Abstract
Alterations in the cytosolic concentration of calcium ions (Ca2+) transmit information that is crucial to the development and function of B cells. Cytosolic Ca2+ concentration is determined by a balance of active transport and gradient driven Ca2+ fluxes, both of which are subject to the influence of multiple receptors and environmental sensing pathways. Recent advances in genomics have allowed for the compilation of an increasingly comprehensive list of Ca2+ transporters and channels expressed by B cells. The accumulating understanding of the function and regulation of these proteins has begun to shift the frontier of Ca2+ physiology in B cells from molecular analysis to determining how diverse inputs to cytosolic Ca2+ concentration are integrated in specific immunological contexts.
Introduction
B cells must respond to diverse chemical and environmental cues. Many of these cues influence the functions of Ca2+ transport proteins, resulting in alterations in cytosolic Ca2+ referred to as Ca2+ signals. Ca2+ signals have the capacity to affect a variety of intracellular processes that are central to cell-fate decisions in B cells, including protein kinase signalling, mitochondrial physiology, apoptosis, nucleocytoplasmic trafficking of transcription factors, chromatin accessibility, and cell adhesion and migration. Here, we review Ca2+ transport and signalling physiology in B cells, placing a special emphasis on the phospholipase C-γ (PLCγ)–inositol-1,4,5-trisphosphate (InsP3)-mediated Ca2+ signalling mechanism used by the B-cell receptor (BCR). We also highlight new mechanisms for the regulation of cytosolic Ca2+ concentration, and discuss how these combined inputs could impact Ca2+-dependent regulation of nuclear factor of activated T cells (NFAT) and nuclear factor-κB (NF-κB) transcription factor pathways and of cell motility in B cells to influence cell-fate choice during humoral immune responses.
The InsP3-mediated Ca2+ signalling pathway
In vertebrate organisms, cytosolic Ca2+ levels are subject to regulation through multiple mechanisms (Table 1), the precise nature and regulation of which vary widely depending on the tissue and cell type. In B cells, many important surface receptors initiate Ca2+ signals through the production and accumulation of the soluble second messenger InsP3 1–3 (Figure 1). InsP3 is produced by the hydrolysis of the membrane lipid phosphatidylinositol-4,-5-bisphosphate (PtdIns(4,5)P2; also known as PIP2), a process that also produces the lipid second messenger diacylglycerol (DAG) (reviewed in 4). PtdIns(4,5)P2 hydrolysis is mediated by members of the PLC enzyme family, which is comprised of several differentially regulated isoforms (reviewed in 5). An important distinction among the various PLC isoforms is that 7-transmembrane spanning G-protein-coupled receptors (GPCRs) activate PLCβ isozymes through the heterotrimeric G-protein Gq and related subunits, whereas tyrosine-kinase-linked receptors, including many growth factor receptors, the T-cell receptor (TCR), the BCR and activating Fc receptors (FcRs), activate PLCγ isozymes through tyrosine phosphorylation.
Table 1.
Cytosolic Ca2+ regulatory mechanisms in lymphocytes
| Mechanism | Biochemical action | Main cell physiological roles | Reference |
|---|---|---|---|
| Active transport | |||
| SERCA pumps | Pumps Ca2+ from the cytosol into ER | Maintain ER stores of Ca2+; shape Ca2+ signals | 114 |
| PMCA pumps | Pumps Ca2+ from the cytosol to outside the cell | Shape Ca2+ signals; limit total cellular Ca2+ accumulation | 18,115 |
| Exchange transport | |||
| Na+/Ca2+ exchangers | Direct mode: exchange cytosolic Ca2+ for Na+ during high cytosolic Ca2+ accumulation | Limit total cellular Ca2+ accumulation | reviewed in 113 |
| Reverse mode: exchange of cytosolic Na+ for extracellular Ca2+ | Mechanism for Ca2+ entry in response to accumulation of cytosolic Na+ | reviewed in 113 | |
| Mitochondrial uptake | |||
| Unknown mitochondrial ion channel | Ion channels that allows primarily electrical gradient-driven diffusion of Ca2+ from ER to mitochondrial matrix | Transient buffering of cytosolic Ca2+ during acute elevations | 116,117, reviewed in 118,119 |
| Mitochondrial extrusion | |||
| Unknown Na+-or H+-gradient-driven active transporters | Exchangers that extrude accumulated Ca2+ from mitochondrial matrix once cytosolic Ca2+ falls | Buffering of the decreasing phase of a Ca2+ signal via gradual release of Ca2+ to the cytosol | reviewed in 119,120 |
| Passive diffusion | |||
| InsP3 receptors | Ion channels which allow passive chemical-gradient-driven diffusion of Ca2+ from ER to cytosol | Transient acute elevations in cytosolic Ca2+ | Reviewed in 121 |
| Plasma membrane Ca2+ channels | Ion channels that allow passive chemical and electrical-gradient-driven diffusion of Ca2+ from ER to cytosol | Transient and sustained elevations in cytosolic Ca2+; replenishment of total cellular Ca2+ | See for example 2,3,6 |
SERCA, sarcoplasmic/endoplasmic reticulum Ca2+ ATPase; PMCA, plasma membrane Ca2+ ATPase; ER, endoplasmic reticulum; InsP3; inositol-1,4,5-triphosphate;
Figure 1. Ca2+ physiology in B cells at rest and during InsP3-mediated Ca2+ signal.
A: In "resting" B cells, cytoplasmic Ca2+ homeostasis is maintained primarily through the actions of plasma membrane Ca2+ ATPase (PMCA) and sarcoplasmic/endoplasmic reticulum Ca2+ ATPase (SERCA) active transporters or pumps. Na+/Ca2+ exchange is limited due to lack of cytoplasmic Ca2+, and plasma membrane channels are primarily in the closed state.
B: A receptor-mediated (inositol-1,4,5-trisphosphate (IP3))-dependent calcium signal is initiated through binding of IP3 to IP3R proteins in the endoplasmic reticulum (ER) membrane. Opening of IP3R channels allows Ca2+ to enter the cytoplasm from ER Ca2+ stores. Once ER Ca2+ stores are sufficiently depleted, ER Stromal interaction molecule (STIM) family proteins are activated to move into proximity to, and open, Ca2+-release activated channels (CRAC channels) in plasma membrane microdomains. Notable aspects of cell physiology in the context of an active Ca2+ signal include: (1) plasma membrane potential provides a significant driving force for Ca2+ entry through ion channels; (2) active Ca2+ transport activities (sarcoplasmic reticulum/endoplasmic reticulum Ca2+ ATPase (SERCA), plasma membrane Ca2+ ATPase (PMCA), and Na+/Ca2+ exchange) increase relative to the resting state in response to the increased availability of Ca2+ on their pumping cytosolic surfaces; and (3) Na+ entry into the cytoplasm may significantly influence cytosolic Ca2+ concentration depending on local changes in Na+ concentrations and the relative flow of Ca2+ through Ca2+ selective ion channels, non-selective cation channels (G), and reverse mode Na+ /Ca2+ exchange (that is, Ca2+ transported to the cytosol from the extracellular environment in exchange for intracellular Na+)111–113.
InsP3 accumulation causes ER Ca2+ release and initiates store operated Ca2+ entry
When a PLC-linked receptor is activated, the InsP3 generated binds to and induces the opening of InsP3 receptor (InsP3R) channels in the endoplasmic reticulum (ER) membrane, allowing Ca2+ to flow from ER stores to the cytosol. This “release” of ER Ca2+ results in the first detectable increase in cytosolic Ca2+ concentration after receptor activation. As stored ER Ca2+ is necessarily limited, ER Ca2+ release can only support a transient increase in cytosolic Ca2+. However, many PLC-linked receptors, including the BCR, are able to sustain increases in cytosolic Ca2+ for many tens of minutes. They are able to do this through a process, known as store operated Ca2+ entry (SOCE; reviewed in 3,6–8), that allows Ca2+ ions to pass from the essentially unlimited extracellular Ca2+ pool to the cytosol. The present SOCE model, as described below, is based on work performed in multiple cell contexts, including DT40 chicken B cells, Drosophila S2 cells, human fibroblasts, and human T cells. Although not every aspect of the model has been tested in all cell types, the bulk of available data suggests that the molecular mechanisms underlying SOCE are largely conserved across species and cell type.
According to the present SOCE model, once InsP3-induced InsP3R opening induces sufficient ER Ca2+ pool depletion, the reduced ER Ca2+ concentration is sensed by members of the stromal interaction molecule (STIM) family of single transmembrane span molecules, which are located as type II transmembrane proteins in the ER membrane9–11. STIM molecules are thought to undergo a Ca2+-dependent conformational coupling [Au: OK?], which leads to direct interaction with and activation of plasma membrane calcium-release activated calcium (CRAC) channels, encoded by multimers of one or more ORAI (also known as CRACM1)-family proteins12–14. Subcellular imaging studies suggest that coupling of STIM proteins with CRAC channels involves interactions between STIM and CRAC channels occurring at discrete puncta located within regions where junctional ER approaches within 10–25 nm of the plasma membrane10,13,15,16. The resulting locally activated CRAC channels allow extracellular Ca2+ influx to the cytoplasm, thus allowing Ca2+ signalling to continue beyond the point of ER-store depletion, as well as providing a source of Ca2+ for refilling of the ER store as the store-depleting stimulus subsides. The protein components, biochemistry, and cell biological properties of puncta generated by store depletion are presently under intense investigation to obtain further insights into the nature and regulation of the SOCE mechanism.
Although initiated entirely by passive Ca2+ flows through ion channels, Ca2+ signals that involve activation of SOCE may be further shaped by active Ca2+ transport though plasma membrane Ca2+ ATPase (PMCA) pumps. In T cells, this shaping occurs because activation of CRAC channels is closely coordinated with activation of PMCA pumps, such that increased rates of Ca2+ entry correlate with increased rates of Ca2+ extrusion independently of changes in the average cellular cytosolic Ca2+ concentration 17,18. This close coordination of CRAC channels and PMCA pumps allows the PMCA pumps to respond rapidly to local changes in Ca2+ concentration, and thereby to significantly influence the frequency and amplitude of intracellular Ca2+ oscillations and/or the peak cytosolic Ca2+ concentration triggered by CRAC channel activation.
SOCE mechanism leads to an ‘InsP3 threshold’ effect
An important characteristic of the SOCE mechanism is that it manifests as an ‘all or nothing’ phenomena: that is either stores are sufficiently empty (depleted) to activate SOCE, in which case SOCE becomes fully activated, or they are not sufficiently empty, in which case SOCE is not activated. This results in an apparent threshold for InsP3 accumulation, below which ER stores are not depleted and SOCE is not activated, and above which InsP3R-dependent release of Ca2+ from ER stores depletes those stores and activates SOCE19. This threshold has the practical effect of allowing small differences in the level or duration of PLC activation to lead to very large differences in Ca2+ signal phenotype. In the simplest case, if the magnitude or duration of a stimulus is such that sufficient InsP3 accumulates to maintain ER Ca2+ store depletion, CRAC-channel-mediated entry of Ca2+ from the vast reservoir of extracellular free Ca2+ will sustain a Ca2+ signal indefinitely, either in the form of elevated cytosolic Ca2+ concentration or oscillations in cytosolic Ca2+ concentration. However, a slightly lower level of stimulation may not lead to sufficient accumulation of InsP3 to deplete Ca2+ stores; in this case ER sarcoplasmic/endoplasmic reticulum Ca2+ (SERCA) pumps will restore and maintain ER Ca2+ stores, SOCE will not be activated, and only a very small and transient Ca2+ signal will be observed. Therefore, for any particular microenvironment, whether overall cellular PLC activation is sufficient to cause InsP3 to accumulate to a level that can maintain ER Ca2+ store depletion is an important determinant of the magnitude of the resulting Ca2+ signal.
BCR-mediated PLCγ activation
Signals mediated by the BCR are central to multiple cell-fate decisions arising during B-cell ontogeny, including the transitions from pro-to-pre-B-cell and pre-to-immature B-cell in the bone marrow; activation of mature B cells in response to antigen; selection into germinal centres; induction of somatic hypermutation; and re-activation of memory B cells. The biochemical mechanisms through which BCR engagement influences B-cell physiology have been studied in detail over the past two decades. A fundamental concept derived from this work is the importance of activation of the B-cell-specific PLCγ isozyme, PLCγ2, as a BCR effector pathway20,21. Furthermore, a detailed model has evolved for how the BCR generates three key biochemical events that lead to positive feedback activation of PLCγ2 (Figure 2), and how this mechanism provides a direct target for highly sensitive co-receptor-mediated positive or negative regulatory influences.
Figure 2. Biochemical events in a BCR signaling complex leading to amplified PLCγ2 activation.
A: Recruitment and phosphorylation (yellow arrows and dots) of B-cell linker (BLNK) protein within the signalosome of the B-cell receptor (BCR) generates binding sites and recruitment (green arrows) of key BCR effectors VAV, GRB2 (growth-factor-receptor-bound protein 2), phospholipase Cg2 (PLCγ2) and Bruton's tyrosine kinase (BTK).
B: P110 Phosphoinositide-3-kinase (PI3K) activation occurs through several parallel pathways (black arrows), and leads to local phosphorylation (yellow arrow) of phosphatidylinositol-4,5-bisphosphate to phosphatidylinositol-3,4,5-trisphosphate (PtdIns(3,4,5)P3). This effect is amplified in part through recruitment (green arrow) of the p85 subunit of PI3K to the CD19 complex.
C: Recruitment of BTK to PtdIns(3,4,5)P3 induces its transphosphorylation by lyn (small yellow arrows), and initiates a positive feedback loop (large yellow arrows): association of BTK with phosphatidyl inositol-4-phosphate 5-kinase (PI5K) leads to increased production of phosphatidylinositol-4,-5- bisphosphate (PtdIns(4,5)P2) and so increased substrate for PtdIns(3,4,5)P3production; enhanced PtdIns(3,4,5)P3 leads to increased BTK recruitment (with additional PI5K) and BTK activation. The overall signal is read out through BTK-dependent tyrosine phosphorylation and activation of PLCγ2 (red arrows), which hydrolyzes (black arrows) locally available and newly synthesized PtdIns(4,5)P2 to IP3 and diacylglycerol (DAG).
A BCR-initiated positive-feedback loop mediates PLCγ2 activation in B cells
The first key biochemical event in BCR-mediated PLCγ2 activation occurs in the context of the formation of a multi-component signalling complex in response to BCR engagement (Figure 2A). The formation of this “signalosome” involves the phosphorylation of tyrosines in the Igα and Igβ chains of immunoreceptor tyrosine-based activation motifs (ITAMs), and subsequent recruitment of SRC-family kinases [Au: OK? Is it not LYN that is recruited along with SYK?] and spleen tyrosine kinase (SYK). The binding and activation of SRC and SYK coordinately results in the recruitment and activation of other signalling proteins including kinases, phosphatases, various G-proteins and their regulatory molecules, adaptor molecules, and lipid hydrolases. Among these targets, the adaptor molecule B-cell linker (BLNK) is rapidly recruited into the signalling complex and phosphorylated22–24. BLNK recruitment is the first crucial biochemical event in PLCγ2 activation23,25. Phosphorylated BLNK is directly responsible for the recruitment of both PLCγ2 — through binding of both of the SRC homology 2 (SH2) domains of PLCγ2 to distinct phosphorylated tyrosines in BLNK22 — and of a PLCγ-activating tyrosine kinase known as Bruton's tyrosine kinase (BTK, a member of the TEC-kinase family of tyrosine kinases)26,27. BLNK recruitment and phosphorylation is absolutely required for BCR-mediated PLCγ2 activation, as PLCγ2 activation is entirely blocked if BLNK is absent23, and severe defects in B-cell development and function result from natural mutations or targeted deletion in the BLNK gene in humans and mice, respectively28,29.
The second key biochemical event in BCR-mediated positive feedback activation of PLCγ2 is the generation and accumulation of a localized pool of phosphatidylinositol-3,4,5-trisphosphate (PtdIns(3,4,5)P3) through several parallel pathways that regulate the activity of p110 isoforms of phosphoinositide 3-kinase (PI3K, Figure 2B). As PtdIns(3,4,5)P3 is produced as a consequence of phosphorylation of PtdIns(4,5)P2 (also the substrate for PLCγ2) by PI3K, PtdIns(3,4,5)P3 preferentially accumulates in membrane areas enriched in PtdIns(4,5)P2. PtdIns(3,4,5)P3 binds with high affinity to the plekstrin-homology domain (PH domain) of BTK, and BTK is therefore rapidly recruited into membrane regions where PtdIns(3,4,5)P3 is being formed30,31. As both BTK and PLCγ2 are bound to phosphorylated BLNK, interaction of the BTK PH domain with PtdIns(3,4,5)P3 also serves to bring PLCγ2 into regions where PtdIns(3,4,5)P3 is being formed, which, as noted above, are also enriched in the PtdIns(4,5)P2 substrate for PLCγ2. In addition, PtdIns(3,4,5)P3 may also have a direct role in activating PLCγ isozymes through an interaction with a functional PH domain formed by apposition in the folded PLCγ protein of two half-domains found widely separated in the primary sequence32. Furthermore, as BTK constitutively interacts with phosphatidylinositol-4-phosphate 5-kinase (PIP5K)33, recruitment of BTK also brings PIP5K into the same complex. PIP5K promotes the formation of additional PtdIns(4,5)P2, thereby ensuring a supply of PtdIns(4,5)P2 substrate for both activated PI3K and PLCγ2. The importance of PI3K recruitment to the signal transduced by the BCR is illustrated by the defects in immune development and function that result from targeted deletions in various PI3K subunits34,35.
In combination, the first two biochemical events result in positive-feedback amplification of upstream signalling events (Figure 2C). The degree of amplification they achieve is subsequently "read out" by the third key biochemical event: BTK-dependent phosphorylation of PLCγ2 on tyrosine residues, Tyr753 and Tyr759, in its SH2–SH3 linker region36. PLCγ2 phosphorylation on these two residues is synergistically promoted by the positive feedback created by the first two events. This is because BTK and PLCγ2 are co-localized by their binding to phosphorylated BLNK, and because PtdIns(3,4,5)P3 binding to the BTK PH domain potentiates tyrosine-phosphorylation-dependent activation of BTK occurring within the signalling complex30,31. The phosphorylation of PLCγ2 on these two regulatory tyrosines is a pivotal output of the signalling complex formed by the BCR, as these phosphorylation events are required to maximally activate the lipid hydrolase activity of PLCγ2, and are specifically mediated by BTK in human B cells37, and by BTK and the related TEC-kinase family TEC (tyrosine kinase expressed in hepatocellular carcinoma) in murine [Au: means both rat and mouse – OK or should it be mouse?] B cells38. Tyrosine kinases not members of the TEC-kinase family present in the BCR signalling complex, such as SYK or LYN, are not able to efficiently access and/or phosphorylate these regulatory tyrosines36,39,40. The importance of TEC-kinase-family-dependent phosphorylation of PLCγ2 as an output of the signal transduced by the BCR is illustrated by the profound defects in calcium flux, and immune development and function in mice or humans which result from targeted deletion or natural mutations in BTK and TEC kinases37,41,42.
The significance to B-cell physiology of the signal amplification achieved by this mechanism is underscored by the striking similarities of the B-cell defects present in mice with individual targeted deletions in the genes encoding BLNK, PI3K, PLCγ2, or BTK, or in humans with natural mutations in the homologous genes. In each case, B-cell development is inhibited at checkpoints where a sufficient amplitude and duration of pre-BCR or BCR signals are required for developing or differentiating B cells to survive and proliferate. The importance of amplified PLCγ2 activation is further underscored by the existence of several co-receptors that function to directly modulate the positive-feedback loop as a means of tuning BCR signal amplitude, as discussed below.
Positive modulation of BCR Ca2+ signalling
Tyrosine phosphorylation-dependent activation of PLCγ2 via the BCR is superficially similar to PLCγ1 and/or PLCγ2 activation that occurs in response to activation of receptors with intrinsic tyrosine kinase activity, such as growth-factor receptors. However, the positive feedback amplification loop created by the BCR signalling complex provides an additional level of regulatory control, the importance of which is instructively illustrated by comparison of the circumstances leading to BCR engagement with those leading to growth-factor-receptor engagement. Co-evolution of interactions between growth factors and their receptors in the tightly regulated context of an organism has lead to many receptors which respond to a single interaction with one or a few types of ligand within a restricted range of ligand concentrations. Because the receptor–ligand interaction affinity is consistent, and the concentration of ligand is controlled, additional mechanisms for tuning receptor response magnitude are typically not required. By contrast, positive-feedback amplification of PLCγ2 activation after BCR engagement creates a means for co-receptors to tune the BCR signal amplitude over a vastly wider dynamic range, and is one key way in which B cells are able to respond to an array of ligands of varying abundance and interaction affinities, and to modulate their response depending on previous experience.
One of the most important examples of BCR signal amplitude tuning by a co-receptor is the role the B-cell specific surface molecule CD19 has in potentiating BCR signals to complement-coated pathogens (Figure 2) 43–45. Complement-coated pathogens enhance the recruitment of a trimolecular complex of CD81–CD19–CD21 (the latter being a receptor for the complement component C3d) into proximity to activated BCRs, leading to the increased recruitment and activation of the guanine-nucleotide-exchange factor (GEF) VAV and the p85 subunit of PI3K to the signalosome, and so synergistic activation of the p110 PI3K subunit46,47. Available data favour a model whereby PI3K is initially recruited to and activated in the signalosome independently of VAV, and the subsequent recruitment of VAV (by immunoreceptor adaptors and/or co-receptors) facilitates further PI3K activation through the proximally located RAC1 (Figure 2B and C). The enhanced PI3K activation produced by recruitment of the CD81-CD19-CD21 complex into proximity to BCR “signalosomes” leads to a marked potentiation of PLCγ2 activation via the positive-feedback mechanism outlined above, and a larger and more sustained increase in cytosolic Ca2+. The signal-amplitude increase provided by the influence of the CD19 complex lowers the threshold of the B-cell response to complement-coated pathogens by two to three orders of magnitude48, providing enhanced immune protection against pathogens at low abundance. These events are also paramount for efficient B-cell activation in the early germinal-centre reaction49.
Limiting BCR-induced Ca2+signaling
A second important example of tuning of the BCR-signal amplitude is the negative-feedback regulation of B-cell activation that occurs in response to co-ligation of the low-affinity receptor for IgG (FcγRIIB1) with the BCR (Figure 3). FcγRIIB1 and BCR co-ligation attenuates the BCR signalling response in a way that markedly inhibits BCR-induced Ca2+ signals50–52. FcγRIIB1 achieves this effect by recruiting SRC-homology-2-domain-containing inositol-5-phosphatase (SHIP)53 and SH2-domain-containing protein tyrosine phosphatase 1 (SHP1) into close proximity to BCR calcium signalling effectors54. Recruitment of SHIP phosphatase by colligation of FcγRIIB1 with the BCR is thought to provide a signal attenuating effect by locally enhancing the rate of degradation of PtdIns(3,4,5)P3 to PtdIns(3,4)P2 31,55, whereas recruitment of SHP1 is thought to result in dephosphorylation of CD19 on residues that are required to mediate PI3K association and activation56,57. Although each of these mechanisms would contribute to interruption of the positive-feedback loop for PLCγ2 activation while allowing other tyrosine-kinase-dependent aspects of the BCR signal to continue unaltered, SHIP recruitment appears to provide the majority of the signal attenuation effect, as FcγRIIB1 inhibitory signalling is significantly compromised in SHIP-deficient cells, and generally intact in SHP1-deficient cells 58,59. Overall, FcγRIIB1-dependent attenuation of PLCγ2 activation leads to decreased InsP3 production, such that InsP3 is not able to accumulate to a level capable of sustaining SOCE, thereby resulting in a relatively specific block in extracellular Ca2+ entry, and consequently a transient Ca2+ signal31,55.
Figure 3. Surface-receptor mechanisms for modulation of BCR-induced Ca2+ signals.
The CD19 complex is a positive regulatory receptor. Its active recruitment of into B-cell receptor (BCR) signaling complexes acts to enhance p110 phosphoinositide-3-kinase (PI3K) activation during BCR signaling. FcγRIIb1 and CD22 are both negative regulators of BCR-mediated calcium signals. When actively recruited into BCR signaling complexes, these receptors both act by recruiting the SH2-containing tyrosine phosphatase-1 (SHP1) and the SH2-containing inositol-5'-phosphatase (SHIP). However, the majority of the impact of FcγRIIb1 on Ca2+ signaling is mediated through the SHIP-dependent attentuation of PI3K signaling, while CD22 appears to have its most significant effect on Ca2+ signaling through SHP-1-dependent enhancement of PMCA-mediated Ca2+ export.
In addition to modulation of PLCγ activation as a means of influencing cytosolic Ca2+ concentration, a third B-cell surface co-receptor, CD22, has been implicated in the direct modulation of cytosolic Ca2+ concentration after BCR stimulation (FIG. 3)60–62. Recent studies suggest that CD22 acts to specifically enhance Ca2+ extrusion by co-localizing the tyrosine phosphatase SHP1 with PMCA463. This regulatory mechanism may be facilitated by co-localization of PMCA pumps and CRAC channels18, as discussed above. CD22-mediated regulation of BCR Ca2+ signals acts as a brake on BCR-mediated B-cell activation, and so dampens humoral immune responses in vivo60,64. Its significance can be seen in the effects of loss of CD22 function, which include hyper-responsive B cells61,62, altered developmental fate of splenic transitional B cells leading to an absence in marginal-zone B cells and their immediate precursors 65–67, and the promotion of humoral autoimmune phenomena 64,68,69.
Interpretation and modulation of BCR-induced Ca2+ signals
Implicit in the above model for regulation of BCR-induced Ca2+ signalling is that extrinsic regulation through co-receptors targets the activation of PLCγ2 and subsequent production of InsP3, with secondary regulation occurring through the influence of PMCA pumps on the rate of Ca2+ export. However, it is important to recognize that other aspects within the ‘cell context’ [Au: OK?] besides co-receptor activity may have important influences on how a given level of BCR-mediated PLCγ activation is ‘interpreted’ into a Ca2+ signal. Such cell context aspects may include various intrinsic or alternative inputs to PLCγ activation and InsP3 metabolism that can modulate PLCγ activation through the BCR or other PLC-linked receptors. Supplementary Table 1 details several such influences and their potential relevance to B-cell physiology. In addition, recent advances in ion channel biology and Ca2+ transport physiology have identified several new channels and regulatory mechanisms that may directly or indirectly influence cytosolic Ca2+ and BCR-mediated Ca2+ signals (FIG. 4).
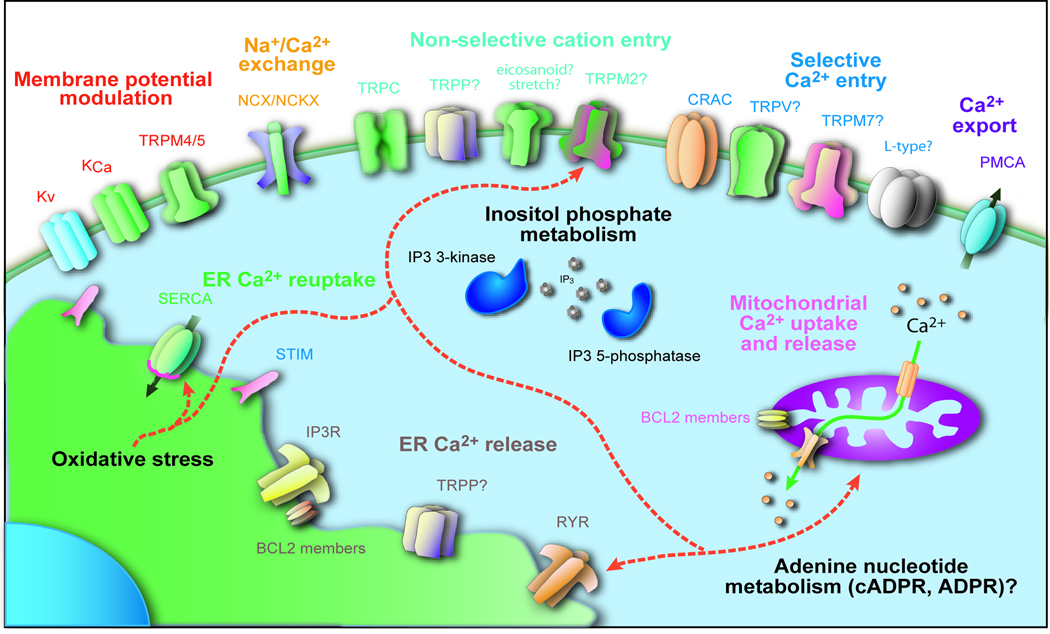
Figure 4. Mechanisms for regulation of cytosolic Ca2+ in B cells.
Pathways with known or potential ability to influence cytosolic Ca2+ in B-cells are grouped by colour according to their mechanism of influence.
Regulation of Ca2+ entry via modulation of plasma membrane potential
Although often overlooked in non-excitable cells, the contribution of membrane potential to the driving force for Ca2+ entry is particularly relevant to Ca2+ entry through highly Ca2+-selective low-conductance channels, such as CRAC channels (reviewed in 2,3,6). B cells express both voltage-operated and Ca2+-activated K+ channels2,3,70–73, and distinct B-cell subsets (for example naive B cells compared with class-switched memory B cells) have been shown express distinct ratios of different types of K+ channels, consistent with a role for K+ channel modulation in the regulation of B-cell physiology74. An additional means for modulating membrane potential has recently been discovered in the form of the monovalent cation permeant transient receptor potential melastatin-related (TRPM) family members, TRPM4 and TRPM5. These channels are activated in response to increases in cytosolic Ca2+ concentrations and modulated by membrane lipid composition75–77, and serve as a means to depolarize the cell membrane. Their activation has been shown to limit the driving force for Ca2+ entry during Ca2+ signals in T cells and mast cells78,79. Consistent with TRPM4 and/or TRPM5 having a similar function in B cells, both channels have been reported to be expressed in bone marrow, splenic and lymph node B cells77. The existence of two distinct methods (K+ and TRPM channels) for subset-dependent and dynamic regulation of membrane potential in B cells suggests that membrane potential modulation may be an unexpectedly rich mechanism for the regulation of B-cell Ca2+ signalling (Figure 5).
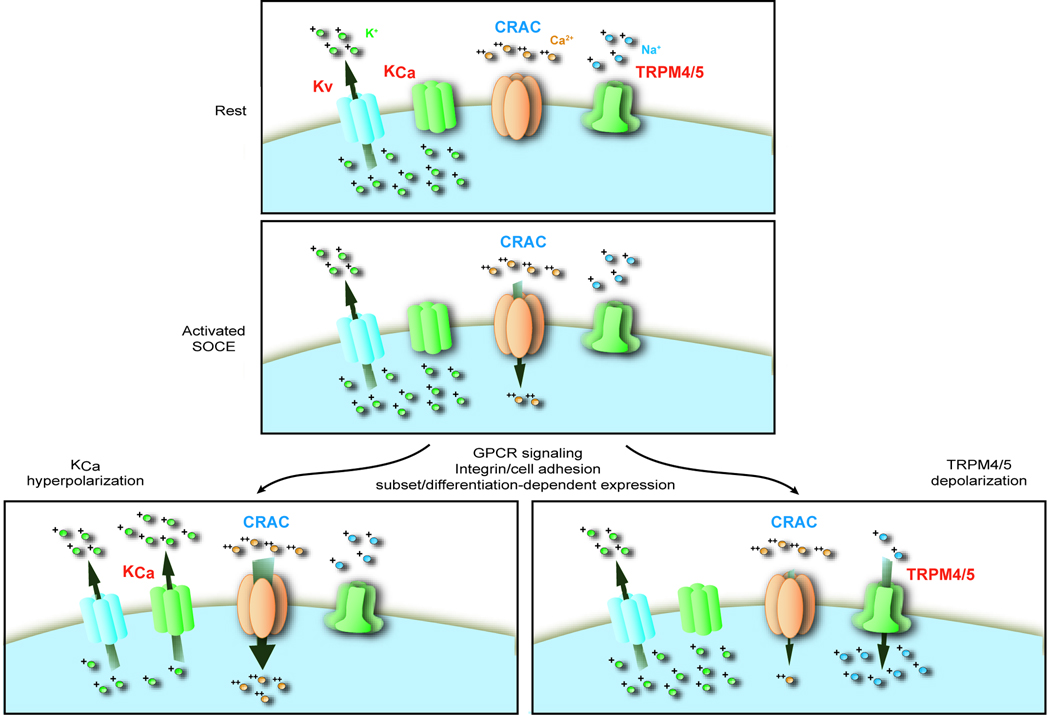
Figure 5. Membrane potential modulation of Ca2+ signals.
Membrane potential can be differentially and dynamically regulated through K+ channel activity and TRPM4/TRPM5 monovalent cation channel activity
Top panel: In resting cells, membrane potential is set at a highly negative potential through the activity of voltage–operated K+ channels. The negative membrane potential is generated through the outward movement of positively charged K+ ions, leaving unpaired intracellular negative charges (not shown). TRPM4/TRPM5 channels are thought to be closed in resting cells.
Middle panel: When a cell stimulus leads to activation of the store-operated Ca2+ entry (SOCE) pathway, opening of highly Ca2+-selective CRAC channels allows Ca2+ to pass across the plasma membrae. The current carried by CRAC channels is very small, and thus the entering positively charged Ca2+ ions have little direct influence on the membrane potential. However, the rise in cytosolic Ca2+ resulting from CRAC channel opening may influence the gating of both Ca2+-activated K+ channels and TRPM4/TRPM5 channels.
Bottom left panel: In cells expressing significant numbers of Ca2+-activated K+ channels, their activation serves to maintain a highly negative membrane potential. Thus, the cell maintains a strong sustained driving force for Ca2+ entry and a large magnitude sustained Ca2+ signal results.
Bottom right panel: In cells expressing significant numbers of TRPM4/TRPM5 monovalent cation channels, their activation allows Na+ entry, thus providing cytosolic positive ions to neutralize the unpaired internal charges. This diminishes the negative membrane potential, thereby attenuating the driving force for Ca2+ entry, and resulting in a reduced magnitude Ca2+ signal.
Beyond Ca2+-dependent activation of K+ and TRPM4/TRPM5 channels, the activity of these channels is variously accessible to receptor mediated stimuli, metabolic mediators, and environmental influences that modulate their gating properties, providing multiple mechanisms through which dynamic regulation of membrane potential could influence B-cell Ca2+ signaling.
Ca2+ entry through non-SOCE mechanisms
In B cells, data have also been generated that implicate various non-SOCE mechanisms in Ca2+ entry. The non-selective Ca2+-permeable transient receptor potential channel (TRPC) family channel, TRPC1, has been implicated in the BCR-mediated Ca2+ response80. While a DT40 B-cell-line deficient in TRPC1 maintains normal levels of receptor-mediated InsP3 production, BCR-induced Ca2+ release and sustained Ca2+ elevations are diminished, and Ca2+ entry induced by thapsigargin (a compound which irreversibly inhibits SERCA-pump function, and thus leads to passive ER store depletion) is diminished80. The ability of several TRPC proteins to bind to InsP3R isoforms and facilitate their optimal activation81–84, and the demonstration of interactions between STIM proteins and both TPRC and ORAI subunits 80,85,86, suggest that the defective transient and sustained Ca2+ responses in TRPC-deficient B cells could be the result of alterations in ER or plasma-membrane cell physiology, which are dependent on TRPC–InsP3R–STIM–ORAI subunit interactions. However, precisely how TRPC proteins could influence STIM1–ORAI interactions during ER-store depletion or other contexts, and the cell biological significance of plasma membrane/ER communication achieved through TRPC–InsP3R–STIM–ORAI-subunit interactions, are not presently well understood. An alternative or additional mechanism for the influence of TRPC1 on cytosolic Ca2+ in B cells is that TRPC channels may function in close coupling with Na+/Ca2+ exchangers such as Na+/Ca2+ exchanger-1 (NCX1) to generate extracellular Ca2+ entry via reverse mode exchange with permeating Na+ ions87.
Other channels potentially found in B cells include other TRPC members, various members of the transient receptor potential-vanilloid receptor-1-related (TRPV) family88,89, TRPM2 and TRPM790–92, and undefined stretch-activated ion channels and eicosanoid-activated channels 93–95. In addition, convincing evidence for expression of mRNA for splice variants of α-subunits of classic voltage-operated Ca2+ channels has been reported in B cells and T cells, although electrophysiological evidence of voltage-operated Ca2+ currents is lacking, suggesting that these splice variants may not be voltage-operated, but instead must have alternative gating mechanisms96–98. Further exploration of the function and physiological regulatory mechanisms of these various channels should begin to provide insight into their distinct roles in B-cell Ca2+ signalling and physiology.
Ca2+ signalling and cell-fate choice
With the accumulation of an increasingly complete picture of the network of inputs regulating B-cell cytosolic Ca2+ concentration and signalling, an important future direction for B-cell immunologists is to understand and define how these inputs influence B-cell physiology and immune responses. Elegant studies by Lewis, Goodnow and colleagues have shown that PLC activation and cytosolic Ca2+ differentially impact several key transcription factor pathways in B cells99,100, as exemplified by the NFAT pathway, which is involved in transcriptional activation of cytokine genes such as interleukin-4 (IL-4), and the NF-κB pathway, which in B cells targets proteins such as B-cell lymphoma-6 (BCL-6) that are involved in protecting the cell from apoptosis and supporting proliferation (Figure 6A). The NFAT pathway is activated in response to sustained Ca2+ elevation or oscillations through induction of phosphorylation-dependent nuclear localization of NFAT family transcription factors (reviewed in 101–103), By contrast, the NF-κB pathway is activated through DAG- and Ca2+-dependent degradation of the inhibitor of NF-κB (IκB) protein (reviewed in 104), and exhibits strong dependence on peak amplitude, rather than duration, of a Ca2+ signal. These observations have provided a useful context to understand how differences in the level of BCR activation, and therefore PLCγ activity, may produce markedly different B-cell-fate choices in the context of tolerizing versus non-tolerizing antigen exposure: non-tolerizing antigens induce large sustained Ca2+ responses which are able to effectively activate both pathways, whereas tolerizing antigens induce low-level sustained responses which are able to active NFAT, but not NF-κB, pathways (see FIG. 6 and 99,100).
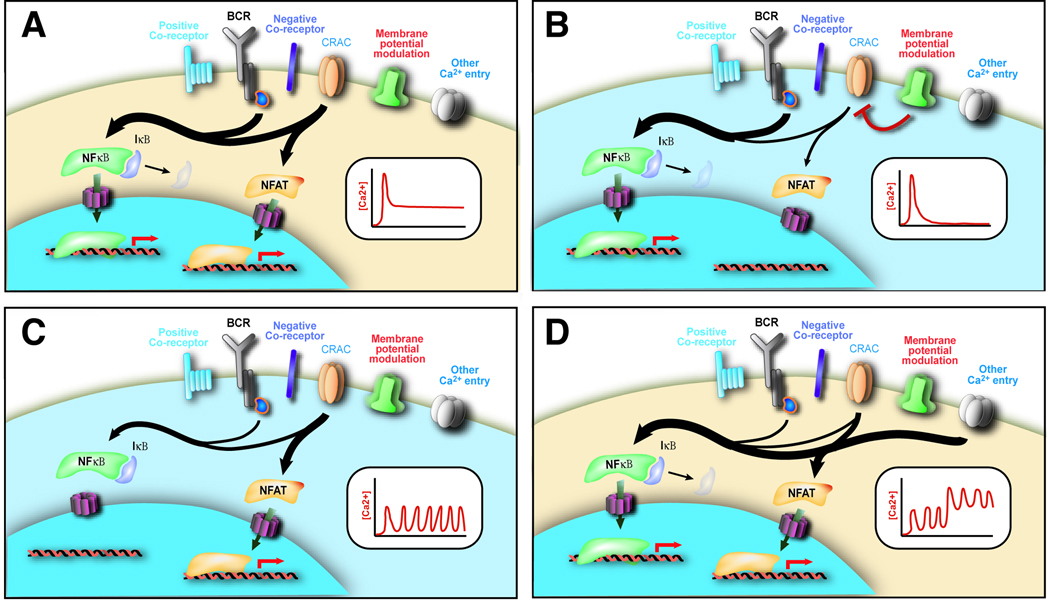
Figure 6. Potential mechanisms for Ca2+-dependent modulation of B-cell fate determination.
Based on present models for Ca2+-dependent regulation of transcription factors such as Nuclear Factor of Activated T-cells (NFAT) and Nuclear Factor kb (NF-κB), membrane potential modulation and direct Ca2+ entry through non-SOCE pathways are predicted to have the capacity to significantly alter B-cell-fate choice.
A: Strong B-cell receptor (BCR) activation fully activates both NF-κB (through inhibitor of kb (IκB) degradation) and NFAT (through calcineurin phosphatase activation) pathways.
B: Modulation of membrane potential attenuates late Ca2+ entry, selectively influencing NFAT activation.
C: A weak but sustained BCR activation signal activates only the NFAT pathway.
D: Late activation of a direct Ca2+ entry pathway restores NF-κB pathway activation despite a weak BCR activation signal.
While influences on the events leading to ER Ca2+ store depletion, such as those outlined in Table 1, [Au: OK?] can be directly integrated with the observations of Lewis, Goodnow and colleagues (such as through their quantitative effects on the BCR Ca2+ signal), future studies will also be challenged to integrate more recently defined Ca2+ regulatory mechanisms with the described properties of the NFAT and NF-κB pathways. Two informative examples are considered in FIG 6. For B cells receiving a strong signal through the BCR, one normally expects activation of both NFAT and NF-κB pathways (FIG.6a), and selection into a maturation pathway appropriate for a high-affinity BCR–antigen interaction. However, if the same B-cell were in a microenvironment in which K+ channel deactivation, non-selective cation-channel activation or both had resulted in cell membrane depolarization, SOCE could be substantially compromised due to a reduction in the driving force for Ca2+ entry. A reduction in SOCE would thus lead to a transient Ca2+ signal, selective loss of NFAT pathway activation, and a cell-fate choice appropriate to a lower affinity BCR–antigen interaction such as death by neglect (as in FIG.6b). In a second example, one can imagine a B-cell in a microenvironment in which a low-level BCR signal is generated, thus leading to a low-level sustained Ca2+ signal capable of NFAT, but not NF-κB, nuclear translocation (FIG.6c). If this B-cell subsequently receives a signal leading to Ca2+ entry through an alternative non-SOCE pathways, NF-κB activation may be recovered, and the B-cell would be diverted into a pathway normally associated with higher affinity BCR–antigen interactions (FIG.6d). In other cases, sustained elevation of cytosolic Ca2+ concentration, occurring as the result of an “excessive” level of receptor engagement or non-SOCE Ca2+ entry in a particular microenvironment, could be of sufficient magnitude to lead to mitochondrial Ca2+ accumulation and eventual activation-induced cell death105,106 — a mechanism that may occur in some forms of B-cell negative selection107–109. Finally, an additional veneer may be added to the above cell-intrinsic regulatory mechanisms, in that increased cytosolic Ca2+ concentration in T-cells has been observed to serve as a “stop” signal, resulting in a marked decrease in the motility of an activated cell. Thus, longer duration elevations of cytosolic Ca2+ are predicted to result in longer residence times in a particular microenvironment, potentially leading to the immobilization of activated T cells within regulatory niches in peripheral tissues and lymphoid organs110. If a similar mechanism operates in B-cells, even a transient compromise of a strong BCR-dependent Ca2+ signal could result in the release of a B cell from a stimulatory environment; conversely, a strong elevation of cytosolic Ca2+ might be sufficient to immobilize a B cell in a particular environment with a normally poorly stimulatory BCR ligand.
Overall, the emerging picture is that while the BCR signal is a crucial determinant of B-cell fate, dynamic inputs from the B-cell membrane potential and direct Ca2+ entry pathways have the potential to significantly influence developmental and activation events within an individual B-cell. Understanding how different B-cell subsets differ in these various Ca2+ regulatory inputs, and how such inputs are influenced by different microenvironmental stimuli, will substantially extend our understanding of B-cell-fate choices in the context of both regulatory homeostasis and active immune responses.
Summary
B cells receive information that is crucial to their physiology and function through cytosolic Ca2+ signals. One of the most important inputs to B-cell cytosolic Ca2+ is derived from BCR-mediated positive-feedback loop activation of PLCγ2. If InsP3 production from this mechanism reaches the threshold required to activate SOCE, sustained elevations in cytosolic Ca2+ are supported. The sensitivity of the positive feedback mechanism renders the amplitude and duration of the resulting changes in cytosolic Ca2+ subject to regulation by B-cell surface molecules at the level of PLCγ activation and at the level of Ca2+ export, allowing the BCR signal to be tuned over a wide dynamic range. Other microenvironmental stimuli may also indirectly influence B-cell cytosolic Ca2+ concentration through contributions to the total pool of activated PLC and InsP3, or through direct effects on Ca2+ fluxes mediated by transporters or channels. Future work will be challenged to integrate these diverse inputs with present models of how Ca2+-dependent signals regulate cell-fate choice in specific immunological contexts.
Supplementary Material
Acknowledgments
Supported by NIH grants AI45901, GM64316, and GM64091 to AMS, and CA81140, HD37091, HL07543, AI044259, and a Leukemia/Lymphoma Society award to DJR. We apologize to any colleagues whose work we have overlooked, or whose work we were not able to cite due to space limitations.
Glossary terms
- Conformational coupling
Refers to ion channel gating regulation by interaction with another protein, as opposed to gating through changes in plasma membrane potential or interaction with a diffusible second messenger.
- Germinal centre
Located in peripheral lymphoid tissues (for example, the spleen or lymph nodes), these structures are sites of B-cell proliferation and selection for clones that produce antigen-specific antibodies of higher affinity.
- Somatic hypermutation
A unique mutation mechanism that is targeted to the variable regions of rearranged immunoglobulin gene segments. Combined with selection for B cells that produce high-affinity antibody, somatic hypermutation leads to affinity maturation of B cells in germinal centres.
- TEC-kinase family
A third class of protein-tyrosine kinases that is required for the activation of haematopoietic cells — the first and second classes being the SRC-and SYK-family kinases. The TEC-family kinase prototypes are ITK (IL-2-inducible T-cell kinase) in T cells and BTK (Bruton’s tyrosine kinase) in B cells. Among other functions, TEC kinases play a crucial role in the activation of phospholipase C enzymes after immunoreceptor ligation.
- Marginal-zone B cells
A static, mature B-cell subset that is enriched mainly in the marginal zone of the spleen, which is located at the border of the white pulp.
- MEMBRANE POTENTIAL
The charge difference (measured in mV) between the two surfaces of a biological membrane that arises from the different concentrations of ions such as H+, Na+ or K+ on either side. The Na+/K+-ATPase creates a membrane potential by using the energy stored in ATP to maintain a low concentration of Na+ and a high concentration of K+ in the cell, against a higher concentration of Na+ and a lower concentration of K+ on the outside.
- Channel conductance
Conductance is defined as current divided by voltage, and in biological systems this means the current flowing across a biological membrane divided by the electrical potential across that membrane. When used in reference to a single open ion channel, (single channel conductance), it provides a measure of the amount of current a single open ion channel is able to carry. The single channel conductance is usually independent of the plasma membrane potential, and thus characteristic of that particular ion channel. Individual ion channels with small single channel conductances carry less current at a given membrane potential than those with large single channel conductances.
- VOLTAGE-OPERATED CHANNELS
Plasma-membrane ion channels whose gating is regulated by changes in plasma membrane potential.
- Stretch-activated ion channels
Plasma-membrane ion channels whose gating is regulated by changes in plasma membrane "stretch" - i.e. forces which are directed within/parallel to the plane of the plasma membrane.
- Eicosanoids
Eicosanoids are fatty-acid derivatives, primarily derived from arachidonic-acid precursors, that have a wide variety of biological activities. There are four main classes of eicosanoid — the prostaglandins, prostacyclins, thromboxanes and leukotrienes — derived from the activities of cyclooxygenases and lipoxygenases on membrane-associated fatty-acid precursors.
- Non-selective cation channels
Ion channels which exhibit a significant selectivity towards a single type of cation are generally designated according to that ionic selectivity - e.g. K+ channel or Ca2+ channels. Cation channels which exhibit little selectivity between monovalent cations, or between monovalent cations and one or more divalent cations such as Ca2+ or Mg2+ are typically grouped together and referred to as non-selective cation channels.
- Reverse mode Na+/Ca2+ exchange
The major physiological role for Na+/Ca2+ exchangers is thought to be removal of Ca2+ from the cytosol in exchange for extracellular Na+. If ionic conditions in the neighborhood of an exchanger are appropriate, it is possible for the exchanger to operate in reverse and exchange intracellular Na+ for extracellular Ca2+ - this is referred to as the reverse mode of the exchanger.
Bibliography
- 1.Quintana A, Griesemer D, Schwarz EC, Hoth M. Calcium-dependent activation of T-lymphocytes. Pflugers Arch. 2005;450:1–12. doi: 10.1007/s00424-004-1364-4. [DOI] [PubMed] [Google Scholar]
- 2.Lewis RS. Calcium signaling mechanisms in T lymphocytes. Annu Rev Immunol. 2001;19:497–521. doi: 10.1146/annurev.immunol.19.1.497. [DOI] [PubMed] [Google Scholar]
- 3.Cahalan MD, Wulff H, Chandy KG. Molecular properties and physiological roles of ion channels in the immune system. J Clin Immunol. 2001;21:235–252. doi: 10.1023/a:1010958907271. [DOI] [PubMed] [Google Scholar]
- 4.Brose N, Betz A, Wegmeyer H. Divergent and convergent signaling by the diacylglycerol second messenger pathway in mammals. Curr Opin Neurobiol. 2004;14:328–340. doi: 10.1016/j.conb.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 5.Rhee SG. Regulation of phosphoinositide-specific phospholipase C. Annu Rev Biochem. 2001;70:281–312. doi: 10.1146/annurev.biochem.70.1.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parekh AB, Penner R. Store depletion and calcium influx. Physiol Rev. 1997;77:901–930. doi: 10.1152/physrev.1997.77.4.901. [DOI] [PubMed] [Google Scholar]
- 7.Lewis RS. The molecular choreography of a store-operated calcium channel. Nature. 2007;446:284–287. doi: 10.1038/nature05637. [DOI] [PubMed] [Google Scholar]
- 8.Cahalan MD, et al. Molecular basis of the CRAC channel. Cell Calcium. 2007 doi: 10.1016/j.ceca.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roos J, et al. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol. 2005;169:435–445. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang SL, et al. STIM1 is a Ca2+ sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane. Nature. 2005;437:902–905. doi: 10.1038/nature04147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liou J, et al. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol. 2005;15:1235–1241. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vig M, et al. CRACM1 multimers form the ion-selective pore of the CRAC channel. Curr Biol. 2006;16:2073–2079. doi: 10.1016/j.cub.2006.08.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yeromin AV, et al. Molecular identification of the CRAC channel by altered ion selectivity in a mutant of Orai. Nature. 2006;443:226–229. doi: 10.1038/nature05108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prakriya M, et al. Orai1 is an essential pore subunit of the CRAC channel. Nature. 2006;443:230–233. doi: 10.1038/nature05122. [DOI] [PubMed] [Google Scholar]
- 15.Luik RM, Wu MM, Buchanan J, Lewis RS. The elementary unit of store-operated Ca2+ entry: local activation of CRAC channels by STIM1 at ER-plasma membrane junctions. J Cell Biol. 2006;174:815–825. doi: 10.1083/jcb.200604015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu MM, Buchanan J, Luik RM, Lewis RS. Ca2+ store depletion causes STIM1 to accumulate in ER regions closely associated with the plasma membrane. J Cell Biol. 2006;174:803–813. doi: 10.1083/jcb.200604014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bautista DM, Hoth M, Lewis RS. Enhancement of calcium signalling dynamics and stability by delayed modulation of the plasma-membrane calcium-ATPase in human T cells. J Physiol. 2002;541:877–894. doi: 10.1113/jphysiol.2001.016154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bautista DM, Lewis RS. Modulation of plasma membrane calcium-ATPase activity by local calcium microdomains near CRAC channels in human T cells. J Physiol. 2004;556:805–817. doi: 10.1113/jphysiol.2003.060004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parekh AB, Fleig A, Penner R. The store-operated calcium current I(CRAC): nonlinear activation by InsP3 and dissociation from calcium release. Cell. 1997;89:973–980. doi: 10.1016/s0092-8674(00)80282-2. [DOI] [PubMed] [Google Scholar]
- 20.Takata M, Homma Y, Kurosaki T. Requirement of phospholipase C-gamma 2 activation in surface immunoglobulin M-induced B cell apoptosis. J Exp Med. 1995;182:907–914. doi: 10.1084/jem.182.4.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang D, et al. Phospholipase Cgamma2 is essential in the functions of B cell and several Fc receptors. Immunity. 2000;13:25–35. doi: 10.1016/s1074-7613(00)00005-4. [DOI] [PubMed] [Google Scholar]
- 22.Fu C, Turck CW, Kurosaki T, Chan AC. BLNK: a central linker protein in B cell activation. Immunity. 1998;9:93–103. doi: 10.1016/s1074-7613(00)80591-9. [DOI] [PubMed] [Google Scholar]
- 23.Taguchi T, et al. Deficiency of BLNK hampers PLC-gamma2 phosphorylation and Ca2+ influx induced by the pre-B-cell receptor in human pre-B cells. Immunology. 2004;112:575–582. doi: 10.1111/j.1365-2567.2004.01918.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baba Y, et al. BLNK mediates Syk-dependent Btk activation. Proc Natl Acad Sci U S A. 2001;98:2582–2586. doi: 10.1073/pnas.051626198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ishiai M, et al. BLNK required for coupling Syk to PLC gamma 2 and Rac1-JNK in B cells. Immunity. 1999;10:117–125. doi: 10.1016/s1074-7613(00)80012-6. [DOI] [PubMed] [Google Scholar]
- 26.Kurosaki T, Tsukada S. BLNK: connecting Syk and Btk to calcium signals. Immunity. 2000;12:1–5. doi: 10.1016/s1074-7613(00)80153-3. [DOI] [PubMed] [Google Scholar]
- 27.Hashimoto S, et al. Identification of the SH2 domain binding protein of Bruton's tyrosine kinase as BLNK--functional significance of Btk-SH2 domain in B-cell antigen receptor-coupled calcium signaling. Blood. 1999;94:2357–2364. [PubMed] [Google Scholar]
- 28.Pappu R, et al. Requirement for B cell linker protein (BLNK) in B cell development. Science. 1999;286:1949–1954. doi: 10.1126/science.286.5446.1949. [DOI] [PubMed] [Google Scholar]
- 29.Minegishi Y, et al. An essential role for BLNK in human B cell development. Science. 1999;286:1954–1957. doi: 10.1126/science.286.5446.1954. [DOI] [PubMed] [Google Scholar]
- 30.Saito K, Scharenberg AM, Kinet JP. Interaction between the Btk PH domain and phosphatidylinositol-3,4,5-trisphosphate directly regulates Btk. J Biol Chem. 2001;276:16201–16206. doi: 10.1074/jbc.M100873200. [DOI] [PubMed] [Google Scholar]
- 31.Scharenberg AM, et al. Phosphatidylinositol-3,4,5-trisphosphate (PtdIns-3,4,5-P3)/Tec kinase-dependent calcium signaling pathway: a target for SHIP-mediated inhibitory signals. Embo J. 1998;17:1961–1972. doi: 10.1093/emboj/17.7.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim SK, et al. Point mutations in the split PLC-gamma1 PH domain modulate phosphoinositide binding. J Biochem Mol Biol. 2004;37:720–725. doi: 10.5483/bmbrep.2004.37.6.720. [DOI] [PubMed] [Google Scholar]
- 33.Saito K, et al. BTK regulates PtdIns-4,5-P2 synthesis: importance for calcium signaling and PI3K activity. Immunity. 2003;19:669–678. doi: 10.1016/s1074-7613(03)00297-8. [DOI] [PubMed] [Google Scholar]
- 34.Fruman DA, et al. Impaired B cell development and proliferation in absence of phosphoinositide 3-kinase p85alpha. Science. 1999;283:393–397. doi: 10.1126/science.283.5400.393. [DOI] [PubMed] [Google Scholar]
- 35.Okkenhaug K, et al. Impaired B and T cell antigen receptor signaling in p110delta PI 3-kinase mutant mice. Science. 2002;297:1031–1034. doi: 10.1126/science.1073560. [DOI] [PubMed] [Google Scholar]
- 36.Humphries LA, et al. Tec kinases mediate sustained calcium influx via site-specific tyrosine phosphorylation of the phospholipase Cgamma Src homology 2-Src homology 3 linker. J Biol Chem. 2004;279:37651–37661. doi: 10.1074/jbc.M311985200. [DOI] [PubMed] [Google Scholar]
- 37.Tsukada S, et al. Deficient expression of a B cell cytoplasmic tyrosine kinase in human X-linked agammaglobulinemia. Cell. 1993;72:279–290. doi: 10.1016/0092-8674(93)90667-f. [DOI] [PubMed] [Google Scholar]
- 38.Yu PW, et al. Sustained correction of B-cell development and function in a murine model of X-linked agammaglobulinemia (XLA) using retroviral-mediated gene transfer. Blood. 2004;104:1281–1290. doi: 10.1182/blood-2003-09-3044. [DOI] [PubMed] [Google Scholar]
- 39.Rodriguez R, et al. Tyrosine residues in phospholipase Cgamma 2 essential for the enzyme function in B-cell signaling. J Biol Chem. 2001;276:47982–47992. doi: 10.1074/jbc.M107577200. [DOI] [PubMed] [Google Scholar]
- 40.Watanabe D, et al. Four tyrosine residues in phospholipase C-gamma 2, identified as Btk-dependent phosphorylation sites, are required for B cell antigen receptor-coupled calcium signaling. J Biol Chem. 2001;276:38595–38601. doi: 10.1074/jbc.M103675200. [DOI] [PubMed] [Google Scholar]
- 41.Kerner JD, et al. Impaired expansion of mouse B cell progenitors lacking Btk. Immunity. 1995;3:301–312. doi: 10.1016/1074-7613(95)90115-9. [DOI] [PubMed] [Google Scholar]
- 42.Rawlings DJ, et al. Mutation of unique region of Bruton's tyrosine kinase in immunodeficient XID mice. Science. 1993;261:358–361. doi: 10.1126/science.8332901. [DOI] [PubMed] [Google Scholar]
- 43.Haas KM, Tedder TF. Role of the CD19 and CD21/35 receptor complex in innate immunity, host defense and autoimmunity. Adv Exp Med Biol. 2005;560:125–139. doi: 10.1007/0-387-24180-9_16. [DOI] [PubMed] [Google Scholar]
- 44.Rickert RC. Regulation of B lymphocyte activation by complement C3 and the B cell coreceptor complex. Curr Opin Immunol. 2005;17:237–243. doi: 10.1016/j.coi.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 45.Carter RH, Barrington RA. Signaling by the CD19/CD21 complex on B cells. Curr Dir Autoimmun. 2004;7:4–32. doi: 10.1159/000075685. [DOI] [PubMed] [Google Scholar]
- 46.Tuveson DA, Carter RH, Soltoff SP, Fearon DT. CD19 of B cells as a surrogate kinase insert region to bind phosphatidylinositol 3-kinase. Science. 1993;260:986–989. doi: 10.1126/science.7684160. [DOI] [PubMed] [Google Scholar]
- 47.Weng WK, Jarvis L, LeBien TW. Signaling through CD19 activates Vav/mitogen-activated protein kinase pathway and induces formation of a CD19/Vav/phosphatidylinositol 3-kinase complex in human B cell precursors. J Biol Chem. 1994;269:32514–32521. [PubMed] [Google Scholar]
- 48.Carter RH, Fearon DT. CD19: lowering the threshold for antigen receptor stimulation of B lymphocytes. Science. 1992;256:105–107. [PubMed] [Google Scholar]
- 49.Wang Y, Carter RH. CD19 regulates B cell maturation, proliferation, and positive selection in the FDC zone of murine splenic germinal centers. Immunity. 2005;22:749–761. doi: 10.1016/j.immuni.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 50.Phillips NE, Parker DC. Fc-dependent inhibition of mouse B cell activation by whole anti-mu antibodies. J Immunol. 1983;130:602–606. [PubMed] [Google Scholar]
- 51.Tony HP, Schimpl A. Stimulation of murine B cells with anti-Ig antibodies: dominance of a negative signal mediated by the Fc receptor. Eur J Immunol. 1980;10:726–729. doi: 10.1002/eji.1830100914. [DOI] [PubMed] [Google Scholar]
- 52.Wilson HA, et al. The B lymphocyte calcium response to anti-Ig is diminished by membrane immunoglobulin cross-linkage to the Fc gamma receptor. J Immunol. 1987;138:1712–1718. [PubMed] [Google Scholar]
- 53.Ono M, Bolland S, Tempst P, Ravetch JV. Role of the inositol phosphatase SHIP in negative regulation of the immune system by the receptor Fc(gamma)RIIB. Nature. 1996;383:263–266. doi: 10.1038/383263a0. [DOI] [PubMed] [Google Scholar]
- 54.D'Ambrosio D, et al. Recruitment and activation of PTP1C in negative regulation of antigen receptor signaling by Fc gamma RIIB1. Science. 1995;268:293–297. doi: 10.1126/science.7716523. [DOI] [PubMed] [Google Scholar]
- 55.Bolland S, Pearse RN, Kurosaki T, Ravetch JV. SHIP modulates immune receptor responses by regulating membrane association of Btk. Immunity. 1998;8:509–516. doi: 10.1016/s1074-7613(00)80555-5. [DOI] [PubMed] [Google Scholar]
- 56.Hippen KL, et al. Fc gammaRIIB1 inhibition of BCR-mediated phosphoinositide hydrolysis and Ca2+ mobilization is integrated by CD19 dephosphorylation. Immunity. 1997;7:49–58. doi: 10.1016/s1074-7613(00)80509-9. [DOI] [PubMed] [Google Scholar]
- 57.Fong DC, et al. Mutational analysis reveals multiple distinct sites within Fc gamma receptor IIB that function in inhibitory signaling. J Immunol. 2000;165:4453–4462. doi: 10.4049/jimmunol.165.8.4453. [DOI] [PubMed] [Google Scholar]
- 58.Fong DC, et al. Selective in vivo recruitment of the phosphatidylinositol phosphatase SHIP by phosphorylated Fc gammaRIIB during negative regulation of IgE-dependent mouse mast cell activation. Immunol Lett. 1996;54:83–91. doi: 10.1016/s0165-2478(96)02654-5. [DOI] [PubMed] [Google Scholar]
- 59.Ono M, et al. Deletion of SHIP or SHP-1 reveals two distinct pathways for inhibitory signaling. Cell. 1997;90:293–301. doi: 10.1016/s0092-8674(00)80337-2. [DOI] [PubMed] [Google Scholar]
- 60.Sato S, Tuscano JM, Inaoki M, Tedder TF. CD22 negatively and positively regulates signal transduction through the B lymphocyte antigen receptor. Semin Immunol. 1998;10:287–297. doi: 10.1006/smim.1998.0121. [DOI] [PubMed] [Google Scholar]
- 61.Nitschke L, Carsetti R, Ocker B, Kohler G, Lamers MC. CD22 is a negative regulator of B-cell receptor signalling. Curr Biol. 1997;7:133–143. doi: 10.1016/s0960-9822(06)00057-1. [DOI] [PubMed] [Google Scholar]
- 62.Otipoby KL, et al. CD22 regulates thymus-independent responses and the lifespan of B cells. Nature. 1996;384:634–637. doi: 10.1038/384634a0. [DOI] [PubMed] [Google Scholar]
- 63.Chen J, et al. CD22 attenuates calcium signaling by potentiating plasma membrane calcium-ATPase activity. Nat Immunol. 2004;5:651–657. doi: 10.1038/ni1072. [DOI] [PubMed] [Google Scholar]
- 64.Nitschke L. The role of CD22 and other inhibitory co-receptors in B-cell activation. Curr Opin Immunol. 2005;17:290–297. doi: 10.1016/j.coi.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 65.Poe JC, et al. Severely impaired B lymphocyte proliferation, survival, and induction of the c-Myc:Cullin 1 ubiquitin ligase pathway resulting from CD22 deficiency on the C57BL/6 genetic background. J Immunol. 2004;172:2100–2110. doi: 10.4049/jimmunol.172.4.2100. [DOI] [PubMed] [Google Scholar]
- 66.Danzer CP, Collins BE, Blixt O, Paulson JC, Nitschke L. Transitional and marginal zone B cells have a high proportion of unmasked CD22: implications for BCR signaling. Int Immunol. 2003;15:1137–1147. doi: 10.1093/intimm/dxg114. [DOI] [PubMed] [Google Scholar]
- 67.Samardzic T, et al. CD22 regulates early B cell development in BOB.1/OBF.1-deficient mice. Eur J Immunol. 2002;32:2481–2489. doi: 10.1002/1521-4141(200209)32:9<2481::AID-IMMU2481>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 68.Asano N. B Lymphocyte signaling established by the CD19/CD22 loop regulates autoimmunity in the tight-skin mouse. Am J Pathol. 2004;165:641–650. doi: 10.1016/S0002-9440(10)63328-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.O'Keefe TL, Williams GT, Batista FD, Neuberger MS. Deficiency in CD22, a B cellspecific inhibitory receptor, is sufficient to predispose to development of high affinity autoantibodies. J Exp Med. 1999;189:1307–1313. doi: 10.1084/jem.189.8.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vianna-Jorge R, Suarez-Kurtz G. Potassium channels in T lymphocytes: therapeutic targets for autoimmune disorders? BioDrugs. 2004;18:329–341. doi: 10.2165/00063030-200418050-00005. [DOI] [PubMed] [Google Scholar]
- 71.Chandy KG, et al. K+ channels as targets for specific immunomodulation. Trends Pharmacol Sci. 2004;25:280–289. doi: 10.1016/j.tips.2004.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Panyi G. Biophysical and pharmacological aspects of K+ channels in T lymphocytes. Eur Biophys J. 2005;34:515–529. doi: 10.1007/s00249-005-0499-3. [DOI] [PubMed] [Google Scholar]
- 73.Panyi G, Varga Z, Gaspar R. Ion channels and lymphocyte activation. Immunol Lett. 2004;92:55–66. doi: 10.1016/j.imlet.2003.11.020. [DOI] [PubMed] [Google Scholar]
- 74.Wulff H, Knaus HG, Pennington M, Chandy KG. K+ channel expression during B cell differentiation: implications for immunomodulation and autoimmunity. J Immunol. 2004;173:776–786. doi: 10.4049/jimmunol.173.2.776. [DOI] [PubMed] [Google Scholar]
- 75.Owsianik G, Talavera K, Voets T, Nilius B. Permeation and Selectivity of TRP Channels. Annu Rev Physiol. 2005;68:685–717. doi: 10.1146/annurev.physiol.68.040204.101406. [DOI] [PubMed] [Google Scholar]
- 76.Launay P, et al. TRPM4 is a Ca2+-activated nonselective cation channel mediating cell membrane depolarization. Cell. 2002;109:397–407. doi: 10.1016/s0092-8674(02)00719-5. [DOI] [PubMed] [Google Scholar]
- 77.Prawitt D, et al. TRPM5 is a transient Ca2+-activated cation channel responding to rapid changes in [Ca2+]i. Proc Natl Acad Sci U S A. 2003;100:15166–15171. doi: 10.1073/pnas.2334624100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Launay P, et al. TRPM4 regulates calcium oscillations after T cell activation. Science. 2004;306:1374–1377. doi: 10.1126/science.1098845. [DOI] [PubMed] [Google Scholar]
- 79.Vennekens R, et al. Increased IgE-dependent mast cell activation and anaphylactic responses in mice lacking the calcium-activated nonselective cation channel TRPM4. Nat Immunol. 2007;8:312–320. doi: 10.1038/ni1441. [DOI] [PubMed] [Google Scholar]
- 80.Mori Y, et al. Transient receptor potential 1 regulates capacitative Ca(2+) entry and Ca(2+) release from endoplasmic reticulum in B lymphocytes. J Exp Med. 2002;195:673–681. doi: 10.1084/jem.20011758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhu MX, Tang J. TRPC channel interactions with calmodulin and IP3 receptors. Novartis Found Symp. 2004;258:44–58. discussion 58–62, 98–102, 263–266. [PubMed] [Google Scholar]
- 82.Tang J, et al. Identification of common binding sites for calmodulin and inositol 1,4,5-trisphosphate receptors on the carboxyl termini of trp channels. J Biol Chem. 2001;276:21303–21310. doi: 10.1074/jbc.M102316200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang Z, et al. Activation of Trp3 by inositol 1,4,5-trisphosphate receptors through displacement of inhibitory calmodulin from a common binding domain. Proc Natl Acad Sci U S A. 2001;98:3168–3173. doi: 10.1073/pnas.051632698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Boulay G, et al. Modulation of Ca(2+) entry by polypeptides of the inositol 1,4, 5-trisphosphate receptor (IP3R) that bind transient receptor potential (TRP): evidence for roles of TRP and IP3R in store depletion-activated Ca(2+) entry. Proc Natl Acad Sci U S A. 1999;96:14955–14960. doi: 10.1073/pnas.96.26.14955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Huang GN, et al. STIM1 carboxyl-terminus activates native SOC, I(crac) and TRPC1 channels. Nat Cell Biol. 2006;8:1003–1010. doi: 10.1038/ncb1454. [DOI] [PubMed] [Google Scholar]
- 86.Ong HL, et al. Dynamic assembly of TRPC1/STIM1/Orai1 ternary complex is involved in store operated calcium influx: Evidence for similarities in SOC and CRAC channel components. J Biol Chem. 2007;282:9105–9116. doi: 10.1074/jbc.M608942200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rosker C, et al. Ca(2+) signaling by TRPC3 involves Na(+) entry and local coupling to the Na(+)/Ca(2+) exchanger. J Biol Chem. 2004;279:13696–13704. doi: 10.1074/jbc.M308108200. [DOI] [PubMed] [Google Scholar]
- 88.Basu S, Srivastava P. Immunological role of neuronal receptor vanilloid receptor 1 expressed on dendritic cells. Proc Natl Acad Sci U S A. 2005;102:5120–5125. doi: 10.1073/pnas.0407780102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Amantini C, et al. Distinct thymocyte subsets express the vanilloid receptor VR1 that mediates capsaicin-induced apoptotic cell death. Cell Death Differ. 2004;11:1342–1356. doi: 10.1038/sj.cdd.4401506. [DOI] [PubMed] [Google Scholar]
- 90.Nadler MJ, et al. LTRPC7 is a Mg.ATP-regulated divalent cation channel required for cell viability. Nature. 2001;411:590–595. doi: 10.1038/35079092. [DOI] [PubMed] [Google Scholar]
- 91.Perraud AL, et al. ADP-ribose gating of the calcium-permeable LTRPC2 channel revealed by Nudix motif homology. Nature. 2001;411:595–599. doi: 10.1038/35079100. [DOI] [PubMed] [Google Scholar]
- 92.Inada H, Iida T, Tominaga M. Different expression patterns of TRP genes in murine B and T lymphocytes. Biochem Biophys Res Commun. 2006;350:762–767. doi: 10.1016/j.bbrc.2006.09.111. [DOI] [PubMed] [Google Scholar]
- 93.Liu X, Zhu P, Freedman BD. Multiple Eicosanoid-Activated Non-Selective Cation Channels Regulate B Lymphocyte Adhesion to Integrin Ligands. Am J Physiol Cell Physiol. 2005;290:c873–c882. doi: 10.1152/ajpcell.00229.2005. [DOI] [PubMed] [Google Scholar]
- 94.Zhu P, Liu X, Labelle EF, Freedman BD. Mechanisms of hypotonicity-induced calcium signaling and integrin activation by arachidonic acid-derived inflammatory mediators in B cells. J Immunol. 2005;175:4981–4989. doi: 10.4049/jimmunol.175.8.4981. [DOI] [PubMed] [Google Scholar]
- 95.Liu QH, et al. Distinct calcium channels regulate responses of primary B lymphocytes to B cell receptor engagement and mechanical stimuli. J Immunol. 2005;174:68–79. doi: 10.4049/jimmunol.174.1.68. [DOI] [PubMed] [Google Scholar]
- 96.Kotturi MF, Jefferies WA. Molecular characterization of L-type calcium channel splice variants expressed in human T lymphocytes. Mol Immunol. 2005;42:1461–1474. doi: 10.1016/j.molimm.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 97.Savignac M, et al. Dihydropyridine receptors are selective markers of Th2 cells and can be targeted to prevent Th2-dependent immunopathological disorders. J Immunol. 2004;172:5206–5212. doi: 10.4049/jimmunol.172.9.5206. [DOI] [PubMed] [Google Scholar]
- 98.Stokes L, Gordon J, Grafton G. Non-voltage-gated L-type Ca2+ channels in human T cells: pharmacology and molecular characterization of the major alpha pore-forming and auxiliary beta-subunits. J Biol Chem. 2004;279:19566–19573. doi: 10.1074/jbc.M401481200. [DOI] [PubMed] [Google Scholar]
- 99.Dolmetsch RE, Lewis RS, Goodnow CC, Healy JI. Differential activation of transcription factors induced by Ca2+ response amplitude and duration. Nature. 1997;386:855–858. doi: 10.1038/386855a0. [DOI] [PubMed] [Google Scholar]
- 100.Healy JI, et al. Different nuclear signals are activated by the B cell receptor during positive versus negative signaling. Immunity. 1997;6:419–428. doi: 10.1016/s1074-7613(00)80285-x. [DOI] [PubMed] [Google Scholar]
- 101.Macian F. NFAT proteins: key regulators of T-cell development and function. Nat Rev Immunol. 2005;5:472–484. doi: 10.1038/nri1632. [DOI] [PubMed] [Google Scholar]
- 102.Liu JO. The yins of T cell activation. Sci STKE 2005. 2005:re1. doi: 10.1126/stke.2652005re1. [DOI] [PubMed] [Google Scholar]
- 103.Hogan PG, Chen L, Nardone J, Rao A. Transcriptional regulation by calcium, calcineurin, and NFAT. Genes Dev. 2003;17:2205–2232. doi: 10.1101/gad.1102703. [DOI] [PubMed] [Google Scholar]
- 104.Guo B, Su TT, Rawlings DJ. Protein kinase C family functions in B-cell activation. Curr Opin Immunol. 2004;16:367–373. doi: 10.1016/j.coi.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 105.Hanson CJ, Bootman MD, Roderick HL. Cell signalling: IP3 receptors channel calcium into cell death. Curr Biol. 2004;14:R933–R935. doi: 10.1016/j.cub.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 106.Butow RA, Avadhani NG. Mitochondrial signaling: the retrograde response. Mol Cell. 2004;14:1–15. doi: 10.1016/s1097-2765(04)00179-0. [DOI] [PubMed] [Google Scholar]
- 107.Jaattela M, Tschopp J. Caspase-independent cell death in T lymphocytes. Nat Immunol. 2003;4:416–423. doi: 10.1038/ni0503-416. [DOI] [PubMed] [Google Scholar]
- 108.Perl A, Gergely P, Jr, Puskas F, Banki K. Metabolic switches of T-cell activation and apoptosis. Antioxid Redox Signal. 2002;4:427–443. doi: 10.1089/15230860260196227. [DOI] [PubMed] [Google Scholar]
- 109.Rathmell JC. Apoptosis and B cell tolerance. Curr Dir Autoimmun. 2003;6:38–60. doi: 10.1159/000066855. [DOI] [PubMed] [Google Scholar]
- 110.Bhakta NR, Oh DY, Lewis RS. Calcium oscillations regulate thymocyte motility during positive selection in the three-dimensional thymic environment. Nat Immunol. 2005;6:143–151. doi: 10.1038/ni1161. [DOI] [PubMed] [Google Scholar]
- 111.Aneiros E, Philipp S, Lis A, Freichel M, Cavalie A. Modulation of Ca2+ signaling by Na+/Ca2+ exchangers in mast cells. J Immunol. 2005;174:119–130. doi: 10.4049/jimmunol.174.1.119. [DOI] [PubMed] [Google Scholar]
- 112.Eder P, Poteser M, Romanin C, Groschner K. Na(+) entry and modulation of Na(+)/Ca(2+) exchange as a key mechanism of TRPC signaling. Pflugers Arch. 2005;451:99–104. doi: 10.1007/s00424-005-1434-2. [DOI] [PubMed] [Google Scholar]
- 113.Guerini D, Coletto L, Carafoli E. Exporting calcium from cells. Cell Calcium. 2005;38:281–289. doi: 10.1016/j.ceca.2005.06.032. [DOI] [PubMed] [Google Scholar]
- 114.Strehler EE, Treiman M. Calcium pumps of plasma membrane and cell interior. Curr Mol Med. 2004;4:323–335. doi: 10.2174/1566524043360735. [DOI] [PubMed] [Google Scholar]
- 115.Wuytack F, Raeymaekers L, Missiaen L. Molecular physiology of the SERCA and SPCA pumps. Cell Calcium. 2002;32:279–305. doi: 10.1016/s0143416002001847. [DOI] [PubMed] [Google Scholar]
- 116.Kirichok Y, Krapivinsky G, Clapham DE. The mitochondrial calcium uniporter is a highly selective ion channel. Nature. 2004;427:360–364. doi: 10.1038/nature02246. [DOI] [PubMed] [Google Scholar]
- 117.Hoth M, Button DC, Lewis RS. Mitochondrial control of calcium-channel gating: a mechanism for sustained signaling and transcriptional activation in T lymphocytes. Proc Natl Acad Sci U S A. 2000;97:10607–10612. doi: 10.1073/pnas.180143997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Saris NE, Carafoli E. A historical review of cellular calcium handling, with emphasis on mitochondria. Biochemistry (Mosc) 2005;70:187–194. doi: 10.1007/s10541-005-0100-9. [DOI] [PubMed] [Google Scholar]
- 119.Jacobson J, Duchen MR. Interplay between mitochondria and cellular calcium signalling. Mol Cell Biochem. 2004;256–257:209–218. doi: 10.1023/b:mcbi.0000009869.29827.df. [DOI] [PubMed] [Google Scholar]
- 120.Gunter TE, Yule DI, Gunter KK, Eliseev RA, Salter JD. Calcium and mitochondria. FEBS Lett. 2004;567:96–102. doi: 10.1016/j.febslet.2004.03.071. [DOI] [PubMed] [Google Scholar]
- 121.Patterson RL, Boehning D, Snyder SH. Inositol 1,4,5-trisphosphate receptors as signal integrators. Annu Rev Biochem. 2004;73:437–465. doi: 10.1146/annurev.biochem.73.071403.161303. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.