Abstract
We report on the analyses of four unrelated patients with de novo, overlapping, hemizygous deletions of the long arm of chromosome 10. These include two small terminal deletions (10q26.2 to 10qter), a larger terminal deletion (10q26.12 to 10qter), and an interstitial deletion (10q25.3q26.13). Single nucleotide polymorphism (SNP) studies (Illumina 550 K) established that these deletions resulted in the hemizygous loss of ∼6.1, ∼6.1, ∼12.5, and ∼7.0 Mb respectively. Additionally, these data establish that Patients 1, 2, and 3 share common, distal, hemizygous deleted regions of 6.09 Mb containing 37 RefSeq genes. Patients 3 and 4 share a 2.52 Mb deleted region corresponding to the proximal deleted region of Patient 3 and the distal deleted region of Patient 4. This common, hemizygous region contains 20 RefSeq genes including two H6 family homeobox genes (HMX2 and HMX3). Based on previous reports that Hmx2/Hmx3 knockout mice have vestibular anomalies, we propose that hemizygous deletions of HMX2 and HMX3 are responsible for the inner ear malformations observed from CT images, vestibular dysfunction, and congenital sensorineural hearing loss found in Patients 3 and 4.
Keywords: 10q deletion, hearing loss, vestibular dysfunction, HMX2, HMX3, SNP microarray
INTRODUCTION
Deletions of chromosome 10qter were first reported by Lewandowski et al. in 1978. Subsequently, Wulfsberg et al. [1989] reported three new patients and reviewed 15 previous patients and proposed a 10qter deletion syndrome. Cytogenetically, these patients had deletions of bands 10q25 or 10q26 to the terminus, as well as interstitial deletions and translocations within this region. Additionally, both familial and de novo patients have been reported [Irving et al., 2003]. Irving et al. [2003] reported 15 new patients involving interstitial and terminal deletions of chromosome bands 10q25.2−26.3, and reviewed 17 patients from the literature, supporting wide clinical variability for deletions in this region yet common features including strabismus, facial asymmetry, prominent chin, down-slanting palpebral fissures, hypertelorism, malformed or large ears, and a thin upper lip. Kehrer-Sawatzki et al. [2005] also described the phenotype of a patient with an interstitial del(10)(q25.2q25.3∼q26.11) and reviewed four previously described interstitial deletion patients involving bands 10q25.2-q26.1. Courtens et al. [2006] reported a subterminal deletion del(10)(q26.2) and reviewed the literature, describing the phenotype of subterminal deletions. Subterminal patients were noted to commonly have low birth weight, microcephaly at birth, short stature in childhood/adulthood, genital anomalies in males, and behavioral problems. Their craniofacial anomalies included broad/prominent nasal bridges, prominent nose, strabismus, thin upper lip, and fifth finger clinodactyly. Overall, there is variability in the sizes and positions of distal 10q deletions, presumably causing haploinsufficiency of many different genes in this large region and therefore contributing to significant variation in phenotype.
How to Cite this Article.
Miller ND, Nance MA, Wohler ES, Hoover-Fong JE, Lisi E, Thomas GH, Pevsner J. 2009. Molecular (SNP) analyses of overlapping hemizygous deletions of 10q25.3 to 10qter in four patients: Evidence for HMX2 and HMX3 as candidate genes in hearing and vestibular function. Am J Med Genet Part A 149A:669−680.
The majority of patients noted in the literature as having interstitial or terminal deletions of distal 10q have been characterized only with standard Giemsa staining (G-banding). In 20 of the approximately 60 patients in the literature, techniques more sensitive than G-banding have been employed [Lukusa and Fryns, 2000; McCandless et al., 2000; Ogata et al., 2000; Irving et al., 2003; Scigliano et al., 2004; Chen et al., 2005; Kehrer-Sawatzki et al., 2005; Courtens et al., 2006; Joshi et al., 2006]. These techniques include subtelomeric fluorescence in situ hybridization (FISH), FISH with BAC probes spanning the deletion region, genotyping with microsatellite markers, and array comparative genome hybridization. Although they refine the breakpoints, these techniques have limited accuracy and/or precision that is important for localizing the breakpoint in these individuals to the resolution needed for genotype–phenotype correlations.
With the resolution of prior cytogenetic techniques, many distal 10q deletion patients were described together without an understanding of how their unique deletions related to their different phenotypes. Now with higher resolution, we can compare and contrast presumably similar cytogenetic anomalies to narrow down to individual genes potentially responsible for specific phenotypes. We report on the analyses of four 10q deletion patients using single nucleotide polymorphism (SNP) microarrays. Three patients have terminal deletions and one has an interstitial deletion. All these probands were extensively phenotyped, and for two probands, we obtained SNP data from their unaffected parents. Such trio data permit additional analyses of genetic mechanisms and inheritance underlying the deletions. Among our findings, we identify a common deleted area shared by two of the four patients and propose candidate genes for vestibular and/or hearing dysfunction.
MATERIALS AND METHODS
Four patients and their parents (available for two of the patients) were studied following the guidelines of the Institutional Review Board at the Johns Hopkins School of Medicine.
Chromosome and FISH Analysis
Chromosome and FISH analyses were performed on thymidine synchronized peripheral blood lymphocyte cultures. At least 20 cells were analyzed from two independent cultures after standard G-banding at the 550 band level or higher. FISH was performed on interphase and metaphase spreads with a commercial probe for the subtelomeric region of 10q (Abott Molecular/Vysis, Des Plaines, IL). FISH was also performed with probes prepared from BAC DNA clones (BacPac, Oakland, CA) mapped to 10q. For FISH probes at least 20 metaphase cells were analyzed. Information regarding BAC clones position and band designation was obtained from NCBI and the Ensembl Genome Browser.
SNP Genotyping
Allelic composition and signal intensity of 550,000 human SNPs were performed with the HumanHap550 Genotyping Beadchip v.1.0 (Illumina, San Diego, CA) at the Johns Hopkins University Genetic Resources Core Facility SNP Center. Samples were processed using the Illumina Infinium II Assay protocol. SNPs were detected using a two-color single base extension reaction [Gunderson et al., 2006]. Approximately 1,125 ng of genomic DNA (15 μl at 75 ng/μl) was used as input for the Infinium II whole genome amplification reaction. After a 20- to 24-hr incubation at 37°C, amplified DNA was fragmented, precipitated, and resuspended according to the manufacturer's protocol except for the robotic platform used, which was the Perkin Elmer® Multiprobe® II HT PLUS robot. Denatured samples were hybridized on prepared HumanHap550 beadchips for 16−24 hr at 48°C. Following hybridization, a single-base extension reaction was performed followed by signal amplification utilizing Tecan instruments. Beadchips were then imaged on Illumina BeadArray Readers. After each batch of chips was processed, the intensity data for all samples were loaded into BeadStudio Genotyping Module v.2.3.41 and analyzed with the Illumina-provided standard definition cluster-file for the Human-Hap500 v.1.0 product. Data analysis was done by examination of signal intensity (log R ratio) and allelic composition (allelic frequency) with the BeadStudio 2.3.41 Loss of Heterozygosity Plus software module (Illumina).
SNP Analysis
With trios of the proband and his or her parents, SNPtrio analysis was done using the default settings on the website (http://pevsnerlab.kennedykrieger.org/SNPtrio.htm) [Ting et al., 2007]. Copy number variants were detected using SNPcnv and SNPlog R but were not verified with FISH. SNPcnv is a copy number analysis tool designed to detect deletions or duplications (including small deletions or duplications, involving ∼5 to 10 SNPs for Illumina 550 K SNP data). SNPcnv identifies blocks of consecutive homozygous SNP calls. Its output includes the number and identity of RefSeq genes in a deletion region (based on links to the UCSC Genome Browser) as well as the copy number (log R ratio). For consecutive homozygous calls or NoCalls, we set SNPcnv to only show blocks with a minimum log2(R ratio) delta of 0.4. We allowed three consecutive SNPs with low log2(R ratio) delta of 0.1 in a block. Results were limited to a minimum window size of seven SNPs, a setting that was optimal in minimizing false positive and false negative results. All chromosomes were analyzed. A synthetic data set was generated to simulate hemizygous deletions. With these settings and a minimum size of 100 kb, 1 out of 20 findings were false positives and 3% of deletions were not detected (false negatives). The SNPlog R algorithm indicates a deletion if three or more SNPs have log R values less than −0.7 and are 10 kb or less from each other. For insertions, a log R threshold of greater than 0.5 is used. Visual inspection is used to determine the copy number state for regions with SNPs spaced further apart, up to 50 kb from each other. Deletions and duplications were reported if any of the algorithms detected them. If they had a single copy number change to 1 or 3, the deletion or duplication minimum size was set at 100 kb to minimize false positives. Homozygous deletions were reported regardless as these are detected more easily.
RESULTS
Patient 1
Patient 1 is a female born at term by vaginal delivery after a pregnancy complicated by decreased fetal movement and maternal anemia. In infancy, there was temperature instability and poor growth. The physical exam was significant for frontal bossing, widow's peak, strabismus, stellate irides and bilateral fifth finger clinodactyly. Milestones were delayed with walking at 20 months and unsteady gait by 3 years. Speech was most significantly delayed with only four words at 3 years. Audiologic testing and brain MRI were normal (Table I).
TABLE I.
Clinical Findings
| Clinical feature | Patient 1 | Patient 2 | Patient 3 | Patient 4 |
|---|---|---|---|---|
| Karyotype | 4G,XXdel(10)(q26.2) | 4G,XXdel(10)(q26.2) | 4G,XYdel(10)(q26.12) | 4G,XY,del(10)(q25.2q26.11 OR q26.11q26.13) |
| Age at last visit | 5 years | 14 months | 4.5 years | 32 months |
| Weight | 10−25th%ile | 10th%ile | <3rd%ile | <3rd%ile |
| Height | 25−50th%ile | <3rd%ile | <3rd%ile | <3rd%ile |
| Head circumference | 50th%ile | 50th%ile | 25th%ile | <3rd%ile |
| Frontal bossing | + | − | − | + |
| Brachycephaly | − | − | + | − |
| Prominent forehead | + | − | + | + |
| Craniosynostosis | ND | ND | − | +, Metopic, sagittal |
| Widow's peak | + | − | − | − |
| Hypotelorism | − | − | + | − |
| Strabismus | + | +, BL | + | + |
| Stellate irides | +, BL | − | − | − |
| Broad nasal bridge | ND | − | + | + |
| Prominent nose | ND | − | + | − |
| Abnormal columella | ND | Recessed | Long | Crease inferior ridge |
| Flat philtrum | ND | − | − | + |
| High arched palate | − | − | − | + |
| Micrognathia | − | − | − | + |
| Hearing loss | − | − | +, BL SN | +, BL SN |
| Temporal bone anomaly | ND | ND | +, BL, Mondini | +, BL vestibule enlarged |
| Heart anomalies | ND | ND | Small VSD | − |
| Kidney anomalies | ND | ND | BL hydronephrosis | − |
| Genital anomalies | − | − | Unilateral undesc. testicle | Ambiguous genitalia |
| Single transverse palmar crease | − | − | ND | +, Unilateral |
| Tapering fingers | − | + | ND | − |
| 5th finger clinodactyly | + | − | + | − |
| Pes planus | ND | ND | + | ND |
| Overlapping toes | ND | ND | + | ND |
| Hypoplastic toe nails | ND | ND | + | ND |
| Feeding difficulties | − | ND | + | − |
| Hypotonia | − | +, Axial | +, Global | +, Global |
| Gait | Mild unsteadiness | NA | Wide-based | Wide-based |
| Age at walking | 20 months | Standing at 14 months | 4 years ? months | 31 months |
| Age at first words | 3 years | None at 14 months | No words at 3 years | One word at 15 months |
| Cognitive level | Mild MR | NA | Mod to severe MR | Mod MR |
| Mother's karyotype | NL | NL | NA | NL |
| Father's karyotype | NL | NL | NA | NL |
| Other normal tests | FXs, PAA, UOA, Williams FISH | UOA, PAA, T4, TSH | PAA, UOA, T4, TSH | FISH SRY+, NL subtelomeres, CK |
Clinical features of the four patients. BL, bilateral; ND, not described; NL, normal; NA, not assessed, MR, mental retardation; SN, sensorineural; VSD, ventriculo-septal defect; uni, unilateral; SRY, sex-determining region Y; T4, thyroxine-4; TSH, thyroid stimulating hormone; UOA, urine organic acids; PAA, plasma amino acids; FISH fluorescence in situ hybridization; CK creatine phosphokinase.
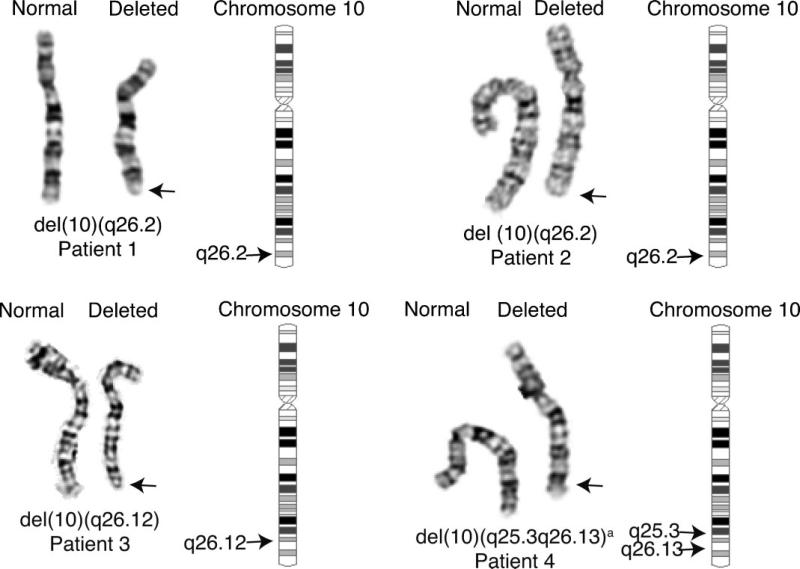
G-banded cytogenetic analysis of cultured lymphocytes revealed a hemizygous deletion of the terminal end of chromosome 10q, interpreted as 46,XX,del(10)(q26.2) (Fig. 1). Subtelomere FISH analysis confirmed a telomeric deletion (data not shown). Cytogenetic studies of the parents found the abnormality to be de novo in the proband.
FIG. 1.
Karyograms of the 10q deletions. Partial karyograms showing normal and deleted chromosomes of three patients (1, 2, and 3) with terminal deletions and Patient 4 with an overlapping interstitial deletion of the long arm of chromosome 10. aThe karyogram for Patient 4 indicated a possible karyotype of del(10)(q25.2q26.11 or q26.11q26.13); analysis with SNP microarrays refined the karyotype to del(10)(q25.3q26.13).
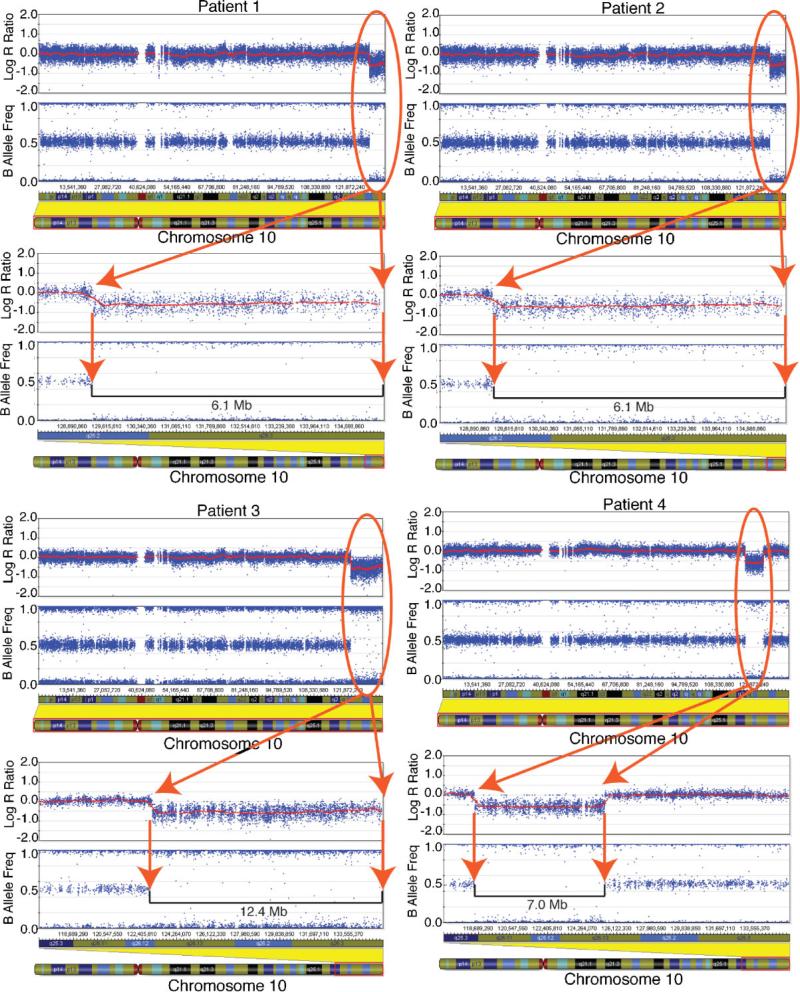
A 6.1 Mb deletion (Figs. 2 and 3, Table II) involving 37 RefSeq genes, with an additional RefSeq gene likely being hemizygous at the terminus (supporting information Table I may be found in the online version of this article), was detected with the Illumina HumanHap550 SNP microarray platform and a suite of analysis software used to determine copy number variations (CNVs) [see Materials and Methods Section and Ting et al., 2007]. (The gene near the terminus is distal to the most distal SNP on 10q. This gene could be present in two copies, but is likely to be deleted based on the cytogenetic findings consistent with a terminal deletion.) SNPtrio analysis of the data from the patient and her parents established a de novo 10q deletion of the proband occurred on the paternal chromosome (supporting information Fig. 1 may be found in the online version of this article). Deletions that overlapped with two previously reported CNVs (Database of Genomic Variants http://projects.tcag.ca/variation/ [Iafrate et al., 2004; Sebat et al., 2004; Sharp et al., 2005; Tuzun et al., 2005; Conrad et al., 2006; Hinds et al., 2006; McCarroll et al., 2006; Redon et al., 2006]) were also detected on chromosomes 14 and 22 in the proband (supporting information Table II may be found in the online version of this article), the former inherited from the mother and the latter de novo.
FIG. 2.
10q Deletions in BeadStudio. Analysis of genotype and copy number data for four 10q deletion patients based on SNP genotyping. Results are shown for Patients 1−4 using BeadStudio software (Illumina). For each patient, results are shown for log R ratio (a measure of chromosomal copy number; y-axis) versus genomic position on chromosome 10. A horizontal smoothing line indicates that the copy number for each is at euploid levels across most of the chromosome but is present in one copy in the deletion region (oval). The B allele frequency panel indicates the presence of BB genotype calls (B allele frequency of 1.0), AB calls (frequency of 0.5), and AA calls (frequency of 0.0); in the deletion region (oval) only homozygous calls are evident. An ideogram illustrates chromosome 10, and the lower panels for each patient provide a detailed view of each deletion region. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
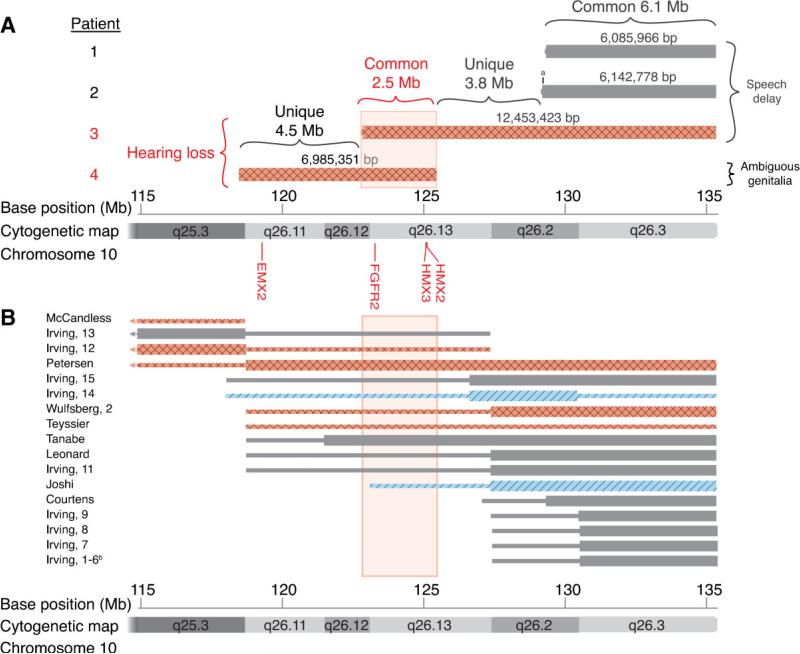
FIG. 3.
Overview of 10q deletion patients and hearing loss. Deletions of 10q for each patient are plotted. Thick bars indicate the minimum deletion. Thin bars indicate the maximum deletion. Diagonal lines (in two of the cases) indicate conductive hearing loss; diamond patterns (in six of the cases) indicate sensorineural hearing loss; and solid gray (in the remaining cases) indicates no hearing loss. A: Positions of the four deletions presented in this paper. B: Patients from the literature that have a pure 10q deletion and report on status of hearing loss. The row labels refer to the first author of the relevant publication and the patient number therein. aThe deletion in Patient 2 is 57 kb larger than in Patient 1. bIrving, Patients 1−6 are familial patients and thus depicted together [Teyssier et al., 1992; Petersen et al., 1998; Leonard et al., 1999; Tanabe et al., 1999]. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
TABLE II.
Breakpoints of the 10q Deletions Determined by SNP Microarray
| Normal SNPs |
Aberrant SNPs |
|||||||
|---|---|---|---|---|---|---|---|---|
| Patient | 10q Deletion | Proximal Location | Distal Location | Proximal Location | Distal Location | Min. size of deletion | Number of altered SNPs | RefSeq genes |
| 1 | q26.2 | 129,325,323 | Not presenta | 129,327,663 | 135,323,184 | 6,085,966 | 1,583 | 37 |
| 2 | q26.2 | 129,263,981 | Not presenta | 129,270,851 | 135,323,184 | 6,142,778 | 1,605 | 37 |
| 3 | q26.12 | 122,956,563 | Not presenta | 122,960,206 | 135,323,184 | 12,453,423 | 3,418 | 78 |
| 4 | q25.3q26.13 | 118,483,053 | 125,493,454 | 118,496,745 | 125,482,095 | 6,985,351 | 1,773 | 45 |
The physical locations are specified in base pairs and based on the May 2004 assembly of the human genome.
Distal normal SNPs were not present because the deletions were terminal (as determined by subtelomeric fish).
Patient 2
Patient 2, a female, was born at term after an uncomplicated pregnancy. She presented at 14 months with strabismus, mild epicanthal folds, a mildly recessed columella, tapered fingers and axial hypotonia (Table I). By 14 months of age, she was not walking independently, babbling, or speaking. There was no clinical record of hearing loss.
Cytogenetic analysis of G-banded blood lymphocyte cultures showed a terminal hemizygous deletion of chromosome 10 in all metaphases examined, determined to be 46,XX,del(10)(q26.2) (Fig. 1).
SNP analysis showed the hemizygous terminal deletion to be approximately 6.1 Mb in length (Figs. 2 and 3, Table II). This deletion was 57 kb larger than that of Patient 1, a difference undetectable by G-band karyotyping. This larger deletion possibly encompasses neuropeptide S (NPS) in addition to the 37 (likely 38) genes that were hemizygous in both Patients 1 and 2 (supporting information Table I may be found in the online version of this article). One deletion and one duplication were also detected elsewhere in the genome in Patient 2 (supporting information Table II may be found in the online version of this article). The former overlapped with a previously reported CNV. Parental samples were not available for study.
Patient 3
Patient 3 is a male who was small for gestational age (3rd centile) after a full term pregnancy. At age 8 months, he exhibited failure to thrive, with weight and height below the 5th percentile. Head circumference was at the 25th centile for age. Dysmorphic features included a triangular face, brachycephaly, a long broad forehead, strabismus, hypotelorism, a small nose with a prominent nasal bridge, and hypoplastic toenails. Later, low-set, abnormally “simple” shaped ears were appreciated (Table I). Other physical findings included a short sternum, contractures of the elbows, 5th finger clinodactyly, and hypotonia. Diagnostic testing revealed a small ventriculo-septal defect, bilateral hydronephrosis which resolved spontaneously, a right undescended testicle, umbilical and inguinal hernias, and a patent urachus. A brain MRI and ophthalmology exam were normal. He was diagnosed with congenital bilateral sensorineural hearing loss with aided pure tone average (PTA) levels of 50−60 dB and unaided levels of 75 dB at five months of age. Due to chronic middle ear effusions, he was treated with myringotomy and placement of middle ear ventilation tubes, first at 1 year of age and replaced multiple times during early childhood. He had moderate to severe developmental delay with no words at 3 years of age and walking with a wide-based gait at 4 years 7 months.
MRI and temporal bone computed tomography (CT) studies revealed inner ear malformations, classified as Mondini type, although they involved both the cochlear and vestibular organs. Specifically, fine cut CT of temporal bones revealed both cochlear and vestibular abnormalities bilaterally, being more severe on the left side (Fig. 4A). On the right, the internal auditory canal (IAC) was narrow, the cochlea having only 1−1.5 turns with a dilated and rudimentary shape, while the cochlear aqueduct was enlarged. The vestibule was massively enlarged and abnormally shaped. While all three semi-circular canals (SCCs) appeared abnormally dilated, the superior SCC was the largest, and the vestibular aqueduct was mildly prominent. On the left, the IAC was very small and narrow while the cochlea was devoid of distinct structure without normal partitioning. The vestibule, as well as the utricle and saccule, were replaced with a 1 cm cyst. The horizontal and posterior SCCs were absent while the superior SCC was abnormally thickened. The vestibular aqueduct could not be identified. Though independent ambulation has been present for greater than 1 year, the wide-based gait persists and is thought to be related to his vestibular anomalies.
FIG. 4.
CT imaging of inner ear abnormalities, Patients 3 and 4. CT images showing the inner ear abnormalities in Patients 3 and 4. A: Axial CT of Patient 3 at the level of the inner ear bilaterally. Right: Abnormally enlarged cystic vestibule (asterisk) and mildly prominent vestibular aqueduct (white arrow) are shown. Left: Abnormally large 1 cm cyst replacing the vestibule (asterisk), and small, narrowed IAC (black arrow) are shown. B: Axial CT of Patient 4 at the level of the right inner ear. The ratio of the transverse dimension of membranous vestibule (white arrow) to the inner diameter of the lateral SCC (black arrow) is increased. Bracket ({) indicates slightly dysplastic cochlea. C: Axial CT of Patient 4 at the level of the left inner ear. The ratio of the transverse dimension of membranous vestibule to the inner diameter of the lateral SCC is slightly increased (arrows same as in panel B). Bracket ({) indicates slightly dysplastic cochlea. Abbreviations: L, patient's left; R, patient's right.
Chromosome analysis of G-banded blood lymphocyte cultures from this patient revealed a large, hemizygous terminal deletion of the long arm of chromosome 10, that is, 46, XY,del(10)(q26.12) in all metaphases examined (Fig. 1). FISH analysis using BAC probe RP11−162A23 (124,795,486−124,978,916 bp) confirmed that the deletion included a region in common with Patient 4.
SNP analysis of DNA isolated from the patient's cultured lymphoblast cells confirmed that the above deletion resulted in the hemizygous loss of a minimum of 12.4 Mb (Figs. 2 and 3, Table II). This region encompassed 78 genes and likely a 79th gene at the terminus (supporting information Tables I, III, and IV may be found in the online version of this article). In addition to the major 10q deletion, one homozygous deletion on chromosome 11 and one duplication on chromosome 16, both previously reported CNVs, were found (supporting information Table II may be found in the online version of this article).
Patient 4
Patient 4 is a male, born at term after an uncomplicated pregnancy (Table I). At birth, he was diagnosed with ambiguous genitalia with bilateral cryptorchidism, a large phallus held down by chordee, a large labial-scrotal fold on the right, and a smaller fold on the left. Ultrasound examination showed the absence of Müllerian ducts. No uterus was seen by genitogram, but there was a large utriculovaginal pouch. At 7 months of age, hearing loss was documented (see below) and physical examination revealed failure to thrive with height, weight and head circumference below the 5th centile, prominent metopic suture, bitemporal narrowing, prominent eyes, bilateral epicanthal folds, down-slanting palpebral fissures, prominent ears, and mild micrognathia with a high arched palate. Other features included a long philtrum, thin upper lip, left single palmar crease and mild 5th finger clinodactyly. At 15 months, the patient underwent left abdominal orchiectomy, right abdominal orchiopexy, and male reconstructive surgery of the external genitalia [Mardo et al., 2008]. The patient returned to our facility at 32 months for hypospadias repair. At that time, his metopic suture was more prominent and a head CT demonstrated metopic and sagittal craniosynostosis; anterior and middle cranial fossa expansion surgery was performed. Height, weight and head circumference remained symmetrically below the 5th centile. New features noted were a depressed nasal tip and crease in the inferior ridge of the columella, flat philtrum, bilateral and symmetric skin dimples at the superolateral scapular border, and hypotonia. Parents described generalized unsteadiness as delayed motor milestones were attained, particularly while reaching for objects and walking (achieved at 31 months). He had no words at the 32-month visit.
Similar to Patient 3, Patient 4 also had congenital sensorineural hearing loss first documented at seven months of age by the absence of otoacoustic emissions. Auditory brainstem response indicated bilateral sensorineural hearing loss (mild/moderate low frequency on the right and mild to severe up-sloping on the left). He was prescribed bilateral hearing aids at 15 months of age. Head CT revealed metopic and sagittal craniosynostosis as well as slightly dysplastic cochlea bilaterally with an enlarged vestibular chamber on the right and a slightly enlarged vestibular chamber on the left (Fig. 4B,C). This was determined by measuring the ratio of the transverse dimension of the membranous vestibule to the inner diameter of the lateral SCC (see Davidson et al. [1999] for analysis method).
In contrast to the previous three patients with terminal deletions, chromosome analysis of this patient revealed a hemizygous interstitial deletion of the long arm of chromosome 10 that was interpreted as 46, XY,del(10)(q25.2q26.11 or q25.3q26.13) in all metaphases examined (Fig. 1). While the precise break points could not be determined by cytogenetic analysis, FISH using BAC probe RP11−162A23 (124,795,486−124,978,916 bp) confirmed that the deletion included band 10q26.13. While the distal breakpoint of this interstitial deletion did not overlap the terminal deletions of Patients 1 and 2, it did overlap the proximal breakpoint of the larger terminal deletion in Patient 3 (Figs. 2 and 3). Chromosome analyses of the parents were normal.
SNP analysis confirmed the patient's interstitial deletion and indicated a 7.0 Mb deletion with physical coordinates approximately 118−125 Mb, refining the karyotype to be 46,XY,del(10)-(q25.3q26.13). This region encompassed 45, possibly 46 genes depending on where the exact breakpoint is located (Fig. 2, Table II, and see supporting information Tables III and V which may be found in the online version of this article). Twenty-one of these genes were in common with the hemizygous deletion in Patient 3, the other patient with an otologic pathology (Supplemental Table III). As with Patient 1, analysis of the family SNP data established that while the deletion was de novo, it involved the paternal chromosome (supporting information Fig. 1 may be found in the online version of this article). Additionally, four duplications and/or deletions were detected, all of which were inherited (supporting information Table II may be found in the online version of this article). One of these is a small deletion in the intron and possibly an exon of the DMD gene. Deletions in exons of this gene can cause Duchenne Muscular Dystrophy (DMD); however, a creatine kinase test was performed on this patient to rule out DMD and was normal.
DISCUSSION
In this study we mapped the breakpoints of four 10q hemizygous deletion patients to within 2.3−25.1 kb by SNP analysis (Figs. 2 and 3, Table II). Through the use of a UCSC Genome Browser BED file (supporting information Table VI may be found in the online version of this article), we displayed the deletions in our patients and selected patients from the literature on a map of chromosome 10 (Fig. 3). This approach established that Patients 1, 2, and 3 have 10q terminal hemizygous deletions of 6.1, 6.1, and 12.4 Mb respectively, thus sharing in common the smallest (6.1 Mb) deletion found in Patient 1 (see Fig. 2 and Table II). In addition, Patients 2 and 3 have a common, small (57 kb) deleted region, not shared with Patient 1 (see the region at position 129 Mb on Fig. 3A, marked a). The proximal end of the deletion present in Patient 3 was also found to overlap the distal end of the interstitial deletion of Patient 4 resulting in a common deletion of 2.5 Mb in these two individuals (Fig. 3 and Table II).
These four patients had a number of common clinical findings. They all shared delayed speech development and had strabismus. While these features have been reported as being common in other patients with deletions in 10q (Table III), the resolution of this study shows that their genetic causes are not in common. Because the hemizygous deletions of Patients 1 and 2 do not overlap with the hemizygous deletion of Patient 4, these findings likely have distinct genetic and environmental causes. The patients also had a number of unique clinical findings. With two entirely separate deletions (Patients 1 and 2 vs. Patient 4), the possibility exists of further refining a broad 10q phenotype into more specific deletion phenotypes, such as a 10q26.3 phenotype. Unfortunately, the few clear differences between these two genetically distinct groups (Table I) do not allow us to further refine a 10q phenotype. Three of the four patients (2, 3, and 4) were <10%ile for weight and height for their age and had some degree of hypotonia. Of note, Patient 4 was the only one of the two males in this group that had ambiguous genitalia. It is possible that a gene important in genital development is present in the 4.5 Mb deleted region at the centromeric end of 10q that is unique to this patient.
TABLE III.
Literature Review of Vestibular–Related Findings in Selected Cohort of 10q Deletion Patients
| Patient information |
Deletion characteristics |
Symptomatology |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Refs. | Patient # | Sex | 10q deletion | cent-q26.12 | 26.12−26.13 | 26.13-ter | Hearing loss | Vestib. disfxn | Strab ismus | Speech/language |
| This study | 1 | F | 26.2ter | D | D | HZ | – | MU | + | SD |
| This study | 2 | F | 26.2ter | D | D | HZ | – | DW | + | SD |
| This study | 3 | M | 26.12ter | D | HZ | HZ | SNHL | DW, WBG | + | SD |
| This study | 4 | M | 25.3−26.13 | HZ | HZ | D | SNHL | DW, WBG | − | SD |
| McCandless et al. [2000] | 1 | M | 25.1−25.3 | HZ | D | D | SNHL | NT | − | NS |
| Irving et al. [2003] | 13 | F | 25.2−26.1 | HZ | U | D | – | − | SD | |
| Irving et al. [2003] | 12 | F | 25.2−26.1 | HZ | U | D | SNHL | PC | + | SD |
| Petersen et al. [1998] | 1 | F | 25.3-ter | HZ | HZ | HZ | SNHL | WBG | + | SD |
| Irving et al. [2003] | 15 | F | 26.1ter | U | HZ | HZ | – | NT | − | |
| Irving et al. [2003] | 14 | M | 26.13−26.3 | D | HZ | HZ | CHL | DW | + | SD |
| Wulfsberg et al. [1989] | 1 | F | 26.1 | D | U | HZ | SNHL | NT | + | |
| Teyssier et al. [1992] | 1 | M | 26 | U | HZ | HZ | SNHL | NT | − | |
| Tanabe et al. [1999] | 1 | M | 26.11 | D | HZ | HZ | – | NT | + | SD |
| Leonard et al. [1999] | 1 | M | 26.1-ter | D | U | HZ | – | WBG | + | SD |
| Irving et al. [2003] | 11 | F | 26.1ter | U | HZ | HZ | – | DW | − | SD |
| Joshi et al. [2006] | 1 | M | 26.13 | D | U | HZ | CHL | + | ||
| Courtens et al. [2006] | 1 | F | 26.2 | D | D | U | – | DW | + | SD |
| Irving et al. [2003] | 9 | F | 26.2ter | D | D | HZ | – | PB | + | Normal |
| Irving et al. [2003] | 8 | F | 26.2ter | D | D | HZ | – | DW | − | SD |
| Irving et al. [2003] | 7 | F | 26.2ter | D | D | HZ | – | − | Normal | |
| Irving et al. [2003] | 6 | F | 26.2ter | D | D | HZ | – | + | SD | |
| Irving et al. [2003] | 5 | F | 26.2ter | D | D | HZ | – | − | SD | |
| Irving et al. [2003] | 4 | F | 26.2ter | D | D | HZ | – | DW | − | SD |
| Irving et al. [2003] | 3 | F | 26.2ter | D | D | HZ | – | DW | + | Normal |
| Irving et al. [2003] | 2 | F | 26.2ter | D | D | HZ | – | DW | − | Normal |
| Irving et al. [2003] | 1 | F | 26.2ter | D | D | HZ | – | + | Normal | |
The patient information refers to the first author of the relevant publication and the patient number therein. F, female; M, male; HZ, heterozygous; U, uncertain; D, diploid; SNHL, sensorineural hearing loss; NT, not tested; MU, mildly unsteady; DW, delayed walking; PC, poor coordination; PB, poor balance; WBG, wide-based gait; SD, speech delay; AS, absent speech; CHL, conductive hearing loss; +, symptom present; -, symptom absent.
The common deleted region in Patients 3 and 4 is of particular interest as these two patients have congenital bilateral sensorineural hearing loss and abnormal balance. In Patient 3, the hearing loss is associated with bilateral inner ear malformations that were classified as Mondini malformations, though both the cochlear and vestibular organs were severely affected (Fig. 4A). For Patient 4, axial head CT images show evidence of abnormal vestibular enlargement (Fig. 4B,C), which often occurs in the setting of congenital hearing loss.
The wide-based gait and delayed development of milestones involved in balance, such as sitting, standing, and walking are clinically recognized features seen with vestibular loss or malfunction [Brown, 2005; Salem-Hartshorne and Jacob, 2005]. Thus, the absence or malformation of vestibular organs in the inner ear such as the SCCs have a profound impact upon the ability to stand up and walk comfortably. Additionally, we have identified four patients from the literature with wide-based gait, poor balance, or poor coordination out of 22 10q deletion patients that were examined for these physical features/findings (Table III). Additionally, seven of these patients were reported with delayed walking. We also note seven with hearing loss (Table III). These 22 patients are depicted in Figure 3 (see supporting information Table VI in the online version of this article for further information on displaying the data).
The hemizygous deleted 10q region shared by Patients 3 and 4 resulted in a single copy of 21 genes. These include two contiguous genes, H6 family homeobox 2 (HMX2) and H6 homeobox family 3 (HMX3), shown in null knockout mice to have key roles in cell fate determination and morphogenesis of vestibular components of the inner ear. These structures include the SCCs, utricle, saccule, and their sensory epithelia as well as the endolymphatic duct and sac. The inner ear, which mediates both hearing and balance, develops from a placode of ectoderm known as the otic placode. HMX2 and HMX3 are coexpressed in the dorsolateral otic epithelium to temporally and spatially signal the embryonic transition of the pars superior of the otocyst to a fully developed vestibular system [Wang et al., 2001]. They are also expressed in the developing central nervous system, including the neural tube and hypothalamus [Wang et al., 2001].
In view of our patient's difficulties with balance, it is of note that Wang et al. [2004] reported complete loss of balance in HMX2/3 null mice with progressive degeneration of the entire vestibular system among other phenotypes. Inner ear imaging revealed that HMX2/3 double knockout mice had altered vestibular development starting from disoriented otic vesicle orientation to absence of SCCs, utriculo-sacular fusion, degeneration of the vestibule, and lack of normal sensory epithelium. Cochlea were found be to be of normal shape with the normal three turns in these mice. Mice with heterozygous mutations in HMX2 or HMX3 were not found to have this vestibular phenotype and were described as normal.
We suggest that the phenotype of Patients 3 and 4 may represent a human correlate to the phenotypes described in HMX2/3 knockout mice. The large 1 cm cyst replacing the vestibule in our Patient 3 correlates with utriculo-sacular fusion seen in the knockout mice (Fig. 4A). Additionally, the enlarged abnormally shaped vestibule in Patient 3 parallels the degenerate vestibules seen in HMX2/3 knockout mice. The human phenotype of dilated, bilateral SCC abnormalities along with the absence of left horizontal and posterior SCCs is comparable to the phenotype of absent SCCs in HMX2/3 knockout mice. Patient 4 also has abnormally large vestibules bilaterally (Fig. 4B,C).
A third gene in the common hemizygous deleted region of Patients 3 and 4 is fibroblast growth factor receptor 2 (FGFR2). Fibroblast growth factors (FGF) and fibroblast growth factor receptors (FGFR) have a vital function in the osteogenesis of long and calvarial bones [Ornitz and Marie, 2002]. In humans, “gain of function” point mutations in FGFR2 are known to induce craniosynostosis (i.e., premature fusion of cranial sutures [Yang et al., 2008]). In mice, the FGFR2 knockout is lethal and the hemizygous model is apparently normal. Patient 4 had metopic and sagittal craniosynostosis which was corrected surgically. This is the first report of a hemizygous deletion of FGFR2 being present in a patient with craniosynostosis. Based on the established gain of function mechanism for point mutations in FGFR2 and lethality in the mouse knockout, the relationship between FGFR2 hemizygosity in humans and craniosynostosis is unclear. FGF are also known signals of induction in the otic placode [Ohyama et al., 2007]. FGFR2 mice knockouts also have various organ abnormalities including small otic vesicles (http://www.informatics.jax.org), a phenotype related to the vestibular defects in Patients 3 and 4.
The deletion in Patient 4, but not Patient 3, results in hemizygosityof the transcription factor empty spiracles homeobox 2 (EMX2). EMX2 has been implicated, along with orthodenticle homeobox 2 (OTX2) (chr14q), in anterior–posterior position signaling in the forebrain [Suda et al., 2001]. OTX2 helps to determine cell fate during otic invagination. In mutant mouse investigations, EMX2 has been reported as necessary for the development of structures in the middle ear and the development and organization of the sensory hair cells of the cochlea. Null mice with EMX2 mutations were found to have sensorineural hearing loss [Rhodes et al., 2003]. Additionally, EMX2 is necessary for mammalian urogenital tract development [Troy et al., 2003]. The hemizygous deletion of EMX2 in Patient 4, discussed further in Mardo et al. [2008], could account for the genito-urinary pathology in that patient.
In conclusion, detailed analyses of the breakpoints in four chromosome 10q deletions patients permit a description of genes that are hemizygous within the region. The 2.5 Mb common deletion region (10q26.12 to 10q26.13) in Patients 3 and 4 is associated with inner ear malformation, vestibular dysfunction, and hearing loss in two of our patients with congenital sensorineural hearing loss. In light of these findings, HMX2 and HMX3 are strong candidate genes for human inner ear vestibular development and have a role in sensorineural hearing.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. David Tunkel for sharing his clinical knowledge and CT scan images. We thank Dr. Ada Hamosh and N. Varg for helpful discussions. The authors wish to acknowledge the excellent services and expertise of Dr. Kim Doheny and her staff of the Johns Hopkins University SNP Center. We thank Alicia Rizzo for bioinformatics work with the candidate genes. This work was supported in part by HD024061 (J.P. and G.H.T.), the Stem Cell Resource Center at Johns Hopkins Institute of Cell Engineering (J.P.), and 1F31NS060388-01 (N.M.). We thank the patients and their parents for their participation in this study.
Grant sponsor: Stem Cell Resource Center at Johns Hopkins Institute of Cell Engineering; Grant numbers: HD024061, 1F31NS060388-01.
Footnotes
Additional supporting information may be found in the online version of this article.
REFERENCES
- Brown D. CHARGE syndrome “behaviors”: Challenges or adaptations? Am J Med Genet Part A. 2005;133A:268–272. doi: 10.1002/ajmg.a.30547. [DOI] [PubMed] [Google Scholar]
- Chen CP, Chern SR, Wang TH, Hsueh DW, Lee CC, Town DD, Wang W, Ko TM. Prenatal diagnosis and molecular cytogenetic analysis of partial monosomy 10q (10q25.3 → qter) and partial trisomy 18q (18q23 → qter) in a fetus associated with cystic hygroma and ambiguous genitalia. Prenat Diagn. 2005;25:492–496. doi: 10.1002/pd.1179. [DOI] [PubMed] [Google Scholar]
- Conrad D, Andrews T, Carter N, Hurles M, Pritchard J. A high-resolution survey of deletion polymorphism in the human genome. Nat Genet. 2006;38:75–81. doi: 10.1038/ng1697. [DOI] [PubMed] [Google Scholar]
- Courtens W, Wuyts W, Rooms L, Pera SB, Wauters J. A subterminal deletion of the long arm of chromosome 10: A clinical report and review. Am J Med Genet Part A. 2006;140A:402–409. doi: 10.1002/ajmg.a.31053. [DOI] [PubMed] [Google Scholar]
- Davidson HC, Harnsberger HR, Lemmerling MM, Mancuso AA, White DK, Tong KA, Dahlen RT, Shelton C. MR evaluation of vestibulocochlear anomalies associated with large endolymphatic duct and sac. Am J Neuroradiol. 1999;20:1435–1441. [PMC free article] [PubMed] [Google Scholar]
- Gunderson KL, Steemers FJ, Ren H, Ng P, Zhou L, Tsan C, Chang W, Bullis D, Musmacker J, King C, Lebruska LL, Barker D, Oliphant A, Kuhn KM, Shen R. Whole-genome genotyping. Methods Enzymol. 2006;410:359–376. doi: 10.1016/S0076-6879(06)10017-8. [DOI] [PubMed] [Google Scholar]
- Hinds D, Kloek A, Jen M, Chen X, Frazer K. Common deletions and SNPs are in linkage disequilibrium in the human genome. Nat Genet. 2006;38:82–85. doi: 10.1038/ng1695. [DOI] [PubMed] [Google Scholar]
- Iafrate A, Feuk L, Rivera M, Listewnik M, Donahoe P, Qi Y, Scherer S, Lee C. Detection of large-scale variation in the human genome. Nat Genet. 2004;36:949–951. doi: 10.1038/ng1416. [DOI] [PubMed] [Google Scholar]
- Irving M, Hanson H, Turnpenny P, Brewer C, Ogilvie CM, Davies A, Berg J. Deletion of the distal long arm of chromosome 10; is there a characteristic phenotype? A report of 15 de novo and familial cases. Am J Med Genet Part A. 2003;123A:153–163. doi: 10.1002/ajmg.a.20220. [DOI] [PubMed] [Google Scholar]
- Joshi C, Dawson AJ, Sanders SR, Prasad C. Congenital indifference to pain and deletion of chromosome 10q-: New association. J Child Neurol. 2006;21:174–177. doi: 10.1177/08830738060210022001. [DOI] [PubMed] [Google Scholar]
- Kehrer-Sawatzki H, Daumiller E, Müller-Navia J, Kendziorra H, Rossier E, du Bois G, Barbi G. Interstitial deletion del(10)(q25.2q25.3 approximately 26.11)—Case report and review of the literature. Prenat Diagn. 2005;25:954–959. doi: 10.1002/pd.1252. [DOI] [PubMed] [Google Scholar]
- Leonard NJ, Harley FL, Lin CC. Terminal deletion of chromosome 10q at band 26.1: Follow-up in an adolescent male with high-output renal failure from congenital obstructive uropathy. Am J Med Genet. 1999;86:115–117. [PubMed] [Google Scholar]
- Lukusa T, Fryns JP. Pure distal monosomy 10q26 in a patient displaying clinical features of Prader-Willi syndrome during infancy and distinct behavioural phenotype in adolescence. Genet Couns. 2000;11:119–126. [PubMed] [Google Scholar]
- Mardo V, Squibb EE, Braverman N, Hoover-Fong JE, Migeon C, Batista DAS, Thomas GH. Molecular cytogenetic analysis of a de novo interstitial deletion of chromosome 10q (q25.3q26.13) in a male child with ambiguous genitalia: Evidence for a new critical region for genital development. Am J Med Genet Part A. 2008;146A:2293–2297. doi: 10.1002/ajmg.a.32316. [DOI] [PubMed] [Google Scholar]
- McCandless SE, Schwartz S, Morrison S, Garlapati K, Robin NH. Adult with an interstitial deletion of chromosome 10 [del(10)(q25.1q25.3)]: Overlap with Coffin-Lowry syndrome. Am J Med Genet. 2000;95:93–98. doi: 10.1002/1096-8628(20001113)95:2<93::aid-ajmg1>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- McCarroll S, Hadnott T, Perry G, Sabeti P, Zody M, Barrett J, Dallaire S, Gabriel S, Lee C, Daly M, Altshuler DM, International HapMap Consortium Common deletion polymorphisms in the human genome. Nat Genet. 2006;38:86–92. doi: 10.1038/ng1696. [DOI] [PubMed] [Google Scholar]
- Ogata T, Muroya K, Sasagawa I, Kosho T, Wakui K, Sakazume S, Ito K, Matsuo N, Ohashi H, Nagai T. Genetic evidence for a novel gene(s) involved in urogenital development on 10q26. Kidney Int. 2000;58:2281–2290. doi: 10.1046/j.1523-1755.2000.00412.x. [DOI] [PubMed] [Google Scholar]
- Ohyama T, Groves AK, Martin K. The first steps towards hearing: Mechanisms of otic placode induction. Int J Dev Bio. 2007;51(6−7):463–472. doi: 10.1387/ijdb.072320to. [DOI] [PubMed] [Google Scholar]
- Ornitz DM, Marie PJ. FGF signaling pathways in endochondral and intramembranous bone development and human genetic disease. Genes Dev. 2002;16:1446–1465. doi: 10.1101/gad.990702. [DOI] [PubMed] [Google Scholar]
- Petersen B, Strassburg HM, Feichtinger W, Kress W, Schmid M. Terminal deletion of the long arm of chromosome 10: A new case with breakpoint in q25.3. Am J Med Genet. 1998;77:60–62. [PubMed] [Google Scholar]
- Redon R, Ishikawa S, Fitch K, Feuk L, Perry G, Andrews T, Fiegler H, Shapero M, Carson A, Chen W, Cho EK, Dallaire S, Freeman JL, González JR, Gratàcos M, Huang J, Kalaitzopoulos D, Komura D, MacDonald JR, Marshall CR, Mei R, Montgomery L, Nishimura K, Okamura K, Shen F, Somerville MJ, Tchinda J, Valsesia A, Woodwark C, Yang F, Zhang J, Zerjal T, Zhang J, Armengol L, Conrad DF, Estivill X, Tyler-Smith C, Carter NP, Aburatani H, Lee C, Jones KW, Scherer SW, Hurles ME. Global variation in copy number in the human genome. Nature. 2006;444(7118):444–454. doi: 10.1038/nature05329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes CR, Parkinson N, Tsai H, Brooker D, Mansell S, Spurr N, Hunter AJ, Steel KP, Brown SD. The homeobox gene Emx2 underlies middle ear and inner ear defects in the deaf mouse mutant pardon. J Neurocytol. 2003;32:1143–1154. doi: 10.1023/B:NEUR.0000021908.98337.91. [DOI] [PubMed] [Google Scholar]
- Salem-Hartshorne N, Jacob S. Adaptive behavior in children with CHARGE syndrome. Am J Med Genet Part A. 2005;133A:262–267. doi: 10.1002/ajmg.a.30546. [DOI] [PubMed] [Google Scholar]
- Scigliano S, Grégoire MJ, Schmitt M, Jonveaux PH, LeHeup B. Terminal deletion of the long arm of chromosome 10. Clin Genet. 2004;65:294–298. doi: 10.1111/j.1399-0004.2004.00218.x. [DOI] [PubMed] [Google Scholar]
- Sebat J, Lakshmi B, Troge J, Alexander J, Young J, Lundin P, Månér S, , Massa H, Walker M, Chi M, Navin N, Lucito R, Healy J, Hicks J, Ye K, Reiner A, Gilliam TC, Trask B, Patterson N, Zetterberg A, Wigler M. Large-scale copy number polymorphism in the human genome. Science. 2004;305(5683):525–528. doi: 10.1126/science.1098918. [DOI] [PubMed] [Google Scholar]
- Sharp A, Locke D, Mcgrath S, Cheng Z, Bailey J, Vallente R, Pertz L, Clark R, Schwartz S, Segraves R. Segmental duplications and copy-number variation in the human genome. Am J Hum Genet. 2005;77:78–88. doi: 10.1086/431652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suda Y, Hossain ZM, Kobayashi C, Hatano O, Yoshida M, Matsuo I, Aizawa S. Emx2 directs the development of diencephalon in cooperation with Otx2. Development. 2001;128:2433–2450. doi: 10.1242/dev.128.13.2433. [DOI] [PubMed] [Google Scholar]
- Tanabe S, Akiba T, Katoh M, Satoh T. Terminal deletion of chromosome 10q: Clinical features and literature review. Pediatr Int. 1999;41(5):565–567. doi: 10.1046/j.1442-200x.1999.01105.x. [DOI] [PubMed] [Google Scholar]
- Teyssier M, Charrin C, Dutruge J, Rousselle C. Monosomy 10qter: A new case. J Med Genet. 1992;29:342–343. doi: 10.1136/jmg.29.5.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting JC, Roberson ED, Miller ND, Lysholm-Bernacchi A, Stephan DA, Capone GT, Ruczinski I, Thomas GH, Pevsner J. Visualization of uniparental inheritance, Mendelian inconsistencies, deletions, and parent of origin effects in single nucleotide polymorphism trio data with SNPtrio. Hum Mutat. 2007;28:1225–1235. doi: 10.1002/humu.20583. [DOI] [PubMed] [Google Scholar]
- Troy PJ, Daftary GS, Bagot CN, Taylor HS. Transcriptional repression of peri-implantation EMX2 expression in mammalian reproduction by HOXA10. Mol Cell Bio. 2003;23:1–13. doi: 10.1128/MCB.23.1.1-13.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuzun E, Sharp A, Bailey J, Kaul R, Morrison V, Pertz L, Haugen E, Hayden H, Albertson D, Pinkel D, Olson MV, Eichler EE. Fine-scale structural variation of the human genome. Nat Genet. 2005;37:727–732. doi: 10.1038/ng1562. [DOI] [PubMed] [Google Scholar]
- Wang W, Chan EK, Baron S, Van de Water T, Lufkin T. Hmx2 homeobox gene control of murine vestibular morphogenesis. Development. 2001;128:5017–5029. doi: 10.1242/dev.128.24.5017. [DOI] [PubMed] [Google Scholar]
- Wang W, Grimmer JF, Van De Water TR, Lufkin T. Hmx2 and Hmx3 homeobox genes direct development of the murine inner ear and hypothalamus and can be functionally replaced by Drosophila Hmx. Dev Cell. 2004;7:439–453. doi: 10.1016/j.devcel.2004.06.016. [DOI] [PubMed] [Google Scholar]
- Wulfsberg EA, Weaver RP, Cunniff CM, Jones MC, Jones KL. Chromosome 10qter deletion syndrome: A review and report of three new cases. Am J Med Genet. 1989;32:364–367. doi: 10.1002/ajmg.1320320319. [DOI] [PubMed] [Google Scholar]
- Yang F, Wang Y, Zhang Z, Hsu B, Jabs EW, Elisseeff JH. The study of abnormal bone development in the Apert syndrome Fgfr2(+/S252W) mouse using a 3D hydrogel culture model. Bone. 2008;43:55–63. doi: 10.1016/j.bone.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.