent initiation through IRES sequence may allow synthesis of proteins when cap-dependent initiation factors are not fully active (Pestova et al., 2001). The seemingly paradoxical effect of phospho-eEF2 and cycloheximide to increase Arc/Arg3.1 protein translation is consistent with a model in which inhibition of global elongation results in a rapid increase in factors that are rate limiting for translation initiation of mRNAs that may be inefficiently initiated under basal conditions (Fernandez et al., 2005; Scheetz et al., 2000; Walden and Thach, 1986). Whether the putative IRES sequence of Arc/Arg3.1 mRNA is required for this response remains to be determined.
mGluR activity also rapidly regulates FMRP-dependent signaling in a manner that can enhance rapid, de novo translation (Muddashetty et al., 2007; Narayanan et al., 2007; Westmark and Malter, 2007). mGluR activation results in dephosphorylation of FMRP, and this reduces its inhibitory action on translation (Narayanan et al., 2007). Dephosphorylation of FMRP occurs within 1 min of mGluR activation and parallels the time course of mGluR-dependent phosphorylation of eEF2. Polysome profiles from WT and Fmr1 KO brain indicate that FMRP is associated with mRNAs that are not in active translation. In the absence of FMRP, specific mRNAs including Arc/Arg3.1 shift to fractions that include actively translating polyribosomes (Zalfa et al., 2003). Group I mGluRs are reported to rapidly reduce the histochemical colocalization of FMRP and Fmr1 mRNA at synapses in association with increased translation of de novo FMRP (Antar et al., 2004). While this is an indirect assay of translational regulation, it supports a model in which mGluR activity reduces FMRP binding to specific mRNAs and thereby provides a dynamic increase in mRNAs in close proximity to synapses for translation. FMRP is not required for mGluR-dependent induction of phospho eEF2 (Figure 7A), suggesting that mGluR-FMRP and mGluR-eEF2K are parallel pathways. Accordingly, the rapid, de novo Arc/Arg3.1 translational response required for normal mGluR-LTD appears to require both a transient reversal of FMRP’s repressive effect on translation and the general inhibition of the elongation step by phospho-eEF2 (Figure 8).
De Novo Translated Arc/Arg3.1 Is Specifically Required for Normal mGluR-LTD
Arc/Arg3.1 protein that mediates mGluR-LTD appears to be functionally distinct from the total pool of Arc/Arg3.1 protein expressed in neurons. For example, the basal expression of Arc/Arg3.1 protein in eEF2K KO neurons is identical to that in WT neurons, yet mGluR-LTD and PP-LFS LTD, which are dependent on Arc/Arg3.1, are absent in the eEF2K KO. Arc/Arg3.1 protein in eEF2K KO neurons is functional, since homeostatic scaling, which is also dependent on Arc/Arg3.1 (Shepherd et al., 2006), is intact. This apparent paradox is resolved with the observations that eEF2K KO neurons selectively lack rapid, de novo Arc/Arg3.1 induction, and this deficit is rescued by brief application of low-dose cycloheximide that also rescues mGluR-LTD. Thus, the eEF2-dependent pool of Arc/Arg3.1 protein appears uniquely capable of mediating LTD. In another example demonstrating the distinct properties of Arc/Arg3.1 related to its mode of translation, Arc/Arg3.1 protein is expressed at near normal levels in Fmr1 KO mice, and Arc/Arg3.1 protein is induced after 40 min of DHPG stimulation; however, the rapid increase of Arc/Arg3.1 at 5 min is absent. In this regard, expression of Arc/Arg3.1 protein in the Fmr1 KO appears identical to the eEF2K KO, but with the technical caveat that we cannot detect changes in basal translation of Arc/Arg3.1 that are predicted from other studies to be increased in Fmr1 KO neurons (Zalfa et al., 2003). Our data suggest that an elevation of constitutive Arc/Arg3.1 translation in Fmr1 KO underlies mGluR LTD that is aberrantly independent of de novo translation but dependent on Arc/Arg3.1. This model rationalizes the distinctly different mGluR-LTD phenotypes in eEK2K and Fmr1 KOs even though they show a similar absence of acute mGluR Arc/Arg3.1 induction. The relative amount of this eEF2K/FMRP-dependent pool of Arc/Arg3.1 protein is typically small compared to the Arc/Arg3.1 level of the delayed response to DHPG or BDNF in neuronal cultures, and we could not detect differences in basal and induced Arc/Arg3.1 in WT and eEF2K KO neurons using conventional histochemical techniques. We infer that proteins translated by the eEF2K/FMRP-dependent mechanism are uniquely available for mGluR-LTD and anticipate that new imagining methods with high spatial and temporal resolution may shed light on this prediction.
Our studies also indicate that mGluR-LTD requires a second signal in addition to rapid, de novo Arc/Arg3.1 protein. Experiments that use cycloheximide to rescue mGluR-LTD in the eEF2K KO demonstrate that cycloheximide alone is sufficient to induce Arc/Arg3.1 protein but does not induce LTD unless accompanied by mGluR activation. Similarly, low-dose cycloheximide induces Arc/Arg3.1 in WT neurons but does not induce LTD without concurrent mGluR activation. These observations rationalize why misregulated expression of Arc/Arg3.1 protein in the Fmr1 KO, and presumably other proteins, does not occlude mGluR-LTD. The dual dependence of Arc/Arg3.1 protein and mGluR activity for aberrant LTD in Fmr1 KO mice provides a supporting rationale for treatment regimens of Fragile X syndrome that include group I mGluR antagonists (Bear et al., 2004; McBride et al., 2005). In other studies, we have reported that mGluR-LTD requires the rapid cleavage of the neuronal pentraxin receptor protein (NPR) in a process that involves the extracellular metalloprotease tumor necrosis factor-α converting enzyme (TACE/ADAM17) (Cho et al., 2008). The pentraxin domain, released of its transmembrane tether, appears to capture AMPA receptors at the site of endocytosis, and this mechanism is required for mGluR-LTD in both the hippocampus and cerebellum. It is possible that this NPR pathway functions in conjunction with the Arc/Arg3.1 endosomal pathway and offers a potential new target for agents to modify mGluR-LTD.
EXPERIMENTAL PROCEDURES
Constructs, cell culture, real-time RT-PCR, metabolic labeling, and fluorescence in situ hybridization assay are included in Supplemental Experimental Procedures.
Antibodies
Anti-phospho-eEF2 (Thr56: rabbit polyclonal) and total-eEF2 (rabbit polyclonal) from Cell Signaling; eEF2K (rabbit polyclonal) and mGluR1 (mouse monoclonal) from BD Biosciences; mGluR5, mGluR2, and PSD-95 from Upstate; mGluR4 from Zymed; horse radish peroxidase (HRP) conjugated HA antibody, HRP-conjugated myc antibody, myc (mouse monoclonal), and actin (mouse monoclonal) from Santa Cruz; Arc/Arg3.1 (Lyford et al., 1995); N-GluR1 antibody (Shepherd et al., 2006).
AMPA Receptor Trafficking Experiments and Immunostaining
Labeling of surface or internalized pool of AMPA receptor was performed as described with minor modifications (Shepherd et al., 2006). Briefly, surface GluR1-containing AMPA receptors were labeled by adding 2.5 μg of GluR1-N JH1816 pAb to the neuronal growth media and subsequently incubated at 37°C for 15 or 60 min after 5 min DHPG application. To visualize surface and internalized GluR1, Alexa 555 secondary was added in excess live at 10°C. Neurons were fixed, permeabilized, and subsequently exposed to Alexa 488 secondary to stain internalized receptors (background in the nonpermeabilized control was negligible). Immunocytochemistry of cultured neurons was performed as described (Shepherd et al., 2006). Immunohistochemistry of phospho-eEF2 in WT and eEF2K mice was performed as described (Ramirez-Amaya et al., 2005) with slight modifications. See Supplemental Experimental Procedures for details.
Electrophysiology
Field recording of excitatory postsynaptic potential (fEPSP) of hippocampal CA1 neurons of postnatal day (P)21–30 male mice as described with minor modifications (Huber et al., 2000). mGluR-LTD was induced by an mGluR1/5 agonist, (R,S)-3,5-DHPG, for 5 min (Tocris, 50 μM, unless otherwise indicated), or by paired-pulse low-frequency stimulation (PP-LFS: 50 ms interstimulus interval, 1 Hz, for 15 min) in the presence of D-APV (Tocris, 50 μM). NMDAR-dependent LTD was induced by using 900 single pulses delivered at 1 Hz (Huber et al., 2000).
LTP was measured in Schaffer collateral-CA1 synapses in hippocampal slices derived from 8- to 10-week-old male mice. Late-phase LTP (L-LTP) was induced by four trains of high-frequency stimulation (HFS) (100 Hz, 1 s) with 3 s of intertrain interval. See Supplemental Experimental Procedures for details.
Western Blotting, Immunoprecipitation Assay, and Surface Biotinylation Assay
Western blotting, IP assay, and surface biotinylation assay were performed as previously described (Chowdhury et al., 2006; Cho et al., 2008). See Supplemental Experimental Procedures for details.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by NIMH grants MH053608 (P.F.W.), MH068830 (R.L.H.), MH51106 (D.J.L.), NIDA grant DA00266 (P.F.W.), NIA grant AG019890 (A.G.R.), GM057300 (A.G.R.), and FRAXA (W.K.). We thank Marlin Dehoff and Glory Harris for animal husbandry and David Lieberman for helpful discussions.
Footnotes
SUPPLEMENTAL DATA The Supplemental Data include Supplemental Experimental Procedures and figures and can be found with this article online at http://www.neuron.org/cgi/content/full/59/1/70/DC1/.
REFERENCES
- Antar LN, Afroz R, Dictenberg JB, Carroll RC, Bassell GJ. Metabotropic glutamate receptor activation regulates fragile x mental retardation protein and FMR1 mRNA localization differentially in dendrites and at synapses. J. Neurosci. 2004;24:2648–2655. doi: 10.1523/JNEUROSCI.0099-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asaki C, Usuda N, Nakazawa A, Kametani K, Suzuki T. Localization of translational components at the ultramicroscopic level at postsynaptic sites of the rat brain. Brain Res. 2003;972:168–176. doi: 10.1016/s0006-8993(03)02523-x. [DOI] [PubMed] [Google Scholar]
- Bear MF, Huber KM, Warren ST. The mGluR theory of fragile X mental retardation. Trends Neurosci. 2004;27:370–377. doi: 10.1016/j.tins.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Begueret J, Perrot M, Crouzet M. Ribosomal proteins in the fungus Podospora anserina: evidence for an electrophoretically altered 60S protein in a cycloheximide resistant mutant. Mol. Gen. Genet. 1977;156:141–144. doi: 10.1007/BF00283486. [DOI] [PubMed] [Google Scholar]
- Browne GJ, Proud CG. Regulation of peptide-chain elongation in mammalian cells. Eur. J. Biochem. 2002;269:5360–5368. doi: 10.1046/j.1432-1033.2002.03290.x. [DOI] [PubMed] [Google Scholar]
- Caudy AA, Myers M, Hannon GJ, Hammond SM. Fragile X-related protein and VIG associate with the RNA interference machinery. Genes Dev. 2002;16:2491–2496. doi: 10.1101/gad.1025202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceman S, O’Donnell WT, Reed M, Patton S, Pohl J, Warren ST. Phosphorylation influences the translation state of FMRP-associated polyribosomes. Hum. Mol. Genet. 2003;12:3295–3305. doi: 10.1093/hmg/ddg350. [DOI] [PubMed] [Google Scholar]
- Cho RW, Park JM, Wolff SBE, Xu D, Hopf C, Kim J.-a., Reddy RC, Petralia RS, Perin MS, Linden DJ, Worley PF. mGluR1/5-dependent long-term depression requires the regulated ectodomain cleavage of neuronal pentraxin NPR by TACE. Neuron. 2008;57:858–871. doi: 10.1016/j.neuron.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chotiner JK, Khorasani H, Nairn AC, O’Dell TJ, Watson JB. Adenylyl cyclase-dependent form of chemical long-term potentiation triggers translational regulation at the elongation step. Neuroscience. 2003;116:743–752. doi: 10.1016/s0306-4522(02)00797-2. [DOI] [PubMed] [Google Scholar]
- Chowdhury S, Shepherd JD, Okuno H, Lyford G, Petralia RS, Plath N, Kuhl D, Huganir RL, Worley PF. Arc/Arg3.1 interacts with the endocytic machinery to regulate AMPA receptor trafficking. Neuron. 2006;52:445–459. doi: 10.1016/j.neuron.2006.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell JC, Jensen KB, Jin P, Brown V, Warren ST, Darnell RB. Fragile X mental retardation protein targets G quartet mRNAs important for neuronal function. Cell. 2001;107:489–499. doi: 10.1016/s0092-8674(01)00566-9. [DOI] [PubMed] [Google Scholar]
- Davidkova G, Carroll RC. Characterization of the role of microtubule-associated protein 1B in metabotropic glutamate receptor-mediated endocytosis of AMPA receptors in hippocampus. J. Neurosci. 2007;27:13273–13278. doi: 10.1523/JNEUROSCI.3334-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez J, Yaman I, Huang C, Liu H, Lopez AB, Komar AA, Caprara MG, Merrick WC, Snider MD, Kaufman RJ, et al. Ribosome stalling regulates IRES-mediated translation in eukaryotes, a parallel to prokaryotic attenuation. Mol. Cell. 2005;17:405–416. doi: 10.1016/j.molcel.2004.12.024. [DOI] [PubMed] [Google Scholar]
- Frey U, Morris RG. Synaptic tagging and long-term potentiation. Nature. 1997;385:533–536. doi: 10.1038/385533a0. [DOI] [PubMed] [Google Scholar]
- Giorgi C, Yeo GW, Stone ME, Katz DB, Burge C, Turrigiano G, Moore MJ. The EJC factor eIF4AIII modulates synaptic strength and neuronal protein expression. Cell. 2007;130:179–191. doi: 10.1016/j.cell.2007.05.028. [DOI] [PubMed] [Google Scholar]
- Gupta KC, Ono E. Stimulation of Sendai virus C’ protein synthesis by cycloheximide. Biochem. J. 1997;321:811–818. doi: 10.1042/bj3210811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzowski JF, McNaughton BL, Barnes CA, Worley PF. Environment-specific expression of the immediate-early gene Arc in hippocampal neuronal ensembles. Nat. Neurosci. 1999;2:1120–1124. doi: 10.1038/16046. [DOI] [PubMed] [Google Scholar]
- Hou L, Antion MD, Hu D, Spencer CM, Paylor R, Klann E. Dynamic translational and proteasomal regulation of fragile X mental retardation protein controls mGluR-dependent long-term depression. Neuron. 2006;51:441–454. doi: 10.1016/j.neuron.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Huber KM, Kayser MS, Bear MF. Role for rapid dendritic protein synthesis in hippocampal mGluR-dependent long-term depression. Science. 2000;288:1254–1257. doi: 10.1126/science.288.5469.1254. [DOI] [PubMed] [Google Scholar]
- Huber KM, Gallagher SM, Warren ST, Bear MF. Altered synaptic plasticity in a mouse model of fragile X mental retardation. Proc. Natl. Acad. Sci. USA. 2002;99:7746–7750. doi: 10.1073/pnas.122205699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacoangeli A, Rozhdestvensky TS, Dolzhanskaya N, Tournier B, Schutt J, Brosius J, Denman RB, Khandjian EW, Kindler S, Tiedge H. On BC1 RNA and the fragile X mental retardation protein. Proc. Natl. Acad. Sci. USA. 2008;105:734–739. doi: 10.1073/pnas.0710991105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizuka A, Siomi MC, Siomi H. A Drosophila fragile X protein interacts with components of RNAi and ribosomal proteins. Genes Dev. 2002;16:2497–2508. doi: 10.1101/gad.1022002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin P, Zarnescu DC, Ceman S, Nakamoto M, Mowrey J, Jongens TA, Nelson DL, Moses K, Warren ST. Biochemical and genetic interaction between the fragile X mental retardation protein and the microRNA pathway. Nat. Neurosci. 2004;7:113–117. doi: 10.1038/nn1174. [DOI] [PubMed] [Google Scholar]
- Kauderer BS, Kandel ER. Capture of a protein synthesis-dependent component of long-term depression. Proc. Natl. Acad. Sci. USA. 2000;97:13342–13347. doi: 10.1073/pnas.97.24.13342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelleher RJ, 3rd, Govindarajan A, Tonegawa S. Translational regulatory mechanisms in persistent forms of synaptic plasticity. Neuron. 2004;44:59–73. doi: 10.1016/j.neuron.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Link W, Konietzko U, Kauselmann G, Krug M, Schwanke B, Frey U, Kuhl D. Somatodendritic expression of an immediate early gene is regulated by synaptic activity. Proc. Natl. Acad. Sci. USA. 1995;92:5734–5738. doi: 10.1073/pnas.92.12.5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyford GL, Yamagata K, Kaufmann WE, Barnes CA, Sanders LK, Copeland NG, Gilbert DJ, Jenkins NA, Lanahan AA, Worley PF. Arc, a growth factor and activity-regulated gene, encodes a novel cytoskeleton-associated protein that is enriched in neuronal dendrites. Neuron. 1995;14:433–445. doi: 10.1016/0896-6273(95)90299-6. [DOI] [PubMed] [Google Scholar]
- Marrone DF, Schaner MJ, McNaughton BL, Worley PF, Barnes CA. Immediate-early gene expression at rest recapitulates recent experience. J. Neurosci. 2008;28:1030–1033. doi: 10.1523/JNEUROSCI.4235-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride SM, Choi CH, Wang Y, Liebelt D, Braunstein E, Ferreiro D, Sehgal A, Siwicki KK, Dockendorff TC, Nguyen HT, et al. Pharmacological rescue of synaptic plasticity, courtship behavior, and mushroom body defects in a Drosophila model of fragile X syndrome. Neuron. 2005;45:753–764. doi: 10.1016/j.neuron.2005.01.038. [DOI] [PubMed] [Google Scholar]
- Mori Y, Imaizumi K, Katayama T, Yoneda T, Tohyama M. Two cis-acting elements in the 3′ untranslated region of alpha-CaMKII regulate its dendritic targeting. Nat. Neurosci. 2000;3:1079–1084. doi: 10.1038/80591. [DOI] [PubMed] [Google Scholar]
- Muddashetty RS, Kelic S, Gross C, Xu M, Bassell GJ. Dysregulated metabotropic glutamate receptor-dependent translation of AMPA receptor and postsynaptic density-95 mRNAs at synapses in a mouse model of fragile X syndrome. J. Neurosci. 2007;27:5338–5348. doi: 10.1523/JNEUROSCI.0937-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nairn AC, Palfrey HC. Identification of the major Mr 100,000 substrate for calmodulin-dependent protein kinase III in mammalian cells as elongation factor-2. J. Biol. Chem. 1987;262:17299–17303. [PubMed] [Google Scholar]
- Narayanan U, Nalavadi V, Nakamoto M, Pallas DC, Ceman S, Bassell GJ, Warren ST. FMRP phosphorylation reveals an immediateearly signaling pathway triggered by group I mGluR and mediated by PP2A. J. Neurosci. 2007;27:14349–14357. doi: 10.1523/JNEUROSCI.2969-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosyreva ED, Huber KM. Metabotropic receptor-dependent long-term depression persists in the absence of protein synthesis in the mouse model of fragile X syndrome. J. Neurophysiol. 2006;95:3291–3295. doi: 10.1152/jn.01316.2005. [DOI] [PubMed] [Google Scholar]
- Obrig TG, Culp WJ, McKeehan WL, Hardesty B. The mechanism by which cycloheximide and related glutarimide antibiotics inhibit peptide synthesis on reticulocyte ribosomes. J. Biol. Chem. 1971;246:174–181. [PubMed] [Google Scholar]
- O’Donnell WT, Warren ST. A decade of molecular studies of fragile X syndrome. Annu. Rev. Neurosci. 2002;25:315–338. doi: 10.1146/annurev.neuro.25.112701.142909. [DOI] [PubMed] [Google Scholar]
- Perlman J, Feldman JF. Cycloheximide and heat shock induce new polypeptide synthesis in Neurospora crassa. Mol. Cell. Biol. 1982;2:1167–1173. doi: 10.1128/mcb.2.10.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestova TV, Kolupaeva VG, Lomakin IB, Pilipenko EV, Shatsky IN, Agol VI, Hellen CU. Molecular mechanisms of translation initiation in eukaryotes. Proc. Natl. Acad. Sci. USA. 2001;98:7029–7036. doi: 10.1073/pnas.111145798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkstaff JK, Chappell SA, Mauro VP, Edelman GM, Krushel LA. Internal initiation of translation of five dendritically localized neuronal mRNAs. Proc. Natl. Acad. Sci. USA. 2001;98:2770–2775. doi: 10.1073/pnas.051623398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plath N, Ohana O, Dammermann B, Errington ML, Schmitz D, Gross C, Mao X, Engelsberg A, Mahlke C, Welzl H, et al. Arc/Arg3.1 is essential for the consolidation of synaptic plasticity and memories. Neuron. 2006;52:437–444. doi: 10.1016/j.neuron.2006.08.024. [DOI] [PubMed] [Google Scholar]
- Ramirez-Amaya V, Vazdarjanova A, Mikhael D, Rosi S, Worley PF, Barnes CA. Spatial exploration-induced Arc mRNA and protein expression: evidence for selective, network-specific reactivation. J. Neurosci. 2005;25:1761–1768. doi: 10.1523/JNEUROSCI.4342-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao VR, Pintchovski SA, Chin J, Peebles CL, Mitra S, Finkbeiner S. AMPA receptors regulate transcription of the plasticity-related immediate-early gene Arc. Nat. Neurosci. 2006;9:887–895. doi: 10.1038/nn1708. [DOI] [PubMed] [Google Scholar]
- Ryazanov AG. Ca2+/calmodulin-dependent phosphorylation of elongation factor 2. FEBS Lett. 1987;214:331–334. doi: 10.1016/0014-5793(87)80081-9. [DOI] [PubMed] [Google Scholar]
- Ryazanov AG. Elongation factor-2 kinase and its newly discovered relatives. FEBS Lett. 2002;514:26–29. doi: 10.1016/s0014-5793(02)02299-8. [DOI] [PubMed] [Google Scholar]
- Ryazanov AG, Shestakova EA, Natapov PG. Phosphorylation of elongation factor 2 by EF-2 kinase affects rate of translation. Nature. 1988;334:170–173. doi: 10.1038/334170a0. [DOI] [PubMed] [Google Scholar]
- Schaeffer C, Bardoni B, Mandel JL, Ehresmann B, Ehresmann C, Moine H. The fragile X mental retardation protein binds specifically to its mRNA via a purine quartet motif. EMBO J. 2001;20:4803–4813. doi: 10.1093/emboj/20.17.4803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheetz AJ, Nairn AC, Constantine-Paton M. NMDA receptor-mediated control of protein synthesis at developing synapses. Nat. Neurosci. 2000;3:211–216. doi: 10.1038/72915. [DOI] [PubMed] [Google Scholar]
- Shepherd JD, Rumbaugh G, Wu J, Chowdhury S, Plath N, Kuhl D, Huganir RL, Worley PF. Arc/Arg3.1 mediates homeostatic synaptic scaling of AMPA receptors. Neuron. 2006;52:475–484. doi: 10.1016/j.neuron.2006.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder EM, Philpot BD, Huber KM, Dong X, Fallon JR, Bear MF. Internalization of ionotropic glutamate receptors in response to mGluR activation. Nat. Neurosci. 2001;4:1079–1085. doi: 10.1038/nn746. [DOI] [PubMed] [Google Scholar]
- Steward O, Wallace CS, Lyford GL, Worley PF. Synaptic activation causes the mRNA for the IEG Arc to localize selectively near activated postsynaptic sites on dendrites. Neuron. 1998;21:741–751. doi: 10.1016/s0896-6273(00)80591-7. [DOI] [PubMed] [Google Scholar]
- Taubenfeld SM, Stevens KA, Pollonini G, Ruggiero J, Alberini CM. Profound molecular changes following hippocampal slice preparation: loss of AMPA receptor subunits and uncoupled mRNA/protein expression. J. Neurochem. 2002;81:1348–1360. doi: 10.1046/j.1471-4159.2002.00936.x. [DOI] [PubMed] [Google Scholar]
- Tu JC, Xiao B, Yuan JP, Lanahan AA, Leoffert K, Li M, Linden DJ, Worley PF. Homer binds a novel proline-rich motif and links group 1 metabotropic glutamate receptors with IP3 receptors. Neuron. 1998;21:717–726. doi: 10.1016/s0896-6273(00)80589-9. [DOI] [PubMed] [Google Scholar]
- Walden WE, Thach RE. Translational control of gene expression in a normal fibroblast. Characterization of a subclass of mRNAs with unusual kinetic properties. Biochemistry. 1986;25:2033–2041. doi: 10.1021/bi00356a030. [DOI] [PubMed] [Google Scholar]
- Wang H, Tiedge H. Translational control at the synapse. Neuroscientist. 2004;10:456–466. doi: 10.1177/1073858404265866. [DOI] [PubMed] [Google Scholar]
- Weiler IJ, Spangler CC, Klintsova AY, Grossman AW, Kim SH, Bertaina-Anglade V, Khaliq H, de Vries FE, Lambers FA, Hatia F, et al. Fragile X mental retardation protein is necessary for neurotransmitter-activated protein translation at synapses. Proc. Natl. Acad. Sci. USA. 2004;101:17504–17509. doi: 10.1073/pnas.0407533101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westmark CJ, Malter JS. FMRP mediates mGluR5-dependent translation of amyloid precursor protein. PLoS Biol. 2007;5:e52. doi: 10.1371/journal.pbio.0050052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan JP, Kiselyov K, Shin DM, Chen J, Shcheynikov N, Kang SH, Dehoff MH, Schwarz MK, Seeburg PH, Muallem S, Worley PF. Homer binds TRPC family channels and is required for gating of TRPC1 by IP3 receptors. Cell. 2003;114:777–789. doi: 10.1016/s0092-8674(03)00716-5. [DOI] [PubMed] [Google Scholar]
- Zalfa F, Giorgi M, Primerano B, Moro A, Di Penta A, Reis S, Oostra B, Bagni C. The fragile X syndrome protein FMRP associates with BC1 RNA and regulates the translation of specific mRNAs at synapses. Cell. 2003;112:317–327. doi: 10.1016/s0092-8674(03)00079-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.