Abstract
Heart valve structures, derived from mesenchyme precursor cells, are composed of differentiated cell types and extracellular matrix arranged to facilitate valve function. Scleraxis (scx) is a transcription factor required for tendon cell differentiation and matrix organization. This study identified high levels of scx expression in remodeling heart valve structures at embryonic day 15.5 through postnatal stages using scx-GFP reporter mice and determined the in vivo function using mice null for scx. Scx−/− mice display significantly thickened heart valve structures from embryonic day 17.5, and valves from mutant mice show alterations in valve precursor cell differentiation and matrix organization. This is indicated by decreased expression of the tendon-related collagen type XIV, increased expression of cartilage-associated genes including sox9, as well as persistent expression of mesenchyme cell markers including msx1 and snai1. In addition, ultrastructure analysis reveals disarray of extracellular matrix and collagen fiber organization within the valve leaflet. Thickened valve structures and increased expression of matrix remodeling genes characteristic of human heart valve disease are observed in juvenile scx−/− mice. In addition, excessive collagen deposition in annular structures within the atrioventricular junction is observed. Collectively, our studies have identified an in vivo requirement for scx during valvulogenesis and demonstrate its role in cell lineage differentiation and matrix distribution in remodeling valve structures.
Keywords: development, extracellular matrix, heart valves, mouse heart development, transcription factors
Congenital cardiovascular defects arising from abnormal formation of cardiac structures are the most common cause of infant mortality, and recent reports suggest that heart valve disease manifested in adults has origins in valve development.1–3 Heart valves are dynamic structures composed of diversified cell types and extracellular matrices (ECMs), organized to facilitate valve function.3 Valve formation initiates during embryogenesis with endothelial-to-mesenchymal transformation in the atrioventricular (AV) canal and outflow tract regions.4–6 Consequently, swellings of endocardial cushions (ECs), composed of highly proliferative mesenchyme cells within matrix-rich cardiac jelly, form the precursor pool for future heart valve structures.6,7 Once established, ECs undergo extensive remodeling involving differentiation of mesenchyme precursor cells and spatially restricted deposition of specialized ECM.3,8 In mature valve leaflets and supporting structures, the order and integrity of connective tissue within valvular compartments is essential for valve function. This is evident in diseased or malfunctioning valves that display aberrations in connective tissue organization and cell lineage distribution.9 Despite substantial clinical implications, the regulatory processes required for valve remodeling are largely unknown, yet essential for normal heart valve formation and function.
Recent studies have elucidated that regulatory hierarchies active during heart valve remodeling are parallel with other connective tissue systems.3 Developing heart valve structures are composed of multiple cell types, including those that express the basic helix–loop–helix transcription factor scleraxis (scx) and other tendon-associated structural proteins.10 Although not exclusive to tendinous tissues, scx is predominantly expressed by tendon progenitors and differentiated tendon cells.11,12 In vivo, scx is required during stages of tenocyte differentiation and is essential for organization of tendinous matrix and, consequently, tendon function.13 Avian somite studies have shown that fibroblast growth factors (FGFs) and intermediate extracellular signal-regulated kinase (ERK)1/2 signaling regulate scx expression.14,15 However, direct downstream targets of scx signaling are largely unknown, although studies show that altered scx function leads to changes in expression of tendinous-structural proteins, including tenascin, type I and XIV collagen, and tenomodulin.16–18 Even though the transcriptional regulation of these potential target genes by scx have not yet been defined, these studies highlight an important role for scx in regulating cell-specific expression of matrix proteins in connective tissue systems.
In the chick heart, scx is expressed in a subset of valve precursor cells following EC formation.10 Expression is maintained during valve remodeling with high levels observed in valve-supporting structures.10 The in vivo function of scx during valvulogenesis has not yet been reported, but insights into its regulation have been gained from previous in vitro studies in chick. In cultured valve precursor cells, scx expression is induced by FGF4 treatment and is associated with increased phospho-ERK1/2 activity, suggesting that heart valve development shares common regulatory pathways with other connective tissue systems.10 Interestingly, induced expression of scx by FGF-ERK1/2 signaling is associated with downregulation of sox9, a transcription factor also expressed in valve precursor cells and common to chondrogenic tissue.10,19–21 Because scx- and sox9-positive cells are derived from a common population of precursor cells of the ECs, these studies suggest that valve precursor cell lineage differentiation is tightly regulated to establish and maintain a balanced distribution of ECM proteins within valve compartments required for normal valve function.
In this study, we use scx−/− mice13 to determine its requirement during stages of valvulogenesis and examine effects of scx loss of function on adult heart valve structure and function in vivo. Scx−/− mice display thickened heart valve structures during embryonic valve remodeling stages in association with defects in valve precursor cell lineage differentiation and ECM organization. Increased ECM production, characteristic of pathological fibrosis, is observed in valve and annular structures of scx−/− mice from postnatal stages. Heart function in juvenile scx−/− mice is subtly, but not significantly, impaired compared to wild-type littermates; however, the reduced viability of mutant mice to reach adult stages hindered our examination of valve degeneration. Collectively, data from this study reveal an important role for scx in normal embryonic heart valve remodeling in vivo.
Materials and Methods
Scx+/− mice back-crossed with scx-GFP reporter mice were genotyped as previously described13,22 and crossbred to generate scx−/−, scx+/− , and scx+/+ control mice. Mendelian ratios were recorded at embryonic times points, as well as shortly after birth. In addition, body weights were reported in viable animals from postnatal stages. Hearts were collected at embryonic day E12.5, E14.5, E16.5, and E17.5, postnatal, and juvenile stages from scx−/− and scx+/+ littermate mice. Whole hearts were fixed for in situ hybridization, immunohisto- and cytochemistry, and electron microscopy, as previously described.9,19,23 Alternatively AV canal regions were collected from E17.5 scx−/− and scx+/+ mice for RNA and protein isolation for subsequent TaqMan low-density array (TLDA) or Western blot analyses, respectively. All animal procedures were approved and performed in accordance with institutional guidelines.
An expanded Materials and Methods section is available in the online data supplement at http://circres.ahajournals.org.
Results
Scleraxis-GFP Is Highly Expressed From Remodeling Stages of Heart Valve Development
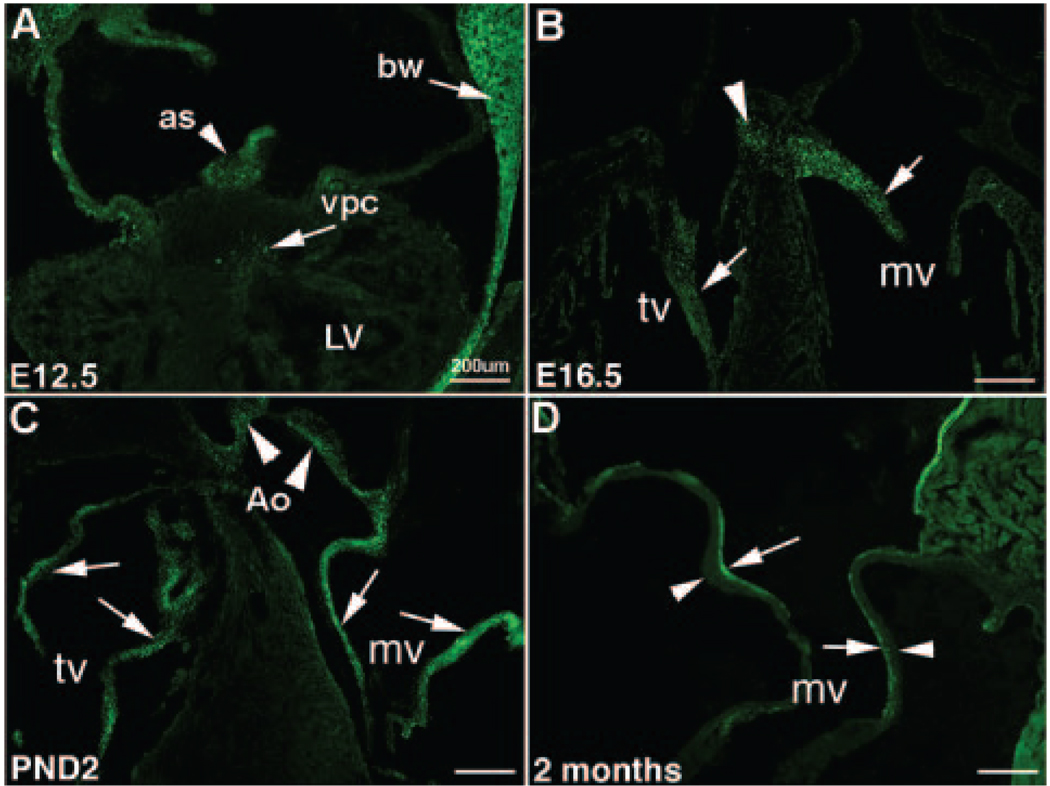
Scx-GFP transgene expression recapitulates endogenous expression,22 and therefore reporter mice were used to indicate scx expression during stages of valve development. At E12.5, scx-GFP is detected in few valve precursor cells within the ECs (arrow, Figure 1A), as well as in the body wall and atrial septum (arrow and arrowhead respectively, Figure 1A). By E16.5, scx-GFP expression is prominent in remodeling tricuspid and mitral valve leaflets (arrows, Figure 1B), as well as in the fibrous continuity (arrowhead, Figure 1B). In postnatal hearts, scx-GFP is expressed throughout the maturing AV (arrows, Figure 1C) and aortic (arrowheads, Figure 1C) valve structures. The widespread expression pattern observed in the mouse is somewhat different from the more restricted expression pattern observed in supporting structures of chick valves.10 This maybe attributed to differences in the complexity of the valve at the cellular level across species.9 At juvenile stages, scx-GFP expression is restricted to the atrial surface of the AV valve leaflets (arrows, Figure 1D), with undetectable expression observed along the ventricular region (arrowhead, Figure 1D). Further in situ hybridization analysis confirms the specificity of the scx-GFP transgene in the AV valves (Figure IA, IC, IE, IG in the online data supplement), and extended analysis includes detection of scx in aortic valves. Scx is detected at very low levels in aortic ECs at E12.5 (supplemental Figure IB), in contrast to high levels observed during remodeling stages from E16.5 to juvenile (supplemental Figure ID, IF, and IH). Collectively, these expression studies demonstrate that scx is most predominantly expressed during remodeling and maturation stages of embryonic valvulogenesis.
Figure 1. Scx-GFP is highly expressed during stages of heart valve remodeling.
A, Scx-GFP expression is detected in a small number of valve precursor cells (vpc) within the endocardial cushion at E12.5 (arrow). Expression is also observed in the body wall (bw) and atrial septum (as) (arrows). B, Scx-GFP is highly expressed in remodeling heart valve structures by E16.5 (arrows), with continued levels of expression observed in mitral (mv), tricuspid (tv) (arrows), and aortic (Ao) (arrowheads) valves of postnatal animals (C). D, Diminished expression levels are apparent in juvenile mice, with noted expression observed along the atrial (arrow) surface, compared to the ventricular (arrowhead) surface of the mature valve leaflet. Scale bars=200 µm.
Scx−/− Mice Are Not Observed at Expected Mendelian Ratios Shortly After Birth and Display Thickened Heart Valve Structures by E17.5
Scx−/− pups were not observed at expected Mendelian ratios shortly after birth; however, ratios during embryogenesis appear normal (Table). Notably, 16% (compared to the expected 25%) of scx−/− mice were observed soon after birth, and only 47% of viable null mice survived to 2 months of age despite efforts to maximize longevity (see expanded Materials and Methods section in the online data supplement). The cause of early lethality is not known and warrants further investigation. In our hands, scx−/− mice recapitulate previously described phenotypes including limited use of all paws and reduced functionality of back muscles (data not shown).13 We also report a significant reduction in body weight of scx−/− mice at 6 weeks (53%) and 2 months of age (31%) compared to scx+/+ control littermates (Figure 2B).
Table.
Mendelian Ratios of scX−/− Mice
| Scx+/+(%) | Scx+/−(%) | Scx−/−(%) | n | |
|---|---|---|---|---|
| E11.5 | 23% | 54% | 23% | 54 |
| E15.5 | 23% | 54% | 23% | 61 |
| E17.5 | 20% | 53% | 27% | 58 |
| Birth | 32% | 52% | 16% | 195 |
Expected Mendelian ratios are observed during embryogenesis; however, expected ratios are not seen shortly after birth.
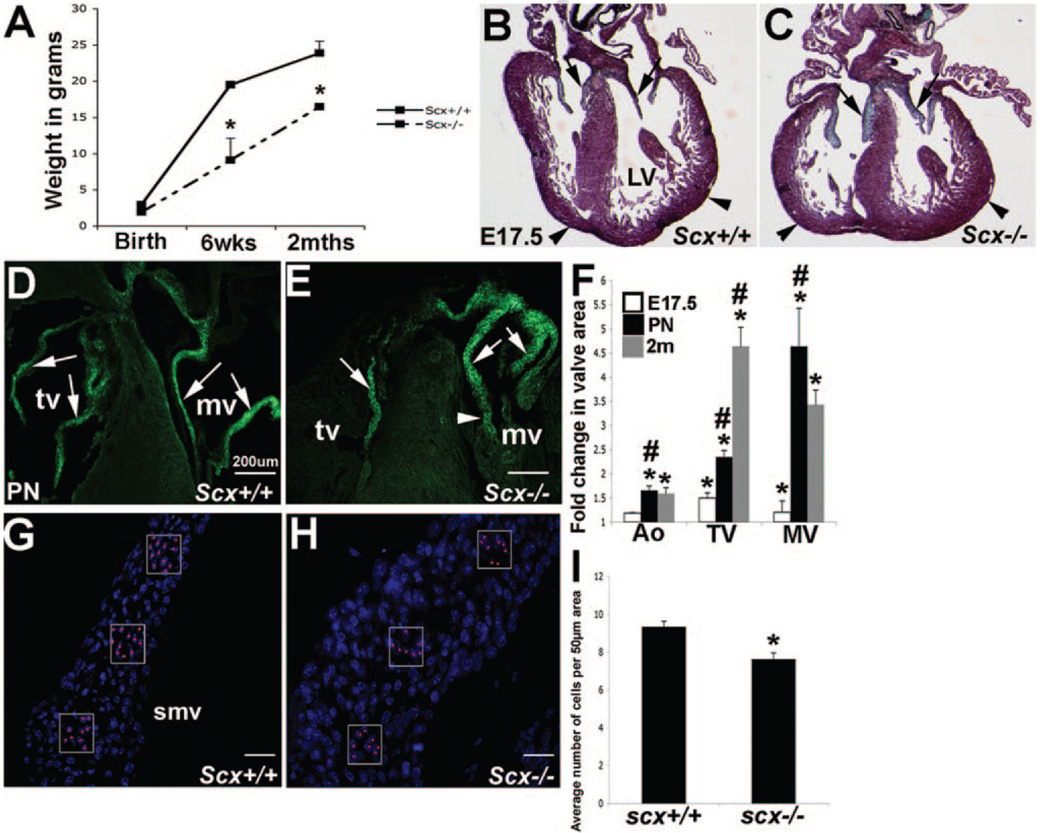
Figure 2. Valve structures from scX−/− mice have increased area from E17.5.
A, At postnatal stages, scX−/− mice display smaller body weights. Pentachrome staining at E17.5 (C and D) and GFP expression at postnatal stages (D and E) show thickened heart valve structures in scX−/− (B and D) animals compared to scX+/+ (C and E). Arrows in D and E show valve leaflet structures, and arrowheads indicate the “globular-shaped” myocardium in B and C and chordae tendineae in D and E. F, Quantitative analysis to show increased aortic (Ao), tricuspid (tv), and mitral (mv) valve area in scX−/− mice. G and H, Cell number counts (shown by red dots), based on nuclear DAPI staining, show decreased cell number per 50 µm2 area in septal mitral valve (smv) leaflets from scx−/− mice at E17.5 compared to controls (I). LV indicates left ventricle.
To determine the effects of scx loss of function on valve formation, we examined gross morphology of developing valve structures during embryogenesis in scx+/+ and scx−/− mice. ECs at E12.5 and valve primordia at E16.5 from scx−/− mice are indistinct from scx+/+ mice (data not shown). However, pentachrome staining at E17.5 indicates that scx−/− mice (Figure 2C, AV valve shown) display significantly thickened valve structures compared to scx+/+ control mice (arrows, Figure 2B). In addition the overall shape of the heart appears “globular” in scx−/− mice (arrowheads, Figure 2C compared to 2B). Comparisons of tissue sections from scx−/− and scx+/+ mice back-crossed with scx-GFP mice demon- strate an increase in valve area likely indicative of thickness in scx−/− mice at postnatal stages (arrows, Figure 2D and 2E). Two-dimensional morphometric analysis from tissue sections collected from scx−/− and scx+/+ mice quantitatively illustrate a significant increase in mitral, tricuspid, and aortic valve area in null animals compared to controls that significantly worsens over time (Figure 2F). Because increased valve area was first observed at E17.5 in scx−/− mice, subsequent molecular analyses was performed at this time point to determine primary effects of scx loss of function on valvulogenesis.
To propose a possible explanation for the increase in valve area, changes in cell proliferation and apoptosis were determined in scx+/+ and scx−/− mice at E17.5; however, no significant differences were observed (data not shown). To further support the lack of observed changes in cell number, the average number of cells within a 50 µm2 area of the septal mitral valve leaflet of E17.5 scx+/+ (Figure 2G and 2I) and scx−/− (Figure 2J and 2I) mice were counted from tissue sections. Interestingly, the average number of cells per area, as depicted by DAPI (4′,6-diamidino-2-phenylindole) nuclear stain, was significantly decreased in scx−/− mice (boxed areas, Figure 2G and 2H). This further suggests that an increase in cell number did not contribute to the increased valve area phenotype but more likely an increase in matrix deposition. These findings show that from stages of valve remodeling, heart valve area in scx−/− mice is increased despite no change in cell number.
Type XIV Collagen Expression Is Downregulated and Collagen Fiber Organization Is Disrupted in Heart Valves From scx−/− Mice at E17.5
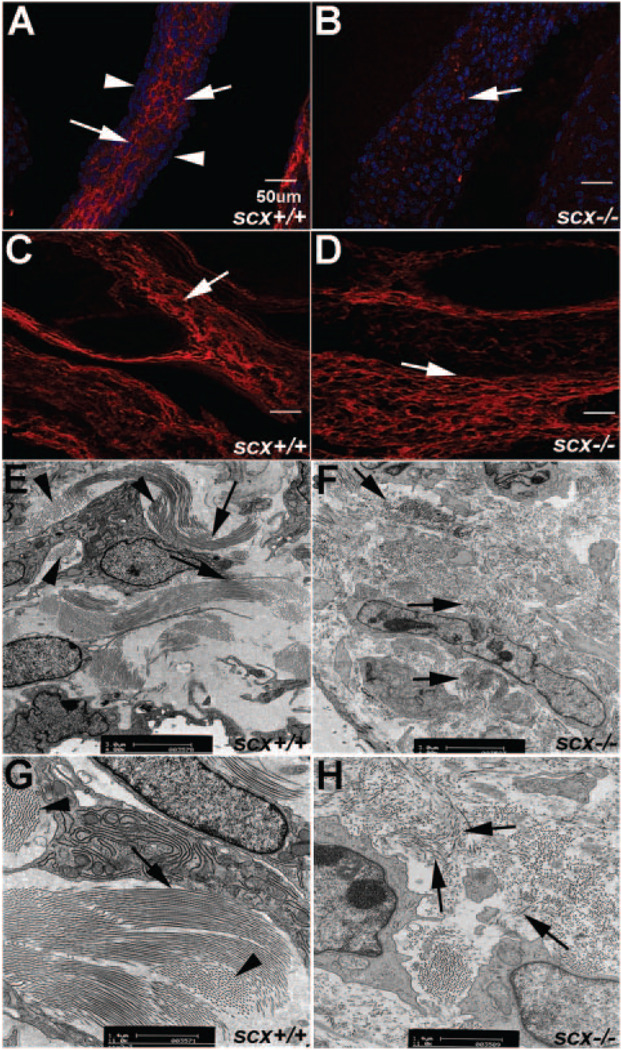
Type XIV collagen is highly expressed in tendon tissue24 and previous studies have shown that type XIV collagen expression is lost in tendon forelimbs in scx−/− mice.13 Type XIV collagen expression has not yet been shown in heart valve structures. Therefore, to determine whether it is present in valvular structures, and to examine similar downregulation in heart valves of scx−/− mice, we performed immunohistochemistry. In scx+/+ mice, type XIV collagen is highly expressed throughout the valve leaflets (arrows, Figure 3A), with notably less expression detected on the leaflet surface (arrowheads, Figure 3A). In contrast, heart valves from scx−/− mice show significantly decreased levels of type XIV collagen expression in both AV (arrows, Figure 3B) and aortic valve regions (data not shown). Downregulation of type XIV collagen expression is restricted to valvular structures, because regions that do not express scx, including the great vessels, continue to express high levels of type XIV collagen (arrows, Figure 3C and 3D). Interestingly, other tendon-associated collagens, including type XI collagen, did not change (data not shown). Ultrastructural analyses using transmission electron microscopy of postnatal aortic valve leaflets reveals highly organized bundles of parallel collagen fibers running longitudinal (arrows, Figure 3E and 3G) and perpendicular (arrowheads, Figure 3E and 3G) within the valve leaflet of scx+/+ mice. In contrast, collagen fiber organization is in disarray in scx−/− mice (Figure 3F and 3H). Organized bundles of parallel collagen fibers are not apparent in mutant animals and fibers appear fragmented and randomly oriented (arrows, Figure 3F and 3H). Collectively, these studies show that heart valves from scx−/− mice have reduced expression of the tendon-associated collagen type XIV and disorganized collagen fiber alignment.
Figure 3. Expression of type XIV collagen is reduced and ECM organization is abrogated in valves from scX−/− mice.
A and B, Immunohistochemistry staining to show decreased type XIV collagen expression in heart valves from scX−/− mice (B) compared to scX+/+ mice (A) at E17.5. Arrows in A indicate type XIV collagen expression within the leaflet, whereas arrowheads show diminished expression on the leaflet surface. C and D, Type XIV collagen expression levels are comparable in vessels from scX+/+ and scX−/− mice. E through H, Transmission electron microscopy to determine collagen fiber organization in aortic valves from postnatal scX−/− mice, compared to scX+/+ mice. E and G, Long parallel collagen fiber bundles (arrows) and fibers running perpendicular (arrowheads) are observed in scX+/+ mice (arrows). F and H, Fragmented and disorganized collagen fibers are prevalent in scX−/− mice (arrows).
Mesenchyme Cell Markers Are Persistently Expressed in Heart Valves From scx−/− Mice at E17.5
Undifferentiated valve precursor cells within the ECs express cell markers associated with mesenchyme cells.3,25–27 As development progresses, valve precursor cells differentiate and expression of mesenchyme cell markers are downregulated.26,27 To determine differentiation of valve precursor cells in scx−/− mice, mesenchyme marker expression was examined in AV canal regions from scx−/− and scx+/+ mice at E17.5 using quantitative TLDA analysis. Findings reveal that genes characteristic of mesenchyme cells are significantly increased in scx−/− mice compared to scx+/+ littermate controls, including Id-1 (2.6-fold), msx1 (5.6-fold), snai1 (3.8-fold), snai2 (5.7-fold), and tbx20 (3.7-fold) (Figure 4A). In support, immunohistochemistry was performed to confirm increased msx1 (Figure 4B and 4C) and snai1 (Figure 4D and 4E) expression in scx−/− mice spatially. In scx+/+ control mice at E17.5, msx1 expression is low and restricted to the distal region of the valve leaflets (arrowheads, Figure 4B). However, msx1 expression is expressed throughout the valve leaflets (arrows, Figure 4C) in scx−/− mice. By E17.5, snai1 expression is observed in cells associated with the valve endothelium of scx+/+ mice (arrows, Figure 4D). In contrast, expression is highly detectable in endothelial (arrows, Figure 4E) and interstitial cells (arrowheads, Figure 4E) within valve leaflets from scx−/− mice. In summary, mesenchyme cell markers are persistently expressed in scx−/− mice during stages of heart valve remodeling.
Figure 4. Mesenchymal cell markers are persistently expressed in valves from scX−/− mice at E17.5.
A, TLDA analysis shows significantly increased fold changes in expression of Id-1, msX1, snai1, snai2, and tbX20 in scX−/− mice compared to scX+/+ mice at E17.5. B and C, Immunohis tochemistry to determine spatial localization of msx1 reveals expression in the distal regions of mitral valves (mv) from scX+/+ mice (arrowheads), in contrast to ectopic expression observed throughout the valve leaflet in scX−/− mice (arrows). D, Snai1 is expressed at low levels in scX+/+ mice at E17.5. Arrows indicate detectable levels in endothelial cells. E, Snai1 expression is observed in the majority of cells throughout the valve leaflet of scX−/− mice (arrows and arrowheads). smv indicates septal mitral valve.
Cartilage-Associated Gene Markers Are Increased in scx−/− Mice at E17.5
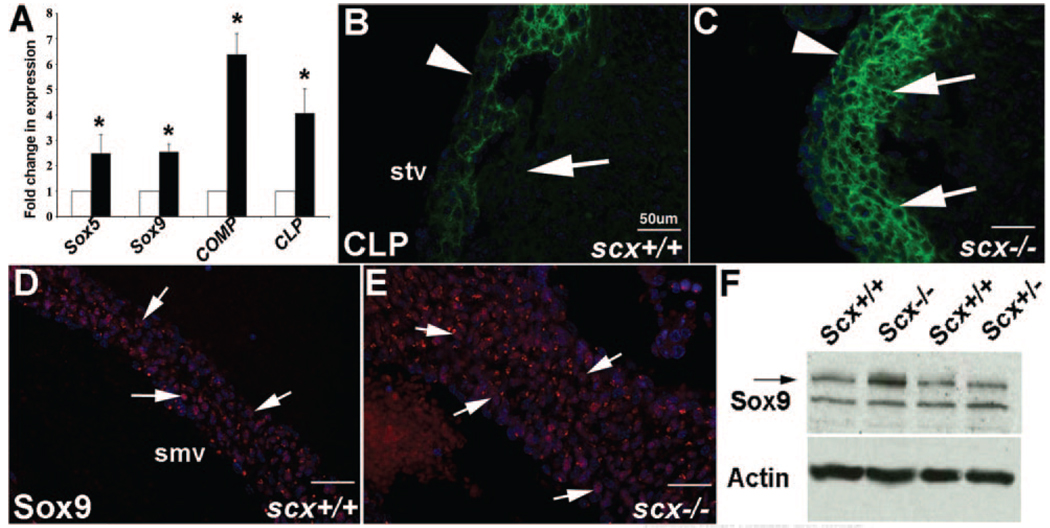
Cartilage- and tendon-like cell types that contribute to mature valve structures are derived from a common population of valve precursor cells within ECs.7,10 To examine effects of scx loss of function on valve precursor cell differentiation into cartilaginous cell types, we used TLDA analyses to quantitatively measure changes in gene expression of cartilage-associated genes previously observed in heart valves at E17.5.19 At E17.5, heart valves from scx−/− mice show increased expression of sox5 (2.5-fold), sox9 (2.5-fold), cartilage oligo matrix protein (COMP) (6.4-fold), and cartilage link protein (CLP) (4.0-fold) compared to scx+/+ mice (Figure 5A). Increased expression of CLP and sox9 in heart valves from scx−/− mice is also seen by immunohistochemistry. In scx+/+ mice at E17.5, CLP is more highly expressed toward the ventricular region of the valve leaflets (arrows, Figure 5B), with lower levels of expression observed along the atrial surface (arrowhead, Figure 5B), whereas in scx−/− mice, CLP is expressed at high levels throughout the valve leaflet (arrows, arrowhead, Figure 5C). Similarly, sox9 expression is also notably higher in valve leaflets from scx−/− mice (arrows, Figure 5E) at E17.5 compared to scx+/+ mice (arrows, Figure 5E). Increased sox9 expression in heart valves from scx−/− mice is also detected by Western blot (Figure 5F). In summary, heart valves from scx−/− mice express higher levels of cartilage-associated genes at E17.5 compared to control mice.
Figure 5. Cartilage-associated markers are increased in valves from scX−/− mice at E17.5.
A, TLDA analysis to show increased fold changes in expression of soX5, soX9, cartilage oligo matrix protein (COMP), and CLP in scX−/− mice compared to scX+/+ mice at E17.5. B, Immunohistochemistry shows the spatial localization of CLP on the ventricular side (arrow), as opposed to atrial regions (arrowhead) of the septal tricuspid valve (stv) in scX+/+ mice at E17.5. C, Scx−/− mice show increased and ectopic CLP expression throughout the valve leaflets (arrows and arrowhead). D, Sox9 is expressed in valves from scX+/+ mice at E17.5 (arrows), although expression is increased in scX−/− mice shown by immunohistochemistry (E) and Western blotting (F). Actin was used as a loading control in F. smv indicates septal mitral valve.
Heart Valves and Annular Structures From Juvenile scx−/− Mice Display Characteristics of Pathological Fibrosis
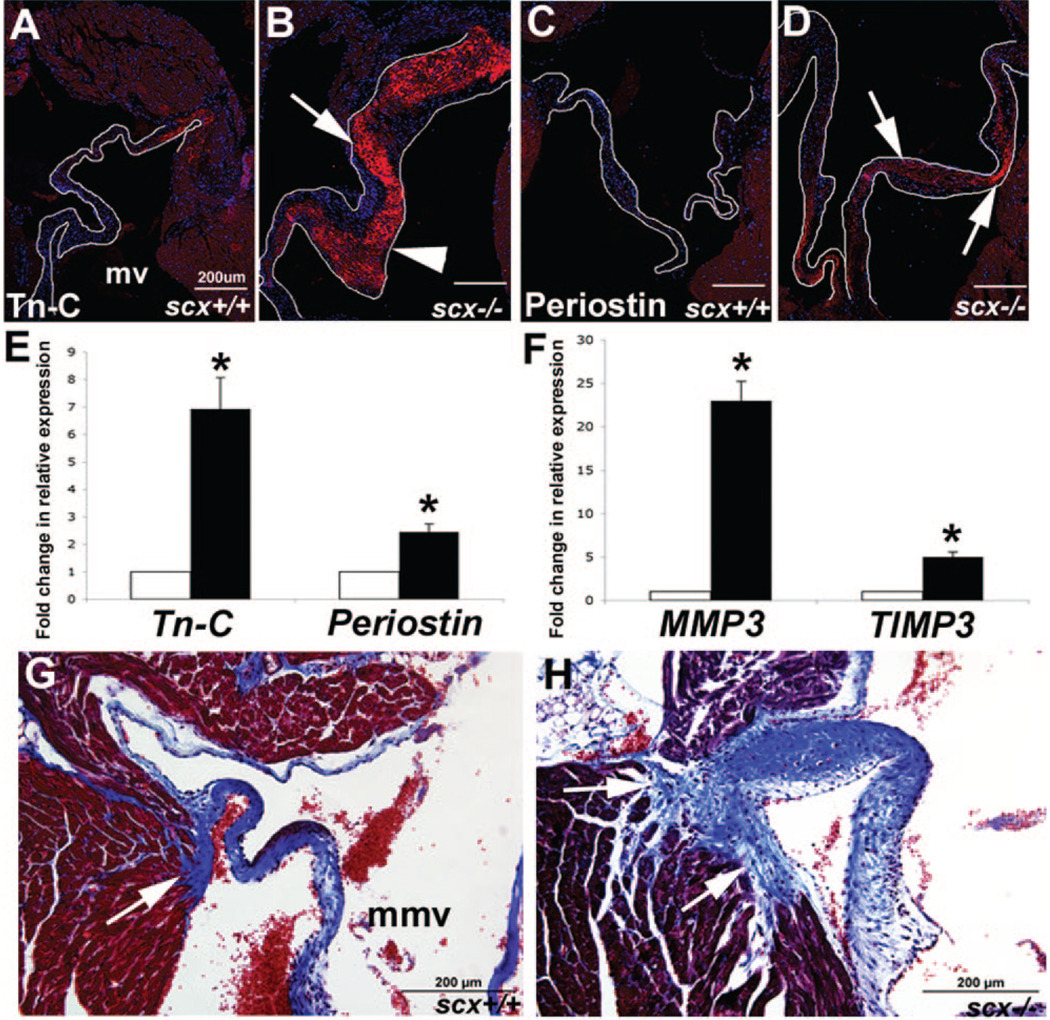
Immunohistochemistry and TLDA analyses were used to determine changes in expression of markers associated with pathological matrix remodeling in scx−/− mice at 2 months of age.28–30 Heart valves from juvenile scx+/+ control mice show low levels of tenascin-C expression (Figure 6A), whereas expression is detected at high levels in thickened valves from scx−/− mice, especially in ventricular regions (arrowhead, compared to arrow, Figure 6B). Likewise, periostin is expressed at low levels in juvenile heart valves from scx+/+ mice (Figure 6C), in contrast to high levels observed in scx−/− mice (arrows, Figure 6D). These findings are supported by increased tenascin-C and periostin expression in scx−/− mice observed at the transcript level (Figure 6E). In addition, increased expression of matrix remodeling enzymes mmp3 and timp3 (Figure 6F) are also detected. Masson’s trichrome staining was used to examine collagen deposition as a measure of fibrosis in scx−/− and scx+/+ juvenile mice. Increased staining was observed in the annular regions of AV and aortic valves in scx−/− mice (mitral valve, arrow, Figure 6H). In summary, loss of scx function is associated with increased deposition of remodeling-associated ECM in valve and annular regions.
Figure 6. Increased ECM deposition and remodeling is observed in the AV region of juvenile scX−/− mice.
A through D, Immunohisto-chemistry was used to determine expression levels of tenascin-C (Tn-C) (A and B) and periostin (C and D) in scX−/− mice compared to scX+/+ mice at 2 months of age. Tn-C (A and B) and periostin (C and D) expression is significantly increased in valve leaflets from scX−/− mice. B, Note increased expression of Tn-C in ventricular regions of valve leaflet from scX−/− mice (arrowhead, compared to arrow). E and F, TLDA analysis shows significantly increased expression of tn-C and periostin at the transcript level in scX−/− mice compared to scX+/+ mice, as well as mmp3 and timp3. G and H, Trichrome staining to show collagen deposition (blue) in AV annular structures. Arrows indicate the posterior paraseptal annulus structure at the left AV groove. Note increased collagen deposition in scX−/− mice (arrows). mv indicates mitral valve; mmv, mural mitral valve.
Discussion
There are increasing reports to suggest that heart valve disease manifested later in life has origins during embryonic development.31,32 Diseased valves are often characterized by excessive ECM production, matrix disorganization, and altered cell distribution, leading to increased thickness and ultimately malfunctioning valves.9 The genetic etiology underlying these pathological phenotypes are largely unknown, but may be related to defects in signaling pathways important for cell lineage differentiation and matrix deposition programs during embryonic valve remodeling. Scx is expressed in tendon progenitor cells and required for formation, organization, and function of tendon tissue.13 This study and others report additional expression in developing heart valve structures.10,33 The requirements for scx during mouse heart valve development were examined in vivo using mice null for scx. Scx expression is not detected during initial stages of valve development, and EC formation appears normal in scx−/− mice, suggesting that scx is not required at this time. However, by E17.5, valve structures are significantly thickened and show defects in cell lineage differentiation and matrix organization. This is marked by decreased expression of the tendon-associated collagen type XIV, and increased expression of cartilage-associated genes, as well as persistent expression of mesenchyme cell markers. Electron microscopy of postnatal valves reveals disorganized connective tissue architecture, including random orientation of shortened collagen fibers in valves from scx−/− mice. Juvenile scx−/− mice display progressively thickened valve structures with increased expression of fibrosis-associated ECM proteins periostin and tenascin-C, as well as remodeling proteases associated with pathological matrix remodeling. Increased collagen deposition was also observed in annular regions. These data provide evidence for a role for scx during remodeling of heart valve structures in vivo and provide insights into molecular mechanisms required for normal heart valve formation and maintenance.
Scx Is Required for Cell Lineage Differentiation and Matrix Distribution During Heart Valve Remodeling
Previous studies have shown that valve remodeling requires differentiation of several cell types including tendon- and cartilage-like cell lineages from multipotential valve precursor cells, as well as deposition of specific ECM.3,10 This process is tightly regulated to establish and maintain normal valve structure and function.19 In this study, we show that in the absence of the tendon-associated gene scx, cell differentiation and matrix deposition are altered in many ways. Firstly, the contribution of a tendon-like connective tissue to the developing valve structures is attenuated, marked by a dramatic decrease in the tendon-associated collagen type XIV; these findings are consistent with previous tendon studies in scx−/− mice,13 Secondly, valve precursor cells in scx−/− mice appear to be preferentially promoted toward the chondrogenic-like differentiation pathway, indicated by increased expression of sox9 and cartilage-associated structural proteins. Thirdly, it appears that without scx, a population of valve precursor cells remain somewhat undifferentiated and continue to express high levels of mesenchymal cell markers including snai1, msx1, and tbx20. In addition to gross valve defects, the ventricles of scx−/− mice appear spherical in shape, a feature associated with myocardial remodeling in the failing heart.34–37 Because scx expression was not observed in the ventricular myocardium, this structural defect is likely an indirect effect from scx loss of function. Collectively, these studies identify a requirement for scx in cell lineage differentiation and matrix deposition during stages of heart valve remodeling in vivo. In addition, these findings provide insights into potential target genes of scx during valvulogenesis that warrant further investigation.
Scx Is Required for Connective Tissue Organization and Homeostasis
Scx−/− mice show significant defects in ECM organization and distribution. Alterations in the organization of collagen fiber bundles observed in these mutant mice may be attributable to the loss of type XIV collagen, a network-forming collagen that has previously been shown to modulate fibril assembly and organization in several connective tissue systems.38,39 Because changes in cell proliferation or apoptosis were not detected in scx−/− mice at E17.5, it seems likely that increased matrix deposition is the cause of increased valve tissue thickness. This is recognized by an increase in cartilaginous matrix at E17.5, as well as excessive deposition of pathological matrix remodeling proteins in valves from scx−/− mice by juvenile stages. In addition, excessive collagen deposition is observed in the annular structures around the AV junction. These observations at later juvenile stages are likely secondary to the primary effects on cell lineage differentiation and matrix organization resulting from the loss of scx function. Nonetheless, findings from juvenile scx−/− mice support the notion that scx is required for connective tissue homeostasis.
Scx and Heart Valve Disease
Heart valve disease is associated with defects in connective tissue remodeling and homeostasis including ECM disorganization and increased deposition.9 However, the signaling pathways important for normal heart valve remodeling are not clear. Mice null for scx exhibit pathological criteria common to diseased valves from embryonic stages and, by juvenile stages, express high levels of fibrosis-associated genes and matrix proteases previously observed in human valve pathology.28–30,40 Echocardiography revealed subtle, but insignificant, differences in cardiac function between scx−/− and scx+/+ mice (data not shown). However, the premature lethality of null mice prevents examination of degenerative valve disease in adult animals. Therefore, we can only speculate that the observed increase in valve area over time, in association with extensive ECM defects, will have detrimental effects on valve structure and function in older viable animals. Utilization of cell-specific loss-of-function models would be appropriate to gain further insights into the long-term effects of scx knockdown on valve pathology and function, as well as determine additional functional roles for scx during valve development and maturation. In conclusion, this study has identified that scx is required for stages of valvular remodeling and adds to increasing evidence that valve disease associated with alterations in ECM has its origins in valve development.
Supplementary Material
Acknowledgments
We thank Drs Joshua M. Hare and Lina Shehadeh for their scientific expertise; Dr Katherine Yutzey for editorial advice; and Drs David Birk, Stan Hoffman, and Michael Wegner for the kind contribution of antibodies.
Sources of Funding
This work was supported by American Heart Association Grant 0735220N (to J.L.) and the Florida Heart Research Institute (to J.L.).
Footnotes
Reprints: Information about reprints can be found online at http://www.lww.com/reprints
Disclosures
None.
References
- 1.Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, Hailpern SM, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O’Donnell C, Roger V, Sorlie P, Steinberger J, Thom T, Wilson M, Hong Y. Heart disease and stroke statistics–2008 update: a report from the Am Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117:e25–e146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 2.Supino PG, Borer JS, Yin A, Dillingham E, McClymont W. The epidemiology of valvular heart diseases: the problem is growing. Adv Cardiol. 2004;41:9–15. doi: 10.1159/000079779. [DOI] [PubMed] [Google Scholar]
- 3.Lincoln J, Lange AW, Yutzey KE. Hearts and bones: shared regulatory mechanisms in heart valve, cartilage, tendon, and bone development. Dev Biol. 2006;294:292–302. doi: 10.1016/j.ydbio.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 4.Armstrong EJ, Bischoff J. Heart valve development: endothelial cell signaling and differentiation. Circ Res. 2004;95:459–470. doi: 10.1161/01.RES.0000141146.95728.da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Lange FJ, Moorman AF, Anderson RH, Manner J, Soufan AT, de Gier-de Vries C, Schneider MD, Webb S, van den Hoff MJ, Christoffels VM. Lineage and morphogenetic analysis of the cardiac valves. Circ Res. 2004;95:645–654. doi: 10.1161/01.RES.0000141429.13560.cb. [DOI] [PubMed] [Google Scholar]
- 6.Person AD, Klewer SE, Runyan RB. Cell biology of cardiac cushion development. Int Rev Cyt. 2005;243:287–335. doi: 10.1016/S0074-7696(05)43005-3. [DOI] [PubMed] [Google Scholar]
- 7.Lincoln J, Alfieri CM, Yutzey KE. Development of heart valve leaflets and supporting apparatus in chicken and mouse embryos. Dev Dyn. 2004;230:239–250. doi: 10.1002/dvdy.20051. [DOI] [PubMed] [Google Scholar]
- 8.Schroeder JA, Jackson LF, Lee DC, Camenisch TD. Form and function of developing heart valves: coordination by extracellular matrix and growth factor signaling. J Mol Med. 2003;81:392–403. doi: 10.1007/s00109-003-0456-5. [DOI] [PubMed] [Google Scholar]
- 9.Hinton RBJ, Lincoln J, Deutsch GH, Osinaka H, Manning PB, Benson DW, Yutzey KE. Extracellular matrix remodeling and organization in developing and diseased aortic valves. Circ Res. 2006;98:1431–1438. doi: 10.1161/01.RES.0000224114.65109.4e. [DOI] [PubMed] [Google Scholar]
- 10.Lincoln J, Alfieri CM, Yutzey KE. BMP and FGF regulatory pathways control cell lineage diversification of heart valve precursor cells. Dev Biol. 2006;292:292–302. doi: 10.1016/j.ydbio.2005.12.042. [DOI] [PubMed] [Google Scholar]
- 11.Cserjesi P, Brown D, Ligon KL, Lyons GE, Copeland NG, Gilbert DJ, Jenkins NA, Olson EN. Scleraxis: a basic helix-loop-helix protein that prefigures skeletal formation during mouse embryogenesis. Development. 1995;121:1099–1110. doi: 10.1242/dev.121.4.1099. [DOI] [PubMed] [Google Scholar]
- 12.Schweitzer R, Chyung JH, Murtaugh LC, Brent AE, Rosen V, Olson EN, Lassar A, Tabin CJ. Analysis of the tendon cell fate using scleraxis, a specific marker for tendons and ligaments. Development. 2001;128:3855–3866. doi: 10.1242/dev.128.19.3855. [DOI] [PubMed] [Google Scholar]
- 13.Murchison ND, Price BA, Conner DA, Keene DR, Olson EN, Tabin CJ, Schweitzer R. Regulation of tendon differentiation by scleraxis distinguishes force-transmitting tendons from muscle-anchoring tendons. Development. 2007;134:2697–2708. doi: 10.1242/dev.001933. [DOI] [PubMed] [Google Scholar]
- 14.Smith TG, Sweetman D, Patterson M, Keyse SM, Munsterberg A. Feedback interactions between MKP3 and ERK MAP kinase control scleraxis expression and the specification of rib progenitors in the developing chick somite. Development. 2005;132:1305–1314. doi: 10.1242/dev.01699. [DOI] [PubMed] [Google Scholar]
- 15.Brent AE, Tabin CJ. FGF acts directly on the somitic tendon progenitors through the Ets transcription factors Pea3 and Erm to regulate scleraxis expression. Development. 2004;131:3885–3896. doi: 10.1242/dev.01275. [DOI] [PubMed] [Google Scholar]
- 16.Shukunami C, Takimoto A, Oro M, Hiraki Y. Scleraxis positively regulates the expression of tenomodulin, a differentiation marker of tenocytes. Dev Biol. 2006;298:234–247. doi: 10.1016/j.ydbio.2006.06.036. [DOI] [PubMed] [Google Scholar]
- 17.Edom-Vovard F, Schuler B, Bonnin MA, Teillet MA, Duprez D. Fgf4 positively regulates scleraxis and tenascin expression in chick limb tendons. Dev Biol. 2002;247:351–366. doi: 10.1006/dbio.2002.0707. [DOI] [PubMed] [Google Scholar]
- 18.Lejard V, Brideau G, Blais F, Salingcarnboriboon R, Wagner G, Roehrl MH, Noda M, Duprez D, Houillier P, Rossert J. Scleraxis and NFATc regulate the expression of the pro-alpha1(I) collagen gene in tendon fibroblasts. J Biol Chem. 2007;282:17665–17675. doi: 10.1074/jbc.M610113200. [DOI] [PubMed] [Google Scholar]
- 19.Lincoln J, Kist R, Scherer G, Yutzey KE. Sox9 is required for precursor cell expansion and extracellular matrix organization during mouse heart valve development. Dev Biol. 2007;305:120–132. doi: 10.1016/j.ydbio.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Akiyama H, Chaboissier MC, Behringer RR, Rowitch DH, Schedl A, Epstein JA, de Crombrugghe B. Essential role of Sox9 in the pathway that controls formation of cardiac valves and septa. Proc Natl Acad Sci U S A. 2004;101:6502–6507. doi: 10.1073/pnas.0401711101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bi W, Deng JM, Zhang Z, Behringer RR, de Crombrugghe B. Sox9 is required for cartilage formation. Nat Genet. 1999;22:85–89. doi: 10.1038/8792. [DOI] [PubMed] [Google Scholar]
- 22.Pryce BA, Brent AE, Murchison ND, Tabin CJ, Schweitzer R. Generation of transgenic tendon reporters, ScxGFP and ScxAP, using regulatory elements of the scleraxis gene. Dev Dyn. 2007;236:1677–1682. doi: 10.1002/dvdy.21179. [DOI] [PubMed] [Google Scholar]
- 23.Humphries SM, Lu Y, Canty EG, Kadler KE. Active negative control of collagen fibrillogenesis in vivo: intracellular cleavage of the type I procollagen propeptides in tendon fibroblasts without intracellular fibrils. J Biol Chem. 2008;283:12129–12135. doi: 10.1074/jbc.M708198200. [DOI] [PubMed] [Google Scholar]
- 24.Young BB, Gordon MK, Birk DE. Expression of type XIV collagen in developing chicken tendons: association with assembly and growth of collagen fibrils. Dev Dyn. 2000;217:430–439. doi: 10.1002/(SICI)1097-0177(200004)217:4<430::AID-DVDY10>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 25.Shelton EL, Yutzey KE. Tbx20 regulation of endocardial cushion cell proliferation and extracellular matrix gene expression. Dev Biol. 2006;317:282–295. doi: 10.1016/j.ydbio.2006.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang J, Sridurongrit S, Dudas M, Thomas P, Nagy A, Schneider MD, Epstein JA, Kaartinen V. Atrioventricular cushion transformation is mediated by ALK2 in the developing mouse heart. Dev Biol. 2005;286:299–310. doi: 10.1016/j.ydbio.2005.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gitler AD, Lu MM, Jiang YQ, Epstein JA, Gruber PJ. Molecular markers of cardiac endocardial cushion development. Dev Dyn. 2003;228:643–650. doi: 10.1002/dvdy.10418. [DOI] [PubMed] [Google Scholar]
- 28.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. 2003;92:827–839. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 29.Imanaka-Yoshida K, Hiroe M, Yoshida T. Interaction between cell and extracellular matrix in heart disease: multiple roles of tenascin-C in tissue remodeling. Histol Histopathol. 2004;19:517–525. doi: 10.14670/HH-19.517. [DOI] [PubMed] [Google Scholar]
- 30.Takayama G, Arima K, Kanaji T, Toda S, Tanaka H, Shoji S, McKenzie AN, Nagai H, Hotokebuchi T, Izuhara K. Periostin: a novel component of subepithelial fibrosis of bronchial asthma downstream of IL-4 and IL-13 signals. J Allergy Clin Immunol. 2006;118:98–104. doi: 10.1016/j.jaci.2006.02.046. [DOI] [PubMed] [Google Scholar]
- 31.Garg V, Muth AN, Ransom JF, Schluterman MK, Barnes R, King IN, Grossfeld PD, Srivastava D. Mutations in NOTCH1 cause aortic valve disease. Nature. 2005;437:270–274. doi: 10.1038/nature03940. [DOI] [PubMed] [Google Scholar]
- 32.Cripe L, Andelfinger G, Martin LJ, Shooner K, Benson DW. Bicuspid aortic valve is heritable. J Am Coll Cardiol. 2004;44:138–143. doi: 10.1016/j.jacc.2004.03.050. [DOI] [PubMed] [Google Scholar]
- 33.Zhao B, Etter L, Hinton RB, Jr, Benson DW. BMP and FGF regulatory pathways in semilunar valve precursor cells. Dev Dyn. 2007;236:971–980. doi: 10.1002/dvdy.21097. [DOI] [PubMed] [Google Scholar]
- 34.Mann DL, Spinale FG. Activation of matrix metalloproteinases in the failing human heart: breaking the tie that binds. Circulation. 1998;98:1699–1702. doi: 10.1161/01.cir.98.17.1699. [DOI] [PubMed] [Google Scholar]
- 35.Jane-Lise S, Corda S, Chassagne C, Rappaport L. The extracellular matrix and the cytoskeleton in heart hypertrophy and failure. Heart Fail Rev. 2000;5:239–250. doi: 10.1023/A:1009857403356. [DOI] [PubMed] [Google Scholar]
- 36.Diez J, Gonzalez A, Lopez B, Querejeta R. Mechanisms of disease: pathologic structural remodeling is more than adaptive hypertrophy in hypertensive heart disease. Nat Clin Pract Cardiovasc Med. 2005;2:209–216. doi: 10.1038/ncpcardio0158. [DOI] [PubMed] [Google Scholar]
- 37.Miner EC, Miller WL. A look between the cardiomyocytes: the extracellular matrix in heart failure. Mayo Clin Proc. 2006;81:71–76. doi: 10.4065/81.1.71. [DOI] [PubMed] [Google Scholar]
- 38.Young BB, Zhang G, Koch M, Birk DE. The roles of types XII and XIV collagen in fibrillogenesis and matrix assembly in the developing cornea. J Cell Biochem. 2002;87:208–220. doi: 10.1002/jcb.10290. [DOI] [PubMed] [Google Scholar]
- 39.Nishiyama T, McDonough AM, Bruns RR, Burgeson RE. Type XII and XIV collagens mediate interactions between banded collagen fibers in vitro and may modulate extracellular matrix deformability. J Biol Chem. 1994;269:28193–28199. [PubMed] [Google Scholar]
- 40.Rabkin-Aikawa E, Farber M, Aikawa M, Schoen FJ. Dynamic and reversible changes of interstitial cell phenotype during remodeling of cardiac valves. J Heart Valve Dis. 2004;13:841–847. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.