Abstract
The synthesis and investigation of 5-[125I]Iodoindol-3-yl-β-D-galactopyranoside ([125I]IBDG) as a radioligand for single photon emission computed tomography (SPECT) imaging of β-galactosidase expression is described. No-carrier-added [125I]IBDG was synthesized by a radioiododestannylation approach in >75% overall radiochemical yield and >99% radiochemical purity. [125I]IBDG was evaluated as a substrate using β-galactosidase-expressing (D54L) and non-expressing (D54) human glioma cell lines. A 24 h incubation of this substrate with cultured cells revealed a 6.5-fold greater intracellular trapping of radioactivity in D54L cells compared to D54 cells. Systemic delivery of [125I]IBDG in nude mice bearing D54L tumors failed to show significant trapping of radioactivity within these tumors by SPECT imaging. In contrast, intratumoral injection of the substrate resulted in efficient trapping of radioactivity in D54L tumors but not D54 tumors resulting in clear SPECT visualization of the former tumor. Based on dynamic SPECT imaging and blood metabolite analysis, we conclude that although [125I]IBDG is an efficient in vivo substrate for β-galactosidase, its rapid renal clearance hampers its intratumoral availability upon systemic administration.
Keywords: gene expression imaging, β-galactosidase, iodine-125, SPECT, PET
INTRODUCTION
Expression of a reporter gene under the control of specific enhancer/promoter sequences is a widely used strategy for monitoring of gene regulation in biological systems. Reporters used for gene expression monitoring include β-galactosidase, chloramphenicol acetyltransferase, β-glucoronidase, firefly luciferase and green fluorescent protein.1 Among these, the bacterial enzyme Escherichia coli β-galactosidase (β-gal), expressed by the lacZ gene is the reporter most commonly used due to its high stability and turnover rate, ease of conjugation and detection and lack of endogenous expression in eukaryotic cells.2 LacZ expression and consequently gene transfection levels can thus be conveniently monitored by utilizing suitable β-gal substrates that exhibit a physical change (e.g. colorimetry, fluorescence) upon enzymatic hydrolysis.3, 4
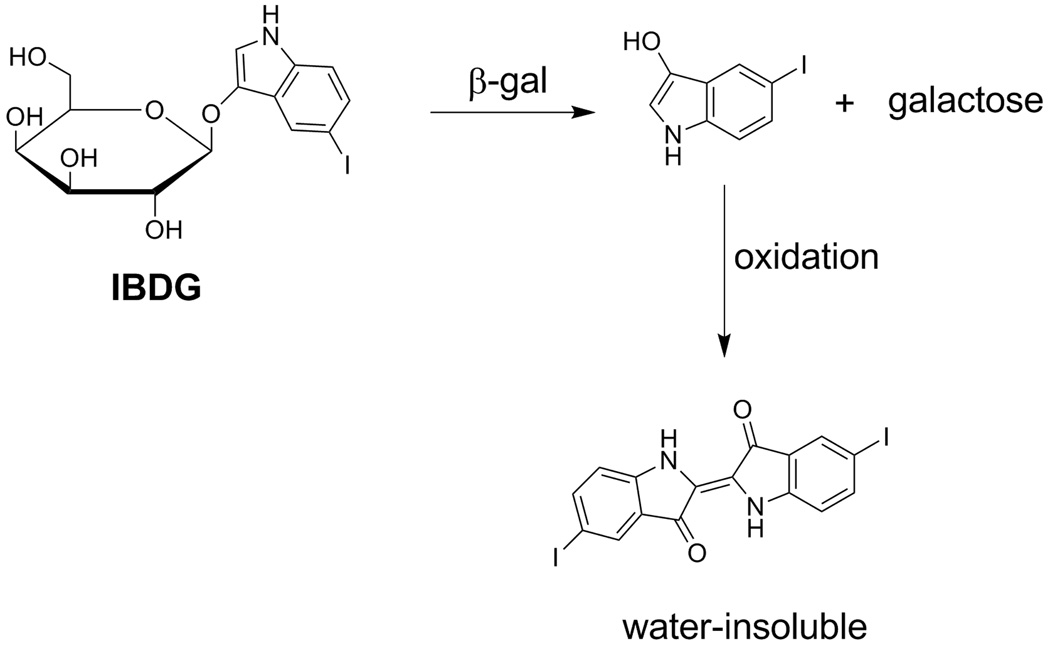
The ability to noninvasively monitor the location, magnitude and persistence of heterologous gene expression in vivo is critical for many studies in medical research.5–7 Consequently, this has led to intense interest in the development of β-gal substrates as imaging probes for in vivo imaging of gene expression. Reports describing a variety of such substrates for far-red fluorescence,2, 8–10 bioluminescence11 and magnetic resonance12 imaging have appeared in the recent literature. In contrast, there have been very few reports concerning the development of radioligands for either single photon emission computed tomography (SPECT) or positron emission tomography (PET) imaging of β-gal expression. A radioiodinated β-gal inhibitor for SPECT imaging of β-gal expression has been described by Choi and coworkers, however, in vivo studies with this radioligand were not encouraging which was attributed, in part, to its low cell membrane permeability.13, 14 From a clinical standpoint, SPECT and PET imaging techniques offer several unique advantages compared to the aforementioned imaging modalities including high sensitivity, improved safety due to administration of subpharmacological doses, ability to visualize gene expression tomographically and ease of external imaging of deep tissues. Furthermore, PET and SPECT imaging techniques of gene expression will likely have greater clinical utility than fluorescence or bioluminescence-based methods due to the wider availability of nuclear medicine imaging facilities. Our efforts to develop a radioiodinated substrate for SPECT/PET imaging of β-gal-expressing tumors focused on the known chromogenic β-gal substrate: 5-Iodoindol-3-yl-β-D-galactopyranoside (Purple-β-D-Gal; IBDG; Figure 1). Intracellular enzymatic hydrolysis of β-gal substrates such as IBDG generates free indoxyl molecules which undergo in situ oxidation and subsequent dimerization to produce chromogenic, water-insoluble, indigo precipitates (Figure 2).15 We hypothesized that use of a radioiodinated IBDG derivative could by a similar process lead to selective retention of the radiolabel in β-gal-expressing cells thus permitting in vivo imaging of β-gal expression by SPECT or PET techniques. As a step towards this goal, the radiosynthesis and preliminary biological evaluation of [125I]IBDG was undertaken and these studies are herein reported.
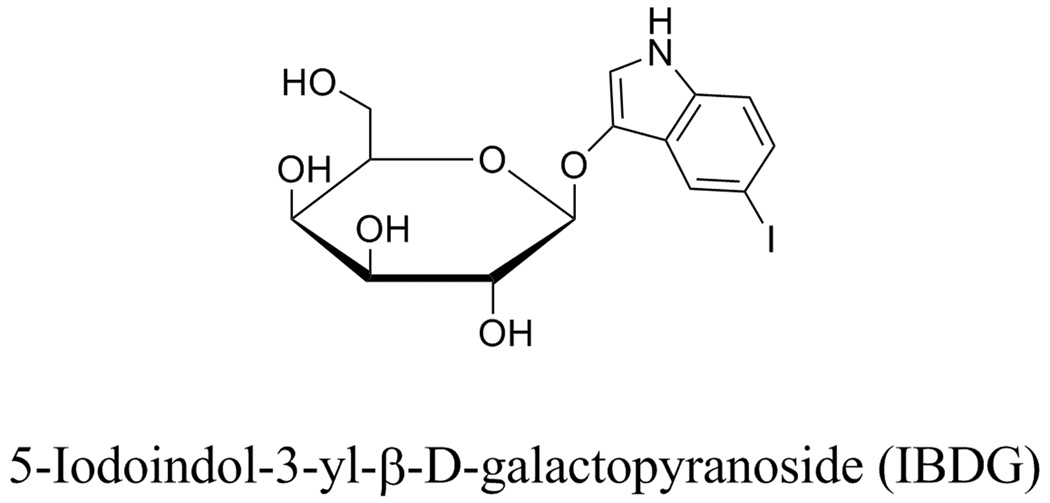
Figure 1.
Structure of IBDG
Figure 2.
Enzymatic hydrolysis of IBDG by β-Galactosidase
MATERIALS AND METHODS
Chemicals and Radiochemicals
N-Acetyl-5-bromoindol-3-ol was purchased from Apollo Scientific Ltd., Cheshire, UK and IBDG was purchased from Biotium, Inc., Hayward, CA. All other chemical reagents were obtained from Aldrich Chemical Co., Milwaukee, WI. Sodium [125I]iodide was obtained from MDS Nordion Inc. (Ottawa, Canada) as a no-carrier-added solution in aqueous 0.01 N NaOH (pH 10 – 12).
Instrumentation and Analyses
Melting points were determined with a Thomas-Hoover melting point apparatus and are uncorrected. Thin-layer chromatography (TLC) was performed using Analtech silica gel GF Uniplates (250 micron). TLC plates were visualized after development using either ultraviolet (UV) light or phosphomolybdic acid reagent with subsequent heating. 1H NMR spectra were recorded on a Varian INOVA instrument operating at 400 MHz with either CDCl3 or DMSO-d6 as solvent and tetramethylsilane (TMS) as internal standard. Chemical shifts (δ) and coupling constants (J) are reported in parts per million (ppm) and Hertz (Hz), respectively. Compound elemental analysis data were obtained at the Department of Chemistry, University of Michigan. High resolution mass spectral analyses were performed at the Department of Chemistry, University of Michigan, using either a VG-70-250-S mass spectrometer for electron impact (EI) and chemical ionization (DCI) modes or a Waters Autospec Ultima instrument with an electrospray interface for electrospray ionization (ESI) mode. HPLC was performed using a Waters Breeze HPLC System (Waters Corporation, Milford, MA) equipped with a Waters 2487 Dual Wavelength Absorbance Detector. Radioactivity was monitored with a Bioscan Flow Count FC-3300 NaI/PMT Radiodetector (Bioscan, Inc., Washington, DC) equipped with a 1.5” × 1.5” NaI(Tl) crystal. Radio-HPLC analysis and purification were conducted with a Supelcosil LC-C18 analytical column (4.6 × 250 mm, 5 µ particle, Supelco, Bellefonte, PA) using HPLC grade water (A) and CH3CN (B) solvent mixtures with either of the following methods: Method I (solvent gradient elution from 55% B to 95% B over 20 min; UV detection at 254 nm); Method II (solvent gradient elution from 10% B to 95% B over 20 min; UV detection at 280 nm). All chromatographic procedures were conducted at ambient temperature using a flow rate of 1 mL/min.
Radioactivity measurements were obtained with a Capintec CRC-15W Radioisotope Dose Calibrator (Ramsey, NJ) and specific activity estimates were determined from a standard curve relating mass to UV absorbance peak area.
Synthetic Chemistry
1-Acetyl-5-bromoindol-3-yl-tetra-O-acetyl-β-D-galactopyranoside (3)
The title compound was synthesized from 2,3,4,6-tetra-O-acetyl-β-D-galactopyranosyl bromide (1) and N-acetyl-5-bromoindol-3-ol (2) according to the method of Horwitz et. al..16 Flash chromatography of the crude product on silica gel (40% EtOAc/hexanes) followed by recrystallization from ethanol provided 3 in 43% yield: mp 175 – 176 °C. 1H NMR (CDCl3): δ 8.26 (br s, 1H), 7.62 (d, 1H, J = 1.7 Hz), 7.47 (dd, 1H, J = 9.0, 1.9 Hz), 7.16 (br s, 1H), 5.56 – 5.48 (m, 2H), 5.12 (dd, 1H, J = 10.5, 3.5 Hz), 4.97 (d, 1H, J = 8.0 Hz), 4.23 (m, 2H), 4.06 (t, 1H, J = 6.9 Hz), 2.60 (s, 3H), 2.21 (s, 3H), 2.15 (s, 3H), 2.07 (s, 3H), 2.04 (s, 3H). HRMS: Calcd for C24H26BrO11NNa: 606.0587. Found: 606.0567. Anal. Calcd for C24H26NBrO11: C, 49.33; H, 4.48; N, 2.40. Found: C, 49.02; H, 4.41; N, 2.30.
1-Acetyl-5-(tributylstannyl)indol-3-yl-tetra-O-acetyl-β-D-galactopyranoside (4)
A solution of the bromoindole analog 3 (0.438 g, 0.75 mmol) and bistributyltin (1.74 g, 3.0 mmol) in anhydrous toluene (12 mL) was degassed with a nitrogen stream for 10 min and treated with tetrakis(triphenylphosphine)Pd(0) (0.087 g, 0.075 mmol) in one portion.17 The mixture was refluxed with stirring under a nitrogen atmosphere for 18 h, at which point, TLC analysis showed completion of reaction. The warm mixture was filtered through a pad of Celite and concentrated in vacuo to give a brown gum. Flash chromatography of the residue on silica gel (40% EtOAc/hexanes) provided 0.21 g (35%) of pure material as an off-white amorphous solid. 1H NMR (CDCl3): δ 8.30 (br s, 1H), 7.59 (s, 1H), 7.46 (d, 1H, J = 8.2 Hz), 7.15 (br s, 1H), 5.60 – 5.56 (m, 1H), 5.49 (m, 1H), 5.13 (dd, 1H, J = 10.5, 3.3 Hz), 5.00 (d, 1H, J = 8.0 Hz), 4.24 (d, 2H, J = 6.4 Hz), 4.08 (t, 1H, J = 6.3 Hz), 2.60 (s, 3H), 2.20 (s, 3H), 2.11 (s, 3H), 2.08 (s, 3H), 2.03 (s, 3H), 1.57 – 1.52 (m, 6H), 1.38 – 1.29 (m, 6H), 1.11 – 1.07 (m, 6H), 0.89 (t, 9H, J = 7.33 Hz). HRMS: Calcd for C36H53O11NSnNa: 818.2538. Found: 818.2545.
1-Acetyl-5-iodoindol-3-yl-tetra-O-acetyl-β-D-galactopyranoside (5)
A stirred solution of the tributylstannyl analog 4 (0.155 g, 0.20 mmol) in anhydrous CHCl3 (15 mL) was treated dropwise with a 0.1 M solution of iodine in CHCl3 until a slight violet color persisted. The solution was stirred a further 3 h at room temperature and quenched with a solution of KF in CH3OH (1 M, 0.5 mL) followed by aqueous 5% NaHSO3 solution (1.5 mL). The mixture was treated with saturated brine (5 mL) and the organic layer separated and dried (Na2SO4). Flash chromatography of the residue on silica gel (40% EtOAc/hexanes) gave 0.10 g (79%) of the title compound 5 as a white solid following recrystallization from ethanol : mp 189 – 191 °C (dec) 1H NMR (CDCl3): δ 8.14 (br s, 1H), 7.82 (d, 1H, J = 1.6 Hz), 7.65 (dd, 1H, J = 8.7, 1.7 Hz), 7.13 (br s, 1H), 5.56 – 5.48 (m, 2H), 5.12 (dd, 1H, J = 10.5, 3.5 Hz), 4.96 (d, 1H, J = 7.8 Hz), 4.23 (m, 2H), 4.06 (t, 1H, J = 5.9 Hz), 2.59 (s, 3H), 2.21 (s, 3H), 2.16 (s, 3H), 2.07 (s, 3H), 2.04 (s, 3H). HRMS: Calcd for C24H26IO11NNa: 654.0448; Found: 654.0453. Anal. Calcd for C24H26NIO11: C, 45.66; H, 4.15; N, 2.22. Found: C, 45.37; H, 4.24; N, 2.33.
5-Iodoindol-3-yl-β-D-galactopyranoside (IBDG)
Sodium methoxide (0.02 mL, 0.01 mmol, 0.5 M in methanol) was added to a stirred solution of the pentaacetate analog 5 (0.025 g, 0.040 mmol) in anhydrous methanol (2 mL) at 5 °C. The clear solution was stirred for an additional 1 h and allowed to stand at 5 °C overnight. The reaction was neutralized with 1 drop of 50% aqueous acetic acid and the mixture concentrated to dryness in vacuo at room temperature. Flash chromatography of the residue on silica gel (15% methanol/dichloromethane) followed by recrystallization from water gave 13 mg (77%) of the title compound as a white solid. mp 180 – 182 °C (dec) 1H NMR (DMSO-d6 + 1 drop D2O): δ 7.94 (d, 1H, J = 1.4 Hz), 7.29 (dd, 1H, J = 8.6, 1.6 Hz), 7.15 (d, 1H, J = 8.6 Hz), 7.06 (s, 1H), 4.46 (d, 1H, J = 7.6 Hz), 3.65 (d, 1H, J = 3.1 Hz), 3.58 – 3.49 (m, 3H), 3.43 (t, 1H, J = 6.2 Hz), 3.35 (dd, 1H, J = 9.6, 3.3 Hz). HRMS: Calcd for C14H16IO6NNa: 443.9920; Found: 443.9918. Anal. Calcd for C14H16NIO6: C, 39.92; H, 3.83; N, 3.33. Found: C, 40.04; H, 3.90; N, 3.27.
Radiosynthetic Chemistry
1-Acetyl-5-[125I]iodoindol-3-yl-tetra-O-acetyl-β-D-galactopyranoside ([125I]5)
A glass vial containing a solution of 4 (30 µg, 38 nmol) in absolute ethanol (30 µL) was treated with a solution of 50 µL of freshly prepared 0.1 N HCl in ethanol. The vial was sealed and crimped, a solution of 6.13 mCi of Na125I in aqueous 0.01 N NaOH (6.0 µL) was added by syringe to the vial and the reaction was initiated by addition of 50 µL of freshly prepared aqueous H2O2 (3% wt./vol.). The vial was shaken periodically for 10 min and the reaction was quenched by addition of 100 µL of aqueous sodium metabisulfite (12 mg/mL). HPLC analysis of the crude product mixture (Method I) showed 95% radiochemical purity for [125I]5 (tR = 10.1 min). The crude product was purified by preparative HPLC (Method I) to afford 5.05 mCi (82%) of [125I]5 (>99% chemical and radiochemical purity) which was concentrated to dryness in vacuo and used directly in the next step.
5-[125I]Iodoindol-3-yl-β-D-galactopyranoside ([125I]IBDG)
A solution of no-carrier-added [125I]5 (5 mCi) in anhydrous methanol (0.5 mL) was cooled to 5 °C (ice-bath) under a nitrogen atmosphere. A 0.5 M solution of sodium methoxide in methanol (15 µL) was then added by syringe and the reaction mixture was maintained at 5 °C for 60 min. The mixture was quenched with 50% aqueous acetic acid (10 µL), concentrated in vacuo and purified by preparative HPLC (Method II; tR of IBDG = 8.5 min). The HPLC fraction containing the product was diluted with water (10 mL) and eluted through a preconditioned C-18 Sep-Pak cartridge. The cartridge was eluted with CH3CN (2 mL) to afford [125I]IBDG which was concentrated under reduced pressure and formulated in PBS:EtOH [95:5] for animal studies. The chemical identity of [125I]IBDG was confirmed using analytical HPLC (Method II) by co-injection with the nonradioactive IBDG standard.
Cell Culture and Transfections
D54 (human glioma) cells were grown in RPMI 1640 medium supplemented with 10% fetal bovine serum, 100 units/ml penicillin, 100 µg/ml streptomycin sulfate, 1 mM sodium pyruvate, 10 mM HEPES, 292 ng/ml L-glutamine (all from Invitrogen, Carlsbad CA) and maintained in a humidified incubator at 37 °C and 5% CO2. Cells were transfected using 4 µg of pEFLacZ and 16 µL of Fugene according to the manufacturer’s protocol. Stable clones were selected using 200 ng/ml G418 (Invitrogen) and characterized for expression of the reporter. Specific clones were identified and selected for further study based on expression levels of the recombinant protein.
Detection of β-gal Activity In Vitro with [125I]IBDG
In vitro studies to detect β-gal activity using [125I]IBDG were conducted using control D54 human glioma cells (D54) and their β-gal expressing counterpart (D54L). Cells were seeded in 6-well dishes and incubated for up to 48 h at 37 °C with no-carrier-added [125I]IBDG (0.1 µCi per well) together with various concentrations of cold IBDG substrate (0 mM, 0.5 mM and 1.0 mM). Cells were collected by scraping after 24 h and 48 h of incubation and pelleted by centrifugation at 1800 rpm for 10 min. After removal of the supernatant, the pellet was rinsed with PBS and re-pelleted. Finally, the pellet was re-suspended in PBS and assayed for radioactivity using a Gamma Counter.
Animal Models
Athymic, CD1 nude mice (nu/nu; 25 – 30 g; 5 – 6 weeks old; N = 6; Charles River Laboratories, IN) were used for the tumor imaging studies. For systemic (i.v., i.p.) injection studies, mice under isoflurane anesthesia were initially injected subcutaneously in each flank with equivalent amounts (2 × 106 cells) of either D54L (right flank) or D54 tumor cells (left flank). Tumors were allowed to grow to approximately 5 – 7 mm in size prior to conducting imaging studies. Animals under isoflurane anesthesia were injected (i.v. or i.p.) with [125I]IBDG (500 µCi in 100 µL of PBS:EtOH [95:5]; specific activity = 5 mCi/mg) and a series of image acquisitions (10 – 60 min) were collected at 5 min, 1 h, 2 h, 4 h, 7 h, 12 h and 24 h post-injection.
For the intratumoral injection studies, mice under isoflurane anesthesia were initially injected subcutaneously in each flank with equivalent amounts (107 cells in 200 µL of PBS) of either D54L (right flank) or D54 tumor cells (left flank). Each tumor implantation site was then directly injected with equivalent doses of [125I]IBDG (500 µCi in 100 µL of PBS:EtOH [95:5]; specific activity = 5 mCi/mg; approximately 0.5 mM concentration in tumor) and a series of image acquisitions (10 – 60 min) were collected at 5 min, 1 h, 2 h, 4 h, 7 h, 12 h and 24 h post-injection.
All animal studies were conducted in accordance with the rules of the University Committee on Use and Care of Laboratory Animals (UCUCA) at the University of Michigan.
SPECT/CT Mouse Imaging Studies
SPECT/CT imaging studies were conducted on a GMI Tri-Modality CT/PET/SPECT small animal scanner (Gamma-Medica Ideas, Inc., Northridge, CA). SPECT images were acquired using a dual-head, high-resolution, low-energy parallel-hole collimator. CT imaging of animals was performed for anatomical co-registration and CT fusion of SPECT images were performed using AMIRA (version 3.1) software.
Analysis of Mouse Blood Metabolites of [125I]IBDG
Tumor-bearing CD1 mice (N = 3) under isoflurane anesthesia were each injected intravenously with approximately 600 µCi of [125I]IBDG in 0.2 mL 0f PBS:EtOH [95:5] and sacrificed 5 min post-injection. Blood samples (approximately 0.6 – 0.8 mL) were collected by cardiac puncture and homogenized with equal volumes of acetonitrile. The mixture was centrifuged at 15,000 g for 20 min, the supernatant was transferred to a fresh centrifuge tube (95% extraction efficiency) and the centrifugation repeated. The clear supernatant was concentrated using a nitrogen stream and analyzed by radio-TLC (silica; CH2Cl2:CH3OH [4:1]) and HPLC analysis (Method II).
Determination of Partition Coefficient of IBDG
The partition coefficient (P) of IBDG was determined by modification of a “shake-flask technique” as described by Rothwell et al.18 n-Octanol and potassium phosphate buffer (20 mM, pH 7.4) were pre-saturated with each other for 24 h prior to use. Solutions of IBDG (1 – 2 mM) in 2 mL of potassium phosphate buffer were combined with an equal volume of n-octanol in a centrifuge tube and vortexed for 1 min. The mixture was then centrifuged at 5000 rpm for 5 min and the layers separated. The concentration of IBDG in each phase was determined from the corresponding UV peak areas following HPLC analysis (Method II; UV analysis at 215 nm). Samples of n-octanol were diluted with 4 volumes of acetonitrile prior to HPLC injection. Partition experiments were conducted in triplicate and HPLC analysis of each phase was conducted in duplicate. The partition coefficient is defined as the ratio of the concentration of substance in n-octanol to that in buffer and lipophilicity (log P) is reported as the logarithm of the partition coefficient. IBDG displayed a log P of 0.8 in these experiments.
RESULTS
Chemistry
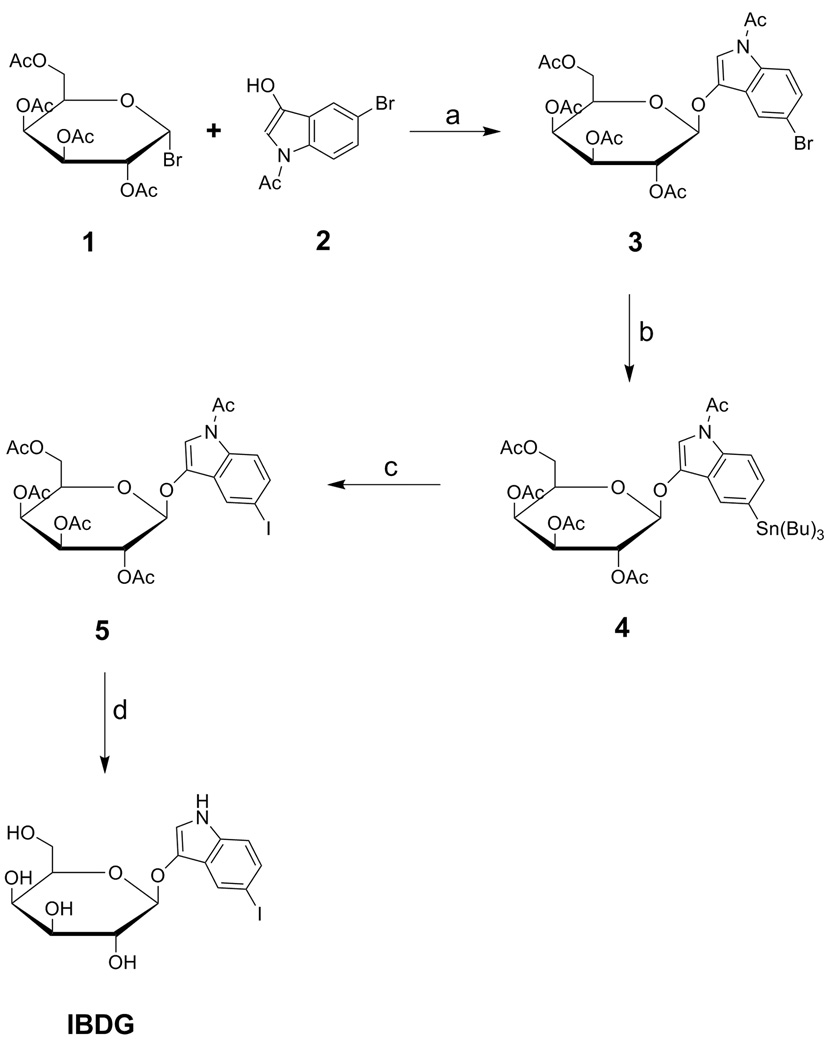
Our synthetic strategy for preparation of radioiodinated IBDG focused on the use of the tributylstannyl analog 4 as a precursor for radiolabeling (Scheme 2). The synthesis of 4 and cold IBDG are shown in Scheme 1. In this approach, we utilized N-acetyl-5-bromoindol-3-ol (2) instead of the corresponding iodo analog as the aglycone in the initial reaction step due to its lower cost and commercial availability. Initial attempts at synthesizing the acetyl-protected galactoside derivative 3 by reaction of 2 with galactopyranosyl bromide (1) under phase-transfer catalysis conditions19, 20 resulted in complex product mixtures and poor isolated yields. Subsequently, compound 3 was prepared in moderate yield (43%) according to the method of Horvitz et al. by a base-catalyzed reaction of 2 with galactopyranosyl bromide (1).16 Replacement of the bromine atom in intermediate 3 with the tributylstannyl group was achieved via a palladium-mediated coupling reaction using bistributyltin and tetrakis(triphenylphosphine)palladium(0) as catalyst to afford 4 in 35% yield. A portion of this material was converted to the corresponding acetyl protected IBDG derivative (5) by an iododestannylation reaction with iodine in chloroform. Deacetylation of the latter intermediate using Zemplen conditions (NaOMe/MeOH) followed by chromatographic purification provided cold IBDG in 77% yield. All of the synthesized compounds displayed H-1 NMR and mass spectra consistent with the assigned structures. The trans configuration of the 1,2 glycoside bond (β-anomer) in the galactoside derivatives was confirmed by the presence of a characteristic doublet for the anomeric proton signal at δ 4.46 – 5.0 (J1,2 7.6 – 8.0 Hz) in their NMR spectra.
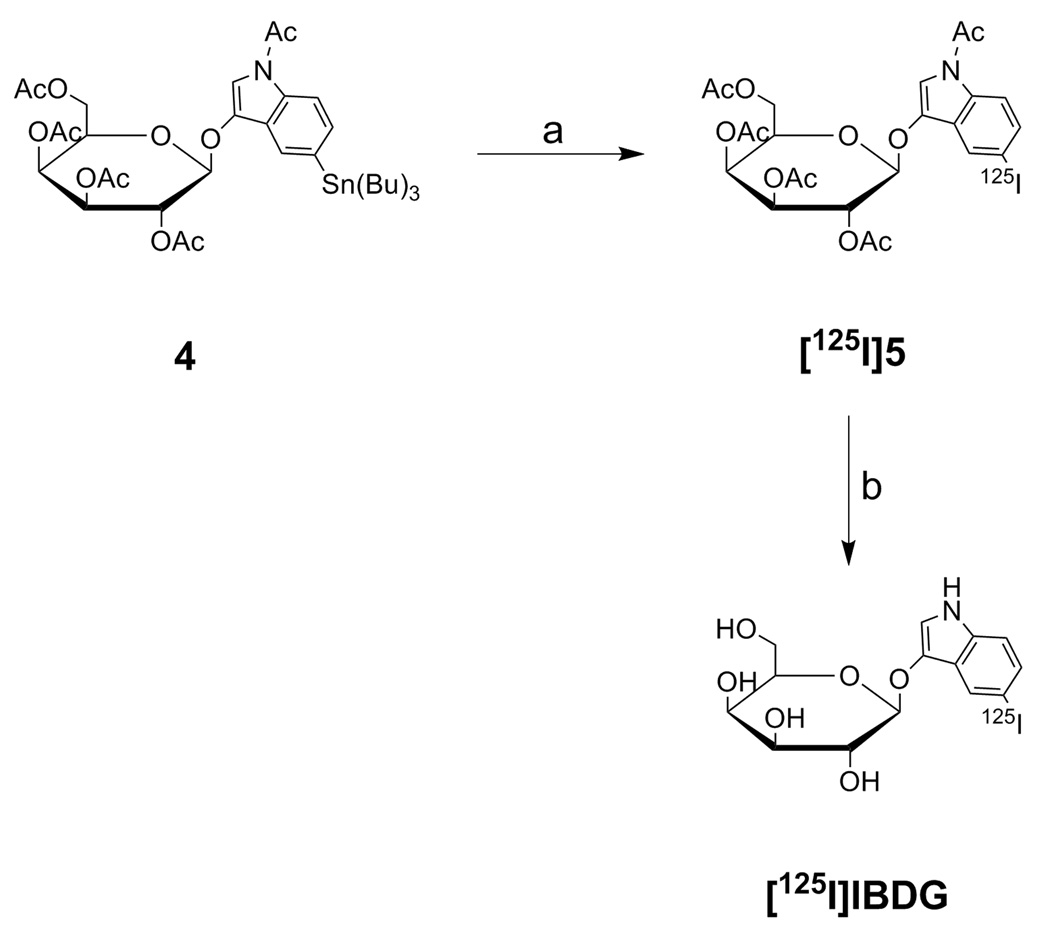
SCHEME 2a.
a Reagents and conditions: (a) Na125I, H2O2, 0.1 N ethanolic HCl; (b) NaOMe, MeOH, 5 °C.
SCHEME 1a.
a Reagents and conditions: (a) 1 N NaOH, acetone, 5 °C; (b) Pd(PPh3)4, Sn2Bu6, toluene, reflux; (c) I2, ChCl3, rt; (d) NaOMe, MeOH, 5 °C.
Radiosynthetic Chemistry
The synthesis of no-carrier-added [125I]IBDG was accomplished using a radioiododestannylation approach (Scheme 2).17 Accordingly, the tributylstannyl analog 4 was treated with Na125I and aqueous H2O2 as oxidizing agent to afford crude [125I]5 in 95% radiochemical purity. Further purification of this material by preparative reversed-phase HPLC provided [125I]5 in 82% overall radiochemical yield and >99% radiochemical purity. Subsequent deacetylation of [125I]5 was conducted using sodium methoxide in methanol at 5 °C to afford [125I]IBDG in 92% radiochemical yield and >99% radiochemical and chemical purity after HPLC purification. The average specific activity of [125I]IBDG was 1685 ± 153 Ci/mmol (N = 5). The radioligand which was formulated in PBS:EtOH [95:5] was stable for at least 1 week when stored at 5 °C (<2% radiolytic decomposition by radio-HPLC analysis).
In Vitro Cell Uptake Studies
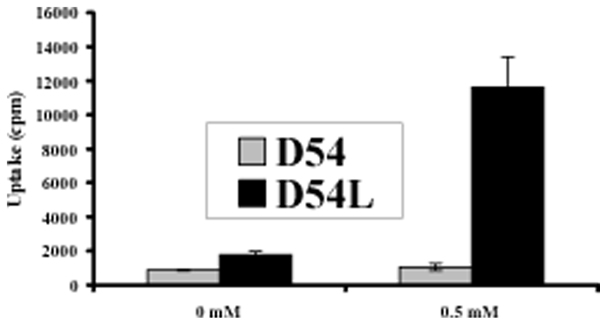
The ability of [125I]IBDG to undergo cellular uptake and retention was investigated in β-gal-expressing D54 cells (D54L) in comparison to control cells (D54).14, 21 Initial validation studies with cold IBDG using a colorimetric assay showed that optimum conversion of this substrate in D54L cells occurs at a 24 – 48 h incubation time with a 0.5 – 1.0 mM substrate concentration (Figure 3). Subsequent studies conducted with [125I]IBDG demonstrated a 6.5- to 7-fold increase in cellular radioactivity in D54L cells compared to D54 controls following a 24 h to 48 h incubation period at 0.5 mM substrate concentrations (Figure 4; 48 h data not shown). Radioactivity uptake ratios were somewhat lower (4.5- to 5.1-fold) at these same time intervals at 1.0 mM substrate concentrations (data not shown). No significant difference in radioactivity uptake was observed between the two cell types for either time interval at no-carrier-added levels of [125I]IBDG (0 mM).
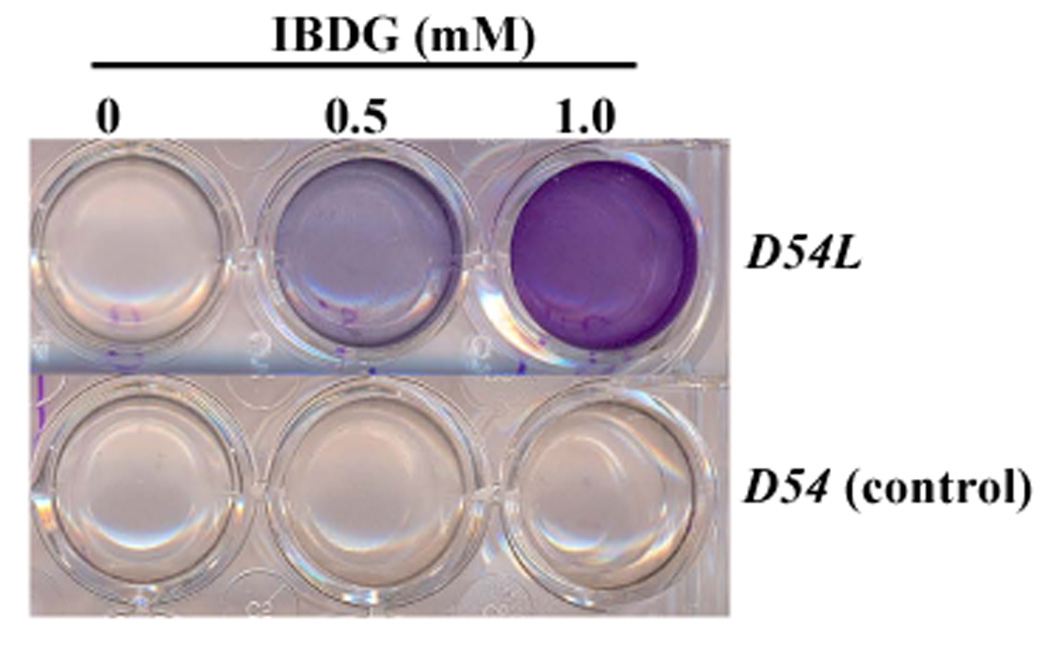
Figure 3.
β-Galactosidase dependent staining of D54 cells in the presence of IBDG. Control D54 human glioma cells (D54) or their β-galactosidase-expressing counterpart (D54L) were cultured in 6-well dishes and treated with 0, 0.5 or 1.0 mM IBDG for 24 h. Experiments were conducted in triplicate. Hydrolysis of IBDG is visualized by virtue of the formation of a violet precipitate in D54L cells but not in D54 cells.
Figure 4.
Comparison of cellular uptake of [125I]IBDG in D54 and D54L cells at 24 h incubation. Control D54 human glioma cells (D54) or their β-galactosidase-expressing counterpart (D54L) were incubated for 24 h in 6-well dishes with various concentrations of cold IBDG (0, 0.5 mM) spiked with [125I]IBDG (0.1 µCi per well). Data points represent the mean ± SD of triplicate determinations. Cellular uptake of radioactivity is reported in units of counts per min (cpm).
SPECT Imaging Studies
A) Systemic [125I]IBDG administration
Mouse SPECT/CT imaging studies were conducted over a 24 h period after intravenous administration of [125I]IBDG. Analysis of the image data showed high initial uptake of radioactivity in kidney within 5 min after injection followed by its excretion into urinary bladder over a 1 – 2 h period. Radioactivity uptake in most major organs were at or near background levels throughout the imaging study. Neither the D54L nor D54 tumor was visualized in the SPECT images during the entire imaging time frame due to insufficient tumor uptake of radioactivity. A similar pharmacokinetic behavior was seen in the SPECT images following i.p. administration of the radioligand.
B) Intratumoral [125I]IBDG administration
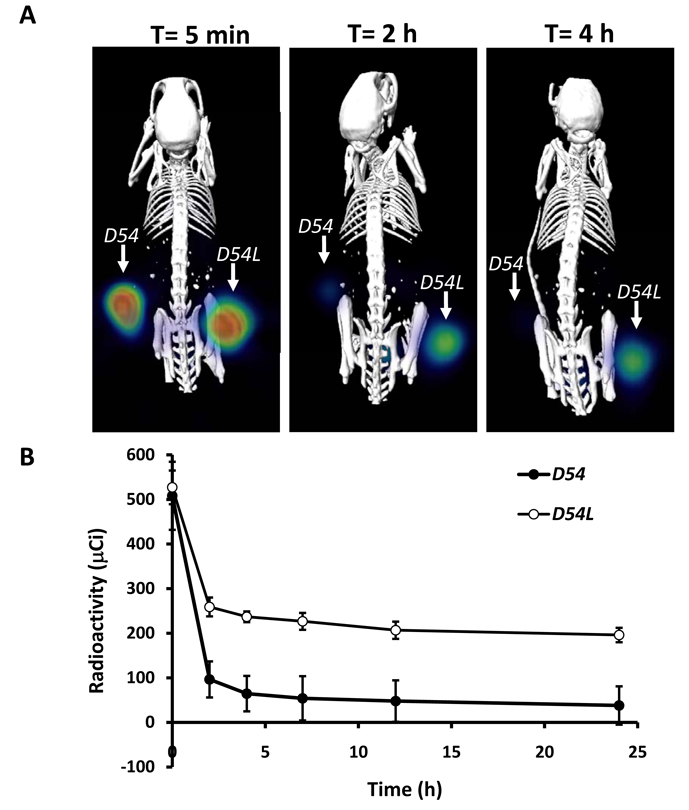
Serial SPECT/CT imaging studies were also conducted as described above following the intratumoral injection of [125I]IBDG. The mouse SPECT image (A) and temporal tumor distribution profile (B) data at 5 min, 2 h and 4 h post-injection are shown in Figure 5. As seen from this data, a sharp decline of radioactivity for the D54 tumor as compared to the D54L tumor is clearly apparent at the 2 h imaging interval. Clearance of radioactivity from the D54 site was essentially complete by 7 h post-injection, while radioactivity levels in the D54L tumor were similar to its 4 h values (data not shown). This differential resulted in clear visualization of the β-gal expressing D54L tumor within the 2 h, 4 h and 7 h images. Radioactivity clearance occurred mainly via the renal pathway as evidenced by high urinary bladder radioactivity and the absence of radioactivity in liver.
Figure 5.
(A). Co-registered SPECT/CT images in a CD1 mouse after [125I]IBDG administration to control D54 tumors (left flank) and β-gal-expressing D54L tumors (right flank). (B). Time-radioactivity curves of administered [125I]IBDG in mouse D54 and D54L tumors.
Mouse Blood Metabolite Analysis
Radio-HPLC analysis of mouse blood radioactivity at 5 min post-injection showed that approximately 39% of the total radioactivity was associated with intact [125I]IBDG. The remaining radioactivity consisted primarily of two polar metabolites eluting at tR = 2.8 min (16%) and tR = 3.3 min (53%), respectively. Neither of the polar metabolites were 125I as confirmed by HPLC analysis with co-injected sodium iodide. A similar metabolite profile was seen by radio-TLC analysis.
DISCUSSION
β-galactosidase is a stable, quantitative and sensitive reporter extensively used for gene expression studies. For example, studies involving promoter function in transgenic animals as well as gene delivery studies in animal models have utilized the β-gal gene.22–24 However, these studies invariably involve sacrifice of the animal and subsequent extraction of the desired tissues for detection of expression. Consequently, such studies only provide a snapshot image of β-gal expression. The use of noninvasive tomographic imaging techniques such as PET or SPECT can provide real-time, quantitative information of biochemical or molecular processes repetitively in the same subject. Such methods when applied to gene expression imaging could therefore provide the means to follow β-gal expression longitudinally in living subjects which would be of great value both for preclinical studies and future clinical gene therapy trials.5
A wide variety of galactoside analogs that are β-gal enzyme substrates are routinely available for spectroscopic analysis of β-galactosidase activity. Enzymatic hydrolysis of these substrates leads to the formation of an intensely-colored, water-insoluble, reaction product which is selectively localized to the site of reporter gene expression and can be conveniently monitored by colorimetry. The goal of the present study was to synthesize and evaluate a suitably radioiodinated β-gal substrate that would exploit this selective trapping mechanism for in vivo imaging of β-gal expression using SPECT. For this purpose, we focused on the chromogenic β-gal substrate: 5-iodoindol-3-yl-β-D-galactopyranoside (IBDG) as a candidate for radioiodination and biological evaluation. For these initial studies, we radioiodinated IBDG with 125I instead of the standard SPECT radioisotope 123I due to its lower cost, longer half-life and its imaging capability with dedicated small animal SPECT scanners. Radiosynthesis of [125I]IBDG was achieved using a radioiododestannylation reaction followed by acetyl deprotection to afford [125I]IBDG in >75% overall radiochemical yield and >99% chemical and radiochemical purity.
Prior to conducting in vivo studies we evaluated the specificity of [125I]IBDG for cell uptake and retention in β-gal expressing cells using an in vitro cell uptake assay. The cellular trapping of [125I]IBDG was evaluated at 3 substrate concentrations (0, 0.5 and 1.0 mM). In these studies, [125I]IBDG displayed optimum cellular trapping of radioactivity (6.5 – 7-fold increase) in β-gal expressing D54 cells (D54L) as compared to control cells (D54) at the 0.5 mM substrate concentration. Importantly, we did not observe a significant difference in radioactivity uptake between the two cell types at either the 24 h or 48 h time interval in the absence of carrier (0 mM). This observation can be attributed to the fact that the IBDG substrate concentration was significantly lower relative to the enzymes’ Km value which is reported to be in the 0.1 – 4 mM range.25
Encouraged by these initial findings, we administered [125I]IBDG intravenously to CD1 mice having D54L and D54 solid tumor xenografts and conducted serial SPECT/CT imaging studies. Since our in vitro cell uptake studies demonstrated optimal trapping of [125I]IBDG at a 0.5 mM substrate concentration, radioligand doses were formulated at a similar IBDG concentration for the imaging studies. Visualization of either tumor was not possible throughout the 24 h imaging interval due to insufficient tumor uptake of radioactivity. Radioactivity clearance occurred mainly via the renal pathway as evidenced by high initial kidney uptake followed by excretion into bladder and the absence of radioactivity in liver. Negligible radioactivity levels were seen in most other organs including thyroid during the entire imaging sequence.
To confirm that the lack of tumor uptake of the radioligand was due to poor delivery to the tumor and not due to lack of β-gal enzyme recognition in vivo, SPECT imaging studies were repeated following direct intratumoral injection of [125I]IBDG to D54L and D54 tumors implanted in the same mouse on opposite flanks. Analysis of the SPECT image data revealed strikingly different kinetics of radioactivity clearance between the two tumor types. The fast clearance of radioactivity from the D54 tumor site relative to the β-gal expressing D54L tumor enabled selective SPECT visualization of the D54L tumor at >4 h post-injection. Radioactivity clearance which occurred predominantly via renal excretion as seen with the intravenous administration route resulted in high radioactivity levels in mouse bladder at early time intervals (2 h – 4 h), which, later declined to near background levels by 7 h post-injection. In addition, near background levels of radioactivity uptake were seen in most other organs including liver and thyroid, the latter indicative that [125I]IBDG is relatively stable to in vivo metabolic deiodination.
Important requirements for a successful in vivo imaging radioligand include a high uptake in target tissues in conjunction with a rapid clearance from non-target tissues. Since β-gal is localized within the cytoplasm, the radioligand must be sufficiently hydrophobic to diffuse through the cell membrane to reach its intended target. Once inside the cell, the radiolabeled product resulting from enzymatic action should also demonstrate low diffusibility to ensure its cellular retention. Our intratumoral injection imaging data confirms that [125I]IBDG (log P = 0.8) undergoes both facile cell permeation and selective intracellular trapping in D54L cells following enzymatic hydrolysis by β-gal. Furthermore, the rapid washout of radioactivity from the D54 control cell site indicates that unprocessed radioligand is being efficiently cleared out of the cell and from the circulation into the renal compartment. In this regard, our biological results underscore the advantage of using radiolabeled enzyme substrates that afford trapping of the product of a catalytic reaction over inhibitors as radioligands for imaging enzyme expression since continuous enzyme processing of such substrates affords high signal amplification at the site of enzymatic processing.
The imaging data from the intratumoral injection studies suggested to us that limited delivery of the radioligand to tumors on systemic injection, which is likely due to high renal clearance, plays a key role in the observed lack of tumor uptake. To further understand the poor tumor-targeting behavior of [125I]IBDG after systemic administration, blood metabolite analysis studies were conducted in tumor-bearing CD1 mice following intravenous injection of the radioligand. In these studies, the intact radioligand ([125I]IBDG) accounted for less than 40% of the total radioactivity present in mouse blood at 5 min post-injection. The remaining radioactivity comprised of two polar metabolites, which we confirmed were not [125I]iodide by radio-HPLC analysis. Importantly, the total blood radioactivity in a mouse at 5 min post-injection was only 13 – 15 µCi following a 600 µCi injection of [125I]IBDG. Since only renal and bladder radioactivity were apparent in the early SPECT images, this data further confirms that the majority of the systemically administered [125I]IBDG is being rapidly excreted in urine either as the intact radioligand or as a metabolite.
In summary, we synthesized and evaluated a radioiodinated β-gal substrate ([125I]IBDG) as a radioligand for in vivo SPECT imaging of tumor β-galactosidase enzyme expression. Although [125I]IBDG showed high differential uptake (6.5 – 7-fold) in β-galactosidase expressing tumor cells over control cells in in vitro studies it demonstrated insufficient uptake in β-gal expressing tumors for SPECT imaging upon systemic injection. However, SPECT imaging of CD1 mice following direct intratumoral injection of [125I]IBDG to β-gal expressing D54L tumors and control D54 tumors co-implanted in the same mouse demonstrated selective retention of radioactivity at the D54L tumor at 2 h through 7 h post-injection resulting in clear visualization of this tumor. Analysis of the imaging and blood metabolite profile data suggest that the poor tumor localization of [125I]IBDG is likely a result of high and rapid renal excretion. Thus, despite useful biological characteristics such as good cell permeability, substrate specificity and fast clearance from non-target tissues, our studies indicate that [125I]IBDG is unsuitable for the in vivo imaging of β-gal expression. We conclude that further structural modification of IBDG to retard its renal clearance and improve cell uptake or the evaluation of alternative β-gal substrates with improved pharmacokinetic properties is warranted to improve the β-gal expression imaging capability of this class of radioligands. These studies are currently underway in our laboratory.
ACKNOWLEDGMENTS
The authors acknowledge support for this work from the following National Institutes of Health Grants: PO1-CA85878, P50-CA093990 and R24-CA083099. We thank Dr. Mahaveer S. Bhojani for helpful discussions and technical assistance with the Figures.
Abbreviations
- β-gal
beta-galactosidase
- IBDG
5-Iodoindol-3-yl-β-D-galactopyranoside
- SPECT
single photon emission computed tomography
- PET
positron emission tomography
REFERENCES
- 1.Schenborn E, Groskreutz D. Reporter gene vectors and assays. Mol Biotechnol. 1999;13:29–44. doi: 10.1385/MB:13:1:29. [DOI] [PubMed] [Google Scholar]
- 2.Kamiya M, Kobayashi H, Hama Y, Koyama Y, Bernardo M, Nagano T, Choyke PL, Urano Y. An enzymatically activated fluorescence probe for targeted tumor imaging. J Am Chem Soc. 2007;129:3918–3929. doi: 10.1021/ja067710a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chilvers KF, Perry JD, James AL, Reed RH. Synthesis and evaluation of novel fluorogenic substrates for the detection of bacterial beta-galactosidase. J Appl Microbiol. 2001;91:1118–1130. doi: 10.1046/j.1365-2672.2001.01484.x. [DOI] [PubMed] [Google Scholar]
- 4.Pocsi I, Taylor SA, Richardson AC, Smith BV, Price RG. Comparison of several new chromogenic galactosides as substrates for various beta-D-galactosidases. Biochim Biophys Acta. 1993;1163:54–60. doi: 10.1016/0167-4838(93)90278-y. [DOI] [PubMed] [Google Scholar]
- 5.Blasberg R. PET imaging of gene expression. Eur J Cancer. 2002;38:2137–2146. doi: 10.1016/s0959-8049(02)00390-8. [DOI] [PubMed] [Google Scholar]
- 6.Haberkorn U, Mier W, Eisenhut M. Scintigraphic imaging of gene expression and gene transfer. Current medicinal chemistry. 2005;12:779–794. doi: 10.2174/0929867053507351. [DOI] [PubMed] [Google Scholar]
- 7.Herschman HR. PET reporter genes for noninvasive imaging of gene therapy, cell tracking and transgenic analysis. Critical reviews in oncology/hematology. 2004;51:191–204. doi: 10.1016/j.critrevonc.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 8.Ho NH, Weissleder R, Tung CH. A self-immolative reporter for beta-galactosidase sensing. Chembiochem. 2007;8:560–566. doi: 10.1002/cbic.200600386. [DOI] [PubMed] [Google Scholar]
- 9.Josserand V, Texier-Nogues I, Huber P, Favrot MC, Coll JL. Non-invasive in vivo optical imaging of the lacZ and luc gene expression in mice. Gene Ther. 2007;14:1587–1593. doi: 10.1038/sj.gt.3303028. [DOI] [PubMed] [Google Scholar]
- 10.Tung CH, Zeng Q, Shah K, Kim DE, Schellingerhout D, Weissleder R. In vivo imaging of beta-galactosidase activity using far red fluorescent switch. Cancer Res. 2004;64:1579–1583. doi: 10.1158/0008-5472.can-03-3226. [DOI] [PubMed] [Google Scholar]
- 11.Wehrman TS, von Degenfeld G, Krutzik PO, Nolan GP, Blau HM. Luminescent imaging of beta-galactosidase activity in living subjects using sequential reporter-enzyme luminescence. Nat Methods. 2006;3:295–301. doi: 10.1038/nmeth868. [DOI] [PubMed] [Google Scholar]
- 12.Louie AY, Huber MM, Ahrens ET, Rothbacher U, Moats R, Jacobs RE, Fraser SE, Meade TJ. In vivo visualization of gene expression using magnetic resonance imaging. Nat Biotechnol. 2000;18:321–325. doi: 10.1038/73780. [DOI] [PubMed] [Google Scholar]
- 13.Choi JH, Choe YS, Lee KH, Choi Y, Kim SE, Kim BT. Synthesis of radioiodine-labeled 2-phenylethyl 1-thio-beta-D-galactopyranoside for imaging of LacZ gene expression. Carbohydr Res. 2003;338:29–34. doi: 10.1016/s0008-6215(02)00359-2. [DOI] [PubMed] [Google Scholar]
- 14.Lee KH, Byun SS, Choi JH, Paik JY, Choe YS, Kim BT. Targeting of lacZ reporter gene expression with radioiodine-labelled phenylethyl-beta- d-thiogalactopyranoside. Eur J Nucl Med Mol Imaging. 2004;31:433–438. doi: 10.1007/s00259-003-1395-7. [DOI] [PubMed] [Google Scholar]
- 15.Kiernan JA. Indigogenic substrates for detection and localization of enzymes. Biotech Histochem. 2007;82:73–103. doi: 10.1080/10520290701375278. [DOI] [PubMed] [Google Scholar]
- 16.Horwitz JP, Chua J, Curby RJ, Tomson AJ, Darooge MA, Fisher BE, Mauricio J, Klundt I. Substrates for Cytochemical Demonstration of Enzyme Activity. I. Some Substituted 3-Indolyl-Beta-D-Glycopyranosides. J Med Chem. 1964;7:574–575. doi: 10.1021/jm00334a044. [DOI] [PubMed] [Google Scholar]
- 17.Van Dort ME, Jung YW, Gildersleeve DL, Hagen CA, Kuhl DE, Wieland DM. Synthesis of the 123I-and 125I-labeled cholinergic nerve marker (−)-5-iodobenzovesamicol. Nuclear medicine and biology. 1993;20:929–937. doi: 10.1016/0969-8051(93)90093-a. [DOI] [PubMed] [Google Scholar]
- 18.Rothwell JA, Day AJ, Morgan MR. Experimental determination of octanol-water partition coefficients of quercetin and related flavonoids. J Agric Food Chem. 2005;53:4355–4360. doi: 10.1021/jf0483669. [DOI] [PubMed] [Google Scholar]
- 19.Dess DK HP, Weinberg DV, Kaufman RJ, Sidhu RS. Phase-Transfer Catalyzed Synthesis of Acetylated Aryl b-D-Glucopyranosides and Aryl b-D-Galactopyranosides. Synthesis. 1981:883–885. [Google Scholar]
- 20.Kleine HPW DV, Kaufman RJ, Sidhu RS. Phase-transfer-catalyzed synthesis of 2,3,4,6-tetra-O-acetyl-b-D-galactopyranosides. Carbohydrate Research. 1985;142:333–337. [Google Scholar]
- 21.Hatch N, Sarid J. Glial fibrillary acidic protein transcriptional regulation is independent of a TFIID-binding downstream initiator sequence. Journal of neurochemistry. 1994;63:2003–2009. doi: 10.1046/j.1471-4159.1994.63062003.x. [DOI] [PubMed] [Google Scholar]
- 22.Cohen JC, Larson JE, Killeen E, Love D, Takemaru K. CFTR and Wnt/beta-catenin signaling in lung development. BMC developmental biology. 2008;8:70. doi: 10.1186/1471-213X-8-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ding Z, Maubach G, Masamune A, Zhuo L. Glial fibrillary acidic protein promoter targets pancreatic stellate cells. Dig Liver Dis. 2008 doi: 10.1016/j.dld.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 24.Jeong JH, Jin JS, Kim HN, Kang SM, Liu JC, Lengner CJ, Otto F, Mundlos S, Stein JL, van Wijnen AJ, Lian JB, Stein GS, Choi JY. Expression of Runx2 transcription factor in non-skeletal tissues, sperm and brain. Journal of cellular physiology. 2008 doi: 10.1002/jcp.21524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weber K, Sund H, Wallenfels K. [on the Nature of the Binding between Subunits in the Molecule of Beta-Galactosidase from E. Coli.] Biochemische Zeitschrift. 1964;339:498–500. [PubMed] [Google Scholar]