SUMMARY
Microgravity induces stress, and the brain is one of the targets that is more influenced in this environment. Alteration in transcription factors can have enormous effect because of discrepancy in the signaling process of the cells. Activator protein-1 (AP-1) is a stress-regulated transcription factor and is involved in the regulation of physiological and pathological stimuli that include cytokines, growth factors, and stress signals. In the present study, an attempt has been made to observe the effect of a microgravity environment on the activation of AP-1 in the mouse brain. Our results show that AP-1 transcription factor is activated in simulated microgravity conditions in different regions of the brain. The activation of the AP-1 is dependent upon the increased kinase activity of c-Jun NH-terminal2 kinase-1. These results suggest that microgravity stress in the brain can elicit AP-1 activity.
Keywords: simulated microgravity, hindlimb unloading, activator protein-1
INTRODUCTION
Microgravity has a profound effect on the brain that results from both direct and indirect factors. The changes encountered under a microgravity environment result in gradual loss of cerebral circulation in the brain. Effects due to such changes have been shown to evoke alteration in the brain electrical activity, coupled with epileptiform discharges triggered primarily in the hippocampus system of the temporal lobe and further spreading into the other brain systems (Adey, 1964, 1972; Harm et al., 2001). Results from these studies have indicated that changes in the brain under induced microgravity might be a useful tool to investigate neurobiological and behavioral responses to stress. This approach may provide insight into the mechanisms underlying development and plasticity of the nervous system. In addition, localization of atrial-natriuretic factor (ANF)-like immunoreactivity was investigated in the brain and heart of the tree frog, Hyla japonica, by indirect immunofluorescence technique. The results from this study showed that amygdala contained highly stained ANF-immunoreactive cells and fibers compared with ground-based controls (Feuilloley et al., 1993). Such observations reveal that prolonged exposure to microgravity affects biosynthesis, release of ANF-related peptides, or both, in discrete regions of the amphibian brain.
Studies with gene expression profiling in the brains of space-flown rats have indicated changes in immediate early genes (IEGs). The gene expression changes in medullary and basal forebrain, which are thought to play an important role in the integration of autonomic and vestibular signals, may regulate neural adaptations in space flights (Pompeiano et al., 2004). Moreover, studies to trace the cytomorphology of glial cells showed alterations in microfilament and intermediate filaments leading to cytoskeletal damage (Uva et al., 2001). Recently, we have shown that in hindlimb suspension model, generating simulated microgravity in mice, induced oxidative stress in the brain and increased lipid peroxidation in various regions of the brain (Wise et al., 2005).
The changes in the neuronal activity result in vegetative deficits that occur during different space flight conditions. Some of the IEGs, namely c-Fos, are indicators of neuronal activity and plasticity (Pompeiano et al., 2001). In the brain, the activity of neuronal functions has been a concern because it relates to the fluid nature of the gray and white matter of the brain and also because of its enclosure within the skull. The activator protein-1 (AP-1) transcription factor is mainly composed of Jun, Fos, and ATF protein dimmers (Wagner, 2001). It mediates gene regulation in response to a large number of physiological and pathological stimuli, including cytokines, growth factors, stress signals, bacterial and viral infections as well as oncogenic stimuli (Hess et al., 2004). AP-1 induces its activity that controls both basal and inducible transcription factors and is best described in several genes containing the consensus sequence named as AP-1 binding sites (5′-TGAG/CTCA-3′) (Karin et al., 1997). AP-1 is activated under oxidative stress in rat cardiomyocytes, thus allowing the additional role of this transcription factor under redox conditions (Wu et al., 2005). Microgravity environment induces stress that implicates that AP-1 transcription factor may be envisaged to be active in the brain as an immediate response-activated protein (Stein, 2002; Wise et al., 2005). In the present study, we have attempted to look for activated AP-1 in brains exposed to simulated microgravity.
MATERIALS AND METHODS
Exposure of mice to simulated microgravity
In small mammals, the tail suspension model has been widely used as a model to simulate microgravity effects (Woodman et al., 1991, 1993). Studies, predominantly in rodents, have demonstrated that tail suspension has good fidelity to many of the changes that occur in larger mammals during real microgravity exposure. Therefore, in the present study, we have used mice as an experimental model to study the effect of simulated microgravity in the hippocampus by using the tail suspension method that was performed as described by Felix et al. (2004) and Wise et al. (2005). We have previously shown in this model that simulated microgravity induced changes in cytokine and reactive oxygen species-mediated nuclear transcription factor activation in the brain (Felix et al., 2004; Wise et al., 2005).
Six- to 8-wk-old male BALB/c mice were obtained from Harlan (Indianapolis, IN). Mice were randomly divided into two groups (n = 20), control and tail suspended, and left for 7 d with free access to drinking water and food. At the end of the experiment, the brain was dissected out, and different regions of the brain were removed, snap frozen in liquid nitrogen, and transferred to −80°C for further experiments.
Electromobility shift assay for AP-1 activation
To determine the activation of AP-1, 20 µg of protein, extracted from different regions of rat brain as indicated above, was incubated with 20 fmol of the γ32P-end-labeled AP-1 consensus oligonucleotide 5′-CGCTTGACCGGAA-3′ (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) for 15 min at 37° C and analyzed by electro-mobility shift assay (Manna et al., 2005). The specificity of binding was examined by competition with unlabeled oligonucleotide and by supershift of the AP-1 band by antibodies against either c-Jun or c-Fos. Visualization and quantitation of radioactive bands were carried out using a Phosphor-Imager (Bio-Rad, Herculus, CA).
c-Jun kinase assay
The c-Jun kinase assay was performed by a modified method as described previously (Manna and Ramesh, 2005). Briefly, brain extracts from control and microgravity-exposed mice (150–250 µg/sample) were immunoprecipitated with 0.3 µg anti-c-Jun NH2 kinase (JNK) antibody for 60 min at 4° C. Immune complexes were collected by incubation with protein A/G-Sepharose heads for 45 min at 4° C. The beads were extensively washed with lysis buffer (four times; 400 µl) and kinase buffer (two times; 400 µl: 20 mM HEPES, pH 7.4, 1 mM dithiothreitol [DTT], and 25 mM NaC1). Kinase assays were performed for 15 min at 30° C with glutathione S-transferase-Jun (1–79) as a substrate in 20 mM HEPES, pH 7.4, 10 mM MgC12, 1 mM DTT, and 10 µCi of [γ32P] ATP. Reactions were stopped with the addition of 15 µl of 2× sodium dodecyl sulfate [SDS] sample buffer, boiled for 5 min, and subjected to SDS-polyacrylamide gel electrophoresis (PAGE) (9%). GST-Jun (1–79) was visualized by staining with Coomassie Blue, and the dried gel was analyzed in a PhosphorImager (Bio-Rad).
RESULTS
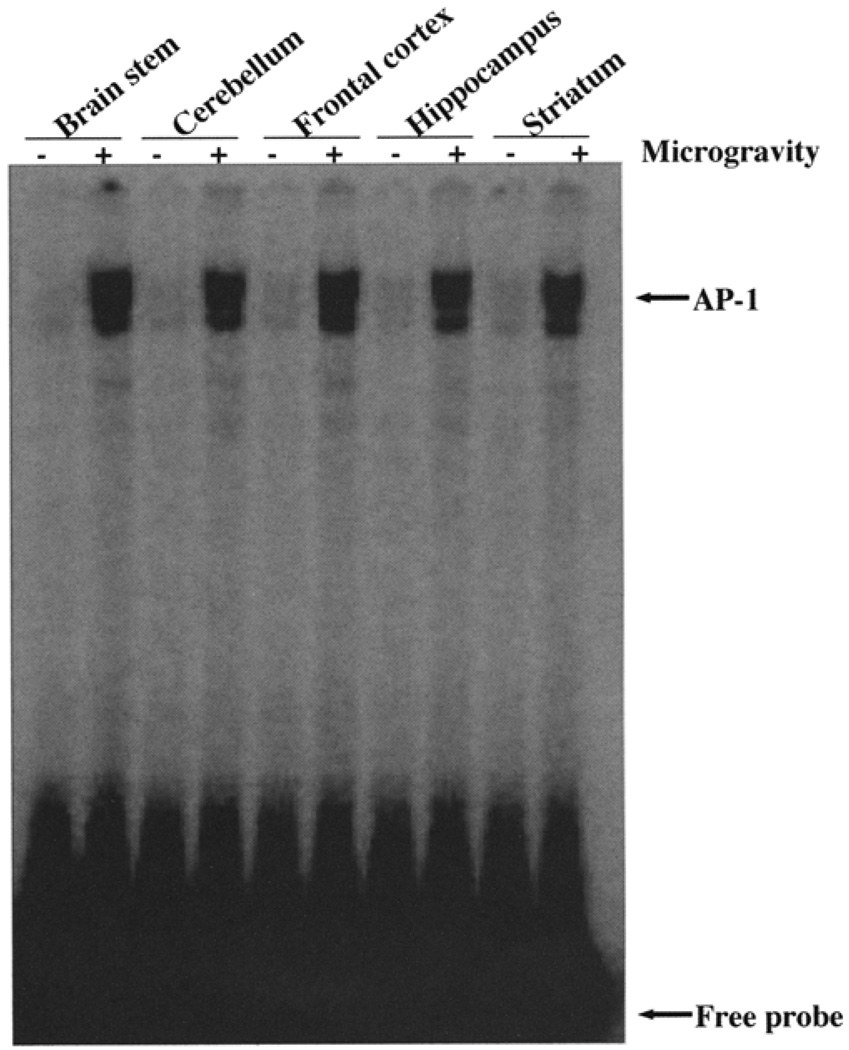
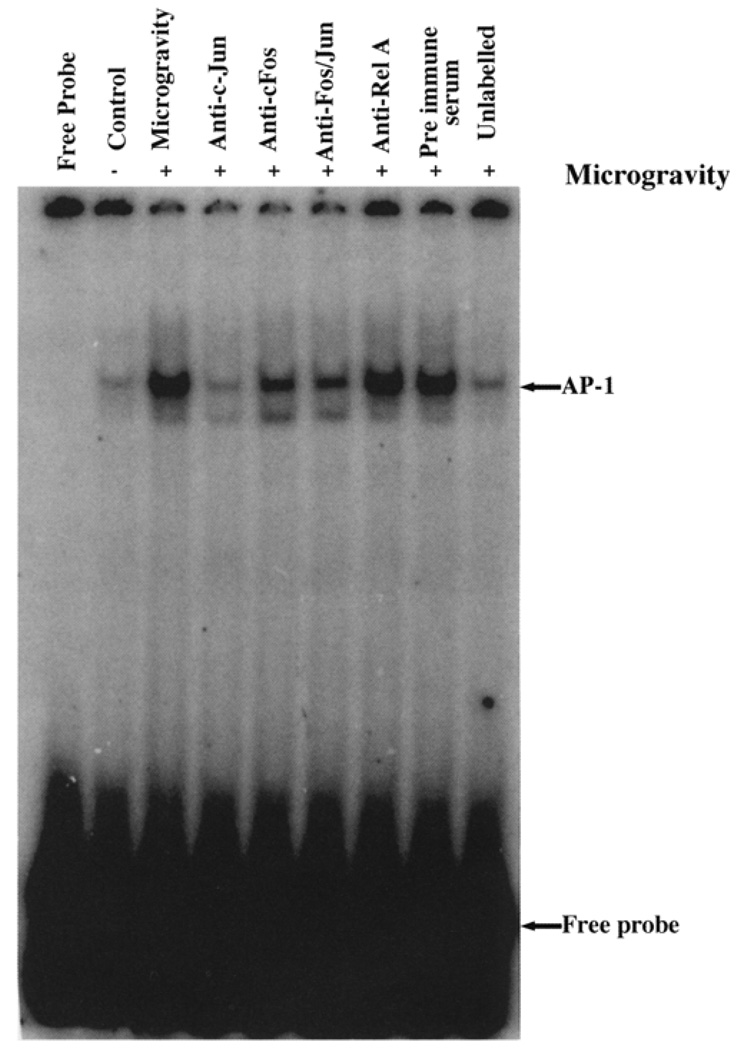
AP-1 activation was observed in all the regions of the brain exposed to microgravity compared with their controls. This finding indicates that microgravity stress induces AP-1 activation in all regions of the brain regardless of the region and function of the varied regions in the brain (Fig. 1). Strong AP-1 binding occurred in the brainstem, cerebellum, frontal cortex, hippocampus, and striatum. The binding of the AP-1 complex to its cognate sequence did not show any difference across all the regions of the brain under simulated microgravity. AP-1 is a complex of c-Jun and c-Fos, and this heterologous complex forms the active AP-1, which then acts as a transcription factor (Wagner, 2001). To validate the specificity of the binding and to identify the proteins in the complex, we have performed supershift assays with antibody to c-Jun and c-Fos. Figure 2 shows that in the presence of c-Jun and c-Fos antibodies, the intensity of the band binding to specific oligonucleotides carrying the AP-1 binding site was significantly down-regulated, suggesting that the binding complex consisted of c-Jun and c-Fos. The decrease in the intensity of the bands signifies that the antibodies were directed to the DNA binding site of the proteins. Under such conditions, it is expected that a masking effect could result in decreased binding of the proteins to the oligonucleotide. Moreover, addition of anti-Rel A and preimmune serum did not inhibit the binding of the AP-1 complex to the specific oligonucleotide. Finally, to show the specificity of the binding, we incubated the labeled oligonucleotide in the presence of 1000-fold excess of unlabeled oligonucleotide to compete out the binding of the labeled oligonucleotide. Indeed, the binding was abolished due to the competition and thus validates the specificity of the assay. Together, these observations suggest that microgravity induces stress, which results in activation of AP-1 in all the regions of the brain.
FIG. 1.
Simulated microgravity activates AP-1 in different regions of the brain. Nuclear extracts were prepared from different regions of the brain and incubated with γ32P AP-1 oligonucleotide and resolved in 6% polyacrylamide gel. The gel was dried, and bound complex was detected by exposing the gel to a PhosphorImager.
FIG. 2.
Supershift assay showing c-Jun and c-Fos as active constituents of the AP-1 complex in nuclear extract obtained from frontal cortex. Nuclear extract was incubated in presence or absence of antibodies as indicated. To validate the specificity of the assay, the binding reaction was performed in presence of nonspecific antibody Rel A, preimmune serum, and 1000-fold excess of unlabeled oligonucleotide. The gel was dried, and bound complex was detected by exposing the gel to a PhosphorImager.
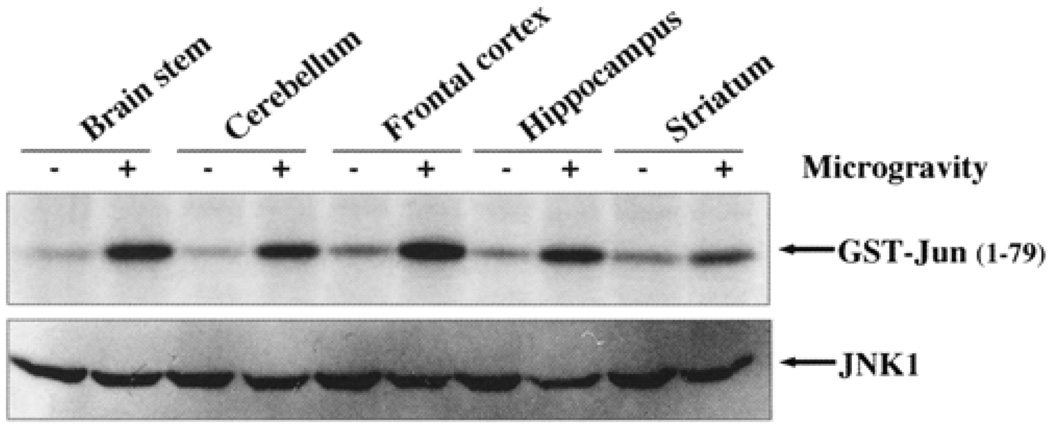
JNK phosphorylates c-Jun to form an active dimer with Fos for transcriptional activity (Wagner, 2001). Therefore, it is important to observe the active phosphorylated state of the protein for AP-1 to act as an active transcription factor (Wagner, 2001). We have therefore assayed the Jun phosphorylation by JNK-1 in brain extracts from different regions of the brain. As shown in Fig. 3, all the regions showed phosphorylation of Jun by JNK-1. The total JNK-1 remains unchanged in the control and microgravity-exposed brain regions.
FIG. 3.
Simulated microgravity activates JNK-1 kinase activity in different regions of the brain. JNK-1 was immunoprecipitated from different regions of the brain tissue extracts, and then kinase assay was performed in presence of [γ32P] ATP and GST-Jun (1–79) substrate. The reaction was terminated by adding 2×SDS sample buffer, boiled for 5 min, and subjected to SDS-PAGE (9%). The gel was dried, and c-Jun phosphorylation was detected by exposing the gel to PhosphorImager.
DISCUSSION
We have shown that microgravity can activate AP-1 in different regions of the brain in a terrestrial model of simulated microgravity. Microgravity can induce oxidative stress, and such stress could transduce multiple signals leading to different responses, depending on the strength of the stress (Stein, 2002). We have reported alteration in the cytokine levels under simulated microgravity conditions in lymph nodes and serum (Felix et al., 2005). It is also observed that AP-1 is regulated by a broad range of physiological and pathological stimuli, including cytokines, growth factors, stress signals, and infections as well as oncogenic stimuli (Hess et al., 2004). It therefore seems likely that microgravity-induced stress is responsible for activating AP-1 in different regions of the brain. Because we did not find any marked difference in the binding of AP-1 to its cognate sequence across different regions of the brain, it could be speculated that a common signaling event is turned on by exposure to microgravity. AP-1 transactivation has been reported under microgravity conditions in differentiating osteoblasts, thus suggesting that AP-1 signaling cascade is influenced by microgravity (Granet et al., 2002).
By using supershift assay, it was confirmed that the active AP-1 is comprised of c-Jun and c-Fos. We performed the supershift assays in nuclear extracts from the frontal cortex, and the results showed the presence of c-Jun and c-Fos as the active AP-1 complex.
We have previously reported the activation of mitogen-activated protein kinase (MAPK) in the brain, which is the upstream regulator for JNK-1 activation, and JNK-1 then can phosphorylate c-Jun, which leads to active Jun (Wise et al., 2005). c-Jun is phosphorylated at two residues proximal to the major transactivation domain. These residues (Ser 63 and Set 73) are required to be phosphorylated for efficient transactivation function (Franklin et al., 1992). The kinases responsible for this modification in vivo are JNKs (Karin et al., 1997; Wagner, 2001). These kinases bind with very high affinity to a region in c-Jun termed the delta domain. Activation of JNK is therefore necessary to phosphorylate c-Jun. Our results show JNK-1 kinase activity was increased in all the regions of the brain, which in turn led to the formation of active AP-1. Based on our previous report and reports from others, we speculate MAPK should be the active signaling molecule that transduces signals to activate JNK-1 (Hess at al., 2004, Manna et al., 2005; Wise et al., 2005). In conclusion, it may be possible to state that the MAPK–JNK–AP-1 signaling cascade may be activated in the different regions of the brain in a terrestrial model for microgravity.
ACKNOWLEDGMENTS
This work was supported by NASA funding NCC 9-165, NCC-1-02038, and NAG 9-1414, and NIH/RCMI RR03045-19 (to G. R.).
REFERENCES
- Adey WR. Effects of gravity on the functions of the central nervous system. Life Sci. Space Res. 1964;2:267–286. [PubMed] [Google Scholar]
- Adey WR. Studies on weightlessness in a primate in the Biosatellite 3 experiment. Life Sci. Space Res. 1972;10:67–85. [PubMed] [Google Scholar]
- Felix K, Wise K, Manna S, Yamauchi K, Wilson BL, Thomas RL, Kulkarni A, Pellis NR, Ramesh GT. Altered cytokine expression in tissues of mice subjected to simulated microgravity. Mol. Cell. Biochem. 2004;266:79–85. doi: 10.1023/b:mcbi.0000049136.55611.dd. [DOI] [PubMed] [Google Scholar]
- Feuilloley M, Yon L, Kawamura K, Kikuyama S, Gutkowska J, Vaudry H. Immunocytochemical localization of atrial natriuretic factor (ANF)-like peptides in the brain and heart of the treefrog Hyla japonica: effect of weightlessness on the distribution of immunoreactive neurons and cardiocytes. J. Comp. Neurol. 1993;33:32–47. doi: 10.1002/cne.903300104. [DOI] [PubMed] [Google Scholar]
- Franklin CC, Sanchez V, Wagner F, Woodgett JR, Kraft AS. Phorbol ester-induced amino terminal phosphorylation of c-Jun but not JunB regulates transcriptional activation. Proc. Natl. Acad. Sci. USA. 1992;89:7247–7251. doi: 10.1073/pnas.89.15.7247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granet C, Vico AG, Alexandre C, Lafage-Proust MH. MAP and src kinases control the induction of AP-1 members in response to changes in mechanical environment in osteoblastic cells. Cell Signal. 2002;14:679–688. doi: 10.1016/s0898-6568(02)00008-6. [DOI] [PubMed] [Google Scholar]
- Harm DL, Jennings RT, Meck JV, et al. Invited review: gender issues related to spaceflight: a NASA perspective. J. Appl. Physiol. 2001;91:2374–2383. doi: 10.1152/jappl.2001.91.5.2374. [DOI] [PubMed] [Google Scholar]
- Hess J, Angel P, Schorpp-Kistner M. AP-1 subunits: quarrel and harmony among siblings. J. Cell Sci. 2004;117:5965–5973. doi: 10.1242/jcs.01589. [DOI] [PubMed] [Google Scholar]
- Karin M, Liu Z, Zandi E. AP-1 function and regulation. Curr. Opin. Cell Biol. 1997;2:240–246. doi: 10.1016/s0955-0674(97)80068-3. [DOI] [PubMed] [Google Scholar]
- Manna SK, Ramesh GT. Interleukin-8 induces nuclear transcription factor-kappaB through a TRAF6-dependent pathway. J. Biol. Chem. 2005;280:7010–7021. doi: 10.1074/jbc.M410994200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manna SK, Sarkar S, Barr J, Wise K, Barrera EV, Jejelowo O, Rice-Ficht AC, Ramesh GT. Single-walled carbon nanotube induces oxidative stress and activates nuclear transcription factor-kappaB in human keratinocytes. Nano Lett. 2005;5:1676–1684. doi: 10.1021/nl0507966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pompeiano O, d’Ascanio P, Balaban E, Centini C, Pompeiano M. Gene expression in autonomic areas of the medulla and the central nucleus of the amygdala in rats during and after space flight. Neuroscience. 2004;124:53–69. doi: 10.1016/j.neuroscience.2003.09.027. [DOI] [PubMed] [Google Scholar]
- Pompeiano O, d’Ascanio P, Centini C, Pompeiano M, Cirelli C, Tononi G. Immediate early gene expression in the vestibular nuclei and related vegetative areas in rats during space flight. Acta Otolaryngol. Suppl. 2001;545:120–126. doi: 10.1080/000164801750388289. [DOI] [PubMed] [Google Scholar]
- Stein TP. Space flight and oxidative stress. Nutrition. 2002;10:867–8671. doi: 10.1016/s0899-9007(02)00938-3. [DOI] [PubMed] [Google Scholar]
- Uva BM, Masini MA, Sturla M, Prato P, Passalacqua M, Giuliani M, Strollo F, Tagliafierro G. Simulated microgravity induces alteration in the central nervous system. J. Gravit. Physiol. 2001;8:93–95. [PubMed] [Google Scholar]
- Wagner EF. AP-1 —Introductory remarks. Oncogene. 2001;20:2334–2335. doi: 10.1038/sj.onc.1204416. [DOI] [PubMed] [Google Scholar]
- Wise KC, Manna SK, Yamauchi K. Activation of nuclear transcription factor-kappaB in mouse brain induced by a simulated microgravity environment. In Vitro Cell Dev. Biol. 2005;41A:118–123. doi: 10.1290/0501006.1. [DOI] [PubMed] [Google Scholar]
- Woodman CR, Stump CS, Stump JA, Sebastian LA, Rahman Z, Tipton CM. Influences of chemical sympathectomy and simulated weightlessness on male and female rats. J. Appl. Physiol. 1991;71:1005–1014. doi: 10.1152/jappl.1991.71.3.1005. [DOI] [PubMed] [Google Scholar]
- Woodman CR, Tipton CM, Evans J, Linderman JK, Gosselink K, Grindeland RE. Metabolic responses to head-down suspension in hypophysectomized rats. J. Appl. Physiol. 1993;75:2718–2726. doi: 10.1152/jappl.1993.75.6.2718. [DOI] [PubMed] [Google Scholar]
- Wu S, Gao J, Ohlemeyer C, Roos D, Niessen H, Kottgen E, Gebetaner R. Activation of AP-1 through reactive oxygen species by angiotensin II in rat cardiomyocytes. Free Radic. Biol. Med. 2005;39:1601–1610. doi: 10.1016/j.freeradbiomed.2005.08.006. [DOI] [PubMed] [Google Scholar]